Recommendations.
Given the balance of harms and benefits, the Canadian Task Force on Preventive Health Care recommends against the use of combined estrogen–progestin therapy and estrogen-only therapy for the primary prevention of chronic diseases in menopausal women (grade D recommendation).
For women who wish to alleviate menopausal symptoms using hormone replacement therapy (HRT), a discussion between the woman and her physician about the potential benefits and risks of HRT is warranted.
In the early 1990s the Canadian Task Force on Preventive Health Care issued a grade B recommendation for counselling perimenopausal women regarding the use of estrogen replacement therapy (ERT) for the primary prevention of osteoporotic fractures.1 At that time, the large observational studies that constituted the best available evidence further indicated the potential for ERT to confer a cardioprotective benefit to women2,3,4 and to prevent bone loss.1,5 The early large observational studies indicated a small but significant risk of breast cancer,6,7,8 and of endometrial cancer among women with an intact uterus taking unopposed estrogen therapy.9
The evidence base has grown in the last decade, as numerous clinical trials have been conducted on the potential positive and negative effects of hormone replacement therapy (HRT) for various chronic conditions. This statement is based on 3 systematic reviews conducted by the task force6,10,11 and by others12 of the potential benefits and harms of HRT, and it incorporates the results of the estrogen-plus-progestin and the estrogen-only trials of the Women's Health Initiative (WHI), stopped early in May 2002 and February 2004 respectively because of safety concerns.13,14 This statement does not review the evidence for use of HRT in the treatment of menopausal symptoms; instead, it provides a brief discussion of considerations that may be useful for clinicians and their patients when deciding whether HRT should be taken for symptom relief as well as a balance sheet of risks and benefits (Table 1).
Table 1
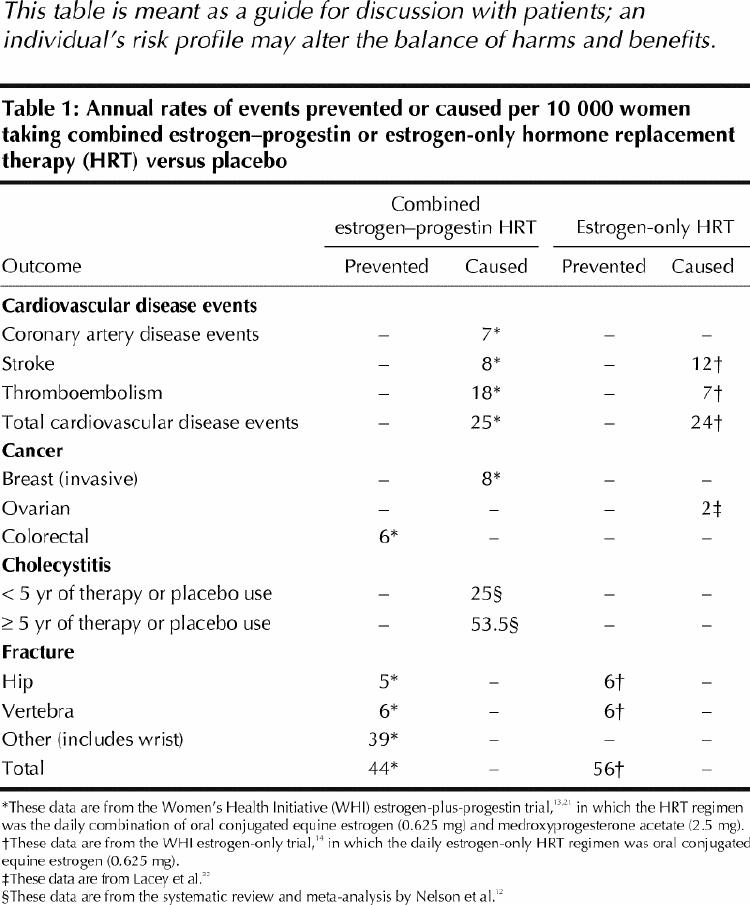
Evidence and clinical summary.
