Abstract
Purpose of review
Interest in the myofibroblast as a key player in propagation of chronic progressive fibrosis continues to elicit many publications, with focus on its cellular origins and the mechanisms underpinning their differentiation and/or transition. The objective of the review is to highlight this recent progress.
Recent findings
The epithelial origin of the myofibroblast in fibrosis has been challenged by recent studies, with the pericyte suggested as a possible precursor instead. Additional signaling pathways, including Notch, Wnt, and hedgehog, are implicated in myofibroblast differentiation. The importance of NADPH oxidase 4 was highlighted recently to suggest a potential link between cellular/oxidative stress and the genesis of the myofibroblast. Recent observations on the importance of lysophosphatidic acid in fibrosis suggest that this may be due, in part, to its ability to regulate myofibroblast differentiation. Finally, there is increasing evidence for the role of epigenetic mechanisms in regulating myofibroblast differentiation, including DNA methylation and miRNA regulation of gene expression.
Summary
These recent discoveries open up a whole new array of potential targets for novel antifibrotic therapies. This is of special importance given the current bleak outlook for chronic progressive fibrotic diseases, such as scleroderma, due to lack of effective therapies.
Keywords: epithelial–mesenchymal transition, epigenetic regulation, fibrosis, myofibroblast
INTRODUCTION
A key feature of myofibroblasts is expression of α-smooth muscle actin (α-SMA) [1■]. They also express other marker genes depending on their anatomic localization and their degree of activation [1■]. Their de-novo emergence in response to tissue injury along with their ability to express high levels of extracellular matrix and fibrogenic cytokines [1■,2] make them key players in the subsequent repair process and wound healing [1■,2,3]. The purpose of this review is to highlight the latest information on the origin and regulation of myofibroblast differentiation, function, and fate in the past year.
ORIGIN OF MYOFIBROBLASTS
Myofibroblasts are rarely found in normal tissue except for some specialized regions [2,4]. However, a large number of myofibroblasts appear de novo in response to tissue injury, with gradual disappearance by apoptosis upon successful repair [1■]. However their persistence is associated with chronic fibrosis that usually progresses to loss of function of the affected organs [1■]. At least three major cellular sources have been proposed for the myofibroblasts that emerge de novo in fibrosis.
RESIDENT FIBROBLASTS OR PERICYTES
Fibroblasts are present in virtually all tissues and organs, albeit in limited numbers under normal conditions [4]. In-situ activation of normally quiescent resident fibroblasts in response to extracellular triggers, such as Transforming Growth Factor β1 [5–7], Wnt [5,8], Jagged/Notch [9■,10], Fizz1 [10], and hedgehog [11■■] are well documented. Direct evidence is obtained from in-vitro tissue culture experiments in which de-novo expression of α-SMA was observed when isolated tissue fibroblasts are appropriately stimulated [5,6,8,9■,11■■]. Transgenic models utilizing elegant gene reporter strategies to define specific myofibroblast lineages determine that these cells are resident fibroblast-like cells or pericytes located exclusively in the perivascular interstitium and not derived from an epithelial source [12,13]. This finding is consistent with a previous kinetic study [14] in which de-novo α-SMA expression in pulmonary fibrosis is first found to localize to the adventitia of blood vessels and airways.
BONE MARROW-DERIVED PROGENITORS
The ability of bone marrow-derived cells to localize and populate distal tissue sites has been demonstrated by bone marrow transplantation studies [15–18], but their ability to differentiate into myofibroblasts is controversial. One study [19] suggests that bone marrow-derived cells contribute to more than 20% of the myofibroblasts in pancreatic injury. Another study [20] suggests derivation from CD14+ monocytes, although the myofibroblast phenotype is lacking in contractile function. In contrast, other studies [15,17,21,22] cannot demonstrate significant contribution of bone marrow-derived cells to the myofibroblast population in lung, liver, kidney, and skin. The basis for these discrepant results remains unclear.
EPITHELIAL AND ENDOTHELIAL ORIGIN OF MYOFIBROBLASTS
Epithelial cells may undergo dedifferentiation and express mesenchymal markers through a process called epithelial–mesenchymal transition (EMT) [23]. Originally proposed in the fibrotic kidney as a source of myofibroblasts, EMT has subsequently been similarly implicated in fibrosis affecting other organs. The importance of endothelial cells as a source of myofibroblasts via EMT has also been suggested using similar approaches [24]. However, despite this abundant evidence, especially in vitro, the in-vivo significance of these processes remains uncertain. Although epithelial cells with myofibroblast features can be identified in cultured epithelial cells, the evidence for EMT in vivo is equivocal and sometimes contradictory [13,25–27,28■■]. In a recent study [28■■] using inducible cell lineage-specific transgenic alleles in a model of pulmonary fibrosis, the authors are unable to show the epithelial origin of myofibroblasts. Moreover, they cannot demonstrate the pericyte as a myofibroblast progenitor but instead suggest other heterogeneous stromal cells as the likely source for myofibroblasts in this model of pulmonary fibrosis [28■■]. In human studies [29–31], a small number of epithelial cells with mesenchymal and myofibroblast markers have been described in biopsies from patients with lung allograft rejection oridiopathic pulmonary fibrosis (IPF). However, another study [32] cannot demonstrate the presence of cells with both epithelial (E-cadherin, ICAM-1, LEA, CD44v9, or SP-A) and myofibroblast markers (α-SMA or vimentin) in lung tissue sections from patients with IPF or nonspecific interstitial pneumonia. As with the controversy on the bone marrow origin of the myofibroblasts, the basis for these discrepancies is not clear and likely will engender further future studies on this topic.
