Case Presentation
A 64-year-old male with a 15-year history of poorly controlled type 2 diabetes and a 10-year history of hypertension and hyperlipidemia had developed multiple diabetes-related complications within the last 5 years. He first developed albuminuria 5 years ago, and over the next several years experienced fairly rapid decline in kidney function, with eGFR of 55 mL/min/1.73m2 noted 2 years ago. He was diagnosed with proliferative retinopathy 5 years ago and underwent laser photocoagulation. Four years ago, he noted symptoms of peripheral neuropathy manifested as shooting pain and numbness with loss of light touch, thermal and vibratory sensation in a stocking distribution. Last year he developed a non-healing ulcer on the plantar aspect of his left foot which was complicated with gangrene and resulted in a below-the-knee amputation of the left leg one year ago. He now reports a new onset of weakness, lightheadedness and dizziness on standing that affects his daily activities. He reports lancinating pain in his right lower extremity, worse in the evening. Medications include: neutral protamine Hagedorn insulin twice daily and regular insulin on a sliding scale, metoprolol 50 mg/d, lisinopril 40 mg/d, atorvastatin 80 mg/d, furosemide 40 mg/d and aspirin 81 mg/d. Blood pressure is 127/69 mm Hg with a pulse rate of 96 bpm while supine and 94/50 mmHg with a pulse rate of 102 bpm while standing. Strength is normal but with a complete loss of all sensory modalities to the knee in his remaining limb and up to the wrists in both upper extremities, and he is areflexic. Today's laboratory evaluations show a serum creatinine of 2.8 mg/dl, an estimated GFR (eGFR) of 24 ml/min/1.73m2, a hemoglobin A1c (HbA1c) of 7.9 % and 2.1 g of urine protein per gram of creatinine. What would be the most appropriate management for this patient?
Introduction
Diabetic nephropathy is the leading cause of end stage renal disease (ESRD) requiring renal replacement therapy in the U.S.1A The progression of diabetes to advanced stages of chronic kidney disease is associated with the progression of multiple other micro and macrovascular complications of diabetes including diabetic neuropathies1. Diabetic peripheral neuropathy is a common complication of diabetes associated with high morbidity, poor quality of life and high risk of lower-extremity amputation. Similarly, cardiac autonomic neuropathy is associated with life-threatening consequences, such as silent myocardial ischemia and high mortality 2-4.
In this review we will examine the characteristics of cardiac autonomic neuropathy and diabetic peripheral neuropathy in diabetic patients with stage 4-5 chronic kidney disease (CKD) or end stage renal disease (ESRD) undergoing dialysis. We will describe the evidence supporting the available therapeutic options and the challenges associated with providing adequate care for these patients and discuss future directions for investigation.
Epidemiology
In patients with diabetes and CKD or ESRD on dialysis, cardiovascular events represent the leading cause of mortality 5-7 with a high incidence of sudden cardiac death. A recent study of the National Institute of Diabetes and Digestive and Kidney Diseases reported an annual death rate of 230 deaths/1000 patient years for US dialysis patients in 2004 8. Forty-three percent of all-cause mortality in hemodialysis and peritoneal dialysis patients was secondary to cardiovascular disease, with approximately 60% of cardiac deaths being secondary to arrhythmic mechanisms 8, 9. Moreover, an association between compromised autonomic function and sudden cardiac death in patients awaiting kidney transplant has been reported 9, 10. The many comorbidities associated with diabetes-induced CKD and the presence of unique metabolic/physiologic alterations of the uremic state make the management of coronary artery disease (CAD) and the prevention of sudden cardiac death challenging in patients with stage 4-5 CKD.
Available evidence demonstrates that patients with stage 4-5 CKD due to diabetic nephropathy present also with multiple neurological complications of diabetes including cardiovascular autonomic neuropathy11 and diabetic peripheral neuropathy. Several studies utilizing measures of heart rate variability for the evaluation of cardiac autonomic neuropathy have established abnormalities in up to 62% of dialysis patients 12-14. These abnormalities frequently occur in the absence of clinical symptoms of autonomic dysfunction and were also described in patients with stage 4-5 CKD prior to dialysis 15. In nondiabetic patients, uremic neuropathy develops at glomerular filtration rates below 12 ml/min/1.73m2 and usually has an insidious onset progressing over months 16; however, in diabetes the presence of peripheral neuropathy may be detected at much earlier stages of decreased kidney function in patients with type 1 diabetes and even at the time of diagnosis in patients with type 2 diabetes 17. The prevalence of neuropathic pain in these patients varies, but was described as high as 50% of all dialysis patients 17.
The presence of cardiac autonomic neuropathy and diabetic peripheral neuropathy further increases the morbidity and mortality risk and negatively impacts quality of life of individuals with late stage CKD.
Pathogenesis
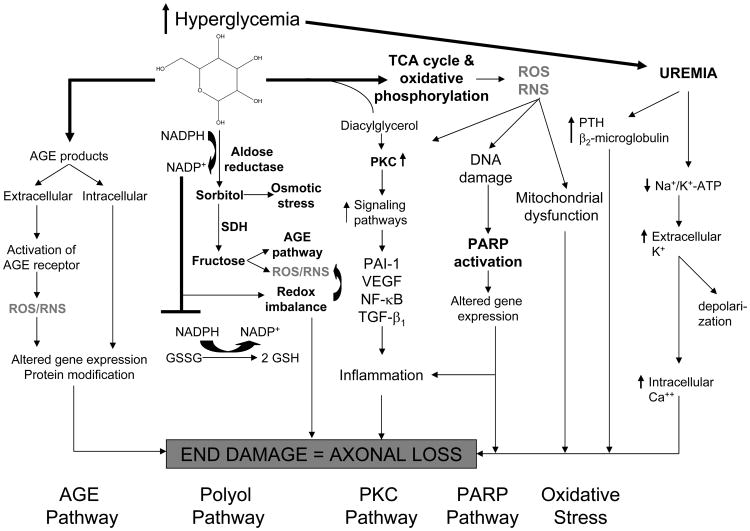
The full spectrum of mechanisms inducing neurotoxicity in diabetic patients with CKD remains unclear. In diabetes, the development of cardiac autonomic neuropathy and diabetic peripheral neuropathy is a function of complex interactions between the degree of hyperglycemia, disease duration, age-related neuronal attrition, and systolic and diastolic blood pressures 18, 19. Hyperglycemia clearly plays a key role in the development and progression of both cardiac autonomic neuropathy and diabetic peripheral neuropathy through activation of biochemical pathways related to the metabolic and/or redox state of the cell. Pathways which are mainly driven by metabolism are: glucose flux through the polyol pathway; the hexosamine pathway; excess/inappropriate activation of protein kinase C (PKC) isoforms; Na+/K+ pump dysfunction 20, 21 and, accumulation of advanced glycation end-products 22, 23. While each pathway may be injurious alone, collectively they cause an imbalance in the mitochondrial redox state of the cell and lead to excess formation of reactive oxygen species (ROS) 24, 25. Increased oxidative stress within the cell leads to activation of the poly(ADP-ribose) polymerase (PARP) pathway, which regulates the expression of genes involved in promoting inflammatory reactions, microvascular deficits and neuronal dysfunction 22(Figure 1). Two articles recently reviewed this topic 22, 23.
Figure 1.
Proposed paradigm of the mechanisms inducing neurotoxicity in diabetic patients with CKD.
Abbreviations: AGE, advanced glycation endproducts; CKD, chronic kidney disease; NF-κB: Nuclear factor kappa B; PARP: poly(ADP-ribose) polymerase; PKC: protein kinase C; PTH, parathyroid hormone; RNS: Reactive nitrogen species; ROS: Reactive oxygen species, GSH: glutathione; GSSG: oxidized glutathione; TCA, tetracylic antidepressant; VEGF: Vascular endothelial growth factor; NADP, nicotinamide adenine dinucleotide phosphate; NADPH, reduced NADP; SDH, sorbitol dehygrogenase; PAI 1, plasminogen activator inhibitor 1.
Adapted and reproduced from Edwards et al22 with permission of Elsevier.
The superimposed uremic state with its constellation of unique metabolic/physiologic alterations contributes to a more rapid onset and progression of both cardiac autonomic neuropathy and diabetic peripheral neuropathy. Some evidence suggests that the presence of a variety of toxins, including parathyroid hormone (PTH) and β2-microglobulin (the levels of which are elevated in patients with ESRD) may underlie the development of uremic neuropathy 26-28. Older experimental evidence proposed that the neurotoxicity associated with the uremic state may be due to alterations in membrane excitability induced by an inhibitory effect on the activity of the axonal Na+/K+ pump, which would abolish the direct contribution of the hyperpolarizing pump current to the membrane potential, leading to an accumulation of extracellular K+ that causes further depolarization 29. The Na+/K+ pump is of critical importance in maintaining normal ionic gradients, which are essential for axonal survival. More recent evidence in humans has shown that hyperkalemia, and not Na+/K+ pump dysfunction, is primarily responsible for uremic depolarization and likely a contributing factor to the development of neuropathy 30. An abnormal pattern of response to ischemia in dialysis patients was however not fully explained by the hyperkalaemic membrane depolarization, suggesting that some other factor, possibly H+ ions, affects nerve excitability in these patients, but that this factor only becomes evident during ischemia31. Disruption of these various ionic gradients may affect the Na+/Ca2+ exchanger, leading to increased levels of intracellular Ca2+ and axonal loss32. In this respect, several studies in CKD patients progressing to ESRD have demonstrated significant alterations in membrane potential prior to hemodialysis, with recovery in the postdialysis period 30, 31, 33.
Measures of motor and sensory nerve excitability have been assessed in relation to changes in serum levels of potential neurotoxins, including K+, Ca2+, urea, uric acid, and middle molecules such as parathyroid hormone (PTH) and β2-microglobumin. Predialysis excitability abnormalities become apparent with serum K+ concentrations in the high normal range, well below the levels required to produce cardiac toxicity. These changes in nerve excitability are strongly correlated with serum K+ in all studies, suggesting that hyperkalemic depolarization may underlie the development of uremic neuropathy 34. The abnormal excitability in dialysis patients is different from that noted in patients with diabetic peripheral neuropathy in the absence of uremia 34, suggesting that the abnormalities noted in dialysis patients are consequences of both structural changes and acute metabolic changes (Figure 1).
The development of cardiac autonomic neuropathy in diabetes patients is characterized by early augmentation of sympathetic tone when compared to healthy individuals. Sympathetic activation also may play a central role in the pathogenesis of CKD 35-37. It is proposed that an imbalance between sympathetic and parasympathetic activities in diabetic dialysis patients is further augmented by the effects and consequences of uremia 38. Enhanced cardiac sympathetic nervous system activity may contribute to myocardial injury.39, 40 Abnormally high myocardial norepinephrine, reflecting sympathetic hyperactivity, results in abnormal norepinephrine signaling and metabolism39, 41, cytotoxic effects to the heart through increased mitochondrial ROS production 40, 42, and calcium-dependent apoptosis 43, 44. Therefore, activation of the sympathetic adrenergic system, which is documented in stage 4-5 CKD patients 35, 36, is likely involved in the pathogenesis of arrhythmias, cardiomyopathy, CAD, heart failure, and sudden death.
Cardiac Autonomic Neuropathy
Clinical manifestations
Clinically, cardiac autonomic neuropathy presents with abnormal heart rate variability, resting tachycardia, exercise intolerance, decreased baroreflex sensitivity and orthostatic hypotension (Box 1). In CKD patients, cardiac autonomic neuropathy often manifests as exercise intolerance due to a reduced response in heart rate and blood pressure, and blunted increases in cardiac output in response to exercise (Box 1) 45, 46.
Box 1. Clinical manifestations of Cardiac Automomic Neuropathy in Patients with CKD and Dialysis.
Abnormal heart rate variability
Resting tachycardia with fixed heart rate
Exercise intolerance
Decreased baroreflex sensitivity
Orthostatic hypotension (a fall >30 mm Hg in systolic or >10 mm Hg in diastolic BP in response to a postural change from supine to standing)
Intradialytic hypotension
Silent myocardial ischemia/cardiac denervation syndrome
Abbreviations: BP, blood pressure; CKD, chronic kidney diease.
Whereas abnormalities in heart rate variability are usually early findings of cardiac autonomic neuropathy, resting tachycardia and a fixed heart rate are characteristic late findings in diabetic patients with vagal impairment 47-49. Heart rate however may not provide a reliable diagnostic criterion of cardiac autonomic neuropathy in the absence of other causes unless it is increased by more than 100 beats per minute (bpm). It is generally accepted that a fixed heart rate that is unresponsive to moderate exercise, stress, or sleep indicates almost complete cardiac denervation 50.