-
Cardiovascular disease
Women in the estrogen–progestin arm of the WHI13,21 had an increased relative risk (RR) of an adverse outcome from cardiovascular disease of 22% (hazard ratio [HR] 1.22, adjusted 95% confidence interval [CI] 1.00– 1.49, or 25 more events per 10 000 person-years of HRT use [157 v. 132 events per 10 000).21 For women in the estrogen-only arm of the WHI, there was no difference in coronary artery disease events (HR 0.91, adjusted 95% CI 0.72–1.15); however, the risk of stroke increased by 39%, with an additional 12 events per 10 000 person-years (HR 1.39, adjusted 95% CI 0.97–1.99). The risk of thromboembolic events increased by 33%, with an additional 7 events per 10 000 person-years. The rate of total cardiovascular disease events, including stroke, was increased by 12% in the estrogen-only group, with an additional 24 events (HR 1.12, adjusted 95% CI 0.97–1.30).14 These findings are consistent with some, but not most, previous observational studies and secondary prevention trials,22,23 as reviewed by Abramson10and summarized in the Apr. 27 issue of CMAJ.24
-
Cancers
Long-term current use of HRT with unopposed estrogen or combination therapy is associated with an increased risk of breast cancer. This risk increases with duration of use.12 The WHI estrogen-plus-progestin results indicated an HR of 1.26 (adjusted 95% CI 1.00–1.59), or an additional 8 cases of invasive breast cancer per 10 000 person-years of HRT use (38 v. 30 events per 10 000 person-years) after 5.2 years.13 The Million Women Study recently showed that, compared with women who never used HRT, those who were using HRT were 1.66 times more likely to develop breast cancer and 1.22 times more likely to die of it.25 Recent data suggest that combination therapy with progestin may confer a higher risk than unopposed estrogen.13,25,26,27
There is some evidence that long-term HRT is associated with an increased risk of ovarian cancer,28,29,30 but studies have shown mixed results.31,32
Both short- and long-term unopposed estrogen therapies are associated with an increased risk of endometrial cancer among women with an intact uterus (RR 2.3, 95% CI 2.1–2.5).33 Combination therapy, especially when progestins are used for more than 10 days, is not associated with any significant increased risk, as indicated by both the WHI results (HR 0.83, adjusted 95% CI 0.29– 2.32) and the meta-analysis of observational studies by Grady and associates33 (overall RR 0.8, 95% CI 0.6– 1.2).
Use of unopposed estrogen or combination therapy (regardless of dose or duration) is associated with a decreased risk of colorectal cancer. The results of the WHI (HR 0.63, adjusted 95% CI 0.32– 1.24, or 6 fewer cancers per 10 000 person-years of HRT use [10 v. 16 events per 10 000]) are consistent with the previous meta-analysis of observational studies by Grodstein and associates,34 which found an RR of 0.80 among women who had ever used HRT (95% CI 0.74– 0.86) and 0.66 among current HRT users (95% CI 0.59– 0.74). How recently HRT was used seems to confer the benefit, rather than merely its use in the past.34,35
The WHI estrogen–progestin arm13 and the estrogen-only arm14 reported composite HRs for all cancers included in their analyses of 1.03 (adjusted 95% CI 0.86– 1.22) and 0.93 (adjusted 95% CI 0.75–1.15) respectively, which is consistent with findings from previous studies.
