Abstract
Objective This study evaluated the feasibility, acceptability, and initial efficacy of an intervention based on Schachter’s externality theory; the Regulation of Cues (ROC) program. Methods 44 overweight and obese 8–12-year-old children and their parents were randomly assigned to a 4-month ROC program or the control group. Outcomes were assessed at baseline, posttreatment, and 4 months posttreatment and included acceptability and feasibility, body weight, and eating behaviors. Results The ROC program had moderate to high acceptability ratings. Significant improvements were found for the ROC group compared with the control group on child food responsiveness at posttreatment and eating in the absence of hunger at 4 months posttreatment. Improvements were seen for the ROC group compared with the control group on body weight measures and food responsiveness, although these only approached significance. Conclusion The ROC intervention may be useful with overweight and obese children. Larger, fully powered studies are needed to further evaluate the efficacy of this model.
Keywords: children, eating and feeding disorders, obesity, weight management
One in three children in the United States is overweight or obese (Ogden, Carroll, Kit, & Flegal, 2012). The primary evidence-based treatment for childhood obesity is family based behavior therapy (FBT), which is typically provided to parents and children weekly for 4–6 months. FBT interventions that combine nutrition education and exercise with behavior therapy techniques are considered the most effective methods for weight loss in children (Epstein, 1996). After completing treatment, approximately one-third of children treated by family based behavioral methods are no longer overweight in adulthood (Epstein, Valoski, Wing, & McCurley, 1990). Although FBT for childhood obesity appears to show long-term efficacy for some children, additional approaches to treat overweight and obese children are needed to maximize the efficacy for a larger number of families.
Today’s ubiquitous food environment, which provides access to hedonic calorically dense foods, could trigger overeating and lead to failure of weight management efforts. FBT teaches parents and children to avoid tempting food cues; to set up a home that has healthy foods; to eliminate high-fat, high-sugar foods from the home; and to self-monitor food intake. Such guidelines are well-grounded in behavioral learning theory. In practice, however, children are often faced with tempting food cues outside of the home (e.g., birthday parties, school lunch, snacks provided at extracurricular activities). The safety of the home environment may not prepare them to manage feelings of craving and food motivation cued by the highly palatable foods they encounter throughout their day. Helping children to resist eating in response to food cues in their natural environment is crucial for successful weight management.
Schachter’s externality theory of obesity (Schachter, 1971; Schachter & Rodin, 1974) could provide a model to develop alternative treatments for obesity. This theory states that obese people are more reactive to external cues to eat and less sensitive to internal hunger and satiety signals than their lean counterparts. According to Schachter’s theory, this over-responsiveness to external food cues and decreased responsivity to hunger and satiety signals could lead to significant overeating in the current environment in vulnerable individuals, given the abundance of palatable food stimuli.
We developed two treatments based on Schachter’s theory to reduce overeating in overweight and obese children; Children’s Appetite Awareness and Cue Exposure Treatment—Food. The focus of Children’s Appetite Awareness Training (CAAT: to address internal cues) is on developing greater sensitivity to hunger and satiety cues, and to use that sensitivity to guide eating behavior. Parents and children are taught to monitor their hunger on a 1–5 scale, using the metaphor of a gas tank, and to eat when physically hungry but stop before they are too full. Research using an appetite awareness program has shown a decrease in binge eating in adults (Craighead & Allen, 1995) and small but significant weight loss in overweight children (Bloom, Sharpe, Mullan, & Zucker, 2013).
The increase in sensitivity to external cues to eat, or food cue reactivity, could be considered a learned response. Cues that accompany food, as well as affective states and cognitions, can be conditioned in vulnerable individuals through Pavlovian conditioning to elicit a physiological response (Bouton, 2011; Jansen, 1994, 1998). Conceptually, physiological and psychological cue reactivity should be amenable to extinction through exposure (Wardle, 1990), although more modern conceptualizations of exposure treatments include improving inhibitory learning (Craske et al., 2008). In Cue Exposure Treatment for Food (CET-Food; to address external cues), children and parents are taught to monitor cravings on a 1–5 scale while exposed to highly palatable foods, to resist eating in response to urges, and to tolerate craving feelings over time. CET-Food directly targets eating when satiated. In theory, CET-Food should decrease cue reactivity, which could lead to a decrease in eating in response to food cues.
We tested the acceptability, feasibility, and initial effects of these two interventions on overeating, weight, and binge eating with overweight and obese children and their parents (Boutelle et al., 2011). Our initial study tested CAAT and CET-Food separately in 8-week groups, and results suggested that both CAAT and CET-Food impacted our study outcomes. Specifically, our data showed that children in the CET-Food arm showed reductions in eating in the absence of hunger (EAH), binge eating, and loss of control eating immediately posttreatment and up to 6 months posttreatment. Children in the CAAT arm also showed significant decreases in binge eating, which were retained up to 6 months posttreatment. Additionally, children in CET-Food had a stable body mass index (BMI) for up to 6 months posttreatment, whereas children in CAAT continued to increase in BMI. Of note, this first trial did not include a control group. Although our results suggested that our CET-Food intervention was promising, in practice, we found that families were often confused about the concept of craving in the context of food cues without education about hunger and satiety mechanisms. We found that children and parents had difficulties understanding and monitoring cravings (wanting to eat when physically full) without practice and mastery of detection of satiety. We decided to integrate CAAT and CET-Food to (1) address both targets of Schachter’s theory in one treatment and (2) to improve understanding and sensitivity of cravings ratings by first training in hunger and satiety.
Regulation of Cues (ROC) combines CAAT with CET-Food interventions, to address both targets of Schachter’s externality theory. The 14-session ROC program includes hunger awareness and cue exposure treatment, as well as psychoeducation, coping skills, parenting skills, and experiential learning. To evaluate feasibility and acceptability of the full ROC treatment protocol, we pilot-tested the ROC program in a randomized control design by comparing it with a waitlist control condition. Our primary aim was to evaluate the feasibility and acceptability of the combined ROC program, and to gain further experience with these novel treatments in a longer treatment trial. Our secondary aim was to examine preliminary efficacy data of the impact of ROC on child weight, caloric consumption, EAH, and parent report of eating behaviors compared with a control group.
