Abstract
Behavioral studies reveal that obese vs. lean individuals show attentional bias to food stimuli. Yet research has not investigated this relation using objective brain imaging or tested whether attentional bias to food stimuli predicts future weight gain, which are important aims given the prominence of food cues in the environment. We used functional magnetic resonance imaging (fMRI) to examine attentional bias in 35 adolescent girls ranging from lean to obese using an attention network task involving food and neutral stimuli. BMI correlated positively with speed of behavioral response to both appetizing food stimuli and unappetizing food stimuli, but not to neutral stimuli. BMI correlated positively with activation in brain regions related to attention and food reward, including the anterior insula/frontal operculum, lateral orbitofrontal cortex (OFC), ventrolateral prefrontal cortex (vlPFC), and superior parietal lobe, during initial orientation to food cues. BMI also correlated with greater activation in the anterior insula/frontal operculum during reallocation of attention to appetizing food images and with weaker activation in the medial OFC and ventral pallidum during reallocation of attention to unappetizing food images. Greater lateral OFC activation during initial orientation to appetizing food cues predicted future increases in BMI. Results indicate that overweight is related to greater attentional bias to food cues and that youth who show elevated reward circuitry responsivity during food cue exposure are at increased risk for weight gain.
INTRODUCTION
Consumption of palatable food results in dopamine (DA) release in the dorsal striatum in lean humans, and the amount released scales with meal pleasantness ratings (1). The dorsal striatum also responds during consumption of chocolate, and this response declines as the chocolate is devalued by eating beyond satiety (2). These data suggest that DA is involved in encoding the pleasure associated with food intake. The incentive-sensitization model of obesity posits that repeated pairings of reward from food intake and cues that predict impending food intake result in a hyper-responsivity of DA-based reward circuitry to food cues, contributing to craving and overeating (3). Animal experiments show that DA response to food shifts from food intake to cues that signal impending food intake after conditioning (4). The evidence that DA is involved in reward learning suggests that abnormalities in responsivity of DA-based reward circuitry could contribute to elevated approach tendencies toward food and food cues.
In line with the thesis that obesity is associated with anomalous responsivity of reward circuitry to food, functional magnetic resonance imaging (fMRI) studies reveal that obese vs. lean individuals show elevated activation of regions that encode the reward value of stimuli (e.g., striatum, orbitofrontal cortex (OFC), and amygdala) in response to pictures of appetizing food (5–7). Obese relative to lean individuals also show elevated activation in the caudate, gustatory regions (anterior insula, frontal operculum), and oral somatosensory regions (parietal operculum, Rolandic operculum) to anticipated receipt of palatable food (8).
The incentive-sensitization model of drug addiction (9) posits that hypersensitivity to motivational stimuli with high incentive salience produces a bias in attentional processing toward drug-related cues, triggering the release of DA and driving consumption. In support, exposure to drug cues results in drug cravings among individuals with substance use disorders, and addicted individuals show attentional bias to drug cues (10). Further, DA antagonists reduce attention bias for drug cues in cigarette smokers and detoxified heroin addicts (11). Results align with the suggestion that DA-based reward circuitry facilitates reward learning by selectively directing attention toward potentially rewarding stimuli (12). DA antagonists in healthy adults delay reaction time to target words (13), and reduce preferential responding to reward-paired cues in a go/no-go task (14). Additionally, DA agonists improve performance on selective attention tasks (15).
The above findings are similar to enhanced neural responses to food cues among obese individuals, leading theorists to propose that individuals who show elevated attentional bias to food images and food cues are at increased risk for overeating and weight gain (16). Behavioral studies reveal that obese vs. lean individuals show attentional bias for food words (e.g., ref. 17). Obese relative to lean children show greater interference with food words on a Stroop task (18). Better memory for food compared to nonfood words has also been demonstrated in obese relative to lean adolescents, though no interference in attention processing for food words emerged (19). Obese vs. lean individuals also orient more quickly toward food pictures and spend more time looking at food than nonfood pictures, as assessed via eye-tracking (16). Another study using event-related potentials as a measure of attention allocation has also found a trend for obese relative to lean individuals to orient more quickly to food cues (20).
If individuals who more rapidly attend to food cues and allocate more attention to these cues are more likely to overeat, then such attentional bias would be difficult to manage in an environment replete with attractive food cues. Although extant data provide behavioral evidence that obese vs. lean individuals tend to show greater attentional bias to food stimuli, few studies have tested for attentional bias for food using food images, and none has used objective brain imaging to investigate attentional bias or tested whether attentional bias to food images increases risk for future weight gain.
Obesity in children and adolescents has increased dramatically (21). Accordingly, the present study uses fMRI to examine attentional bias in adolescents ranging from lean to obese using a food-specific paradigm. We adapted the fMRI version of the attention network test (ANT) (22), which examines three attention systems: alerting, orienting, and executive control. Alerting refers to achieving and maintaining an alert state. Orienting involves allocation of attention to a location in space when a spatially informative cue is given (23). Executive control refers to the ability to direct attention toward task-relevant stimuli and inhibit the processing of distractor items (23). Our adapted version of the ANT task (food ANT) uses images of food instead of symbols and was designed to focus on the orienting and executive control aspects of attention (see Methods and Procedures). We hypothesize that obese relative to lean participants will: (i) respond more quickly to pictures of food that appear where cues originally appear, signaling great initial orienting of attention to food stimuli, (ii) respond more quickly to pictures of food that appear opposite from the side originally cued, which suggests more rapid reallocation of attention to food stimuli, (iii) show greater activation during food images in regions responsible for orienting attention (e.g., fusiform, postcentral gyri) (22) and in regions responsible for executive control during attention reallocation (e.g, superior and middle frontal gyri and anterior cingulate gyrus (22). We also expect that each of these effects will be stronger for appetizing vs. unappetizing foods, based on evidence that obese vs. lean individuals report a greater liking of high-fat and high-sugar foods (24). It is important to note that there is contradictory evidence on whether obese and lean individuals differ in terms of “liking” (pleasure/palatability) and more consistent evidence that they differ in terms of “wanting” (appetite/incentive motivation). However, Snyder and Bartoshuk (25) report in their review of this literature that there is consistent evidence that obese vs. lean individuals differ in terms of liking, when the experiment is performed properly. However, we also included images of water as control stimuli because of evidence that obese vs. lean individuals show a bias for both appetizing and unappetizing foods (26,27).