-
Osteoporotic fractures
-
Other outcomes
-
Women who use HRT are at increased risk of:
venous thromboembolic events (deep vein thrombosis and pulmonary embolism) (HR 2.11, adjusted 95% CI 1.26–3.55)13
cholecystitis in the first 5 years (RR 1.8, 95% CI 1.6–2.0); this risk is increased with sustained use (RR 2.5, 95% CI 2.0–2.9)37
probable dementia, with the recent WHI Memory Study results indicating an HR of 2.05 (95% CI 1.21–3.48, or an additional 23 cases of dementia per 10 000 person-years (45 v. 22 cases per 10 000 person-years)38
worsening urinary incontinence in women with existing incontinence (summary odds ratio 1.51, 95% CI 1.26–1.82).39
HRT has not been found to improve health-related quality of life, especially in asymptomatic women,40 nor to prevent mild cognitive impairment.41
-
-
Clinical implications
Before the publication of the WHI estrogen–progestin trial results, it was estimated that 22% of Canadian women aged 45–64 were currently using HRT, with highest use (33%) among those 50–54 years of age.42 Although many women have been taking combination HRT for the prevention of chronic diseases, the current evidence indicates that the harms outweigh the benefits, demonstrating an increased risk of breast cancer, venous thromboembolism, pulmonary embolism, stroke, myocardial infarction, probable dementia, cholecystitis and worsening incontinence, and a decreased risk of osteoporotic fractures and colorectal cancer. In addition, HRT use by postmenopausal women without menopausal symptoms does not improve health-related quality-of-life outcomes, including depression, sleep, sexual functioning and overall self-rated quality of life.40 The recently released results of the WHI estrogen-only trial,14 showing an increased risk of stroke and a decreased risk of hip fractures, further support the notion that HRT, whether unopposed or in combination with progestin, should not be used for the prevention of chronic diseases.
Many women, however, especially those in early menopause, seek HRT to control menopausal symptoms,43 in particular vasomotor effects, an outcome for which there is demonstrated benefit.44For women who wish to alleviate menopausal symptoms using HRT, a discussion between the woman and her physician of the potential benefits and risks is warranted (Table 1). If the risks are acceptable to the woman and her physician, therapy of as short a duration as possible, and at as low a dose as possible, may be indicated.15,16,17,18
While scientific debate and subanalysis of existing data continue, the available evidence indicates that specific adverse outcomes may occur at different times after initiation of HRT. For combined estrogen–progestin therapy, the risk of certain cardiovascular events would appear to increase soon after therapy is begun, within the first few months for coronary artery disease and venous thromboembolism and by about 18 months for stroke. The elevated risk for all 3 persists at least through the first 5 years of HRT. On the other hand, the risk for invasive breast cancer does not become elevated until later in therapy, around year 4. For estrogen-only therapy, the increased risk of stroke within the first year continues to increase throughout the 6.8 years of follow-up, whereas the slightly increased risk of coronary artery disease in the early follow-up period diminishes over time. This information may help women in deciding whether to initiate HRT for the relief of menopausal symptoms and, if initiated, in deciding how long to take it.
Recommendations by others
The US Preventive Services Task Force,15 the American College of Obstetricians and Gynecologists,16 the North American Menopause Society (NAMS),17 Health Canada,18 the US Food and Drug Administration (FDA)19 and, in a joint statement, the Heart and Stroke Foundation of Canada, the Society of Obstetricians and Gynaecologists of Canada and the Canadian Cardiovascular Society20 all have recommended that asymptomatic women should not use combination estrogen– progestin therapy for the prevention of cardiovascular disease or other chronic diseases, because the risks outweigh the benefits. They advocate that women considering HRT should discuss their individual risks with their physician. These groups also recommend that women who choose to take HRT to relieve menopausal symptoms should use as low a dose as possible and for as short a time as possible, with periodic re-evaluation of whether HRT is still required. The FDA and NAMS have extended these recommendations to include all estrogen preparations, including unopposed estrogen. Their stance is that, until there is evidence from randomized controlled trials showing benefit, other methods of lowering cardiovascular disease and cancer risk (e.g., smoking cessation, and lifestyle and diet changes) should be used.
Supplementary Material
Footnotes
The references and the list of task force members are available online at www.cmaj.ca/cgi/content/full/170/10/1535.