Method
Participants
Participants consisted of 44 overweight and obese 8–12-year-old children who met study criteria for EAH, and their parents. We targeted children who were high on EAH because we wanted to be able to measure changes in overeating, as it is directly targeted in the treatment protocol. Participants were recruited from schools, after-school day care programs, medical clinics, newspaper and online advertisements, community postings, and mailings to homes with 8–12-year-old children. We recruited children who were overweight (BMI percentile ≥85th%) and ate 10% of their daily caloric needs in the EAH free access session (see Measures section). Inclusion criteria also included liking cheese pizza (the dinner provided). Exclusion criteria included non-English speakers/readers, participation in a formal weight loss program, having a medical condition or taking any medication that could influence growth/weight and eating, food allergies or dietary restrictions, or having any sort of disability that would prevent them from being able to participate in group sessions or assessment visits.
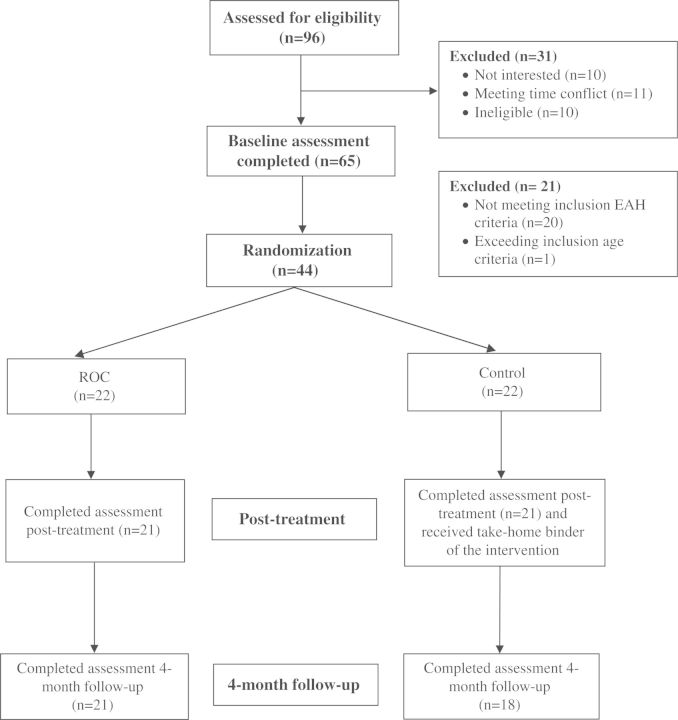
Of the 96 participants who completed the phone screen, 65 (68%) were seen in clinic for an EAH evaluation, and 44 (68%) were eligible for randomization (Figure 1). Following the baseline assessment, the project coordinator used a computer-generated randomization table to assign participants to one of two possible treatment conditions (ROC or control) by sex (Table I). The University of Minnesota Institutional Review Board approved the study protocol. All parents provided written consent and all children provided written assent.
Figure 1.
Study recruitment, randomization, and completion of parent–child pairs in intervention and control conditions.
Table I.
Characteristics of Study Sample (n = 44)
| Total | Intervention | Control | |
|---|---|---|---|
| Visit 1 (baseline), n | 44 | 22 | 22 |
| Visit 2 (end of treatment), n | 42 | 21 | 21 |
| Visit 3 (4-month follow-up), n | 40 | 21 | 19 |
| Child BMI percentile, mean (SD) | 97.4 (2.6) | 97.6 (2.6) | 97.2 (2.5) |
| Child age, mean (SD) | 10.2 (1.3) | 10.5 (1.5) | 9.9 (1.1) |
| Children female, % | 50.0 | 45.5 | 54.5 |
| Children White non-Hispanic, % | 69.1 | 68.2 | 70.0 |
| Parent age, mean (SD) | 43.3 (10.3) | 44.5 (5.7) | 42.1 (4.6) |
| Parents female, % | 93.2 | 95.5 | 90.9 |
| Parents with college degree, % | 59.1 | 54.5 | 63.6 |
| Parents married/partnered, % | 70.5 | 77.3 | 63.6 |
Description of the ROC Intervention
The ROC intervention provided weekly treatment for 12 weeks, and biweekly for an additional two visits (total treatment duration = 4 months). The ROC program was provided in separate, but simultaneous, parent and child groups of 8–10 members for approximately 45 min, and both parents and children were given study-specific workbooks and handouts. The content of the groups was similar for children and parents, except that the child-specific materials were presented in the form of games and discussion in an age-appropriate manner. In addition, following the separate groups, parents and children participated in an experiential exercise together for an additional 30 min at each session.
If a parent–child pair missed an intervention group meeting, they were called by the group leader and the missed materials were mailed to the family. All the groups were led by doctoral-level psychologists and assisted by masters-level co-therapists and several undergraduate volunteers. All therapists attended a 1-day training regarding the treatments and attended weekly supervision with the first author.
ROC Core Components
Psychoeducation
Ten of the sessions (sessions 2–11) in ROC include a “Tricky Hunger,” which represents ways that the environment “tricks” the body into overeating past nutritional needs. The overall goal of psychoeducation was to increase parents’ and children’s awareness of the reasons why they may overeat and to relieve parents and children from guilt regarding overeating by helping them understand the processes by which these phenomena occur. The concepts were taught using a chronic disease model in which the child is considered to have the biological vulnerability to overeat that is amplified by the current obesogenic food environment. Participants were provided information about basic learning theory and how physiological responses to food cues develop and can be managed.
Parenting Skills
Parents were taught positive parenting skills, including the use of praise, motivation systems, daily meetings, self-monitoring, modeling, shaping behaviors, and logical consequences to assist their child in implementing the ROC program.
Coping Skills
Along with each tricky hunger, a coping skill was taught that was designed to fit within the session and to address that particular type of tricky hunger. Coping skills addressed changing the physical state of the body (e.g., deep breathing, relaxation), increasing behavioral alternatives to eating (e.g., behavioral activation, delay, problem-solving), changing the attentional focus (e.g., distraction, imagery, self-motivational statements), and enhancing motivation to resist cues (e.g., decision balance, cost–benefit analyses).
Self-Monitoring of Hunger
Parents and children were taught about hunger, satiety, and craving. Families were taught to monitor their hunger in a self-monitoring booklet on a 1–5 scale, with 1 = starving and 5 = stuffed. Parents and children were instructed to self-monitor hunger and satiety before, during, and after each meal, as well as 10 and 20 min after eating for a minimum of two meals/snacks per day.
Self-Monitoring of Craving
Later in the program (session 6), parents and children learned to assess and rate their cravings (defined as urges to eat when not physically hungry). Craving was monitored with a 5-point scale, 1 = not craving it at all and 5 =craving is explosive. Families were taught to rate any and all cravings they had each day (ideally one craving a day at minimum).
