Abstract
Electronic cigarettes (e-cigarettes) are becoming increasingly popular worldwide and their cellular effects warrant further evaluation. In this study, we investigated the effects of an e-cigarette cartridge solution on allergen related asthmatic airway inflammation (AI) and airway hyperresponsiveness (AHR), when it is delivered by intratracheal route in mice. Asthmatic AI and AHR were induced by systemic sensitization to ovalbumin (OVA) followed by intratracheal, intraperitoneal, and aerosol allergen challenges in BALB/c mice. The cartridge solution of e-cigarette (containing 16 mg/ml nicotine) was diluted 50 times and 100 μl of the diluted solution was intratracheally instilled to OVA-sensitized (OVA-S) mice two times a week for 10 weeks. Long-term e-cigarette inhalation elicited no remarkable changes in the activities of alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase enzymes in serum, however, increased infiltration of inflammatory cells including eosinophils, into airways from blood, aggravated the asthmatic AI and AHR, and stimulated the production of cytokines such as interleukin (IL)-4, IL-5 and IL-13, and OVA-specific IgE production. Our data suggest that the inhalation of e-cigarette solutions can function as an important factor to exacerbate the allergy-induced asthma symptoms. Further studies are needed to address the effects of e-cigarette solutions on human health.
Keywords: Electronic cigarettes, Airway inflammation, Eosinophils, IgE, Cytokine, Airway hyperresponsiveness
INTRODUCTION
Recently, the use of electronic cigarettes (e-cigarettes), for smoking cessation, as an alternative to smoking, or for nicotine replacement has rapidly increased in Korea. E-cigarettes look like cigarettes, but do not contain tobacco leaf and do not require combustion and oxidation reactions. They consist of three essential components-: a cartridge that serves as a mouthpiece and as a liquid reservoir, an atomizer that serves as the heating element, and a battery that serves as the power supply. Some e-cigarettes also contain a light-emitting diode at the tip that glows when the user puffs, to resemble the burning end of the cigarette. The liquid reservoir of a cartridge contains diverse chemical additives and flavors such as tobacco, chocolate, coffee, mint, or fruit, and it may contain nicotine -or may be nicotine-free, in addition to containing some humectants (1). When the user puffs, the e-cigarette is activated, the heating element vaporizes the liquid, and the steam is absorbed orally (2,3).
The e-cigarette concept first appeared in a patent acquired by Herbert A. Gilbert in 1963. The modern e-cigarette was invented by the Chinese pharmacist Hon Lik in 2003 and introduced into the market the following year. The export of e-cigarettes started in 2005~2006, and the first international patent was obtained in 2007 (4). In Korea, e-cigarettes were imported from China at the beginning of 2007 and were sold through various online distributors. However, in November 2008, the Ministry for Strategy and Finance made an authoritative interpretation that regarded e-cigarettes as a type of cigarette, and therefore only allowed marketing of e-cigarettes within the existing regulatory framework for tobacco. After that judgment, the marketing of e-cigarettes through the Internet was banned (5).
To date, few studies on the analysis and user surveys of ecigarettes have been published (6-10). The Food and Drug Administration (FDA) of United States reported that the nicotine-labeled cartridge in e-cigarettes contains toxic nitrosamines and diethylene glycol, as well as harmful alkaloids such as anabasine and myosmine (7,10). The FDA also detected low levels of nicotine in cartridges that were labeled as “nicotine-free” (7). Some manufacturers do not disclose the ingredients in their products (10). Laugesen et al. (2008) reported that the mist puffed from the Ruyan ecigarette contains acetaldehyde and mercury (6). Results from recent surveys, indicate that e-cigarettes have been used mainly to quit smoking, but several respondents were concerned about their potential toxicity (8,10). The surveys also indicate that e-cigarettes may be effective in helping smokers quit (11). E-cigarettes were also used to replace nicotine medications to avoid relapse of smoking by former smokers or as an aid to reduce the frequency or to cease smoking (12). In clinical studies, e-cigarettes appear to attenuate the craving for smoking, despite delivering very little nicotine to the blood (13,14).
