Abstract
The human cytochrome P450 2J2 catalyzes an epoxygenase reaction to oxidize various fatty acids including arachidonic acid. In this study, three recombinant enzyme constructs of P450 2J2 were heterologously expressed in Escherichia coli and their P450 proteins were successfully purified using a Ni2+-NTA affinity column. Deletion of 34 amino acid residues in N-terminus of P450 2J2 enzyme (2J2-D) produced the soluble enzyme located in the cytosolic fraction. The enzymatic analysis of this truncated protein indicated the typical spectral characteristics and functional properties of P450 2J2 enzyme. P450 2J2-D enzymes from soluble fraction catalyzed the oxidation reaction of terfenadine to the hydroxylated product. However, P450 2J2-D enzymes from membrane fraction did not support the P450 oxidation reaction although it displayed the characteristic CO-binding spectrum of P450. Our finding of these features in the N-terminal modified P450 2J2 enzyme could help understand the biological functions and the metabolic roles of P450 2J2 enzyme and make the crystallographic analysis of the P450 2J2 structure feasible for future studies.
Keywords: P450 2J2, N-terminal truncation, Terfenadine
INTRODUCTION
Cytochrome P450s (CYPs, P450s) are an enzyme superfamily containing the Cys-coordinated heme as a prosthetic group and they are found in various organisms including animals, plants, fungi and bacteria (1). In humans, P450s are involved in the metabolism of various endogenous and exogenous compounds such as sterols, fatty acids, carcinogens or xenobiotic toxins (2). Since it was first characterized by Omura and Sato, the functional roles of P450s in the human body and specific organs have been extensively examined (3).
Human P450 2J2 has been well known as an unsaturated fatty acid epoxygenase found in extrahepatic tissues including human heart (4). Specially, P450 2J2 has attracted much attention due to its metabolism of arachidonic acid (AA). It converts arachidonic acid to four different epoxyeicosatrienoic acids (EETs) with various biological effects (5). Moreover, P450 2J subfamily enzymes are well conserved among the various mammalian species and they displayed similar enzymatic functions and biochemical activities (6-8). Aiba et al. reported that P450 2J enzymes catalyzed the hydroxylation reaction of vitamin D and therefore it could play an important role of vitamin D metabolism (9). Recently, the drug metabolism by human P450 2J2 was determined and its clinical relevance was reported. Several antihistamine drugs including terfenadine, ebastine, and astemizole have been identified as substrates for P450 2J2 (10,11).
In this study, we have constructed the recombinant P450 2J2 enzyme clones with N-terminus modifications and purified them. The purified P450 2J2 enzymes have been characterized in terms of their spectroscopic and catalytic properties. These results could provide valuable clues to define the functional roles of P450 2J2 enzyme.
MATERIALS AND METHODS
Chemicals. Terfenadine, sodium dithionite, glucose-6-phosphate, glucose-6-phosphate dehydrogenase, and NADP+ were purchased from Sigma-Aldrich (St. Louis, MO). Other chemicals were of the highest grade commercially available.
Construction of expression plasmids for truncated P450 2J2 enzymes. The cDNA of human P450 2J2 gene was generously provided by Professor Sang Seop Lee (Inje University, Korea). The open reading frame of P450 2J2 gene was amplified using PCR with the forward and reverse primers describes in Table 1 (94℃ for 10 min, 35 cycles of 94℃ for 30 s, 55℃ for 60 s, 72℃ for 90 s, and followed by 72℃ for 10 min). MALLLAVF amino acids sequences was introduced into N-terminus of P450 2J2 to generate P450 2J2-M clone and the truncation of 34 amino acids at N-terminus region of native enzyme produced P450 2J2-D clone (12). The amplified PCR fragments were cloned into the pCW vector using the BamHI and XbaI restriction sites with 6×His-tag at C-terminus of enzyme (13). The constructed expression vectors for P450 2J2 were verified by nucleotide sequencing analysis.
Table 1.
