Abstract
Nociceptin/orphanin FQ (N/OFQ), added in vitro to murine spleen cells in the picomolar range, suppressed antibody formation to sheep red blood cells in a primary and a secondary plaque-forming cell (PFC) assay. The activity of the peptide was maximal at 10−12 M, with an asymmetric U-shaped dose response curve that extended activity to 10−14 M. Suppression was not blocked by pretreatment with naloxone. Specificity of the suppressive response was shown using affinity purified rabbit antibodies against two N/OFQ peptides, and with a pharmacological antagonist. Antisera against both peptides were active, in a dose related manner, in neutralizing N/OFQ -mediated immunosuppression, when the peptide was used at concentrations from 10−12.3 to 10−11.6 M. In addition, nociceptin given in vivo by osmotic pump for 48 hr suppressed the capacity of spleen cells placed ex vivo to make an anti-sheep red blood cell response. These studies show that nociceptin directly inhibits an adaptive immune response, i.e. antibody formation, both in vitro and in vivo.
Keywords: Nociceptin/orphanin FQ (N/OFQ), immunosuppression, mouse, plaque-forming assay cell assay, Anti-N/OFQ antibodies, neutralizing antibodies, RIA
Introduction
Nociceptin/orphanin FQ (N/OFQ) is a heptadecapeptide encoded by a full-length cDNA, which was first identified in mammalian brain tissues (Meunier et al, 1995; Reinscheid et al, 1995). N/OFQ is processed from a polypeptide precursor (PPNOC), and shares a high structural homology with the opioid peptide, dynorphin A (Meunier et al, 1995; Reinscheid et al, 1995; Houtani et al, 1996). However, N/OFQ does not bind to the delta opioid receptor, or to either of the two other opioid receptors, mu and kappa (Mollereau et al, 1994; Pan et al, 1995). N/OFQ was found to be the natural ligand for the orphan ORL1 receptor (opioid receptor-like 1) which was cloned from the neural tissue of humans (Mollereau et al, 1994), rats (Bunzow et al, 1994; Chen et al, 1994; Wick et al, 1994; Fukuda et al, 1994), and mice (Halford et al, 1995). N/OFQ and ORL-1 were initially linked to the opioid system because of: 1) the 60% homology of N/OFQ to other opioid peptides; 2) the similarity of the precursor proteins in the two systems; and 3) the observations that the ORL-1 receptor, like the opioid receptors, was a G-protein coupled, seven transmembrane protein, which when bound to N/OFQ resulted in inhibition of forskolin-induced cAMP accumulation via a pertussis toxin-sensitive Gi protein (Chen et al, 1994; Reinscheid et al, 1995; Civelli, 2008). However, ligands for opioid receptors were not active at ORL-1 (Bunzow et al, 1994; Mollereau et al, 1994; Wang et al, 1994; Reinscheid et al, 1998; Meng et al, 1996), and the activity of ORL-1 in neuronal tissue was found to be naloxone insensitive in vitro (Knoflach et al, 1996; Reinscheid et al, 1995), and in vivo (Chen et al, 2001). These latter findings indicated that ORL-1 is not a classical opioid receptor. Using in situ hybridization and immunohistochemistry, studies showed that N/OFQ and ORL-1 are widely expressed in the brain and peripheral nervous system of mammals (Neal et al, 1999b; Peluso et al, 1998; Bunzow et al, 1994; Mollereau et al, 1994; Fukuda et al, 1994; Neal et al, 1999a; Houtani et al, 1996; Anton et al, 1996; Reinscheid and Civelli, 2002), as well as peripherally in the intestines, skeletal muscle, vas deferens, and the spleen (Wang et al, 1994). Studies on the function of N/OFQ discovered a broad spectrum of bioactivities in a variety of complex neural functions, such as nociception (Mogil and Pasternak, 2001), neuroendocrine control (Bryant et al, 1998), water-electrolyte balance (Kapusta et al, 1997), sexual behavior (Sinchak et al, 2007), alimentary responses (Olszewski and Levine, 2004; Polidori et al, 2000), learning and memory (Mogil and Pasternak, 2001), kindling and epilepsy (Gutiérrez et al, 2001), stress and anxiogenic activity (Green et al, 2007), locomotor activity and reward (Mogil and Pasternak, 2001), and drinking behavior (Ciccocioppo et al, 2002).
An interesting observation is that the N/ORL-1 message is highly expressed in cells of the immune system and in several cases these cells have been found to produce N/ORL-1 peptide. Human peripheral blood leukocytes and spleen cells, as well as mouse splenocytes, have been shown to express message for N/ORL (Halford et al, 1995; Wick et al, 1995; Hazum et al, 1979; Peluso et al, 1998). Initially, T-cells were identified as positive for message, which was shown to be significantly up-regulated after treatment with mitogens (Wick et al, 1995; Arjomand et al, 2002). Subsequently, message was also demonstrated in human monocytes (Serhan et al, 2001), in monocytic cell lines (THP and U937) (Peluso et al, 2001; Peluso et al, 1998) and human peripheral blood polymorphonuclear (PMN) leukocytes (Peluso et al, 1998; Serhan et al, 2001; Fiset et al, 2003). In addition, human B-cell (Hom et al, 1999) and T-cell lines were shown to express N/ORL message constitutively (Wick et al, 1995; Peluso et al, 1998). A functional role for the receptors is implied by the observation that monocytic, T-cell, and B-cell lines, as well as primary human PMNs, bind N/OFQ at levels comparable to those exhibited by human SH-SY5Y neuroblastoma cells (Peluso et al, 2001; Hom et al, 1999; Serhan et al, 2001; Krüger et al, 2006). It is present in human neutrophil granules (PMNs), and excreted upon PMN degranulation (Fiset et al, 2003). Rat splenocytes have also been shown to excrete N/OFQ when stimulated with mitogens, pro-inflammatory cytokines, and other classes of compounds, indicating that leukocytes can be a source of this neuropeptide (Miller and Fulford, 2007). However, a clear role for N/OFQ in the immune system is still to be determined. Exogenous N/OFQ has been shown to be both pro-inflammatory and anti-inflammatory, immunoenhancing and immunosuppressive. Evidence suggesting a pro-inflammatory role includes the following. Mice lacking the gene for the precursor for N/OFQ have decreased proinflammatory cytokine responses to Staphylococcal Enterotoxin A (SEA) (Goldfarb et al, 2006), and its absence decreases murine experimental colitis (Horvath et al, 2004; Szalay et al, 2004; Kato et al, 2005). Mice injected with N/OFQ have increased levels of mRNA for TNF-α and IFN-γ in their spleens when subsequently injected with SEA (Goldfarb et al, 2006). N/OFQ is chemotactic for PMNs and human monocytes in vitro and in vivo (Serhan et al, 2001; Fiset et al, 2003; Trombella et al, 2005). Human T cells activated with Staphylococcal Enterotoxin B (SEB) show increased proliferation in response to nociceptin and up-regulated expression of the co-stimulatory molecule CD28 (Waits et al, 2004). Further, antisense to ORL-1 was shown to suppress the polyclonal IgM and IgG responses of mouse spleen cells stimulated with lipopolysaccharide by 50%, suggesting that the peptide exerts a functional role in immunocompetence (Halford et al, 1995). In contrast to the proinflammatory role suggested by these observations, N/ORL has also been reported to suppress the production of chemokine proteins (i.e, CCL2/MCP-1 and CCL5/RANTES) in human monocytes and monocyte-like cell lines (Kaminsky and Rogers, 2008), and to inhibit the proliferation of Con A activated rat splenocytes, as well as the production of IL-2 in vitro (Miller and Fulford, 2007). The peptide has been reported to decrease expression of mRNA for TNF-α, IL-1β, and IL-6 in astrocytes activated in vivo by peripheral administration of an inflammatory stimulus, and also by astrocyte cultures stimulated in vitro with lipopolysaccharide (Fu et al, 2007).
Most of the above studies on the effect of N/ORL on immune responses measured parameters of innate immune responses or of nonspecific immune responses, for example responses to mitogens, cytokine levels, or polyclonal immunoglobulin responses. The present studies were undertaken to examine the effect of nociceptin on an adaptive immune parameter, namely the capacity of mouse spleen cells to mount an in vitro or ex vivo antibody response to sheep red blood cells. Previous studies from our laboratory have shown that mu, kappa, and delta opioid agonists are suppressive in these assays (Bussiere et al, 1993; Eisenstein et al, 1995) (unpublished observation). N/ORL was tested by application directly to spleen cells in vitro, and also by in vivo infusion using Alzet pumps and testing of spleen cells from treated mice ex vivo. In both paradigms, suppression of antibody responses by N/ORL was observed.