E-cigarettes are of great interest worldwide owing to their efficacy in assisting with smoking cessation and reduction; however, their popularity may also pose significant challenges and thorough evaluation of their safety is warranted, with regard to their effects on human health, particularly for addressing the concerns of regulatory authorities. Little is known about the health effects of e-cigarettes, and it is of the utmost importance to evaluate the effects of nicotinecontaining and nicotine-free e-cigarettes on the respiratory system. In a previous study, we investigated the effect of ecigarette on airway inflammation (AI) and airway hyperresponsiveness (AHR) in mice. We found that long-term intratracheal instillation of the cartridge diluted solution of e-cigarettes in mice tended to increase on AI and AHR, however, the changes observed were not statistically significant( data not shown). Therefore, the aim of this study was to investigate the effects of nicotine-containing e-cigarettes on allergen-related asthmatic AI and AHR in mice. Using a murine model of asthma, we evaluated the changes in serum enzymes, accumulation of airway inflammatory cells, AHR, and Th2 cytokine production upon intratracheal instillation of e-cigarettes.
MATERIALS AND METHODS
Animals and breeding conditions. Five-week-old female BALB/c mice were obtained from DaehanBiolink Co. LTD. (Eumsung, Korea). The study protocols were approved by the Committee for Animal Welfare at Daejeon University. All animal procedures were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee of the Korea Research Institute of Bioscience and Biotechnology (Daejeon, Korea). The mice were divided into the following groups: the Normal group (n = 8) given drinking water (N); Ovalbumin (OVA)-sensitized group (n = 8), given OVA (OVA-S), and OVA sensitized e-cigarette (EC) treated group (n = 8), given OVA and EC (OVAS + EC) groups. The breeding conditions were as follows : 20℃ ± 2℃ temperature and 40~60% humidity, illumination at 200~300 lux for 12 hr per day, and ventilation for 12~15 times per hour. The mice had access to food and water ad libitum.
OVA sensitization and E-cigarette treatment. As per a modification of a previously described protocol (15,16). OVA (500 μg ml-1) in PBS was mixed with an equal volumes of 10% (w/v) aluminum potassium sulfate (alum; Sigma) in distilled water, incubated for 60 min at room temperature after adjustment to pH 6.5 with 10 N NaOH, and centrifuged at 750 ×g for 5 min. The OVA/alum pellet was resuspended to the original volume in distilled water. All mice were immunized on three different days (first day of week 2, 3, and 4 before inhalation exposure) by intraperitoneal (i.p) injections of 0.2 ml alum-precipitated antigen containing 100 μg of OVA (Sigma-Aldrich, Korea) bound to 4 mg of aluminum hydroxide (Sigma-Aldrich, Korea) in PBS. Seven days after the second sensitization with an intratracheal injection of 250 μg of OVA (on day 21) on the back of the tongue, mice were exposed to aerosolized OVA for 30 min/day, 3 days/week for 10 weeks (at a flow rate of 250 L/min, 1% OVA in normal saline for first 9 weeks followed by 2% OVA in normal saline for the last week). The cartridge solution of e-cigarette (containing 16 mg/ml nicotine) was purchased in Korea (Z-company, Korea) and diluted 50 times with 0.9% saline solution. One hundred microliters of the diluted solution was intratracheally administered to mice two times a week for 10 weeks. This concentration as of nicotine corresponds to 1.28 mg of nicotine/kg body weight. One day after the last OVA exposure (2% OVA inhalation), the extent of AHR was determined, and bronchoalveolar lavage fluid (BALF), lung cell, and serum samples were collected for further molecular analyses.