Contributors: Nadine Wathen drafted the current article and made subsequent revisions. Denice Feig, Beth Abramson and Angela Cheung authored the original systematic evidence reviews, critically reviewed the current article and reviewed subsequent revisions. John Feightner critically reviewed the current article and reviewed subsequent revisions. The Canadian Task Force on Preventive Health Care critically reviewed the evidence and developed the recommendations according to its methodology and consensus development process.
The Canadian Task Force on Preventive Health Care is an independent panel funded through a partnership of the federal and provincial/territorial governments of Canada.
Competing interests: Nadine Wathen has received research funding from Wyeth Canada. Angela Cheung has received honoraria to participate in CME events partially or fully supported by Eli Lilly, Merck, Proctor & Gamble, Aventis and Novartis. These companies have also contributed unrestricted educational grants in support of Toronto City-wide Osteoporosis Rounds, which Angela Cheung chairs. No competing interests declared for Beth Abramson or the members of the Canadian Task Force on Preventive Health Care.
Correspondence to: Canadian Task Force on Preventive Health Care, 117–100 Collip Circle, London ON N6G 4X8; fax 519 858-5181; ctf@ctfphc.org
References
- 1.Feig DS. Prevention of osteoporotic fractures in women by estrogen replacement therapy. In: Canadian Task Force on the Periodic Health Examination. Canadian guide to clinical preventive health care. Ottawa: Health Canada; 1994. p. 620-31.
- 2.Wolf PH, Madans JH, Finucane FF, Higgins M, Kleinman JC. Reduction of cardiovascular disease-related mortality amongpostmenopausal women who use hormones: evidence from a national cohort. Am J Obstet Gynecol 1991;164:489-94. [DOI] [PubMed]
- 3.Stampfer MJ, Colditz GA. Estrogen replacement therapy and coronary heart disease: a quantitative assessment of the epidemiologic evidence. Prev Med 1991;20:47-63. [DOI] [PubMed]
- 4.Barrett-Connor E. Estrogen and estrogen-progestogen replacement: therapy and cardiovascular diseases. Am J Med 1993;95(Suppl 5A):40S-3S. [DOI] [PubMed]
- 5.Ettinger B, Genant HK, Cann CE. Long-term estrogen replacement therapy prevents bone loss and fractures. Ann Intern Med 1985;102:319-24. [DOI] [PubMed]
- 6.Cheung AM, Proctor WR, Stewart DE, with the Canadian Task Force on Preventive Health Care. The effect of hormone replacement therapy on cancer in postmenopausal women: systematic review [CTFPHC Technical Report no 03-3]. London (ON): The Task Force; 2003.
- 7.Steinberg KK, Thacker SB, Smith SJ, Stroup DF, Zack MM, Flanders WE et al. A meta-analysis of the effect of estrogen replacement therapy on the risk of breast cancer. JAMA 1991; 265: 1985-90. [PubMed]
- 8.WHO Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet 1997;350:1047-59. [PubMed]
- 9.Gambrell RD Jr, Massey FM, Castaneda TA, Ugenas AJ, Ricci CA, Wright JM. Use of the progestogen challenge test to reduce the risk of endometrial cancer. Obstet Gynecol 1980; 55(6):732-38. [PubMed]
- 10.Abramson B, with the Canadian Task Force on Preventive Health Care. Postmenopausal hormone replacement therapy for the primary prevention of cardiovascular and cerebrovascular disease. Systematic Review and Recommendations. CTFPHC Technical Report no 03-2. London (ON): The Task Force; 2003. [DOI] [PMC free article] [PubMed]
- 11.Cheung AM, Feig DS, Kapral MK, Diaz-Granados N, Dodin S, with the Canadian Task Force on Preventive Health Care. Prevention of osteoporosis and osteoporotic fractures in postmenopausal women: Systematic review & recommendations. CTFPHC Technical Report no 03-1. London (ON): The Task Force; 2003. [DOI] [PMC free article] [PubMed]
- 12.Nelson HD, Humphrey LL, Nygren P, Teutsch SM, Allan JD. Postmenopausal hormone replacement therapy: scientific review. JAMA 2002; 288 (7): 872-81. [DOI] [PubMed]
- 13.Writing Group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA 2002; 288 (3): 321-33. [DOI] [PubMed]
- 14.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, et al; Women's Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA 2004;291 (14): 1704-12. [DOI] [PubMed]
- 15.US Preventive Services Task Force. Postmenopausal hormone replacement therapy for the primary prevention of chronic conditions: recommendations and rationale. Ann Intern Med 2002;137(10):834-9. [DOI] [PubMed]
- 16.American College of Obstetricians and Gynecologists. Questions and answers on hormone therapy [press release]. Washington: The College; August 2002. Available: www.acog.org/from_home/publications/press_releases/nr08-30-02.cfm (accessed 2004 Mar 03).