Materials and Methods
Animals
Six week-old, specific pathogen-free C3HeB/FeJ female mice were purchased from Jackson Laboratories (Bar Harbor, Maine). New Zealand White male rabbits (2.5 kg) were purchased from Harlan S.A., Mexico.
Primary and secondary in vitro plaque-forming cell (PFC) antibody responses
Immune function was assessed using in vitro plaque-forming cell (PFC) assays that measure the capacity of spleen cells to mount an antibody response to sheep red blood cells (SRBCs), according to the method of Mishell and Dutton (Mishell and Dutton, 1967). For the primary PFC assay, mice were sacrificed and their spleens aseptically removed. A single cell suspension of spleen cells was obtained by pushing the spleen through nylon mesh bags in HEPES-buffered RPMI-1640 (InVitrogen GIBCO, Grand Island, NY) supplemented with 5% fetal bovine serum (FBS). Red blood cells were lysed by hypotonic shock with sterile water. Cells were washed twice and resuspended in tissue culture medium consisting of RPMI-1640 supplemented with 10% FBS, 2mM L-glutamine, 50 μg/ml gentamicin, 1 mM nonessential amino acids, 1 mM sodium pyruvate, 10 μg/ml of adenosine, uridine, cytosine, and guanosine, and 0.05 mM of 2-mercaptoethanol. The cells were counted and resuspended to 1.0 × 107 cells/ml and dispensed into flat-bottom 24-well tissue culture plates (Corning Costar, Corning, NY). For assays of immunosuppression by N/OFQ, replicate wells of spleen cells received N/OFQ (Multiple Peptide Systems, San Diego, CA) at concentrations ranging from 10−7 M to 10−14 M. Each condition was done in triplicate. Cultures then received 3.5 × 106 sheep red blood cells (SRBC) (Rockland, Gilbertsville, PA) in 50 μl of supplemented RPMI-1640. Cultures for determining background levels of PFCs did not receive SRBCs. Cultures were incubated for 5 days at 37°C in 10% CO2, 7% O2, and 83% N2. For antagonist studies, naloxone (Endo Pharmaceuticals, Chadds Ford, PA) or PG-Nociceptin ([Phe1Ψ(CH2-NH)Gly2]-nociceptin 1,13-NH2, Tocris, Baldwin, MO) were added to the appropriate wells, at the desired concentration, and incubated as indicated above for 2 hours before addition of N/OFQ and SRBC. For assays of neutralizing antibodies, spleen cells were pretreated for 2 hours with 2-fold serial dilutions of antibody, from 10 μg/ml to 0.078 μg/ml. Normal rabbit IgG (BD Biosciences, Franklin Lakes, NJ) was used as a pretreatment control. N/OFQ was then added at a concentration of 10−12 M to all wells, including control wells that received no antibody. On day 5, cells were harvested, washed in RPMI-1640, and the number of direct PFCs (cells producing IgM antibodies against SRBCs) quantitated using the Cunningham modification of the Jerne hemolytic plaque assay (Cunningham and Szenberg, 1968). The number of PFCs detected in the background cultures were subtracted from all control and experimental cultures. Unfacilitated plaques were counted, which represents cells producing IgM antibodies to SRBC. Results are expressed as a Suppression Index, where untreated spleen cells are given a value of 1.00 (100%), and responses of cultures receiving treatment with EMs are calculated as:
For the secondary PFC assay, mice were immunized in vivo 14 days before spleen harvest with a 10% suspension of SRBC given i.p. in 0.2 ml of phosphate-buffered saline (Eisenstein et al, 1995). The spleens of the SRBC-primed mice were removed and processed in the same manner as for the primary PFC assay described above. Unfacilitated plaques were counted, which represents cells producing IgM antibodies to SRBC. The number of plaques in spleen cell cultures which did not receive treatment with N/OFQ was in the range of 1000 to 2500 for the primary PFC assay and 3000 to 5000 for the secondary PFC assay. If less than 500 plaques were detected in control wells, the experiment was discarded.
Viability of cultures was assessed by trypan blue exclusion at the time of cell harvest on day 5 after culture.
In vivo treatment with N/OFQ
Alzet® osmotic minipumps, Model 1003D, were used to administer agonists in vivo. The pumps used deliver 1 μl/hour, therefore the dose in mg/kg/day had to be calculated on the basis of mg/kg/ 24 μl. The doses of N/OFQ or morphine were calculated by weighing the mice to obtain a mean weight in kgs, then making solution strengths such that the desired dose/kg/day was contained in 24 μl, which was given over the course of one day. Mice were anesthetized with methoxyflurane and an area of the back was shaved. A 1-cm incision was made in the skin and a pump filled with either saline, N/OFQ in saline, or morphine sulfate in saline, was implanted subcutaneously. The incision was closed with Auto-Clip® wound clips (Becton Dickenson, Sparks, MD). After 48 h, mice were sacrificed and spleens removed, and placed in vitro in single cell suspension to assess immune function by the Mishell-Dutton plaque-forming cell assay described above.
Synthesis of peptides for antibody production
Peptide antigens used for immunization and assays consisted of the C-termini truncated N/OFQ1-13 (F-11-K) and the N/OFQ1-15 [F-13-A] peptide fragments and the full length N/OFQ1-17 [F-15-Q] nociceptin sequence. Peptides were synthesized on 2-chlorotrityl resin (Anaspec Inc.) using standard Fmoc solid phase procedures (Hockfield et al., 1993). Purity was achieved with RP-HPLC and structural integrity verified by LC-MS-MS. The N/OFQ peptide analogue Y-15-Q was used as the 125I-labeled tracer in RIAs for N/OFQ1-17. The synthetic Dynorphin A1-17 (Dyn A1-17), Met-Enkephalin (ME), Leu-Enkephalin (LE) and BAM-18 were used as competitive peptide antigens in the assays.
Production of polyclonal antibodies to N/OFQ
Adult (2kg) New Zealand white male rabbits were used to generate specific antisera against N/OFQ1-13 (F-11-K), N/OFQ1-15 [F-13-A] and N/OFQ1-17 [F-15-Q] peptides as haptenized antigens. Synthetic peptides were covalently conjugated to KLH (Sigma-Aldrich) as the protein carrier using standard aldehyde cross-linking procedures (Harlow et al., 1988). Antisera generated against each peptide fragment were raised according to standard immunization protocols (Harlow et al., 1988) and standard solid-phase antibody capture ELISA assays (Harlow et al., 1988) were used for identification and titering of productive sera. High titers (>1:100,000) of reactive sera were detected from rabbits C-1 and C-4, being the C-1 rabbit immunized with a conjugate mixture of N/OFQ1-13 [F-11-K] plus N/OFQ1-17 (F-15-Q) and the C-4 rabbit with N/OFQ1-15 [F-13-A] plus N/OFQ1-17 (F-15-Q). Whole reactive antisera were antigen affinity-purified through standard affinity chromatography procedures (Harlow et al., 1988) using N/OFQ peptide antigens coupled to epoxy-activated Sepharose 6B resin columns (AmershamPharmacia Biotec, NJ). The recovery of specific C-1 and C-4 antigen affinity-purified antisera (A-APA) verified by the standard antibody capture ELISA assay (data not shown).
Characterization of cross-reactivity of both C-1 and C-4 A-APA for structurally related peptides to N/OFQ
A standard dot-blot assay (Gutiérrez et al, 2001) was used to screen the specificities of both C-1 and C-4 A-APA against membrane-spotted N/OFQ synthetic peptide antigens and structurally related neuropeptides such as Dynorphin A1-17, Met-Enkephalin, Leu-Enkephalin and BAM-18 tested as potential cross-reactive antigens. Complementary testing for A-APA specificities was carried out using a solid-phase radioimmunoassay (RIA) for N/OFQ1-17 peptide adapted from Hockfield (Hockfield et al.,1993). The N/OFQ1-17 peptide analogue, Y-15-Q, was radioiodinated using the chloramine-T method. The solid phase of the assay was prepared by adding sequentially Protein-A and C-1 or C-4 A-APA to Immulon II removable wells at dilutions previously detemined to provide approximately 20–30% of the peptide tracer binding. Competitive peptides (1 × 10−6 M – 1.5 × 10−17 M in quadruplicate) plus 125I-G-15-Q peptide tracer (5000 c.p.m.) were incubated for generation of typical standard displacement curves. Treated wells were washed out and counted for 4 minutes in a ten-channel gamma counter (ISO 199 DATA 500, Hewlett-Packard).