Assessment of AHR. AHR in mice was estimated using a previously described method with some modifications (17,18). A Buxco system (Biosystem XA; Buxco Electronics Inc., Troy, Conn) was used to evaluate the extent of airway constriction in different groups of mice following the previously described protocol. The enhanced pause, Penh, is defined by the formula: Pause × PEF/PIF, where Pause = (Te - eTr)/Tr; (PIF, is the peak inspiratory flow; PEF: is the peak expiratory flow; Te, is the expiratory time; and Tr, is the relaxation time). In this experiment, mice were aerosolized with OVA for 30 min/day, 3 days/week for 10 weeks. Twenty-four hours after the final inhalation, mice were given aerosolized normal saline, followed by 3.15, 6.25, 12.5 and 25 50 mg ml-1 of methacholine in successive steps. Airway reactivity was then monitored for 30 min. Differences in the Penh value between groups were evaluated using the unpaired Student’s t-test.
Blood chemistry and analysis of BALF. Immediately following the assessment of AHR, mice were sacrificed with an i.p injection of sodium pentobarbitone (100 mg kg-1). Blood was collected by cardiac puncture, and the serum was separated by centrifugation. Serum was divided into small vials and stored at -70℃ until further analysis. Levels of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and lactate dehydrogenase (LDH) were measured using a commercially available kit (A-san Pharmaceutical, Korea). The trachea was cannulated and BAL samples were obtained by washing the airway lumina. Briefly, cells in the lungs were recovered by flushing 1 ml of BALF [1 mM EDTA, and 10% fetal bovine serum (FBS) in PBS] into the lungs via the trachea. Total cell counts were determined, and 100 μl of the BALF was processed onto glass slides by using a Cytospin centrifuge (400 g x for 4 min; Cellspin, Hanil, Korea). Differential cell counts were obtained after staining with a Diff-Quik Stain Set (Baxter Healthcare Corp., Miami, FL, USA). The BALF supernatant was stored at -25℃ for determination of cytokine levels.
Digestion of pulmonary tissue and cells preparations. Single cell suspensions from lung tissues and BALF were isolated by mechanical disruption in RPMI 1640 medium supplemented with 2 mM L-glutamine, 100 U/ml penicillin, 100 μg ml-1 streptomycin, 50 μM 2-mercaptoethanol, 20 mM HEPES, and 2% heat-inactivated FBS, (GIBCO, Grand Island, NY). Briefly, the lungs were removed from the thoracic cavity. After mincing using sterile scalpels, the tissue was incubated in pre-RPMI 1640 medium (pH 7.4) containing 1 mg ml-1 collagenase IV. The incubation was repeated four times at 37℃ for 25 min. After incubation, lung tissue was vigorously pipetted up and down to further dissolve remaining tissue clumps and then filtered using a 70 μm cells trainer (Falcon, Le Pont de Claix, France). The total number of cells was counted manually using a hemocytometer chamber (Fisher). Approximately 2 to 4 × 103 cells were spun onto glass slides (in a Cytospin centrifuge). Differential counts were obtained according to standard morphologic criteria.
Enzyme-linked immunosorbent assay (ELISA). Production of interleukins (ILs), including IFN-γ, IL-4, IL-5, and IL-13, in BALF and production of anti-OVA IgE in the sera of the mice (n = 5) was measured by ELISA according to the manufacturer’s instructions with a monoclonal antibodybased mouse IL ELISA kit (R&D system).
Statistical analysis. Data were analyzed by one-way ANOVA or unpaired Student’s t-test followed by Dunnett’s multiple comparison test (using SPSS version 14.0 statistical software). Differences were considered statistically significant if P-values were < 0.05 and < 0.1 by using ANOVA and the unpaired Student’s t-test, respectively The difference between the N and OVA-S groups was clearly distinguishable, and therefore, statistical significance if comparisons between the N and OVA-S groups has not been indicated. The differences between the OVA-S and OVA-S + EC groups, are emphasized in the discussions of the data.