Finally, because we use a food-specific ANT task, we expect elevated activation in regions that encode the reward value of stimuli in response to food images vs. control images for obese vs. lean individuals (e.g, striatum, amygdala, insula, opercular regions, anterior cingulate cortex, and OFC). Obese vs. lean individuals have consistently shown greater activation in response to appetizing food images vs. control images in these regions (5–7).
METHODS AND PROCEDURES
Participants
Participants were 39 adolescent girls (mean age = 15.6; s.d. = 0.96; mean BMI = 24.2; s.d. = 4.5, BMI range = 17.3–38.8 (it is important to note that BMI values are age dependent and that a value of 17.3 is not in the anorexic range for an adolescent); 2.3% African Americans, 84.1% European Americans, 4.5% Native Americans, and 9.1% mixed racial heritage). Participants were recruited from a larger study of female high schools students with body image concerns. Individuals in this larger study who gave consent to be contacted about other studies were asked to participate in a study on the neural response to presentation of food. A pre-scan eligibility assessment verified inclusion and exclusion criteria. Those who reported binge eating or compensatory behaviors in the past 3 months, any use of psychoactive drugs, head injury with a loss of consciousness, color blindness, or current Axis I psychiatric disorder (including attention deficit hyperactivity disorder and anorexia or bulimia nervosa) were excluded. fMRI data from 5 of the 39 subjects were not analyzed due to technical difficulties (e.g., excess movement during scanning). Excessive movement was determined using realignment parameters and was defined as movement >1 mm in any direction during any run. Ninety percent of subjects were right-handed. The local institutional review board approved this project. Participants and parents provided written consent.
Measures
Body mass
The BMI (kg/m2) was used to reflect adiposity. After removal of shoes and coats, height was measured to the nearest millimeter using a stadiometer and weight was assessed to the nearest 0.1 kg using a digital scale. Two measures of height and weight were obtained and averaged at baseline and at 1-, 6- and 12-month follow-up. BMI correlates with direct measures of total body fat such as dual energy X-ray absorptiometry (r = 0.80 to .90) and with health measures including blood pressure, adverse lipoprotein profiles, atherosclerotic lesions, serum insulin levels, and diabetes mellitus in adolescent samples (28).
Food ANT
The fMRI ANT examines the effects of cues and targets within a single reaction time task to examine individual differences in attention (22). The ANT has been widely used in studies of normal performance and various forms of psychopathology in adults (e.g., ref. 29). The ANT discriminates between youth with vs. without attention deficit hyperactivity disorder (30). A version of the ANT was adapted for the present study to test whether BMI and change in BMI over a 1-year follow-up are correlated with activations in brain regions during initial orientation to food stimuli and reallocation of attention to food images.
Procedure
Participants were asked to consume a typical breakfast or lunch, but to refrain from eating or drinking (except water) for 4–6 hours immediately preceding their scan for standardization. We selected this deprivation period to capture the hunger state that most individuals experience as they approach their next meal, which is a time when individual differences in food reward would logically impact caloric intake. Most participants completed the paradigm between 16:00 and 18:00 (~5 hours after eating a typical lunch), but a subset completed scans between 11:00 and 13:00 (~ 5 hours after eating a typical breakfast).
Prior to scanning, participants rated how appetizing in general they found foods shown in 103 pictures using a visual analog scale (range: “least appetizing” = −395 to “most appetizing” = 395). Pictures included processed foods (e.g., cheeseburger), fruits (e.g., grapes), and vegetables (e.g., cauliflower). During the fMRI paradigm, each participant was exposed to the 20 pictures of food she rated as most appetizing and the 20 pictures she rated as least appetizing. Participants rated appetizing food images (mean = 313.5, s.d. = 62.8) significantly as more appetizing than unappetizing food images (mean = −330.5, s.d. = 107.5; t[33] = 21.8, P < 0.001). We included pictures of a glass of water and a cue signaling the appearance of this image, as it seemed possible that overweight individuals would show hyper-responsivity of the attentional network toward both appetizing and unappetizing food images (26,27).
