Abstract
Background. Plasmodium falciparum placental infection primes the fetal immune system and alters infant immunity. Mechanisms leading to these outcomes are not completely understood. We focused on Vγ2Vδ2 cells, which are part of the immune response against many pathogens, including P. falciparum. These unconventional lymphocytes respond directly to small, nonpeptidic antigens, independent of major histocompatibility complex presentation. We wondered whether placental malaria, which may increase fetal exposure to P. falciparum metabolites, triggers a response by neonatal Vγ2Vδ2 lymphocytes that can be a marker for the extent of fetal exposure to malarial antigens.
Methods. Cord blood mononuclear cells were collected from 15 neonates born to mothers with P. falciparum infection during pregnancy (8 with placental malaria) and 25 unexposed neonates. Vγ2Vδ2 cell phenotype, repertoire, and proliferative responses were compared between newborns exposed and those unexposed to P. falciparum.
Results. Placental malaria–exposed neonates had increased proportions of central memory Vγ2Vδ2 cells in cord blood, with an altered Vγ2 chain repertoire ex vivo and after stimulation.
Conclusion. Our results suggest that placental malaria affects the phenotype and repertoire of neonatal Vγ2Vδ2 lymphocytes. Placental malaria may lower the capacity for subsequent Vγ2Vδ2 cell responses and impair the natural resistance to infectious diseases or the response to pediatric vaccination.
Keywords: gammadelta, neonatal, cord blood, Plasmodium, placental malaria, repertoire, innate, phosphoantigen, aminobisphosphonate
Prenatal exposure to microbial agents (eg, Plasmodium falciparum, human immunodeficiency virus [HIV], and helminthes) primes fetal immunity [1], often impairing responses to pediatric vaccines [2–4] and resistance to infections among infants [5–7]. Fetal immune priming also influences immunity to unrelated pathogens [3, 4, 8], suggesting that broadly reactive cell subsets, including γδ T cells, are affected by fetal exposure to maternal infectious diseases.
Vγ2Vδ2 T cells, a subset of γδ lymphocytes (also known as Vγ9Vδ2 cells), respond to a broad array of infectious agents, including P. falciparum and Mycobacterium tuberculosis. Vγ2Vδ2 cell cross-reactivity reflects their major histocompatibility complex (MHC)–unrestricted recognition [9] of small precursors of isoprenoid biosynthesis, collectively named phosphoantigens (PAg) [10, 11]. All eukaryotic cells and many microorganisms produce similar PAg, and all individuals respond to them regardless of MHC haplotype. Vγ2Vδ2 lymphocytes are also triggered by aminobisphosphonates, which block isoprenoid biosynthesis and cause accumulation of PAg to stimulatory concentrations [12]. Aminobisphosphonates are potent stimulators of adult and neonatal Vγ2Vδ2 lymphocytes [13–15].
Activated Vγ2Vδ2 cells produce T-helper type 1 cytokines [16, 17], promote dendritic cell maturation [18, 19], mediate antibody-dependent cellular cytotoxicity [20, 21], and increase natural killer (NK) cell cytotoxicity [22]. Rapid Vγ2Vδ2 lymphocyte responses to infection, which can be sustained by cytokines of myeloid origin, such as interleukin 23 [15] or interleukin 15 (IL-15) [23], may be critical for disease resistance in infants in whom CD4+ T cells have not fully matured. Neonatal Vγ2Vδ2 lymphocytes are a significant component of immune responses to the tuberculosis vaccine, BCG [13, 24, 25], that is administered routinely to neonates in sub-Saharan Africa, and damage to this cell subset is likely to impair responses to the vaccine.
Several studies showed that Vγ2Vδ2 lymphocytes react to Plasmodium infection. Vγ2Vδ2 cells proliferate in vitro [26] in response to Plasmodium PAg [27], and they inhibit replication of the blood-stage parasite [28–30]. Episodes of malaria in adults with no previous exposure are characterized by decreases in the PAg-specific Vγ2Vδ2 lymphocyte count during paroxysm [31], followed by a sustained expansion during convalescence [32, 33]. Vγ2Vδ2 cells may be most important in early immune responses, during which they express the proinflammatory cytokines interferon γ (IFN-γ) and tumor necrosis factor α (TNF-a) shortly after exposure to P. falciparum–infected erythrocytes [34, 35], independent of simultaneous CD4+ T-cell activation [34].
Since the first P. falciparum infection perturbs Vγ2Vδ2 cells in malaria-naive adults, we tested whether prenatal exposure to P. falciparum similarly affects neonatal Vγ2Vδ2 lymphocytes. Damage to neonatal Vγ2Vδ2 cells might decrease infant immunity to malaria and modulate early immune responses to BCG. In a previous study, we compared the Vγ2 repertoire in cord blood specimens from Italy and Nigeria. Nigerian samples had lower levels of PAg-reactive Vγ2 chains [36]; we hypothesized that environmental exposure (including via maternal P. falciparum infection during pregnancy) might contribute to these differences. The current study compares cord blood samples from neonates born to mothers with or without malaria at delivery and relies on T-cell receptor (TCR) repertoire analysis to detail the composition of cord blood (fetal) Vγ2Vδ2 cell populations.
METHODS
Sample Collection and Cord Blood Mononuclear Cell (CBMC) Isolation
Women were enrolled and provided written informed consent in the maternity division of Hôpital Central de Yaoundé before onset of active labor. The study was approved by the Ethical Committee of the Centre International de Référence Chantal Biya, Yaoundé, and by the Division for Health Operations Research in Cameroon.
Maternal blood (5–8 mL), cord blood, and small fragments of placenta were collected after delivery. Thick blood smears (for maternal blood and cord blood) and impression smears (for placenta) were stained with Giemsa, and parasites were counted against 200 leukocytes. Two technicians in independent laboratories analyzed the smears. Rapid diagnostic test– or polymerase chain reaction (PCR)–based screenings were not performed, because of logistic reasons. Mothers were undergoing intermittent preventive treatment with sulfadoxine-pyrimethamine during pregnancy, but the number of doses and timing of treatment varied. Maternal HIV status was known at enrollment (all HIV-positive women were enrolled in the Cameroonian Prevention of Mother to Child Transmission Program) and was confirmed by rapid test (Determine, Abbot).
Cord blood (20–30 mL) was collected soon after uncomplicated, full-term deliveries, using a sterile syringe and transferring the blood quickly into 50-mL collection tubes with anticoagulant. Cord blood was diluted with Roswell Park Memorial Institute (RPMI) 1640 medium and layered over a Ficoll-hypaque density gradient to purify CBMCs. A fraction of CBMCs (8 × 106) was reserved for cell culture, and 0.5 × 107–1 × 107 were lysed for RNA extraction; remaining cells were frozen at a concentration of 1 × 107 CBMCs/mL in 90% fetal bovine serum and 10% dimethyl sulfoxide freezing medium.
Cell Culture
Vγ2Vδ2 lymphocytes were expanded in vitro for 16 unexposed and all 15 P. falciparum-exposed neonates. Freshly isolated CBMCs were resuspended at 106 cells/mL in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS; Gibco, Life Technologies), 2 mM L-glutamine, and 100 IU/mL penicillin-streptomycin (Lonza). To expand Vγ2Vδ2 lymphocytes, cultures were treated with alendronate sodium trihydrate (Sigma) at 5 μM, in the presence of 100 IU/mL of human recombinant interleukin 2 (IL-2; Tecin, NIH reagent program) or 10 ng/mL of human recombinant IL-15 (Thermo Scientific). Medium with IL-2 or IL-15 and without alendronate was the control condition. Cells were cultured for 14 days at 37°C with 5% CO2 as described elsewhere [23]. A fraction of the lymphocytes was used to determine Vδ2 cell frequency and phenotype, and 5 × 106 cells were lysed for RNA extraction and stored as cell lysates at −20°C.