RESULTS
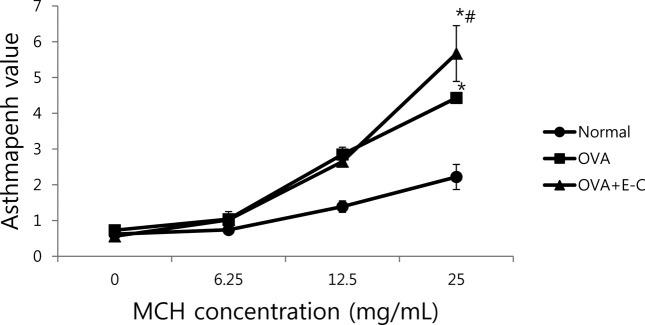
Effects of e-cigarettes on AHR. To evaluate the effect of e-cigarettes on AHR, we estimated total pulmonary airflow by using whole-body plethysmography, and we measured the Penh value on day 1 after final inhalation. Dosedependent methacholine treatment in mice is useful to evaluate the degree of AHR induction, as indicated by the Penh value. In OVA-S mice with or without intratracheal instillation of cartridge liquid solution of e-cigarette for 10 weeks, the Penh value with methacholine concentration greatly increased in a dose-dependent manner, compared that of normal mice (Fig. 1). The Penh value upon treatment with 12.5 mg ml-1 of methacholine was not significantly different between the OVA-S and OVA-S + E-C groups; however, the Penh value with 25.0 mg/ml of methacholine in the OVA-S + E-C group was significantly higher than that in the OVA-S mice (p<0.05) (Fig. 1).
Fig. 1. Effects of e-cigarettes on airway hyper-responsiveness. N, normal group; OVA-S, OVA-sensitized group; OVA-S + E-C, group sensitized with OVA and instilled with nicotine solution from e-cigarettes; Penh, enhanced pause value in asthma assessment; MCH, methacholine. *p< 0.01 compared to the N group and #p < 0.05 compared to the OVA-S group, as assessed by, respectively.
Effects of e-cigarette on serum enzyme activities. The changes in ALT, AST, and LDH activities in serum after intratracheal instillation of cartridge liquid solution of e-cigarette to OVA-S mice for 10 weeks are listed in Table 1. There was no change in the activity of ALT, which is one of the indices of liver damage. However, AST activity, another liver damage index, in OVA-S + E-C group was increased compared to that in the OVA-S group (p<0.01), and LDH activity, which is an index of liver damage and inflammation, exhibited a tendency to increase without becoming statistically significant.
Table 1.
Effects of e-cigarette on serum enzyme activities
| Group | ALT (U/L) | AST (U/L) | LDH (U/L) |
|---|---|---|---|
|
| |||
| N | 26.0 ± 1.7 | 52.6 ± 4.1 | 205.0 ± 16.7 |
| OVA-S | 30.2 ± 1.7 | 81.5 ± 9.3* | 225.0 ± 34.3 |
| OVA-S + E-C | 27.8 ± 1.6 | 108.3 ± 18.5* | 240.0 ± 39.2 |
N. normal group: OVA-S. OVA-sensitized group: OVA-S + E-C. group sensitized with OVA and instilled with nicotine solution from e-cigarettes. *: p < 0.01compared to the N group.
Effects of e-cigarettes on airway influx of inflammatory cells. The changes in airway eosinophil accumulation and influx of inflammatory cells into lung and BALF in OVA-S mice with or without intratracheal instillation of cartridge nicotine liquid solution of e-cigarette for 10 weeks, are listed in Table 2. The number of total leukocytes in the BALF obtained from OVA-S + E-C group was significantly higher than that in the BALF from the OVA-S group (p<0.01) (Table 2). Moreover, the eosinophil numbers in total leukocytes in the BALF and the total lung cells from the OVA-S + EC group ware also higher than that from the OVA-S group (p<0.01) (Table 2).
Table 2.
