Abstract
In this study, we investigated the hepatoprotective effects of aged black garlic (ABG) in rodent models of liver injury. ABG inhibited carbon tetrachloride-induced elevation of aspartate transaminase (AST) and alanine transaminase (ALT), which are markers of hepatocellular damage, in SD rats. D-galactosamineinduced hepatocellular damage was also suppressed by ABG treatment. However, ABG does not affect the elevation of alkaline phosphatase (ALP), a marker of hepatobilliary damage, in rats treated with carbon tetrachloride or D-galactosamine. We also examined the effect of ABG on high-fat diet (HFD)-induced fatty liver and subsequent liver damage. ABG had no significant effect on body weight increase and plasma lipid profile in HFD-fed mice. However, HFD-induced increase in AST and ALT, but not ALP, was significantly suppressed by ABG treatment. These results demonstrate that ABG has hepatoprotective effects and suggest that ABG supplementation might be a good adjuvant therapy for the management of liver injury.
Keywords: Aged black garlic, Liver injury, Carbon tetrachloride, D-galactosamine, Non-alcoholic fatty liver disease
INTRODUCTION
Garlic has long been used as a medicinal food and recent studies have validated a variety of its biological activities, including cancer prevention, anti-thrombosis and cardioprotection (1-3). In spite of these beneficial effects, consumption of raw garlic is not prevalent in many areas because of its unpleasant odor and adverse effects, such as gastrointestinal disorder and hemolytic anemia (4,5). Allicin and lipidsoluble sulfur compounds derived from allicin have been known to be involved in these adverse effects (4,6). Aged garlic extract (AGE, Kyolic) is a preparation produced by long-term extraction of fresh garlic in aqueous ethanol at room temperature (7). AGE has been known to contain water-soluble allyl amino acid derivatives, stable lipid-soluble allyl sulfides, flavonoids and saponins, but not allicin anymore (8). AGE has also been shown to exert various biological activities, including anti-cancer, antioxidant and immune-potentiating effects without already known adverse effects of raw garlic (7-10).
Recently, another garlic preparation, called aged black garlic (ABG), was marketed (11,12). ABG is produced by ageing whole garlic at high temperature and high humidity (13,14). It has been reported that unstable compounds of raw garlic are converted into stable compounds, such as Sallylcysteine (SAC), after this processing. ABG was shown to have stronger antioxidant activity than raw garlic both in vitro and in vivo (11,15). It was also reported that ABG is beneficial for prevention of cancer and diabetes (11,16,17). Moreover, ABG was reported to have a protective effect against chronic alcohol-induced liver injury in rats (12).
Based on these reports, we hypothesized that ABG might also have protective effects against liver injuries induced by various insults. In the present study, we examined the effects of ABG on carbon tetrachloride (CCl4)- or D-galactosamine-induced liver damage. We also investigated the protective effect of ABG on high-fat diet (HFD)-induced hepatic steatosis and subsequent liver injury.
MATERIALS AND METHODS
Reagents and animals. All reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise noted. Seven weeks old male SD (Sprague Dawley) rats and C57BL/6 mice were purchased from Koatech (Pyungtaek, Gyeonggi, Korea) and Biomedical Mouse Resource Center in KRIBB (Cheongwon, Chungbuk, Korea), respectively, and cared for as described previously (18). Animals were allowed to acclimate to the local environment for at least 1 week before use. All animal experiments were conducted using protocols approved by Institutional Animal Care and Use Committee at Korea Research Institute of Bioscience and Biotechnology.
ABG preparation. The raw material of garlic was cultivated in Namhae, Gyeongnam, Republic of Korea. The raw garlic was extracted using water at 95 ± 2℃ for 12 hr, which was filtrated three times and then vacuum evaporated at 55℃~58℃ to get 60 brix. The residue was homogenized and sterilized to yield ABG used in this study. ABG used in this study contained ~61.9% of solid materials.
