Abstract
Introduction:
In recent years, a class of products marketed as “tobacco-free” alternatives for the “health conscious user” has become widely available for waterpipe (hookah, narghile, or shisha) smoking. Their adoption may be in part driven by regulations banning tobacco smoking in public places and by an increasing awareness of the hazards of waterpipe tobacco smoking. Although these products are presented in advertising as a “healthier” choice, very little is known about their health effects.
Methods:
In this study, we compared the effects of smoke generated with tobacco-free and conventional tobacco-derived products on human alveolar cells. Smoke was generated with a smoking machine that precisely mimicked the puffing behavior of 15 experienced waterpipe smokers when they used conventional waterpipe tobacco products of their choice and flavor-matched tobacco-free products. Human alveolar epithelial cells (A549) were treated with particulate matter sampled from the smoke, and the effects on cell cycle, proliferation, and doubling time were measured during the subsequent 72hr.
Results:
We found that smoke from both types of waterpipe products markedly reduced cell proliferation, caused cell cycle arrest at G0/G1, and increased cell doubling time. There were no significant differences across product in any measure.
Conclusion:
Tobacco-free and tobacco-based waterpipe products exert substantial and similar deleterious effects on human lung cells. This study adds to the nascent evidence base indicating that except for exposure to nicotine and its derivatives, use of tobacco-free waterpipe products does not present a reduced health risk relative to the use of conventional tobacco-based products.
INTRODUCTION
Waterpipe (also known as hookah, narghile, or shisha) tobacco smoking has spread globally in recent years, and it now includes tobacco-free “herbal” preparations marketed “for the health conscious” user (http://www.soex.com). Adoption of these tobacco-free products may be enhanced by efforts of hospitality venues to exploit ambiguities in tobacco control laws (Government of Alberta, 2012) and to argue that there is little evidence linking herbal waterpipe smoke to negative health effects. Previous work (Shihadeh et al., 2012) has highlighted the similarities in toxicant yields between tobacco-based and tobacco-free waterpipe products, demonstrating that tobacco-free products yield essentially the same quantities of carbon monoxide (CO), nitric oxide (NO), volatile aldehydes, and polycyclic aromatic hydrocarbons; among the quantified toxicants, only nicotine differed significantly. Although these constituents are considered major causative agents in diseases suffered by cigarette smokers, they nonetheless account for a small mass fraction of the total mainstream smoke emissions. Other unidentified components of waterpipe smoke may also be relevant to disease risk for waterpipe smokers, including those using tobacco-free preparations. One way to examine this possibility is to study how the smoke from a waterpipe loaded with tobacco-containing or tobacco-free preparations influences disease risk as measured by established in vitro toxicology assays.
Among other methods, in vitro assays are used to identify potential health risks from exposure to chemically unresolved mixtures, including tobacco smoke (Johnson, Schilz, Djordjevic, Rice, & Shields, 2009) and can provide information about the effects of toxicant exposure on target organs in the body. We have shown that waterpipe tobacco smoke elicits deleterious effects on cell function of human alveolar epithelial and vascular endothelial cells (Rammah, Dandachi, Salman, Shihadeh, & El-Sabban, 2012, 2013). This work also provided lines of evidence for mechanistic pathways by which impaired cellular growth and inflammation in alveolar cells can lead to chronic obstructive pulmonary disease(Rammah et al., 2012) and by which compromised vasodilatory function and repair mechanisms in vascular cells can lead to vascular disease (Rammah et al., 2013) in regular waterpipe smokers.
In our previous work, we evaluated smoke from a single tobacco-based waterpipe product, using a single standard smoking protocol with a fixed number of puffs of given volume to produce the smoke. The current study extends this analysis by examining the effects of smoke generated using a variety of tobacco-based waterpipe preparations on human alveolar epithelial cells in which the smoke was generated in a manner that mimics human smoking behavior; in addition, we compare results observed with smoke from tobacco-containing waterpipe preparations with those observed when the smoke is generated from a tobacco-free preparation. Thus, this study addresses the extent to which the tobacco content of the product used to generate waterpipe smoke influences smoke-induced alveolar cell injury.
METHODS
The smoke generated for this study was produced by a machine that mimicked the smoking behavior (i.e., puff topography) of experienced waterpipe tobacco smokers when they were using tobacco-based or tobacco-free waterpipe products in a clinical laboratory setting (Blank et al., 2011).
Population
Puff topography was recorded from 33 healthy participants, 18–50 years of age, who reported smoking waterpipe tobacco 2–5 times per month for ≥6 months. No participant reported a history of chronic health problems or psychiatric conditions, regular use of prescription medications (other than vitamins or birth control), or current pregnancy or breast feeding. They also did not report use of >5 cigarettes/month, any other tobacco products, marijuana (>5 days in past month), or other illicit drugs.