Effects of e-cigarettes on airway eosinophil accumulation, and influx of inflammatory cells into lung and BALF
| Group | Total lung cells (× 106) | Total BALF cells (× 104) | Eosinophils in BALF (× 400) |
|---|---|---|---|
|
| |||
| Nl | 1.17 ± 0.09 | 12.5 ± 2.5 | 5.25 ± 1.11 |
| OVA-S | 2.70 ± 0.06* | 39.5 ± 4.4* | 64.25 ± 7.76* |
| OVA-S + E-C | 4.26 ± 0.36*# | 52.5 ± 6.2*# | 103.00 ± 23.90*# |
N. normal group: OVA-S. OVA-sensitized group: OVA-S + E-C. group sensitized with ova and instilled with nicotine solution from e-cigarettes: BALF, bronchoalveolar lavage fluid. *p<0.01 compared to the N group and #p < 0.01 compared to the OVA-S group.
Effects of e-cigarettes on Th2 cytokines and OVA-specific Ig-E production. The changes in Th2 cytokines levels in BALF and OVA-specific Ig-E production in the sera of OVA-S mice with or without intratracheal instillation of cartridge liquid nicotine solution of e-cigarette for 10 weeks are shown in Table 3. OVA-specific Ig-E level in the serum, and the levels of all Th2 cytokine in BALF, but not IFN-γ levels, were significantly higher in the OVA-S than in the N group (p < 0.01). The production of OVA specific Ig-E, an important component of allergic asthma, from the OVA-S + EC group also significantly increased compared to that in the OVA-S group. Of the Th2 cytokines, IL-13 and IL-4 levels from the OVA-S + EC group were also higher than that in the OVA-S group (p < 0.01), However, although IL- 5 levels in the OVA-S + EC group showed an elevated trend compared with to the OVA-S group, the increase was not significant. IFN-γ levels in the OVA-S + EC group were lower than those in the OVA-S group but the differences were not statistically significant.
Table 3.
Effects of e-cigarettes on Th2 cytokines and OVA-specific Ig-E production in BALF and serum
| Group | OVA-S IgE (U/ml) | IFN-γ(pg/ml) | IL-13 (pg/ml) | IL-4 (pg/ml) | IL-5 (pg/ml) |
|---|---|---|---|---|---|
|
| |||||
| N | 144.9 ± 12.5 | 40.1 ± 16.4 | 3.3 ± 2.2 | 32.1 ± 2.0 | 0.45 ± 0.01 |
| OVA-S | 604.0 ± 62.7* | 57.9 ± 16.9 | 18.1 ± 2.0* | 77.9 ± 16.2* | 9.95 ± 3.44* |
| OVA-S + E-C | 1148.3 ± 1.3*# | 32.6 ± 11.0 | 28.4 ± 4.3*# | 155.7 ± 3.4*# | 18.86 ± 5.06*# |
N, normal group; OVA-S, OVA-sensitized group; OVA-S + E-C, group sensitized with OVA and instilled with nicotine solution from e-cigarettes;BALF, bronchoalveolar lavage fluid; IFN-γ, interferon-γ; IL, interleukin. *p < 0.01 compared to the N group and #p<0.01 compared to the OVA-S group.
DISCUSSION
Bronchial asthma is characterized by airway obstruction, which is variable and reversible. In addition, chronic inflammation of the respiratory tract can occur, which is mediated by the increased expression of multiple inflammatory proteins, including cytokines, chemokines, adhesion molecules, inflammatory enzymes, and receptors (19). In our previous studies, we demonstrated through histological analysis and profiles of BALF and lung cells that an allergen, OVA, induced pulmonary inflammation characterized by increased accumulation of lymphocytes and eosinophil around the airways (15,16). In addition, OVA also induced AHR to methacholine treatment (15,16). In the present study, we investigated whether the cartridge solution of e-cigarettes has an effect on OVA -induced AI and AHR because the safety of e-cigarettes remains controversial.