REGULATION OF MYOFIBROBLAST DIFFERENTIATION
Regulation of myofibroblast differentiation is primarily investigated in terms of the regulation of myofibroblast marker genes, especially the key marker of differentiation, the α-SMA gene [1■]. The DNA sequence and promoter analysis have identified a series of cis-acting elements and their corresponding trans-acting factors [1■]. Many of them function in combinatorial fashion as reviewed previously [1■]. The list of factors and their interactions capable of regulating myofibroblast differentiation continue to grow, and recent progress will be discussed in the following sections. They will be organized on the basis of signaling pathways, downstream transcriptional, and epigenetic regulation.
TGFβ signaling
The stimulation of myofibroblast differentiation by TGFβ is well documented and mediated by Smads and relevant Ras/ERK/MAPK kinases in conjunction with other transcription factors, such as Sp1/Sp3, TEF-1, and KLF4 [1■,2,33]. Additionally, recent studies [34,35] indicate that TGFβ also induces NADPH oxidase 4 (Nox4), a source for reactive oxygen species, thus providing a link between oxidative stress and myofibroblast differentiation. Moreover, expression of Nox4 induces Smad2/3 phosphorylation that promotes myofibroblast differentiation [34,35]. Elevated expression of Nox4 is reported in hyperplastic alveolar type II cells and fibroblasts in the lungs of patients with IPF [34,36], thus suggesting a potential role in pathogenesis. This possibility is supported by animal model studies [37■■,38] showing deficient fibrosis in Nox4 knockout mice or by treatment with Nox inhibitors. Another recent study [39] confirms the importance of MyoD in TGFβ-induced myofibroblast differentiation and concludes that differentiation is reversible. However, other studies [40,41] suggest that disappearance of myofibroblasts in successful wound healing occurs via apoptosis rather than a process of dedifferentiation. Interestingly, bFGF or FGF-2 is found to inhibit myofibroblast differentiation in the latter study and is likely mediated by enhanced expression of Nkx2.5, a repressor of α-SMA gene expression [42]. Another modulator of TGFβ signaling is Cx43, which is found to mediate the activation of the α-SMA gene by TGFβ [43] by competing with Smads for binding to microtubules [44]. Finally, another soluble agonist capable of inducing myofibroblast differentiation is lysophosphatidic acid [45], which activates a chloride channel and depends on autocrine TGFβ to induce differentiation [46]. The importance of lysophosphatidic acid in fibrosis [47] may be mediated in part through this ability to promote myofibroblast differentiation.
Wnt signaling
The importance of Wnt signaling in fibrosis [48,49■] suggests its potential importance in myofibroblast differentiation. Moreover, its importance in EMT [50,51] suggests another way in which this signaling pathway can participate in genesis of the myofibroblast. Indeed, several recent studies indicate that Wnt signaling is important in induction of myofibroblast differentiation [5] and in part by being activated by TGFβ [52]. However, Wnt3a is also reported to enhance TGFβ expression and signaling [53], suggesting a potential positive feedback loop on its effect on myofibroblast differentiation.
Notch signaling
Four members of Notch signaling have been identified in mammalian cells [54]. All of them except for Notch4 are capable of regulating myofibroblast differentiation [10,55–58]. Notch1 and Notch3 are known to stimulate α-SMA gene expression in lung fibroblasts [10] and hepatic stellate cells [55], whereas Notch2 inhibits TGFβ-induced α-SMA and collagen I gene expression through downregulation of Notch3 in myoblasts [57]. However, in 10T1/2 fibroblasts, Notch3 represses expression of smooth muscle target genes including α-SMA by inhibition of the activation of Smad3 and p38 mitogen-activated protein kinase [58]. In contrast, in alveolar epithelial cells, Notch1 induces the phosphorylation of Smad3 and activates α-SMA gene transcription in a SRF-binding site [CC(A/T)6GG, termed CArG box]-dependent and TGFβ control element-dependent manner [59]. Other experiments also suggest that Notch1 suppresses fibroblast proliferation that depends on Wnt11-dependent WISP-1 expression [60]. The importance of Notch signaling in fibrosis [61] including in scleroderma may be due to the activating effects of this signaling pathway on myofibroblast differentiation, including that via EMT and endothelial–mesenchymal transition.
Hedgehog signaling
Hedgehog signaling is primarily known for its critical function in development and cell differentiation as well as in cancer [62–66]. Sonic hedgehog (Shh) is the most widely expressed and recently shown to be implicated in fibrotic disorders [62]. It is highly induced in epithelial cells at sites of fibrotic disease [67]. Activation of hedgehog induces, whereas its inhibition with either siRNA or inhibitors suppresses, myofibroblast differentiation markers of gene expression including α-SMA, desmin, fibronectin, and collagen I expression [68]. Additionally Shh can mediate EMT in liver fibrosis [69]. In vivo, Gli1-deficient mice exhibit reduced interstitial fibrosis in kidneys after obstructive injury [68]. Suppressing the Shh signal with inhibitor against either Shh or its downstream mediator Smo prevented myofibroblast differentiation, reduced extracellularmatrix expression, and mitigated fibrotic lesions [68,70■■,71■■].
EPIGENETIC REGULATION
The epigenetic regulation of gene expression includes DNA methylation, histone modification and their interaction with DNA, as well as small interfering RNA-mediated gene regulation [2,3,72]. All these factors are found to be involved in the regulation of myofibroblast differentiation.
