Abstract
Background
We previously demonstrated that donor treatment with inhaled hydrogen protects lung grafts from cold ischemia/reperfusion (I/R) injury during lung transplantation. To elucidate the mechanisms underlying hydrogen’s protective effects, we conducted a gene array analysis to identify changes in gene expression associated with hydrogen treatment.
Methods
Donor rats were exposed to mechanical ventilation with 98% oxygen and 2% nitrogen or 2% hydrogen for 3 h before harvest; lung grafts were stored for 4 h in cold Perfadex. Affymetrix gene array analysis of mRNA transcripts was performed on the lung tissue prior to implantation.
Results
Pretreatment of donor lungs with hydrogen altered the expression of 229 genes represented on the array (182 upregulated; 47 downregulated). Hydrogen treatment induced several lung surfactant-related genes, ATP synthase genes and stress-response genes. The intracellular surfactant pool, tissue adenosine triphosphate (ATP) levels and heat shock protein 70 (HSP70) expression increased in the hydrogen-treated grafts. Hydrogen treatment also induced the transcription factors C/EBPα and C/EBPβ, which are known regulators of surfactant-related genes.
Conclusion
Donor ventilation with hydrogen significantly increases expression of surfactant-related molecules, ATP synthases and stress-response molecules in lung grafts. The induction of these molecules may underlie hydrogen’s protective effects against I/R injury during transplantation.
Keywords: Hydrogen, Lung transplantation, Gene array, Ischemia/reperfusion injury, Lung surfactant, Adenosine triphosphate
1. Introduction
Ischemia/reperfusion (I/R) injury, which affects 10–20% of lung transplant recipients, is a major complication of lung transplantation and can lead to primary graft dysfunction, the main cause of early posttransplantation morbidity and mortality [1]. Furthermore, I/R injury increases the risk of bronchiolitis obliterans syndrome and contributes to late mortality.
Inhaled therapeutic gas therapy may be a straightforward and reasonable approach for lung disease [2–4], and hydrogen is a promising therapeutic gaseous agent. Hydrogen can reduce cellular oxidation and has potent anti-inflammatory and antiapoptotic properties [5]. Recently, our group demonstrated that preloading hydrogen gas into donor lungs during ventilation prior to organ procurement effectively protected lung grafts from I/R-induced injury in a rat lung transplantation model [6]. One possible explanation was the ability of hydrogen to induce heme oxygenase-1 (HO-1), an antioxidant enzyme, in the lung grafts prior to implantation [6]. However, the mechanisms underlying hydrogen’s protective effects against lung I/R injury remain largely unknown.
In this study, we conducted a gene array analysis to begin elucidating the mechanisms underlying the protective effects of preloading hydrogen into lung grafts prior to procurement. We then examined the expression of several of the upregulated genes and the transcription factors regulating their expression in our established rat orthotopic lung transplant model.
2. Materials and methods
2.1. Animals
Inbred male Lewis (LEW, RT1l) rats and Brown Norway (BN, RT1n) rats weighing 250–300 g were purchased from Harlan Sprague Dawley, Inc., (Indianapolis, IN) and maintained in laminar flow cages in a specific pathogen-free animal facility at the University of Pittsburgh. Animals were fed a standard diet and provided water ad libitum. All procedures were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee at the University of Pittsburgh and the National Research Council’s Guide for the Humane Care and Use of Laboratory Animals.
2.2. Donor treatment
Donor rats underwent tracheotomy and were mechanically ventilated for 3 h with isoflurane (Aerrane; Baxter, Deerfield, IL) and either mixed gas with 98% oxygen and 2% nitrogen (N2), or mixed gas with 98% oxygen and 2% hydrogen (H2) with a tidal volume of 10 ml/kg and a respiratory rate of 60 breaths/min and a positive end expiratory pressure (PEEP) of 3.0 cm H2O. The lungs were flushed through the main pulmonary artery with 20 ml of cold (4 °C) low potassium dextran (Perfadex®, Vitrolife, Gothenburg, Sweden) and excised. After excision of the heart–lung block, cuffs were attached to the pulmonary artery, pulmonary vein, and bronchus; then, the graft was placed in low potassium dextran and stored at 4 °C for 4 h. Sham-operated animals underwent anesthesia, tracheotomy, and mechanical ventilation for 5 min with 100% oxygen. The lungs of the sham animals were then immediately removed by thoracotomy for analysis.
2.3. Gene array analysis
We studied the differential gene-expression pattern of allografts from LEW donors. Graft tissue was collected for analysis after mechanical ventilation of the donor for 3 h with either 2% hydrogen or 2% nitrogen and 4 h of cold storage, but prior to implantation into a recipient. Changes in gene expression in the allografts exposed to hydrogen were compared with changes in allografts exposed to nitrogen using Affymetrix arrays (Illumina RatRef-12; Affymetrix, Inc., Santa Clara, CA). Optimization of perfect-match probe set data was conducted using an efficiency analysis (EA) [7]. In the EA, technical and biological replicates were subsampled repeatedly to create data sets that were then analyzed independently. Various methods for data transformation, normalization and testing for differentially expressed genes were applied in combination to the two independent subsamples. Combinations of methods were identified that exhibited the highest internal consistency (reproducibility). In this study, the optimal combination was z-transformation (within array/sample) followed by the use of the J5 test [8]. The J5 test is the ratio of the between-group difference (δ) in average expression for a given gene to the average absolute between-group δ of all of the genes on the array. Standard data quality measures, such as box-and-whiskers plots, global correlation, coefficient of variation, and confounding index, were also used to rule out undesired effects of data transformation; no such indicators were found. A threshold of 13.72 was selected for the J5 test using the EA criterion.