N-terminal sequences of human P450 2J2 constructs for heterologous expression in E. coli
| P450 2J2 constructs | N-terminal amino acids sequences | DNA sequences of forward PCR primers* |
|---|---|---|
|
| ||
| 2J2-native | MLAAMGSLAAALWAVVHPRTLLLGTVAFLLAADFLKRRRPKNYPPGP | 5'-AAACAGGATCCATCGATGCTTAGGAGGTCATatgctcgcggcgatggg-3' |
| 2J2-M | MALLLAVF - - - - - - - - - - - - LLLGTVAFLLAADFLKRRRPKNYPPGP | 5'-CGAATCATatggctctgttattagcagtttttctcctactgggcactgtcgccttt-3' |
| 2J2-D | MA - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - KRRRPKNYPPGP | 5'-CGAATCATatggctaaacgtcgtcgcccaaagaactacccg-3' |
*DNA sequences for reverse PCR primer: 5'-TGCGCTGTTCCTCAGGTGCATCACCATCACCATCACTAAAAGCTTCCGGTCTAGAAATAAT-3'.
Expression and purification. Expression of native and modified P450 2J2 enzymes (2J2-M and 2J2-D) were carried out as previously described with some modifications (14). In brief, the constructed plasmids were transformed into E. coli DH5α cells. A single colony of DH5α cells containing the plasmid was inoculated into Luria Broth (LB) media containing 50 μg/ml of ampicillin and grown at 37℃ for overnight. The culture was transferred to 1 L of Terrific Broth (TB) media (supplemented with 50 μg/ml of ampicillin, 1 mM thiamine, 0.5 mM of δ-aminolevulenic acid, and trace elements) and further incubated at 28℃ for 48 h after addition of 1 mM isopropyl β-D-thiogalactopyranoside (IPTG). Cells were harvested by centrifugation at 5,000 g for 15 min. TB media were discarded and the bacterial pellets were resuspended in 100 mM Tris-acetate buffer (pH 7.4) containing 500 mM sucrose and 0.5 mM EDTA. The spheroplasts was prepared with addition of lysozyme and then ultra-sonicated in 100 mM phosphate buffer (pH 7.4) containing 20% glycerol, 6 mM magnesium acetate, 0.1 mM DTT, 1 mM phenylmethanesulphonyl fluoride (PMSF), and Complete protease inhibitor cocktail tablet (Roche, Branford, CT). The resulting fractions were centrifuged at 10,000 g for 20 min to remove the debris. The supernatant was transferred to the new ultra-centrifuge tubes and then centrifuged at 100,000 g for 3 hr. The membrane pellets were resuspended 100 mM potassium phosphate buffer (pH 7.4) containing 20% glycerol (v/v) and homogenized with a dounce homogenizer.
Bacterial inner membrane fractions containing P450 2J2 were purified using by a Ni2+-nitrilotriacetate (NTA) column as described previously (13). Membranes were resuspend in 100 mM potassium phosphate buffer (pH 7.4) containing 20% glycerol (v/v), 0.5M NaCl and added 1% CHAPS (w/v). The solution was solubilized by gently stirring over 12 hr in the cold box and the solution was centrifuged at 100,000 g for 1.5 hr to remove the insoluble materials. The supernatant containing 6×His-tagged P450 2J2 enzyme was collected and loaded onto pre-requilibrated Ni2+-NTA column which equilibrated with 10 column volume (cv) of equilibrium buffer [100 mM potassium phosphate buffer (pH 7.4) containing 0.5 M NaCl, 20% glycerol (w/v) and 5 mM imidazole]. The brown-red colored column was washed with 10 cv of washing buffer [100 mM potassium phosphate buffer (pH 7.4) containing 0.5 M NaCl, 20% glycerol and 40 mM imidazole]. Brown-red colored fractions containing P450 2J2 enzymes were eluted by 3 cv of elution buffer [100 mM potassium phosphate buffer (pH 7.4) containing 0.5 M NaCl, 20% glycerol and 300 mM imidazole]. The removal of imidazole and CHAPS was achieved by dialysis [100 mM potassium phosphate buffer (pH 7.4) containing 20% glycerol, 1 mM EDTA and 0.1 mM DTT].
Spectroscopic characterization. P450 contents were determined using Omura & Sato method (15). Sodium dithionite was added to reduce the purified ferric P450. The CO-ferrous P450 complexes were generated by passing CO gas through solutions of the ferrous P450. UV-visible spectra were collected on a Varian CARY 100 spectrophotometer in 100mM potassium phosphate buffer (pH 7.4) at room temperature.