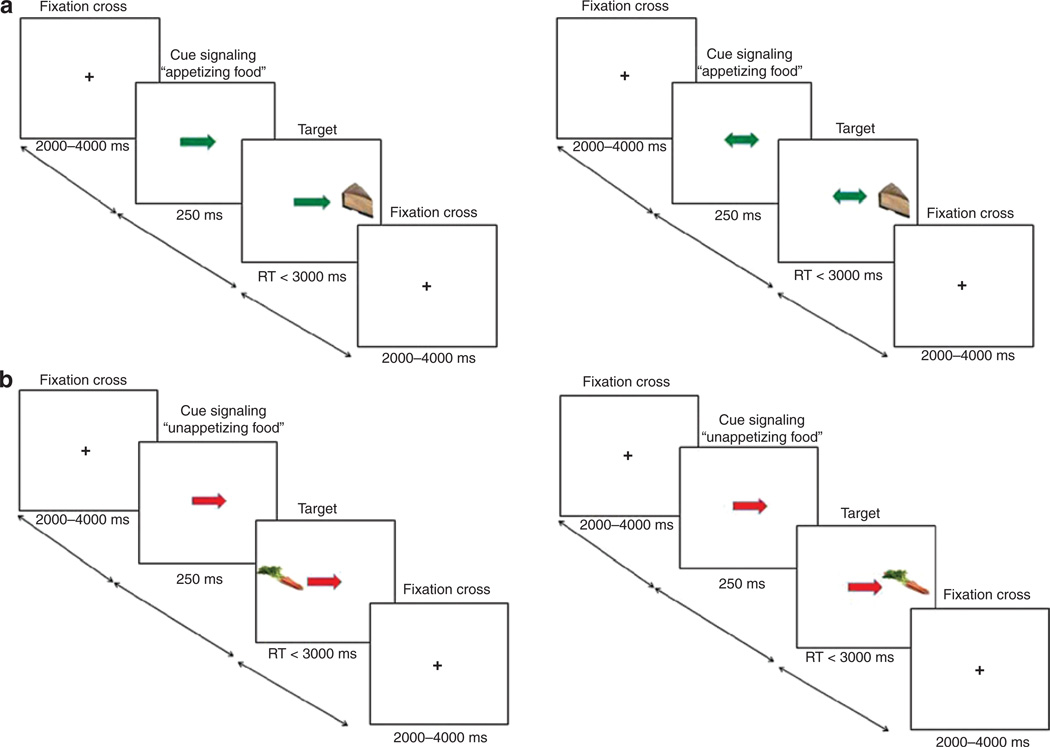
The details of the paradigm are illustrated in Figure 1. Participants saw a single arrow at fixation that was either green (signaling the appearance of an appetizing food image) or red (signaling the appearance of a unappetizing food image) pointing to the left or right. On 30% of the trials a double arrow indicated that the two sides were equally likely. On single arrow trials the target image appeared 80% on the side of the arrow (valid) and 20% on the opposite side (invalid). For double arrow trials, the target image appeared 50% on each side. The participants’ task was to identify the location of the targets by pressing one of two buttons as rapidly as possible, using their left index finger for leftward targets and right index finger for rightward targets. The target was presented until a response was made, with a maximum duration of 3,000 ms. The experiment consisted of 1 run with a total of 156 events: (i) appetizing food images appearing on the side of the single green arrow (48 events), (ii) appetizing food images appearing on the opposite side of the single green arrow (12 events), (iii) appetizing food images after double green arrows (12 events), (iv) unappetizing food images appearing on the side of the single red arrow (48 events), (v) unappetizing food images appearing on the opposite side of the single red arrow (12 events), (vi) unappetizing food images after double red arrows (12 events), and (vii) images of glasses of water after a double black arrow (12 events).
Figure 1.
Time course of events during the food attention network test (ANT) to (a) time course of events to measure initial orientation to food images with appetizing food images appearing after a single green arrow contrasted to appetizing food images appearing after a double green arrow; and (b) time course of events to measure reallocation of attention to food images with unappetizing food images appearing on the opposite side of the single red arrow contrasted to unappetizing food images appearing on the concurrent side of the single red arrow.
Before scanning, participants were familiarized with the fMRI paradigm through practice on a separate computer. Stimuli were presented visually using the Presentation software package (version 9; Neurobehavioral Systems, Davis, CA) and were displayed using a video projector that illuminated a rear projection screen at the end of the head-bore. Images and arrows were of similar luminosity and resolution. Participants viewed stimuli through an adjustable mirror attached to the head coil.
Behavioral analyses
For each participant, median reaction times to the images after valid, invalid, or double arrows were calculated. Spearman’s ρ was used to calculate the correlations of reaction time to both BMI and change in BMI over a 1-year period. BMI slope over 1 year (N = 35, range: −0.97–1.42) was calculated based on BMI measurements taken at baseline, 1-, 6-, and 12-month follow-up visits, to model change in BMI.
Acquisition of anatomical and functional MRI data
Scanning was performed by a Siemens Allegra 3 Tesla head-only MRI scanner. A standard birdcage coil was used to acquire data from the entire brain. Functional scans used a T2 × weighted gradient single-shot echo planar imaging sequence (echo time (TE) = 30 ms, repetition time (TR) = 2,000 ms, flip angle = 80°) with an in-plane resolution of 3.0 × 3.0 mm2 (64 × 64 matrix; 192 × 192 mm2 field of view). To cover the whole brain, 32 4-mm slices (interleaved acquisition, no skip) were acquired along the AC-PC transverse, oblique plane as determined by the midsagittal section. Structural scans were collected using an inversion recovery T1 weighted sequence (MP-RAGE) in the same orientation as the functional sequences to provide detailed anatomic images aligned to the functional scans. High-resolution structural MRI sequences (FOV = 256 × 256 mm2, 256 × 256 matrix, thickness = 1.0 mm, slice number ≈160) were acquired.
Image pre-processing
Data were pre-processed and analyzed using SPM5 software (Wellcome Department of Imaging Neuroscience, London, UK) in MATLAB (Mathworks, Sherborn, MA (31). Images were time-acquisition corrected to the slice obtained at 50% of the TR. Functional images were realigned to the mean. Images were normalized to the standard MNI template brain implemented in SPM5 (ICBM152, based on an average of 152 normal MRI scans), resulting in a voxel size of 3 mm3 for functional images and 1 mm3 for structural images. Functional images were smoothed with a 6 mm FWHM isotropic Gaussian kernel. We included temporal derivatives of the hemodynamic function to obtain a better model of the data. A 128-second high-pass filter (per SPM5 convention) removed low-frequency noise and slow drifts in the signal.
fMRI analyses
fMRI data were analyzed as an event-related design using the general linear model approach in a two-level procedure. On the first level in the single subject SPM models, responses to stimuli were modeled as events and convolved with the canonical hemodynamic response function to account for the lag between event onset and expected increase of the blood oxygenation level-dependent (BOLD) signal. To account for variance caused by head movement, realignment parameters were included as additional regressors in the model. At the second level, a random effects analysis was used to test the relation between baseline BMI and regional neural response during the events of interest by using the BMI score as a covariate.
To test our hypotheses that BMI is correlated with increased activation in response to food stimuli in regions associated with initial orienting attention, reallocation of attention, and food reward, small volume correction (SVC) analysis was performed in the fusiform gyrus, precentral gyrus, superior parietal lobe, thalamus, superior, inferior, and middle frontal gyri, anterior cingulate gyrus, striatum, hippocampus, insula, OFC, and pallidum. We performed SVC with activation peaks found in previous fMRI studies of attention and response to food images (22,1,5–8) as centroids to define 10-mm diameter spheres. T-map threshold was set at P uncorrected = 0.005 and a 3-voxel cluster size. Predicted activations were considered significant at P < 0.05 after correcting for multiple comparisons (pFDR) across the voxels within the a priori defined small volumes. FDR corrects for multiple comparisons, decreasing the likelihood of Type I errors. We derived the effect sizes (r) from the Z-values (Z/√N).