Orthostatic hypotension, defined by the American Autonomic Society and the American Academy of Neurology as >30 mm Hg decrease in systolic or >10 mm Hg decrease in diastolic BP in response to a postural change from supine to standing,51 occurs in diabetic patients largely as a consequence of efferent sympathetic vasomotor denervation, causing reduced vasoconstriction of the splanchnic and other peripheral vascular beds. Symptoms associated with orthostatic hypotension include: lightheadedness, weakness, faintness, dizziness, visual impairment, and syncope on standing. The contribution of autonomic dysfunction to the development of hypotension during dialysis remains a matter of ongoing debate, with some studies suggesting a possible association 52, 53 and others suggesting no significant relationship 54.
Another clinical manifestation of cardiac autonomic neuropathy with important prognostic implications is the silent myocardial ischemia/cardiac denervation syndrome. Patients present with reduced appreciation for ischemic pain, which can impair timely recognition of myocardial ischemia or infarction, and thereby delay appropriate therapy50. It has been shown that diabetic subjects with alterations of cardiac sympathetic innervation, tone, and/or responsiveness exhibit abnormal myocardial blood flow regulation 55-60, which may increase mortality associated with myocardial ischemia. Regional cardiac sympathetic imbalance may promote malignant arrhythmogenesis and cardiac death, particularly when accompanied by reduced parasympathetic tone and myocardial ischemia 61-63. Consequently, sudden cardiac death is the ultimate severe clinical consequence of cardiac autonomic neuropathy and is the single greatest cause of mortality in CKD patients on dialysis 9.
A meta-analysis of studies of diabetic patients concluded that the mortality of subjects without cardiac autonomic neuropathy during 5.5 years of observation was 5%, but that this increased to 27% with onset of cardiac autonomic neuropathy 64. When impairment of cardiovascular autonomic function is combined with left ventricular hypertrophy, an independent risk factor for shortened survival in CKD patients as well as for cardiovascular disease 65, 66, the mortality of CKD patients increased further.67
Evaluation
Evaluation of Heart Rate Variability
Variability in the instantaneous beat-to-beat heart rate intervals is a function of sympathetic and parasympathetic activity that regulates the cardiac functional response to the body's level of metabolic activity. The autonomic nervous system transmits impulses from the central nervous system to peripheral organs and is responsible for controlling the heart rate, blood pressure and respiratory activity. In healthy individuals the heart rate has a high degree of beat-to-beat variability. Assessment of heart rate variability provides a non-invasive method for investigating autonomic input into the heart. It quantifies the amount by which the R–R interval or heart rate changes from one cardiac cycle to the next. heart rate variability fluctuates with respiration: it increases with inspiration and decreases with expiration and is primarily mediated by parasympathetic activity.
Heart rate variability studies should be performed as a battery of autonomic tests (i.e., R-R response to deep breathing, Valsalva maneuver, and R-R response to postural changes), and ideally under paced breathing (Box 2). Incorporating respiratory signal analysis enables one to independently measure each branch of the autonomic nervous system. These validated tests, described in detail in a Statement by the American Diabetes Association 68 are summarized in Box 2.
Box 2. Heart rate variability studies for the Diagnosis of Cardiac Autonomic Neuropathy.
Clinical Tests
Note: all but orthostatic hypotension measure cardiovagal function
Beat-to-beat variation with deep breathing (E:I ratio)1
Changes in heart rate with standing (30:15 ratio)a
Changes in heart rate with Valsalve maneuver (Valsalva ratio)a
Orthostatic hypotensionb
ECG recordingsc
Time Domain Indices
(measure cardiovagal function)
mean normal-to-normal R-R intervala
mean heart rate
standard deviation of all normal R–R intervals
standard deviation of 5-min average of normal R-R intervals
root-mean square of the difference of successive R-R intervals
Frequency Domain Indices
Spectral analysis: high-frequency (0.15–0.40 Hz) power (measures parasympathetic function)
Spectral analysis of heart rate variation, very-low-frequency (0.003–0.04 Hz) power (measures sympathetic function)
Very-low-frequency power/high-frequency power (measures sympathetic/parasympathetic balance)
Other
QT/QTc intervals
aNormative cutpoints had been recommended for interpretation of the various heart rate variability (heart rate variability) indices More recent studies though demonstrate that heart rate variability is affected by several factors, mainly age and gender 158, 159. Therefore, adjustments for these variables are recommended for higher accuracy 50, 158, 159.
bOrthostatic hypotension is defined as a fall >30 mm Hg in systolic or >10 mm Hg in diastolic BP in response to a postural change from supine to standing)
c24-hour ECG recordings are in general recommended although shorter recordings can be used 160.
Heart rate variability also can be analyzed by using different indices in the time and frequency domains. Time-domain analysis measures normal R–R intervals and various measures are computed from these intervals usually during a 24-h ECG recording, although these measures can be computed from shorter recordings if necessary. The most commonly used measurements are described in Box 2. Frequency-domain analysis splits the heart rate signal into constituent frequency components, mainly using fast Fourier transformation that decomposes the signal into a set of sine and cosine waves. The sympathetic system primarily generates the very low-frequency components (0.003–0.04Hz) and the parasympathetic system primarily generates the high-frequency components (0.15 to 0.4 Hz) (Box 2).
Evaluation by Scintigraphic and Other Techniques
Quantitative scintigraphic assessment of sympathetic innervation of the human heart is possible with either [123I]meta-iodobenzylguanidine (MIBG) or [11C]HED. Deficits of LV [123I]MIBG and [11C]HED retention have been identified in type 1 57, 60, 69-74 and type 2 75, 76 diabetic subjects with 57, 71, 74 and without 77 abnormal cardiovascular reflex testing. [11C]HED undergoes highly specific uptake into sympathetic nerve varicosities via norepinephrine transporters (“uptake-1”) 78. [11C]HED is metabolically stable and its neuronal retention requires intact vesicular storage 78. Therefore, [11C]HED retention correlates with myocardial neuronal norepinephrine content and norepinephrine transporter density 79, 80. Evaluation of these scintigraphic techniques in stage 4-5 CKD or dialysis patients has not yet been reported.
Microneurographic techniques are based on recording electrical activity emitted by peroneal, tibial, or radial muscle sympathetic nerves and identification of muscle sympathetic bursts, which reflect the centrally generated postganglionic sympathetic nerve activity towards the human skeletal muscle vasculature. Recently developed, fully automated sympathetic neurogram techniques provide a rapid and objective method that is minimally affected by signal quality and preserves beat-by-beat sympathetic neurograms 81.
Diabetic Peripheral Neuropathy
Clinical Manifestations
Diabetic peripheral neuropathy in stage 4-5 of CKD presents as a distal symmetrical polyneuropathy with greater lower-limb than upper-limb involvement. The most frequent clinical features of uremic neuropathy are those of large-fiber involvement, with paresthesias, impaired or absent deep tendon reflexes, impaired vibration sense, distal foot muscle wasting and weakness 34. In patients with diabetes on dialysis, however, symptoms and signs of small fiber involvement can dominate, with patients experiencing severe burning and shooting pains with impaired pain and temperature perception. Commonly, both large and small fibers can be affected in CKD patients with diabetic peripheral neuropathy. Sensory deficits overshadow motor nerve dysfunction and appear first in the distal portions of the extremities and progress proximally in a “stocking-glove” distribution. In patients with CKD, the severity of diabetic peripheral neuropathy is directly correlated with the duration of diabetes, the degree of glycemic control, and the degree of uremia 22. In rare cases, diabetic peripheral neuropathy may first present with late complications such as ulceration or neuroarthropathy (Charcot's joints) of the foot; both of these complications are more prevalent in patients with ESRD82.
Lower Limb Amputation
The diabetic population with stage 4-5 CKD is at extremely high risk of lower limb amputation. A prospective study reported that rate of lower limb amputation increased during a four-year period of follow-up from 4.8 per 100 person years to 6.2 per 100 person years 83. The rate among diabetic patients with stage 4-5 CKD was 10 times greater than in the diabetic population at large, and two-thirds died within two years following the first amputation 83. In a cohort of 29,838 patients in DOPPS (Dialysis Outcomes and Practice Patterns Study) followed from 1996 to 2004, diabetic patients on dialysis had more than a 9-fold greater incidence of new amputation, a higher mortality risk and shorter survival after their first amputation compared with nondiabetic dialysis patients 84
Evaluation
A consensus statement from the San Antonio Conference on Diabetic Neuropathy recommended that the diagnosis and classification of diabetic peripheral neuropathy for research and clinical trials be based on at least one standardized measure from each of the following categories: clinical symptoms, clinical examination, electrophysiology and quantitative sensory testing 85. It is important to note that the diagnosis of subclinical or clinical diabetic peripheral neuropathy requires that signs (e.g., abnormal quantitative tests for neuropathy) and symptoms (for clinical neuropathy) are not attributable to a nondiabetic etiology. Because there are no distinguishing features unique to diabetic peripheral neuropathy, all other possible causes of the observed neuropathic disorders must be ruled out by careful history and physical examination. Considering that the uremic state per se is associated with direct pathological effects on peripheral nerve fibers, as mentioned above, the neurological deficits found in the diabetic patients are in general more advanced and include all types of fibers.
A simple neurological examination is usually sufficient to diagnosis diabetic peripheral neuropathy in patients with CKD or on dialysis. The examiner carefully inspects the patient's feet, looking for evidence of dryness, fissures or skeletal abnormalities such as hammer toes, all of which suggest neuropathy. Small fiber function is assessed with a 10 gram monofilament, a pin and a cotton wasp on the dorsum of the great toe and first forefinger. The 10 gram monofilament can also be used on the plantar surface of the foot. Large fiber function is assessed using a 256 Hz tuning fork to determine vibratory sensation on the dorsum of the great toe and forefinger; if altered, proprioception is assessed using small up/down movements of the distal joints of the toe and first finger. Reflexes are assessed, with special emphasis on the Achilles reflex. A simple screening tool for the diagnosis of diabetic peripheral neuropathy, the Michigan Neuropathy Screening Instrument, is publicly available (www.pnrd.umich.edu) and incorporates these measures with a high degree of sensitivity and specificity.
More quantitative techniques exist to assess warm and cold perception thresholds and current perception thresholds. These techniques have been developed for research purposes, are generally time-consuming and require specialized equipment, and thus are not routinely employed in a clinical setting. In contrast, nerve conduction studies can be used to quantify the degree of nerve injury in diabetic peripheral neuropathy in CKD and dialysis patients in an outpatient setting 86. While not usually required for diagnosis, nerve conduction studies can help the patient and physician monitor diabetic peripheral neuropathy progression over a long period of time, particularly if the patient is asymptomatic. Nerve conduction studies are also useful to identify superimposed mononeuropathies, e.g., carpal tunnel syndrome, which are also a common problem in dialysis patients with diabetic peripheral neuropathy.
Therapeutic Strategies
In diabetic patients with CKD, as in all patients with diabetes, therapies for cardiac autonomic neuropathy and diabetic peripheral neuropathy may be divided into treatments that target the underlying pathogenic mechanisms and those aimed at relieving symptoms22, 23, 68, 87.
Therapies to Interrupt Pathogenic Mechanisms
In spite of significant efforts undertaken over the last few decades to develop effective agents targeting principal pathways in the diabetic patient that are involved in nervous system dysfunction, the only proven method currently available to prevent cardiac autonomic neuropathy and diabetic peripheral neuropathy or to slow their progression is strict glycemic control. Other than strict glycemic control, disease modifying treatments for neuropathies are presently only experimental and are as such beyond the scope of this review. However, a recent review discusses this topic 22.
The benefits of strict glycemic control were demonstrated in type 1 diabetes by large randomized controlled trials such as the DCCT (Diabetes Control and Complications Trial) 88-90 and by observational clinical trials such as EURODIAB.91, 92 Tight control of blood glucose remains the optimal treatment for prevention of diabetic nephropathy, as well. The EDIC (Epidemiology of Diabetes Interventions and Complications), a prospective observational study of the DCCT cohort, has shown that the differences in diabetic peripheral neuropathy and cardiac autonomic neuropathy between the intensive and conventional treatment groups observed at the end of the DCCT have persisted for a decade despite the loss of glycemic separation between the groups following the end of the DCCT 93, 94, 95. However, there are no similar data demonstrating benefit of tight glucose control on cardiac autonomic neuropathy or diabetic peripheral neuropathy outcomes in type 1 diabetic patients with significant CKD or those on renal replacement therapies. Thus, strict glycemic control should be pursued actively in type 1 diabetic patients before they manifest a substantial reduction in GFR, but it is unclear whether this remains a good strategy in patients with advanced CKD or on dialysis.
The evidence linking good glycemic control with prevention or delay of progression of diabetic neuropathy is more limited in patients with type 2 diabetes. A randomized prospective 6-year study in 110 Japanese patients with type 2 diabetes, demonstrated that intensive insulin therapy prevents the progression of diabetic peripheral neuropathy96. In the UKPDS (UK Prospective Diabetes Study), intensive treatment significantly reduced the risk of an aggregate endpoint of microvascular complications mainly comprised of retinopathy and nephropathy; however, there was only a trend for reduction in the risk of amputation in the intensively treated patients 97. There was a significant reduction in the risk of sudden death in which it is possible that cardiac autonomic neuropathy plays a role 98.