Data Analysis
Suppression index data was analyzed using analysis of variance for fixed effects (between groups and doses). Data from multiple replications and multiple wells were pooled after testing for differences. The suppression index data was transformed to normalized ranks prior to analysis due to failing the Wilk-Shapiro test of normality. Because of the exploratory nature of the experiments, no adjustments were made for multiple comparisons. Comparisons made to the unitary suppression index were based on 95% confidence intervals of the observed means. Data was analyzed using SAS V9.1 (Cary, NC).
Results
Effect of N/OFQ on in vitro spleen cell primary and secondary plaque-forming cell (PFC) responses
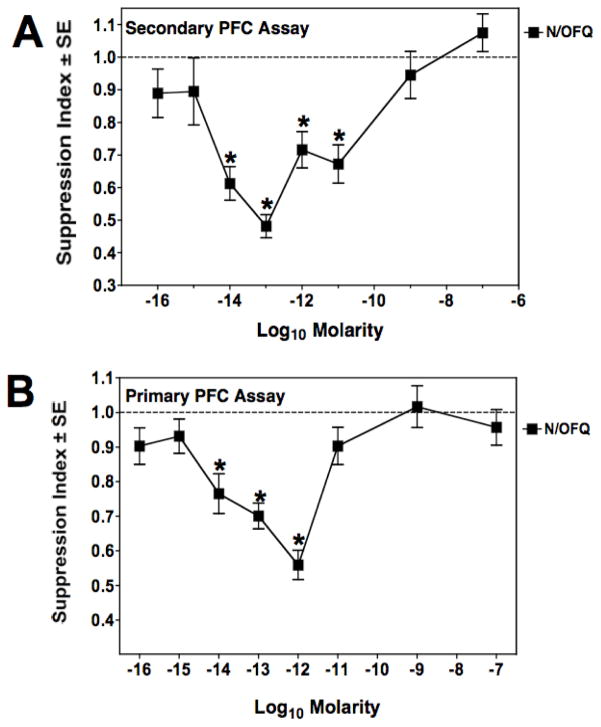
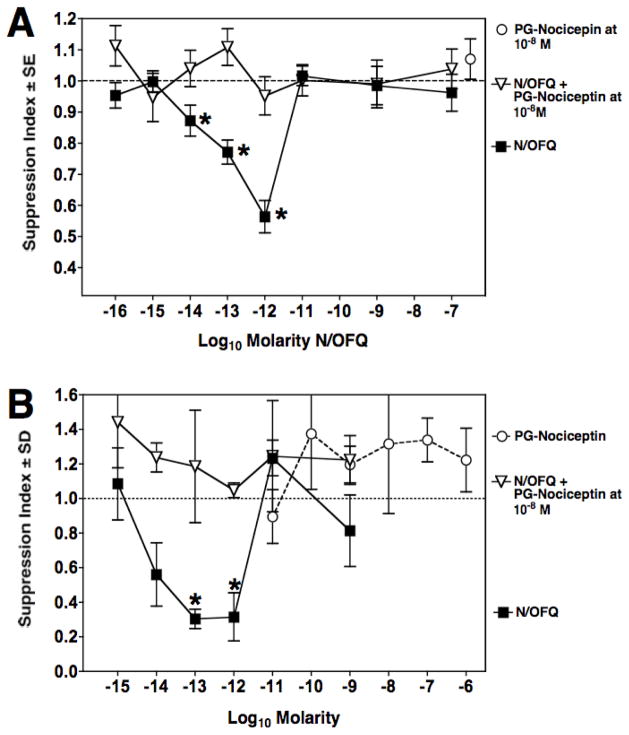
Figure 1A shows a titration of N/OFQ to determine its immunosuppressive capacity on mouse spleen cells placed in vitro in a secondary PFC assay. In this paradigm, mice are immunized 14 days before spleen cell harvest with sheep red blood cells. N/OFQ was added to these spleen cells in culture over a wide range of doses from 10−7 to 10−16 M. Immunosuppression was observed at doses between 10−11 to 10−14 M, with maximal activity at 10−13 M. Similar experiments were carried out using a primary PFC assay, where spleen cells were harvested without prior in vivo priming of mice with sheep red blood cells. The results presented in Fig. 1B show that in the primary PFC assay, N/OFQ exhibited an asymmetric, U-shaped dose response curve, with immunosuppression in the range of 10−12 to 10−14 M, which was maximal at 10−12 M. No immunosuppression was observed at 10−11 M. Note that the graph represents a pool of 6 individual experiments. To assess the receptor specificity of ORL-1 on spleen cells, studies were continued using the primary PFC assay. Previous studies in our laboratory had shown that morphine is suppressive only in the secondary PFC assay (Bussiere et al, 1993; Eisenstein et al, 1995). Spleen cells were pre-treated with naloxone, a non-specific opioid receptor antagonist, and as shown in Fig. 2, it did not block the in vitro immunosuppressive effect of N/OFQ in the primary PFC assay. In contrast, pre-incubation of the cells with 10−8 M PG-nociceptin, an antagonist specific to the ORL receptor, completely abrogated the immunosuppressive effect produced by N/OFQ (Fig. 3A). As immune cells are reported to produce N/OFQ, the effect of PG-nociceptin alone on the PFC response was assessed (Fig. 3B), and found to have no effect. Neither nociceptin, nor PG-nociceptin, were toxic alone, or in combination, at the highest concentrations of each that were used (data not shown).
Figure 1.
N/OFQ suppresses both the secondary and primary plaque-forming cell assay of mouse spleen cells. Panel A: Secondary PFC assay. Mice were primed in vivo with sheep red blood cells 2 weeks prior to harvest of spleen cells. Spleen cells were treated in vitro with N/OFQ at doses ranging from 10−7 M to 10−16 M, and their capacity to mount a IgM antibody response determined. The graph is the average of 3 experiments, with each concentration tested in triplicate in each experiment. Mean ± SEM of control PFC/culture = 3753 ± 465. *p< 0.05 compared to responses of control cultures (dotted line) with no peptide added. Panel B: Primary PFC assay. Nociceptin added to naïve mouse spleen cells in vitro over a wide range of concentrations suppressed the primary IgM plaque-forming cell response in the range of 10−12 M to 10−14 M. The line is an average of 6 experiments, with each concentration tested in triplicate in each experiment. Mean ± SEM of control PFC/culture = 4166 ± 594. *p<0.05 compared to responses of control cultures (dotted line) with no peptide added.
Figure 2.
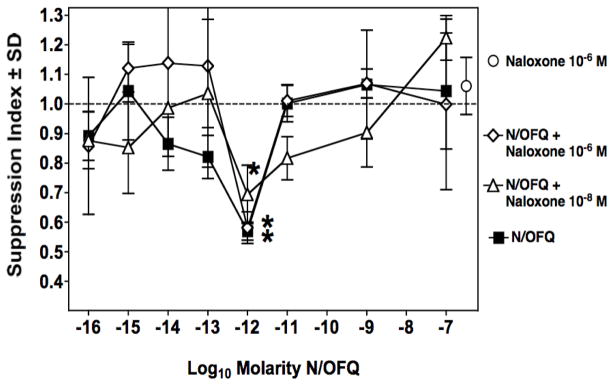
Immunosuppression by N/OFQ in vitro is not blocked by naloxone. Primary PFC assay showing immunosuppression produced by N/OFQ alone (■); pretreatment with naloxone for 2 hrs, at two concentrations (10−6 M:◆ and 10−8 M:▲) plus N/OFQ tested over a wide range of concentrations. Data are the means of triplicate cultures per point, of a single experiment. Mean ± SEM of control PFC/culture = 4017 ± 123. *p<0.05 between experimental groups and control cultures.
Figure 3.
PG-nociceptin blocks the immunosuppressive effect of N/OFQ. Panel A: Primary PFC assay showing immunosuppression produced by N/OFQ alone (■); pretreatment with PG-nociceptin for 2 hrs at 10−8 M (▽) plus N/OFQ tested over a wide range of concentrations. Data are means of triplicate cultures from three experiments. Mean ± SEM of control PFC/culture = 3936 ± 954. *p<0.05 between experimental groups and cultures with N/OFQ and PG-nociceptin. Panel B: Primary PFC assay showing immunosuppression produced by N/OFQ alone (■); pretreatment with PG-nociceptin for 2 hrs at 10−8 M (▽) plus N/OFQ tested from 10−9 to 10−15 M, and PG-nociceptin alone (❍) over a range of concentrations from 10−6 to 10−11 M. Data are means of triplicate cultures from a single experiment. Mean ± SEM of control PFC/culture = 2259 ± 223. *p<0.05 between experimental groups and control cultures.