Experiential Learning
In each session, parents and children joined together to complete an experiential learning exercise. The first four visits included appetite awareness training, and parents and children brought a dinner meal to clinic and started off each session by eating dinner and monitoring their hunger with prompting from the interventionist. In visits 6–11, parents and children were taught cue exposure treatment, and they created an individualized hierarchy of highly craved foods, and completed an “exposure” at each session. During the exposure, parents and children rated their cravings while looking at the food, holding the food, smelling the food, after taking two small bites of the food, and then rated their cravings at 30-s intervals for the duration of the exposure. After the child and parent habituated to the craving (i.e., cravings were reduced to a 2 or lower on a 5-point scale), the families disposed of the food without eating it and the exposure ended. The last two sessions included both appetite awareness training and cue exposure treatment.
Description of Control Condition
Participants randomized to the control group did not have any intervention during the 4 months of treatment, and at the posttreatment assessment, were given a binder for the program including an at-home version of the curriculum with handouts with a brief 5-min orientation to the program. No other information was provided to the control group.
Procedure
Participants who completed an initial phone screen were scheduled for an on-site assessment meeting to provide informed consent and assess study eligibility. As part of the EAH assessment, children and parents ate an ad libitum dinner together (i.e., cheese pizza, applesauce, carrots, milk, juice, and water) and were encouraged to eat until satiated (see Measures section for further description). The child completed a survey for 10 min, and then completed the taste test and the free access EAH assessment without the parent present. Child participants also completed a survey and one 24-hr dietary recall in clinic. Two other dietary recalls were completed by phone during the subsequent 2 weeks. Parents completed their self-administered questionnaire by computer. Assessments were conducted at baseline, posttreatment, and 4-month follow-up.
Child Measures
Treatment Acceptability
At the posttreatment assessment visit, each child participant in the intervention group completed a treatment evaluation form that asked “How much did you like the ROC program?” Responses included 1 = did not like, 2 = liked a little, 3 = liked OK, 4 = liked it a lot, 5 = loved it. Children were also asked to respond how true the following statements were for them “Because of ROC, I feel more in control of my eating.” Responses included 1 = not true for me, 2 = sort of true for me, 3 = very true for me. Additionally, children responded to the following question with a yes or no answer “Do you think other kids your age would like the ROC program?”
EAH Free Access Paradigm
The assessment measure of EAH was initially described by Birch and colleagues (Birch & Fisher, 2000; Fisher & Birch, 2002) and has been used in the literature with children and adolescents (Fisher et al., 2007; Kral et al., 2010, 2012; Pieper & Laugero, 2013; Shomaker, et al., 2010a, 2010b). Each child participated in a standard ad libitum pizza dinner with their parents. Self-reported postmeal satiety was assessed with a cartoon representation of three levels of fullness (Faith et al., 2006) along with two questions regarding each child’s level of hunger (How hungry do you feel right now?) and fullness (How full do you feel right now?) via a 1–5 scale (1 = not at all and 5 = extremely). Ten minutes after the completion of the meal, each child tasted and rated palatability of small samples of 11 sweet and savory snack foods (popcorn, Cheez-its, Cheetos, potato chips, pretzels, Skittles, Hershey bars, chocolate chip cookies, Fig Newtons, jelly beans, and M&M’s) using cartoon illustrations of faces depicting “yummy,” “just ok,” and “yucky” (Faith et al., 2006). Following the rating of foods, the child was told that the coordinator had work in the adjacent room, and the child was left alone in a room with containers holding generous pre-weighed portions of the snack foods as well as toys and games. The child was told that s/he could eat whatever s/he wanted and/or play with games or books while waiting. After 10 min, the coordinator returned to the room and ended the free access session. The amounts of remaining food items were measured. The total calories consumed by each child was calculated from the amount consumed data, and this total was divided by child’s estimated daily calorie needs to derive the percent of calorie needs consumed during the free access period (EAH%). Daily calorie needs were estimated using age-specific formulas for calculating estimates of energy requirements according to weight, age, height, and physical activity level. A physical activity level of “low active” was considered for all children in this study to be conservative (National Academy of Sciences, 2005).
Child Usual Dietary Intake
Dietary intake of the child was assessed with three 24-hr dietary recalls at each assessment point on 3 nonconsecutive days. Studies have provided support for the use of this method of dietary assessment for youth (Collins, Watson, & Burrows, 2010; Lytle, Murray, Perry, & Eldridge, 1998). Dietary intake data for each child were collected and analyzed using Nutrition Data System for Research software version 2007. Utilizing the multiple-pass system of the Nutrition Data System for Research interview methodology, a trained interviewer conducted one 24-hr recall in-person at the assessment visit, along with two subsequent recalls over the phone within the following 2 weeks. During the in-person interview, children used both food models and a food amount booklet to help them estimate quantities of foods/drinks consumed; the booklet alone was used for the recalls conducted by phone. Parents were consulted to verify aspects of the food (e.g., butter or margarine, brand names). The three dietary recalls were averaged to generate the dietary intake variables associated with that assessment period. Average total daily caloric intake was used as a measure of outcome.
Anthropometry
Child height was measured using a standard stadiometer in duplicate. Children’s weight was measured in duplicate on a calibrated slide scale without jackets, outerwear, or shoes. The average of the two values was used for analysis. Children’s heights and weights were translated to BMI-for-age percentile scores using the Centers for Disease Control and Prevention growth charts (Kuczmarski et al., 2000) and to BMI-Z scores. Recent recommendations suggest that BMI-Z scores are useful for indexing adiposity at any one time, and that BMI is recommended for indexing change over time, so both are presented (Cole, Faith, Pietrobelli, & Heo, 2005). Percent overweight was derived by calculating the child's percent over the median BMI for age and sex ([child's BMI − median BMI for age and sex]/median BMI for age and sex × 100) using Centers for Disease Control and Prevention growth charts. For this study, we report BMI, BMI-Z, and percent overweight.
Parent Measures
Demographic Characteristics and Weight History (Baseline Only)
Each parent completed a demographic and weight history questionnaire.
Treatment Acceptability
At the posttreatment assessment visit, each parent participant in the intervention group completed a treatment evaluation form that asked “How much did you like the ROC program?” and “How much do you think your child liked the ROC program?” Responses included 1 = did not like, 2 = liked a little, 3 = liked OK, 4 = liked it a lot, 5 = loved it. Additionally, parents reported how much they agreed or disagreed with the following statement “The ROC program has taught my child to have more control of their eating.” Responses included 1 = strongly disagree, 2 = disagree, 3 = neither agree nor disagree, 4 = agree, 5 = strongly agree.