2.4. Orthotopic left lung transplantation in rats
Orthotopic left lung transplantation was performed (allogeneic: LEW to BN) utilizing a cuff technique as previously described [9,10]. After 4 h of cold storage, the lung grafts were transplanted into the recipients. The recipients were evaluated 2 h after reperfusion. No animals exhibited overinflation of the native lung or macroscopic atelectasis of the transplanted lung.
2.5. Immunohistochemical staining
Prior to implantation, lung graft tissues were fixed in 10% formalin, embedded in paraffin, and sectioned to 4-µm thickness. Anti-clara cell secretory protein antibodies (cat. no. 07–623; Millipore, Billerica, MA; dilution, 1:2000) were used, as described previously [6].
2.6. Western blot analysis
Western blot analysis was performed on 30 µg of whole cell protein from each lung graft, as described previously [10]. The following primary antibodies were used: anti-phospho-p38 (Thr180/Tyr182); anti-total p38; anti-phospho-extracellular signal-regulated protein kinase (ERK)1/2 (Thr202/Tyr204); anti-total-ERK1/2; anti-phospho-c-Jun N-terminal kinase (JNK) (Thr183/Tyr185); anti-total-JNK (all from Cell Signaling Technology, Beverly, MA); anti-clara cell protein 16 (CC16); anti-palate, lung, nasal epithelium clone (PLUNC); anti-CCAAT/enhancer binding protein (C/EBP) α; anti-C/EBP β (all from Santa Cruz Biotechnology, Santa Cruz, CA); anti-heat shock protein (HSP)70 (Abcam, Cambridge, MA); and anti-β-actin (Sigma–Aldrich, St. Louis, MO). Expression of CC16, PLUNC, CEBP α, CEBP β, HSP70 and β-actin was examined in lung graft tissues were taken prior to implantation. Expression and phosphorylation of p38, JNK and ERK were examined in lung graft tissues taken 2 h after reperfusion.
2.7. SYBR Green real-time RT-PCR
The mRNAs for CC16; surfactant protein A (SP-A); dual specificity phosphatase 1 (DUSP1); secretoglobin, family 3A, member 2 (SCGB3A2); hydroxysteroid 11-β dehydrogenase 1 (HSD11β1); heat shock protein 5 (HSPA5); PLUNC; adenosine triphosphate (ATP) synthase H+ transporting mitochondrial F1 complex beta polypeptide (ATP5b); ATP synthase H+ transporting mitochondrial F1 complex O subunit (ATP5o); ATP synthase H+ transporting mitochondrial F0 complex subunit F6 (ATP5j); ATP synthase H+ transporting mitochondrial F0 complex subunit G (ATP5 l); and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were quantified in duplicate using SYBR Green two-step, real-time reverse transcriptase (RT-PCR), as described previously [10], on frozen lung graft tissues taken prior to implantation.
2.8. Measurement of tissue ATP levels
ATP level in the lung graft tissue prior to implantation was quantified using the ENLITEN ATP luciferin/luciferase bioluminescence assay system (Promega, Madison, WI) according to manufacturer’s instructions. Luminescence was measured at a set lag time of 1 s using a 1420 VICTOR multi-label counter (PerkinElmer Life Sciences, Waltham, MA).
2.9. Transmission electron microscopy
Prior to implantation, lung grafts were harvested and immersion-fixed in 2.5% glutaraldehyde overnight at 4 °C. Following fixation, the tissue was dehydrated through a graded series of 30–100% ethanol, 100% propylene oxide, and then infiltrated with a 1:1 mixture of propylene oxide: Polybed 812 epoxy resin. After several changes of 100% resin, the tissue was embedded and cured. Ultrathin (70 nm) sections were collected on 200 mesh copper grids and stained with 2% uranyl acetate and 1% lead citrate. Sections were visualized using a JEOL JEM 1210 transmission electron microscope at 80 kV.
2.10. Statistical analysis
Results are expressed as mean ± standard error of the mean (SEM). The data were analyzed with one-way analysis of variance followed by post hoc analysis with the Bonferroni correction. A probability level of p < 0.05 was considered statistically significant.
3. Results
3.1. Gene array analysis
We previously demonstrated that preloading hydrogen gas into donor lungs during ventilation prior to organ procurement effectively protected lung grafts from I/R-induced injury in a rat lung transplantation model [6]. To begin elucidating the mechanisms underlying the protective effects of preloading hydrogen into lung grafts prior to procurement, we employed a global approach to quantify changes in gene expression associated with H2 inhalation prior to organ harvest. To this end, we compared the lung transcriptomes of rats treated with 2% hydrogen with those of rats treated with 2% nitrogen (control) using Affymetrix arrays with 45,101 unique probe sets representing 36,512 target genes. Of these, 229 genes were differentially expressed. Hydrogen treatment reduced the expression of 47 genes and increased the expression of 182 genes, as compared with N2-treated lungs (Table 1, Supplemental Tables 1 and 2).
Table 1.