Characterization of affinity-purified antisera to N/OFQ
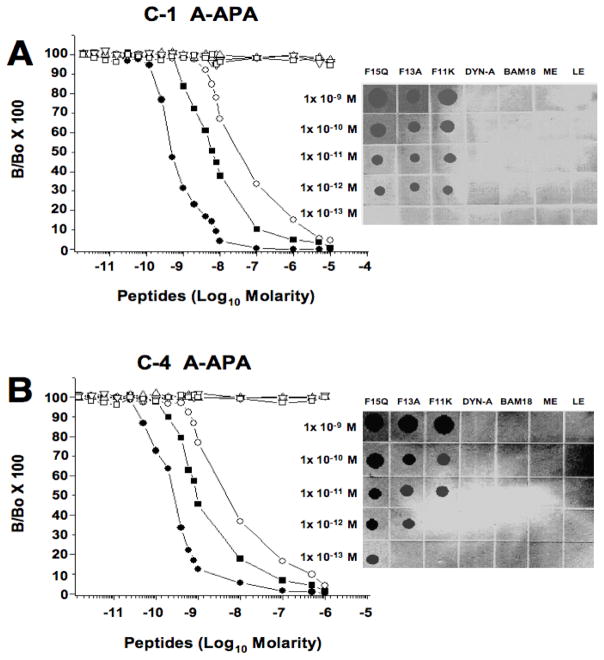
To further show that the immunosuppression induced by the peptide was specific, two antigen-affinity purified rabbit IgG antibodies were generated, and their specificity was tested by two complementary immunological assays for synthetic N/OFQ1-17, in order to assess their use in pharmacological assays for neutralizing the suppressive effect of this peptide. Insets in Figure 4 shows representative dot-blots of both C-1 (A) and C4 (B) antisera targeted to the C-terminus of N/OFQ which were tested for reactivitiy to the full-length N/OFQ (lane labeled F-15-Q) and both N/OFQ peptide fragments truncated by either 4 or 2 amino acids at the C-terminal end of the N/OFQ1-17 (lanes labeled F-11-K or F-13-A, respectively), as well as to classical opioid peptides such as dynorphin1-17 (Dyn-A), BAM-18 (an adrenal medulla peptide), Leu-enkephalin (LE) or Met-enkephalin (ME). As described previously in Methods, both antisera were raised against a mixture of N/OFQ1-17 and its truncated peptide analogs F-11-K and F-15-Q and showed reactivity to the three nociceptin peptides which share common structural homology at both core and C-terminal domains, but not to classical opioid encoding structurally related N-terminal opioid peptide motifs with N/OFQ1-17 (i.e., N/OFQ1-17 = Phe-Gly-Gly-Phe-Thr; DynA and Leu-enk = Tyr-Gly-Gly-Phe-Leu and Met-enk = Tyr-Gly-Gly-Gly-Met). This data support the specificity of both C-1 and C-4 A-APA reacting against the C-terminus domain of the N/OFQ1-17 peptide as epitope. To further verify and assess the specificity of both C-1 and C-4 A-APA antisera, a sensitive and specific solid-phase RIA to N/OFQ1-17 peptide was developed using as labeled tracer the N/OFQ1-17 peptide analogue, (125I)-Y-15-Q. Representative RIAs showing typical displacement standard curves by synthetic N/OFQ1-17 peptide using the C-1 A-APA is shown in Fig. 4A. This antigen-affinity purified N/OFQ1-17 antiserum was capable of detecting synthetic N/OFQ1-17 peptide at approximately 4.5 × 10−10 M at the IC50 reference value. Moreover, the smallest measurable displacement detected of synthetic N/OFQ1-17 at the IC50 was as low as 2.3 × 10−10 M in the same assay, and 2.9 × 10−9 M at the IC80. Reactivity of C-1 A-APA antisera for both C-terminal N/OFQ truncated antigen peptides were 3.4 × 10−8 M and 6.1 × 10−9 M for N/OFQ-F-11-K and N/OFQ-F-13-A at the IC50 reference value, respectively. In addition, no significant cross-reactivity against Dynorphin A, BAM-18 and Leu-enkephalin was observed using 1 × 10−6 M to 1.5 × 10−12 M of competitive peptides. Fig. 4B shows a typical displacement standard curve of labeled N/OFQ1-17 peptide tracer by the synthetic N/OFQ1-17 peptide using the C-4-A-APA. This reactive antiserum was capable of detecting synthetic N/OFQ1-17 peptide at approximately 2.7 × 10−10 M at the IC50 reference value. The smallest measurable displacement detected of synthetic N/OFQ1-17 at the IC20 was as low as 6.2 × 10−11 M and 7.1 × 10−10 M at the IC80 in the same assay. Complementary reactivity of C-4 A-APA antisera for both C-terminal N/OFQ truncated antigen peptides were 4.6 × 10−9 M and 9.3 × 10−10 M for N/OFQ-F-11-K and N/OFQ-F-13-A at the IC50 reference value, respectively. Similar to C-1 A-APA, this antiserum did not show significant cross-reactivity against Dynorphin A, BAM-18 or Leu-enkephalin using 1 × 10−6 M to 1.5 × 10−12 M of competitive peptides.
Figure 4.
Representative solid-phase radioimmunoassays and dot-blot assays for C-1 and C-4 A-APA. Displacement curves for N/OFQ (F-15-Q) and truncated N/OFQ [F-11-K] and N/OFQ [F-13-A] peptides fragments tested in a solid-phase RIA, using either the C-1 antiserum (A) or C-4 antiserum (B). In both graphs, synthetic [N/OFQ (F-15-Q)] (-●-) as well as truncated N/OFQ [F-11-K] (-○-) and N/OFQ [F-13-A] (-■-) peptides sequences were used as competitive peptide antigens to test specificity of both antisera in the assays. No significant cross-reactivities were detected against structurally-related synthetic peptides to N/OFQ (F-15-Q) such as Dynorphin A1-17 (-△-), Leu-Enkephalin (-□-) and BAM-18 (-▽-) when tested in a concentration range from 10−12 to M-10−6 M as competitive antigens. Complementary immunoblot-dot assays showing specificities of both C-1 (upper inset) and C-4 A-APA (lower inset) for filter-spotted N/OFQ F-15-Q, N/OFQ F-13-A, N/OFQ F-11-K, Dyn-A), BAM-18, Leu-enkephalin (LE) and Met-Enkephalin (ME) using the indicated concentrations (10−9 M- X10−13 M).
Neutralization of immunosuppression by N/OFQ-specific affinity-purified antisera
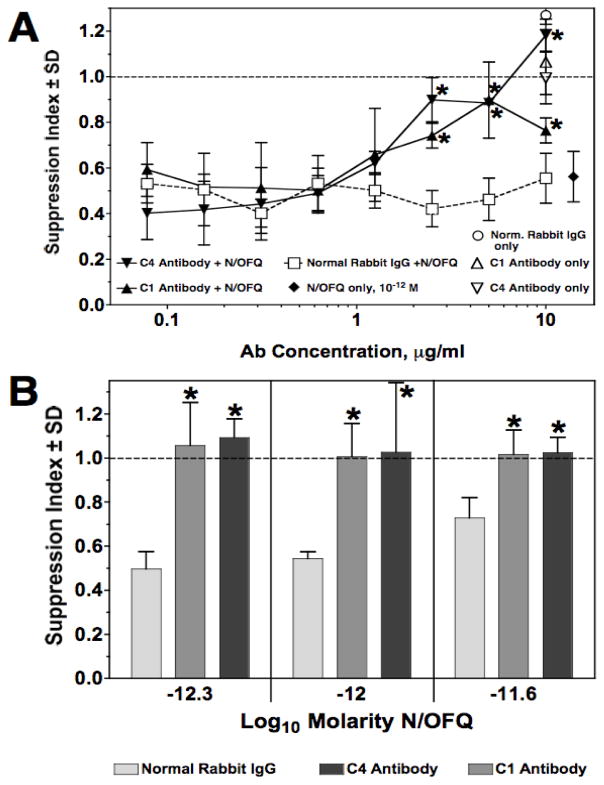
Both antisera were tested for capacity to neutralize N/OFQ (10−12 M), in the PFC assay. As shown in Fig. 5A, both antibodies significantly neutralized N/OFQ in a concentration related fashion. Neither antiserum alone (at concentrations from 10 μg to 0.16 μg/ml), nor normal rabbit serum, had any effect on the PFC response. (Data for 10 μg/ml shown in Fig 5A.) A further experiment was carried out to assess the efficacy of the two antibodies using three different concentrations of N/OFQ, bracketing the 10−12 M concentration. As shown in Fig. 5B, both antibodies neutralized the immunosuppressive effect of N/OFQ at all three concentrations of the peptide.