Flow Cytometry
Ex vivo CBMCs or expanded Vδ2 lymphocytes were resuspended in phosphate-buffered saline (PBS) and 10% FBS and were stained at 4°C with directly conjugated monoclonal antibodies. After 15 minutes, cells were washed with PBS and 10% FBS and were resuspended in PBS and 10% FBS with 1% paraformaldehyde. At least 5 × 104 lymphocytes (gated on the basis of forward- and side-scatter profiles) were collected for each sample on a FACSCalibur (BD Biosciences), and results were analyzed with FlowJo software (Tristar).
The following monoclonal antibodies, all purchased from BD/Pharmingen, were used for 4-color staining: anti-Vδ2 (clone B6), anti-Vγ9 (clone B3), anti-CD3 (clone SP34-2 and UCHT1), anti-CD25 (clone M-A251), anti-CD45-RA (clone HI100), anti-NKG2D (clone 1D11), and anti CD56 (clone B159). Anti-CD56 (clone N901) and anti-NKG2A (clone Z199) were purchased from Beckman-Coulter. Anti-CD27 (clone O323) was purchased from eBioscience, and anti-Vδ1 (clone TS8.2) was purchased from Thermo Scientific.
RNA Extraction, Reverse-Transcription PCR, and PCR
Total RNA was extracted from 0.5 × 107–1 × 107 CBMCs, using the RNeasy mini kit (Qiagen). Total RNA (1 μg) conversion into complementary DNA (using the reverse-transcription system kit, Promega) and PCR were performed as described in the Supplementary Materials.
Runoff Reaction
Primer extension reactions were performed and prepared for loading on a 3130 genetic analyzer (Applied Biosystems) as previously described [23, 37]. Molecular size and relative abundance of extension products were determined using GeneMapper software (Applied Biosystems). To standardize the data irrespective of the runoff primer position, the CDR3 length variation is expressed in terms of the total Vγ2 and Vδ1 coding region lengths.
Cloning and Sequencing of Vγ2 Chains
A library of Vγ2 chain sequences for each specimen were sequenced as described elsewhere [23] and in the Supplementary Materials.
Statistical Analysis
Statistical analyses were performed using the software GraphPad Prism. For each variable, D'Agostino and Pearson omnibus normality tests were performed to assess whether experimental values were normally distributed. Differences between groups were evaluated using t tests or Mann–Whitney tests for normally or nonnormally distributed variables, respectively. Whenever the sample size was too low to perform a test specific for normally distributed data, nonparametric tests were used.
RESULTS
Placental Malaria Increases Central Memory Vγ2Vδ2 T Cells in Cord Blood
Cord blood samples were collected from deliveries to 2 groups of mothers (Table 1): those who were P. falciparum negative (neonates were unexposed to P. falciparum) and those who were P. falciparum positive (neonates were exposed to P. falciparum); a subset of the P. falciparum–positive mothers had active placental infection at delivery (neonates were exposed to placental malaria). All cord blood specimens were P. falciparum negative by microscopy. All mothers were HIV negative by rapid test for peripheral blood antibodies. To test whether malaria during pregnancy influences fetal Vγ2Vδ2 T cells, we compared γδ T-cell levels and expression of common phenotypic markers in fresh CBMCs among exposed and unexposed neonates.
Table 1.
Study Population Characteristics
| Maternal Status | Subjects Enrolled, No. | Age, y, Mean ± SD |
|---|---|---|
| HIV negative, P. falciparum negative | 25 | 28.2 ± 5.3 |
| HIV negative, P. falciparum positivea | ||
| All P. falciparum infections | 15 | 25.2 ± 7.4 |
| Placental malariab | 8 | 24.6 ± 7.9 |
a P. falciparum infection was diagnosed by microscopy by 2 independent laboratories.
b Women with placental malaria (8) are a subset of all women with P. falciparum infection (15)
The proportions of Vδ2+, Vδ1+, and Vγ2+ T cells in CBMCs were similar across groups (Figure 1A and Supplementary Figure 1). The mean frequencies of Vδ2+ cells (±SD) were comparable to our previous results for specimens collected in Rome, Italy, and in Abidjan, Cote d'Ivoire (0.44% ± 0.29% and 0.47 + 0.3%, respectively). The current groups were also similar in terms of Vδ2 cell phenotype. NK receptors CD56, NKG2A, and NKG2D were present on a small fraction of Vδ2 T cells (usually <10% of Vδ2 lymphocytes for CD56 and NKG2A and <30% for NKG2D; Table 2). The frequency of NKG2A+ Vδ2 lymphocytes in this study compared well with the frequency of CD94+ Vδ2 T cells that we measured for CBMCs from Abidjan (mean [±SD], 9.1% ± 6.1% and 8.4% ± 5.5%, respectively) [13].
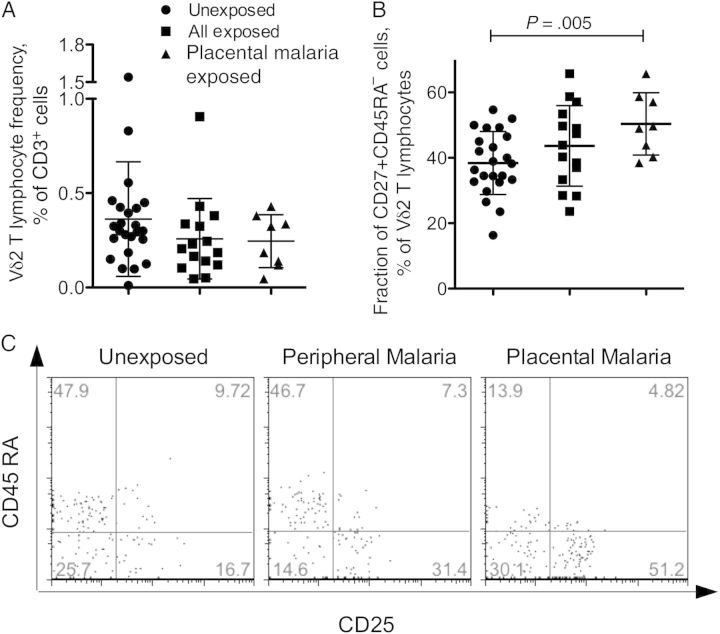
Figure 1.
Neonates exposed to placental malaria have a higher proportion of central memory Vδ2 lymphocytes in cord blood, compared with the unexposed group. A, The frequency of Vδ2+ lymphocytes was analyzed by flow cytometry as fraction of CD3+ cells in freshly isolated cord blood mononuclear cells for unexposed neonates (n = 25), all Plasmodium falciparum–exposed neonates (n = 15), and the subset of neonates exposed to placental malaria. B, The proportion of cord blood central memory (CD45RA−CD27+) Vδ2 lymphocytes is shown for unexposed neonates (n=25), all P. falciparum–exposed neonates (n = 15), and neonates exposed to placental malaria only (n = 8). The scatterplots show individual values and means ± SDs for each group of neonates. Differences between means were analyzed by the unpaired t test. C, The fraction of CD25+ Vδ2 lymphocytes is shown for a neonate born to an uninfected mother, a neonate born to a mother with peripheral malaria, and a neonate born to a mother with placental malaria.