- 17.North American Menopausal Society. Estrogen and progestogen use in peri- and post-menopausal women: September 2003 position statement from the North American Menopause Society. Cleveland: The Society; 2003. Available: www.menopause.org/aboutmeno/HTpositionstatement.pdf (accessed 2004 Mar 3).
- 18.Benefits and risks of combined (estrogen and progestin) hormone replacement therapy. In: It's Your Health. Ottawa: Health Canada; updated 2004 Jan 21. Available: www.hc-sc.gc.ca/english/iyh/medical/estrogen.html (accessed 2004 Mar 3).
- 19.US Food and Drug Administration (FDA). FDA approves new labeling and provides new advice to postmenopausal women who use or who are considering using estrogen and estrogen with progestin [FDA Fact Sheet]. Washington: FDA; 2003 Jan 8. Available: www.fda.gov/oc/factsheets/WHI.html (accessed 2004 Mar 3).
- 20.Abramson BL, Derzko C, Lalonde A, Reid R, Turek M, Weilgosz A. Hormone replacement therapy and cardiovascular disease: a joint statement from the Heart and Stroke Foundation of Canada, the Society of Obstetricians and Gynaecologists of Canada and the Canadian Cardiovascular Society. Can J Cardiol 2000;18(7):723-4. [PubMed]
- 21.Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med 2003;349(6):523-34. [DOI] [PubMed]
- 22.Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA 1998;280:605-13. [DOI] [PubMed]
- 23.Herrington DM, Reboussin DM, Brosnihan KB, Sharp PC, Shumaker SA, Snyder TE, et al. Effects of estrogen replacement on the progression of coronary-artery atherosclerosis. N Engl J Med 2000;343(8):522-9. [DOI] [PubMed]
- 24.Postmenopausal hormone replacement therapy for primary prevention of cardiovascular and cerebrovascular disease. Recommendation statement from the Canadian Task Force on Preventive Health Care. CMAJ 2004; 170 (9): 1388-9. [DOI] [PMC free article] [PubMed]
- 25.Beral V; Million Women Study Collaborators. Breast cancer and hormone-replacement therapy in the Million Women Study [published erratum in Lancet 2003;362:1160]. Lancet 2003;362:419-27. [DOI] [PubMed]
- 26.Schairer C, Lubin J, Troisi R, Sturgeon S, Brinton L, Hoover R. Menopausal estrogen and estrogen-progestin replacement therapy and breast cancer risk. JAMA 2000;283:485-91. [DOI] [PubMed]
- 27.Persson I, Weiderpass E, Bergkvist L, Bergstrom R, Schairer C. Risks of breast and endometrial cancer after estrogen and estrogen-progestin replacement. Cancer Causes Control 1999;10(4):253-60. [DOI] [PubMed]
- 28.Tavani A, Ricci E, La Vecchia C, Surace M, Benzi G, Parazzini F, et al. Influence of menstrual and reproductive factors on ovarian cancer risk in women with and without family history of breast or ovarian cancer. Int J Epidemiol 2000;29:799-802. [DOI] [PubMed]
- 29.Riman T, Dickman PW, Nilsson S, Correia N, Nordlinder H, Magnusson CM, et al. Hormone replacement therapy and the risk of invasive epithelial ovarian cancer in Swedish women. J Natl Cancer Inst 2002;94:497-504. [DOI] [PubMed]