RESULTS
Paradigm validation: activation in regions related to food reward
To test whether this paradigm activates regions previously implicated in food reward, we contrasted BOLD response during (i) appetizing food images appearing on the congruent side vs. unappetizing food images appearing on the congruent side, (ii) appetizing food images appearing on the congruent side vs. images of glasses of water, and (iii) unappetizing food images appearing on the congruent side vs. images of glasses of water. Activations were assessed with one-sample t-tests (see Table 1). We found greater activation in the (i) right opercular regions in response to appetizing food images vs. unappetizing food images, (ii) left anterior cingulate cortex and left hippocampus in response to appetizing food images vs. images of glasses of water, and (iii) left opercular regions and left ACC during unappetizing food images vs. images of glasses of water. These regions have all been implicated in encoding the reward value of food (2,5–8). These data suggest that the attention network task involving food stimuli is suitable for studying food reward.
Table 1.
Main effects: regions showing activation during target conditions across subjects, independent of BMI (N = 34)
| Region and regression condition |
Reference coordinatesa |
x | y | z | Cluster | Z |
P value < 0.05 FDR corrected |
Effect size |
|---|---|---|---|---|---|---|---|---|
| Appetizing food images after valid arrows > unappetizing food images after valid arrows | ||||||||
| Rolandic operculum | 54, −9, 30 | 54 | −15 | 24 | 33 | 3.39 | 0.02 | 0.57 |
| Frontal operculum | 39, −15, 18 | 42 | −21 | 24 | 20 | 3.59 | 0.03 | 0.61 |
| Appetizing food images after valid arrows > glasses of water | ||||||||
| ACC | −6, 3, 45 | −6 | 12 | 48 | 20 | 3.48 | 0.01 | 0.59 |
| Hippocampus | 24, −30, −10 | −27 | −21 | −6 | 8 | 3.50 | 0.03 | 0.59 |
| Unappetizing food images after valid arrows > glasses of water | ||||||||
| Rolandic operculum | 54, −3, 27 | −51 | −3 | 24 | 3 | 3.35 | 0.03 | 0.57 |
| Frontal operculum | −48, −6, 3 | −45 | −6 | 12 | 3 | 3.28 | 0.04 | 0.55 |
| ACC | −6, 3, 45 | −9 | 12 | 42 | 7 | 4.16 | 0.002 | 0.70 |
| −6 | 6 | 54 | 5 | 3.65 | 0.007 | 0.62 | ||
Correlation of BMI with reaction times
BMI was negatively related to median reaction times to appetizing food images appearing on the congruent side, appetizing food images appearing on the opposite side, appetizing food images after double arrows, unappetizing food appearing on the congruent side, unappetizing food appearing on the opposite side, and unappetizing food after double arrows (Table 2). There were no significant correlations between BMI and the median reaction time to images of a glass of water and between BMI and error rate. Behavioral responses also did not predict change in BMI over the 1-year follow-up. That is, reaction time to food and water images and error rate did not correlate significantly with change in BMI over 1-year follow-up.
Table 2.
Correlation between BMI and median reaction times to valid, invalid, and neutral targets (N = 35)
| BMI | |
|---|---|
| Appetizing food after valid arrow | −0.45** |
| Appetizing food after invalid arrow | −0.38* |
| Appetizing food after double arrow | −0.47** |
| Unappetizing food after valid arrow | −0.44** |
| Unappetizing food after invalid arrow | −0.43** |
| Unappetizing food after double arrow | −0.41* |
| Glass of water | −0.05 |
Correlation is significant at P < 0.05.
Correlation is significant at P < 0.01.
Relation of BMI to initial orientation to food
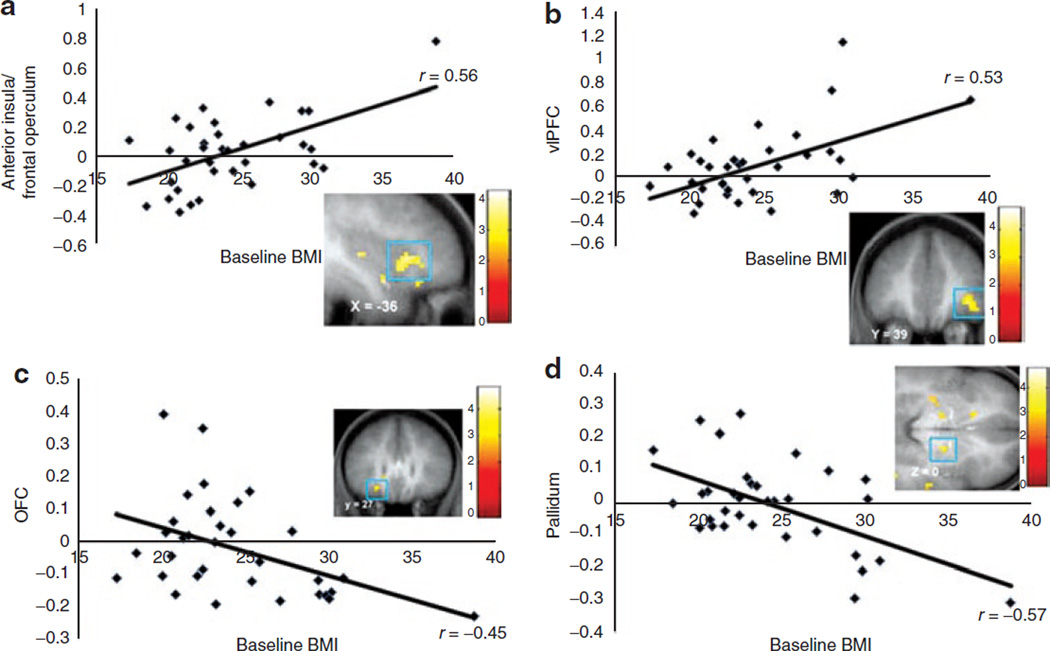
To measure initial orientation to appetizing food images, we contrasted BOLD responses during a single green arrow vs. a double green arrow. To measure initial orientation to unappetizing food images, we contrasted BOLD responses during a single red arrow vs. a double red arrow. BMI was positively correlated with activation in the right mid insula, left frontal operculum, left anterior insula/frontal operculum (Figure 2a), and left lateral OFC (Table 3) during initial orientation to appetizing food images. (We re-estimated the relations between BMI and initial orientation and between BMI and reallocation of attention while controlling for handedness; the relations between BMI and BOLD responses to initial orientation and reallocation of attention remained significant.) No significant deactivations were observed. BMI was positively correlated with activation in the right ventrolateral prefrontal cortex (vlPFC) (Figure 2b) and right frontal operculum as well as in bilateral parietal lobe (Table 3) during initial orientation to unappetizing food images. No significant deactivations were observed. Although there was one apparent outlier that may have driven the baseline BMI-BOLD correlations, the effects remained significant at P < 0.05 FDR corrected when the outlier was excluded.