Table 2.
Cord Blood Vγ2Vδ2 T-Cell Phenotype
| Phenotype | Unexposed (n = 25) |
All P. falciparum Exposed (n = 15) |
Placental Malaria Exposed (n = 8) |
|||
|---|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | |
| CD56+ | 10.3 ± 10.7 | 0–50 | 7.3 ± 8.3 | 0–33.3 | 4.5 ± 3.5 | 0–9.1 |
| NKG2A+ | 9.2 ± 6.2 | 0–21.9 | 7.4 ± 6.2 | 0–21 | 5.1 ± 2.9 | 1.8–11.5 |
| NKG2D+ | 34.9 ± 9.9 | 15.2–55.5 | 32.9 ± 14.7 | 11.6–62.2 | 27.2 ± 11.0 | 11.6–41.3 |
| CD25+ | 29.1 ± 11.0 | 6.3–55 | 32.4 ± 16.8 | 7.4–61 | 38.8 ± 15.0 | 14.6–61 |
| CD45RA−CD27+ | 38.4 ± 9.6 | 16.3–54.7 | 43.6 ± 12.3 | 23.6–65.7 | 50.4 ± 9.5a | 38.5–65.7 |
| CD45RA−CD27− | 3.4 ± 4.5 | 0–20.6 | 3.4 ± 3.3 | 0–12.3 | 4.0 ± 3.9 | 0.7–12.3 |
Data represent the percentage of Vδ2+ T cells with a specific phenotype.
a P < 0.05.
There were no significant differences in cord blood Vδ2 cell expression of memory/naive markers (CD27 and CD45RA) between all P. falciparum–exposed and unexposed neonates. Approximately 40%–45% of Vδ2 cells had a naive phenotype (CD45RA+CD27+), 40% had a central memory phenotype (CD45RA−CD27+), and a small fraction had effector memory phenotype (CD45RA−CD27−; Table 2). However, the subset of 8 neonates exposed to placental malaria had significantly higher central memory Vδ2 cells, compared with unexposed neonates (Figure 1B). In general, the samples in this study had higher proportions of central memory Vδ2 T cells than the specimens previously collected in Rome (mean [±SD], 25.9% ± 15.6%) and Abidjan (mean [±SD], 16.3% ± 12.2%) [13].
The IL-2 receptor β chain (CD25) was present on approximately 30% of Vδ2 T cells in unexposed neonates and on a larger fraction of Vδ2 cells (40%) in neonates exposed to placental malaria (Figure 1C), but the difference did not reach significance (Table 2). The population of CD25+ Vδ2 cells in CBMCs was not explained by contaminating maternal peripheral blood mononuclear cells, because CD25+ Vδ2 T cells were not detected in the mother's blood sample (data not shown). The unexpectedly high frequency of CD25+ cells was limited to the Vδ2 cell subset; on average <5% of the cells in the lymphocyte gate expressed this marker (data not shown).
Plasmodium-Exposed Neonates Have a Smaller Fraction of Jγ1.2 + Vγ2 Chains Ex Vivo
The subset of γδ cells most reactive to PAg and pathogens expresses the Vγ2 gene rearranged with the Jγ1.2 segment (also named Vγ9 and JγP in an alternate nomenclature) [38]. Changes in the Vγ2 repertoire are linked to antigen-driven selection [37]. Among healthy adults, the majority of peripheral blood γδ cells express PAg-reactive Vγ2Vδ2 TCR [37, 39]. In cord blood, both the absolute number and frequency of this subset are lower [36, 40, 41]. PAg-specific Vγ2Vδ2 lymphocytes increase in blood after birth [41] because of PAg stimulation and positive selection, leading eventually to the Jγ1.2-biased adult repertoire [39, 41].
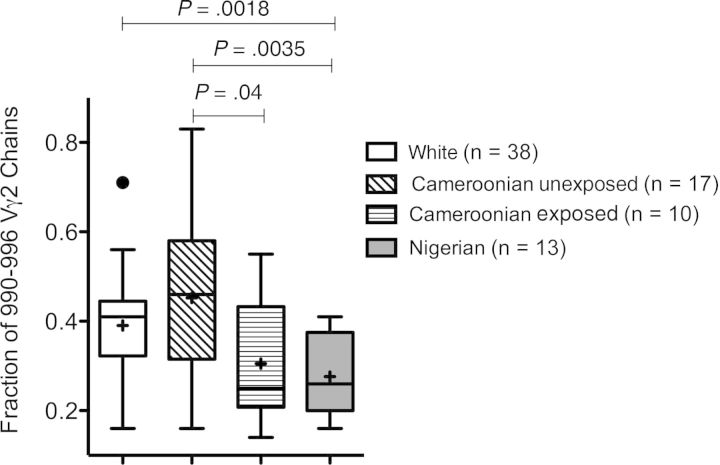
In a previous study, we observed that CBMCs collected in Nigeria had a lower proportion of Jγ1.2+ chains among all Vγ2+ cells, compared with CBMCs from Italy [36]. Even though mothers enrolled in the Nigerian group had not been screened for malaria, these samples were collected during the rainy season, when a high prevalence of Plasmodium infection would be expected, and differences might have been due to environmental factors (ie, maternal malaria) [36]. To test this possibility, we analyzed (by spectratyping) the pattern of Vγ2 chain lengths for control and P. falciparum–exposed neonates in Cameroon. Vγ2 lengths are correlated with J segment use. Among adult Vγ2 lymphocytes, most of the chains between 990–996 nucleotides include the Jγ1.2 segment [37], and this value (%990–996) is highly correlated with the frequency of Jγ1.2+ chains. Spectratyping was done with 17 unexposed and 10 of all P. falciparum–exposed (6 belonging to the placental malaria subset) CBMC specimens; the %990–996 was significantly lower for exposed neonates (Figure 2). Importantly, the %990–996 for all P. falciparum–exposed Cameroonian neonates was similar to the %990–996 for Nigerian neonates, while the values for unexposed Cameroonian neonates were comparable to the values for Italian neonates who had no exposure to maternal malaria (Figure 2). Thus, fetal exposure to maternal Plasmodium infection seems to alter the neonatal Vγ2 repertoire. We also analyzed length distributions for Vδ1 chains, which do not recognize PAg. We found no differences among the groups of Cameroonian neonates (data not shown), indicating that the Vδ1 repertoire was not affected by maternal malaria.
Figure 2.
Neonates exposed to Plasmodium falciparum have lower values for the percentage of Vγ2 chains with a length between 990–996 nucleotides (%990–996) than unexposed neonates. Vγ2 chain length distributions for cord blood mononuclear cell specimens were determined by spectratyping. The proportions of Vγ2 chains with a length of 990–996 nucleotides (the most common lengths for Jγ1.2+ chains) were compared for white, Cameroonian unexposed, Cameroonian P. falciparum–exposed, and Nigerian neonates. Box plots show interquartile ranges, medians, and means for each group. Differences between means were analyzed by the unpaired t test.