CCl4- or D-galactosamine-induced liver injury model. SD rats were pretreated orally with vehicle (saline) or indicated concentrations of ABG (100 or 200 mg/kg) once a day for 7 days. SD rats were fasted for 16 hr and CCl4 (20% in olive oil, 2 ml/kg) or D-galactosamine (in saline, 400 mg/kg) was administrated orally to induce liver injury. After 48 hr, SD rats were sacrificed, blood was collected from the posterior vena cava, plasma was prepared and stored at –70℃ for biochemical analysis. Liver was also removed, weighed and fixed in formalin and liquid nitrogen for histopathology.
High-fat diet-induced liver steatosis and injury model. C57BL/6 mice were used for HFD-induced liver steatosis and injury model. The control mice were fed with a control diet (CD, D12450B, Research Diets, Inc., New Brunswick, NJ, USA) and the other mice were fed with a high-fat diet (HFD, D12492, Research Diets, Inc.) for 4 weeks before sample treatment. Vehicle or indicated concentrations of ABG (100, 200 or 400 mg/kg) were give five days a week for 4 weeks. The body weight was recorded once a week. At the end of experiment, blood was collected from the posterior vena cava and plasma was prepared for biochemical analysis. Liver, subcutaneous fat, epididymal fat and mesenteric fat were removed surgically, weighed and stored in formalin and liquid nitrogen for further analysis. Frozen liver was homogenized and liver TG was measured using Triglyceride Quantitation Kit (BioVision Inc., San Francisco, USA) according to manufacturer’s instructions.
Biochemical analysis. The levels of aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), albumin (ALB), total cholesterol (TC), lowdensity lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C) or triglycerides (TG) in plasma were measured using a chemistry analyzer (AU400, Olympus, Tokyo, Japan) according to manufacturer’s instructions.
Histopathology. Fixed livers were embedded in paraffin and thin sections were made. The sections were stained with hematoxylin and eosin and the slides were photographed. For detection of lipid deposition, liver sections were prepared from frozen liver and stained with Oil Red O as described previously (19).
Statistical analysis. Results are expressed as mean ± S.D. Two-way ANOVA and Bonferroni’s multiple comparison test were used for body weight and One-way ANOVA and Dunnett’s t-test were used for another results for statistical analysis using GraphPad Prism (GraphPad Software; La Jolla, CA, USA). The criterion for statistical significance was set at p < 0.05.
RESULTS
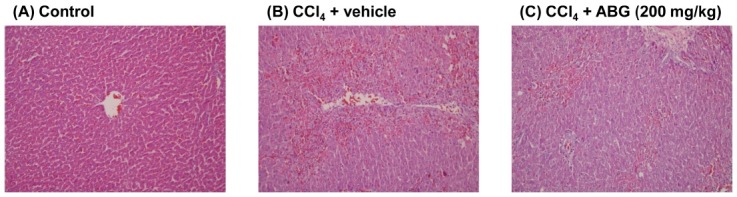
Hepatoprotective effect of ABG on CCl4-induced hepatic injury. To investigate the hepatoprotective effect of ABG, we examined the effect of ABG on CCl4-induced liver damage in SD rats. As shown in Table 1, liver weight was increased significantly by CCl4 administration, which is in consistent with previous reports (20,21). However, treatment of rats with ABG inhibited CCl4-induced increase in liver weight in a concentration-dependent manner, although the effect was not statistically significant (Table 1). The level of AST and ALT, which are known as a marker of hepatocellular damage, was also elevated significantly by CCl4 treatment, and this was dose-dependently suppressed by ABG treatment (Table 1). In addition, the hepatobiliary damage, which is manifested by plasma ALP level, was increased significantly, and concentration-dependent, but statistically insignificant, inhibition of CCl4-induced increase in ALP by ABG was observed (Table 1). Plasma ALB level was not changed in all groups examined in this experiment (Table 1). Histopathological analysis demonstrated that CCl4 induced alterations in hepatocyte morphology and infiltration of immune cells, but not severe necrotic cell death in our experimental condition (Fig. 1). However, the changed induced by CCl4 treatment were suppressed by ABG treatment (Fig. 1).
Table 1.