Clinical Laboratory Procedures
Each participant completed two counterbalanced, 2-h sessions that differed by product used: waterpipe tobacco or a flavor-matched tobacco-free product. Prior to the first session, participants indicated their preferred brand and flavor of waterpipe tobacco (Al Fakher, Nakhle, and Starbuzz brands, and these choices spanned eight different flavors). These products were paired with same-flavored Soex brand tobacco-free waterpipe products. The waterpipe design and preparation procedure was identical to that used in the study by Blank et al. (2011). Recording of smoking behavior commenced after participants were presented with a waterpipe containing 10g of product in the foil-covered head with a lit charcoal briquette placed on top (additional half briquettes available on request). The waterpipe head was always packed and emptied by a researcher who had no participant contact. Aluminum foil covering obscured the head contents, and neither participants nor study staff were informed of the product used on a particular day. Participants were given a minimum of 45min to smoke the waterpipe ad lib while watching a video of their choice. Topography was measured via a flow sensor integrated into the waterpipe hose (Shihadeh, Antonius, & Azar, 2005). Although not relevant to the current report, blood, breath, and subjective response also were measured periodically during and/or after the smoking episode (see Blank et al., 2011, for details).
Smoke Generation and Sampling
A digitally controlled puff-replicating waterpipe smoking machine (Shihadeh & Azar, 2006) was used to reproduce the flow data recorded for 15 participants in the clinical laboratory. Each of the 30 smoking records (15 participants × 2 sessions per participant) was reproduced one time. The participant pool for this study was determined by sampling every second participant enrolled in the study, in chronological order until N = 15 was reached.
Procedures identical to those in the clinical laboratory were followed to prepare each waterpipe, and smoke was generated and sampled using the procedures described in the study by Shihadeh et al. (2012). In brief, tobacco and nontobacco preparations drawn from the same batches used in the clinical laboratory were stored in the dark at −4 °C until 24hr prior to their use, at which time they were placed in a 23 °C environment at 50%–60% relative humidity. Charcoal and waterpipes identical to those used in the clinical laboratory were used, and waterpipe hose infiltration rates (as determined in the report by Saleh & Shihadeh, 2008) were comparable (1–1.8 liters per minute). Mainstream smoke exiting the mouthpiece was split into four parallel streams, and each stream was drawn through a glass fiber filter (Gelman type A/E). NO, CO, and total particulate matter yields were quantified for each machine smoking session.
Waterpipe Smoke Extraction
The filters collected from smoking sessions were stored in airtight containers at −20 °C. Smoke extract was prepared as described by Rammah et al. (2012). Briefly, filters were eluted with cell culture media (without fetal bovine serum), to yield a particulate mass concentration of 40mg/ml. The resulting solution from any given smoking session was pooled and sterilized using 0.22-µm filters (Costar).
Cell Culture and Proliferation Assays
Human alveolar epithelial (A549) cells were cultured in Dulbecco's modified Eagle high-glucose (4.5g/l) media supplemented with penicillin G 100U/ml and streptomycin 100 µg/ml (Gibco), 1% sodium pyruvate (Lonza), and 10% fetal bovine serum (Sigma Aldrich).
Cells were seeded at a density of 104 cells/cm2. Treatment started 24hr postseeding and was performed for 24hr by exposure to extract diluted with complete media to 4mg/ml of tobacco and nontobacco smoke extract to avoid cytotoxic effects (see Rammah et al., 2012). This treatment was repeated for three consecutive days (repeated exposure). Dead and live cells were counted after the 72-hr treatment using the Trypan blue dye exclusion assay.
Cell Cycle Analysis
Cells were plated at a density of 104 cells/cm2 and treated repeatedly for three consecutive days. Data analysis and percentage of cells in different phases of the cell cycle were determined using DNA content analysis using propidium iodide staining and flow cytometry (Beckton Dickensson). The percentages of the cells in the S phase were obtained and then compared with that in the control using Flow Jo software. The reduction in the percentage of the S-phase cells in treated conditions was then calculated relative to the control (Rammah et al., 2012).
Doubling Time (Cell Proliferation) by Real-Time Cell Impedance Analysis
Real-Time Cell Analyzer xCELLigence System (Roche Applied Science) was used to monitor cell proliferation rates dynamically. A549 cells were seeded at a density of 7,000 cells per well on an E-Plate™. Treatment started 24hr postseeding by mixing extract and complete media to the desired final concentration (4mg/ml). Dynamic impedance parameters were monitored at 45-min intervals for 24hr after treatments (Rammah et al., 2012).
Statistical Analysis
Differences in means between measures for tobacco and nontobacco preparations were analyzed for significance using a two-tailed t test. Probability values below 0.05 were taken as significant. Pearson product correlation coefficients, r, were also tested for significance using a two-tailed t distribution.