Placental Malaria Alters the Neonatal Vγ2 Repertoire Responses
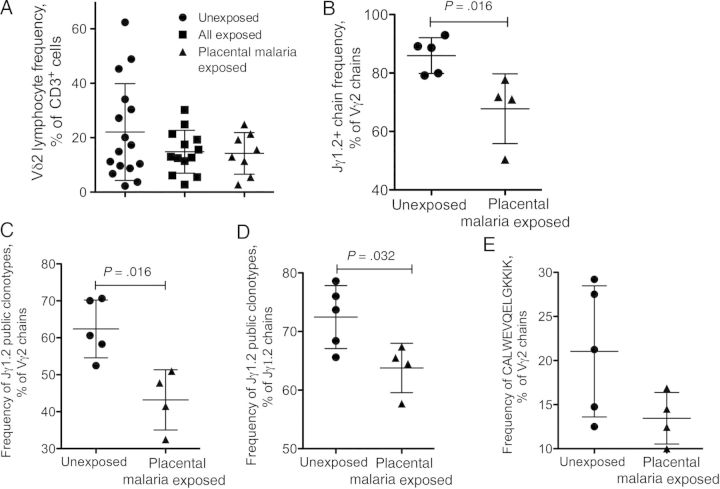
To test whether maternal infection affected fetal Vγ2Vδ2 cell responses, we stimulated cord blood Vγ2Vδ2 lymphocytes and evaluated proliferation and phenotype after 14 days. The aminobisphosphonate alendronate (ALN) was used as stimulus, and IL-2 or IL-15 was added to sustain Vγ2Vδ2 cell proliferation. After 14-day stimulations, there were no major differences between unexposed and exposed specimens in terms of phenotype (Supplementary Table 1). Proliferation (measured as increased frequencies of Vδ2 lymphocytes among all CD3+ cells) for all P. falciparum–exposed neonates tended to be lower, but the difference between groups was not significant (Figure 3A).
Figure 3.
Antigen-specific proliferation of cord blood Vδ2 cells is attenuated for all Plasmodium falciparum–exposed neonates, and neonates exposed to placental malaria accumulate lower proportions of phosphoantigen (PAg)–reactive Vγ2 chains. A, The proportions of Vδ2 lymphocytes among CD3+ cells were monitored by flow cytometry 14 days after alendronate stimulation for unexposed neonates, all P. falciparum–exposed neonates, and neonates exposed to placental malaria. B, The fractions of Jγ1.2+ chains among all Vγ2 14 days after alendronate stimulation were determined by sequence analysis for unexposed neonates (n = 5) and neonates exposed to placental malaria (n = 4). At least 200 productively rearranged Vγ2 chains were analyzed for each specimen. The proportions of Jγ1.2+ chains encoding public clonotypes in the Vγ2 pool (C) or in the Jγ1.2+ subset (D) 14 days after alendronate stimulation are shown for unexposed neonates and neonates exposed to placental malaria. E, Proportions of the single most abundant and most common nucleotype (CALWEVQELGKKIK) are shown for unexposed neonates and neonates exposed to placental malaria. Scatterplots show individual values and means ± SD for each group of neonates. Differences between medians were analyzed by the Mann–Whitney U test.
Since most Vγ2-Jγ1.2 cells respond to PAg, proliferation assays can obscure significant differences in the Vγ2 repertoire, as we discovered for HIV-infected patients [42]. To have the best chance for finding changes in neonatal Vγ2 repertoire associated with maternal disease, we concentrated on neonates exposed to placental malaria.
Starting with CBMCs from 5 unexposed subjects and 4 subjects exposed to placental malaria, we generated for each specimen Vγ2 chain cDNA plasmid libraries representing cells cultured with ALN + IL-2 or ALN + IL-15. We sequenced 150 Vγ2 cDNA clones from each library to obtain a representative sample of the Vγ2 repertoire. For each specimen, we pooled ALN + IL-2 and ALN + IL-15 sequences, because there were no substantial differences between these data sets [23] and pooling improved statistical power.
After ALN stimulation and proliferation, the fraction of Jγ1.2 chains increased in all samples. However, the resulting proportion of Vγ2-Jγ1.2 chains, as a percentage of all Vγ2 sequences (Figure 3B), was significantly lower for neonates exposed to placental malaria (P = .016).
The next step was to identify public Vγ2 clonotypes in each set of sequence data. Public Vγ2 clonotypes are identical amino acid sequences found in >1 donor. Both the neonatal and the adult Vγ2-Jγ1.2+ lymphocyte pools are dominated by public clonotypes [36, 38, 43, 44]. Table 3 lists the 30 most common public clonotypes in order of abundance (number of repeats for each sequence among all specimens). Tables 4 and 5 show only public clonotypes comprising ≥1% of all Jγ1.2 chains for the unexposed and placental malaria groups; clonotypes are ordered according to their frequency within the Jγ1.2 subset or within the Vγ2 pool. We identified 85 public clonotypes; the most common and abundant were present in both unexposed and placental malaria groups, and their profile was similar but not identical between the 2 groups (data not shown). Seven clonotypes were present in every specimen, and 6 other clonotypes were present in every unexposed sample but were less common in the placental malaria group (Table 3). For the unexposed group, we identified 67 public Jγ1.2 clonotypes, 14 of which were present in 5 of 5 donors and 12 that each represented ≥1% of total Jγ1.2+ chains (Tables 3–5). In the placental malaria group, there were 52 Jγ1.2 public clonotypes, 8 were present in 4 of 4 specimens, and 9 others represented ≥1% of the total Jγ1.2+ chains (Tables 3–5).
Table 3.
Abundance of Public Clonotypes After Alendronate Stimulation for All Neonates Analyzed
| Clonotype |
Unexposed |
Placental Malaria Exposed |
Totala | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| V | N | Jγ1.2 | A | B | C | D | E | F | G | H | I | |
| CALWEV | ND | QELGKKIK | 88 | 81 | 42 | 42 | 63 | 38 | 33 | 44 | 53 | 484 |
| CALWEV | R | ELGKKIK | 11 | 17 | 7 | 16 | 12 | 3 | 14 | 10 | 6 | 96 |
| CALWE | ND | QELGKKIK | 6 | 10 | 4 | 9 | 8 | 5 | 5 | 11 | 10 | 68 |
| CALWE | A | QELGKKIK | 4 | 7 | 6 | 3 | 2 | 2 | 5 | 8 | 10 | 47 |
| CALWEV | K | ELGKKIK | 9 | 3 | 5 | 3 | 5 | ND | 3 | 6 | 3 | 37 |
| CALWEV | ND | ELGKKIK | 3 | 1 | 4 | 8 | 8 | 5 | 2 | 1 | 4 | 36 |
| CALWE | E | ELGKKIK | 4 | 1 | 4 | 5 | 2 | 4 | 2 | 4 | 2 | 28 |
| CALWEV | G | ELGKKIK | 5 | 2 | 5 | 4 | 2 | 1 | 4 | ND | 2 | 25 |
| CALWEV | L | ELGKKIK | 3 | 7 | 3 | 5 | 2 | 1 | 1 | 1 | 2 | 25 |
| CALWE | P | QELGKKIK | 3 | ND | 2 | 11 | ND | ND | 3 | ND | 1 | 20 |
| CALWEV | H | ELGKKIK | 2 | 5 | 2 | 3 | 3 | ND | 1 | 4 | ND | 20 |
| CALWEV | E | ELGKKIK | 1 | 2 | 5 | ND | 2 | ND | 1 | ND | 5 | 16 |
| CALWEV | P | QELGKKIK | 4 | 1 | 3 | 3 | 4 | ND | ND | ND | ND | 15 |
| CALWE | L | QELGKKIK | 3 | 1 | 2 | 1 | 2 | 1 | 3 | ND | 1 | 14 |
| CALW | D | QELGKKIK | ND | 1 | ND | 8 | ND | 3 | ND | 1 | ND | 13 |
| CALWE | G | QELGKKIK | ND | 5 | 2 | 1 | 1 | ND | 2 | ND | 2 | 13 |
| CALWEV | P | ELGKKIK | 1 | 2 | 1 | 1 | ND | ND | 1 | 7 | ND | 13 |
| CALWEV | QG | ELGKKIK | 1 | 3 | 2 | ND | ND | ND | 2 | 4 | 1 | 13 |
| CALWE | ND | ELGKKIK | 2 | 2 | 1 | 3 | 1 | ND | ND | 1 | 2 | 12 |
| CALWE | T | QELGKKIK | 2 | 2 | ND | ND | 2 | 1 | 3 | 1 | 1 | 12 |
| CALWEV | R | QELGKKIK | 2 | 2 | 3 | 1 | 1 | 1 | 1 | 1 | ND | 12 |
| CALWE | AL | QELGKKIK | ND | 1 | 1 | 3 | 1 | ND | ND | 4 | 1 | 11 |
| CALWEV | Q | LGKKIK | ND | 3 | ND | ND | 3 | 1 | 2 | ND | 2 | 11 |
| CALWEV | T | ELGKKIK | 1 | 2 | 5 | ND | ND | ND | 2 | ND | 1 | 11 |
| CALWE | S | QELGKKIK | 2 | 3 | ND | ND | 1 | ND | ND | 3 | 1 | 10 |
| CALWE | F | QELGKKIK | 1 | 2 | ND | 4 | ND | 1 | 1 | ND | ND | 9 |
| CALWE | G | ELGKKIK | ND | 1 | ND | 3 | ND | 2 | ND | ND | 2 | 8 |
| CALWE | ND | LGKKIK | ND | 1 | 2 | ND | 2 | 1 | ND | 1 | ND | 7 |
| CALWEV | H | QELGKKIK | 1 | ND | ND | ND | 4 | ND | 1 | ND | 1 | 7 |
| CALWEV | V | ELGKKIK | 1 | ND | 1 | 1 | 1 | ND | 2 | ND | 1 | 7 |
Data are total no. of repeats per sequence in the pool of 9 donors analyzed. Clonotypes are aligned in order of decreasing abundance. Bold face indicates that the clonotype is present in every specimen. Italics indicates that the clonotype is present in all specimens within one of the two groups.