Effect of ABG on liver weight and markers for liver damage in carbon tetrachloride-induced liver injury model
| Untreated | CCl4 + vehicle | CCl4 + ABG (100mg/kg) | CCl4 + ABG (200 mg/kg) | |
|---|---|---|---|---|
|
| ||||
| Liver weight (g) | 8.0 ± 0.7* | 10.0 ± 1.0 | 9.6 ± 1.1 | 9.3 ± 0.7 |
| AST (IU/L) | 278.2 ± 543.5* | 8,530.0 ± 3,239.0 | 6,296.0 ± 2,184.2 | 5,904.0 ± 1,681.8* |
| ALT (IU/L) | 93.4 ± 157.7* | 3,288.0 ± 1,093.2 | 2,500.0 ± 1,095.0 | 2,128.0 ± 896.3* |
| ALP (IU/L) | 524.6 ± 115.5* | 1,910.3 ± 295.4 | 1,733.0 ± 321.2 | 1,615.5 ± 259.3 |
| ALB (g/dl) | 2.3 ± 0.1 | 2.3 ± 0.1 | 2.3 ± 0.2 | 2.2 ± 0.1 |
Each row shows the mean ± S.D. of ten determinations. Statistical significant was analyzed by one-way ANOVA and Dunnett’s t-test (* p < 0.05 vs. vehicle).
Fig. 1. Histopathological analysis of liver. SD rats were pre-treated with vehicle or ABG (200mg/kg) and CCl4 (20% in olive oil, 2ml/kg) was administrated orally to induce liver injury. After 48 hr, livers were removed and fixed in formalin. Liver sections were prepared and analyzed by hematoxylin and eosin staining (magnification ×100).
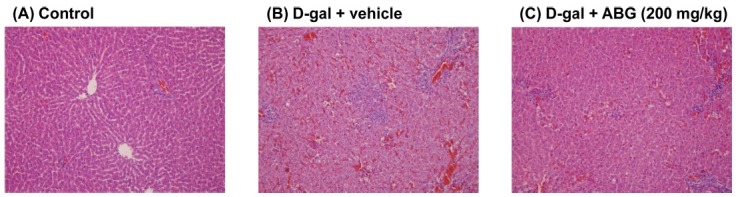
Hepatoprotective effect of ABG on D-galactosamineinduced hepatic injury. To further investigate the protective effect of ABG against hepatotoxicant-induced liver damage, we examined the effect of ABG on D-galactosamineinduced liver damage in SD rats. In consistent with the results of CCl4-induced liver damage model, the administration of D-galactosamine caused significant increase in liver weight in SD rats. However, ABG had no effect on Dgalactosamine-induced increase in liver weight in SD rats (Table 2). Plasma level of AST, ALT and ALP was also increased significantly by D-galactosamine treatment, but only AST and ALT were suppressed by ABG treatment (Table 2). The level of plasma ALB was neither affected by D-galactosamine treatment nor ABG treatment (Table 2). In addition, Fig. 2 also shows that D-galactosamine treatment induced immune cell infiltration, but this was reversed by ABG treatment.
Table 2.
Effect of ABG on liver weight and markers for liver damage in D-galactosamine-induced liver injury model
| Untreated | D-galactosamine + vehicle | D-galactosamine + ABG (100 mg/kg) | D-galactosamine + ABG (200 mg/kg) | |
|---|---|---|---|---|
|
| ||||
| Liver weight (g) | 7.6 ± 0.8* | 8.6 ± 1.1 | 8.2 ± 0.8 | 8.2 ± 0.5 |
| AST (IU/L) | 128.2 ± 22.3* | 1,311.6 ± 582.7 | 951.9 ± 441.0 | 737.0 ± 205.6* |
| ALT (IU/L) | 44.1 ± 4.6* | 714.1 ± 427.3 | 520.0 ± 339.1 | 277.0 ± 109.5* |
| ALP (IU/L) | 538.8 ± 138.3* | 821.8 ± 70.3 | 854.4 ± 106.6 | 796.1 ± 98.4 |
| ALB (g/dl) | 2.5 ± 0.1 | 2.4 ± 0.1 | 2.4 ± 0.1 | 2.5 ± 0.1 |
Each row shows the mean ± S.D. (n = 10). Statistical significant was analyzed by one-way ANOVA and Dunnett’s t-test (* p < 0.05 vs. vehicle).