In the preliminary study, we determined the nicotine concentration in cartridge solutions of e-cigarettes and established the appropriate protocol for intratracheal instillation concentration of the cartridge solution to mice. Our preliminary analyses indicated that nicotine concentrations in cartridge solutions made by the Z-company in Korea were 16.64 ± 0.37 mg ml-1. At the beginning, we diluted the cartridge solution 10 times with 0.9% saline solution, and then, administered 100 μl of this solution intratracheally to eight mice. However, all of the mice died within 2 days. In our second attempt, we diluted the catridge sloution 20 times and instilled 100 μl intratracheally to eight mice. Four mice of the eight mice died within 7 days. In our last attempt, we diluted the cartridge solution 50 times and instilled 100 μl intratracheally to eight mice. None of the mice died, therefore, we determined that a 50-fold dilution of the cartridge solution was appropriate for conducting further studies. In reference, LD50 of pure nicotine solution in mice was 0.3 mg/kg i.v., 9.5 mg/kg i.p. and 230 mg/kg orally (20). The actual amount of nicotine administered to the mice according to the measured nicotine concentration corresponds to 1.28 mg of nicotine/kg of body weights. This dose of the e-cigarette solution given to mice is almost the same that consumed by human smokers weighing 60 kg body who smoke the equivalent of 18 cigarettes of standard reference cigarette (CORESTA Approved Monitor Cigarette No. 7; tar: 13.34 mg/cig, nicotine 1.236 mg/cig) a day.
Asthmatic individuals typically experience exaggerated decrements in their ability to breathe after receiving standard doses of a smooth muscle agonist, a phenomenon known as AHR (21). Breathing difficulties are caused by excessive narrowing of the pulmonary airways, which is instigated by shortening of the airway smooth muscle (22). To document and quantitate AHR, methacholine and histamine bronchoprovocation challenges have been used widely (23). In this study, we hypothesized that cartridge-derived nicotine solution combined with OVA sensitization might exacerbate AHR in response to methacholine treatment. Our results support this hypothesis. To our knowledge, this is the first empirical estimation of the enhancement of allergic asthma by e-cigarettes. Bronchial asthma produces immune abnormalities in a wide variety of cells. Eosinophils preferentially accumulate at sites of allergic inflammation and are believed to play important roles in the pathophysiology of asthma by releasing a variety of inflammatory mediators, including platelet-activating factor, oxygen radicals, leukotrienes, and major basic protein (24). In the present study, the numbers of total cells in BALF and lung were significantly increased by combination treatment with cartridge nicotine solution and OVA sensitization. Airway eosinophil accumulation was also dramatically elevated by the combined treatment.
The preferential recruitment of large numbers of eosinophils is regulated in various ways by Th2 cytokines (25). The Th2 cytokines IL-4, IL-5, and IL-13, which are responsible for allergic inflammation, are produced not only by Th2 lymphocytes but also by other effector cells such as eosinophil and mast cells and by structural cells such as epithelial cells and fibroblasts (26). Others cytokines such as transforming growth factor β and IFN-γ may counteract the Th2-dominated inflammatory response (27). An important role for these cytokine in the pathogenesis of bronchial asthma and AHR is well established (27). It can be concluded that the Th2 cytokines IL-4, IL-5 and IL-13 have a key pathogenic roles in asthma. In this study, the levels of these Th2 cytokines, in BALF were elevated by long-term intratracheal instillation of cartridge liquid nicotine solution from e-cigarettes, Therefore, we surmised that AI and AHR induced by the combination of cartridge nicotine solution administration and OVA sensitization were concomitant with the increased expression of Th2 cytokines IL-4, IL-5 and IL-13 in the BALF. OVA-specific IgE antibody levels were also increased in the sera of the OVA-S mice with intratracheal instillation of e-cigarette solution. These results indicate that the nicotine containing solution of ecigarettes accelerates the proliferation of lung Th2-cells in our murine AI and AHR model.