DNA methylation
DNA methylation is commonly associated with repression of the affected genes and is catalyzed by DNA methyl transferases (DNMTS) [72]. There is mounting evidence to suggest its importance in the regulation of myofibroblast differentiation [1■,6]. A recent study [73■] reveals widespread differences in global DNA methylation patterns between lung tissue from IPF patients when compared with those from controls [73■]. Interestingly these altered patterns of DNA methylation in IPF lung show some similarities to the changes observed in lung cancer samples. Although no significant alterations in overall global DNA methylation are observed, differentially methylated CpG islands and RNA expression of their affected genes have been identified between IPF and control lungs [73■]. However, global hypomethylation of genomic DNA is observed in cancer-associated myofibroblasts and in early-stage liver fibrosis [74,75]. For the α-SMA gene, differential DNA methylation has been identified between fibroblasts and lung alveolar epithelial type II cells [6]. Although the α-SMA gene promoter region is highly methylated in both cell types, the first intronic region is only highly methylated in the epithelial cells, which do not express this gene. Moreover induced overexpression or underexpression of DNMTS suppresses or activates α-SMA gene expression, respectively, consistently with inhibition of myofibroblast differentiation by DNA methylation. This is also supported by in-vitro evidence that DNA hypermethylation of the α-SMA promoter abolished its activity [6]. However, DNA methylation will also affect expression of genes other than α-SMA, which may also affect myofibroblast differentiation indirectly. For example, in hepatic stellate cells, inhibition of DNA methylation leads to activation of Peroxisome Proliferator-Activated Receptor γ (PPARγ) [76], a repressor for α-SMA gene expression [77,78], resulting in inhibition of myofibroblast differentiation. The specific mechanism by which DNA methylation affects α-SMA gene expression is not clear; however, it does enhance binding of the trans-acting factor MeCP2 to the methylated α-SMA DNA fragments [79■]. Although methylation of the α-SMA gene increases binding of MeCP2 and inhibits myofibroblast differentiation, paradoxically MeCP2 is found to be essential for fibrosis and enhances myofibroblast differentiation. This may indicate that additional effects of MeCP2 on other target genes also significantly influence myofibroblast differentiation, perhaps via repression of PPARγ expression [78]. Another relevant gene target subject to regulation by DNA methylation is Thy-1 [80] whose expression and interaction with αVβ5 integrin disrupt contraction-dependent TGFβ activation and myofibroblast differentiation [81,82].
Histone modification and their interaction with DNA
The importance of histone acetylation in regulating myofibroblast differentiation is initially suggested by evidence that trichostatin A, a histone deacetylase (HDAC) inhibitor, is an inhibitor of TGFβ1-induced α-SMA and type I collagen expression, but has since been confirmed in multiple studies of fibrosis in other organ systems [83]. For example, knockdown of HDAC4 inhibits TGFβ-induced α-SMA expression through phosphorylation of Akt [84]. Another HDAC inhibitor, spiruchostatin A, is also found to be effective in suppressing TGFβ-induced human lung myofibroblast differentiation [85]. It is noteworthy that HDAC inhibition also activates Thy-1 expression, in part by reducing DNA methylation status of this gene with expected consequences on myofibroblast differentiation [86■]. Thus, it is likely that future studies will yield further insights into these complex interactions between these two modes of epigenetic regulation.
Regulation by small interfering RNAs
Small interfering RNAs are small noncoding RNAs (approximately 22 nucleotides) that lead to silencing of genetic information through posttranscriptional degradation of messenger RNA and/or translational inhibition of protein expression [87]. These are primarily microRNAs, many of which were found recently to regulate myofibroblast differentiation and fibrosis [88]. Despite their broad range of targets, their overall effect on myofibroblast differentiation has begun to be identified. For example, miR-21, which targets Smad7 [89] and programmed cell death 4 [90], enhances myofibroblast differentiation and lung fibrosis. On the contrary, miR-146a by targeting SMAD4 [91■], miR-132 by targeting MeCP2 [78], andmir-155 by inhibiting ERK1/2 phosphorylation [92] have a suppressive effect on myofibroblast differentiation. Other microRNAs such as miR-29 also may play a role in myofibroblast differentiation and fibrosis, but their relevant target genes remain unclear. There is some evidence that miR-29 targets collagen types I and IV mRNAs [93], but appears to enhance collagen gene transcription by targeting DNMTs and consequent inhibiting DNA methylation [94]. Further studies are necessary to resolve these apparently conflicting effects of miR-29 on a key phenotypic property of the myofibroblast.
SIGNIFICANCE OF MYOFIBROBLAST DIFFERENTIATION
Myofibroblast differentiation represents a key event during wound healing, tissue repair, as well as chronic fibrosis [1■,2,3]. The high contractile force generated by myofibroblasts is beneficial for physiological tissue remodeling but detrimental for tissue function when it becomes excessive such as in hypertrophic scars, in virtually all fibrotic diseases, and during stromal reaction to tumors [3]. The myofibroblast are shown to be the major extracellular matrix producing cells in fibrotic diseases in a variety of organs [1■,8]. However, despite evidence suggesting that suppression of myofibroblast differentiation correlates with reduced fibrosis [1■,2,3], direct proof is lacking that this is due specifically to the suppression of de-novo genesis of the myofibroblast. More direct evidence was obtained recently in a study [95■■] using mesenchymal cell/fibroblast-specific conditional CCAAT/Enhancer Binding Protein β (C/EBPβ) knock out mice. These mice had reduced myofibroblasts and pulmonary fibrosis but an intact inflammatory/immune cell response when endotracheally injected with bleomycin [95■■]. Thus, despite the broad spectrum of C/EBPβ target genes in multiple cell types, its selective depletion in fibroblasts results in diminished myofibroblast differentiation and fibrosis.