Fig. 2. Histopathological analysis of liver. SD rats were pre-treated with vehicle or ABG (200 mg/kg) and D-galactosamine (400 mg/kg) was administrated orally to induce liver injury. After 48 hr, livers were removed and fixed in formalin. Liver sections were prepared and analyzed by hematoxylin and eosin staining (magnification ×100).
ABG protects liver injury induced by hepatic steatosis in HFD-induced obesity model. We also investigated the effect of ABG on obesity-related hepatic steatosis and subsequent liver damage in HFD-induced obesity model. As shown in Table 3, HFD-induced increase in body weight was significantly affected by ABG treatment. However, the weight of adipose tissues, including subcutaneous fat, epididymal fat and mesenteric fat, were significantly reduced by ABG treatment in a concentration-dependent manner (Table 3). Moreover, Table 3 shows that HFD-induced plasma levels of TC and HDL-C were not changed by ABG treatment, whereas the level of LDL-C were not significantly affected by both HFD consumption and ABG treatment in our model. Plasma TG was slightly induced by HFD and this was suppressed by ABG treatment although the levels of TG between groups are not statistically different.
Table 3.
Effect of ABG on body weight, adipose tissue weight and plasma lipid profile in HFD-induced obesity model
| CD | HFD + vehicle | HFD + ABG (100 mg/kg) | HFD + ABG (200 mg/kg) | |
|---|---|---|---|---|
|
| ||||
| Body weight (g) | ||||
| Initial | 26.5 ± 1.4* | 35.1 ± 2.5 | 34.1 ± 2.5 | 33.8 ± 2.1 |
| Final | 26.9 ± 1.1* | 39.3 ± 2.6 | 36.6 ± 2.5 | 36.8 ± 3.0 |
| Adipose tissue (mg) | ||||
| Subcutaneous | 357.3 ± 66.3* | 2063.6 ± 253.6 | 1602.5 ± 324.9* | 1569.7 ± 373.0* |
| Epididymal | 428.3 ± 56.2* | 2347.1 ± 337.3 | 2054.3 ± 345.7 | 1896.3 ± 443.2* |
| Mesenteric | 96.4 ± 18.7* | 938.7 ± 98.8 | 848.6 ± 124.2 | 733.2 ± 134.3* |
| Lipid profile (mg/dl) | ||||
| TC | 136.5 ± 9.5* | 199.5 ± 10.3 | 201.6 ± 15.6 | 194.4 ± 9.5 |
| HDL-C | 75.1 ± 5.5* | 98.1 ± 4.4 | 101.5 ± 3.4 | 99.3 ± 5.8 |
| LDL-C | 6.6 ± 1.1 | 6.1 ± 1.1 | 7.5 ± 1.9 | 7.5 ± 1.5 |
| TG | 71.5 ± 8.8 | 80.1 ± 23.3 | 62.1 ± 20.3 | 66.3 ± 23.4 |
Each row shows the mean ± S.D. (n = 8). Statistical significant was analyzed by one-way ANOVA and Dunnett’s t-test (* p < 0.05 vs. vehicle).
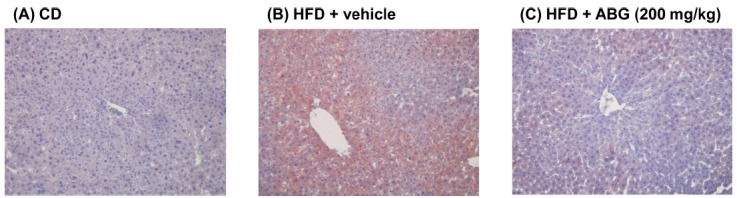
Further analysis demonstrated that HFD-induced increase in liver weight was significantly inhibited by treatment with ABG. In contrast, liver TG was increased by HFD consumption, but this was not significantly suppressed by ABG treatment (Table 4). The markers of hepatocellular damage, including AST and ALT, were up-regulated by HFD, and ABG treatment significantly suppressed the plasma levels of these markers. However, ALP was decreased by HFD consumption and HFD-induced change in ALP level was not affected by ABG treatment (Table 4). The level of plasma ALB was not changed in all groups examined in this experiment (Table 4). Oil-Red O staining revealed that high-fat diet feeding induced fatty liver (Fig. 3). However, ABG treatment markedly suppressed the accumulation of lipids in high-fat diet-fed mice (Fig. 3).