- 30.Lacey JV Jr, Mink PJ, Lubin JH, Sherman ME, Troisi R, Hartge P, et al. Menopausal hormone replacement therapy and risk of ovarian cancer. JAMA 2002;288:334-41. [DOI] [PubMed]
- 31.Chiaffarino F, Pelucchi C, Parazzini F, Negri E, Franceschi S, Talamini R, et al. Reproductive and hormonal factors and ovarian cancer. Ann Oncol 2001; 12: 337-41. [DOI] [PubMed]
- 32.Sit AS, Modugno F, Weissfeld JL, Berga SL, Ness RB. Hormone replacement therapy formulations and risk of epithelial ovarian carcinoma. Gynecol Oncol 2002;86:118-23. [DOI] [PubMed]
- 33.Grady D, Gebretsadik T, Kerlikowske K, Ernster V, Petitti D. Hormone replacement therapy and endometrial cancer risk: a meta-analysis. Obstet Gynecol 1995;85:304-13. [DOI] [PubMed]
- 34.Grodstein F, Newcomb PA, Stampfer MJ. Postmenopausal hormone therapy and the risk of colorectal cancer: a review and meta-analysis. Am J Med 1999;106:574-82. [DOI] [PubMed]
- 35.Nanda K, Bastian LA, Hasselblad V, Simel DL. Hormone replacement therapy and the risk of colorectal cancer: a meta-analysis. Obstet Gynecol 1999;93:880-8. [DOI] [PubMed]
- 36.Cheung AM, Feig D, Kapral M, Diaz-Granados N, Dodin S, and the Canadian Task Force on Preventive Health Care. Prevention of osteoporosis and osteoporotic fractures in postmenopausal women. Recommendation statement from the Canadian Task Force on Preventive Health Care. CMAJ. In press. [DOI] [PMC free article] [PubMed]
- 37.Grodstein F, Colditz GA, Stampfer MJ. Postmenopausal hormone use and cholecystectomy in a large prospective study. Obstet Gynecol 1994; 83 (1): 5-11. [PubMed]
- 38.Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, et al; WHIMS Investigators. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA 2003;289(20):2651-62. [DOI] [PubMed]
- 39.Grady D, Brown JS, Vittinghoff E, Applegate W, Varner E, Snyder T; HERS Research Group. Postmenopausal hormones and incontinence: the Heart and Estrogen/ Progestin Replacement Study. Obstet Gynecol 2001; 97 (1): 116-20. [DOI] [PubMed]
- 40.Hays J, Ockene JK, Brunner RL, Kotchen JM, Manson JE, Patterson RE, et al. Effects of estrogen plus progestin on health-related quality of life. N Engl J Med 2003;348(19):1-16. [DOI] [PubMed]
- 41.Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Manson JE, et al; WHIMS Investigators. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA 2003; 289 (20): 2663-72. [DOI] [PubMed]
- 42.Beaudet MP, Walop W, Le Petit C. Characteristics of women on hormone replacement therapy. Health Rep 1997; 9(2). Ottawa: Statistics Canada. Cat no 82-003-XPB.
- 43.Kaufert P, Boggs PP, Ettinger B, Woods NF, Utian WH. Women and menopause: beliefs, attitudes, and behaviors. The North American Menopause Society 1997 Menopause Survey. Menopause 1998;5:197-202. [PubMed]
- 44.MacLennan A, Lester S, Moore V. Oral estrogen replacement therapy versus placebo for hot flushes: a systematic review. Climacteric 2001; 4 (1): 58-74. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