CONCLUSION
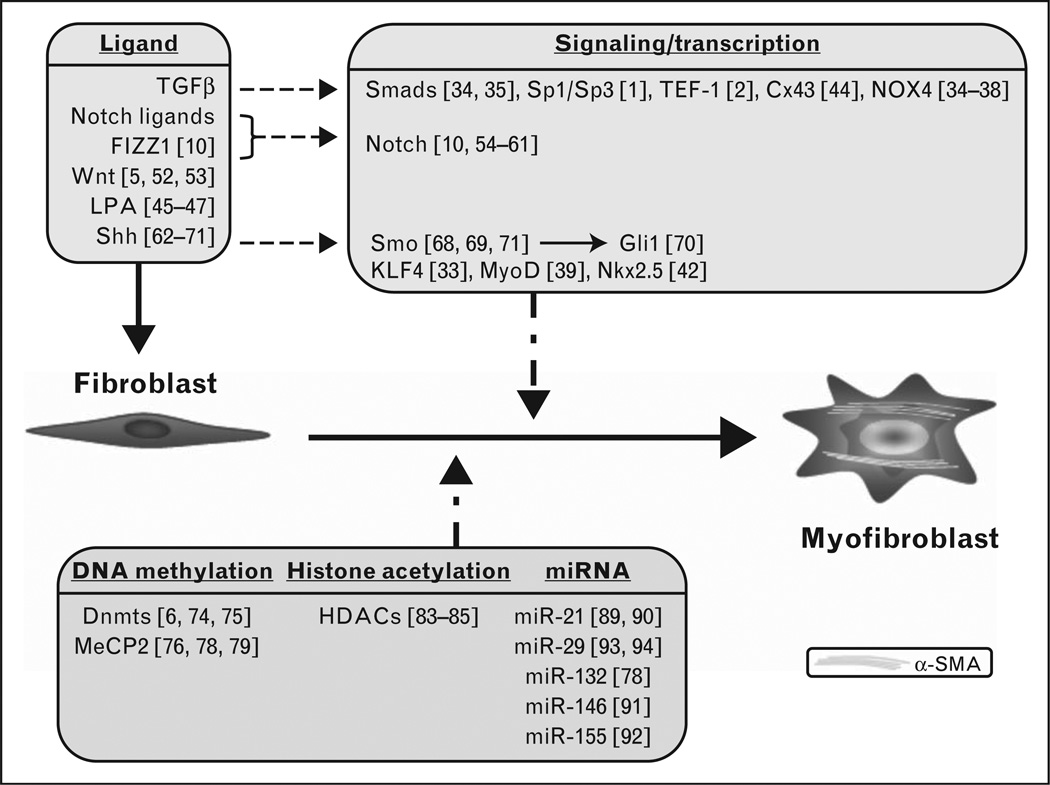
The focus of recent studies is on critical mechanisms underlying genesis of myofibroblasts (summarized in Fig. 1). These studies elucidate the importance of the major signaling pathways, including TGFβ, Wnt, Notch, and hedgehog pathways along with their downstream transcription factor targets that mediate their effects on gene expression. Additionally, mounting evidence for epigenetic regulatory mechanisms has been identified in the control of myofibroblast differentiation. Future studies should reveal more of the complexities underlying these mechanisms and how they interact to ultimately regulate myofibroblast differentiation and fate.
FIGURE 1.
Regulation of myofibroblast differentiation. Recently reported diverse ligands, signaling pathways, transcription, and epigenetic factors are summarized in this cartoon. The numbers within the square brackets refer to the relevant references. The respective factors are primarily reviewed from the standpoint of α-SMA as the target myofibroblast marker gene, but are also relevant to other genes associated with myofibroblast differentiation and function as described in the text. The fibroblast is indicated as the myofibroblast progenitor cell, but many of these factors play similar roles in differentiation from other progenitor cell types as discussed in the relevant sections. DNMTS, DNA methyl transferases; HDAC, histone deacetylase; LPA, lysophosphatidic acid; SMA, smooth muscle actin.
KEY POINTS.
Increasing evidence for local stromal origin of myofibroblasts.
Importance of the hedgehog, Notch, and Wnt signaling pathways highlighted.
NOX4 mediates myofibroblast differentiation.
Epigenetic mechanisms regulate myofibroblast differentiation.
Acknowledgements
None.
This work was supported in part by grants HL28737, HL52285, HL77297 and HL91775 from the National Institute of Health.
Footnotes
Conflicts of interest
The authors report no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 151).
- 1. Hinz B, Phan SH, Thannickal VJ, et al. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol. 2012;180:1340–1355. doi: 10.1016/j.ajpath.2012.02.004. This recent review summarizes the key regulators of myofibroblast differentiation identified in the past few years.
- 2.Hinz B, Phan SH, Thannickal VJ, et al. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526–537. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 4.Meran S, Steadman R. Fibroblasts and myofibroblasts in renal fibrosis. Int J Exp Pathol. 2011;92:158–167. doi: 10.1111/j.1365-2613.2011.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu J, Wang Y, Pan Q, et al. Wnt/beta-catenin pathway forms a negative feedback loop during TGF-beta1 induced human normal skin fibroblast-to-myofibroblast transition. J Dermatol Sci. 2012;65:38–49. doi: 10.1016/j.jdermsci.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Hu B, Gharaee-Kermani M, Wu Z, Phan SH. Epigenetic regulation of myofibroblast differentiation by DNA methylation. Am J Pathol. 2010;177:21–28. doi: 10.2353/ajpath.2010.090999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chapman HA. Epithelial-mesenchymal interactions in pulmonary fibrosis. Annu Rev Physiol. 2011;73:413–435. doi: 10.1146/annurev-physiol-012110-142225. [DOI] [PubMed] [Google Scholar]
- 8.George SJ. Regulation of myofibroblast differentiation by convergence of the Wnt and TGF-beta1/Smad signaling pathways. J Mol Cell Cardiol. 2009;46:610–611. doi: 10.1016/j.yjmcc.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 9. Dees C, Tomcik M, Zerr P, et al. Notch signalling regulates fibroblast activation and collagen release in systemic sclerosis. Ann Rheum Dis. 2011;70:1304–1310. doi: 10.1136/ard.2010.134742. An up-to-date review that summarizes the progress on Notch signaling regulation of myofibroblast differentiation in the past few years.