Eating in the Absence of Hunger Questionnaire Parent Report of Child
The Eating in the Absence of Hunger Questionnaire for Children and Adolescents–Parent Report of Child (EAH-PC; Shomaker et al., 2010a; Tanofsky-Kraff et al., 2008) includes three subscales: Negative Affect, External Eating, and Fatigue/Boredom Eating. Parents read a prompt stating, “Imagine that your child is eating a meal or snack at home, work, or at a restaurant. Imagine that your child eats enough of his/her meal so that he/she is no longer hungry.” Parents are then asked questions about whether their child would start or keep eating for various reasons, such as “because the food looks, tastes, or smells so good” or “because s/he is feeling sad or depressed.” Parents choose from the following responses: never, rarely, sometimes, often, or always. For this study, investigators also added an “I don’t know” response option for parents. In one study, EAH-PC scale demonstrated good internal reliability (α = .85–.94) and temporal stability (rs = .63–.70; ps < .01) over a mean of 10.5 weeks with youth 8–17 years of age (Shomaker et al., 2013). Furthermore, convergent validity is supported by associations between EAH-PC and overeating and disordered eating behaviors (Shomaker et al., 2010a).
Child Eating Behavior Questionnaire
Parents completed two scales from the Child Eating Behaviour Questionnaire (CEBQ; Wardle, Guthrie, Sanderson, & Rapoport, 2001) regarding their child’s eating patterns: Food Responsiveness and Satiety Responsiveness. The CEBQ was developed to assess a range of eating behavior traits in children and has been shown to have good internal consistency and adequate construct validity (Wardle et al., 2001). The food responsiveness scale reflects eating in response to environmental food cues. Satiety responsiveness represents the ability of a child to reduce food intake after eating to regulate energy intake. The CEBQ is internally valid (α = .72–.91) and has good test–retest reliability (Carnell & Wardle, 2007; Wardle et al., 2001).
Statistical Analysis
Treatment acceptability outcomes were assessed for the treatment group using descriptive statistics. Differences between treatment groups in demographic characteristics and baseline scores on study outcome measures were assessed using independent-samples t-tests for continuous variables and chi-squared tests for dichotomous variables. Linear regression models were conducted, with posttreatment and 4-month follow-up outcome scores regressed on treatment group, adjusted for baseline status on the outcome. This approach to investigating intervention effects was chosen because it accounts for any differences in study outcomes at baseline. Outcome variables were observed to have normal distributions. Follow-up t-tests were conducted to investigate within-group changes in outcome scores from baseline to posttreatment and from baseline to 4-month follow-up.
Results
Completion Rates
As can be seen in Figure 1, treatment completion rate was high for the ROC intervention. Of the 14 sessions, 63.6% (n = 14) attended 10–14 sessions, 22.7% (n = 5) attended 5–9 sessions, and 13.6% (n = 3) attended <5 sessions. One parent–child dyad did not attend the posttreatment assessment in the ROC arm, and one parent–child dyad did not attend the posttreatment assessment in the control condition. Twenty-two families were assigned to the control group, and 21 attended the posttreatment assessment and were given a binder with an at-home version of the program. At the 4-month follow-up assessment, families were queried as to whether they used any of the materials in the binder. Of the 21 families who received the binder, 42.8% (n = 9) acknowledged looking through the workbook, 19.0% (n = 4) reported trying any of the activities, and 38.1% (n = 8) reported not using the materials at all or were unknown.
Examination of characteristics of study noncompleters revealed that the one participant in the treatment group who did not complete the posttreatment assessment was a boy whose age was approximately 2 standard deviations (SDs) above the sample mean and whose BMI was approximately 3 SDs above the sample mean. The one participant in the control group who did not complete posttreatment assessment was a boy with an age and a BMI within 1 SD of the sample mean. These two participants were excluded from the study analyses. The two participants who did not complete the 4-month follow-up were in the control group; one was a boy, the other a girl; both had similar characteristics as the overall sample and both showed little-to-no change in BMI from baseline to posttreatment.
Treatment Acceptability
Half of the children in the ROC intervention reported liking the program a lot or loving it (vs. liked okay, liked a little, or did not like), 62% believed the treatment helped them control their eating (very true vs. sort of true or not true), and 85% believed other kids would like the ROC program (yes vs. no). Sixty-seven percent of parents reported liking the program a lot or loving it, 47% believed that their child liked the program a lot or loved it, and 81% believed the program taught their child to be more in control of his or her eating.
Treatment Outcomes
Participant demographic characteristics did not differ between intervention groups (ps = .141–.899; Table I). T-tests of baseline scores on study outcomes revealed no between-group differences: child BMI t(40) = −0.56, p = .579; child BMI z-score t(40) = −0.31, p = .758; EAH task t(40) = 0.74, p = .464; mean calories consumed per day t(40) = −0.37, p = .717; external eating t(38) = −1.04, p = .303; negative affect eating t(28) = −0.31, p = .760; fatigue/boredom eating t(30) = 0.60, p = .553; food responsiveness t(40) = −0.52, p = .608; and satiety responsiveness t(40) = −0.90, p = .372.
Between-group effects were found at posttreatment for food responsiveness (p = .010) and at 4-month follow-up for the EAH task (p = .033; Table II). We found marginally significant between-group effects for external eating (p = .077) and BMI, BMI z-score, and percent overweight (ps = .101–.125) at posttreatment.
Table II.