Substrate binding titrations. Purified enzymes were diluted to 1 μM in 100 mM potassium phosphate buffer (pH 7.4) and then divided between two glass cuvettes. Spectral changed (from 350 to 500 nm) were recorded with subsequent additions of terfenadine using a Varian CARY 100 spectrophoto meter. The difference in absorbance between the wavelength maximum and minimum was plotted versus the substrate concentration. Binding affinities were estimated by fitting the data of absorbance difference to the quadratic equation using nonlinear regression analysis with Graph-Pad Prism software (Graph-Pad, San Diego, CA, USA).
Measurement of P450 2J2 enzymatic activity. Enzymatic activity for terfenadine hydroxylation was determined by HPLC as previously reported with some modifications (16). The reaction mixtures consisted of 100 pmol P450 2J2, 200 pmol of rat NADPH-P450 reductase, and 30 μg of DLPC, an NADPH-generating system [0.5 mM NADP+, 10 mM glucose 6-phosphate, and 1.0 IU glucose 6-phos-phate dehydrogenase ml−1], and varying concentrations of terfenadine in a total volume of 0.5 ml of 100 mM potassium phosphate buffer (pH 7.4). Incubations were done for 30 min at 37℃ and terminated with addition of 0.5 ml of the ice-cold MeOH. The incubation mixtures were centrifuged at 3,000 rpm for 10 min and then 50 μl of supernatants were injected into the HPLC system (Younglin 9100, Korea) equipped with an Alltima C18 column (4.6 × 150 mm, 5 μm; Alltech, Albany, OR). Reaction products were analyzed with a gradient from 30% buffer A (0.1 M Tris acetate, pH 4.6) and 70% buffer B (CH3CN/CH3OH/H2O = 7 : 2 : 1, v/v) to 100% Buffer A for 10 min at a flow rate of 1.5 ml/min. Metabolites were detected at 254 nm using UV/Vis detector.
RESULTS AND DISCUSSION
Expression of recombinant P450 2J2 constructs. Expression levels of native and truncated P450 2J2 enzymes were spectrally determined in the whole cell cultures (Table 2). There was no detectable P450 in the expression of a native P450 2J2 while two truncated constructs P450 2J2-M and P450 2J2-D yielded the expression level of 125~320 nmol P450 holoenzyme per liter culture (Table 2). This result suggests that the N-terminus of P450 2J2 interferes the expression of recombinant P450 2J2 and its deletion could accommodate the successful expression of homologous P450 in E. coli. The previous study with P450 2J enzymes showed the similar result that the only truncated constructs produced the P450 holoenzymes in E. coli (9). After ultracentrifugation separation, all the detectable P450 content of P450 2J2-M construct was located in the membrane fraction (Table 2). However, the major P450 content of P450 2J2-D construct was observed in the cytosolic fraction (Table 2). This result suggests that the first 34 amino acid residues in the N-terminus of P450 2J2 may be associated with the membrane and its truncation can induce the dissociation of P450 2J2-D protein from the membrane. Higher expression level of P450 holoenzyme was observed in P450 2J2-D than in P450 2J2-M construct (Table 2). This result suggests that the sequence “MALLLAVF” did not effectively increase the expression of P450 2J2 (12).
Table 2.
Expression, distribution, and purification levels of P450 2J2 constructs
| P450 2J2 constructs | Whole cell level (nmol/liter culture) | After ultracentrifugation (nmol/L culture) | After Ni2+-NTA column (nmol/L culture) | |
|---|---|---|---|---|
|
| ||||
| Cytosolic fraction | Membrane fraction | |||
|
| ||||
| 2J2-native | ND* | ND* | ND* | ND* |
| 2J2-M | 125 | ND* | 18.6 | 8.3 |
| 2J2-D | 320 | 136 | 16 | 14/6.8** |
*Not detected, **from cytosolic fraction/from membrane fraction.
Purification of truncated P450 2J2 constructs. Purification of P450 2J2 protein was performed using Ni2+-NTA affinity column. After ultracentrifugation separation, the membrane fraction from 2J2-M and 2J2-D was successfully solubilized with an anionic surfactant, CHAPS. The cytosolic fraction from 2J2-D and the solubilized membrane fractions from 2J2-M and 2J2-D were eluted from Ni2+-NTA column and the SDS-PAGE analysis showed the eluted protein bands with the expected molecular weights (56.3 and 54.9 kDa) (Fig. 2).