- 10.Liu T, Hu B, Choi YY, et al. Notch1 signaling in FIZZ1 induction of myofibroblast differentiation. Am J Pathol. 2009;174:1745–1755. doi: 10.2353/ajpath.2009.080618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Horn A, Palumbo K, Cordazzo C, et al. Hedgehog signaling controls fibroblast activation and tissue fibrosis in systemic sclerosis. Arthritis Rheum. 2012;64:2724–2733. doi: 10.1002/art.34444. The importance of hedgehog in the fibrosis is suggested for both human disease and an animal model.
- 12.Lin SL, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol. 2008;173:1617–1627. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humphreys BD, Lin SL, Kobayashi A, et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang K, Rekhter MD, Gordon D, Phan SH. Myofibroblasts and their role in lung collagen gene expression during pulmonary fibrosis: a combined immunohistochemical and in situ hybridization study. Am J Pathol. 1994;145:114–125. [PMC free article] [PubMed] [Google Scholar]
- 15.Hashimoto N, Jin H, Liu T, et al. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest. 2004;113:243–252. doi: 10.1172/JCI18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mori L, Bellini A, Stacey MA, et al. Fibrocytes contribute to the myofibroblast population in wounded skin and originate from the bone marrow. Exp Cell Res. 2005;304:81–90. doi: 10.1016/j.yexcr.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Kisseleva T, Uchinami H, Feirt N, et al. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J Hepatol. 2006;45:429–438. doi: 10.1016/j.jhep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Deng C, Wang J, Zou Y, et al. Characterization of fibroblasts recruited from bone marrow-derived precursor in neonatal bronchopulmonary dysplasia mice. J Appl Physiol. 2011;111:285–294. doi: 10.1152/japplphysiol.00201.2010. [DOI] [PubMed] [Google Scholar]
- 19.Akita S, Kubota K, Kobayashi A, et al. Role of bone marrow cells in the development of pancreatic fibrosis in a rat model of pancreatitis induced by a choline-deficient/ethionine-supplemented diet. Biochem Biophys Res Commun. 2012;420:743–749. doi: 10.1016/j.bbrc.2012.03.060. [DOI] [PubMed] [Google Scholar]
- 20.Binai N, O’Reilly S, Griffiths B, et al. Differentiation potential of CD14+ monocytes into myofibroblasts in patients with systemic sclerosis. PLoS One. 2012;7:e33508. doi: 10.1371/journal.pone.0033508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yokota T, Kawakami Y, Nagai Y, et al. Bone marrow lacks a transplantable progenitor for smooth muscle type alpha-actin-expressing cells. Stem Cells. 2006;24:13–22. doi: 10.1634/stemcells.2004-0346. [DOI] [PubMed] [Google Scholar]
- 22.Barisic-Dujmovic T, Boban I, Clark SH. Fibroblasts/myofibroblasts that participate in cutaneous wound healing are not derived from circulating progenitor cells. J Cell Physiol. 2010;222:703–712. doi: 10.1002/jcp.21997. [DOI] [PubMed] [Google Scholar]
- 23.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Piera-Velazquez S, Li Z, Jimenez SA. Role of endothelial-mesenchymal transition (EndoMT) in the pathogenesis of fibrotic disorders. Am J Pathol. 2011;179:1074–1080. doi: 10.1016/j.ajpath.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanjore H, Xu XC, Polosukhin VV, et al. Contribution of epithelial-derived fibroblasts to bleomycin-induced lung fibrosis. Am J Respir Crit Care Med. 2009;180:657–665. doi: 10.1164/rccm.200903-0322OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scholten D, Osterreicher CH, Scholten A, et al. Genetic labeling does not detect epithelial-to-mesenchymal transition of cholangiocytes in liver fibrosis in mice. Gastroenterology. 2010;139:987–998. doi: 10.1053/j.gastro.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chapman HA. Epithelial responses to lung injury: role of the extracellular matrix. Proc Am Thorac Soc. 2012;9:89–95. doi: 10.1513/pats.201112-053AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rock JR, Barkauskas CE, Cronce MJ, et al. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci USA. 2011;108:E1475–E1483. doi: 10.1073/pnas.1117988108. A more recent study using cell fate tracking demonstrates local lung stromal cells as the likely origin for myofibroblasts in pulmonary fibrosis, disputing EMT and pericytes as their progenitors.