The top 25 genes upregulated by hydrogen pretreatment of lung grafts and other interesting genes regulated by hydrogen treatment.
| Rank | Gene name | Accession number |
J5 score |
|---|---|---|---|
| 1a | Secretoglobin family 1A member 1 (uteroglobin) (Scgb1a1) | NM_013051.1 | 112.181 |
| 2 | P:myosin light polypeptide 7 regulatory (Myl7_) | XM_214074.4 | 82.586 |
| 3 | Collagen type I alpha 2 (Col1a2) | NM_053356.1 | 58.645 |
| 4a | Surfactant pulmonary-associated protein A1 (Sftpa1) | NM_017329.1 | 58.545 |
| 5 | Hemoglobin alpha adult chain 2 (Hba-a2) | NM_013096.1 | 57.481 |
| 6 | Niemann-Pick disease type C2 (Npc2) | NM_173118.1 | 54.093 |
| 7 | Peptidylprolyl isomerase A (cyclophilin A) (Ppia) | NM_017101.1 | 53.375 |
| 8 | S100 calcium binding protein A11 (calizzarin) (S100a11) | NM_001004095.1 | 52.942 |
| 9 | Myosin heavy polypeptide 6 cardiac muscle alpha (Myh6) | NM_017239.1 | 51.154 |
| 10 | P:RGD1561181 (RGD1561181_) | XM_001058739.1 | 50.147 |
| 11 | P:ribosomal protein L23a transcript variant 2 (Rpl23a) | XM_001060002.1 | 49.884 |
| 12 | P:actin gamma cytoplasmic (Actg_) | XM_213540.3 | 48.852 |
| 13 | P:similar to RIKEN cDNA 2410116I05 (LOC363377) | XM_343712.2 | 48.235 |
| 14a | Dual specificity phosphatase 1 (Dusp1) | NM_053769.2 | 47.277 |
| 15 | Ribosomal protein S4 X-linked (Rps4×) | NM_001007600.1 | 46.703 |
| 16 | Lysosomal-associated protein transmembrane 4A (Laptm4a) | NM_199384.1 | 46.299 |
| 17 | Insulin-like growth factor binding protein 7 (Igfbp7) | NM_001013048.1 | 45.933 |
| 18 | Keratin 19 (Krt19) | NM_199498.1 | 44.734 |
| 19 | S100 calcium binding protein A6 (S100a6) | NM_053485.2 | 43.624 |
| 20 | Ribosomal protein L32 (Rpl32) | NM_013226.2 | 43.613 |
| 21 | Tumor protein translationally-controlled 1 (Tpt1) | NM_053867.1 | 43.531 |
| 22 | Defensin beta 4 (Defb4) | NM_022544.2 | 41.889 |
| 23 | Calmodulin 2 (Calm2) | NM_017326.1 | 41.262 |
| 24 | Hemoglobin beta chain complex (Hbb) | NM_033234.1 | 40.688 |
| 25 | ATPase Ca++ transporting cardiac muscle slow twitch 2 (Atp2a2)_GI_8392934-S | NM_017290.1 | 40.471 |
| Other surfactant-related genes | |||
| 27a | Secretoglobin family 3A member 2 (Scgb3a2) | NM_001024283.1 | 39.612 |
| 50a | Hydroxysteroid 11-beta dehydrogenase 1 (Hsd11b1) | NM_017080.2 | 31.318 |
| 68 | Secretoglobin family 3A member 1 (Scgb3a1)_GI_61557201-S | NM_001013180.1 | 24.925 |
| −1a,b | Palate lung and nasal epithelium associated (Plunc)_GI_62645787-A | NM_172031.1 | −324.0 |
| ATP synthase subunits | |||
| 32a | ATP synthase H+ transporting mitochondrial F1 complex beta polypeptide (Atp5b) nuclear gene encoding mitochondrial protein | NM_134364.1 | 37.291 |
| 74a | ATP synthase H+ transporting mitochondrial F1 complex O subunit (Atp5o) nuclear gene encoding mitochondrial protein_GI_20302060 | NM_138883.1 | 23.893 |
| 157a | ATP synthase H+ transporting mitochondrial F0 complex subunit F6 (Atp5j) nuclear gene encoding mitochondrial protein_GI_16758387-S | NM_053602.1 | 15.428 |
| 165a | ATP synthase H+ transporting mitochondrial F0 complex subunit G (Atp5 l) nuclear gene encoding mitochondrial protein_GI_47058993-S | NM_212516.1 | 15.18 |
| Stress-response gene | |||
| 66a | Heat shock protein 5 (Hspa5)_GI_25742762-S | NM_013083.1 | 25.314 |
Indicates genes chosen for further analysis.
Downregulated by hydrogen treatment.