In the Veterans Administration Cooperative Study on Type 2 Diabetes, there was no improvement in clinical diabetic peripheral neuropathy or cardiac autonomic neuropathy in the intensive treatment group 99. The Steno-2 Study compared the effect of a targeted, intensified, multifactorial intervention with that of conventional treatment on modifiable risk factors for cardiovascular disease including intensive treatment of hyperglycemia in patients with type 2 diabetes and microalbuminuria. After a mean follow-up of 7.8 years, patients receiving the intensive intervention had a significant reduction in the risk of cardiac autonomic neuropathy 100. However, the risk reduction was mainly driven by the reduction in systolic and diastolic blood pressure.100.
In summary, the interventional evidence targeting hyperglycemia to prevent diabetic peripheral neuropathy and cardiac autonomic neuropathy in patients with type 2 diabetes is less conclusive than that in patients with type 1 diabetes. Yet, in view of the strong associations between glucose control as measured by HbA1c and the incidence, prevalence, and progression of neuropathy in all forms of diabetes, as well as the overwhelming evidence demonstrating the beneficial effects of tight glucose control on diabetic nephropathy, aggressive treatment aiming for normalization of both fasting and postprandial glucose levels remains the first and most important step in treating patients with cardiac autonomic neuropathy and diabetic peripheral neuropathy in patients with diabetes and earlier stages (1-3) of CKD.
Management of Cardiac Autonomic Neuropathy
Therapies for Sympathetic Hyperactivity
Activation of the sympathetic adrenergic system is well documented in stage 4-5 CKD patients as outlined above, and is likely involved in the pathogenesis of cardiovascular events including sudden death. Various pharmacological approaches that modify sympathetic/parasympathetic balance have been shown to reduce the incidence of arrhythmias, and consequently of cardiovascular death, in several patient populations, including post-myocardial infarction patients or patients with congestive heart failure. Therefore, it would seem logical that the use of agents that reduce sympathetic activity and interfere with the renin system would have beneficial effects. However, the clinical evidence in stage 4-5 CKD and dialysis populations is limited and somewhat controversial (Table 1).
Table 1. Management of cardiac autonomic neuropathy is Stage 4-5 CKD.
| Class and Drug | Patient Population (N) | Design | Outcome | Evidence in CKD | Reference |
|---|---|---|---|---|---|
| ACEi/ARBs | |||||
| Ramipril | Dialysis, diabetes (11) | Prospective pilot, not controlled, 4 wks | HRV | Worsening HRV | 113 |
| Telmisartan | Stage 4-5 CKD, diabetes (10) | Prospective pilot, not controlled, 12 mos | HRV | Progressive impairment in HRV | 114 |
| ACEi | Dialysis, diabetes (187) | Retrospective analysis | HRV | No effect | 112 |
| Enalapril | CKD, nondiabetic (21) | Randomized, control vs. amlodipine | Muscle Sympathetic Activity | Muscle sympathetic activity improved with enalapril vs. amlodipine | 116 |
| ACEi/ARB | CKD, nondiabetic (31) | enalapril vs. losartan vs. eprosartan | Muscle Sympathetic Activity | Modest improvement in muscle sympathetic activity from baseline with all | 115 |
| BETA-BLOCKER | |||||
| All agents | Dialysis, diabetic and nondiabetic (43,200) | Observational cohort | Cardiac arrest | Indirect; improved odds of survival | 109 |
| Carvedilol | Dialysis with cardiomyopathy, diabetic and nondiabetic (114) | Randomized controlled | Two year all-cause and cardiovascular mortality | Indirect; increased survival | 107 |
| Beta-blockers | Dialysis, diabetic and nondiabetic (2,286) | Prospective observational cohort | All-cause mortality | Indirect; increased survival in nondiabetic but not in diabetic | 108 |
| Beta-blockers | Dialysis, diabetic and nondiabetic (11,142) | Prospective observational cohort | All-cause mortality | Indirect; increased survival overall | 103 |
| Human erythropoietin | Dialysis (27) | Prospective, not placebo controlled | HRV | No effect | 120 |
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blockers; CKD, chronic kidney disease; HRV, heart rate variability.
The benefits of beta-blockers in patients with normal kidney function and increased cardiovascular risk have been amply demonstrated101-104. However, the benefits of beta-blockade in patients with stage 4-5 CKD or in patients on renal replacement therapy remain unclear. Concerns for potential higher rates of adverse effects, including hypotension during dialysis, hyperkalemia, and glycemic abnormalities, have somewhat limited their use, although there is no clear evidence that the risk is any greater than other antihypertensive agents. A few small studies of short duration have shown improvement in heart rate variability with propranolol in nondiabetic dialysis patients 105. However, studies directly evaluating the effects of beta–blockers on cardiac autonomic neuropathy and sympathetic overactivity in diabetic patients with stage 4-5 CKD are lacking. A recent review argued that the “old” beta -blockers such as propranolol are a source of concern because they reduce insulin sensitivity and may worsen glucose control and aggravate dyslipidemia, with negative effects on both nerve and kidney function 101. The combined alpha/beta-blocker carvedilol is metabolically neutral, has beneficial effects on kidney perfusion, and could be used in diabetic patients 101. In addition to being a potent beta1-adrenergic receptor blocking agent, carvedilol blocks beta2- and alpha1-receptors at therapeutic doses and slightly reduces cardiac adrenergic drive with a more “comprehensive” degree of adrenergic inhibition 106. A prospective study of 114 dialysis patients with cardiomyopathy randomized to receive either carvedilol or placebo in addition to standard therapy, showed that carvedilol significantly increased two-year survival and decreased both all-cause and cardiovascular mortality 107. A recent study evaluating the association between beta-blocker use and all cause mortality in a large cohort from the Japan Dialysis Outcomes and Practice Patterns Study showed highly significant associations between treatment with beta-blockers and a lower risk for all-cause mortality after adjustment for multiple risk factors 108. Furthermore, in a prospective observational analysis of the United States Renal Data System Dialysis Morbidity and Mortality Study, Foley et al 103 found that the use of beta-blockers was the only class of antihypertensive agents associated with a significant reduction in all-cause mortality after adjustment of multiple comorbidities. A benefit from beta-blockade in improving the odds ratio for survival is also reported in an observational study of 729 cases of cardiac arrest in 43,200 prevalent dialysis patients between 2002 and 2005 109. Although the majority of these studies did not directly assess the changes in heart rate variability with beta-blockers, sympathetic hyperactivity has been shown to increase susceptibility to increased cardiovascular complications such as arrhythmias and sudden cardiac death 110, 111. Thus, on the basis of current evidence, it is likely that beta-blockers provide some degree of protection from cardiovascular events to advanced CKD and dialysis patients and that their beneficial survival effects are due in part to the attenuation of sympathetic hyperactivity and subsequent improvement in electrocardiographic abnormalities.
Angiotensin converting enzyme inhibitors (ACEI) and angiotensin receptor blockers (ARB) have been shown to improve heart rate variability in asymptomatic patients with diabetes or in patients with heart failure 50. In contrast, the evidence available in stage 4-5 CKD suggests no or opposite effects on heart rate variability (Table 1). A retrospective study analyzing determinants of heart rate variability in 187 dialysis patients found no associations with the use of ACEI 112. A more recent study evaluating the effects of a 4-week treatment with the ACEI ramipril on dialysis patients described a deleterious shift in several major time domain measures of heart rate variability that were consistent with an increased cardiac sympathetic tone with the use of ramipril in this patient population 113. Another small study evaluating the effects of telmisartan, an angiotensin receptor blocker, on blood pressure in patients with diabetes and stage 4-5 CKD found a deterioration of heart rate variability with an increase in the sympathetic tone after 12 months of treatment, in spite of a significant reduction in blood pressure 114. On the contrary, treatment with ACEI or ARB was shown to decrease muscle sympathetic nerve activity as assessed by nerve microneurography in nondiabetic patients with CKD and hypertension. 115, 116. It is possible that in patients with both diabetes and CKD, the decrease in the muscle sympathetic nerve activity induced by ACEI/ARB induces a baroreflex-mediated increase in sympathetic activity which may explain the changes described in heart rate variability. The use of ACEIs and ARBs is standard of care in CKD patients. Since the few prospective studies evaluating their direct effect on heart rate variability were small and not controlled, studies evaluating the longer term implications of the described changes in heart rate variability on cardiovascular events in this patient population are needed.
The benefits and risks of rHuEPO administration in dialysis patients in general are well known and beyond the scope of this review. A small collection of studies have reported that some patients with severe symptomatic cardiac autonomic neuropathy from type 1 diabetes have a normocytic anemia associated with erythropoietin deficiency prior to the point where they develop significant CKD. In these patients, treatment of with rHu EPO rapidly corrects their anemia and improves their symptoms 117-119. However, only a few investigators have evaluated the effects of rHu-EPO on measures of heart rate variability in patients with stage 4-5 CKD. One study using the standard battery of cardiovascular reflex tests (deep-breathing, Valsalva maneuver, handgrip exercise, heart rate response to standing, post-Valsalva rise in blood pressure and postural drop in blood pressure) randomized two groups of patients on maintenance hemodialysis to rHu-EPO for either one or two years of treatment and compared these patients to an untreated group. The results of the tests were compared before and after the correction of anemia by rHu-EPO in each group. There was no improvement in heart rate variability with the rHu-EPO in these patients in spite of correction of anemia 120.
Therapies for Orthostatic Hypotension
The treatment of orthostatic hypotension is challenging in patients with CKD. Non-pharmacological treatments include: avoidance of sudden changes in body posture to the head-up position; avoiding medications that aggravate hypotension, such as tricyclic antidepressants and phenothiazines; eating small, frequent meals to avoid postprandial hypotension; and avoiding activities that involve straining, since increased intra-abdominal and intra-thoracic pressure decrease venous return 50. In addition, adjusting the doses of diuretics and the timing and doses of ACEIs/ARBs needs to be considered.
Midodrine is a peripheral-selective α1-adrenoreceptor agonist and the only FDA approved agent for the treatment of orthostatic hypotension. It is widely used for dialysis-associated hypotension. Dosing regimens ranging from 2.5 to 10 mg of midodrine given 15-30 min before dialysis are safe and effective in these patients. A recent review of the literature on the use of the midodrine in the treatment of intradialytic hypotension suggested a beneficial effect in improving symptoms and signs, although the authors drew attention to the fact that most studies were not randomized and had small sample sizes 121. In addition, there have been no randomized trials examining the effects of maintenance midodrine therapy during interdialytic periods and concerns exist regarding possible adverse effects due to supine hypertension 122. There have been no reported trials of midodrine for cardiac autonomic neuropathy in stage 4-5 CKD patients.
Sertraline hydrochloride was reported to improve hemodynamic parameters of patients with dialysis-induced hypotension (DIH). A small study evaluated measures of heart rate variability response to tilt-table testing in dialysis patients with and without DIH and in healthy control subjects. Four-week treatment with 50 mg of sertraline daily induced a paradoxical reduction in indices of sympathetic modulation and sympathovagal balance in patients with DIH while there was an increase in these indices in patients without DIH and in healthy controls, suggesting that the effects of sertraline on DIH might be related to the improvement in the regulation of the autonomic response to hypovolemia 123. There have been no reported trials of sertraline for cardiac autonomic neuropathy in stage 4-5 CKD patients.
Effects of Dialysis on Measures of Cardiac Autonomic Neuropathy
The effects of hemodialysis therapy on heart rate variability parameters in diabetic patients during the 24 h period surrounding dialysis are still a matter of debate. Some investigators have not observed any change in autonomic function as a result of hemodialysis treatment 124, 125, while others have found significant improvement 126, 127. Rubinger et al. compared dialysis patients who presented with intradialytic hypotension with patients who maintained a stable blood pressure during hemodialysis and found that while on dialysis, R-R variation increased in both stable and unstable patients, and that both groups of patients demonstrated a decrease in sympathetic activity 126. They also noted that sympathetic/parasympathetic balance was significantly lower in women compared with men 126. Tong et al 127 studied 35 dialysis patients and noted significant reductions in some measures of heart rate variability during dialysis, which recovered 2 hours post dialysis to values similar to the pre-dialytic period 127. They reported that the ratio between low- and high-frequency power assessed by spectral analysis of heart rate variability was negatively correlated with ultrafiltration rate and positively correlated with Kt/V suggesting that better dialysis adequacy can improve heart rate variability.
In a study comparing heart rate variability parameters in diabetic patients to non-diabetic patients immediately before, during, and after dialysis, Giordano et al. found that diabetic patients showed cardiac sympathetic hyperactivity in the pre-dialytic period compared to non-diabetic patients 46. During the dialytic period, the sympathetic tone increased further in diabetic, although increased in the non-diabetic patients as well. Post-dialysis, in the non-diabetic group the sympathetic activity decreased significantly so that the cardiac autonomic balance shifted towards a parasympathetic predominance whereas in the diabetic group the sympathetic activity decreased only to the pre-dialytic level 46.