- 29.Carvajal G, Droguett A, Burgos ME, et al. Gremlin: a novel mediator of epithelial mesenchymal transition and fibrosis in chronic allograft nephropathy. Transplant Proc. 2008;40:734–739. doi: 10.1016/j.transproceed.2008.02.064. [DOI] [PubMed] [Google Scholar]
- 30.Tyler JR, Robertson H, Booth TA, et al. Chronic allograft nephropathy: intraepithelial signals generated by transforming growth factor-beta and bone morphogenetic protein-7. Am J Transplant. 2006;6:1367–1376. doi: 10.1111/j.1600-6143.2006.01339.x. [DOI] [PubMed] [Google Scholar]
- 31.Kim KK, Kugler MC, Wolters PJ, et al. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci USA. 2006;103:13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamada M, Kuwano K, Maeyama T, et al. Dual-immunohistochemistry provides little evidence for epithelial-mesenchymal transition in pulmonary fibrosis. Histochem Cell Biol. 2008;129:453–462. doi: 10.1007/s00418-008-0388-9. [DOI] [PubMed] [Google Scholar]
- 33.Phan SH. Biology of fibroblasts and myofibroblasts. Proc Am Thorac Soc. 2008;5:334–337. doi: 10.1513/pats.200708-146DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amara N, Goven D, Prost F, et al. NOX4/NADPH oxidase expression is increased in pulmonary fibroblasts from patients with idiopathic pulmonary fibrosis and mediates TGFbeta1-induced fibroblast differentiation into myofibroblasts. Thorax. 2010;65:733–738. doi: 10.1136/thx.2009.113456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bondi CD, Manickam N, Lee DY, et al. NAD(P)H oxidase mediates TGF-beta1-induced activation of kidney myofibroblasts. J Am Soc Nephrol. 2010;21:93–102. doi: 10.1681/ASN.2009020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carnesecchi S, Deffert C, Donati Y, et al. A key role for NOX4 in epithelial cell death during development of lung fibrosis. Antioxid Redox Signal. 2011;15:607–619. doi: 10.1089/ars.2010.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jiang JX, Chen X, Serizawa N, et al. Liver fibrosis and hepatocyte apoptosis are attenuated by GKT137831, a novel NOX4/NOX1 inhibitor in vivo. Free Radic Biol Med. 2012;53:289–296. doi: 10.1016/j.freeradbiomed.2012.05.007. A significant translational study reports on the in-vivo effects of NOXx4/NOX1 inhibitor on fibrosis and shown to have pharmaceutical potential.
- 38.Hecker L, Vittal R, Jones T, et al. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med. 2009;15:1077–1081. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hecker L, Jagirdar R, Jin T, Thannickal VJ. Reversible differentiation of myofibroblasts by MyoD. Exp Cell Res. 2011;317:1914–1921. doi: 10.1016/j.yexcr.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Desmouliere A, Chaponnier C, Gabbiani G. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen. 2005;13:7–12. doi: 10.1111/j.1067-1927.2005.130102.x. [DOI] [PubMed] [Google Scholar]
- 41.Ishiguro S, Akasaka Y, Kiguchi H, et al. Basic fibroblast growth factor induces down-regulation of alpha-smooth muscle actin and reduction of myofibroblast areas in open skin wounds. Wound Repair Regen. 2009;17:617–625. doi: 10.1111/j.1524-475X.2009.00511.x. [DOI] [PubMed] [Google Scholar]
- 42.Hu B, Wu YM, Wu Z, Phan SH. Nkx2.5/Csx represses myofibroblast differentiation. Am J Respir Cell Mol Biol. 2010;42:218–226. doi: 10.1165/rcmb.2008-0404OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Asazuma-Nakamura Y, Dai P, Harada Y, et al. Cx43 contributes to TGF-beta signaling to regulate differentiation of cardiac fibroblasts into myofibroblasts. Exp Cell Res. 2009;315:1190–1199. doi: 10.1016/j.yexcr.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 44.Dai P, Nakagami T, Tanaka H, et al. Cx43 mediates TGF-beta signaling through competitive Smads binding to microtubules. Mol Biol Cell. 2007;18:2264–2273. doi: 10.1091/mbc.E06-12-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jeon ES, Moon HJ, Lee MJ, et al. Cancer-derived lysophosphatidic acid stimulates differentiation of human mesenchymal stem cells to myofibroblastlike cells. Stem Cells. 2008;26:789–797. doi: 10.1634/stemcells.2007-0742. [DOI] [PubMed] [Google Scholar]
- 46.Yin Z, Watsky MA. Chloride channel activity in human lung fibroblasts and myofibroblasts. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1110–L1116. doi: 10.1152/ajplung.00344.2004. [DOI] [PubMed] [Google Scholar]
- 47.Shea BS, Tager AM. Role of the lysophospholipid mediators lysophosphatidic acid and sphingosine 1-phosphate in lung fibrosis. Proc Am Thorac Soc. 2012;9:102–110. doi: 10.1513/pats.201201-005AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim TH, Kim SH, Seo JY, et al. Blockade of the Wnt/beta-catenin pathway attenuates bleomycin-induced pulmonary fibrosis. Tohoku J Exp Med. 2011;223:45–54. doi: 10.1620/tjem.223.45. [DOI] [PubMed] [Google Scholar]
- 49. Akhmetshina A, Palumbo K, Dees C, et al. Activation of canonical Wnt signalling is required for TGF-beta-mediated fibrosis. Nat Commun. 2012;3:735. doi: 10.1038/ncomms1734. In this study, the interaction between the Wnt and TGFβ pathway is highlighted.