Figure 5.
Rabbit antibodies to N/OFQ neutralize immunosuppression induced by the peptide. Panel A: Primary plaque-forming cell responses of by 10−12 M N/OFQ alone (◆), and when pretreated for 2 hrs with specific anti-N/OFQ antibodies C-1 (▲) and C-4 (▼) titrated over a 100-fold concentration range, followed by addition of 10−12 M N/OFQ. Pretreatment with normal rabbit IgG (□) did not abrogate immunosuppression. Treatment with C1 (△) or C4 (▽) antibody, without addition of N/OFQ, did not suppress the primary PFC response. Data are the means of triplicate cultures per point, of a single experiment. Mean ± SEM of control PFC/culture = 4092 ± 291. * p< 0.05 for C-1 or C-4 antibody treatment vs. normal rabbit IgG. Panel B: Effect of pretreatment with a single concentration of anti-N/OFQ antibodies C-1 or C-4 on several concentrations of N/OFQ. Either C-1 A-APA, C-4 A-APA, or normal rabbit IgG, all at a concentration of 5 μg/ml, were added to PFC cultures 2 hrs prior to addition to one of three doses of N/OFQ. Data are means of triplicate cultures per group, from a single experiment. Mean ± SEM of control PFC/culture = 2897 ± 198. *p < 0.05 anti-C1 or anti-C4 vs normal IgG.
Effect of in vivo administration of N/OFQ on primary PFC responses
The immunomodulatory capacity of N/OFQ when given in vivo by an Alzet® pump was assessed. Doses ranging from 0.02 to 2 mg/kg/day were tested. After 48 hours of infusion of the peptide, animals were sacrificed and spleens removed and placed ex vivo to examine their capacity to mount a primary PFC response. The results show (Fig. 6), that immunosuppression was observed over a wide range of doses, with a maximal effect at 0.05 mg/kg/day. Morphine, at 0.5 mg/kg/day, was included in these experiments as a positive control, as this dose had been shown previously by our laboratories to produce maximal immunosuppression (Rahim et al, 2001).
Fig. 6.
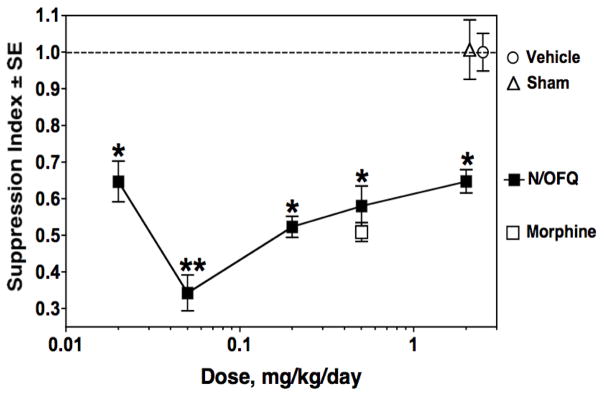
N/OFQ given in vivo via Alzet® pumps suppresses ex vivo plaque-forming cell responses. Mice were implanted s.c. with pumps delivering N/OFQ (
 ), morphine (■), or saline vehicle (❍), at indicated doses, for 48 hr before harvest of spleen cells which were placed ex vivo and tested in the PFC assay. Data are means of triplicate cultures from 2 experiments. In each experiment, spleens of 2 animals were pooled and for each treatment group there were a total of 6 animals. Mean ± SEM of control PFC/culture = 4366 ± 876. *p<0.05 for mice receiving N/OFQ versus mice receiving saline (dotted line). **p<0.05 for 0.05 N/OFQ mg/kg/day versus other doses of the peptide.
), morphine (■), or saline vehicle (❍), at indicated doses, for 48 hr before harvest of spleen cells which were placed ex vivo and tested in the PFC assay. Data are means of triplicate cultures from 2 experiments. In each experiment, spleens of 2 animals were pooled and for each treatment group there were a total of 6 animals. Mean ± SEM of control PFC/culture = 4366 ± 876. *p<0.05 for mice receiving N/OFQ versus mice receiving saline (dotted line). **p<0.05 for 0.05 N/OFQ mg/kg/day versus other doses of the peptide.
Discussion
Cells of the immune system have been shown to produce and have receptors for a variety of neuropeptides including β-endorphin, enkephalins, Substance P, and Vasoactive Intestinal Peptide (Smith et al, 1986; Rosen et al, 1989; Lai et al, 1998; Delgado et al, 2004; Mousa et al, 2004). As documented in the Introduction, leukocytes have been shown to express message for the ORL-1 receptor, to bind N/OFQ, and even to produce N/OFQ.
The current studies add to the activities of nociceptin in the immune system, demonstrating that the peptide suppresses the capacity of mouse spleen cells to make an antibody plaque-forming response to sheep red blood cells. The current results are novel because they are the first to show that nociceptin modulates an adaptive immune response, antibody production, using a classic assay to assess this parameter. Previous studies have examined the role of nociceptin in inflammation, in innate immune responses, and in nonspecific immune responses, such as responses to mitogens or the capacity to make a polyclonal IgG or IgM response. Halford et al. had previously shown that polyclonal IgG and IgM production by mouse spleen cells could be suppressed by antisense to ORL-1 (Halford et al, 1995). In the current experiments, the peptide was shown to be active in vivo in suppressing spleen cell function assayed ex vivo, as well as to have in vitro activity. The immunosuppressive activity of N/OFQ was found to be in the femtomolar range in vitro (10−9 to 10−14M). The validity of the findings is supported by the reproducibility of the data. The dose titration of N/OFQ alone was done 13 times if the number of experiments in figures 1 through 3 are totaled. A narrower 3-dose titration experiment, shown in figure 5, brings the total number of iterations to 14, with each point in each experiment having been done in triplicate. Activity at these low concentrations has also been reported by other investigators using nociceptin in several assays of immune function. Peluso (Peluso et al, 2001) found that nociceptin inhibited proliferation of PHA stimulated human PBMCs at doses of 10−8 to 10−13 M. Maximal chemotaxis for human PMNs and monocytes was observed between 10−8 to 10−10 M (Serhan et al, 2001; Trombella et al, 2005). Inhibition of proliferation of Con A activated rat splenocytes, and production of IL-2, was observed at 10−10 to 10−14 M (Miller and Fulford, 2007), the same concentration range as the results presented in the present studies using mice. Waits et al. reported that N/OFQ up-regulated CD28 on fresh human T cells at concentrations of 10−12 to 10−14 M (Waits et al, 2004). Studies on binding of N/OFQ to cells of the immune system have demonstrated high affinity binding sites which would be compatible with the activity of the peptide at low concentrations. Hom et al (Hom et al, 1999) showed that Raji cells, a human B cell line, had a high affinity site with a Kd of 68.4 pM. Peluso et al (Peluso et al, 2001) reported Kds of 0.014 nM for the CEM T cell line and 0.024 nM for the U937 monocyte cell line. Serhan et al (Serhan et al, 2001) found that the Kd for human neutrophils was significantly higher, 1.5 nM. However, the biologic activity of N/OFQ in activating chemotaxis of human neutrophils was maximal at 100 pM. Other neuropeptides, including endomorphins 1 and 2, have also been reported to act on cells of the immune system at these ultra-low concentrations (Anton et al, 2008). Microglia also respond to Dynorphin A and to endomorphin 1 in the femtomolar range (Liu et al, 2001; Peterson et al, 1999).