3.2. Surfactant-related genes and C/EBPs in the lung grafts prior to implantation
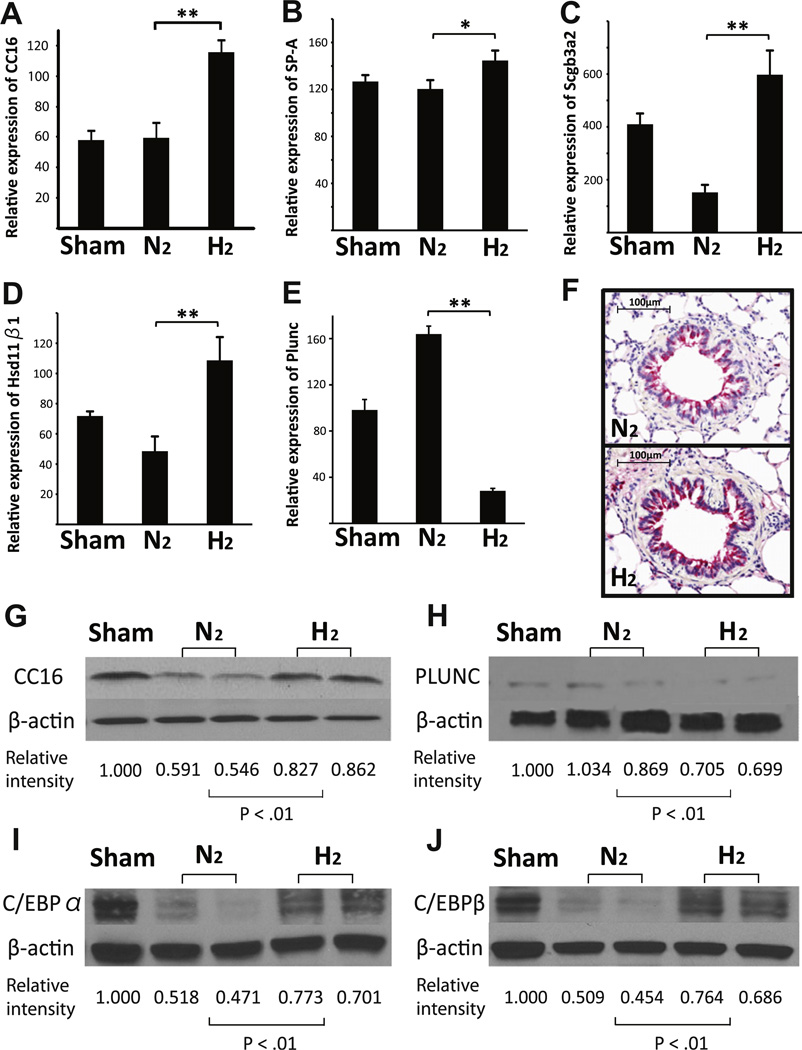
Clara cell protein 16 (CC16) was the most upregulated gene after hydrogen pretreatment of the donor lungs (Table 1, Fig. 1A). CC16, 15.8-kDa homodimeric protein, is a secretoglobin family member (also called CC10 or uteroglobin). CC16 is one of the major proteins produced by the nonciliated cells of the tracheobronchial epithelial tree and has anti-inflammatory and antioxidant properties. (7) Preloading hydrogen by mechanical ventilation also significantly increased other surfactant-related mRNAs including SP-A, SCGB3A2 and HSD11β1 (Fig. 1B–D). Interestingly, the mRNA most dramatically downregulated by hydrogen pretreatment also encoded a surfactant-related protein, PLUNC, a secretory product of the epithelium with airway surfactant properties (Table 1, Supplemental Table 2, Fig. 1E). Consistent with mRNA expression, CC16 protein levels were increased and PLUNC protein levels were decreased in the allografts prior to implantation. Immunohistochemical analysis revealed more prominent CC16-positive epithelial cells in the hydrogen-preloaded allografts than in control allografts, although there were no obvious histopathological changes (Fig. 1F–H).
Fig. 1.
Quantitative RT-PCR for (A) CC16, (B) SP-A, (C) SCGB3A2, (D) HSD11β1 and (E) PLUNC (n = 4 for each group; **p < 0.01 and *p < 0.05). (F) Representative immunohistochemistry for CC16 in the lung grafts before transplantation. Western blots for (G) CC16, (H) PLUNC, (I) C/EBPα, (J) C/EBP β, and β-actin on protein extracts from lung grafts taken after 3 h of mechanical ventilation and 4 h cold storage. The images are representative of 3 independent experiments.
The CC16, SP-A, SCGB3A2 and HSD11β1 genes are regulated by same transcription factor family, the C/EBPs. Although C/EBPs were not identified in the microarray as hydrogen-regulated mRNAs, preloading hydrogen increased C/EBPα and β protein levels in the lung allografts prior to implantation as compared with control allografts (Fig. 1I and J).
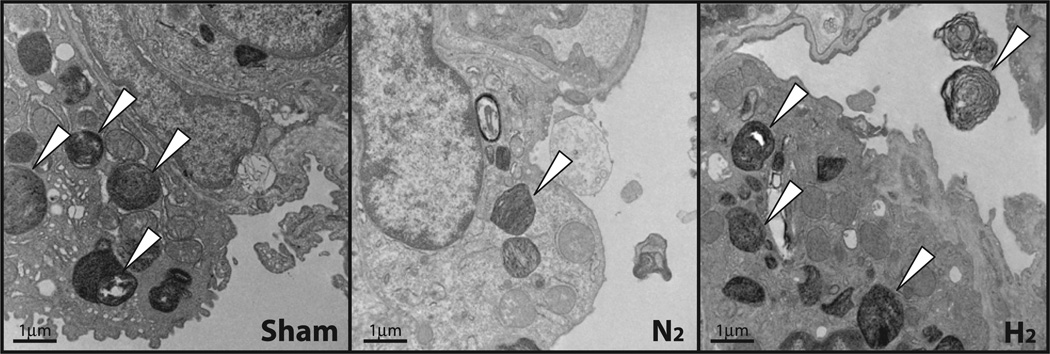
Morphological analysis of alveolar surfactant in the grafts prior to transplant was performed using transmission electron microscopy. The intracellular surfactant pool, indicated by the number of lamellar bodies, was increased in H2-treated grafts (Fig. 2). However, no obvious changes in the morphology of the alveolar epithelial type II cells were observed between H2-treated grafts and N2-treated grafts.