Observed Means of Child Outcomes at Each Time Point and Intervention Effects (n = 44)
| Observed mean (SD) |
Group by time interaction p value |
||||
|---|---|---|---|---|---|
| Outcome | Baseline | Posttreatment | 4-month follow-up | Posttreatment | 4-month follow-up |
| Obesity | |||||
| BMI | |||||
| Intervention | 28.0 (5.0) | 27.0 (4.2) | 27.9 (4.4) | .140 | .229 |
| Control | 26.5 (4.5) | 26.8 (4.8) | 27.1 (4.8) | ||
| BMI z-score | |||||
| Intervention | 2.13 (0.40) | 2.00 (0.48) | 2.03 (0.45) | .153 | .158 |
| Control | 2.06 (0.40) | 2.02 (0.43) | 2.01 (0.41) | ||
| Percent overweight | |||||
| Intervention | 63.32 (26.43) | 56.32 (22.40) | 59.16 (22.54) | .136 | .166 |
| Control | 58.48 (25.31) | 58.07 (26.88) | 58.28 (27.35) | ||
| EAH taska | |||||
| Percent of daily calories consumed during free access | |||||
| Intervention | 15.8 (5.0) | 13.2 (7.5) | 15.2 (4.9) | .863 | .326 |
| Control | 18.2 (8.0) | 15.0 (9.1) | 20.4 (8.3) | ||
| Diet | |||||
| Kcal per day | |||||
| Intervention | 2067 (704) | 1848 (634) | 1705 (515) | .833 | .420 |
| Control | 1972 (434) | 1805 (525) | 1740 (436) | ||
| EAH scale (parent report of child) | |||||
| External eating | |||||
| Intervention | 15.29 (1.62) | 12.85 (2.46) | 13.35 (2.41) | .045 | .804 |
| Control | 14.71 (2.47) | 14.37 (4.00) | 12.61 (2.87) | ||
| Negative affect eating | |||||
| Intervention | 12.72 (3.38) | 11.21 (3.58) | 10.84 (4.09) | .080 | .650 |
| Control | 12.00 (4.12) | 14.31 (8.77) | 9.33 (4.23) | ||
| Fatigue/boredom eating | |||||
| Intervention | 10.61 (2.12) | 9.11 (2.08) | 9.11 (2.18) | .713 | .336 |
| Control | 11.20 (3.51) | 10.79 (5.01) | 9.18 (3.09) | ||
| CEBQ scale (parent report of child) | |||||
| Food responsiveness | |||||
| Intervention | 3.94 (0.58) | 3.33 (0.77) | 3.35 (0.76) | .009 | .374 |
| Control | 3.81 (0.73) | 3.65 (0.83) | 3.40 (0.95) | ||
| Satiety responsiveness | |||||
| Intervention | 2.06 (0.53) | 2.29 (0.51) | 2.32 (0.51) | .564 | .448 |
| Control | 1.95 (0.63) | 2.07 (0.63) | 2.27 (0.66) | ||
Note. EAH = eating in the absence of hunger; CEBQ = Child Eating Behaviour Questionnaire.
aAdjusted for percent of daily calories consumed at ad libitum dinner.
Follow-up within-group t-tests revealed that the ROC group showed significant improvements at posttreatment and 4-month follow-up on BMI-Z, external eating, negative affect eating, fatigue/boredom eating, food responsiveness, and satiety responsiveness (all p < .05). ROC participants also showed significant improvements from baseline to 4-month follow-up on mean calories consumed per day (p < .05). Additionally, the control group showed significant improvements at posttreatment on fatigue/boredom eating (p < .05). Control participants also showed significant improvements from baseline to 4-month follow-up on BMI, mean calories consumed per day, external eating, fatigue/boredom eating, food responsiveness, and satiety responsiveness (all p < .05).
Discussion
We present a new intervention for the treatment of childhood overeating based on Schachter’s externality theory. The ROC program includes both appetite awareness training and cue exposure treatment, as well as parenting, self-monitoring, and coping skills. Overall, the intervention was well tolerated and had moderate to high acceptability ratings from both parents and children. Most importantly, parents believed that the program allowed their children to be more in control of their eating. Moreover, we evaluated the intervention effects on EAH in children, child weight outcomes, and reported overeating. There were significant improvements for the ROC compared with the waitlist control group at posttreatment on preoccupation with food (Food Responsiveness subscale) and a trend toward a decrease in weight status (BMI, BMI-Z, and percent overweight), and in eating in response to external cues (External Eating). At 4 months posttreatment, there were significant improvements for the ROC compared with waitlist control on the amount they ate in the EAH paradigm.
We developed this treatment specifically for children who overeat when they are not physically hungry (i.e., have high EAH). We chose this specific behavioral phenotype because we hypothesized that these children might not respond to traditional FBT, where parents are taught to work with their child on reducing caloric consumption and increasing physical activity, but it does not directly target mastery of cravings related to available and omnipresent palatable food stimuli or does not seek to enhance internal satiety responsiveness. Children with high EAH may not be as sensitive to experiences of fullness or satiety; thus, restricting their caloric intake in traditional treatment programs may be especially challenging for these children to perform and challenging for their parents to consistently implement. Schachter’s externality theory offers a framework to develop treatments for overeating in children, and our preliminary treatment data suggest that targeting enhanced awareness to internal responses to external cues can affect eating behaviors. The acceptability ratings from our study were high, suggesting that this treatment is a good fit for overweight children with high EAH. While we used a cut-off score to identify children for enrollment in the current study, EAH is a continuous variable that has been consistently associated with overweight more generally. Thus, while our intervention targeted the extreme tails of this distribution, it is reasonable to evaluate the future efficacy of this intervention with more general samples of obese children.
The theory underpinning the intervention was relatively easy for both children and parents to understand. We were able to integrate the concepts of appetite awareness training with cue exposure treatment relatively easily, explaining a model whereby the children’s bodies and minds may overreact to food stimuli and may under-react to sensations of fullness. Based on our previous work (Boutelle et al., 2011, p. 468), we found that parent–child dyads had difficulty differentiating the sensation of craving from that of hunger. Providing appetite awareness training before offering cue exposure treatment provided parents and children with a base of knowledge about hunger and satiety cues and practice with regulating eating based on appetite and thus addressed the “under-reaction” part of the model. Cue exposure treatment was introduced as one way of managing the “over-reaction” part of the model. Thus, training was focused on helping the parents and children to resist completely consuming hedonic foods placed directly in front of them. We practiced resisting highly craved foods in our clinic such that these skills could be used in the real world when needed. Because this was new learning that competed with, but did not replace, former cue–food consumption associations (Bouton, 2011), dyads were warned that they would continue to experience cravings in the real world—they would just feel more competent in resisting them.
There are several possible mechanisms of interventions effects. We developed CET-Food using the conceptual framework of Pavlovian conditioning. We initially conceptualized the CET-Food treatment as extinction of the relationship between highly enticing food and craving cues (i.e., urges to eating something when not physically full). We emphasized to parent–child dyads the importance of learning to break this relationship between the cue and overeating to extinguish eating in response to food stimuli and other triggering cues. However, another interpretation of the mechanism of CET-Food is that instead of extinguishing the relationship between food and overeating, we are increasing the strength of inhibitory mechanisms (Craske et al., 2008). Recent studies suggest that overweight and obese children have deficits in inhibitory responding (Nederkoorn, Braet, Van Eijs, Tanghe, & Jansen, 2006; Nederkoorn, Coelho, Guerrieri, Houben, & Jansen, 2012; Pauli-Pott, Albayrak, Hebebrand, & Pott, 2010). Because we did not assess inhibition in the current study, future studies should assess inhibitory strength as an additional outcome and potential mechanism of EAH.