Fig. 2. SDS-PAGE analysis of the purified P450 2J2 proteins. Lane 1, 10 pmol protein from membrane fraction of P450 2J2-M; lane 2, 10 pmol protein from membrane fraction of P450 2J2-D; 3 and 4, 5 and 10 pmol protein from cytosolic fraction of P450 2J2-D, respectively.
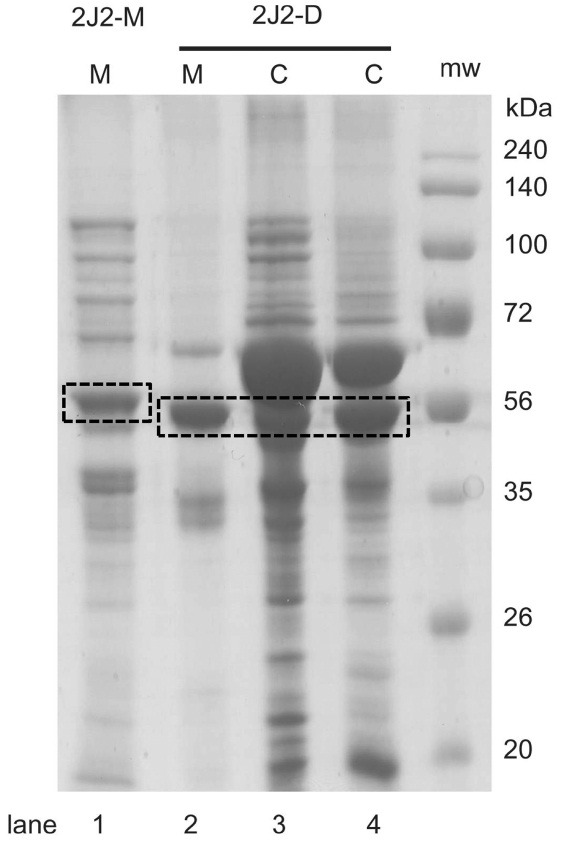
Fig. 1. The cloning scheme of P450 2J2 into pCW expression vector.
Spectral characterization of native and truncated P450 2J2 proteins. The reduced CO difference spectra of all three P450 2J2 proteins showed a maximum absorption at 450 nm (Fig. 3). P450 2J2-D displayed the spectra containing peaks at 420 nm, indicating the incorrectly folding enzyme species (Fig. 3B and Fig. 3C). Binding analysis of purified P450 2J2-D with terfenadine showed the typical Type I binding substrate binding spectral change (Fig. 4). The calculated binding affinity (Kd) is 3.3 ± 0.6 μM. This result suggests that the binding of terfenadine displaces water molecule upon the heme iron in P450 2J2 enzyme.
Fig. 3. CO-binding difference spectra of purified P450 2J2 proteins. (A) purified P450 2J2-M, (B) purified P450 2J2-D (from membrane fraction), (C) purified P450 2J2 (from soluble fraction).