Fig. 2.
Transmission electron microscopy of lung grafts. Cell structure of alveolar epithelial type II cells in hydrogen-treated and nitrogen-treated grafts. Lamellar bodies (white arrowheads) contain intracellular surfactant.
3.3. Expression of ATP synthase genes in the lung grafts
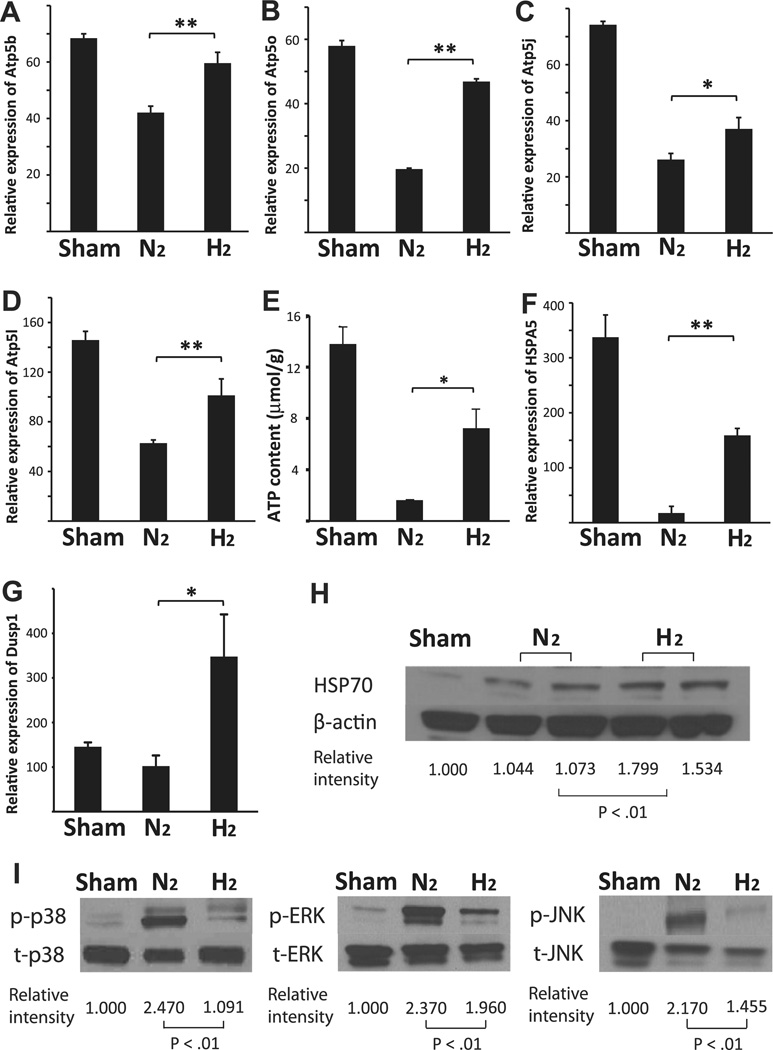
The gene array analysis indicated that four ATP synthase genes (ATP5b ATP5o ATP5j and ATP5l) were upregulated by hydrogen treatment of the lung grafts prior to harvest (Table 1). Because maintenance of tissue ATP is associated with reduced I/R in lung grafts [11], we chose to further investigate these genes. Significant increases in the mRNA levels for these 4 ATP synthase genes after hydrogen treatment were confirmed with real-time RT-PCR (Fig. 3A–D). The lung grafts pretreated with hydrogen prior to harvest also had significantly higher tissue ATP levels after cold storage than those treated with nitrogen (Fig. 3E).
Fig. 3.
Quantitative RT-PCR for (A) ATP5b, (B) ATP5o, (C) ATP5j and (D) ATP5 l (n = 4 for each group; **p < 0.01 and *p < 0.05). (E) Lung ATP content prior to implantation. (n = 4 per group *p < 0.05). Quantitative RT-PCR for (F) HSPA5 and (G) DUSP1 (n = 4 for each group; **p < 0.01 and *p < 0.05). (H) Western blots for HSP70 and β-actin on protein extracts from lung grafts taken after 3 h of mechanical ventilation and 4 h of cold storage. (I) Western blots for phosphorylated (p) and total (t) p38, ERK1/2 and JNK on protein extracts from lung grafts taken 2 h after reperfusion. The images are representative of 3 independent experiments (n = 3 for each group).
3.4. Expression of stress-response genes in the lung grafts
Changes in the expression of several stress-response proteins were noted in the gene-expression array. Specifically, the mRNA for HSPA5, which encodes a member of the HSP70 protein family, and the mRNA for DUSP1, a dual specificity phosphatase that decreases mitogen-activated protein kinase (MAPK) phosphorylation/activation [12], were upregulated after hydrogen inhalation. We confirmed these results using real-time RT-PCR (Fig. 3F and G). We continued our analysis by examining the expression of the stress-response proteins encoded by these mRNAs in hydrogen pretreated lung grafts prior to implantation. Consistent with mRNA expression, HSP70 protein levels increased in the allografts (Fig. 3H). To assess DUSP1 expression, activation of the 3 major MAPKs (p38, ERK1/2 and JNK), as indicated by phosphorylation, was examined in the lung tissue 2 h after reperfusion. Preloading hydrogen into the lung significantly reduced activation of p38 MAPK, ERK 1/2 and JNK in the grafts as compared with nitrogentreated controls (Fig. 3I).