Figure 2.
Greater activation in (a) the anterior insula/frontal operculum (MNI coordinates: 36, 21, 6, Z =3.42, pFDR = 0.02) during orientation to appetizing food and (b) the ventrolateral prefrontal cortex (MNI coordinates: 39, 39, −3, Z = 3.24, pFDR = 0.03) during orientation to unappetizing food cues as a function of BMI. The effects remain significant at the pFDR = 0.05 level when data from the participant with the greatest BMI were excluded. Decreased activation in (c) orbitofrontal cortex (MNI coordinates: −24, 27, −12, Z = 3.13, pFDR = 0.04) and (d) ventral pallidum (MNI coordinates: 18, −6, 0, Z = 3.45, pFDR = 0.04) in response to reallocation of attention to unappetizing food images as a function of BMI.
Table 3.
Regions showing brain activation in response to orientation to food and reallocation of attention to food correlated with BMI (N = 34)
| Region and regression condition | Reference coordinatesa |
x | y | z | Cluster | Z |
P value < 0.05 FDR corrected |
Effect size |
|---|---|---|---|---|---|---|---|---|
| Orientation to appetizing food (green single arrow > green double arrow) | ||||||||
| Mid insula | −38, −14, −8 | 39 | −12 | −6 | 18 | 3.14 | 0.03 | 0.54 |
| Frontal operculum | −38, 18, 0 | −42 | 15 | −3 | 6 | 3.46 | 0.02 | 0.59 |
| Anterior insula/frontal operculum | −38, 18, 0 | −36 | 21 | 6 | 4 | 3.42 | 0.02 | 0.59 |
| Lateral OFC | 36, 27, −15 | −39 | 30 | −12 | 5 | 3.24 | 0.05 | 0.56 |
| Orientation to unappetizing food (red single arrow > red double arrow) | ||||||||
| vlPFC | 45, 45, 0 | 39 | 39 | −3 | 3 | 3.24 | 0.03 | 0.56 |
| Frontal operculum | 54, 26, 2 | −42 | 27 | 6 | 3 | 3.61 | 0.009 | 0.62 |
| Superior parietal lobe | −36, −46, 50 | −36 | −45 | 51 | 6 | 3.75 | 0.01 | 0.64 |
| −36, −46, 50 | −39 | −45 | 51 | 3 | 3.23 | 0.02 | 0.55 | |
| Reallocation of attention to appetizing food (appetizing food after invalid arrow > appetizing food after valid arrow) | ||||||||
| Anterior insula/frontal operculum | −38, 18, 0 | −42 | 18 | 6 | 10 | 3.06 | 0.05 | 0.53 |
| Reallocation of attention to unappetizing food (unappetizing after invalid arrow > unappetizing food after valid arrow) | ||||||||
| Medial OFC | −20, 16, −14 | −24 | 27 | −12 | 9 | 3.13 | 0.04 | −0.54 |
| Ventral pallidum | −24, −8, 0 | 18 | −6 | 0 | 17 | 3.45 | 0.04 | −0.59 |
Relation of BMI to reallocation of attention to food images
To identify brain regions activated in response to reallocation of attention to appetizing food images, we contrasted BOLD responses during appetizing food images appearing on the opposite side vs. appetizing food images appearing on the congruent side. To identify brain regions activated in response to reallocation of attention to unappetizing food images, we contrasted BOLD responses during unappetizing food images appearing on the opposite side vs. unappetizing food images appearing on the congruent side. BMI was correlated with greater activation in the left anterior insula/frontal operculum during reallocation of attention to appetizing food images (Table 3). There were no positive correlations between BMI and brain activation during reallocation of attention to unappetizing food images; however, BMI was negatively correlated to activation in the left OFC (Figure 2c) and right ventral pallidum (Figure 2d; Table 3).