RESULTS
Puff Topography and Toxicant Yields
Puff topography parameters are summarized in Table 1, as are yields of total particulate matter, CO, and NO. There were no significant differences in puff topography or toxicant yields between the tobacco-based and tobacco-free products. The topography and yields are consistent with previously reported values for the same products (Shihadeh et al., 2012) and confirm that waterpipe smoke contains significant quantities of toxicants. Although puff topography varied widely across individuals, puff topography parameters were well correlated within an individual when comparing tobacco and nontobacco conditions (e.g., for total smoke volume, r = .64, p < .05).
Table 1.
Puff Topography and Toxicant Yields for 15 Waterpipe Users Who Smoked Flavor-Matched Tobacco and Nontobacco Products in the Clinical Laboratory
| Mean (SEM) | ||
|---|---|---|
| Tobacco | Nontobacco | |
| Puffs drawn | 78 (25) | 76 (23) |
| Puff duration, s | 4.0 (0.9) | 3.6 (0.6) |
| Interpuff interval, s | 44 (14) | 49 (22) |
| Total smoke volume, l | 75 (28) | 66 (20) |
| Product consumed, mg | 3,910 (540) | 4,030 (420) |
| TPM yield, mg | 1,150 (450) | 1,080 (400) |
| CO yield, mg | 190 (60) | 170 (40) |
| NO yield, mg | 470 (140) | 400 (130) |
Note. SEM = standard error of the mean; TPM = total particulate matter. No significant difference was found across product types in any measure.
Cell Cycle Parameters
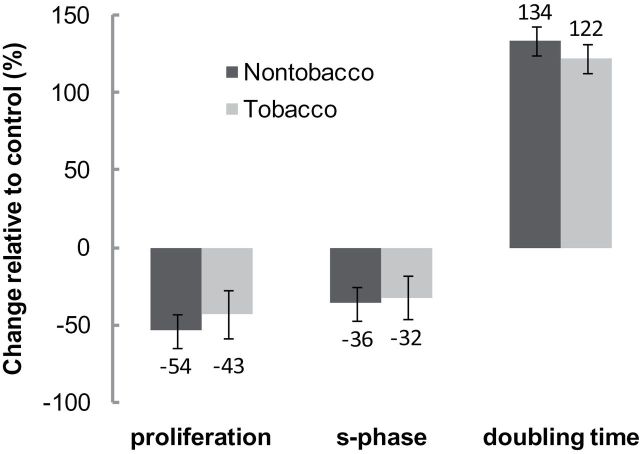
Tobacco-based and tobacco-free smoke extract markedly reduced cell proliferation relative to control by 43% and 54%, respectively, after 72hr of treatment; this reduction likely occurs through inhibition of cell cycle entry. This effect was confirmed further by using Real-Time Cell Analyzer, a more sensitive technique that allows monitoring changes in the properties of A549 cells after 12hr of exposure to the smoke extract. Real-Time Cell Analyzer results show that tobacco and nontobacco smoke extract induced an increase in the doubling time by 22% and 34%, respectively, after 12hr of exposure (Figure 1).
Figure 1.
Effect of tobacco-based and tobacco-free waterpipe smoke extract on cell proliferation, cell cycle, and doubling time. Error bars correspond to 95% confidence interval. No significant difference was found across product types in any outcome measure.
This reduction was accompanied by a decrease in the percentage of cells in the S phase, consistent with our previous findings (Rammah et al., 2012) that waterpipe tobacco smoke particulate matter induces cell cycle arrest at G0/G1. Overall, there were no significant differences in outcomes between smoke extract derived from tobacco-based and tobacco-free waterpipe preparations. These results show that tobacco-free waterpipe products, as tobacco-based waterpipe products, impair alveolar epithelial cellular growth, thus leading to abnormal cell repair in case of injury. Disruption of such physiological processes would facilitate and contribute to the progression of pulmonary diseases, suggesting that smoking tobacco-free waterpipe products is also a pulmonary disease risk factor.
DISCUSSION
This study complements previous work regarding the potential health risks of waterpipe smoking in two important ways. First, by using a variety of waterpipe tobacco brands and smoke generated in a manner that mimics human waterpipe tobacco smoking, it extends previous findings demonstrating that waterpipe tobacco smoking is likely to pose significant health risk, at least as indexed by the standard toxicological assays reported here. Second, it demonstrates that the smoke produced by tobacco-free waterpipe preparations also may be associated with the same health risks. Limitations of this study include the fact that the topography records used to generate smoke were produced using a sample of “occasional” waterpipe users; more experienced users may smoke more intensely, especially when using tobacco-free preparations (Cobb, Sahmarani, Eissenberg, & Shihadeh, 2012). Another limitation is that tobacco-specific nitrosamines, a class of powerful carcinogens, were not measured in this study. It is unlikely that these would be found in the tobacco-free products.