Abbreviation: ND, not detected.
Table 4.
Fraction of Jγ1.2+ Chains Coding Public Clonotypes and Frequencies of the Most Abundant Public Jγ1.2 Clonotypes After Alendronate Stimulation Among Unexposed Neonates
| Clonotype |
Fraction of Jγ1.2+ Chains Coding Public Clonotypes, % |
Frequency of Most-Abundant Public Jγ1.2 Clonotypes, % of Total Vγ2 Chains |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| V | N | Jγ1.2 | A | B | C | D | E | Average | A | B | C | D | E | Average |
| CALWEV | … | QELGKKIK | 37.0 | 32.4 | 21.5 | 17.9 | 30.7 | 27.9 | 33.0 | 30.1 | 17.2 | 15.9 | 24.3 | 24.1 |
| CALWEV | R | ELGKKIK | 4.6 | 6.8 | 3.6 | 6.8 | 5.9 | 5.5 | 4.1 | 6.3 | 2.9 | 6.1 | 4.6 | 4.8 |
| CALWE | … | QELGKKIK | 2.5 | 4.0 | 2.1 | 3.8 | 3.9 | 3.3 | 2.2 | 3.7 | 1.6 | 3.4 | 3.1 | 2.8 |
| CALWEV | K | ELGKKIK | 3.8 | 1.2 | 2.6 | 1.3 | 2.4 | 2.3 | 3.4 | 1.1 | 2.0 | 1.1 | 1.9 | 1.9 |
| CALWEV | … | ELGKKIK | 1.3 | 0.4 | 2.1 | 3.4 | 3.9 | 2.2 | 1.1 | 0.4 | 1.6 | 3.0 | 3.1 | 1.9 |
| CALWE | A | QELGKKIK | 1.7 | 2.8 | 3.1 | 1.3 | 1.0 | 2.0 | 1.5 | 2.6 | 2.5 | 1.1 | 0.8 | 1.7 |
| CALWEV | L | ELGKKIK | 1.3 | 2.8 | 1.5 | 2.1 | 1.0 | 1.7 | 1.1 | 2.6 | 1.2 | 1.9 | 0.8 | 1.5 |
| CALWEV | G | ELGKKIK | 2.1 | 0.8 | 2.6 | 1.7 | 1.0 | 1.6 | 1.9 | 0.7 | 2.0 | 1.5 | 0.8 | 1.4 |
| CALWE | E | ELGKKIK | 1.7 | 0.4 | 2.1 | 2.1 | 1.0 | 1.4 | 1.5 | 0.4 | 1.6 | 1.9 | 0.8 | 1.2 |
| CALWEV | P | QELGKKIK | 1.7 | 0.4 | 1.5 | 1.3 | 2.0 | 1.4 | 1.5 | 0.4 | 1.2 | 1.1 | 1.5 | 1.2 |
| CALWE | P | QELGKKIK | 1.3 | ND | 1.0 | 4.7 | ND | 1.4 | 1.1 | ND | 0.8 | 4.2 | ND | 1.2 |
| CALWEV | H | ELGKKIK | 0.8 | 2.0 | 1.0 | 1.3 | 1.5 | 1.3 | 0.7 | 1.9 | 0.8 | 1.1 | 1.2 | 1.1 |
| CALWEV | E | ELGKKIK | 0.4 | 0.8 | 2.6 | ND | 1.0 | 1.0 | 0.4 | 0.7 | 2.0 | ND | 0.8 | 0.8 |
| Total no. of sequences | 238a | 250 | 195 | 234 | 205 | ND | 267b | 269 | 244 | 264 | 259 | ND | ||
Clonotypes are listed in order of decreasing abundance.
Abbreviation: ND, not detected.
aTotal no. of Jγ1.2+ chains
bTotal no. of Vγ2 chains
Table 5.
Fraction of Jγ1.2+ Chains Coding Public Clonotypes and Frequencies of the Most Abundant Public Jγ1.2 Clonotypes After Alendronate Stimulation Among Neonates Exposed to Placental Malaria
| Clonotype |
Fraction of Jγ1.2+ Chains Coding Public Clonotypes, % |
Frequency of Most-Abundant Public Jγ1.2 Clonotypes, % of Total Vγ2 Chains |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| V | N | Jγ1.2 | F | G | H | I | Average | F | G | H | I | Average |
| CALWEV | ND | QELGKKIK | 31.4 | 17.0 | 24.3 | 26.1 | 24.7 | 15.8 | 12.2 | 17.3 | 20.3 | 16.4 |
| CALWEV | R | ELGKKIK | 2.5 | 7.2 | 5.5 | 3.0 | 4.5 | 1.2 | 5.2 | 3.9 | 2.3 | 3.2 |
| CALWE | A | QELGKKIK | 1.7 | 2.6 | 4.4 | 4.9 | 3.4 | 0.8 | 1.9 | 3.1 | 3.8 | 2.4 |
| CALWE | ND | QELGKKIK | 4.1 | 2.6 | 6.1 | 4.9 | 4.4 | 2.1 | 1.9 | 0.4 | 3.8 | 2.0 |
| CALWEV | ND | ELGKKIK | 4.1 | 1.0 | 0.6 | 2.0 | 1.9 | 2.1 | 0.7 | 0.4 | 1.5 | 1.2 |
| CALWE | E | ELGKKIK | 3.3 | 1.0 | 2.2 | 1.0 | 1.9 | 1.7 | 0.7 | 1.6 | 0.8 | 1.2 |
| CALWEV | K | ELGKKIK | ND | 1.5 | 3.3 | 1.5 | 1.6 | ND | 1.1 | 2.4 | 1.1 | 1.2 |
| CALWEV | P | ELGKKIK | ND | 0.5 | 3.9 | ND | 1.1 | ND | 0.4 | 2.7 | ND | 0.8 |
| CALWEV | G | ELGKKIK | 0.8 | 2.1 | ND | 1.0 | 1.0 | 0.4 | 1.5 | ND | 0.8 | 0.7 |
| Total no. of sequences | 121a | 194 | 181 | 203 | ND | 241b | 270 | 255 | 261 | ND | ||
Clonotypes are listed in order of decreasing abundance.