Some studies reported that beneficial effects of hemodialysis on heart rate variability appear to be the most pronounced during the first day of the inter-dialytic period persisting for up to 24 hours post dialysis in non-diabetic patients 127. Other long term studies showed a persistent effect. A longitudinal study comparing 20 dialysis patients (13 on hemodialysis and 7 on continuous peritoneal dialysis) to 15 healthy controls found a significant improvement in time-domain indices of heart rate variability after 12 months of dialysis, suggesting that longer term dialysis may improve autonomic dysfunction in these patients 128.
Others have explored the effects of dialysate sodium concentration, an important determinant of interdialytic weight gain and fluid balance, on blood pressure and heart rate variability. A longitudinal study comparing dialysis patients who underwent increased sodium profiling during dialysis with those who underwent conventional dialysis, found that the sodium modeling may be associated with increased blood pressure and abnormal heart rate variability over time suggestive of increased sympathetic activity 129.
Management of Painful Diabetic Peripheral Neuropathy
Pain may be managed through the use of non-opioids, opioids, and adjuvants (Table 2) 130. The treatment effect is usually assessed by the reduction in pain intensity on a visual analogue scale or an 11-point numerical rating scale ranging from ‘no pain’ to ‘worst possible pain’. This measure is often supplemented with the degree of pain relief on similar scales 131. Various measures of life quality and the patient's/assessor's impression of change can be added.
Table 2. Pharmaceutical therapies for painful diabetic peripheral neuropathy.
| Class and Drug | No. | Design | Outcome | Evidence in CKD | Reference |
|---|---|---|---|---|---|
| TCAs | |||||
| Amitriptyline | 29 | Cross over, 2×6 wks, amitriptyline > placebo | 50% pain reduction | No, requires dose adjustment | 161 |
| Desipramine | 20 | Cross over, 2×6 wks, desipramine > placebo | pain reduction | No, requires dose adjustment | 162 |
| SSRI | |||||
| Paroxetine | 29 | Randomized Cross over, 2×2× 2 wks, paroxetine 40 mg > Imipramine> placebo | pain reduction | No, requires dose adjustment | 163 |
| SNRIs | |||||
| Duloxetine | 457 | Parallel, 12 wks, duloxetine (60mg, 120 mg) > placebo | 50% pain reduction | No, requires dose adjustment | 146 |
| Duloxetine | 348 | Parallel, 12 wks, duloxetine (60mg, 120 mg > placebo | 50% pain reduction | No | 147 |
| Duloxetine | 334 | Parallel, 12 wks, duloxetine (60mg, 120 mg > placebo | 50% pain reduction | No | 148 |
| Calcium channel α2-δagonists | |||||
| Gabapentin | 165 | Parallel, 8 wks, gabapentin > placebo | 50% pain reduction | No, requires dose adjustment | 138 |
| Pregabalin | 146 | Parallel, 8 wks, pregabalin > placebo | 50% pain reduction | No, requires dose adjustment | 134 |
| Pregabalin | 338 | Parallel, 5 wks, pregabalin (300, 600 mg) > placebo | 50% pain reduction | No, requires dose adjustment | 132 |
| Pregabalin | 246 | Parallel, 6 wk, pregabalin (600 mg) > placebo | 50% pain reduction | No, requires dose adjustment | 133 |
| μ receptor agonists | |||||
| Tramadol | 127 | Parallel, 6 wks, tramadol > placebo | 50% pain reduction | No dose adjustments required | 150 |
Abbreviations: TCA, Tricyclic and tetracyclic antidepressants; SNRIs, Serotonin-Norepinephrine Reuptake Inhibitors.
Adapted and reproduced from Edwards et al22 with permission of Elsevier.
However, for patients with stage 4-5 CKD or on dialysis, dosages and dosing intervals often need to be adjusted, and side effect profiles can be distinct and severe. In addition, clinical evidence regarding the effects of the agents discussed below to treat painful diabetic peripheral neuropathy in patients on dialysis and those with stage 4-5 CKD is virtually nonexistent. Therefore in selecting the drug regimen for painful diabetic peripheral neuropathy in dialysis patients and those with stage 4-5 CKD, recommendations should take into account the specific dosing restrictions for each agent, as well as the constellation of potential side effects of these agents.
Calcium Channel α2-δ Ligands
Pregabalin is one of the two agents currently approved for the treatment of pain associated with diabetic peripheral neuropathy and acts by binding to the α2-δ subunit of L-type voltage gated calcium channels and decreasing calcium influx. As demonstrated in four randomized placebo control trials, 300 -600 mg/day pregabalin is significantly more effective in alleviating diabetic peripheral neuropathy than placebo 132-134. Unlike gabapentin, pregabalin has better GI absorption and can be administered twice per day. Its linear pharmacokinetics provide a rapid (< two weeks) onset of maximal pain relief 135. A recent review of the Cochrane database found that the number needed to treat (NNT) benefit for at least 50 % pain relief for 600 mg pregabalin vs. placebo is 5.0 (95% confidence interval 4.0-6.6) 136. The same review found that 150 mg daily dose was generally ineffective. However, side effects such as dizziness, ataxia, sedation, euphoria, ankle edema, and weight gain may limit its use. Pregabalin dosage adjustment should be considered for patients with CrCl < 60 mL/min. A 50% reduction in pregabalin daily dose is recommended for patients with CrCl between 30 and 60 mL/min compared to those with CrClr > 60 mL/min 137. Daily doses should be further reduced by an additional 50% for each additional 50% decrease in CrCl 137. Pregabalin is rapidly cleared by hemodialysis. Supplemental pregabalin doses should be given to patients after each hemodialysis treatment to patients on maintenance hemodialysis to maintain steady-state plasma pregabalin concentrations within desired ranges. It was shown that each 4-hour hemodialysis session removes approximately 50% of the drug from the body, therefore 50 mg will need to be replaced after each dialysis session 137.
Gabapentin is probably the most commonly prescribed drug for painful diabetic neuropathy due to its effectiveness and low side effect profile. Gabapentin produces analgesia also via binding to the α2-δ site of L-type voltage gated calcium channels. In general, gabapentin ≤ 2400 mg/day is effective in treating diabetic peripheral neuropathy in patients with normal kidney function, according to data obtained in several randomized control clinical trials 138, 139. However, since gabapentin is cleared solely by renal excretion and is not bound to plasma proteins, in stage 4-5 CKD dosing restrictions apply based on the creatinine clearance (CrCl) 140. It is recommended that total dose does not exceed 1400 mg/day for a CrCl of 30-60 ml/min given in two doses, 700 mg/day for a CrCl of 16-29 ml/min given in one or two doses and 300 mg/day for Cr Cl of 15 ml/min taken in one dose. For patients with a CrCl less than 15 ml/min, the dose should be decreased in proportion to the decrease in the CrCl. It is recommended that patients on maintenance dialysis receive an initial 300-mg to 400-mg gabapentin loading dose and then maintain plasma concentrations by receiving 200-300 mg after every 4 hours of hemodialysis 140. Titration to the maximal dose should be done gradually to avoid side effects, which include dizziness, ataxia, sedation, euphoria, ankle edema, and weight gain 141. The risks of myoclonus and altered consciousness due to gabapentin are increased in patients with stage 4-5 CKD and in dialysis patients 135, 142, 143. Myoclonus in these individuals is more disabling than in patients with normal kidney function, and when it occurs, discontinuation of gabapentin is mandatory135.
Tricylic and Tetracyclic Antidepressive Agents
In clinical practice, often the tricylic and tetracylic antidepressants (TCAs) are the first line treatment for neuropathic pain. They are inexpensive and effective. Their therapeutic actions are mediated by inhibition of the reuptake of norepinephrine and serotonin and therefore these agents control pain and pain related symptoms such as insomnia and depression. Pooled data from several small placebo-controlled trials suggests that approximately 1 in 3 patients experience at least 50% relief from pain by using these drugs 144. The use of TCAs is however limited by their important side effects 145. Overall, secondary amines (nortriptyline, desipramine) are better tolerated than tertiary amines (amytriptyline, imipramine), but TCAs are not well tolerated in older patients 135, 145. The TCAs should be used with great caution (or avoided altogether) in patients with cardiac arrhythmias, congestive heart failure, orthostatic hypotension, and urinary retention, which further limits their use in dialysis patients. An electrocardiogram is mandatory before the initiation of treatment. In addition, their analgesic effects require several weeks to develop which limits their utility for acute pain. TCAs are contraindicated in patients taking monoamine oxidase (MAO) inhibitors. The usual dosage schedule for TCAs is 10 to 25 mg at bedtime initially, titrating as tolerated up to 100 or 150 mg as a single bedtime dose and no dose adjustments are needed in dialysis or CKD patients.
SSRIs
Selective serotonin reuptake inhibitors (SSRIs) are newer antidepressants that have largely replaced TCAs for the treatment of depression because they are better tolerated. However, in contrast to TCAs, the effects of SSRIs on neuropathic pain associated with diabetic peripheral neuropathy are limited. Although most studies specifically evaluating diabetic peripheral neuropathy pain as a primary outcome were small and short in duration, pooled data for SSRI treatment of diabetic peripheral neuropathy demonstrates no significant difference between SSRIs and placebo 144.
Serotonin-norepinephrine Reuptake Inhibitors
Duloxetine, a serotonin-norepinephrine reuptake inhibitor, is the only other agent besides pregabalin that has been approved by the FDA for treating painful diabetic peripheral neuropathy. In three large randomized placebo control trials 146-148, duloxetine 60 mg and 120 mg daily provided significant relief from diabetic peripheral neuropathy. The higher dose, 120 mg daily provides greater relief from diabetic peripheral neuropathy pain, but is associated with increased side effects, including orthostatic hypotension, tremor, anxiety and elevated blood pressure. The presence of kidney impairment requires a lower starting dose of 30 mg and a more gradual titration with a maximum suggested dose of 60 mg daily. The use of duloxetine is not recommended for a Cr Cl of < 30 ml/min.
Anticonvulsants
Anticonvulsants control neuronal excitability by blocking sodium and/or calcium channels 149. Originally developed for preventing seizures, they are in broad use for the treatment of neuropathic pain. Phenytoin and carbamazepine primarily block the voltage gated sodium channel. At doses between 200-600 mg/day, both reduce the pain associated with diabetic peripheral neuropathy compared to placebo. However, due to quite serious side effects and newer improved therapies, we do not recommend to use these compounds in patients with stage 4-5 CKD or on renal replacement therapies.
Opioids
Opioids inhibit noxious transmission via multiple mechanisms including peripheral, presynaptic, and postsynaptic-located opioid receptors in the dorsal horn and at sites in the brain. Several randomized controlled trials have shown that opioids are effective in relieving pain in painful diabetic peripheral neuropathy 131. The most common side effects are constipation, cognitive side effects, and nausea. The risk of drug abuse and immunological side effects is a limiting factor for using these drugs in nonmalignant pain. There is no general agreement on how opioids should be dosed, but usually, these drugs are dosed with short-acting opioids every 4–6 h followed by a switch to long-acting opioids after 1–2 weeks.
Tramadol is a weak μ -receptor agonist that inhibits re-uptake of serotonin. A few small double blind, randomized, placebo controlled trials have shown that tramadol (200-400 mg/day) over short periods of time significantly reduced pain scores in patients with diabetic peripheral neuropathy 150, 151. Tramadol is well tolerated with a modest side effect profile that includes nausea, constipation, headache, and dyspepsia. In patients with stage 4-5 CKD it is recommended that treatment should start at a lower dose and carefully titrate up to a maximum of 200 mg/day, with a dosing interval of 12 hours. Since only 7% of an administered dose is removed by hemodialysis, dialysis patients can receive their regular dose on the day of dialysis.
Short term clinical trials report that 20-80 mg/day slow release oxycodone relieves pain associated with diabetic peripheral neuropathy 152. Although opioids are an effective alternative against diabetic peripheral neuropathy pain, their long term use often results in side effects including constipation, urinary retention, impaired cognitive function, impaired immune function, and issues associated with tolerance and addiction. In general, lower doses of opiates should be used in CKD and dialysis patients although a maximal daily dose is not yet well established for oxycodone.
Combination Treatment
The dynamic and plastic nature of the pain system suggests participation of several mechanisms in generation and maintenance of chronic pain 131. In neuropathic pain, the pain-signaling system is distorted and the plastic changes become increasingly complex. Hence a multifaceted treatment of these pains is a reasonable approach, but surprisingly few attempts have been made to address this issue. A recent trial showed that a combination of an opioid and gabapentin in patients with painful diabetic peripheral neuropathy resulted in improved pain relief in comparison to treatment with either agent alone allowing for successful lowering of opiate dosing 153. Another more recent randomized crossover trial evaluated the effects of oral treatment with the TCA nortriptyline, gabapentin, and their combination at maximum tolerated doses in participants with chronic pain associated with painful diabetic neuropathy 154. The results showed that pain intensity and pain-related sleep disturbance were lower with combined treatment than with each drug alone 154. Although no patients with stage 4-5 CKD were included in these trials, the use of a combination therapy in the management of pain could help achieve beneficial effects on pain reduction in this patient population.