Table 4.
Effect of ABG on liver weight, liver TG and markers for liver damage in HFD-induced obesity model
| CD | HFD + vehicle | HFD + ABG (100 mg/kg) | HFD + ABG (200 mg/kg) | |
|---|---|---|---|---|
|
| ||||
| Liver weight (g) | 1.1 ± 0.1* | 1.4 ± 0.2 | 1.3 ± 0.2 | 1.2 ± 0.1* |
| Liver TG (mmole/g liver) | 21.3 ± 13.2* | 64.7 ± 14.9 | 51.4 ± 17.8 | 40.6 ± 13.1 |
| AST (IU/L) | 42.4 ± 2.3* | 63.1 ± 3.6 | 55.6 ± 3.1* | 55.3 ± 4.9* |
| ALT (IU/L) | 14.5 ± 1.4* | 35.5 ± 4.3 | 28.1 ± 4.1* | 28.5 ± 3.2* |
| ALP (IU/L) | 274.9 ± 22.1* | 190.9 ± 19.5 | 182.5 ± 15.1 | 176.3 ± 22.9 |
| ALB (g/dl) | 1.9 ± 0.1 | 1.9 ± 0.1 | 1.9 ± 0.0 | 1.9 ± 0.1 |
Each row shows the mean ± S.D. (n = 8). Statistical significant was analyzed by one-way ANOVA and Dunnett’s t-test (* p < 0.05).
Fig. 3. Histopathological analysis of liver. C57BL/6 mice were treated with vehicle or 200 mg/kg of ABG by oral administration. On day 28, livers were removed and fixed in liquid nitrogen. Liver sections were prepared from frozen liver and stained with Oil-Red O (magnification ×100).
DISCUSSION
We investigated the hepatoprotective effects of ABG on liver injuries induced by hepatotoxicants, including CCl4 and D-galactosamine, and HFD-induced hepatic steatosis. In this study, ABG was shown to inhibit both hepatotoxicants- and hepatic steatosis-induced liver injuries in rodents. Previously, it was reported that ABG exert a hepatoprotective effect against chronic alcohol consumption-induced liver injury in rats (12). Therefore, these results suggest that ABG is a hepatoprotectant against broad spectrum of hepatic injuries.
It has been reported that acute liver injury induced by single administration of CCl4 involves centrizonal necrosis and steatosis (22). However, chronic CCl4 administration induces liver fibrosis, cirrhosis and hepatocellular carcinoma (23). Reactive metabolites, such as trichloromethyl radical or trichloromethyl peroxyl radical, were known to be involved in CCl4-induced hepatotoxicity by adduct formation, lipid peroxidation and loss of calcium homeostasis and, ultimately, cell death (24). A variety of herbal extracts have been shown to exert hepatoprotective effects via their antioxidant activities (25-28). D-galactosamine is also a wellknown hepatotoxic agent used in the assessment of hepatoprotective potential of herbal extracts. The hepatotoxic effect of D-galactosamine was known to be mediated by an insufficiency of UDP-glucose and UDP-galactose and the loss of intracellular calcium homeostasis, leading to cell death (29). The activity of anti-oxidant enzymes were decreased after D-galactosamine treatment and the treatment of herbal extracts with anti-oxidant activities reversed anti-oxidant status and hepatic damage induced by D-galactosamine (30-32). In this report, we also demonstrated that ABG, which was known to have strong antioxidant effects, protected rats from CCl4- and D-galactosamine-induced hepatic injury. Our results and above-mentioned previous reports strongly suggest that the treatment with anti-oxidants might be beneficial for protection from liver injuries induced by CCl4 and D-galactosamine.