4. Discussion
In this study, we demonstrated that preloading hydrogen into lung allografts prior to harvest was associated with upregulation of lung surfactant-related molecules, ATP synthases and stress-response molecules. Hydrogen is an important physiologic regulatory gas molecule with antioxidant, anti-inflammatory and antiapoptotic effects on cells and organs that underlie its potential therapeutic benefits for the prevention of lung injuries [5]. Our previous studies demonstrated that treating donor lungs with hydrogen prior to harvest and cold storage ameliorated lung cold I/R injury during transplantation [6]. We expand on this observation in the current study, revealing previously unknown molecular changes that may underlie the beneficial effects of inhaled hydrogen therapy for lung disease.
Extracellular surfactants play important roles in innate host defense by binding and clearing infectious pathogens and regulating immune cell activity through multiple cellular pathways [13–16]. Likewise, there is increasing evidence that the pulmonary surfactant system is altered after lung transplantation. Lung perfusion with preservation solution or prolonged storage of donor lungs impairs the integrity of the surfactant system, and these modifications are associated with early primary graft dysfunction. Recent work in a rat lung transplantation model showed that pulmonary surfactant continued to be deficient 1 week after transplantation [17]. Exogenous surfactant treatment not only increases the quantity of surfactant but also enhances recovery from reperfusion injury after lung transplantation [15,18]. Exogenous administration of surfactant prior to reperfusion improved oxygenation and dynamic compliance in grafts stored for 20 h and was beneficial for endogenous surfactant metabolism [17]. A clinical retrospective review also revealed that bronchoscopic instillation of surfactant improved oxygenation and prognosis in lung transplant recipients after severe I/R injury [19]. Thus, it is likely that maintenance of surfactant in the pulmonary graft is critical for successful outcomes after lung transplantation.
In our gene array analysis, CC16 was the most upregulated gene in response to preloading hydrogen in lung grafts. CC16 (also known as CC10, CCSP and uteroglobin) is one of the major proteins secreted by the respiratory epithelium. It modulates the production and activity of various inflammatory mediators including phospholipase A2 (PLA2), interferon-γ and TNF-α. CC16 also increases lung surfactant protein levels and prevents lung surfactant degradation by PLA2 inhibition [20,21]. In addition to the prominent upregulation of CC16 mRNA, the mRNAs for SP-A, SCGB3A2 and HSD11β1 were upregulated by preloading hydrogen in the lung grafts. SP-A is a surfactant protein and has a significant role in surfactant homeostasis and protection of the alveoli against inflammation [16,22]. SCGB3A2 (also known as uteroglobin-related protein 1) is a novel cytokine-like molecule. It belongs to the secretoglobin family, as does CC16, and exhibits anti-inflammatory effects in the lung [23,24]. HSD11β1 is highly expressed in liver and lung and increases intracellular glucocorticoid activity by converting glucocorticoids from inactive to active forms. HSD11β1 has been implicated in xenobiotic detoxification and surfactant production in lung [25]. Thus, it is likely that these proteins contribute to mitigation of I/R injury after lung transplantation and expand the possible mechanisms underlying inhaled hydrogen therapy. PLUNC was the only surfactant-related gene with decreased expression after hydrogen treatment. PLUNC is a secretory product of the epithelium and has airway surfactant properties that may interfere with biofilm formation by the airway pathogen Pseudomonas aeruginosa [26]. Further study will be needed to determine whether downregulation of PLUNC contributes to the attenuation of I/R injury.
ATP synthase synthesizes ATP from adenosine diphosphate (ADP) and inorganic phosphate (P) using the energy of proton gradients [27]. Decreasing adenine nucleotides caused edema in the isolated, perfused rat lung and adding ATP to the preservation solution mitigated cold I/R injury in rats [11]. In our gene array analysis, 4 ATP synthase subunits genes were upregulated by hydrogen and higher ATP levels were observed in the hydrogen pretreated lung grafts. This upregulation of ATP might contribute to mitigation of I/R injury after lung transplantation.
Our gene array analysis also showed upregulation of stress-response molecules. DUSP1 can dephosphorylate and inactivate mitogen-activated protein kinases (MAPKs) and it may play an important role in suppressing the inflammatory response [12]. HSPA5 encodes an HSP70 protein that is induced by various types of cellular stress and has anti-inflammatory and antiapoptotic properties [28]. Upregulation of DUSP1 and HSPA5 by hydrogen treatment may explain the hydrogen-dependent decreases in MAPK phosphorylation/activation seen after reperfusion and increases in HSP70 expression prior to implantation in vivo.
CC16, SP-A, SCGB3A2, DUSP1 and HSD11β1 are all induced by the C/EBP transcription factor family [12,22,23,25]. There are 6 C/EBP family members (C/EBPα-ζ) that modulate numerous cellular responses including differentiation, inflammation, liver regeneration, and metabolism [22,29]. C/EBPα, -β, and -δ are expressed in the lung epithelium and regulate proliferation, differentiation and response to acute inflammation or injury. In our study, treatment with 2% hydrogen by mechanical ventilation prior to harvest significantly increased C/EBPα and C/EBPβ protein expression in the donor lungs. These observations suggest that amelioration of I/R injury with inhaled hydrogen is conveyed, at least partially, by increasing C/EBP expression, which results in the transcriptional upregulation of CC16, SP-A, SCGB3A2, DUSP1 and HSD11β1. Other C/EBP-regulated genes may also contribute to the protective effects of hydrogen treatment and require further investigation.