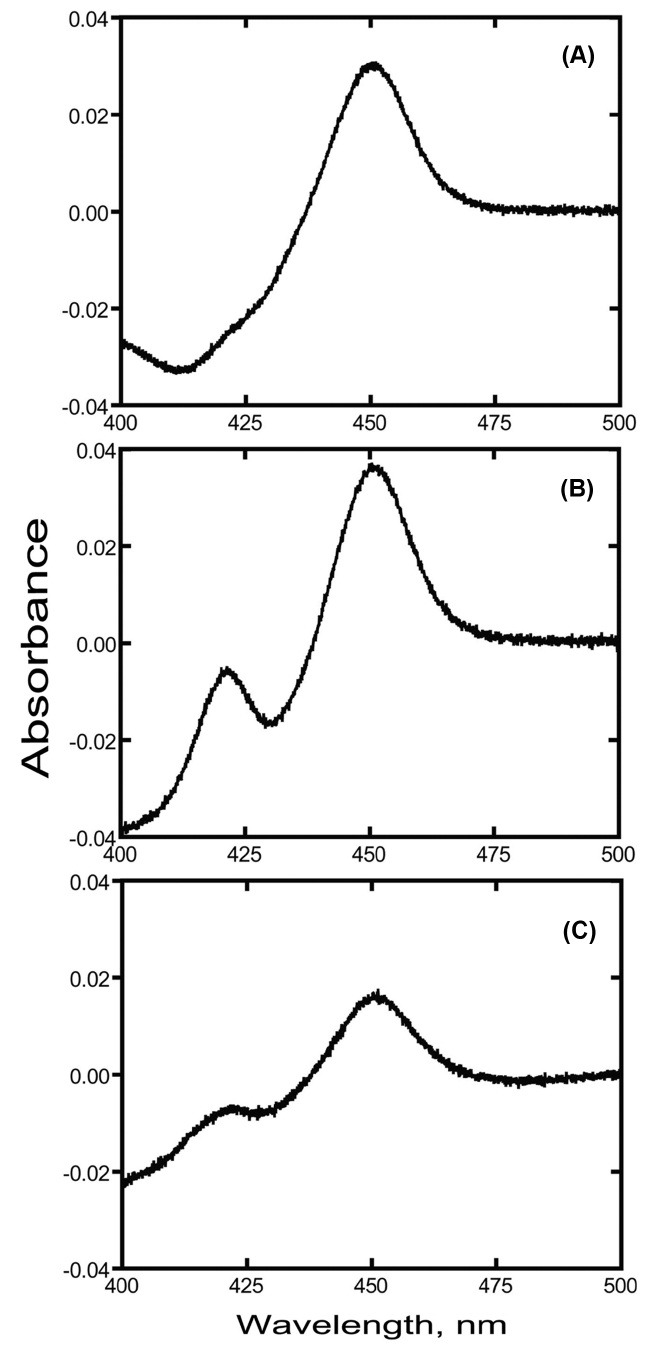
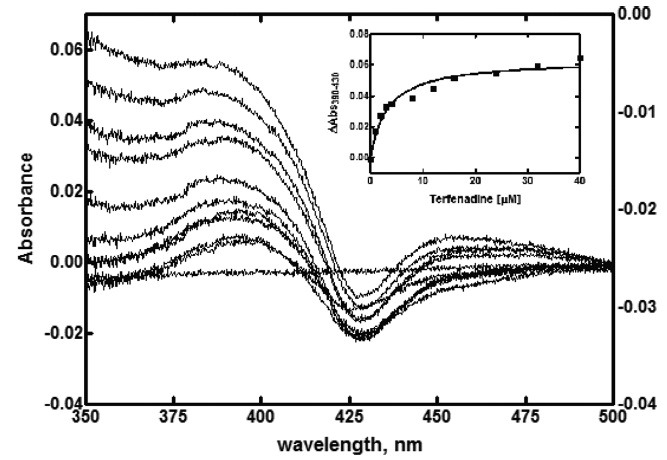
Fig. 4. Binding titration of purified P450 2J2 with terfenadine. Increasing concentration of terfenadine was added to the sample and equal volume of solvent to the reference cuvettes. The inset shows the plot of 390~430 nm vs. concentration of terfenadine.