We also assessed the hepatoprotective effect of ABG in HFD-induced obesity model. Our results demonstrated that ABG had no effect on HFD-induced body weight gain and plasma lipid profile, although plasma TG was slightly decreased by ABG treatment. However, HFD-induced increase in body fat was significantly suppressed by ABG treatment. In contrast to our results, AGE was previously shown to suppress the increase in body weight and body fat and enhance plasma lipid profile in HFD-fed rats (15). In addition, Jung et al. (16) reported that the administration of yeast (Saccharomyces cerevisiae)-fermented black garlic extract also attenuated HFD-induced increase in body weight, body fat and plasma lipids, including TC, LDL-C, HDL-C and TG, in mice. These results suggest that the composition of ABG might be different from that of AGE or yeast-fermented black garlic extract. Our unpublished experiments, in which ABG was treated from the start of HFD feeding for 4 weeks, also support this notion by showing that HFD-mediated changes in plasma cholesterols were not affected by ABG treatment although the increases in body weight, body fat and plasma TG were significantly suppressed in HFD-fed mice. However, our results showed that the hepatoprotective effect of ABG is in consistent with the results of AGE and yeast-fermented black garlic extract, suggesting that the hepatoprotective potential against obesity-related hepatic steatosis are universal characteristics of all these garlic preparations.
Collectively, our results demonstrated that ABG exerts a hepatoprotective effects in various models of hepatic injury. Our results also suggested that ABG might have unique characteristics compared to other garlic preparations. Overall, these results suggest that ABG might be beneficial for the prophylactic and therapeutic management of liver injury.
Acknowledgments
This work was supported by a grant (No. A101836) of the Korean Health Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea, a grant from the KRIBB Research Initiative Program and a grant from the Chungbuk Bioinfrastructure Project supporting cooperation of Industry, Research Institute and Government.
References
- 1.Amagase H., Milner J.A. Impact of various sources of garlic and their constituents on 7,12-dimethylbenz[a]anthracene binding to mammary cell DNA. Carcinogenesis. (1993);14:1627–1631. doi: 10.1093/carcin/14.8.1627. [DOI] [PubMed] [Google Scholar]
- 2.Block E. The chemistry of garlic and onions. Sci. Am. (1985);252:114–119. doi: 10.1038/scientificamerican0385-114. [DOI] [PubMed] [Google Scholar]
- 3.Isensee H., Rietz B., Jacob R. Cardioprotective actions of garlic (Allium sativum). Arzneimittelforschung. (1993);43:94–98. [PubMed] [Google Scholar]
- 4.Hoshino T., Kashimoto N., Kasuga S. Effects of garlic preparations on the gastrointestinal mucosa. J. Nutr. (2001);131:1109S–1113S. doi: 10.1093/jn/131.3.1109S. [DOI] [PubMed] [Google Scholar]
- 5.Oboh G. Prevention of garlic-induced hemolytic anemia using some tropical green leafy vegetables. J. Med. Food. (2004);7:498–501. doi: 10.1089/jmf.2004.7.498. [DOI] [PubMed] [Google Scholar]
- 6.McRae M.P. A review of studies of garlic (Allium sativum on serum lipids and blood pressure before and after 1994: does the amount of allicin released from garlic powder tablets play a role? J. Chiropr. Med. (2005);4:182–190. doi: 10.1016/S0899-3467(07)60149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishikawa H., Saeki T., Otani T., Suzuki T., Shimozuma K., Nishino H., Fukuda S., Morimoto K. Aged garlic extract prevents a decline of NK cell number and activity in patients with advanced cancer. J. Nutr. (2006);136:816S–820S. doi: 10.1093/jn/136.3.816S. [DOI] [PubMed] [Google Scholar]