In accord with other reports in the literature, using ORL1 in a variety of nonimmune assays, we found that the immunosuppression induced by ORL1 was not inhibited by naloxone. Only one other study of immune parameters tested the effect of naloxone on a functional measure of ORL1 activity (Trombella et al, 2005), and these investigators reported that 10−6 M naloxone did not block human monocyte chemotaxis induced by the peptide. In support of the inefficacy of naloxone at the ORL1 receptor, Hom (Hom et al, 1999) reported that naloxone did not inhibit binding of radiolabeled N/OFQ in Raji cells. We have previously shown that pretreatment for 2 hrs with either naloxone or with other mu or kappa receptor selective antagonists completely blocked the immunosuppressive effect of morphine and of the kappa agonist, U50488, in the same PFC assay (Eisenstein et al, 1995), indicating that the protocol is effective in ability to demonstrate receptor antagonism. In the present studies, in contrast to the inactivity of naloxone, the same incubation protocol using PG-nociceptin was effective in inhibiting the immunosuppressive activity of N/OFQ. To further confirm the specificity of the immunosuppression, two different rabbit antigen-affinity purified antisera generated in the current study, (namely C-1 and C-4 A-APA), each with demonstrated specificity for the C-terminus domain of the N/OFQ1-17 peptide by both immune dot blot and solid-phase RIA, both inhibited the activity of nociception. It is interesting to note that this study used a vaccination strategy that involved mixing of immunogens containing haptenized both full length N/OFQ1-17 and C-terminal truncated N/OFQ peptides (F-11-K and F-13-K) in order to enhance the targeting of the N/OFQ C-terminus as the dominant epitope for antibody recognition. Although a detailed N/OFQ1-17 epitope mapping characterization for either C-1 or C-4 A-APA reactive antisera was not approached in the current study, the aforementioned vaccination strategy was successful as confirmed by the dot blot and RIA assays, since no cross-reactivity of either C-1 or C-4 was observed against classical opioid peptides (Mogil and Pasternak, 2001) encoding structurally related N-terminal opioid peptide motifs also present in N/OFQ1-17 (i.e., N/OFQ1-17 = Phe-Gly-Gly-Phe-Thr; DynA and Leu-enk = Tyr-Gly-Gly-Phe-Leu and Met-enk = Tyr-Gly-Gly-Gly-Met). The fact that the data demonstrate that the antibodies predominantly recognize C-terminal domains of the N/OFQ1-17 peptide, fits with the report of Rossi et al. who showed a critical functional role for the last four C-terminal amino acids encoded into the N/OFQ1-17 peptide, a structural domain targeted by our C-1 and C-4 A-APA reactive antisera (Rossi et al, 1997). The data are consistent with an antibody-dependent neutralization of immunosuppression by the N/OFQ1-17 peptide mediated through the C-terminus of the molecule which induces steric-hindrance effects capable of altering peptide binding to ORL-1 receptors expressed on cultured spleen cells.
Another issue to be considered is the fact that the dot-blot and RIAs assays were designed to determine both sensitivity and specificity of detection of C-1 and C-4 A-APA for the N/OFQ1-17 peptide. Data presented in Figure 4 shows a very high reactivity capacity in the low picomolar range of C-1 A-APA (4.5 × 10−10 M) and C-4 (2.7 × 10−10 M) A-APA, respectively, at the IC50 reference value for the N/OFQ1-17 synthetic peptide. However, from this data is not possible to extrapolate quantitative affinity parameters (i.e., Kds) for the A-APA antisera. Equilibrium dialysis experiments using radiolabeled N/OFQ1-17 synthetic peptide would be required to determine the Kd values. At the present time we do not have a definitive explanation for why at least two orders of magnitude more antibody (i.e., 6.6 × 10−10–4.6 × 10−11 for either C-1 or C-2 A-APA) were required to neutralize the immunosuppressive activity of the N/OFQ1-17 synthetic peptide in the low to high picomolar range (i.e., 10−9 to 10−14M). One plausible explanation is that our antigen affinity chromatography procedures used for whole reactive C-1 and C-4 sera purification are yielding antigen reactive antibodies contaminated with significant amounts of nonspecific immunoglobulins, which may contribute to UV measurements of apparent specific purified antibodies in chromatographic fractions. Therefore, antisera neutralizing doses in our study could actually be overestimated in the antibody-dependent neutralization assays of immunosuppressive activity of N/OFQ1-17. Another possible explanation is that spleen cells produced N/OFQ that bound to the antisera (Miller and Fulford, 2007).
The PFC assay used in the present experiments is a measure of antibody formation, an adaptive immune response. The assay requires B-cells, T-cells, and antigen presenting cells for a functional antibody response. The current experiments do not indicate which cells are the target of ORL1, leading to inhibition of the immune response to sheep red blood cells, nor is a mechanism for the inhibition identified. However, it is noteworthy that ORL1 inhibited both the primary and the secondary PFC responses. While a mechanism for the inhibition has not been established in the present studies, Waits et al. found that N/OFQ could upregulate CTLA-4 on T cells restimulated wit anti-CD3/CD80 (Waits et al, 2004). Further, N/OFQ induced PGE2 in human peripheral T cells stimulated with SEB. Both CTLA-4 and PGE2 are known to have immunosuppressive properties. In the PFC system, there is a complex interaction between three types of cells, and the mechanism of immunosuppression by N/OFQ has not been investigated.
The significance of finding ORL1 and its receptor in abundance in the immune system is still to be defined. As noted by the literature cited in the Introduction and above, the majority of the rather limited number of studies on the effects of this peptide in the immune system indicate that ORL1 is immunopotentiating. However, there are several other reports, including the present one, where it has been found to be immunosuppressive. These seemingly conflicting results may be due to dosage, time of exposure to the neuropeptide (and N/OFQ-induced ORL-1 receptor down-regulation), species, or assays. ORL1 may have a different effect on an adaptive immune response than on inflammatory and nonspecific immune responses. It is well documented that opioid peptides are immunosuppressive in some assays of immune function and stimulatory in others (Wybran et al, 1979; Sharp et al, 1985; Johnson et al, 1982), in contrast to morphine, which has been found by almost all investigators to be immunosuppressive in a variety of assays of immune status (Eisenstein and Hilburger, 1998). The current results add to the body of literature showing that the N/OFQ peptide is immunomodulatory, and present new data showing that it inhibits antibody formation in a naloxone-insensitive manner. These observations extend the evidence of intimate connections between the neural and immune systems. Future studies are needed to examine the mechanisms by which nociceptin is immunosuppressive. Exploration of this type of neuro-immune connection remains a fertile area for future research, with a promise of avenues to develop therapeutics to modulate immune status.
Acknowledgments
This work was supported by NIDA grants DA06650 and DA13429, and by SEP-CONACyT-2004-CO1-47804, UNAM MacroProject MP6-18, INPRFM-Subcuenta-100.
Footnotes
Conflict of Interest Disclosures
The authors have no known conflicts of interest concerning the results reported in this manuscript.