In conclusion, donor treatment with inhaled hydrogen was associated with the upregulation of pulmonary surfactant-related molecules, ATP synthases and stress-response molecules in lung allografts. These molecules may play pivotal roles in hydrogen-induced cytoprotective mechanisms. Although the therapeutic efficacies of hydrogen have been extensively studied, there is very limited information on the pathways and processes regulated in vivo by the hydrogen molecule. Considering the potential for wide clinical application of hydrogen treatment, it will become important to understand the mechanisms underlying the protective effects of hydrogen. To our knowledge, our study is the first gene-array analysis on hydrogen-treated lung tissue and the first demonstration of induction of lung surfactant-related genes by hydrogen treatment. In the future, further studies on hydrogen’s protective effects including the role of the C/EBPs will be essential. This study paves the way for understanding the mechanisms underlying the protective effects of hydrogen treatment during lung transplantation.
Supplementary Material
Acknowledgments
We thank Lisa Chedwick and Jonathan M. Franks for their excellent technical support, and Dr Shannon L. Wyszomierski for editing the manuscript.
The Illumina gene expression data used in this study were generated by the University of Pittsburgh Genomics and Proteomics Core Laboratory. This publication was made possible in part by Grant No. 5 UL1 RR024153 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research, R21 HL102528-01, and the research funds of the Department of Cardiothoracic Surgery. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on the NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
T.K is a recipient of the Thomas E. Starzl postdoctoral fellowship.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bbrc.2012.08.005.
References
- 1.Christie JD, Edwards LB, Aurora P, Dobbels F, Kirk R, Rahmel AO, Taylor DO, Kucheryavaya AY, Hertz MI. Registry of the international society for heart and lung transplantation: twenty-fifth official adult lung and heart/lung transplantation report–2008. Journal of Heart and Lung Transplantation. 2008;27:957–969. doi: 10.1016/j.healun.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 2.Huang CS, Kawamura T, Lee S, Tochigi N, Shigemura N, Buchholz BM, Kloke JD, Billiar TR, Toyoda Y, Nakao A. Hydrogen inhalation ameliorates ventilator-induced lung injury. Critical Care. 2010;14:R234. doi: 10.1186/cc9389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawamura T, Huang CS, Tochigi N, Lee S, Shigemura N, Billiar TR, Okumura M, Nakao A, Toyoda Y. Inhaled hydrogen gas therapy for prevention of lung transplant-induced ischemia/reperfusion injury in rats. Transplantation. 2010;90:1344–1351. doi: 10.1097/TP.0b013e3181fe1357. [DOI] [PubMed] [Google Scholar]
- 4.Nakao A, Sugimoto R, Billiar TR, McCurry KR. Therapeutic antioxidant medical gas. Journal of Clinical Biochemistry and Nutrition. 2009;44:1–13. doi: 10.3164/jcbn.08-193R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang CS, Kawamura T, Toyoda Y, Nakao A. Recent advances in hydrogen research as a therapeutic medical gas. Free Radical Research. 2010;44:971–982. doi: 10.3109/10715762.2010.500328. [DOI] [PubMed] [Google Scholar]
- 6.Kawamura T, Huang CS, Peng X, Masutani K, Shigemura N, Billiar TR, Okumura M, Toyoda Y, Nakao A. The effect of donor treatment with hydrogen on lung allograft function in rats. Surgery. 2011;150:240–249. doi: 10.1016/j.surg.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 7.Jordan R, Patel S, Hu H, Lyons-Weiler J. Efficiency analysis of competing tests for finding differentially expressed genes in lung adenocarcinoma. Cancer Information. 2008;6:389–421. doi: 10.4137/cin.s791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel S, Lyons-Weiler J. CaGEDA: a web application for the integrated analysis of global gene expression patterns in cancer. Applied Bioinformatics. 2004;3:49–62. doi: 10.2165/00822942-200403010-00007. [DOI] [PubMed] [Google Scholar]
- 9.Sugimoto R, Nakao A, Nagahiro I, Kohmoto J, Sugimoto S, Okazaki M, Yamane M, Inokawa H, Oto T, Tahara K, Zhan J, Sano Y, McCurry KR. Experimental orthotopic lung transplantation model in rats with cold storage. Surgery Today. 2009;39:641–645. doi: 10.1007/s00595-008-3929-x. [DOI] [PubMed] [Google Scholar]
- 10.Kohmoto J, Nakao A, Kaizu T, Tsung A, Ikeda A, Tomiyama K, Billiar TR, Choi AM, Murase N, McCurry KR. Low-dose carbon monoxide inhalation prevents ischemia/reperfusion injury of transplanted rat lung grafts. Surgery. 2006;140:179–185. doi: 10.1016/j.surg.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Hirata T, Fukuse T, Ishikawa S, Miyahara R, Wada H. Addition of ATP and MgCl2 to the preservation solution attenuates lung reperfusion injury following cold ischemia. Respiration; International Review of Thoracic Diseases. 2001;68:292–298. doi: 10.1159/000050513. [DOI] [PubMed] [Google Scholar]