- 8.Borek C. Antioxidant health effects of aged garlic extract. J. Nutr. (2001);131:1010S–1015S. doi: 10.1093/jn/131.3.1010S. [DOI] [PubMed] [Google Scholar]
- 9.Uda N., Kashimoto N., Sumioka I., Kyo E., Sumi S., Fukushima S. Aged garlic extract inhibits development of putative preneoplastic lesions in rat hepatocarcinogenesis. J. Nutr. (2006);136:855S–860S. doi: 10.1093/jn/136.3.855S. [DOI] [PubMed] [Google Scholar]
- 10.Lau B.H., Yamasaki T., Gridley D.S. Garlic compounds modulate macrophage and T-lymphocyte functions. Mol. Biother. (1991);3:103–107. doi: 10.1007/BF02172082. [DOI] [PubMed] [Google Scholar]
- 11.Lee Y.M., Gweon O.C., Seo Y.J., Im J., Kang M.J., Kim M.J., Kim J.I. Antioxidant effect of garlic and aged black garlic in animal model of type 2 diabetes mellitus. Nutr. Res. Pract. (2009);3:156–161. doi: 10.4162/nrp.2009.3.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim M.H., Kim M.J., Lee J.H., Han J.I., Kim J.H., Sok D.E., Kim M.R. Hepatoprotective effect of aged black garlic on chronic alcohol-induced liver injury in rats. J. Med. Food. (2011);14:732–738. doi: 10.1089/jmf.2010.1454. [DOI] [PubMed] [Google Scholar]
- 13.Jang E.K., Seo J.H., Lee S.P. Physiological activity and antioxidative effects of aged black garlic (Allium sativum L.) extract. Korean J. Food Sci. Technol. (2008);40:443–448. [Google Scholar]
- 14.Kang M.J., Lee S.J., Shin J.H., Kang S.K., Kim J.G., Sung N.J. Effect of garlic with different procesing on lipid metabolism in 1% cholesterol fed rats. J. Korean Soc. Food Sci. Nutr. (2008);37:162–169. doi: 10.3746/jkfn.2008.37.2.162. [DOI] [Google Scholar]
- 15.Seo Y.J., Gweon O.C., Lee Y.M., Kang M.J., Kim J.I. Effect of garlic and aged black garlic on hyperglycemis and dyslipidemia in animal model of type 2 diabetes mellitus. J. Food Sci. Nutr. (2009);14:1–7. doi: 10.3746/jfn.2009.14.1.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung Y.M., Lee S.H., Lee D.S., You M.J., Chung I.K., Cheon W.H., Kwon Y.S., Lee Y.J., Ku S.K. Fermented garlic protects diabetic obese mice when fed a highfat diet by antioxidant effects. Nutr. Res. (2011);31:387–396. doi: 10.1016/j.nutres.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Wang X., Jiao F., Wang Q.W., Wang J., Yang K., Hu R.R., Liu H.C., Wang H.Y., Wang Y.S. Aged black garlic extract induces inhibition of gastric cancer cell growth in vitro and in vivo. Mol. Med. Rep. (2012);5:66–72. doi: 10.3892/mmr.2011.588. [DOI] [PubMed] [Google Scholar]
- 18.Kang J.S., Lee W.K., Lee C.W., Yoon W.K., Kim N., Park S.K., Lee H.S., Park H.K., Han S.B., Yun J., Lee K., Lee K.H., Park S.K., Kim H.M. Improvement of highfat diet-induced obesity by a mixture of red grape extract, soy isoflavone and L-carnitine: implications in cardiovascular and non-alcoholic fatty liver diseases. Food Chem. Toxicol. (2011);49:2453–2458. doi: 10.1016/j.fct.2011.06.071. [DOI] [PubMed] [Google Scholar]
- 19.Foretz M., Guichard C., Ferre P., Foufelle F. Sterol regulatory element binding protein-1c is a major mediator of insulin action on the hepatic expression of glucokinase and lipogenesis-related genes. Proc. Natl. Acad. Sci. U. S. A. (1999);96:12737–12742. doi: 10.1073/pnas.96.22.12737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uemitsu N., Nishimura C., Nakayoshi H. Evaluation of liver weight changes following repeated administration of carbon tetrachloride in rats and body-liver weight relationship. Toxicology. (1986);40:181–190. doi: 10.1016/0300-483X(86)90077-6. [DOI] [PubMed] [Google Scholar]