Relation between brain activation and change in BMI
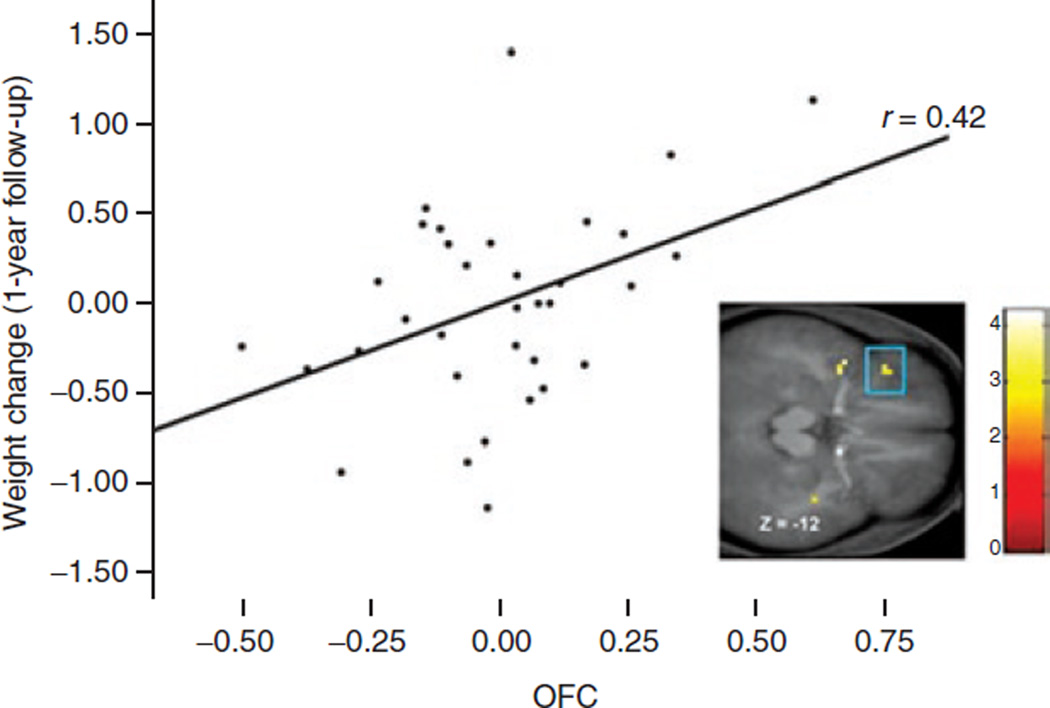
For the longitudinal analyses, parameter estimates of significant peaks were entered in SPSS to calculate the correlation of these peaks with change in BMI over follow-up, controlling for baseline BMI. The average increase in BMI over the 1-year follow-up period was 0.20, with s.d. = 0.56 and range = −0.97–1.42, suggesting that there was sufficient variation in weight change over follow-up. Activation in the OFC (MNI coordinates: −39, 30, −12, Z = 3.24, pFDR = 0.05) during initial orientation to appetizing food cues correlated with increases in BMI over the 1-year follow-up (r partial = 0.42, P = 0.016; Figure 3).
Figure 3.
Activation in the OFC (MNI coordinates: −39, 30, −12, Z = 3.24, pFDR = 0.05) in response to orientation to appetizing food cues was related to future increases in BMI. The effect remained significant when the participant with the greatest increase in BMI was excluded.
Although there was one apparent outlier that may have driven the correlation, the effect remained significant when the outlier was excluded (r partial = 0.37, P = 0.037). No other peaks correlated significantly with BMI slope.
DISCUSSION
As hypothesized, participants with higher BMI scores responded behaviorally more quickly to food images, but not to neutral images (glasses of water). These results dovetail with previous evidence that obese vs. lean individuals show greater attentional bias to food stimuli compared to control stimuli (16–18). These results also converge with evidence that addicted vs. nonaddicted individuals show attentional bias for drug cues vs. control cues (10), suggesting possible parallels in attentional neural functioning in substance abuse and obesity. Interestingly, BMI was positively correlated with behavioral response to both appetizing and unappetizing food images, implying that food cues in general trigger greater attention in overweight vs. lean individuals. Behavioral responses did not predict change in BMI over the 1-year follow-up. It is possible that we observed only one prospective activation effect because we had power to detect only moderately large effects.
We hypothesized that BMI would correlate with greater activation in brain regions related to attention and encoding of reward value of stimuli during the initial orientation to food stimuli. As predicted, BMI correlated positively with activation in the mid insula, frontal operculum, anterior insula/frontal operculum, and OFC during initial orientation to appetizing food images. Generally, the insula processes gustatory information, especially that with emotional valence (32). In particular, the anterior insula has been implicated in attention and emotional responding (33) and is thought to identify the most relevant among internal and external stimuli in order to guide behavior (32). BMI also correlated positively with activation in the vlPFC, frontal operculum, and superior parietal lobe during the initial orientation to unappetizing food images. The insula, frontal operculum, OFC, and vlPFC have found to be activated by memory of the rewarding effects of food (e.g., 34), and taste of palatable food (7,8). The superior parietal lobe has found to be activated during orientation toward the spatial location of visual signals (22). The vlPFC forms an important part of the circuitry in which associations between visual cues and the actions or choices they specify are formed and is thought to play a role in selecting the correct course of action out of multiple behavioral choices (35). Results could suggest that overweight participants more readily orient to the spatial location of the food stimuli. However, the vlPFC has also found to be activated during food reward (8). It is therefore possible that the positive correlation found between BMI and vlPFC refers to greater food reward activation. Consistent with the behavioral data, results suggest that individuals with higher BMIs show greater attention to food cues in general, not just highly appetizing food cues.
We hypothesized that individuals with higher vs. lower BMIs would show greater activation in the attentional network as well as regions activated by food reward during reallocation of attention to food images. BMI was positively correlated with activation in the anterior insula/frontal operculum during reallocation to appetizing food images. The anterior insula has found to be engaged by working-memory processes and attentional shifting (36). This result may suggest that overweight subjects more readily shift their attention to appetizing food cues. This finding accords with our behavioral results as individuals with a higher vs. lower BMI identified the location of the appetizing food images appearing on the opposite side of the arrows faster. This result dovetails with previous evidence that obese vs. lean individuals orient more quickly toward food pictures (16). However, this region is also referred to as the primary taste cortex, representing the identity and intensity of a taste (37), and has been found to be activated during food reward in obese compared to lean individuals (5,8).
We found that BMI was negatively correlated with activation in the OFC and ventral pallidum during attention reallocation to unappetizing food images. Several studies found activation in the OFC during experiments measuring disengagement from targets (e.g., ref. 1). The OFC also plays a key role in decision-making involving reward and response cost (38) and plays a role in assessing the reward value of taste (3). The ventral pallidum encodes reward “liking” of tastes (3,39) and has been implicated in food reward in humans (5). The evidence that overweight individuals showed less activation in these regions during reallocation of attention to unappetizing food images might indicate that they experience a greater ‘dislike’ when viewing unappetizing food. Although BMI was correlated with less activation in brain regions related to food reward during unappetizing food images, it was correlated with greater activation in regions related to both attention and food reward during cues signaling unappetizing food images. Thus, it is possible that even though individuals with high BMIs experience a greater “dislike” when viewing unappetizing food images, food cues in general exert a powerful motivational effect, resulting in overeating. This finding may be analogous to the role of incentive salience, which suggests that cues associated with a substance (in this case food) may begin triggering the release of DA and driving consumption because of the learned associations between cues and their reward (9).