In conclusion, the results of this study indicate that the inhalation of cartridge nicotine solution in e-cigarettes is likely to exacerbate asthmatic symptoms by elevating infiltration of inflammatory cells including eosinophils into airways This process, in turn, enhances allergic AI and AHR, likely driven by the increase in the production of IL-4, IL-5, IL-13 and OVA-specific IgE. Further studies are needed to elucidate whether these effects of e-cigarette are solely dependent on the nicotine component, or whether they may also be caused by other components, or by mixture of nicotine and other constituents, of e-cigarettes.
References
- 1.Uryupin A.B., Peregudov A.S., Kochetkov K.A., Bulatnikova L.N., Kiselev S.S., Nekrasov Y.S. Qualitative and quantitative compositions of fluids for electronic cigarettes. Pharm. Chem. J. (2013);46:687–692. doi: 10.1007/s11094-013-0871-z. [DOI] [Google Scholar]
- 2.Polosa R., Caponnetto P., Morjaria J.B., Papale G., Campagna D., Russo C. Effect of an electronic nicotine delivery device (e-Cigarette) on smoking reduction and cessation: a prospective 6-month pilot study. BMC Public Health. (2011);11:786. doi: 10.1186/1471-2458-11-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ingebrathsen B.J., Cole S.K., Alderman S.L. Electronic cigarette aerosol particle size distribution measurements. Inhalation Toxicol. 2012;24:976–984. doi: 10.3109/08958378.2012.744781. [DOI] [PubMed] [Google Scholar]
- 4.Kuschner W.G., Reddy S., Mehrotra N., Paintal H.S. Electronic cigarettes and thirdhand tobacco smoke: two emerging health care challenges for the primary care provider. Int. J. Gen. Med. (2011);4:115–120. doi: 10.2147/IJGM.S16908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee S., Kimm H., Yun J.E., Jee S.H. Public health challenges of electric cigarettes in South Korea. J. Prev. Med. Public Health. (2011);44:235–241. doi: 10.3961/jpmph.2011.44.6.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laugesen M. Safety report on the Ruyan e-cigarette cartridge and inhaled aerosol, Health New Zealand Ltd. New Zealand; (2008). pp. 1–23. [Google Scholar]
- 7.Food and Drug Administration. Summary of Results: Laboratory Analysis of Electronic Cigarettes Conducted By FDA. FDA; US: (2010). p. 1. [Google Scholar]
- 8.Etter J.F. Electronic cigarettes: a survey of users. BMC Public Health. (2010);10:231. doi: 10.1186/1471-2458-10-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caponnetto P., Auditore R., Russo C., Cappello G.C., Polosa R. Impact of an electronic cigarette on smoking reduction and cessation in schizophrenic smokers: A prospective 12-month pilot study. Int. J. Environ. Res. Public Health. (2013);10:446–461. doi: 10.3390/ijerph10020446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flouris A.D., Chorti M.S., Poulianiti K.P., Jamurtas A.Z., Kostikas K., Tzatzarakis M.N., Wallace Hayes A., Tsatsakis A.M., Koutedakis T. Acute impact of active and passive electronic cigarette smoking on serum cotinin and lung function. Inhalation Toxicol. 3;25:91–101. doi: 10.3109/08958378.2012.758197. [DOI] [PubMed] [Google Scholar]
- 11.Siegel M.B., Tanwar K.L., Wood K.S. Electronic cigarettes as a smoking-cessation tool. Results from an online survey. Am. J. Prev. Med. (2011);40:472–475. doi: 10.1016/j.amepre.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Etter J.F., Bullen C. Electronic cigarette: users profile, utilization, satisfaction and perceived efficacy. Addiction. (2011);106:2017–2028. doi: 10.1111/j.1360-0443.2011.03505.x. [DOI] [PubMed] [Google Scholar]