Topical agents
Capsaicin is an extract of capsicum peppers. Capsaicin binds to TRPV1 receptor and exhausts substance P in the peripheral nerves to achieve it analgesic effects. In the study published by the Capsaicin Study Group, 0.075% capsaicin cream applied three times a day for 6 weeks was more effective in alleviating diabetic peripheral neuropathy than placebo 155. Burning was the most common side effect which tended to decrease as therapy was continued. The therapeutic effects of capsaicin are however modest and usually manifest weeks after the cream application. Recently, a patch containing high concentrated capsaicin has demonstrated promising effects in treating painful diabetic peripheral neuropathy.
Topical lidocaine 5% patches have been reported by several studies to relieve diabetic peripheral neuropathy. Lidocaine blocks voltage-gated sodium channels, and topical application is thought to silence ectopic discharges on small afferent fibers by blocking sodium channels unspecifically 131. In an open labeled study, up to four 5% lidocaine patches applied for up to 18 h/day are well tolerated in patients with painful diabetic peripheral neuropathy. Lidocaine patches significantly improved pain and quality-of-life ratings, and may allow tapering of concomitant analgesic therapy. However data from randomized controlled trials in patients with painful diabetic peripheral neuropathy and CKD are not available.
Botulinum toxin, which has been shown to inhibit vanilloid receptors to inhibit release of glutamate and substance P, has also been shown to have a pain-relieving effect in one randomized trial in patients with painful diabetic peripheral neuropathy 156, but no data is available for stage 4-5 CKD.
Non Pharmacologic Treatment of Neuropathic Pain
Because drug treatment is associated with numerous side effects and may be ineffective in many CKD and hemodialysis patients, nonpharmacologic strategies such as electrotherapy are a potential recourse. Among various forms of electrostimulation, high-tone external muscle stimulation may be a promising alternative treatment for stage 4-5 CKD and dialysis patients with symptomatic diabetic peripheral neuropathy. A recent small pilot studying 25 patients on maintenance dialysis diagnosed with symptomatic diabetic peripheral neuropathy showed that high-tone external muscle stimulation can ameliorate the discomfort and pain associated with both diabetic and uremic neuropathy and could be a valuable supplement in the treatment of pain and neuropathic discomfort in these patients 157. Finally, coordinated programs to screen and provide regular foot care for those patients at high risk due to diabetic peripheral neuropathy, combined with guidelines for treatment and referral of ulceration, are needed and will assist in amputation prevention and quality of life.
Case Review
The patient discussed in the opening vignette has stage 4 CKD secondary to diabetic nephropathy and presents with painful diabetic peripheral neuropathy and new-onset autonomic neuropathy manifested as orthostatic hypotension. Several therapeutic interventions are recommended. First, his insulin regimen should be changed to a basal/bolus algorithm with insulin glargine or detemir and a short acting insulin analog such as aspart or lispro based on carbohydrate counting. The pharmacokinetic profiles of these insulin analogs and the use of carbohydrate counting for calculation of prandial insulin requirements ensure a superior blood glucose control with a lower incidence of hypo and hyperglycemic peaks. Second, to reduce symptomatic orthostasis the dose of furosemide should be reduced as tolerated and lisinopril should be taken at bedtime with a possible reduction in dose to 20 mg/day. In case of persistent symptomatic orthostatic hypotension, midodrine 5-10 mg could be added, up to 3 times/day. However, a careful monitoring of supine blood pressure should be performed as supine hypertension is a possible side effect of this agent. Additional interventions to reduce orthostatic hypotension include elevating the head of the bed while sleeping and wearing compression stockings. Finally, to treat his painful neuropathy, he should be started on gabapentin with slow titration of dosage up to 700 mg BID. If he tolerates this maximum dose, but is still experiencing pain, duloxetine could be added beginning at 20 mg/day slowly titrating up to 60 mg/day. Careful foot care will be critical.
Acknowledgments
Support: Dr Pop-Busui is supported by grants from the American Diabetes Association (1-08-CR-48), the Juvenile Diabetes Research Foundation (1-2008-1025 and 4-200-421 [for the study of complications of diabetes], and the Michigan Diabetes Research and Training Center (National Institutes of Health [NIH] 5P60-DK020572). Dr Feldman is supported by grants from the Michigan Diabetes Research and Training Center (NIH 5P60-DK020572), Animal Models of Diabetic Complications Consortium (NIH U01-DK076160), the Taubman Institute, and the Program for Neurology Research and Discovery. Dr Pennathur is supported by a Clinician Scientist Development Award from the Doris Duke Foundation and NIH grants HL092237 and HL092237-02S109.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1A.Kamal-Bahl SJ, Pantely S, Pyenson B. Employer-paid nonmedical costs for patients with diabetes and end-stage renal disease. Prev Chronic Dis. 2006;3(3):A83. http://www.cdc.gov/pcd/issues/2006/jul/05_0210.htm. [PMC free article] [PubMed] [Google Scholar]
- 1.Fernando DJ, Hutchison A, Veves A, Gokal R, Boulton AJ. Risk factors for non-ischaemic foot ulceration in diabetic nephropathy. Diabet Med. 1991 Apr;8(3):223–225. doi: 10.1111/j.1464-5491.1991.tb01576.x. [DOI] [PubMed] [Google Scholar]
- 2.Navarro X, Kennedy WR, Sutherland DE. Autonomic neuropathy and survival in diabetes mellitus: effects of pancreas transplantation. Diabetologia. 1991 Aug;34(Suppl 1):S108–112. doi: 10.1007/BF00587633. [DOI] [PubMed] [Google Scholar]
- 3.Rathmann W, Ziegler D, Jahnke M, Haastert B, Gries FA. Mortality in diabetic patients with cardiovascular autonomic neuropathy. Diabet Med. 1993 Nov;10(9):820–824. doi: 10.1111/j.1464-5491.1993.tb00173.x. [DOI] [PubMed] [Google Scholar]
- 4.Sampson MJ, Wilson S, Karagiannis P, Edmonds M, Watkins PJ. Progression of diabetic autonomic neuropathy over a decade in insulin- dependent diabetics. Q J Med. 1990;75(278):635–646. [PubMed] [Google Scholar]
- 5.Manjunath G, Levey AS, Sarnak MJ. How can the cardiac death rate be reduced in dialysis patients? Semin Dial. 2002 Jan-Feb;15(1):18–20. doi: 10.1046/j.1525-139x.2002.0006a.x. [DOI] [PubMed] [Google Scholar]
- 6.Locatelli F, Marcelli D, Conte F. Dialysis patient outcomes in Europe vs the USA. Why do Europeans live longer? Nephrol Dial Transplant. 1997 Sep;12(9):1816–1819. doi: 10.1093/ndt/12.9.1816. [DOI] [PubMed] [Google Scholar]
- 7.Locatelli F, Marcelli D, Conte F, et al. Survival and development of cardiovascular disease by modality of treatment in patients with end-stage renal disease. J Am Soc Nephrol. 2001 Nov;12(11):2411–2417. doi: 10.1681/ASN.V12112411. [DOI] [PubMed] [Google Scholar]
- 8.National Institutes of Health NIoDaDaKD. U.S. Renal Data System. USRDS 2006 Annual Data Report: Atlas of End-Stage Renal Disease in the United States [Google Scholar]
- 9.Ranpuria R, Hall M, Chan CT, Unruh M. Heart rate variability (HRV) in kidney failure: measurement and consequences of reduced HRV. Nephrol Dial Transplant. 2008 Feb;23(2):444–449. doi: 10.1093/ndt/gfm634. [DOI] [PubMed] [Google Scholar]
- 10.Hathaway DK, Cashion AK, Milstead EJ, et al. Autonomic dysregulation in patients awaiting kidney transplantation. Am J Kidney Dis. 1998 Aug;32(2):221–229. doi: 10.1053/ajkd.1998.v32.pm9708605. [DOI] [PubMed] [Google Scholar]
- 11.Zander E, Schulz B, Heinke P, Grimmberger E, Zander G, Gottschling HD. Importance of cardiovascular autonomic dysfunction in IDDM subjects with diabetic nephropathy. Diabetes Care. 1989 Apr;12(4):259–264. doi: 10.2337/diacare.12.4.259. [DOI] [PubMed] [Google Scholar]
- 12.Chang MH, Chou KJ. The role of autonomic neuropathy in the genesis of intradialytic hypotension. Am J Nephrol. 2001 Sep-Oct;21(5):357–361. doi: 10.1159/000046274. [DOI] [PubMed] [Google Scholar]
- 13.Laaksonen S, Voipio-Pulkki L, Erkinjuntti M, Asola M, Falck B. Does dialysis therapy improve autonomic and peripheral nervous system abnormalities in chronic uraemia? J Intern Med. 2000 Jul;248(1):21–26. doi: 10.1046/j.1365-2796.2000.00690.x. [DOI] [PubMed] [Google Scholar]
- 14.Spallone V, Mazzaferro S, Tomei E, et al. Autonomic neuropathy and secondary hyperparathyroidism in uraemia. Nephrol Dial Transplant. 1990;5(Suppl 1):128–130. doi: 10.1093/ndt/5.suppl_1.128. [DOI] [PubMed] [Google Scholar]
- 15.Johansson M, Gao SA, Friberg P, et al. Reduced baroreflex effectiveness index in hypertensive patients with chronic renal failure. Am J Hypertens. 2005 Jul;18(7):995–1000. doi: 10.1016/j.amjhyper.2005.02.002. discussion 1016. [DOI] [PubMed] [Google Scholar]
- 16.Brouns R, De Deyn PP. Neurological complications in renal failure: a review. Clin Neurol Neurosurg. 2004 Dec;107(1):1–16. doi: 10.1016/j.clineuro.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Innis J. Pain assessment and management for a dialysis patient with diabetic peripheral neuropathy. CANNT J. 2006 Apr-Jun;16(2):12–17. 20–16. quiz 18-19, 27-18. [PubMed] [Google Scholar]
- 18.Stella P, Ellis D, Maser RE, Orchard TJ. Cardiovascular autonomic neuropathy (expiration and inspiration ratio) in type 1 diabetes. Incidence and predictors. J Diabetes Complications. 2000 Jan-Feb;14(1):1–6. doi: 10.1016/s1056-8727(00)00054-4. [DOI] [PubMed] [Google Scholar]
- 19.Witte DR, Tesfaye S, Chaturvedi N, Eaton SE, Kempler P, Fuller JH. Risk factors for cardiac autonomic neuropathy in type 1 diabetes mellitus. Diabetologia. 2005 Jan;48(1):164–171. doi: 10.1007/s00125-004-1617-y. [DOI] [PubMed] [Google Scholar]
- 20.Krishnan AV, Lin CS, Kiernan MC. Activity-dependent excitability changes suggest Na+/K+ pump dysfunction in diabetic neuropathy. Brain. 2008 May;131(Pt 5):1209–1216. doi: 10.1093/brain/awn052. [DOI] [PubMed] [Google Scholar]
- 21.Greene DA, Lattimer SA. Impaired energy utilization and Na-K-ATPase in diabetic peripheral nerve. Am J Physiol. 1984;246:E311–E318. doi: 10.1152/ajpendo.1984.246.4.E311. [DOI] [PubMed] [Google Scholar]
- 22.Edwards JL, Vincent AM, Cheng HT, Feldman EL. Diabetic neuropathy: mechanisms to management. Pharmacol Ther. 2008 Oct;120(1):1–34. doi: 10.1016/j.pharmthera.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pop-Busui R, Sima A, Stevens M. Diabetic neuropathy and oxidative stress. Diabetes Metab Res Rev. 2006 Jul-Aug;22(4):257–273. doi: 10.1002/dmrr.625. [DOI] [PubMed] [Google Scholar]
- 24.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001 Dec 13;414(6865):813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 25.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005 Jun;54(6):1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 26.Massry SG. Parathyroid hormone: a uremic toxin. Adv Exp Med Biol. 1987;223:1–17. doi: 10.1007/978-1-4684-5445-1_1. [DOI] [PubMed] [Google Scholar]