Abbreviation: ND, not detected.
aTotal no. of Jγ1.2+ chains
bTotal no. of Vγ2 chains
The fractions of Jγ1.2 sequences identified as public clonotypes were significantly lower for the placental malaria group (Figure 3C and 3D). For the unexposed group, the fraction of public Jγ1.2 clonotypes ranged from 65.6% to 78.6% of Jγ1.2 chains and from 52.5% to 70.6% of all Vγ2 sequences. For the placental malaria group, these ranges were from 57.75% to 65.5% and from 32.5% to 51%, respectively.
Within the entire Vγ2 pool, the 2 most abundant public Jγ1.2 clonotypes were the germ-line sequence CALWEVQELGKKIK and the closely related sequence CALWEVRELGKKIK (Tables 3–5). Both of these important clonotypes were less frequent in the Vγ2 repertoire of neonates exposed to placental malaria, even though differences were not significant. The germ-line nucleotype comprised a mean (±SD) of 13.5% ± 2.9% of all Vγ2 chains in neonates exposed to placental malaria, compared with 21% ± 7.4% in unexposed neonates (Figure 3E).
DISCUSSION
In this study, we describe a direct effect of placental malaria on fetal innate immunity. Newborns prenatally exposed to maternal malaria (peripheral and/or placental) had a lower proportion of PAg reactive Vγ2 chains (%990–996) in cord blood when compared to unexposed newborns. Moreover, cord blood Vγ2Vδ2 T cells in neonates exposed to placental malaria were shifted toward a central memory phenotype and had reduced proportions of PAg-reactive Jγ1.2+ clonotypes (especially public sequences) in response to stimulation in vitro. These differences were not observed for neonates born to HIV-positive mothers without malaria (data not shown). The pattern of cell differentiation and effects on public repertoire are consistent with antigen-driven, clonal deletion resulting from strong stimulation during placental malaria. Placental malaria creates a potent source of stimulatory PAg in close proximity to the fetus; these low-molecular-weight compounds may cross the placental barrier, overstimulate Vγ2Vδ2 cells, and deplete highly reactive clones. However, the mechanism and efficiency of PAg transplacental transfer are currently unknown. A similar outcome was observed for European adults upon their initial exposure to P. falciparum infection (ie, traveler's malaria), where potent activation of Vγ2Vδ2 lymphocytes led to transient depletion of Vγ2-Jγ1.2+ cells [31]. In both traveler's malaria and prenatal exposure to placental malaria, reactive Vγ2-Jγ1.2+ cells in P. falciparum–naive individuals may undergo activation-induced cell death. Nevertheless, we cannot exclude that in neonates born to mothers with malaria, the Vγ2-Jγ1.2+ lymphocytes were sequestered in lymphoid organs as a consequence of in utero exposure to PAg and, therefore, were underrepresented in the circulating compartment at birth.
Normally, the neonatal Vγ2 repertoire is dominated by public clonotypes that significantly contribute to antigen responses. These clonotypes (in particular, CALWEVQELGKKIK and CALWEVRELGKKIK) persist and remain abundant throughout healthy adult life [38, 43]. Neonates exposed to placental malaria display altered maturation of the Vγ2 repertoire, with reduced expansion of public Jγ1.2 clonotypes in response to stimulation in vitro. This is likely a direct consequence of depletion (or exhaustion) of Vγ2-Jγ1.2+ clones. Altered APC function or suppression of proliferation by plasmodium-specific T regulatory cells may also hamper Vγ2Vδ2 cell responses, but it would not cause a selective defect in the proliferation of public Jγ1.2+ clones.
Our study suggests that effects of placental malaria have a strong impact in vivo, by impairing or delaying the peripheral selection of PAg-reactive Vγ2Vδ2 clones. This may have broad consequences since Vγ2Vδ2 cells respond to both P. falciparum and BCG, which is administered routinely to neonates within the first few days of life. Because of antigen-driven depletion, Vγ2Vδ2 cells in neonates exposed to placental malaria may respond less efficiently to BCG vaccine.
There are some important limitations to our present study. During the project period, we enrolled 8 women with placental malaria. Women were only tested for P. falciparum infection at delivery, rather than throughout the course of pregnancy. For malaria cases, we have limited information about disease timing relative to pregnancy and peak parasitemia. Vγ2Vδ2 cells are highly susceptible to activation-induced cell death [45]; therefore, the duration and intensity of PAg stimulation will influence the outcome of fetal Vγ2Vδ2 T-cell priming. While strong and prolonged prenatal stimulation may induce overactivation and deletion of reactive Vγ2Vδ2 lymphocytes, a mild stimulation caused by low-parasitemia infections or successfully treated maternal malaria might result in appropriate activation of fetal Vγ2Vδ2 cells with potentially protective effects after birth. Results published in 2 studies by Engelmann et al support this hypothesis. CB γδ cells in unexposed Gabonese neonates have limited ability to produce T-helper type 1 cytokines (IFN-γ) and cytotoxic mediators ex vivo or in response to polyclonal stimulation [46]. However, neonates born to mothers successfully treated for malaria during pregnancy have a higher proportion of CB γδ cells producing IFN-γ than neonates born to mothers without malaria, and the fraction of γδ expressing CD25 after polyclonal stimulation is higher in the former group [47]. The time of exposure is also likely to influence the outcome of fetal Vγ2Vδ2 cell priming, and published studies suggest that the Plasmodium infection history during pregnancy is critical in determining the effects on other neonatal innate [48] and adaptive [49] cell subsets. Larger sample size, detailed P. falciparum infection history during pregnancy, and better characterization of pregnancy-associated malaria cases are essential to dissect the potentially diverse effects of maternal malaria on fetal Vγ2Vδ2 cells.
Studies focusing on repertoire perturbations, possibly including activation status (CD25 expression) or differentiation of naive cells to memory, may be important measures of placental malaria if they can be validated in larger studies. In the context of expanding efforts to treat malaria during pregnancy and programs to reintroduce older drugs like chloroquine [50], quantitative markers of fetal immunity will be valuable to assess drug effects on placental malaria. Our studies also help to understand how maternal infectious disease can alter the fetal immune system. An important challenge in this area is to understand why maternal infection may affect infant immunity to seemingly unrelated pathogens or vaccines [3, 4, 8]. Vγ2Vδ2 lymphocytes, through TCR recognition of PAg from a variety of microbial species, are important for broad pathogen resistance. Defects in this cell population might explain some of the complex relationships between maternal infections, infant susceptibility to infectious diseases, and responses to pediatric BCG vaccination.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the Hôpital Central de Yaoundé, Maternity Division, for their invaluable assistance with cord blood collection; and Prof Pierre Joseph Fouda, CIRCB, for facilitating the research efforts of Dr Cairo in Cameroon.