Interestingly, for both initial orientation to food cues and reallocation of attention to food images, most of the brain responses were found in neural regions associated with food reward processing rather than simple attention processing. This may imply in overweight individuals, attention is overridden by food reward when confronted with food stimuli. However, it is important to note that by using actual food pictures instead of a simple symbol (the asterisk symbol (“*”) is often used in the attentional network task), the food attentional network task is less of a spatial attention task.
Our results also extend knowledge regarding risk for weight gain. Activation in the lateral OFC during initial orientation to appetizing food predicted increases in BMI over 1-year follow-up. This finding converges with evidence that activation in the lateral OFC in response to imagined intake of appetizing foods increases risk for future weight gain (7), though that study found that genotypes associated with DA signaling capacity moderated those predictive effects. Thus, this finding of lateral OFC activation predicting weight gain may suggest that those at risk for unhealthy weight gain are more attuned to the hedonic reward aspect of appetizing food stimuli and that cues associated with food may be driving consumption by triggering the release of DA, providing prospective support for an incentive model of obesity. The fact that activation showed six relations to BMI but only one to change in BMI suggests that the latter effects are smaller. However, this could also be due to different analytic methods used for cross-sectional vs. prospective analyses.
When interpreting the results, it is important to consider certain limitations. First, instruction type (button press) and stimulus (images) were confounded. Activation during the images may have been caused either by the processing of the stimuli or by behavioral response. However, subjects were instructed to press buttons to all stimuli. Therefore, the activation caused by behavioral response should not affect our findings. Second, given that the effects decreased when removing the outlier (participant with the greatest BMI) and the fact that this is one of the first prospective fMRI studies to predict future increases in BMI, results should be considered provisional until replicated in a larger sample. Third, all food images were repeated 3 times and the image of the glass of water 12 times. Repetition of the images could have resulted in some habituation effects, affecting BOLD activation. Fourth, events during which food images appeared on the opposite side of the arrow occurred only 12 times, potentially limiting our statistical power to detect effects. However, the fact that we did find activation in the hypothesized areas, suggest that we had adequate sensitivity. Fifth, it is possible that the found activations in regions related to attention and food reward are not food specific, but rather suggest elevated general reward sensitivity. There is evidence that receipt of food, alcohol, nicotine, and money activate similar regions of the brain (e.g., refs. 1,40). Further, BMI correlates with self-reported general reward sensitivity (41). In addition, normal-weight adolescents at high- vs. low-risk for obesity showed aberrant activation of reward circuitry in response to both food reward and monetary reward (42). Future studies should explore these relations further. Finally, our sample is limited to adolescent girls and thus results may not be generalizable to other demographic groups. This is important given the evidence of gender and age-related differences in brain activation (e.g., refs. 43,44). For example, sex-specific brain responses to a meal have been found (43).
In sum, our behavioral and fMRI findings may suggest that individuals with a higher BMI vs. lower BMI show greater attentional bias to food stimuli. There was also evidence that individuals who experience greater activation in the lateral OFC are at increased risk for overeating and weight gain. Results may suggest that elevated attention to food cues contributes to overeating, particularly given the plethora of food cues in our current environment.
Acknowledgments
This research was supported by a Roadmap Supplement for Interdisciplinary Research in Behavioral and Biological Sciences (R1MH64560A).
Footnotes
Disclosure
The authors declared no conflict of interest.
REFERENCES
- 1.Small DM, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage. 2003;19:1709–1715. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- 2.Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain. 2001;124:1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- 3.Berridge KC. ‘Liking’ and ‘wanting’ food rewards: brain substrates and roles in eating disorders. Physiol Behav. 2009;97:537–550. doi: 10.1016/j.physbeh.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schultz W, Apicella P, Ljungberg T. Responses of monkey dopamine neurons to reward and conditioned stimuli during successive steps of learning a delayed response task. J Neurosci. 1993;13:900–913. doi: 10.1523/JNEUROSCI.13-03-00900.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rothemund Y, Preuschhof C, Bohner G, et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage. 2007;37:410–421. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Stoeckel LE, Weller RE, Cook EW, 3rd, et al. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage. 2008;41:636–647. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 7.Stice E, Yokum S, Bohon C, Marti N, Smolen A. Reward circuitry responsivity to food predicts future increases in body mass: moderating effects of DRD2 and DRD4. Neuroimage. 2010;50:1618–1625. doi: 10.1016/j.neuroimage.2010.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol. 2008;117:924–935. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- 10.Franken IH. Drug craving and addiction: integrating psychological and neuropsychopharmacological approaches. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:563–579. doi: 10.1016/S0278-5846(03)00081-2. [DOI] [PubMed] [Google Scholar]
- 11.Franken IH, Hendriks VM, Stam CJ, Van den Brink W. A role for dopamine in the processing of drug cues in heroin dependent patients. Eur Neuropsychopharmacol. 2004;14:503–508. doi: 10.1016/j.euroneuro.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Leyton M. The neurobiology of desire: dopamine and the regulation of mood and motivational states in humans. In: Kringelbach ML, Berridge KC, editors. Pleasures of the Brain. Oxford, UK: Oxford University Press; 2010. pp. 222–243. [Google Scholar]