- 13.Vansickel A.R., Cobb C.O., Weaver M.F., Eissenberg T.E. A clinical laboratory model for evaluating the acute effects of electronic ‘'cigarettes’': nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiol. Biomarkers Prev. (2010);19:1945–1953. doi: 10.1158/1055-9965.EPI-10-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bullen C., McRobbie H., Thornley S., Glover M., Lin R., Laugesen M. Effect of an electronic nicotine delivery device (e cigarette) on desire to smoke and withdrawal, user preferences and nicotine delivery: randomised cross-over trial. Tob. Control. (2010);19:98–103. doi: 10.1136/tc.2009.031567. [DOI] [PubMed] [Google Scholar]
- 15.Kim D.S., Kim S.H., Kim B.K., Yang M.C., Ma J.Y. Antiasthmatic effects of herbal complex MA and its fermented product MA128. Evid. Based Complement. Altern. Med. (2012);2012:769508. doi: 10.1155/2012/769508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim S.H., Kim B.K., Lee Y.C. Antiasthmatic effects of hesperidin, a potential Th2 cytokine antagonist, in a mouse model of allergic asthma. Mediators Inflammation. (2011);2011:485402. doi: 10.1155/2011/485402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cieslewicz G., Tomkinson A., Adler A., Duez C., Schwarze J., Takeda K., Larson K.A., Lee J.J., Irvin C.G., Gelfand E.W. The late, but not early, asthmatic response is dependent on IL-5 and correlates with eosinophil infiltration. J. Clin. Invest. 9;104:301–308. doi: 10.1172/JCI7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee Y.C., Kim S.H., Seo Y.B., Roh S.S., Lee J.C. Inhibitory effects of Actinidia polygama extract and cyclosporine A on OVA-induced eosinophilia and bronchial hyperresponsiveness in a murine model of asthma. Int. Immunopharmacol. (2006);6:703–713. doi: 10.1016/j.intimp.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Barnes P.J. Pathophysiology of allergic inflammation. Immunol. Rev. (2011);242:31–50. doi: 10.1111/j.1600-065X.2011.01020.x. [DOI] [PubMed] [Google Scholar]
- 20.Barlow R.B., McLeod L.J. Some studies on cytosine and its methylated derivatives. Br. J. Pharmacol. (1969);35:161–174. doi: 10.1111/j.1476-5381.1969.tb07977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomkinson A., Duez C., Cieslewicz G., Pratt J.C., Joetham A., Shanafelt M.C., Gundel R., Gelfand E.W. A murine IL-4 receptor antagonist that inhibits IL-4- and IL-13- induced responses prevents antigen-induced airway eosinophilia and airway hyperresponsiveness. J. Immunol. (2001);166:5792–5800. doi: 10.4049/jimmunol.166.9.5792. [DOI] [PubMed] [Google Scholar]
- 22.Bates J.H., Maksym G.N. Mechanical determinants of airways. (2011). [DOI] [PubMed] [Google Scholar]
- 23.Cockcroft D.W. Direct challenge tests: airway hyperresponsiveness in asthma: its measurement and clinical significance. Chest. (2010);138:18S–24S. doi: 10.1378/chest.10-0088. [DOI] [PubMed] [Google Scholar]
- 24.Nakagome K., Nagata M. Pathogenesis of airway inflammation in bronchial asthma. Auris Nasus Larynx. (2011);38:555–563. doi: 10.1016/j.anl.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Brightling C.E. Eosinophils, bronchitis and asthma; Pathogenesis of cough and air flow obstruction. Pulm. Pharmacol. Ther. (2011);24:324–327. doi: 10.1016/j.pupt.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Amin K. The role of mast cells in allergic inflammation. Respir. Med. (2012);106:9–14. doi: 10.1016/j.rmed.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Bosnjak B., Stelzmueller B., Erb K.J., Epstein M.M. Treatment of allergic asthma: Modulation of Th2 cells and their responses. Respir. Res. (2011);12:114. doi: 10.1186/1465-9921-12-114. [DOI] [PMC free article] [PubMed] [Google Scholar]