Financial support. This work was supported by UNESCO (Families First Africa program); the Istituto Superiore di Sanita’ (ISS/MAE AID 7999.03.6); the US Public Health Service (grants AI068508 [to C. P. D.] and 1R01AI104702 [to C. C.]); and the Faculty Development Program, Institute of Human Virology, University of Maryland, School of Medicine (to C. C.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Dauby N, Goetghebuer T, Kollmann TR, Levy J, Marchant A. Uninfected but not unaffected: chronic maternal infections during pregnancy, fetal immunity, and susceptibility to postnatal infections. Lancet Infect Dis. 2012;12:330–40. doi: 10.1016/S1473-3099(11)70341-3. [DOI] [PubMed] [Google Scholar]
- 2.Labeaud AD, Malhotra I, King MJ, King CL, King CH. Do antenatal parasite infections devalue childhood vaccination? PLoS Negl Trop Dis. 2009;3:e442. doi: 10.1371/journal.pntd.0000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miles DJ, Gadama L, Gumbi A, Nyalo F, Makanani B, Heyderman RS. Human immunodeficiency virus (HIV) infection during pregnancy induces CD4 T-cell differentiation and modulates responses to Bacille Calmette-Guerin (BCG) vaccine in HIV-uninfected infants. Immunology. 2010;129:446–54. doi: 10.1111/j.1365-2567.2009.03186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walther B, Miles DJ, Waight P, et al. Placental malaria is associated with attenuated CD4 T-cell responses to tuberculin PPD 12 months after BCG vaccination. BMC Infect Dis. 2012;12:6. doi: 10.1186/1471-2334-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Hesran JY, Cot M, Personne P, et al. Maternal placental infection with Plasmodium falciparum and malaria morbidity during the first 2 years of life. Am J Epidemiol. 1997;146:826–31. doi: 10.1093/oxfordjournals.aje.a009200. [DOI] [PubMed] [Google Scholar]
- 6.Malhotra I, Dent A, Mungai P, et al. Can prenatal malaria exposure produce an immune tolerant phenotype? A prospective birth cohort study in Kenya. PLoS Med. 2009;6:e1000116. doi: 10.1371/journal.pmed.1000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malhotra I, Mungai PL, Wamachi AN, et al. Prenatal T cell immunity to Wuchereria bancrofti and its effect on filarial immunity and infection susceptibility during childhood. J Infect Dis. 2006;193:1005–13. doi: 10.1086/500472. [DOI] [PubMed] [Google Scholar]
- 8.Malhotra I, Mungai P, Wamachi A, et al. Helminth- and Bacillus Calmette-Guerin-induced immunity in children sensitized in utero to filariasis and schistosomiasis. J Immunol. 1999;162:6843–8. [PubMed] [Google Scholar]
- 9.Morita CT, Beckman EM, Bukowski JF, et al. Direct presentation of nonpeptide prenyl pyrophosphate antigens to human gamma delta T cells. Immunity. 1995;3:495–507. doi: 10.1016/1074-7613(95)90178-7. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka Y, Morita CT, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. Nature. 1995;375:155–8. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 11.Eberl M, Hintz M, Reichenberg A, Kollas AK, Wiesner J, Jomaa H. Microbial isoprenoid biosynthesis and human gammadelta T cell activation. FEBS Lett. 2003;544:4–10. doi: 10.1016/s0014-5793(03)00483-6. [DOI] [PubMed] [Google Scholar]
- 12.Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, De Libero G. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197:163–8. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cairo C, Mancino G, Cappelli G, et al. Vdelta2 T-lymphocyte responses in cord blood samples from Italy and Cote d'Ivoire. Immunology. 2008;124:380–7. doi: 10.1111/j.1365-2567.2007.02784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kunzmann V, Bauer E, Feurle J, Weissinger F, Tony HP, Wilhelm M. Stimulation of gammadelta T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood. 2000;96:384–92. [PubMed] [Google Scholar]
- 15.Moens E, Brouwer M, Dimova T, Goldman M, Willems F, Vermijlen D. IL-23R and TCR signaling drives the generation of neonatal V{gamma}9V{delta}2 T cells expressing high levels of cytotoxic mediators and producing IFN-{gamma} and IL-17. J Leukoc Biol. 2011;89:743–52. doi: 10.1189/jlb.0910501. [DOI] [PubMed] [Google Scholar]
- 16.Lang F, Peyrat MA, Constant P, et al. Early activation of human V gamma 9 V delta 2 T cell broad cytotoxicity and TNF production by nonpeptidic mycobacterial ligands. J Immunol. 1995;154:5986–94. [PubMed] [Google Scholar]
- 17.Barnes PF, Abrams JS, Lu S, Sieling PA, Rea TH, Modlin RL. Patterns of cytokine production by mycobacterium-reactive human T-cell clones. Infect Immun. 1993;61:197–203. doi: 10.1128/iai.61.1.197-203.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conti L, Casetti R, Cardone M, et al. Reciprocal activating interaction between dendritic cells and pamidronate-stimulated gammadelta T cells: role of CD86 and inflammatory cytokines. J Immunol. 2005;174:252–60. doi: 10.4049/jimmunol.174.1.252. [DOI] [PubMed] [Google Scholar]
- 19.Martino A, Casetti R, D'Alessandri A, Sacchi A, Poccia F. Complementary function of gamma delta T-lymphocytes and dendritic cells in the response to isopentenyl-pyrophosphate and lipopolysaccharide antigens. J Clin Immunol. 2005;25:230–7. doi: 10.1007/s10875-005-4080-8. [DOI] [PubMed] [Google Scholar]
- 20.Gertner-Dardenne J, Bonnafous C, Bezombes C, et al. Bromohydrin pyrophosphate enhances antibody-dependent cell-mediated cytotoxicity induced by therapeutic antibodies. Blood. 2009;113:4875–84. doi: 10.1182/blood-2008-08-172296. [DOI] [PubMed] [Google Scholar]
- 21.Capietto AH, Martinet L, Fournie JJ. Stimulated gammadelta T cells increase the in vivo efficacy of trastuzumab in HER-2+ breast cancer. J Immunol. 2011;187:1031–8. doi: 10.4049/jimmunol.1100681. [DOI] [PubMed] [Google Scholar]
- 22.Maniar A, Zhang X, Lin W, et al. Human gammadelta T lymphocytes induce robust NK cell-mediated antitumor cytotoxicity through CD137 engagement. Blood. 2010;116:1726–33. doi: 10.1182/blood-2009-07-234211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cairo C, Sagnia B, Cappelli G, et al. Human cord blood gammadelta T cells expressing public Vgamma2 chains dominate the response to bisphosphonate plus interleukin-15. Immunology. 2013;138:346–60. doi: 10.1111/imm.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazzola TN, Da Silva MT, Moreno YM, et al. Robust gammadelta+ T cell expansion in infants immunized at birth with BCG vaccine. Vaccine. 2007;25:6313–20. doi: 10.1016/j.vaccine.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 25.Tastan Y, Arvas A, Demir G, Alikasifoglu M, Gur E, Kiray E. Influence of Bacillus Calmette-Guerin vaccination at birth and 2 months old age on the peripheral blood T-cell subpopulations [gamma/delta and alpha-beta T cell] Pediatr Allergy Immunol. 2005;16:624–9. doi: 10.1111/j.1399-3038.2005.00329.x. [DOI] [PubMed] [Google Scholar]