References
- Anton B, Fein J, To T, Li X, Silberstein L, Evans CJ. Immunohistochemical localization of ORL-1 in the central nervous system of the rat. J Comp Neurol. 1996;368:229–251. doi: 10.1002/(SICI)1096-9861(19960429)368:2<229::AID-CNE5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Anton B, Leff P, Calva JC, Acevedo R, Salazar A, Matus M, Pavón L, Martinez M, Meissler JJ, Adler MW, Gaughan JP, Eisenstein TK. Endomorphin 1 and endomorphin 2 suppress in vitro antibody formation at ultra-low concentrations: Anti-peptide antibodies but not opioid antagonists block the activity. Brain Behav Immun. 2008;22:824–832. doi: 10.1016/j.bbi.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arjomand J, Cole S, Evans CJ. Novel orphanin FQ/nociceptin transcripts are expressed in human immune cells. J Neuroimmunol. 2002;130:100–108. doi: 10.1016/s0165-5728(02)00217-5. [DOI] [PubMed] [Google Scholar]
- Bryant W, Janik J, Baumann M, Callahan P. Orphanin FQ stimulates prolactin and growth hormone release in male and female rats. Brain Res. 1998;807:228–233. doi: 10.1016/s0006-8993(98)00802-6. [DOI] [PubMed] [Google Scholar]
- Bunzow JR, Saez C, Mortrud M, Bouvier C, Williams JT, Low M, Grandy DK. Molecular cloning and tissue distribution of a putative member of the rat opioid receptor gene family that is not a mu, delta or kappa opioid receptor type. FEBS Lett. 1994;347:284–288. doi: 10.1016/0014-5793(94)00561-3. [DOI] [PubMed] [Google Scholar]
- Bussiere JL, Adler MW, Rogers TJ, Eisenstein TK. Cytokine reversal of morphine-induced suppression of the antibody response. J Pharmacol Exp Ther. 1993;264:591–597. [PubMed] [Google Scholar]
- Chen X, McClatchy DB, Geller EB, Liu-Chen L-Y, Tallarida RJ, Adler MW. Possible mechanism of hypothermia induced by intracerebroventricular injection of orphanin FQ/nociceptin. Brain Res. 2001;904:252–258. doi: 10.1016/s0006-8993(01)02467-2. [DOI] [PubMed] [Google Scholar]
- Chen Y, Fan Y, Liu J, Mestek A, Tian M, Kozak CA, Yu L. Molecular cloning, tissue distribution and chromosomal localization of a novel member of the opioid receptor gene family. FEBS Lett. 1994;347:279–283. doi: 10.1016/0014-5793(94)00560-5. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Polidori C, Antonelli L, Salvadori S, Guerrini R, Massi M. Pharmacological characterization of the nociceptin receptor which mediates reduction of alcohol drinking in rats. Peptides. 2002;23:117–125. doi: 10.1016/s0196-9781(01)00587-3. [DOI] [PubMed] [Google Scholar]
- Civelli O. The orphanin FQ/nociceptin (OFQ/N) system. Results Prob Cell Differ. 2008;46:1–25. doi: 10.1007/400_2007_057. [DOI] [PubMed] [Google Scholar]
- Cunningham A, Szenberg A. Further improvements in the plaque technique for detecting single antibody producing cells. Immunology. 1968;14:599–601. [PMC free article] [PubMed] [Google Scholar]
- Delgado M, Pozo D, Ganea D. The significance of vasoactive intestinal peptide in immunomodulation. Pharmacol Rev. 2004;56:249–290. doi: 10.1124/pr.56.2.7. [DOI] [PubMed] [Google Scholar]
- Eisenstein TK, Hilburger ME. Opioid modulation of immune responses: effects on phagocyte and lymphoid cell populations. J Neuroimmunol. 1998;83:36–44. doi: 10.1016/s0165-5728(97)00219-1. [DOI] [PubMed] [Google Scholar]
- Eisenstein TK, Meissler JJ, Jr, Rogers TJ, Geller EB, Adler MW. Mouse strain differences in immunosuppression by opioids in vitro. J Pharmacol Exp Ther. 1995;275:1484–1489. [PubMed] [Google Scholar]
- Fiset ME, Gilbert C, Poubelle PE, Pouliot M. Human neutrophils as a source of nociceptin: a novel link between pain and inflammation. Biochem. 2003;42:10498–10505. doi: 10.1021/bi0300635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Zhu ZH, Wang YQ, Wu GC. Regulation of proinflammatory cytokine gene expression by nociceptin/orphanin FQ in the spinal cord and the cultured astrocytes. Neurosci. 2007;144:275–285. doi: 10.1016/j.neuroscience.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Kato S, Mori K, Nishi M, Takeshima H, Iwabe N, Miyata T, Houtani T, Sugimoto T. cDNA cloning and regional distribution of a novel member of the opioid receptor family. FEBS Lett. 1994;343:42–46. doi: 10.1016/0014-5793(94)80603-9. [DOI] [PubMed] [Google Scholar]
- Goldfarb Y, Reinscheid RK, Kusnecov AW. Orphanin FQ/nociceptin interactions with the immune system in vivo: gene expression changes in lymphoid organs and regulation of the cytokine response to staphylococcal enterotoxin A. J Neuroimmunol. 2006;176:76–85. doi: 10.1016/j.jneuroim.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Green MK, Barbieri EV, Brown BD, Chen KW, Devine DP. Roles of the bed nucleus of stria terminals and of the amygdala in N/OFQ-mediated anxiety and HPA axis activation. Neuropeptides. 2007;41:399–410. doi: 10.1016/j.npep.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Gutiérrez R, Leff P, Romo-Parra H, Acevedo R, Anton B. Orphanin-FQ/nociceptin inhibits kindling epileptogenesis and enhances hippocampal feed-forward inhibition. Neurosci. 2001;105:325–333. doi: 10.1016/s0306-4522(01)00196-8. [DOI] [PubMed] [Google Scholar]
- Halford WP, Gebhardt BM, Carr DJJ. Functional role and sequence analysis of a lymphocyte orphan opioid receptor. J Neuroimmunol. 1995;59:91–101. doi: 10.1016/0165-5728(95)00030-6. [DOI] [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies, A Laboratory Manual. Cold Spring Harbor Press; Cold Spring Harbor, NY: 1988. [Google Scholar]
- Hazum E, Chang KJ, Cuatrecasas P. Specific nonopiate receptors for beta-endorphin. Science. 1979;205:1033–1035. doi: 10.1126/science.224457. [DOI] [PubMed] [Google Scholar]
- Hockfield S, Carlson S, Evans C, Levitt P, Pintar J, Silberstein L. Selected methods for antibody and nucleic acid probes. Cold Spring Harbor Press; Cold Spring Harbor, NY: 1993. Molecular Probes of the Nervous System I. [Google Scholar]
- Hom JSH, Goldberg I, Mathis J, Pan YX, Brooks AI, Ryan-Moro J, Scheinberg DA, Pasternak GW. [125I]orphanin FQ/nociceptin binding in Raji cells. Synapse. 1999;34:187–191. doi: 10.1002/(SICI)1098-2396(19991201)34:3<187::AID-SYN3>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Horvath A, Folhoffer A, Lakatos P, Halosz J, Illyes G, Schaff Z. Rising plasma nociceptin level during development of HCC: a case report. World J Gastroent. 2004;10:152–154. doi: 10.3748/wjg.v10.i1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtani T, Nishi M, Takeshima H, Nukada T, Sugimoto T. Structure and regional distribution of nociceptin/orphanin FQ precursor. Biochem Biophys Res Comm. 1996;219:714–719. doi: 10.1006/bbrc.1996.0300. [DOI] [PubMed] [Google Scholar]
- Johnson HM, Smith EM, Torres BA, Blalock JE. Regulation of the in vitro antibody response by neuroendocrine hormones. Proc Natl Acad Sci USA. 1982;79:4171–4174. doi: 10.1073/pnas.79.13.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminsky DE, Rogers TJ. Suppression of CCL2/MCP-1 and CCL5/RANTES expression by nociceptin in human monocytes. J Neuroimm Pharmacol. 2008;3:75–82. doi: 10.1007/s11481-007-9086-y. [DOI] [PubMed] [Google Scholar]
- Kapusta DR, Sezen SF, Chang JK, Lippton H, Kenigs VA. Diuretic and antinatriuretic responses produced by the endogenous opioid-like peptide, nociceptin (orphanin FQ) Life Sci. 1997;60:L15–L22. doi: 10.1016/s0024-3205(96)00593-0. [DOI] [PubMed] [Google Scholar]
- Kato S, Tsuzuki Y, Hokari R, Okada Y, Miyazaki J, Matsuzaki K. Role of nociceptin/orphanin FQ (Noc/oFQ) in murine experimental colitis. J Neuroimmunol. 2005;161:21–28. doi: 10.1016/j.jneuroim.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Knoflach F, Reinscheid RK, Civelli O, Kemp JA. Modulation of voltage-gated calcium channels by orphanin FQ in freshly dissociated hippocampal neurons. J Neurosci. 1996;16:6657–6664. doi: 10.1523/JNEUROSCI.16-21-06657.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger C, Köthe L, Struppert A, Pietruck C, Simm A, Grond S. Expression and function of the ORL-1 receptor on human leukocytes. Schmertz. 2006;20:509–518. doi: 10.1007/s00482-006-0488-1. [DOI] [PubMed] [Google Scholar]