It is important to note that this program differs from FBT in several distinct and important ways, including format, dietary prescription, and inclusion of instructions to increase physical activity. FBT includes parent and child in separate but concurrent groups combined with individual parent–child behavioral coaching. Thus, from a pure dose perspective, FBT is more intensive than the ROC intervention. FBT provides a specific dietary prescription, and both parents and children self-monitor dietary intake and caloric intake and work toward decreasing caloric intake using this nutrition program (Epstein, Myers, Raynor, & Saelens, 1998; Epstein, Roemmich, & Raynor, 2001). In this model, children are taught to avoid or decrease consumption of “red foods” (i.e., foods with high caloric density). Additionally, FBT focuses on increasing energy expenditure via shaping increases in physical activity and decreases in sedentary behavior via positive reinforcement. The ROC program does not prescribe any diet or any changes in physical activity. ROC is based on altering conditioning of internal cues (hunger, satiety, or craving) and behavioral eating responses. ROC targets changes in adaptive associations that could potentially lead to decreased caloric intake, and potentially improved weight management in the future.
The trend toward significant differences between groups on the weight outcomes in this study was not surprising, considering the small sample size. However, improvements in the control group were unexpected. The mean scores on the BMI-Z, percentage overweight, EAH, nutritional intake variables, and many of the questionnaire scales decreased in the control group as well as in the ROC group, which made it unlikely to find intervention effects. In previous studies reporting on control groups of overweight and obese children (Boutelle, Norman, Rock, Rhee, & Crow, 2013; Epstein, Wing, Koeske, & Valoski, 1984; Flodmark, Ohlsson, Ryden, & Sveger, 1993; Owens et al., 1999; Robinson et al., 2003), control groups typically gain weight throughout the assessment period, which was not the case in this study. There are a few possibilities to explain the control group’s responses on outcomes, including (1) that the control group in this study was an anomaly, (2) that families with overweight children with high EAH respond to demand characteristics of enrolling in a randomized clinical trial, (3) that weight and eating variability in overweight children with high EAH is greater than in studies with overweight children in general, or (4) that the binder with study materials influenced parents and children in the control group. Finally, pilot studies such as this one should not be used to estimate effect sizes, as because of the size of the studies, the estimates are considered unstable (Kraemer et al., 2003; Kraemer & Kupfer, 2006). Studies with larger sample sizes are needed to evaluate these hypotheses further.
Responses to ROC were comparable with other pediatric obesity interventions in several respects. Of note, the children in ROC decreased their BMI by .3 BMI points and their BMI-Z by .10. This reduction is similar to some programs studying 4–6 months of FBT (Foster et al., 2012; Janicke et al., 2008) and lower than others (Boutelle, Cafri, & Crow, 2011; Epstein, Paluch, Roemmich, & Beecher, 2007; Wilfley et al., 2007). Critically, weight change occurred despite the fact that the ROC program did not prescribe a diet or physical activity, both of which are major components of FBT. However, the rebound effects of the weight variables in ROC suggest, like FBT, that continued contact may be needed with families and/or that the ROC program may need to be paired with additional components, such as those focusing on physical activity.
A number of strengths and weaknesses need to be considered in interpreting the results of this study. As noted earlier, the improvements made by the control group were unexpected and limited our ability to evaluate intervention efficacy. The small sample size (N = 44) and large SDs in outcome measures also limited our ability to detect intervention effects. Based on our limited sample size and its potential impact on statistical power, the effects should be interpreted with caution (Kraemer, Mintz, Noda, Tinklenberg, & Yesavage, 2006). In addition, the ROC intervention was only 4 months long, and the protocol, specifically the CET-Food protocol, is relatively new and may not be optimized, which may have contributed to lower effects. Our EAH measure, using the free access paradigm, the 24-hr recalls, and the surveys, could be susceptible to a social desirability bias or a response bias. It is also possible that children who have high EAH may be more challenging to treat, as they are typically screened out of current obesity treatment studies. The strengths of this study include the use of a conceptually derived novel treatment for children who overeat, based on theoretical models of causal and maintenance mechanisms of overeating. Additionally, we used standardized assessment measures, and were able to follow the children for 4 months after completing treatment. This study is also novel in that it targeted a specific behavioral phenotype of overweight and obese children, those who eat in the absence of hunger. Considering these strengths and weaknesses, the interventions tested in this study may serve as the basis for future research examining interventions that target appetite and food cue reactivity in overweight and obese children or adults.
Our main purpose for conducting this study was to evaluate the feasibility and acceptability of these treatments, and to discern whether the model would be accepted by parent–child dyads. Based on their evaluation and the preliminary efficacy data reported in this article, we are positioned to revise our treatment model and intervention strategy as needed. For example, given the preliminary nature of this investigation, future, larger studies should further evaluate the efficacy of both the ROC treatment and the underlying model in overweight and obese children. This pilot study suggests that the ROC intervention is promising, but that more research including larger randomized controlled trials is needed to determine both efficacy and mechanisms of treatment effects.
Funding
University of Minnesota, Faculty Development Grant to K.B. and L.H., R01DK094475 and K02HL112042 (K.B.). Clinicaltrials.gov identifier (NCT01442142).
Conflicts of interest: None declared.