- 50.Chen HC, Zhu YT, Chen SY, Tseng SC. Wnt signaling induces epithelial-mesenchymal transition with proliferation in ARPE-19 cells upon loss of contact inhibition. Lab Invest. 2012;92:676–687. doi: 10.1038/labinvest.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Howard S, Deroo T, Fujita Y, Itasaki N. A positive role of cadherin in Wnt/beta-catenin signalling during epithelial-mesenchymal transition. PLoS One. 2011;6:e23899. doi: 10.1371/journal.pone.0023899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen JH, Chen WL, Sider KL, et al. beta-catenin mediates mechanically regulated, transforming growth factor-beta1-induced myofibroblast differentiation of aortic valve interstitial cells. Arterioscler Thromb Vasc Biol. 2011;31:590–597. doi: 10.1161/ATVBAHA.110.220061. [DOI] [PubMed] [Google Scholar]
- 53.Carthy JM, Garmaroudi FS, Luo Z, McManus BM. Wnt3a induces myofibroblast differentiation by upregulating TGF-beta signaling through SMAD2 in a beta-catenin-dependent manner. PLoS One. 2011;6:e19809. doi: 10.1371/journal.pone.0019809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hansson EM, Lendahl U, Chapman G. Notch signaling in development and disease. Semin Cancer Biol. 2004;14:320–328. doi: 10.1016/j.semcancer.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 55.Chen S, Xu L, Lin N, et al. Activation of Notch1 signaling by marrow-derived mesenchymal stem cells through cell-cell contact inhibits proliferation of hepatic stellate cells. Life Sci. 2011;89:975–981. doi: 10.1016/j.lfs.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 56.Chen YX, Weng ZH, Zhang SL. Notch3 regulates the activation of hepatic stellate cells. World J Gastroenterol. 2012;18:1397–1403. doi: 10.3748/wjg.v18.i12.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ono Y, Sensui H, Okutsu S, Nagatomi R. Notch2 negatively regulates myofibroblastic differentiation of myoblasts. J Cell Physiol. 2007;210:358–369. doi: 10.1002/jcp.20838. [DOI] [PubMed] [Google Scholar]
- 58.Kennard S, Liu H, Lilly B. Transforming growth factor-beta (TGF-1) downregulates Notch3 in fibroblasts to promote smooth muscle gene expression. J Biol Chem. 2008;283:1324–1333. doi: 10.1074/jbc.M706651200. [DOI] [PubMed] [Google Scholar]
- 59.Aoyagi-Ikeda K, Maeno T, Matsui H, et al. Notch induces myofibroblast differentiation of alveolar epithelial cells via transforming growth factor- {beta}-Smad3 pathway. Am J Respir Cell Mol Biol. 2011;45:136–144. doi: 10.1165/rcmb.2010-0140oc. [DOI] [PubMed] [Google Scholar]
- 60.Liu ZJ, Li Y, Tan Y, et al. Inhibition of fibroblast growth by notch1 signaling is mediated by induction of Wnt11-dependent WISP-1. PLoS One. 2012;7:e38811. doi: 10.1371/journal.pone.0038811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kavian N, Servettaz A, Weill B, Batteux F. New insights into the mechanism of notch signalling in fibrosis. Open Rheumatol J. 2012;6:96–102. doi: 10.2174/1874312901206010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Choi SS, Omenetti A, Syn WK, Diehl AM. The role of Hedgehog signaling in fibrogenic liver repair. Int J Biochem Cell Biol. 2011;43:238–244. doi: 10.1016/j.biocel.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McMillan R, Matsui W. Molecular pathways: the hedgehog signaling pathway in cancer. Clin Cancer Res. 2012;18:4883–4888. doi: 10.1158/1078-0432.CCR-11-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Epstein DJ. Regulation of thalamic development by sonic hedgehog. Front Neurosci. 2012;6:57. doi: 10.3389/fnins.2012.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.VanHook AM. Focus issue: fine-tuning Hedgehog signaling in development and disease. Sci Signal. 2011;4:eg10. doi: 10.1126/scisignal.2002653. [DOI] [PubMed] [Google Scholar]
- 66.Carpenter RL, Lo HW. Hedgehog pathway and GLI1 isoforms in human cancer. Discov Med. 2012;13:105–113. [PMC free article] [PubMed] [Google Scholar]
- 67.Stewart GA, Hoyne GF, Ahmad SA, et al. Expression of the developmental sonic hedgehog (Shh) signalling pathway is up-regulated in chronic lung fibrosis and the Shh receptor patched 1 is present in circulating T lymphocytes. J Pathol. 2003;199:488–495. doi: 10.1002/path.1295. [DOI] [PubMed] [Google Scholar]
- 68.Ding H, Zhou D, Hao S, et al. Sonic hedgehog signaling mediates epithelial-mesenchymal communication and promotes renal fibrosis. J Am Soc Nephrol. 2012;23:801–813. doi: 10.1681/ASN.2011060614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Syn WK, Jung Y, Omenetti A, et al. Hedgehog-mediated epithelial-to-mesenchymal transition and fibrogenic repair in nonalcoholic fatty liver disease. Gastroenterology. 2009;137:1478–1488. e1478. doi: 10.1053/j.gastro.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fabian SL, Penchev RR, St-Jacques B, et al. Hedgehog-Gli pathway activation during kidney fibrosis. Am J Pathol. 2012;180:1441–1453. doi: 10.1016/j.ajpath.2011.12.039. This is the first detailed description of paracrine hedgehog signaling in renal fibrosis.
- 71. Horn A, Kireva T, Palumbo-Zerr K, et al. Inhibition of hedgehog signalling prevents experimental fibrosis and induces regression of established fibrosis. Ann Rheum Dis. 2012;71:785–789. doi: 10.1136/annrheumdis-2011-200883. The inhibitor used in this study may have therapeutic potential.
- 72.Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33(Suppl):245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 73. Rabinovich EI, Kapetanaki MG, Steinfeld I, et al. Global methylation patterns in idiopathic pulmonary fibrosis. PLoS One. 2012;7:e33770. doi: 10.1371/journal.pone.0033770. Global methylation patterns and their alterations are investigated in IPF lung samples and compared with those with lung cancer.