- 26.Goerlich R, Hacker G, Pfeffer K, Heeg K, Wagner H. Plasmodium falciparum merozoites primarily stimulate the V gamma 9 subset of human gamma/delta T cells. Eur J Immunol. 1991;21:2613–6. doi: 10.1002/eji.1830211045. [DOI] [PubMed] [Google Scholar]
- 27.Behr C, Poupot R, Peyrat MA, et al. Plasmodium falciparum stimuli for human gammadelta T cells are related to phosphorylated antigens of mycobacteria. Infect Immun. 1996;64:2892–6. doi: 10.1128/iai.64.8.2892-2896.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costa G, Loizon S, Guenot M, et al. Control of Plasmodium falciparum erythrocytic cycle: gammadelta T cells target the red blood cell-invasive merozoites. Blood. 2011;118:6952–62. doi: 10.1182/blood-2011-08-376111. [DOI] [PubMed] [Google Scholar]
- 29.Elloso MM, van der Heyde HC, vande Waa JA, Manning DD, Weidanz WP. Inhibition of Plasmodium falciparum in vitro by human gamma delta T cells. J Immunol. 1994;153:1187–94. [PubMed] [Google Scholar]
- 30.Troye-Blomberg M, Worku S, Tangteerawatana P, et al. Human gamma delta T cells that inhibit the in vitro growth of the asexual blood stages of the Plasmodium falciparum parasite express cytolytic and proinflammatory molecules. Scand J Immunol. 1999;50:642–50. doi: 10.1046/j.1365-3083.1999.00647.x. [DOI] [PubMed] [Google Scholar]
- 31.Martini F, Paglia MG, Montesano C, et al. V gamma 9 V delta 2 T-cell anergy and complementarity-determining region 3-specific depletion during paroxysm of nonendemic malaria infection. Infect Immun. 2003;71:2945–9. doi: 10.1128/IAI.71.5.2945-2949.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roussilhon C, Agrapart M, Guglielmi P, Bensussan A, Brasseur P, Ballet JJ. Human TcR gamma delta+ lymphocyte response on primary exposure to Plasmodium falciparum. Clin Exp Immunol. 1994;95:91–7. doi: 10.1111/j.1365-2249.1994.tb06020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwartz E, Shapiro R, Shina S, Bank I. Delayed expansion of V delta 2+ and V delta 1+ gamma delta T cells after acute Plasmodium falciparum and Plasmodium vivax malaria. J Allergy Clin Immunol. 1996;97:1387–92. doi: 10.1016/s0091-6749(96)70208-7. [DOI] [PubMed] [Google Scholar]
- 34.D'Ombrain MC, Hansen DS, Simpson KM, Schofield L. gammadelta-T cells expressing NK receptors predominate over NK cells and conventional T cells in the innate IFN-gamma response to Plasmodium falciparum malaria. Eur J Immunol. 2007;37:1864–73. doi: 10.1002/eji.200636889. [DOI] [PubMed] [Google Scholar]
- 35.Horowitz A, Newman KC, Evans JH, Korbel DS, Davis DM, Riley EM. Cross-talk between T cells and NK cells generates rapid effector responses to Plasmodium falciparum-infected erythrocytes. J Immunol. 2010;184:6043–52. doi: 10.4049/jimmunol.1000106. [DOI] [PubMed] [Google Scholar]
- 36.Cairo C, Propp N, Auricchio G, et al. Altered cord blood gammadelta T cell repertoire in Nigeria: possible impacts of environmental factors on neonatal immunity. Mol Immunol. 2008;45:3190–7. doi: 10.1016/j.molimm.2008.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Evans PS, Enders PJ, Yin C, Ruckwardt TJ, Malkovsky M, Pauza CD. In vitro stimulation with a non-peptidic alkylphosphate expands cells expressing Vgamma2-Jgamma1.2/Vdelta2 T-cell receptors. Immunology. 2001;104:19–27. doi: 10.1046/j.0019-2805.2001.01282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davodeau F, Peyrat MA, Hallet MM, et al. Close correlation between Daudi and mycobacterial antigen recognition by human gamma delta T cells and expression of V9JPC1 gamma/V2DJC delta-encoded T cell receptors. J Immunol. 1993;151:1214–23. [PubMed] [Google Scholar]
- 39.Davodeau F, Peyrat MA, Hallet MM, Houde I, Vie H, Bonneville M. Peripheral selection of antigen receptor junctional features in a major human gamma delta subset. Eur J Immunol. 1993;23:804–8. doi: 10.1002/eji.1830230405. [DOI] [PubMed] [Google Scholar]
- 40.Morita CT, Parker CM, Brenner MB, Band H. TCR usage and functional capabilities of human gamma delta T cells at birth. J Immunol. 1994;153:3979–88. [PubMed] [Google Scholar]
- 41.Parker CM, Groh V, Band H, et al. Evidence for extrathymic changes in the T cell receptor gamma/delta repertoire. J Exp Med. 1990;171:1597–612. doi: 10.1084/jem.171.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riedel DJ, Sajadi MM, Armstrong CL, et al. Natural viral suppressors of HIV-1 have a unique capacity to maintain gammadelta T cells. AIDS. 2009;23:1955–64. doi: 10.1097/QAD.0b013e32832ff1ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cairo C, Armstrong CL, Cummings JS, et al. Impact of age, gender, and race on circulating gammadelta T cells. Hum Immunol. 2010;71:968–75. doi: 10.1016/j.humimm.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Delfau MH, Hance AJ, Lecossier D, Vilmer E, Grandchamp B. Restricted diversity of V gamma 9-JP rearrangements in unstimulated human gamma/delta T lymphocytes. Eur J Immunol. 1992;22:2437–43. doi: 10.1002/eji.1830220937. [DOI] [PubMed] [Google Scholar]
- 45.Li B, Bassiri H, Rossman MD, et al. Involvement of the Fas/Fas ligand pathway in activation-induced cell death of mycobacteria-reactive human gamma delta T cells: a mechanism for the loss of gamma delta T cells in patients with pulmonary tuberculosis. J Immunol. 1998;161:1558–67. [PubMed] [Google Scholar]
- 46.Engelmann I, Moeller U, Santamaria A, Kremsner PG, Luty AJ. Differing activation status and immune effector molecule expression profiles of neonatal and maternal lymphocytes in an African population. Immunology. 2006;119:515–21. doi: 10.1111/j.1365-2567.2006.02466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Engelmann I, Santamaria A, Kremsner PG, Luty AJ. Activation status of cord blood gamma delta T cells reflects in utero exposure to Plasmodium falciparum antigen. J Infect Dis. 2005;191:1612–22. doi: 10.1086/429336. [DOI] [PubMed] [Google Scholar]
- 48.Adegnika AA, Kohler C, Agnandji ST, et al. Pregnancy-associated malaria affects toll-like receptor ligand-induced cytokine responses in cord blood. J Infect Dis. 2008;198:928–36. doi: 10.1086/591057. [DOI] [PubMed] [Google Scholar]
- 49.Broen K, Brustoski K, Engelmann I, Luty AJ. Placental Plasmodium falciparum infection: causes and consequences of in utero sensitization to parasite antigens. Mol Biochem Parasitol. 2007;151:1–8. doi: 10.1016/j.molbiopara.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 50.Laufer MK, Thesing PC, Eddington ND, et al. Return of chloroquine antimalarial efficacy in Malawi. N Engl J Med. 2006;355:1959–66. doi: 10.1056/NEJMoa062032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.