- 13.Clark CR, Geffen GM, Geffen LB. Role of monoamine pathways in attention and effort: effects of clonidine and methylphenidate in normal adult humans. Psychopharmacology (Berl) 1986;90:35–39. doi: 10.1007/BF00172868. [DOI] [PubMed] [Google Scholar]
- 14.Leyton M, aan het Rot M, Booij L, et al. Mood-elevating effects of d-amphetamine and incentive salience: the effect of acute dopamine precursor depletion. J Psychiatry Neurosci. 2007;32:129–136. [PMC free article] [PubMed] [Google Scholar]
- 15.Servan-Schreiber D, Carter CS, Bruno RM, Cohen JD. Dopamine and the mechanisms of cognition: Part II. D-amphetamine effects in human subjects performing a selective attention task. Biol Psychiatry. 1998;43:723–729. doi: 10.1016/s0006-3223(97)00449-6. [DOI] [PubMed] [Google Scholar]
- 16.Castellanos EH, Charboneau E, Dietrich MS, et al. Obese adults have visual attention bias for food cue images: evidence for altered reward system function. Int J Obes (Lond) 2009;33:1063–1073. doi: 10.1038/ijo.2009.138. [DOI] [PubMed] [Google Scholar]
- 17.Schachter S, Rodin J. Obese Humans and Rats. Washington DC: Halsted Press; 1974. [Google Scholar]
- 18.Braet C, Crombez G. Cognitive interference due to food cues in childhood obesity. J Clin Child Adolesc Psychol. 2003;32:32–39. doi: 10.1207/S15374424JCCP3201_04. [DOI] [PubMed] [Google Scholar]
- 19.Soetens B, Braet C. Information processing of food cues in overweight and normal weight adolescents. Br J Health Psychol. 2007;12:285–304. doi: 10.1348/135910706X107604. [DOI] [PubMed] [Google Scholar]
- 20.Nijs IM, Muris P, Euser AS, Franken IH. Differences in attention to food and food intake between overweight/obese and normal-weight females under conditions of hunger and satiety. Appetite. 2010;54:243–254. doi: 10.1016/j.appet.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2010;303:242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 22.Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. J Cogn Neurosci. 2002;14:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- 23.Dye MW, Green CS, Bavelier D. The development of attention skills in action video game players. Neuropsychologia. 2009;47:1780–1789. doi: 10.1016/j.neuropsychologia.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drewnowski A, Brunzell JD, Sande K, Iverius PH, Greenwood MR. Sweet tooth reconsidered: taste responsiveness in human obesity. Physiol Behav. 1985;35:617–622. doi: 10.1016/0031-9384(85)90150-7. [DOI] [PubMed] [Google Scholar]
- 25.Snyder DJ, Bartoshuk LM. The logic of sensory and hedonic comparisons: are the obese different? In: Blass EM, editor. Obesity: Causes, Mechanisms, Prevention, and Treatment. Sunderland, MA: Sinauer Associates, Inc.; 2008. pp. 139–161. [Google Scholar]
- 26.Batterink L, Yokum S, Stice E. Body mass correlates inversely with inhibitory control in response to food among adolescent girls: an fMRI study. Neuroimage. 2010;52:1696–1703. doi: 10.1016/j.neuroimage.2010.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Craeynest M, Crombez G, De Houwer J, et al. Explicit and implicit attitudes towards food and physical activity in childhood obesity. Behav Res Ther. 2005;43:1111–1120. doi: 10.1016/j.brat.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 28.Dietz WH, Robinson TN. Use of the body mass index (BMI) as a measure of overweight in children and adolescents. J Pediatr. 1998;132:191–193. doi: 10.1016/s0022-3476(98)70426-3. [DOI] [PubMed] [Google Scholar]
- 29.Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. Neuroimage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Shafritz KM, Marchione KE, Gore JC, Shaywitz SE, Shaywitz BA. The effects of methylphenidate on neural systems of attention in attention deficit hyperactivity disorder. Am J Psychiatry. 2004;161:1990–1997. doi: 10.1176/appi.ajp.161.11.1990. [DOI] [PubMed] [Google Scholar]
- 31.Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited–again. Neuroimage. 1995;2:173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- 32.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hutcherson CA, Goldin PR, Ochsner KN, et al. Attention and emotion: does rating emotion alter neural responses to amusing and sad films? Neuroimage. 2005;27:656–668. doi: 10.1016/j.neuroimage.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 34.Pelchat ML, Johnson A, Chan R, Valdez J, Ragland JD. Images of desire: food-craving activation during fMRI. Neuroimage. 2004;23:1486–1493. doi: 10.1016/j.neuroimage.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 35.Fillmore MI, Rush CR. Alcohol effects on inhibitory and activational response strategies in the acquisition of alcohol and other reinforcers: priming the motivation to drink. J Stud Alcohol. 2001;62:646–656. doi: 10.15288/jsa.2001.62.646. [DOI] [PubMed] [Google Scholar]
- 36.Wager TD, Jonides J, Reading S. Neuroimaging studies of shifting attention: a meta-analysis. Neuroimage. 2004;22:1679–1693. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- 37.Grabenhorst F, Rolls ET, Bilderbeck A. How cognition modulates affective responses to taste and flavor: top-down influences on the orbitofrontal and pregenual cingulate cortices. Cereb Cortex. 2008;18:1549–1559. doi: 10.1093/cercor/bhm185. [DOI] [PubMed] [Google Scholar]
- 38.Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000;123(Pt 11):2189–2202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- 39.Tindell AJ, Smith KS, Berridge KC, Aldridge JW. Dynamic computation of incentive salience: “wanting” what was never “liked”. J Neurosci. 2009;29:12220–12228. doi: 10.1523/JNEUROSCI.2499-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahern AL, Field M, Yokum S, Bohon C, Stice E. Relation of dietary restraint scores to cognitive biases and reward sensitivity. Appetite. 2010;55:61–68. doi: 10.1016/j.appet.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 42.Stice E, Yokum S, Burger KS, Epstein LH, Small DM. Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. J Neurosci. 2011;31:4360–4366. doi: 10.1523/JNEUROSCI.6604-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Del Parigi A, Chen K, Gautier JF, et al. Sex differences in the human brain’s response to hunger and satiation. Am J Clin Nutr. 2002;75:1017–1022. doi: 10.1093/ajcn/75.6.1017. [DOI] [PubMed] [Google Scholar]
- 44.Bjork JM, Smith AR, Chen G, Hommer DW. Adolescents, adults and rewards: comparing motivational neurocircuitry recruitment using fMRI. Neuroimage. 2010;15:643–657. doi: 10.1371/journal.pone.0011440. [DOI] [PMC free article] [PubMed] [Google Scholar]