- 74.Jiang L, Gonda TA, Gamble MV, et al. Global hypomethylation of genomic DNA in cancer-associated myofibroblasts. Cancer Res. 2008;68:9900–9908. doi: 10.1158/0008-5472.CAN-08-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Komatsu Y, Waku T, Iwasaki N, et al. Global analysis of DNA methylation in early-stage liver fibrosis. BMC Med Genomics. 2012;5:5. doi: 10.1186/1755-8794-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mann J, Oakley F, Akiboye F, et al. Regulation of myofibroblast transdifferentiation by DNA methylation and MeCP2: implications for wound healing and fibrogenesis. Cell Death Differ. 2007;14:275–285. doi: 10.1038/sj.cdd.4401979. [DOI] [PubMed] [Google Scholar]
- 77.Burgess HA, Daugherty LE, Thatcher TH, et al. PPARgamma agonists inhibit TGF-beta induced pulmonary myofibroblast differentiation and collagen production: implications for therapy of lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1146–L1153. doi: 10.1152/ajplung.00383.2004. [DOI] [PubMed] [Google Scholar]
- 78.Mann J, Chu DC, Maxwell A, et al. MeCP2 controls an epigenetic pathway that promotes myofibroblast transdifferentiation and fibrosis. Gastroenterology. 2010;138:705–714. doi: 10.1053/j.gastro.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hu B, Gharaee-Kermani M, Wu Z, Phan SH. Essential role of MeCP2 in the regulation of myofibroblast differentiation during pulmonary fibrosis. Am J Pathol. 2011;178:1500–1508. doi: 10.1016/j.ajpath.2011.01.002. A profibrotic role is established for MeCP2 that may be mediated by enhancement of myofibroblast differentiation.
- 80.Sanders YY, Pardo A, Selman M, et al. Thy-1 promoter hypermethylation: a novel epigenetic pathogenic mechanism in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2008;39:610–618. doi: 10.1165/rcmb.2007-0322OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou Y, Hagood JS, Lu B, et al. Thy-1-integrin alphav beta5 interactions inhibit lung fibroblast contraction-induced latent transforming growth factor-beta1 activation and myofibroblast differentiation. J Biol Chem. 2010;285:22382–22393. doi: 10.1074/jbc.M110.126227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ramirez G, Hagood JS, Sanders Y, et al. Absence of Thy-1 results in TGFbeta induced MMP-9 expression and confers a profibrotic phenotype to human lung fibroblasts. Lab Invest. 2011;91:1206–1218. doi: 10.1038/labinvest.2011.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Niki T, Rombouts K, De Bleser P, et al. A histone deacetylase inhibitor, trichostatin A, suppresses myofibroblastic differentiation of rat hepatic stellate cells in primary culture. Hepatology. 1999;29:858–867. doi: 10.1002/hep.510290328. [DOI] [PubMed] [Google Scholar]
- 84.Guo W, Shan B, Klingsberg RC, et al. Abrogation of TGF-beta1-induced fibroblast-myofibroblast differentiation by histone deacetylase inhibition. Am J Physiol Lung Cell Mol Physiol. 2009;297:L864–L870. doi: 10.1152/ajplung.00128.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Davies ER, Haitchi HM, Thatcher TH, et al. Spiruchostatin A inhibits proliferation and differentiation of fibroblasts from patients with pulmonary fibrosis. Am J Respir Cell Mol Biol. 2012;46:687–694. doi: 10.1165/rcmb.2011-0040OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sanders YY, Tollefsbol TO, Varisco BM, Hagood JS. Epigenetic regulation of thy-1 by histone deacetylase inhibitor in rat lung fibroblasts. Am J Respir Cell Mol Biol. 2011;45:16–23. doi: 10.1165/rcmb.2010-0154OC. This study describes interacting epigenetic mechanisms in regulation of Thy-1 gene expression with implications for myofibroblast differentiation.
- 87.Hamilton AJ, Baulcombe DC. A species of small antisense RNA in posttranscriptional gene silencing in plants. Science. 1999;286:950–952. doi: 10.1126/science.286.5441.950. [DOI] [PubMed] [Google Scholar]
- 88.Pandit KV, Milosevic J, Kaminski N. MicroRNAs in idiopathic pulmonary fibrosis. Transl Res. 2011;157:191–199. doi: 10.1016/j.trsl.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 89.Liu G, Friggeri A, Yang Y, et al. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J Exp Med. 2010;207:1589–1597. doi: 10.1084/jem.20100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Asangani IA, Rasheed SA, Nikolova DA, et al. MicroRNA-21 (miR-21) posttranscriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27:2128–2136. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- 91. Liu Z, Lu CL, Cui LP, et al. MicroRNA-146a modulates TGF-beta1-induced phenotypic differentiation in human dermal fibroblasts by targeting SMAD4. Arch Dermatol Res. 2012;304:195–202. doi: 10.1007/s00403-011-1178-0. A novel function of miR-146a is identified.
- 92.Zheng L, Xu CC, Chen WD, et al. MicroRNA-155 regulates angiotensin II type 1 receptor expression and phenotypic differentiation in vascular adventitial fibroblasts. Biochem Biophys Res Commun. 2010;400:483–488. doi: 10.1016/j.bbrc.2010.08.067. [DOI] [PubMed] [Google Scholar]
- 93.Kwiecinski M, Noetel A, Elfimova N, et al. Hepatocyte growth factor (HGF) inhibits collagen I and IV synthesis in hepatic stellate cells by miRNA-29 induction. PLoS One. 2011;6:e24568. doi: 10.1371/journal.pone.0024568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fabbri M, Garzon R, Cimmino A, et al. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc Natl Acad Sci USA. 2007;104:15805–15810. doi: 10.1073/pnas.0707628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hu B, Wu Z, Nakashima T, Phan SH. Mesenchymal-specific deletion of C/EBPbeta suppresses pulmonary fibrosis. Am J Pathol. 2012;180:2257–2267. doi: 10.1016/j.ajpath.2012.02.010. This is the first demonstration of the mesenchymal cell-specific importance of C/EBPβ in pulmonary fibrosis, presumably due to promotion of myofibroblast differentiation.