- 27.Slatopolsky E, Martin K, Hruska K. Parathyroid hormone metabolism and its potential as a uremic toxin. Am J Physiol. 1980 Jul;239(1):F1–12. doi: 10.1152/ajprenal.1980.239.1.F1. [DOI] [PubMed] [Google Scholar]
- 28.Vanholder R, De Smet R, Hsu C, Vogeleere P, Ringoir S. Uremic toxicity: the middle molecule hypothesis revisited. Semin Nephrol. 1994 May;14(3):205–218. [PubMed] [Google Scholar]
- 29.Kaji R, Sumner AJ. Ouabain reverses conduction disturbances in single demyelinated nerve fibers. Neurology. 1989 Oct;39(10):1364–1368. doi: 10.1212/wnl.39.10.1364. [DOI] [PubMed] [Google Scholar]
- 30.Krishnan AV, Phoon RK, Pussell BA, Charlesworth JA, Bostock H, Kiernan MC. Altered motor nerve excitability in end-stage kidney disease. Brain. 2005 Sep;128(Pt 9):2164–2174. doi: 10.1093/brain/awh558. [DOI] [PubMed] [Google Scholar]
- 31.Krishnan AV, Phoon RK, Pussell BA, Charlesworth JA, Bostock H, Kiernan MC. Ischaemia induces paradoxical changes in axonal excitability in end-stage kidney disease. Brain. 2006 Jun;129(Pt 6):1585–1592. doi: 10.1093/brain/awl099. [DOI] [PubMed] [Google Scholar]
- 32.Craner MJ, Lo AC, Black JA, Waxman SG. Abnormal sodium channel distribution in optic nerve axons in a model of inflammatory demyelination. Brain. 2003 Jul;126(Pt 7):1552–1561. doi: 10.1093/brain/awg153. [DOI] [PubMed] [Google Scholar]
- 33.Kiernan MC, Walters RJ, Andersen KV, Taube D, Murray NM, Bostock H. Nerve excitability changes in chronic renal failure indicate membrane depolarization due to hyperkalaemia. Brain. 2002 Jun;125(Pt 6):1366–1378. doi: 10.1093/brain/awf123. [DOI] [PubMed] [Google Scholar]
- 34.Krishnan AV, Kiernan MC. Uremic neuropathy: clinical features and new pathophysiological insights. Muscle Nerve. 2007 Mar;35(3):273–290. doi: 10.1002/mus.20713. [DOI] [PubMed] [Google Scholar]
- 35.Converse RL, Jr, Jacobsen TN, Toto RD, et al. Sympathetic overactivity in patients with chronic renal failure. N Engl J Med. 1992 Dec 31;327(27):1912–1918. doi: 10.1056/NEJM199212313272704. [DOI] [PubMed] [Google Scholar]
- 36.Siddiqi L, Joles JA, Grassi G, Blankestijn PJ. Is kidney ischemia the central mechanism in parallel activation of the renin and sympathetic system? J Hypertens. 2009 Jul;27(7):1341–1349. doi: 10.1097/HJH.0b013e32832b521b. [DOI] [PubMed] [Google Scholar]
- 37.Axelrod S, Lishner M, Oz O, Bernheim J, Ravid M. Spectral analysis of fluctuations in heart rate: an objective evaluation of autonomic nervous control in chronic renal failure. Nephron. 1987;45(3):202–206. doi: 10.1159/000184117. [DOI] [PubMed] [Google Scholar]
- 38.Campese VM, Romoff MS, Levitan D, Lane K, Massry SG. Mechanisms of autonomic nervous system dysfunction in uremia. Kidney Int. 1981 Aug;20(2):246–253. doi: 10.1038/ki.1981.127. [DOI] [PubMed] [Google Scholar]
- 39.Paulson DJ, Light KE. Elevation of serum and ventricular norepinephrine content in the diabetic rat. Res Commun Chem Pathol Pharmacol. 1981;33(3):559–562. [PubMed] [Google Scholar]
- 40.Givertz MM, Sawyer DB, Colucci WS. Antioxidants and myocardial contractility: illuminating the “Dark Side” of beta-adrenergic receptor activation? Circulation. 2001 Feb 13;103(6):782–783. doi: 10.1161/01.cir.103.6.782. [DOI] [PubMed] [Google Scholar]
- 41.Felten SY, Peterson RG, Shea PA, Besch HR, Jr, Felten DL. Effects of streptozotocin diabetes on the noradrenergic innervation of the rat heart: a longitudinal histofluorescence and neurochemical study. Brain Res Bull. 1982;8(6):593–607. doi: 10.1016/0361-9230(82)90086-7. [DOI] [PubMed] [Google Scholar]
- 42.Scacco S, Vergari R, Scarpulla RC, et al. cAMP-dependent phosphorylation of the nuclear encoded 18-kDa (IP) subunit of respiratory complex I and activation of the complex in serum-starved mouse fibroblast cultures. J Biol Chem. 2000 Jun 9;275(23):17578–17582. doi: 10.1074/jbc.M001174200. [DOI] [PubMed] [Google Scholar]
- 43.Iwai-Kanai E, Hasegawa K, Araki M, Kakita T, Morimoto T, Sasayama S. alpha-and beta-adrenergic pathways differentially regulate cell type-specific apoptosis in rat cardiac myocytes. Circulation. 1999 Jul 20;100(3):305–311. doi: 10.1161/01.cir.100.3.305. [DOI] [PubMed] [Google Scholar]
- 44.Communal C, Singh K, Pimentel DR, Colucci WS. Norepinephrine stimulates apoptosis in adult rat ventricular myocytes by activation of the beta-adrenergic pathway. Circulation. 1998 Sep 29;98(13):1329–1334. doi: 10.1161/01.cir.98.13.1329. [DOI] [PubMed] [Google Scholar]
- 45.Colberg SR, Swain DP, Vinik AI. Use of heart rate reserve and rating of perceived exertion to prescribe exercise intensity in diabetic autonomic neuropathy. Diabetes Care. 2003 Apr;26(4):986–990. doi: 10.2337/diacare.26.4.986. [DOI] [PubMed] [Google Scholar]
- 46.Giordano M, Manzella D, Paolisso G, Caliendo A, Varricchio M, Giordano C. Differences in heart rate variability parameters during the post-dialytic period in type II diabetic and non-diabetic ESRD patients. Nephrol Dial Transplant. 2001 Mar;16(3):566–573. doi: 10.1093/ndt/16.3.566. [DOI] [PubMed] [Google Scholar]
- 47.Ewing DJ, Campbell IW, Clarke BF. The natural history of diabetic autonomic neuropathy. Q J Med. 1980 Winter;49(193):95–108. [PubMed] [Google Scholar]
- 48.Ewing DJ, Clarke BF. Diagnosis and management of diabetic autonomic neuropathy. Br Med J (Clin Res Ed) 1982;285(6346):916–918. doi: 10.1136/bmj.285.6346.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ewing DJ, Clarke BF. Diabetic autonomic neuropathy: present insights and future prospects. Diabetes Care. 1986 Nov-Dec;9(6):648–665. doi: 10.2337/diacare.9.6.648. [DOI] [PubMed] [Google Scholar]
- 50.Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation. 2007 Jan 23;115(3):387–397. doi: 10.1161/CIRCULATIONAHA.106.634949. [DOI] [PubMed] [Google Scholar]
- 51.Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Neurology. 1996 May;46(5):1470. doi: 10.1212/wnl.46.5.1470. [DOI] [PubMed] [Google Scholar]
- 52.Kersh ES, Kronfield SJ, Unger A, Popper RW, Cantor S, Cohn K. Autonomic insufficiency in uremia as a cause of hemodialysis-induced hypotension. N Engl J Med. 1974 Mar 21;290(12):650–653. doi: 10.1056/NEJM197403212901203. [DOI] [PubMed] [Google Scholar]
- 53.Sato M, Horigome I, Chiba S, et al. Autonomic insufficiency as a factor contributing to dialysis-induced hypotension. Nephrol Dial Transplant. 2001 Aug;16(8):1657–1662. doi: 10.1093/ndt/16.8.1657. [DOI] [PubMed] [Google Scholar]
- 54.Ligtenberg G, Blankestijn PJ, Boomsma F, Koomans HA. No change in automatic function tests during uncomplicated haemodialysis. Nephrol Dial Transplant. 1996 Apr;11(4):651–656. doi: 10.1093/oxfordjournals.ndt.a027354. [DOI] [PubMed] [Google Scholar]
- 55.Di Carli MF, Bianco-Batlles D, Landa ME, et al. Effects of autonomic neuropathy on coronary blood flow in patients with diabetes mellitus. Circulation. 1999;100(8):813–819. doi: 10.1161/01.cir.100.8.813. [DOI] [PubMed] [Google Scholar]
- 56.Di Carli MF, Tobes MC, Mangner T, et al. Effects of cardiac sympathetic innervation on coronary blood flow. N Engl J Med. 1997;336(17):1208–1215. doi: 10.1056/NEJM199704243361703. [DOI] [PubMed] [Google Scholar]
- 57.Stevens MJ, Dayanikli F, Raffel DM, et al. Scintigraphic assessment of regionalized defects in myocardial sympathetic innervation and blood flow regulation in diabetic patients with autonomic neuropathy. J Am Coll Cardiol. 1998;31(7):1575–1584. doi: 10.1016/s0735-1097(98)00128-4. [DOI] [PubMed] [Google Scholar]
- 58.Nitenberg A, Valensi P, Sachs R, Dali M, Aptecar E, Attali JR. Impairment of coronary vascular reserve and ACh-induced coronary vasodilation in diabetic patients with angiographically normal coronary arteries and normal left ventricular systolic function. Diabetes. 1993 Jul;42(7):1017–1025. doi: 10.2337/diab.42.7.1017. [DOI] [PubMed] [Google Scholar]
- 59.Nitenberg A, Antony I, Foult JM. Coronary vasoconstriction induced by acetylcholine in young smokers with normal angiographic coronary vessels. Arch Mal Coeur Vaiss. 1993 Aug;86(8):1133–1136. [PubMed] [Google Scholar]
- 60.Langer A, Freeman MR, Josse RG, Armstrong PW. Metaiodobenzylguanidine imaging in diabetes mellitus: assessment of cardiac sympathetic denervation and its relation to autonomic dysfunction and silent myocardial ischemia. J Am Coll Cardiol. 1995;25(3):610–618. doi: 10.1016/0735-1097(94)00459-4. [DOI] [PubMed] [Google Scholar]
- 61.Schwartz PJ, Randall WC, Anderson EA, et al. Sudden cardiac death. Nonpharmacologic interventions. Circulation. 1987;76(1 Pt 2):I215–219. [PubMed] [Google Scholar]
- 62.Willich SN, Maclure M, Mittleman M, Arntz HR, Muller JE. Sudden cardiac death. Support for a role of triggering in causation. Circulation. 1993;87(5):1442–1450. doi: 10.1161/01.cir.87.5.1442. [DOI] [PubMed] [Google Scholar]
- 63.Lown B, Verrier RL. Neural activity and ventricular fibrillation. N Engl J Med. 1976;294(21):1165–1170. doi: 10.1056/NEJM197605202942107. [DOI] [PubMed] [Google Scholar]
- 64.Maser RE, Mitchell BD, Vinik AI, Freeman R. The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: a meta-analysis. Diabetes Care. 2003 Jun;26(6):1895–1901. doi: 10.2337/diacare.26.6.1895. [DOI] [PubMed] [Google Scholar]
- 65.Brown DW, Giles WH, Croft JB. Left ventricular hypertrophy as a predictor of coronary heart disease mortality and the effect of hypertension. Am Heart J. 2000 Dec;140(6):848–856. doi: 10.1067/mhj.2000.111112. [DOI] [PubMed] [Google Scholar]
- 66.Levy D, Anderson KM, Savage DD, Balkus SA, Kannel WB, Castelli WP. Risk of ventricular arrhythmias in left ventricular hypertrophy: the Framingham Heart Study. Am J Cardiol. 1987 Sep 1;60(7):560–565. doi: 10.1016/0002-9149(87)90305-5. [DOI] [PubMed] [Google Scholar]
- 67.Nishimura M, Hashimoto T, Kobayashi H, et al. Association between cardiovascular autonomic neuropathy and left ventricular hypertrophy in diabetic haemodialysis patients. Nephrol Dial Transplant. 2004 Oct;19(10):2532–2538. doi: 10.1093/ndt/gfh361. [DOI] [PubMed] [Google Scholar]
- 68.Boulton AJ, Vinik AI, Arezzo JC, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005 Apr;28(4):956–962. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- 69.Allman KC, Wieland DM, Muzik O, Degrado TR, Wolfe ER, Jr, Schwaiger M. Carbon-11 hydroxyephedrine with positron emission tomography for serial assessment of cardiac adrenergic neuronal function after acute myocardial infarction in humans. J Am Coll Cardiol. 1993;22(2):368–375. doi: 10.1016/0735-1097(93)90039-4. [DOI] [PubMed] [Google Scholar]
- 70.Stevens MJ, Raffel DM, Allman KC, et al. Cardiac sympathetic dysinnervation in diabetes: implications for enhanced cardiovascular risk. Circulation. 1998;98(10):961–968. doi: 10.1161/01.cir.98.10.961. [DOI] [PubMed] [Google Scholar]
- 71.Stevens MJ, Raffel DM, Allman KC, Schwaiger M, Wieland DM. Regression and progression of cardiac sympathetic dysinnervation complicating diabetes: an assessment by C-11 hydroxyephedrine and positron emission tomography. Metabolism. 1999;48(1):92–101. doi: 10.1016/s0026-0495(99)90016-1. [DOI] [PubMed] [Google Scholar]