References
- Birch L L, Fisher J O. Mothers' child-feeding practices influence daughters' eating and weight. American Journal of Clinical Nutrition. 2000;71:1054–1061. doi: 10.1093/ajcn/71.5.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom T, Sharpe L, Mullan B, Zucker N. A pilot evaluation of appetite-awareness training in the treatment of childhood overweight and obesity: A preliminary investigation. The International Journal of Eating Disorder. 2013;46:47–51. doi: 10.1002/eat.22041. doi:10.1002/eat.22041. [DOI] [PubMed] [Google Scholar]
- Boutelle K N, Cafri G, Crow S J. Parent-only treatment for childhood obesity: A randomized controlled trial. Obesity. 2011;19:574–580. doi: 10.1038/oby.2010.238. doi:oby2010238 [pii]10.1038/oby.2010.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutelle K N, Norman G N, Rock C L, Rhee K E, Crow S J. Guided self-help for the treatment of pediatric obesity. Pediatrics. 2013;131:e1435–e1442. doi: 10.1542/peds.2012-2204. doi:10.1542/peds.2013-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutelle K N, Zucker N L, Peterson C B, Rydell S A, Cafri G, Harnack L. Two novel treatments to reduce overeating in overweight children: A randomized controlled trial. Journal of Consulting and Clinical Psychology. 2011;79:759–771. doi: 10.1037/a0025713. doi:10.1037/a0025713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton M E. Learning and the persistence of appetite: Extinction and the motivation to eat and overeat. Physiology and Behaviour. 2011;103:51–58. doi: 10.1016/j.physbeh.2010.11.025. doi:S0031-9384(10)00425-7 [pii]10.1016/j.physbeh.2010.11.025. [DOI] [PubMed] [Google Scholar]
- Carnell S, Wardle J. Measuring behavioural susceptibility to obesity: Validation of the Child Eating Behaviour Questionnaire. Appetite. 2007;48:104–113. doi: 10.1016/j.appet.2006.07.075. doi:S0195-6663(06)00503-4 [pii]10.1016/j.appet.2006.07.075. [DOI] [PubMed] [Google Scholar]
- Cole T J, Faith M S, Pietrobelli A, Heo M. What is the best measure of adiposity change in growing children: BMI, BMI %, BMI z-score or BMI centile? European Journal of Clinical Nutrition. 2005;59:419–425. doi: 10.1038/sj.ejcn.1602090. doi:1602090 [pii]10.1038/sj.ejcn.1602090. [DOI] [PubMed] [Google Scholar]
- Collins C E, Watson J, Burrows T. Measuring dietary intake in children and adolescents in the context of overweight and obesity. International Journal of Obesity. 2010;34:1103–1115. doi: 10.1038/ijo.2009.241. doi:ijo2009241 [pii]10.1038/ijo.2009.241. [DOI] [PubMed] [Google Scholar]
- Craighead L W, Allen H N. Appetite awareness training—A cognitive-behavioral intervention for binge-eating. Cognitive and Behavioral Practice. 1995;2:249–270. [Google Scholar]
- Craske M G, Kircanski K, Zelikowsky M, Mystkowski J, Chowdhury N, Baker A. Optimizing inhibitory learning during exposure therapy. Behaviour Research and Therapy. 2008;46:5–27. doi: 10.1016/j.brat.2007.10.003. doi:S0005-7967(07)00205-7 [pii]10.1016/j.brat.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Epstein L H. Family-based behavioural intervention for obese children. Research Support, U.S. Gov't, P.H.S. International Journal of Obesity and Related Metabolic Disorders. 1996;20(Suppl. 1):S14–S21. [PubMed] [Google Scholar]
- Epstein L H, Myers M D, Raynor H A, Saelens B E. Treatment of pediatric obesity. Pediatrics. 1998;101(3 Pt. 2):554–570. [PubMed] [Google Scholar]
- Epstein L H, Paluch R A, Roemmich J N, Beecher M D. Family-based obesity treatment, then and now: Twenty-five years of pediatric obesity treatment. Health Psychology. 2007;26:381–391. doi: 10.1037/0278-6133.26.4.381. doi:2007-09406-001 [pii]10.1037/0278-6133.26.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein L H, Roemmich J N, Raynor H A. Behavioral therapy in the treatment of pediatric obesity. Pediatrics Clinics of North America. 2001;48:981–993. doi: 10.1016/s0031-3955(05)70352-7. [DOI] [PubMed] [Google Scholar]
- Epstein L H, Valoski A, Wing R R, McCurley J. Ten-year follow-up of behavioral, family-based treatment for obese children. Journal of the American Medical Association. 1990;264:2519–2523. [PubMed] [Google Scholar]
- Epstein L H, Wing R R, Koeske R, Valoski A. Effects of diet plus exercise on weight change in parents and children. Journal of Consulting and Clinical Psychology. 1984;52:429–437. doi: 10.1037//0022-006x.52.3.429. [DOI] [PubMed] [Google Scholar]
- Faith M S, Berkowitz R I, Stallings V A, Kerns J, Storey M, Stunkard A J. Eating in the absence of hunger: A genetic marker for childhood obesity in prepubertal boys? Obesity. 2006;14:131–138. doi: 10.1038/oby.2006.16. [DOI] [PubMed] [Google Scholar]
- Fisher J O, Birch L L. Eating in the absence of hunger and overweight in girls from 5 to 7 y of age. American Journal of Clinical Nutrition. 2002;76:226–231. doi: 10.1093/ajcn/76.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J O, Cai G, Jaramillo S J, Cole S A, Comuzzie A G, Butte N F. Heritability of hyperphagic eating behavior and appetite-related hormones among Hispanic children. Obesity. 2007;15:1484–1495. doi: 10.1038/oby.2007.177. doi:10.1038/oby.2007.177. [DOI] [PubMed] [Google Scholar]
- Flodmark C E, Ohlsson T, Ryden O, Sveger T. Prevention of progression to severe obesity in a group of obese schoolchildren treated with family therapy. Pediatrics. 1993;91:880–884. [PubMed] [Google Scholar]
- Foster G D, Sundal D, McDermott C, Jelalian E, Lent M R, Vojta D. Feasibility and preliminary outcomes of a scalable, community-based treatment of childhood obesity. Pediatrics. 2012;130:652–659. doi: 10.1542/peds.2012-0344. doi:10.1542/peds.2012-0344. [DOI] [PubMed] [Google Scholar]
- Janicke D M, Sallinen B J, Perri M G, Lutes L D, Huerta M, Silverstein J H, Brumback B. Comparison of parent-only vs family-based interventions for overweight children in underserved rural settings: Outcomes from project STORY. Archives of Pediatrics and Adolescent Medicine. 2008;162:1119–1125. doi: 10.1001/archpedi.162.12.1119. doi:162/12/1119 [pii]10.1001/archpedi.162.12.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen A. The learned nature of binge eating. In: Legg C, Booth DA, editors. Appetite, neural and behavioral bases. Oxford: Oxford University Press; 1994. pp. 193–211. [Google Scholar]
- Jansen A. A learning model of binge eating: Cue reactivity and cue exposure. Behaviour Research and Therapy. 1998;36:257–272. doi: 10.1016/s0005-7967(98)00055-2. [DOI] [PubMed] [Google Scholar]