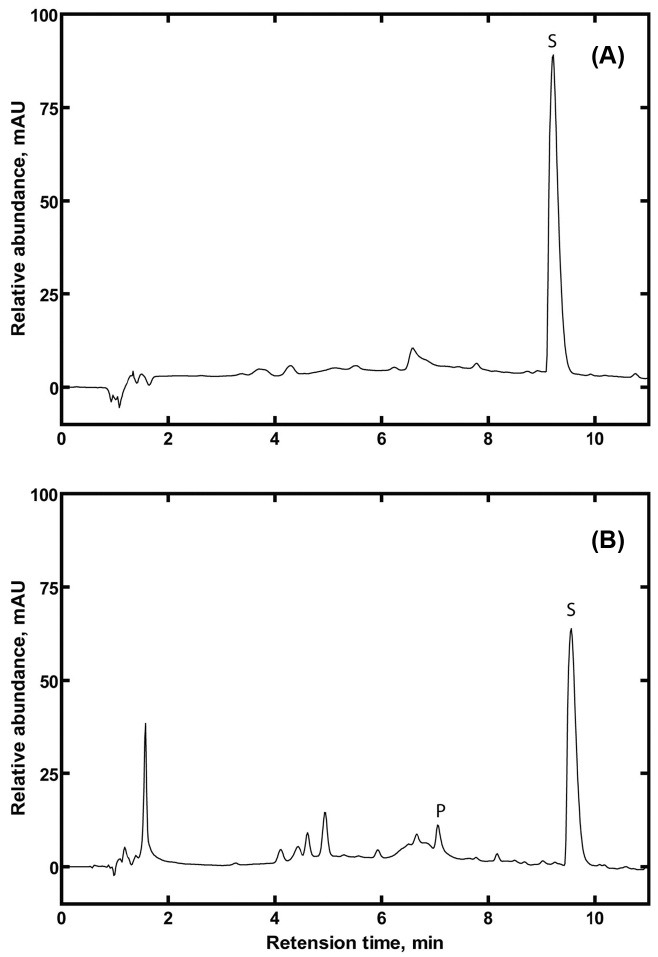
Terfenadine hydroxylation by P450 2J2. Catalytic activities of purified P450 2J2-D enzymes were evaluated in the terfenadine hydroxylation reaction using a reconstitution system including NADPH-P450 reductase. The retention time of the hydroxylated product of terfenadine was observed at ~7 min in the HPLC chromatogram (Fig. 5). Interestingly, the hydroxylated product was observed only in the reaction by P450 2J2-D enzymes from soluble fraction (Fig. 5A), not by P450 2J2-D enzymes from membrane fraction (Fig. 5B). This result indicates that the purified enzyme of P450 2J2-D from the membrane fraction displayed a correctly folded P450 but it lacked of the enzymatic function. However, the purified enzyme of P450 2J2-D from the soluble fraction showed a functional enzymatic catalysis of P450 2J2, therefore it can be used for the further studies of P450 2J2 metabolism and the structurefunction relationship using the possible crystal structural analysis of P450 2J2 enzymes.
Fig. 5. HPLC analysis of terfenadine hydroxylation by purified P450 2J2 enzymes. (A) Enzyme reaction by P450 2J2-D from membrane fraction, (B) Enzyme reaction by P450 2J2-D from soluble fraction.
Three recombinant enzyme constructs of P450 2J2 were heterologously expressed in E. coli and their P450 proteins were successfully purified. Deletion of 34 amino acid residues in N-terminus of P450 2J2 enzyme produced the soluble enzyme located in the cytosolic fraction. The enzymatic analysis of this truncated protein indicated the spectral characteristics and functional properties of P450 2J2 enzyme. These features of the N-terminal truncated enzyme can provide an important insight for understanding the biological functions and metabolic roles of P450 2J2 enzyme. In addition, its modification could make the crystallographic analysis of the P450 2J2 structure possible.
Acknowledgments
This paper was written as part of Konkuk University’s research support program for its faculty on sabbatical leave in 2012.