- 72.Kreiner G, Wolzt M, Fasching P, et al. Myocardial m-[123I]iodobenzylguanidine scintigraphy for the assessment of adrenergic cardiac innervation in patients with IDDM. Comparison with cardiovascular reflex tests and relationship to left ventricular function. Diabetes. 1995;44(5):543–549. doi: 10.2337/diab.44.5.543. [DOI] [PubMed] [Google Scholar]
- 73.Schnell O, Kirsch CM, Stemplinger J, Haslbeck M, Standl E. Scintigraphic evidence for cardiac sympathetic dysinnervation in long- term IDDM patients with and without ECG-based autonomic neuropathy. Diabetologia. 1995;38(11):1345–1352. doi: 10.1007/BF00401768. [DOI] [PubMed] [Google Scholar]
- 74.Schnell O, Muhr D, Weiss M, Dresel S, Haslbeck M, Standl E. Reduced myocardial 123I-metaiodobenzylguanidine uptake in newly diagnosed IDDM patients. Diabetes. 1996;45(6):801–805. doi: 10.2337/diab.45.6.801. [DOI] [PubMed] [Google Scholar]
- 75.Shimabukuro M, Chibana T, Yoshida H, Nagamine F, Komiya I, Takasu N. Increased QT dispersion and cardiac adrenergic dysinnervation in diabetic patients with autonomic neuropathy. Am J Cardiol. 1996;78(9):1057–1059. doi: 10.1016/s0002-9149(96)00538-3. [DOI] [PubMed] [Google Scholar]
- 76.Turpeinen AK, Vanninen E, Kuikka JT, Uusitupa MI. Demonstration of regional sympathetic denervation of the heart in diabetes. Comparison between patients with NIDDM and IDDM. Diabetes Care. 1996;19(10):1083–1090. doi: 10.2337/diacare.19.10.1083. [DOI] [PubMed] [Google Scholar]
- 77.Pop-Busui R, Kirkwood I, Schmid H, et al. Sympathetic dysfunction in type 1 diabetes: association with impaired myocardial blood flow reserve and diastolic dysfunction. J Am Coll Cardiol. 2004 Dec 21;44(12):2368–2374. doi: 10.1016/j.jacc.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 78.DeGrado TR, Hutchins GD, Toorongian SA, Wieland DM, Schwaiger M. Myocardial kinetics of carbon-11-meta-hydroxyephedrine: retention mechanisms and effects of norepinephrine. J Nucl Med. 1993;34(8):1287–1293. [PubMed] [Google Scholar]
- 79.Schwaiger M, Hutchins GD, Kalff V, et al. Evidence for regional catecholamine uptake and storage sites in the transplanted human heart by positron emission tomography. J Clin Invest. 1991;87(5):1681–1690. doi: 10.1172/JCI115185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ungerer M, Hartmann F, Karoglan M, et al. Regional in vivo and in vitro characterization of autonomic innervation in cardiomyopathic human heart. Circulation. 1998 Jan 20;97(2):174–180. doi: 10.1161/01.cir.97.2.174. [DOI] [PubMed] [Google Scholar]
- 81.Hamner JW, Taylor JA. Automated quantification of sympathetic beat-by-beat activity, independent of signal quality. J Appl Physiol. 2001 Sep;91(3):1199–1206. doi: 10.1152/jappl.2001.91.3.1199. [DOI] [PubMed] [Google Scholar]
- 82.Foster AV, Snowden S, Grenfell A, Watkins PJ, Edmonds ME. Reduction of gangrene and amputations in diabetic renal transplant patients: the role of a special foot clinic. Diabet Med. 1995 Jul;12(7):632–635. doi: 10.1111/j.1464-5491.1995.tb00555.x. [DOI] [PubMed] [Google Scholar]
- 83.Eggers PW, Gohdes D, Pugh J. Nontraumatic lower extremity amputations in the Medicare end-stage renal disease population. Kidney Int. 1999 Oct;56(4):1524–1533. doi: 10.1046/j.1523-1755.1999.00668.x. [DOI] [PubMed] [Google Scholar]
- 84.Combe C, Albert JM, Bragg-Gresham JL, et al. The burden of amputation among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2009 Oct;54(4):680–692. doi: 10.1053/j.ajkd.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 85.Report and recommendations of the San Antonio Conference on Diabetic Neuropathy. American Diabetes Association. Muscle Nerve. 1988 Jul;11(7):661–667. doi: 10.1002/mus.880110702. [DOI] [PubMed] [Google Scholar]
- 86.Boulton AJ, Malik RA, Arezzo JC, Sosenko JM. Diabetic somatic neuropathies. Diabetes Care. 2004 Jun;27(6):1458–1486. doi: 10.2337/diacare.27.6.1458. [DOI] [PubMed] [Google Scholar]
- 87.Adriaensen H, Plaghki L, Mathieu C, Joffroy A, Vissers K. Critical review of oral drug treatments for diabetic neuropathic pain-clinical outcomes based on efficacy and safety data from placebo-controlled and direct comparative studies. Diabetes Metab Res Rev. 2005 May-Jun;21(3):231–240. doi: 10.1002/dmrr.552. [DOI] [PubMed] [Google Scholar]
- 88.DCCT. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 89.DCCT. Effect of intensive diabetes treatment on nerve conduction in the Diabetes Control and Complications Trial. Ann Neurol. 1995;38(6):869–880. doi: 10.1002/ana.410380607. [DOI] [PubMed] [Google Scholar]
- 90.DCCT. The effect of intensive diabetes therapy on measures of autonomic nervous system function in the Diabetes Control and Complications Trial (DCCT) Diabetologia. 1998;41(4):416–423. doi: 10.1007/s001250050924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tesfaye S, Stevens LK, Stephenson JM, et al. Prevalence of diabetic peripheral neuropathy and its relation to glycaemic control and potential risk factors: the EURODIAB IDDM Complications Study. Diabetologia. 1996 Nov;39(11):1377–1384. doi: 10.1007/s001250050586. [DOI] [PubMed] [Google Scholar]
- 92.Tesfaye S, Chaturvedi N, Eaton SE, et al. Vascular risk factors and diabetic neuropathy. N Engl J Med. 2005 Jan 27;352(4):341–350. doi: 10.1056/NEJMoa032782. [DOI] [PubMed] [Google Scholar]
- 93.DCCT. Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA. 2002 May 15;287(19):2563–2569. doi: 10.1001/jama.287.19.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.DCCT/EDIC, Writing Group. Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA. 2003 Oct 22;290(16):2159–2167. doi: 10.1001/jama.290.16.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pop-Busui R, Evans G, Gerstein HC, et al. Cardiac Autonomic Dysfunction Predicts Cardiovascular Mortality in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial. Diabetes. 2009 Jun;58(Suppl 1) doi: 10.2337/dc10-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995 May;28(2):103–117. doi: 10.1016/0168-8227(95)01064-k. [DOI] [PubMed] [Google Scholar]
- 97.UKPDS. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998 Sep 12;352(9131):837–853. [PubMed] [Google Scholar]
- 98.UKPDS. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998 Sep 12;352(9131):854–865. [PubMed] [Google Scholar]
- 99.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009 Jan 8;360(2):129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 100.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003 Jan 30;348(5):383–393. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 101.Bakris GL, Hart P, Ritz E. Beta blockers in the management of chronic kidney disease. Kidney Int. 2006 Dec;70(11):1905–1913. doi: 10.1038/sj.ki.5001835. [DOI] [PubMed] [Google Scholar]
- 102.Ebbehoj E, Poulsen PL, Hansen KW, Knudsen ST, Molgaard H, Mogensen CE. Effects on heart rate variability of metoprolol supplementary to ongoing ACE-inhibitor treatment in Type I diabetic patients with abnormal albuminuria. Diabetologia. 2002 Jul;45(7):965–975. doi: 10.1007/s00125-002-0869-7. [DOI] [PubMed] [Google Scholar]
- 103.Foley RN, Herzog CA, Collins AJ. Blood pressure and long-term mortality in United States hemodialysis patients: USRDS Waves 3 and 4 Study. Kidney Int. 2002 Nov;62(5):1784–1790. doi: 10.1046/j.1523-1755.2002.00636.x. [DOI] [PubMed] [Google Scholar]
- 104.Kamalesh M, Subramanian U, Sawada S, Eckert G, Temkit M, Tierney W. Decreased survival in diabetic patients with heart failure due to systolic dysfunction. Eur J Heart Fail. 2006 Jun;8(4):404–408. doi: 10.1016/j.ejheart.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 105.Tory K, Horvath E, Suveges Z, et al. Effect of propranolol on heart rate variability in patients with end-stage renal disease: a double-blind, placebo-controlled, randomized crossover pilot trial. Clin Nephrol. 2004 May;61(5):316–323. doi: 10.5414/cnp61316. [DOI] [PubMed] [Google Scholar]
- 106.Bristow MR. What type of beta-blocker should be used to treat chronic heart failure? Circulation. 2000 Aug 1;102(5):484–486. doi: 10.1161/01.cir.102.5.484. [DOI] [PubMed] [Google Scholar]
- 107.Cice G, Ferrara L, D'Andrea A, et al. Carvedilol increases two-year survivalin dialysis patients with dilated cardiomyopathy: a prospective, placebo-controlled trial. J Am Coll Cardiol. 2003 May 7;41(9):1438–1444. doi: 10.1016/s0735-1097(03)00241-9. [DOI] [PubMed] [Google Scholar]
- 108.Nakao K, Makino H, Morita S, et al. beta-Blocker Prescription and Outcomes in Hemodialysis Patients from The Japan Dialysis Outcomes and Practice Patterns Study. Nephron Clin Pract. 2009 Aug 12;113(3):c132–c139. doi: 10.1159/000232593. [DOI] [PubMed] [Google Scholar]
- 109.Pun PH, Lehrich RW, Smith SR, Middleton JP. Predictors of survival after cardiac arrest in outpatient hemodialysis clinics. Clin J Am Soc Nephrol. 2007 May;2(3):491–500. doi: 10.2215/CJN.02360706. [DOI] [PubMed] [Google Scholar]
- 110.Orth SR, Amann K, Strojek K, Ritz E. Sympathetic overactivity and arterial hypertension in renal failure. Nephrol Dial Transplant. 2001;16(Suppl 1):67–69. doi: 10.1093/ndt/16.suppl_1.67. [DOI] [PubMed] [Google Scholar]
- 111.Paoletti E, Specchia C, Di Maio G, et al. The worsening of left ventricular hypertrophy is the strongest predictor of sudden cardiac death in haemodialysis patients: a 10 year survey. Nephrol Dial Transplant. 2004 Jul;19(7):1829–1834. doi: 10.1093/ndt/gfh288. [DOI] [PubMed] [Google Scholar]
- 112.Tamura K, Tsuji H, Nishiue T, Yajima I, Higashi T, Iwasaka T. Determinants of heart rate variability in chronic hemodialysis patients. Am J Kidney Dis. 1998 Apr;31(4):602–606. doi: 10.1053/ajkd.1998.v31.pm9531175. [DOI] [PubMed] [Google Scholar]
- 113.Ondocin PT, Narsipur SS. Influence of angiotensin converting enzyme inhibitor treatment on cardiac autonomic modulation in patients receiving haemodialysis. Nephrology (Carlton) 2006 Dec;11(6):497–501. doi: 10.1111/j.1440-1797.2006.00680.x. [DOI] [PubMed] [Google Scholar]
- 114.Weinbergova O, Metelka R, Vymetal J, Konecny K, Kosatikova Z. Telmisartan in the treatment of hypertension in patients with chronic renal insufficiency. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2004 Jul;148(1):69–73. [PubMed] [Google Scholar]
- 115.Neumann J, Ligtenberg G, Klein IH, et al. Sympathetic hyperactivity in hypertensive chronic kidney disease patients is reduced during standard treatment. Hypertension. 2007 Mar;49(3):506–510. doi: 10.1161/01.HYP.0000256530.39695.a3. [DOI] [PubMed] [Google Scholar]
- 116.Ligtenberg G, Blankestijn PJ, Oey PL, et al. Reduction of sympathetic hyperactivity by enalapril in patients with chronic renal failure. N Engl J Med. 1999 Apr 29;340(17):1321–1328. doi: 10.1056/NEJM199904293401704. [DOI] [PubMed] [Google Scholar]
- 117.Bosman DR, Winkler AS, Marsden JT, Macdougall IC, Watkins PJ. Anemia with erythropoietin deficiency occurs early in diabetic nephropathy. Diabetes Care. 2001 Mar;24(3):495–499. doi: 10.2337/diacare.24.3.495. [DOI] [PubMed] [Google Scholar]
- 118.Hoeldtke RD, Streeten DH. Treatment of orthostatic hypotension with erythropoietin. N Engl J Med. 1993 Aug 26;329(9):611–615. doi: 10.1056/NEJM199308263290904. [DOI] [PubMed] [Google Scholar]