- Lai J-P, Douglas SD, Ho W-Z. Human lymphocytes express substance P and its receptor. J Neuroimmunol. 1998;86:80–86. doi: 10.1016/s0165-5728(98)00025-3. [DOI] [PubMed] [Google Scholar]
- Liu B, Qin L, Yang S-N, Wilson BC, Liu Y, Hong J-S. Femtomolar concentrations of dynorphins protect rat mesencephalic dopaminergic neurons against inflammatory damage. J Pharmacol Exp Ther. 2001;298:1133–1141. [PubMed] [Google Scholar]
- Meng F, Taylor LP, Hoversten MT, Ueda Y, Ardati A, Reinscheid RK, Monsma FJ, Watson SJ, Civelli O, Akil H. Moving from the orphanin FQ receptor to an opioid receptor using four point mutations. J Biol Chem. 1996;271:32016–32020. doi: 10.1074/jbc.271.50.32016. [DOI] [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B. Isolation and structure of the endogenous agonist of opioid receptor-like ORL-1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- Miller TR, Fulford AJ. Regulation of nociceptin/orphanin FQ secretion by immune cells and functional modulation of interleukin-2. Peptides. 2007;28:2243–2252. doi: 10.1016/j.peptides.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Mishell RI, Dutton RW. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967;126:423–424. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS, Pasternak GW. The molecular and behavioral pharmacology of the orphanin FQ/nociceptin peptide and receptor family. Pharmacol Rev. 2001;53:381–415. [PubMed] [Google Scholar]
- Mollereau C, Parmentier M, Mailleux P, Butour J-L, Moisand C, Chalon P, Caput D, Vassart G, Meunier J-C. ORL1, a novel member of the opioid receptor family. Cloning, functional expression and localization. FEBS Lett. 1994;341:33–38. doi: 10.1016/0014-5793(94)80235-1. [DOI] [PubMed] [Google Scholar]
- Mousa SA, Shakibaei M, Sitte N, Schäfer M, Stein C. Subcellular pathways of β-endorphin synthesis, processing, and release from immunocytes in inflammatory pain. Endocrinology. 2004;145:1331–1341. doi: 10.1210/en.2003-1287. [DOI] [PubMed] [Google Scholar]
- Neal CR, Jr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Akil H, Watson SJ., Jr Opioid receptor-like (ORL1) receptor distribution in the rat central nervous system: comparison of ORL1 receptor with 125I-[14Tyr]-orphanin FQ binding. J Comp Neurol. 1999a;412:563–605. [PubMed] [Google Scholar]
- Neal CR, Jr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Watson SJ., Jr Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system of the rat. J Comp Neurol. 1999b;406:503–547. [PubMed] [Google Scholar]
- Olszewski PK, Levine AS. Characterization of influence of central nociceptin/orphanin FQ on consummatory behavior. Endocrinology. 2004;145:2627–2632. doi: 10.1210/en.2004-0016. [DOI] [PubMed] [Google Scholar]
- Pan YX, Cheng J, Xu J, Rossi G, Jacobson E, Ryan-Moro J. Cloning and functional characterization through antisense mapping of a kappa 3-related opioid receptor. Mol Pharmacol. 1995;47:1180–1188. [PubMed] [Google Scholar]
- Peluso J, Gavériaux-Ruff C, Matthes HWD, Filliol D, Kieffer BL. Orphanin FQ/nociceptin binds to functionally coupled ORL1 receptors on human immune cell lines and alters peripheral blood mononuclear cell proliferation. Brain Res Bull. 2001;54:655–660. doi: 10.1016/s0361-9230(01)00482-8. [DOI] [PubMed] [Google Scholar]
- Peluso J, LaForge KS, Matthes HW, Kreek MJ, Kieffer BL, Gavériaux-Ruff C. Distribution of nociceptin/orphanin FQ receptor transcript in human central nervous system and immune cells. J Neuroimmunol. 1998;81:184–182. doi: 10.1016/s0165-5728(97)00178-1. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Gekker G, Hu S, Lokensgard J, Portoghese PS, Chao CC. Endomorphin-1 potentiates HIV-1 expression in human brain cell cultures: implications of an atypical μ-opioid receptor. Neuropharmacology. 1999;38:273–278. doi: 10.1016/s0028-3908(98)00167-1. [DOI] [PubMed] [Google Scholar]
- Polidori C, de Caro G, Massi M. The hyperphagic effect of nociceptin/orphanin FQ in rats. Peptides. 2000;21:1051–1062. doi: 10.1016/s0196-9781(00)00243-6. [DOI] [PubMed] [Google Scholar]
- Rahim RT, Meissler JJ, Jr, Cowan A, Rogers TJ, Geller EB, Gaughan J, Adler MW, Eisenstein TK. Administration of mu-, kappa- or delta2-receptor agonists via osmotic minipumps suppresses murine splenic antibody responses. Int Immunopharm. 2001;1:2001–2009. doi: 10.1016/s1567-5769(01)00128-x. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Civelli O. The orphanin FQ/nociceptin knockout mouse: a behavioral model for stress responses. Neuropeptides. 2002;36:72–76. doi: 10.1054/npep.2002.0901. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Higelin J, Henningsen RA, Monsma FJ, Jr, Civelli O. Structures that delineate orphanin FQ and dynorphin A pharmacological activities. J Biol Chem. 1998;273:1490–1495. doi: 10.1074/jbc.273.3.1490. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, Civelli O. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- Rosen H, Behar O, Abramsky O, Ovadia H. Regulated expression of proenkephalin A in normal lymphocytes. J Immunol. 1989;143:3703–3707. [PubMed] [Google Scholar]
- Rossi G-C, Leventhal L, Bolan E, Pasternak GW. Pharmacological characterization of orphanin FQ/nociceptin and its fragments. J Pharmacol Exp Ther. 1997;282:858–865. [PubMed] [Google Scholar]
- Serhan CN, Fierro IM, Chiang N, Pouliot M. Cutting Edge: Nociceptin stimulates neutrophil chemotaxis and recruitment: inhibition by aspirin-triggered-15-epi-lipotoxin A4. J Immunol. 2001;166:3650–3654. doi: 10.4049/jimmunol.166.6.3650. [DOI] [PubMed] [Google Scholar]
- Sharp BM, Keane WF, Suh HJ, Gekker G, Tsukayama D, Peterson PK. Opioid peptides rapidly stimulate superoxide production by human polymorphonuclear leukocytes and macrophages. Endocrinology. 1985;117:793–795. doi: 10.1210/endo-117-2-793. [DOI] [PubMed] [Google Scholar]
- Sinchak K, Dewing P, Cook M, Micevych P. Release of orphanin FQ/nociceptin in the medial preoptic nucleus and ventromedial nucleus of the hypothalamus facilitates lordosis. Horm Behav. 2007;51:406–412. doi: 10.1016/j.yhbeh.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EM, Morrill AC, Meyer WJI, Blalock JE. Corticotropin releasing factor induction of leukocyte-derived immunoreactive ACTH and endorphins. Nature. 1986;321:881–882. doi: 10.1038/321881a0. [DOI] [PubMed] [Google Scholar]
- Szalay F, Hantos MB, Horvath A, Lakatos PL, Folhoffer A, Dunkel K. Increased nociceptin/orphanin FQ plasma levels in hepatocellular carcinoma. World J Gastroent. 2004;10:42–45. doi: 10.3748/wjg.v10.i1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombella S, Vergura R, Falzarano S, Guerrini R, Calo G, Spisani S. Nociceptin/orphanin FQ stimulates human monocyte chemotaxis via NOP receptor activation. Peptides. 2005;26:1497–1502. doi: 10.1016/j.peptides.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Waits PS, Purcell WM, Fulford AJ, McLeod JD. Nociceptin/orphanin FQ modulates human T cell function in vitro. J Neuroimmunol. 2004;149:110–120. doi: 10.1016/j.jneuroim.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Wang JB, Johnson PS, Imai Y, Persico AM, Ozenberger BA, Eppler CM, Uhl GR. cDNA cloning of an orphan opiate receptor gene family member and its splice variant. FEBS Lett. 1994;348:75–79. doi: 10.1016/0014-5793(94)00557-5. [DOI] [PubMed] [Google Scholar]
- Wick MJ, Minnerath SR, Lin X, Elde R, Law PY, Loh HH. Isolation of a novel cDNA encoding a putative membrane receptor with high homology to the cloned mu, delta, and kappa opioid receptors. Brain Res Mol Brain Res. 1994;27:37–44. doi: 10.1016/0169-328x(94)90181-3. [DOI] [PubMed] [Google Scholar]
- Wick MJ, Minnerath SR, Roy S, Ramakrishnan S, Loh HH. Expression of alternate forms of brain opioid ‘orphan’ receptor mRNA in activated human peripheral blood lymphocytes and lymphocytic cell lines. Mol Brain Res. 1995;32:342–347. doi: 10.1016/0169-328x(95)00096-b. [DOI] [PubMed] [Google Scholar]
- Wybran J, Appelboom T, Famaey J-P, Govaerts A. Suggestive evidence for receptors for morphine and methionine-enkephalin on normal human blood T lympohcytes. J Immunol. 1979;123:1068–1070. [PubMed] [Google Scholar]