- 12.Johansson-Haque K, Palanichamy E, Okret S. Stimulation of MAPK-phosphatase 1 gene expression by glucocorticoids occurs through a tethering mechanism involving C/EBP. Journal of Molecular Endocrinology. 2008;41:239–249. doi: 10.1677/JME-08-0015. [DOI] [PubMed] [Google Scholar]
- 13.Wright JR. Pulmonary surfactant: a front line of lung host defense. Journal of Clinical Investigation. 2003;111:1453–1455. doi: 10.1172/JCI18650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goerke J. Pulmonary surfactant: functions and molecular composition. Biochimica et Biophysica Acta. 1998;1408:79–89. doi: 10.1016/s0925-4439(98)00060-x. [DOI] [PubMed] [Google Scholar]
- 15.Struber M, Fischer S, Niedermeyer J, Warnecke G, Gohrbandt B, Gorler A, Simon AR, Haverich A, Hohlfeld JM. Effects of exogenous surfactant instillation in clinical lung transplantation: a prospective, randomized trial. Journal of Thoracic and Cardiovascular Surgery. 2007;133:1620–1625. doi: 10.1016/j.jtcvs.2006.12.057. [DOI] [PubMed] [Google Scholar]
- 16.Kingma PS, Whitsett JA. In defense of the lung: surfactant protein A and surfactant protein D. Current Opinion in Pharmacology. 2006;6:277–283. doi: 10.1016/j.coph.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Erasmus ME, Hofstede GJ, Petersen AH, Haagsman HP, Oetomo SB, Prop J. Effects of early surfactant treatment persisting for one week after lung transplantation in rats. American Journal of Respiratory and Critical Care Medicine. 1997;156:567–572. doi: 10.1164/ajrccm.156.2.9607005. [DOI] [PubMed] [Google Scholar]
- 18.Hohlfeld JM, Tiryaki E, Hamm H, Hoymann HG, Krug N, Haverich A, Fabel H. Pulmonary surfactant activity is impaired in lung transplant recipients. American Journal of Respiratory and Critical Care Medicine. 1998;158:706–712. doi: 10.1164/ajrccm.158.3.9708063. [DOI] [PubMed] [Google Scholar]
- 19.Kermeen FD, McNeil KD, Fraser JF, McCarthy J, Ziegenfuss MD, Mullany D, Dunning J, Hopkins PM. Resolution of severe ischemia-reperfusion injury post-lung transplantation after administration of endobronchial surfactant. Journal of Heart and Lung Transplantation. 2007;26:850–856. doi: 10.1016/j.healun.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Broeckaert F, Bernard A. Clara cell secretory protein (CC16): characteristics and perspectives as lung peripheral biomarker. Clinical and Experimental Allergy. 2000;30:469–475. doi: 10.1046/j.1365-2222.2000.00760.x. [DOI] [PubMed] [Google Scholar]
- 21.Wolfson MR, Funanage VL, Kirwin SM, Pilon AL, Shashikant BN, Miller TL, Shaffer TH. Recombinant human Clara cell secretory protein treatment increases lung mRNA expression of surfactant proteins and vascular endothelial growth factor in a premature lamb model of respiratory distress syndrome. American Journal of Perinatology. 2008;25:637–645. doi: 10.1055/s-0028-1090587. [DOI] [PubMed] [Google Scholar]
- 22.Cassel TN, Nord M. C/EBP transcription factors in the lung epithelium, American Journal of Physiology. Lung Cellular and Molecular Physiology. 2003;285:L773–L781. doi: 10.1152/ajplung.00023.2003. [DOI] [PubMed] [Google Scholar]
- 23.Kido T, Tomita T, Okamoto M, Cai Y, Matsumoto Y, Vinson C, Maru Y, Kimura S. FOXA1 plays a role in regulating secretoglobin 1a1 expression in the absence of CCAAT/enhancer binding protein activities in lung in vivo, American Journal of Physiology. Lung Cellular and Molecular Physiology. 2011;300:L441–L452. doi: 10.1152/ajplung.00435.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiba Y, Kurotani R, Kusakabe T, Miura T, Link BW, Misawa M, Kimura S. Uteroglobin-related protein 1 expression suppresses allergic airway inflammation in mice. American Journal of Respiratory and Critical Care Medicine. 2006;173:958–964. doi: 10.1164/rccm.200503-456OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruley C, Lyons V, Worsley AG, Wilde MD, Darlington GD, Morton NM, Seckl JR, Chapman KE. A novel promoter for the 11beta-hydroxysteroid dehydrogenase type 1 gene is active in lung and is C/EBPalpha independent. Endocrinology. 2006;147:2879–2885. doi: 10.1210/en.2005-1621. [DOI] [PubMed] [Google Scholar]
- 26.Gakhar L, Bartlett JA, Penterman J, Mizrachi D, Singh PK, Mallampalli RK, Ramaswamy S, McCray PB., Jr PLUNC is a novel airway surfactant protein with anti-biofilm activity. PLoS ONE. 2010;5:e9098. doi: 10.1371/journal.pone.0009098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elston T, Wang H, Oster G. Energy transduction in ATP synthase. Nature. 1998;391:510–513. doi: 10.1038/35185. [DOI] [PubMed] [Google Scholar]
- 28.Yenari MA, Liu J, Zheng Z, Vexler ZS, Lee JE, Giffard RG. Antiapoptotic and anti-inflammatory mechanisms of heat-shock protein protection. Annals of the New York Academy of Sciences. 2005;1053:74–83. doi: 10.1196/annals.1344.007. [DOI] [PubMed] [Google Scholar]
- 29.Ramji DP, Foka P. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochemical Journal. 2002;365:561–575. doi: 10.1042/BJ20020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.