- Kraemer H C, Kupfer D J. Size of treatment effects and their importance to clinical research and practice. Biological Psychiatry. 2006;59:990–996. doi: 10.1016/j.biopsych.2005.09.014. doi:10.1016/j.biopsych.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Kraemer H C, Mintz J, Noda A, Tinklenberg J, Yesavage J A. Caution regarding the use of pilot studies to guide power calculations for study proposals. Archives of General Psychiatry. 2006;63:484–489. doi: 10.1001/archpsyc.63.5.484. doi:10.1001/archpsyc.63.5.484. [DOI] [PubMed] [Google Scholar]
- Kraemer H C, Morgan G A, Leech N L, Gliner J A, Vaske J J, Harmon R J. Measures of clinical significance. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42:1524–1529. doi: 10.1097/00004583-200312000-00022. [DOI] [PubMed] [Google Scholar]
- Kral T V, Allison D B, Birch L L, Stallings V A, Moore R H, Faith M S. Caloric compensation and eating in the absence of hunger in 5- to 12-y-old weight-discordant siblings. American Journal of Clinical Nutrition. 2012;96:574–583. doi: 10.3945/ajcn.112.037952. doi:10.3945/ajcn.112.037952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral T V, Moore R H, Stunkard A J, Berkowitz R I, Stettler N, Stallings V A, Faith M S. Adolescent eating in the absence of hunger and relation to discretionary calorie allowance. Journal of American Dietetic Association. 2010;110:1896–1900. doi: 10.1016/j.jada.2010.09.009. doi:10.1016/j.jada.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczmarski R J, Ogden C L, Grummer-Strawn L M, Flegal K M, Guo S S, Wei R, Johnson C L. CDC growth charts: United States. Advance Data. 2000;314:1–27. [PubMed] [Google Scholar]
- Lytle LA, Murray DM, Perry CL, Eldridge AL. Validating fourth-grade students' self-report of dietary intake: Results from the 5 A day power plus program. Journal of American Dietetic Association. 1998;98:570–572. doi: 10.1016/S0002-8223(98)00127-8. doi:S0002-8223(98)00127-8 [pii] [DOI] [PubMed] [Google Scholar]
- National Academy of Sciences. Institute of medicine, food and nutrition board. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients) 2005 Retrieved from http://fnic.nal.usda.gov/nal_dispaly/index.php? [Google Scholar]
- Nederkoorn C, Braet C, Van Eijs Y, Tanghe A, Jansen A. Why obese children cannot resist food: The role of impulsivity. Eating Behaviours. 2006;7:315–322. doi: 10.1016/j.eatbeh.2005.11.005. doi:10.1016/j.eatbeh.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Nederkoorn C, Coelho J S, Guerrieri R, Houben K, Jansen A. Specificity of the failure to inhibit responses in overweight children. Appetite. 2012;59:409–413. doi: 10.1016/j.appet.2012.05.028. doi:10.1016/j.appet.2012.05.028. [DOI] [PubMed] [Google Scholar]
- Ogden C L, Carroll M D, Kit B K, Flegal K M. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA. 2012;307:483–490. doi: 10.1001/jama.2012.40. doi:10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens S, Gutin B, Allison J, Riggs S, Ferguson M, Litaker M, Thompson W. Effect of physical training on total and visceral fat in obese children. Medicine and Science in Sports and Exercise. 1999;31:143–148. doi: 10.1097/00005768-199901000-00022. [DOI] [PubMed] [Google Scholar]
- Pauli-Pott U, Albayrak O, Hebebrand J, Pott W. Association between inhibitory control capacity and body weight in overweight and obese children and adolescents: Dependence on age and inhibitory control component. Child Neuropsychology. 2010;16:592–603. doi: 10.1080/09297049.2010.485980. doi:10.1080/09297049.2010.485980. [DOI] [PubMed] [Google Scholar]
- Pieper J R, Laugero K D. Preschool children with lower executive function may be more vulnerable to emotional-based eating in the absence of hunger. Appetite. 2013;62:103–109. doi: 10.1016/j.appet.2012.11.020. doi:10.1016/j.appet.2012.11.020. [DOI] [PubMed] [Google Scholar]
- Robinson T N, Killen J D, Kraemer H C, Wilson D M, Matheson D M, Haskell W L, Varady A. Dance and reducing television viewing to prevent weight gain in African-American girls: The Stanford GEMS pilot study. Ethnicity and Disease. 2003;13(Suppl. 1):S65–S77. [PubMed] [Google Scholar]
- Schachter S. Some extraordinary facts about obese humans and rats. American Psychologist. 1971;26:129–144. doi: 10.1037/h0030817. [DOI] [PubMed] [Google Scholar]
- Schachter S, Rodin J. Obese humans and rats. Washington, DC: Erlbaum/Halsted; 1974. [Google Scholar]
- Shomaker L B, Tanofsky-Kraff M, Elliott C, Wolkoff L E, Columbo K M, Ranzenhofer L M, Yanovski J A. Salience of loss of control for pediatric binge episodes: Does size really matter? Internayional Journal of Eating Disorder. 2010a;43:707–716. doi: 10.1002/eat.20767. doi:10.1002/eat.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomaker L B, Tanofsky-Kraff M, Mooreville M, Reina S A, Courville A B, Field S E, Yanovski J A. Links of adolescent- and parent-reported eating in the absence of hunger with observed eating in the absence of hunger. Obesity. 2013;21:1243–1250. doi: 10.1002/oby.20218. doi:10.1002/oby.20218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomaker L B, Tanofsky-Kraff M, Zocca J M, Courville A, Kozlosky M, Columbo K M, Yanovski J A. Eating in the absence of hunger in adolescents: Intake after a large-array meal compared with that after a standardized meal. American Journal of Clinical Nutrition. 2010b;92:697–703. doi: 10.3945/ajcn.2010.29812. doi:10.3945/ajcn.2010.29812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Ranzenhofer L M, Yanovski S Z, Schvey N A, Faith M, Gustafson J, Yanovski J A. Psychometric properties of a new questionnaire to assess eating in the absence of hunger in children and adolescents. Appetite. 2008;51:148–155. doi: 10.1016/j.appet.2008.01.001. doi:S0195-6663(08)00072-X [pii]10.1016/j.appet.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle J. Conditioning processes and cue exposure in the modification of excessive eating. Addictive Behaviours. 1990;15:387–393. doi: 10.1016/0306-4603(90)90047-2. [DOI] [PubMed] [Google Scholar]
- Wardle J, Guthrie C A, Sanderson S, Rapoport L. Development of the Children's Eating Behaviour Questionnaire. Journal of Child Psychology and Psychiatry. 2001;42:963–970. doi: 10.1111/1469-7610.00792. [DOI] [PubMed] [Google Scholar]
- Wilfley D E, Stein R I, Saelens B E, Mockus D S, Matt G E, Hayden-Wade H A, Epstein L H. Efficacy of maintenance treatment approaches for childhood overweight: A randomized controlled trial. JAMA. 2007;298:1661–1673. doi: 10.1001/jama.298.14.1661. doi:298/14/1661 [pii]10.1001/jama.298.14.1661. [DOI] [PubMed] [Google Scholar]