References
- 1.Ortiz de Montellano P.R. in Cytochrome P450: Structure, Mechanism, and Biochemistry. (Ortiz de Montellano, P.R. ed.). Plenum Press; New York: (2005). pp. 1–689. [Google Scholar]
- 2.Guengerich F.P. in Cytochrome P450: Structure, Mechanism, and Biochemistry. (Ortiz de Montellano, P.R. ed.). Plenum Press; New York: (2005). pp. 377–551. [Google Scholar]
- 3.Omura T. Forty years of cytochrome P450. Biochem. Biophys. Res. Commun. (1999);266:690–698. doi: 10.1006/bbrc.1999.1887. [DOI] [PubMed] [Google Scholar]
- 4.Wu S., Moomaw C.R., Tomer K.B., Falck J.R., Zeldin D.C. Molecular cloning and expression of CYP2J2, a human cytochrome P450 arachidonic acid epoxygenase highly expressed in heart. J. Biol. Chem. (1996);271:3460–3468. doi: 10.1074/jbc.271.48.30548. [DOI] [PubMed] [Google Scholar]
- 5.Xu M., Ju W., Hao H., Wang G., Li P. Cytochrome P450 2J2: distribution, function, regulation, genetic polymorphisms and clinical significance. Drug Metab. Rev. (2013);45:311–352. doi: 10.3109/03602532.2013.806537. [DOI] [PubMed] [Google Scholar]
- 6.Wu S., Chen W., Murphy E., Gabel S., Tomer K.B., Foley J., Steenbergen C., Falck J.R., Moomaw C.R., Zeldin D.C. Molecular cloning, expression, and functional significance of a cytochrome P450 highly expressed in rat heart myocytes. J. Biol. Chem. (1997);272:12551–12559. doi: 10.1074/jbc.272.19.12551. [DOI] [PubMed] [Google Scholar]
- 7.Ma J., Qu W., Scarborough P.E., Tomer K.B., Moomaw C.R., Maronpot R., Davis L.S., Breyer M.D., Zeldin D.C. Molecular cloning, enzymatic characterization, developmental expression, and cellular localization of a mouse cytochrome P450 highly expressed in kidney. J. Biol. Chem. (1999);274:17777–17788. doi: 10.1074/jbc.274.25.17777. [DOI] [PubMed] [Google Scholar]
- 8.Messina A., Nencioni S., Gervasi P.G., Gotlinger K.H., Schwartzman M.L., Longo V. Molecular cloning and enzymatic characterization of sheep CYP2J. Xenobiotica. (2010);40:109–118. doi: 10.3109/00498250903410590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aiba I., Yamasaki T., Shinki T., Izumi S., Yamamoto K., Yamada S., Terato H., Ide H., Ohyama Y. Characterization of rat and human CYP2J enzymes as Vitamin D 25-hydroxylases. Steroids. (2006);71:849–856. doi: 10.1016/j.steroids.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Lee C.A., Neul D., Clouser-Roche A., Dalvie D., Wester M.R., Jiang Y., Jones J.P. 3rd, Freiwald S., Zientek M., Totah R.A. Identification of novel substrates for human cytochrome P450 2J2. Drug Metab. Dispos. (2010);38:347–356. doi: 10.1124/dmd.109.030270. [DOI] [PubMed] [Google Scholar]
- 11.Lee C.A., Jones J.P. 3rd, Katayama J., Kaspera R., Jiang Y., Freiwald S., Smith E., Walker G.S., Totah R.A. Identifying a selective substrate and inhibitor pair for the evaluation of CYP2J2 activity. Drug Metab. Dispos. (2012);40:943–951. doi: 10.1124/dmd.111.043505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong M.S., Bell L.C., Guo Z., Phillips D.R., Blair I.A., Guengerich F.P. Identification of retained N-formylmethionine in bacterial recombinant mammalian cytochrome P450 proteins with the N-terminal sequence MALLLAVFL...: roles of residues 3-5 in retention and membrane topology. Biochemistry. (1996);35:10031–10040. doi: 10.1021/bi960873z. [DOI] [PubMed] [Google Scholar]
- 13.Choi S., Han S., Lee H., Chun Y.J., Kim D. Evaluation of luminescent P450 analysis for directed evolution of human CYP4A11. Biomol. Ther. (2013);21:487–492. doi: 10.4062/biomolther.2013.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim J., Lim Y.R., Han S., Han J.S., Chun Y.J., Yun C.H., Lee C.H., Kim D. Functional influence of human CYP2D6 allelic variations: P34S, E418K, S486T, and R296C. Arch. Pharm. Res. (2013);36:1500–1506. doi: 10.1007/s12272-013-0212-5. [DOI] [PubMed] [Google Scholar]
- 15.Omura T., Sato R. The Carbon Monoxide-Binding Pigment of Liver Microsomes. I. Evidence for Its Hemoprotein Nature. J. Biol. Chem. (1964);239:2370–2378. [PubMed] [Google Scholar]
- 16.Lee S.S., Jeong H.E., Liu K.H., Ryu J.Y., Moon T., Yoon C.N., Oh S.J., Yun C.H., Shin J.G. Identification and functional characterization of novel CYP2J2 variants: G312R variant causes loss of enzyme catalytic activity. Pharmacogenet. Genomics. (2005);15:105–113. doi: 10.1097/01213011-200502000-00006. [DOI] [PubMed] [Google Scholar]