- 21.Domitroviæ R., Jakovac H., Tomac J., Sain I. Liver fibrosis in mice induced by carbon tetrachloride and its reversion by luteolin. Toxicol. Appl. Pharmacol. (2009);241:311–321. doi: 10.1016/j.taap.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Fujii T., Fuchs B.C., Yamada S., Lauwers G.Y., Kulu Y., Goodwin J.M., Lanuti M., Tanabe K.K. Mouse model of carbon tetrachloride induced liver fibrosis: Histopathological changes and expression of CD133 and epidermal growth factor. BMC Gastroenterol. (2010);10:79. doi: 10.1186/1471-230X-10-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin Y., Tian Y.P. Protective effects of total glucosides of paeony and the underlying mechanisms in carbon tetrachloride- induced experimental liver injury. Arch. Med. Sci. (2011);7:604–612. doi: 10.5114/aoms.2011.24129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boll M., Weber L.W., Becker E., Stampfl A. Mechanism of carbon tetrachloride-induced hepatotoxicity. Hepatocellular damage by reactive carbon tetrachloride metabolites. Z. Naturforsch. C. (2001);56:649–659. doi: 10.1515/znc-2001-7-826. [DOI] [PubMed] [Google Scholar]
- 25.Nagi M.N., Alam K., Badary O.A., al-Shabanah O.A., al-Sawaf H.A., al-Bekairi A.M. Thymoquinone protects against carbon tetrachloride hepatotoxicity in mice via an antioxidant mechanism. Biochem. Mol. Biol. Int. (1999);47:153–159. doi: 10.1080/15216549900201153. [DOI] [PubMed] [Google Scholar]
- 26.Ajith T.A., Janardhanan K.K. Antioxidant and antihepatotoxic activities of Phellinus rimosus (Berk) Pilat. J. Ethnopharmacol. (2002);81:387–391. doi: 10.1016/S0378-8741(02)00042-9. [DOI] [PubMed] [Google Scholar]
- 27.Kuriakose G.C., Kurup M.G. Antioxidant and antihepatotoxic effect of Spirulina laxissima against carbon tetrachloride induced hepatotoxicity in rats. Food Funct. (2011);2:190–196. doi: 10.1039/c0fo00163e. [DOI] [PubMed] [Google Scholar]
- 28.Hassan M.H., Edfawy M., Mansour A., Hamed A.A. Antioxidant and antiapoptotic effects of capsaicin against carbon tetrachloride-induced hepatotoxicity in rats. Toxicol. Ind. Health. (2012);28:428–438. doi: 10.1177/0748233711413801. [DOI] [PubMed] [Google Scholar]
- 29.Decker K., Keppler D. Galactosamine induced liver injury. Prog. Liver Dis. (1972);4:183–199. [PubMed] [Google Scholar]
- 30.Shanmugarajan T.S., Sivaraman D., Somasundaram I., Arunsundar M., Krishnakumar E., Balaji R., Ravichandiran V. Influence of alpha lipoic acid on antioxidant status in D-galactosamine-induced hepatic injury. Toxicol. Ind. Health. (2008);24:635–642. doi: 10.1177/0748233708101215. [DOI] [PubMed] [Google Scholar]
- 31.Yoo Y.M., Nam J.H., Kim M.Y., Choi J., Park H.J. Pectolinarin and pectolinarigenin of Cirsium setidens prevent the hepatic injury in rats caused by D-galactosamine via an antioxidant mechanism. Biol. Pharm. Bull. (2008);31:760–764. doi: 10.1248/bpb.31.760. [DOI] [PubMed] [Google Scholar]
- 32.Seo D.Y., Lee S., Figueroa A., Kwak Y.S., Kim N., Rhee B.D., Ko K.S., Bang H.S., Baek Y.H., Han J. Aged garlic extract enhances exercise-mediated improvement of metabolic parameters in high fat diet-induced obese rats. Nutr. Res. Pract. (2012);6:513–519. doi: 10.4162/nrp.2012.6.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]