- 119.Winkler AS, Marsden J, Chaudhuri KR, Hambley H, Watkins PJ. Erythropoietin depletion and anaemia in diabetes mellitus. Diabet Med. 1999 Oct;16(10):813–819. doi: 10.1046/j.1464-5491.1999.00172.x. [DOI] [PubMed] [Google Scholar]
- 120.Stojcheva-Taneva OO, Polenakovic MH. Autonomic neuropathy in hemodialysis patients treated with recombinant human erythropoietin. Int J Artif Organs. 1996 Oct;19(10):574–577. [PubMed] [Google Scholar]
- 121.Prakash S, Garg AX, Heidenheim AP, House AA. Midodrine appears to be safe and effective for dialysis-induced hypotension: a systematic review. Nephrol Dial Transplant. 2004 Oct;19(10):2553–2558. doi: 10.1093/ndt/gfh420. [DOI] [PubMed] [Google Scholar]
- 122.Rubinstein S, Haimov M, Ross MJ. Midodrine-induced vascular ischemia in a hemodialysis patient: a case report and literature review. Ren Fail. 2008;30(8):808–812. doi: 10.1080/08860220802249025. [DOI] [PubMed] [Google Scholar]
- 123.Yalcin AU, Kudaiberdieva G, Sahin G, et al. Effect of sertraline hydrochloride on cardiac autonomic dysfunction in patients with hemodialysis-induced hypotension. Nephron Physiol. 2003 Jan;93(1):P21–28. doi: 10.1159/000066655. [DOI] [PubMed] [Google Scholar]
- 124.Agarwal A, Anand IS, Sakhuja V, Chugh KS. Effect of dialysis and renal transplantation on autonomic dysfunction in chronic renal failure. Kidney Int. 1991 Sep;40(3):489–495. doi: 10.1038/ki.1991.236. [DOI] [PubMed] [Google Scholar]
- 125.Allon M, Rodriguez M, Llach F. Insulin in the acute renal adaptation to dietary phosphate restriction in the rat. Kidney Int. 1990 Jan;37(1):14–20. doi: 10.1038/ki.1990.2. [DOI] [PubMed] [Google Scholar]
- 126.Rubinger D, Revis N, Pollak A, Luria MH, Sapoznikov D. Predictors of haemodynamic instability and heart rate variability during haemodialysis. Nephrol Dial Transplant. 2004 Aug;19(8):2053–2060. doi: 10.1093/ndt/gfh306. [DOI] [PubMed] [Google Scholar]
- 127.Tong YQ, Hou HM. Alteration of heart rate variability parameters in nondiabetic hemodialysis patients. Am J Nephrol. 2007;27(1):63–69. doi: 10.1159/000099013. [DOI] [PubMed] [Google Scholar]
- 128.Dursun B, Demircioglu F, Varan HI, et al. Effects of different dialysis modalities on cardiac autonomic dysfunctions in end-stage renal disease patients: one year prospective study. Ren Fail. 2004 Jan;26(1):35–38. doi: 10.1081/jdi-120028541. [DOI] [PubMed] [Google Scholar]
- 129.Rubinger D, Backenroth R, Pollak A, Sapoznikov D. Blood pressure and heart rate variability in patients on conventional or sodium-profiling hemodialysis. Ren Fail. 2008;30(3):277–286. doi: 10.1080/08860220701857308. [DOI] [PubMed] [Google Scholar]
- 130.Finnerup NB, Otto M, McQuay HJ, Jensen TS, Sindrup SH. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain. 2005 Dec 5;118(3):289–305. doi: 10.1016/j.pain.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 131.Jensen TS, Madsen CS, Finnerup NB. Pharmacology and treatment of neuropathic pains. Curr Opin Neurol. 2009 Oct;22(5):467–474. doi: 10.1097/WCO.0b013e3283311e13. [DOI] [PubMed] [Google Scholar]
- 132.Freynhagen R, Strojek K, Griesing T, Whalen E, Balkenohl M. Efficacy of pregabalin in neuropathic pain evaluated in a 12-week, randomised, double-blind, multicentre, placebo-controlled trial of flexible- and fixed-dose regimens. Pain. 2005 Jun;115(3):254–263. doi: 10.1016/j.pain.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 133.Richter RW, Portenoy R, Sharma U, Lamoreaux L, Bockbrader H, Knapp LE. Relief of painful diabetic peripheral neuropathy with pregabalin: a randomized, placebo-controlled trial. J Pain. 2005 Apr;6(4):253–260. doi: 10.1016/j.jpain.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 134.Rosenstock J, Tuchman M, LaMoreaux L, Sharma U. Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trial. Pain. 2004 Aug;110(3):628–638. doi: 10.1016/j.pain.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 135.Barrett AM, Lucero MA, Le T, Robinson RL, Dworkin RH, Chappell AS. Epidemiology, public health burden, and treatment of diabetic peripheral neuropathic pain: a review. Pain Med. 2007 Sep;8(Suppl 2):S50–62. doi: 10.1111/j.1526-4637.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- 136.Moore RA, Straube S, Wiffen PJ, Derry S, McQuay HJ. Pregabalin for acute and chronic pain in adults. Cochrane Database Syst Rev. 2009;(3):CD007076. doi: 10.1002/14651858.CD007076.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Randinitis EJ, Posvar EL, Alvey CW, Sedman AJ, Cook JA, Bockbrader HN. Pharmacokinetics of pregabalin in subjects with various degrees of renal function. J Clin Pharmacol. 2003 Mar;43(3):277–283. doi: 10.1177/0091270003251119. [DOI] [PubMed] [Google Scholar]
- 138.Backonja M, Beydoun A, Edwards KR, et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. Jama. 1998 Dec 2;280(21):1831–1836. doi: 10.1001/jama.280.21.1831. [DOI] [PubMed] [Google Scholar]
- 139.Backonja M, Glanzman RL. Gabapentin dosing for neuropathic pain: evidence from randomized, placebo-controlled clinical trials. Clin Ther. 2003 Jan;25(1):81–104. doi: 10.1016/s0149-2918(03)90011-7. [DOI] [PubMed] [Google Scholar]
- 140.Wong MO, Eldon MA, Keane WF, et al. Disposition of gabapentin in anuric subjects on hemodialysis. J Clin Pharmacol. 1995 Jun;35(6):622–626. doi: 10.1002/j.1552-4604.1995.tb05020.x. [DOI] [PubMed] [Google Scholar]
- 141.Zhang C, Glenn DG, Bell WL, O'Donovan CA. Gabapentin-induced myoclonus in end-stage renal disease. Epilepsia. 2005 Jan;46(1):156–158. doi: 10.1111/j.0013-9580.2005.20804.x. [DOI] [PubMed] [Google Scholar]
- 142.Hung TY, Seow VK, Chong CF, Wang TL, Chen CC. Gabapentin toxicity: an important cause of altered consciousness in patients with uraemia. Emerg Med J. 2008 Mar;25(3):178–179. doi: 10.1136/emj.2007.053470. [DOI] [PubMed] [Google Scholar]
- 143.Jones H, Aguila E, Farber HW. Gabapentin toxicity requiring intubation in a patient receiving long-term hemodialysis. Ann Intern Med. 2002 Jul 2;137(1):74. doi: 10.7326/0003-4819-137-1-200207020-00029. [DOI] [PubMed] [Google Scholar]
- 144.Collins SL, Moore RA, McQuay Hj, Wiffen P. Antidepressants and anticonvulsants for diabetic neuropathy and postherpetic neuralgia: a quantitative systematic review. J Pain Symptom Manage. 2000 Dec;20(6):449–458. doi: 10.1016/s0885-3924(00)00218-9. [DOI] [PubMed] [Google Scholar]
- 145.Jann MW, Slade JH. Antidepressant agents for the treatment of chronic pain and depression. Pharmacotherapy. 2007 Nov;27(11):1571–1587. doi: 10.1592/phco.27.11.1571. [DOI] [PubMed] [Google Scholar]
- 146.Goldstein DJ, Lu Y, Detke MJ, Lee TC, Iyengar S. Duloxetine vs. placebo in patients with painful diabetic neuropathy. Pain. 2005 Jul;116(1-2):109–118. doi: 10.1016/j.pain.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 147.Raskin J, Wang F, Pritchett YL, Goldstein DJ. Duloxetine for patients with diabetic peripheral neuropathic pain: a 6-month open-label safety study. Pain Med. 2006 Sep-Oct;7(5):373–385. doi: 10.1111/j.1526-4637.2006.00207.x. [DOI] [PubMed] [Google Scholar]
- 148.Wernicke JF, Pritchett YL, D'Souza DN, et al. A randomized controlled trial of duloxetine in diabetic peripheral neuropathic pain. Neurology. 2006 Oct 24;67(8):1411–1420. doi: 10.1212/01.wnl.0000240225.04000.1a. [DOI] [PubMed] [Google Scholar]
- 149.Wiffen P, Collins S, McQuay H, Carroll D, Jadad A, Moore A. Anticonvulsant drugs for acute and chronic pain. The Cochrane Database of Systematic Reviews. 2005 Jul 20;(3):CD001133. doi: 10.1002/14651858.CD001133.pub2. [DOI] [PubMed] [Google Scholar]
- 150.Harati Y, Gooch C, Swenson M, et al. Double-blind randomized trial of tramadol for the treatment of the pain of diabetic neuropathy. Neurology. 1998 Jun;50(6):1842–1846. doi: 10.1212/wnl.50.6.1842. [DOI] [PubMed] [Google Scholar]
- 151.Sindrup SH, Andersen G, Madsen C, Smith T, Brosen K, Jensen TS. Tramadol relieves pain and allodynia in polyneuropathy: a randomised, double-blind, controlled trial. Pain. 1999 Oct;83(1):85–90. doi: 10.1016/s0304-3959(99)00079-2. [DOI] [PubMed] [Google Scholar]
- 152.Watson CP, Moulin D, Watt-Watson J, Gordon A, Eisenhoffer J. Controlled-release oxycodone relieves neuropathic pain: a randomized controlled trial in painful diabetic neuropathy. Pain. 2003 Sep;105(1-2):71–78. doi: 10.1016/s0304-3959(03)00160-x. [DOI] [PubMed] [Google Scholar]
- 153.Gilron I, Bailey JM, Tu D, Holden RR, Weaver DF, Houlden RL. Morphine, gabapentin, or their combination for neuropathic pain. N Engl J Med. 2005 Mar 31;352(13):1324–1334. doi: 10.1056/NEJMoa042580. [DOI] [PubMed] [Google Scholar]
- 154.Gilron I, Bailey JM, Tu D, Holden RR, Jackson AC, Houlden RL. Nortriptyline and gabapentin, alone and in combination for neuropathic pain: a double-blind, randomised controlled crossover trial. Lancet. 2009 Oct 10;374(9697):1252–1261. doi: 10.1016/S0140-6736(09)61081-3. [DOI] [PubMed] [Google Scholar]
- 155.Effect of treatment with capsaicin on daily activities of patients with painful diabetic neuropathy. Capsaicin Study Group. Diabetes Care. 1992 Feb;15(2):159–165. doi: 10.2337/diacare.15.2.159. [DOI] [PubMed] [Google Scholar]
- 156.Yuan RY, Sheu JJ, Yu JM, et al. Botulinum toxin for diabetic neuropathic pain: a randomized double-blind crossover trial. Neurology. 2009 Apr 28;72(17):1473–1478. doi: 10.1212/01.wnl.0000345968.05959.cf. [DOI] [PubMed] [Google Scholar]
- 157.Klassen A, Di Iorio B, Guastaferro P, Bahner U, Heidland A, De Santo N. High-tone external muscle stimulation in end-stage renal disease: effects on symptomatic diabetic and uremic peripheral neuropathy. J Ren Nutr. 2008 Jan;18(1):46–51. doi: 10.1053/j.jrn.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 158.Low PA, Denq JC, Opfer-Gehrking TL, Dyck PJ, O'Brien PC, Slezak JM. Effect of age and gender on sudomotor and cardiovagal function and blood pressure response to tilt in normal subjects. Muscle Nerve. 1997 Dec;20(12):1561–1568. doi: 10.1002/(sici)1097-4598(199712)20:12<1561::aid-mus11>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 159.Gelber DA, Pfeifer M, Dawson B, Schumer M. Cardiovascular autonomic nervous system tests: determination of normative values and effect of confounding variables. J Auton Nerv Syst. 1997 Jan 12;62(1-2):40–44. doi: 10.1016/s0165-1838(96)00107-5. [DOI] [PubMed] [Google Scholar]
- 160.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996 Mar 1;93(5):1043–1065. [PubMed] [Google Scholar]
- 161.Max MB, Culnane M, Schafer SC, et al. Amitriptyline relieves diabetic neuropathy pain in patients with normal or depressed mood. Neurology. 1987 Apr;37(4):589–596. doi: 10.1212/wnl.37.4.589. [DOI] [PubMed] [Google Scholar]
- 162.Max MB, Lynch SA, Muir J, Shoaf SE, Smoller B, Dubner R. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992 May 7;326(19):1250–1256. doi: 10.1056/NEJM199205073261904. [DOI] [PubMed] [Google Scholar]
- 163.Sindrup SH, Gram LF, Brosen K, Eshoj O, Mogensen EF. The selective serotonin reuptake inhibitor paroxetine is effective in the treatment of diabetic neuropathy symptoms. Pain. 1990 Aug;42(2):135–144. doi: 10.1016/0304-3959(90)91157-E. [DOI] [PubMed] [Google Scholar]