In line with previous findings that nonnicotine toxicant emissions and exposure, in addition to changes in heart rate variability, are similar regardless of the tobacco content of the product smoked, we found clear evidence that tobacco-free waterpipe smoke involves similar intrinsic risk of damage to lung cells as does the smoke of tobacco-based products. These findings are also consistent with the notion that an important fraction of the proinflammatory content of waterpipe smoke is derived from the burning charcoal, which does not depend on the tobacco content of the product smoked. Indeed, previous work (Monzer, Sepetdjian, Saliba, & Shihadeh, 2008) has demonstrated that more than 90% of the polycyclic aromatic hydrocarbon and CO content of waterpipe tobacco smoke is derived from the charcoal.
To date, all available evidence on toxicant emissions, biological activity, and acute physiological responses to tobacco-free waterpipe products points in the same direction: except for nicotine addiction, tobacco-free waterpipe preparations entail similar health hazards as tobacco-based preparations and should therefore be subject to the same controls, including restrictions on advertising that conveys a reduced-harm message in association with tobacco-free products.
FUNDING
This work was supported by U.S. Public Health Service (R01CA120142, R01DA025659).
DECLARATION OF INTERESTS
None declared.
REFERENCES
- Blank M. D., Cobb C. O., Kilgalen B., Austin J., Weaver M. F., Shihadeh A., Eissenberg T. (2011). Acute effects of waterpipe tobacco smoking: a double-blind, placebo-control study. Drug and Alcohol Dependence, 116, 102–109. 10.1016/j.drugalcdep.2010.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb C. O., Sahmarani K., Eissenberg T., Shihadeh A. (2012). Acute toxicant exposure and cardiac autonomic dysfunction from smoking a single narghile waterpipe with tobacco and with a “healthy” tobacco-free alternative. Toxicology Letters, 215, 70–75. 10.1016/ j.toxlet.2012.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Government of Alberta. (2012). Waterpipe smoking in Alberta: a report by the chief medical officer of health. Retrieved from http://www.health.alberta.ca/documents/CMOH-Waterpipe-Smoking-2012.pdf
- Johnson M. D., Schilz J., Djordjevic M. V., Rice J. R., Shields P. G. (2009). Evaluation of in vitro assays for assessing the toxicity of cigarette smoke and smokeless tobacco. Cancer Epidemiology, Biomarkers & Prevention 18, 3263–3304.10.1158/1055–9965.EPI-09-0965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzer B., Sepetdjian E., Saliba N., Shihadeh A. (2008). Charcoal emissions as a source of CO and carcinogenic PAH in mainstream narghile waterpipe smoke. Food & Chemical Toxicology, 46, 2991–2995. 10.1016/j.fct.2008.05.031 [DOI] [PubMed] [Google Scholar]
- Rammah M., Dandachi F., Salman R., Shihadeh A., El-Sabban M. (2012). In vitro cytotoxicity and mutagenicity of mainstream waterpipe smoke and its functional consequences on alveolar type II derived cells. Toxicology Letters, 211, 220–231. 10.1016/j.toxlet.2012.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rammah M., Dandachi F., Salman R., Shihadeh A., El-Sabban M. (2013). In vitro effects of waterpipe smoke condensate on endothelial cell function: a potential risk factor for vascular disease. Toxicology Letters, 219, 133–142. 10.1016/j.toxlet.2013.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh R., Shihadeh A. (2008). Elevated toxicant yields with narghile waterpipes smoked using a plastic hose. Food & Chemical Toxicology:46, 1461–1466. 10.1016/j.fct.2007.12.007 [DOI] [PubMed] [Google Scholar]
- Shihadeh A., Antonios C., Azar S. (2005). A portable, low-resistance puff topography instrument for pulsating, high-flow smoking devices. Behavior Research Methods, 37, 186–191. 10.3758/BF03206414 [DOI] [PubMed] [Google Scholar]
- Shihadeh A., Azar S. (2006). A closed-loop control “playback” smoking machine for generating mainstream smoke aerosols. Journal of Aerosol Medicine 19, 137–147.10.1089/jam.2006.19.137 [DOI] [PubMed] [Google Scholar]
- Shihadeh A., Salman R., Jaroudi E., Saliba N., Sepetdjian E., Blank M., …Eissenberg T. (2012). Does switching to a tobacco-free waterpipe product reduce toxicant intake? A crossover study comparing CO, NO, PAH, volatile aldehydes, “tar” and nicotine yields. Food & Chemical Toxicology, 50, 1494–1498. 10.1016/ j.fct.2012.02.041 [DOI] [PMC free article] [PubMed] [Google Scholar]