Abstract
Rheumatoid arthritis (RA) is a chronic debilitating disease of the joints. Both the innate and adaptive immune responses participate in the development and progression of RA. While several therapeutic reagents, such as TNF-α agonists, have been successfully developed for the clinical use in the treatment of RA, more than half of the patients do not respond to anti-TNF therapy. Therefore, new therapeutic reagents are needed. Recent studies have shown that sirtuin 1 (Sirt1), a nicotinamide adenine dinucleotide (NAD)-dependent histone deacetylase, is a critical negative regulator of both the innate and adaptive immune response in mice, and its altered functions are likely to be involved in autoimmune diseases. Small molecules that modulate Sirt1 functions are potential therapeutic reagents for autoimmune inflammatory diseases. This review highlights the role of Sirt1 in immune regulation and RA.
Keywords: Rheumatoid arthritis, Sirt1, Epigenetic
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic debilitating disease of the joints characterized by leukocyte infiltration, hyper-proliferation of synovial cells and bone destruction. The exact causes of RA are unknown. However, it is well accepted that a combination of factors, including abnormal autoimmune response, genetic susceptibility, and some environmental or biologic triggers, such as viral infection or hormonal changes, is involved in RA development.
T cell immune responses to self-antigens play important roles in RA development and progression. Several auto-antigens have been identified in RA, including Ra33 (hnRNP A2), fibrinogen, fibronectin, α-enolase, type II collagen, immunoglobulin binding protein (BiP), annexins and glucose-6-phosphate isomerase (GPI). T cell activation is initiated by the binding of antigenic peptides presented by the major histocompatibility complexes (MHCs) to the T cell receptor (TCR)/CD3 complex, which results in T cell proliferation and IL-2 production (Bach et al., 1976). In addition to antigen-specific interaction with the TCR, full-scale T cell activation requires a co-stimulatory signal provided by the engagement of the T cell co-receptor, CD28, with its ligands, B7 on antigen presenting cells (APCs) (Kang et al., 1992; Powell et al., 1999). Activation of the IL-2 promoter in T cells requires the cooperative interactions of several transcription factors, including activator protein 1 (AP-1), nuclear factor of kappa-B (NF-κB) and nuclear factor of activated T cells (NFAT) (Jain et al., 1992a, 1992b, 1992c; Ullman et al., 1993; Rincon and Flavell, 1994; Jung et al., 1995).
In addition to T cells, all other types of immune cells are either directly or indirectly involved in RA both in human and in experimental arthritis rodent models. In particular, macrophages appear to be a key mediator of inflammation in RA. Toll-like receptor (TLR)-mediated signaling, when triggered by endogenous ligands, such as fibrinogen, and heat-shock proteins 22, 60 and 70, initiates the production of inflammatory cytokines by macrophages during RA (Roelofs et al., 2006; Sutmuller et al., 2007; Hu et al., 2008; Yavuz et al., 2008; Huang et al., 2009). Macrophages have been used as therapeutic targets, either by inhibiting TLR-mediated signaling or by blocking their trafficking into synovial tissues, for RA treatment both in rodents and in humans with some success (Stamp et al., 2004; McInnes et al., 2005;Morand, 2005; Sen, 2005; Tak, 2006; Ohori, 2008; Simmonds and Foxwell, 2008; Bartok and Firestein, 2011; Fiocco et al., 2011). In addition to leukocytes, chondrocytes and synoviocytes can also contribute to the inflammatory phenotype in RA. Interestingly, recent studies suggest that sirtuin 1 (Sirt1) also functions in chondrocytes and synoviocytes during inflammatory arthritis (Niederer et al., 2011; Huang et al., 2012;Moon et al., 2013). Therefore, it is likely that Sirt1 modulates a variety of cell types during arthritis disease development and progression.
The mammalian Sirtuin family proteins, which were initially identified as orthologs of the yeast sir2 (silent information regulatory 2), have seven members, named Sirt1 to Sirt7. Like sir2, Sirtuins possess NAD+-dependent deacetylase activity and belong to the type III histone deacetylase (HDAC) (Imai et al., 2000). In addition, Sirt6 and Sirt4 have adenosine diphosphate (ADP)-ribosyltransferase activity (Liszt et al., 2005). Besides histones, the Sirtuin family can deacetylate a variety of non-histone substrates including transcription factors, heat-shock proteins and metabolic enzymes. The substrates of Sirt1 are particularly abundant and include p53, Nijmegen breakage syndrome 1 (NBS1), NF-κB transcription factor RelA/p65, AP-1 family transcription factor c-Jun and c-Myc (Yeung et al., 2004; Solomon et al., 2006; Yuan et al., 2007; Gao and Ye, 2008; Yuan et al., 2009). Sirt1 is highly expressed in heart, brain and skeletal muscle and is expressed at very low levels in kidney and lung (Afshar and Murnane, 1999). In the immune system, it is highly expressed in thymus, particularly in the CD4+CD8+ stage, suggesting an involvement of Sirt1 in T cell development (Cheng et al., 2003). CD4+CD8+ thymocytes from Sirt1−/− mice exhibit increased sensitivity to γ-irradiation-induced apoptosis (Afshar and Murnane, 1999). We have found that Sirt1 is expressed in the thymus, spleen and lymph nodes as well as purified CD4+ T cells. As summarized below, recent studies have shown that Sirt1 is a critical immune suppressor of both T cell and macrophage activation. Genetic deletion of Sirt1 in mice leads to lupus-like autoimmunity. Conversely, activation of Sirt1 by its activators, such as resveratrol, has been shown to hold great therapeutic potential in the treatment of autoimmune inflammatory diseases including RA.
Sirt1 IN T CELL ACTIVATION AND TOLERANCE
T cells that recognize self-antigens such as collagen have been considered to be initiators for RA. Collagen-specific T cell clones have been isolated from the peripheral blood leucocytes of RA patients (Ofosu-Appiah et al., 1989). Accumulated evidence indicates that Sirt1 is a crucial negative regulator of T cell immunity. Although the inflammatory phenotype of Sirt1-null mice varies in severity depending on genetic background (McBurney et al., 2003), on certain genetic backgrounds such as DBA1, mice develop severe joint inflammation when immunized with bovine collagen, an established arthritic experimental model known as collagen-induced arthritis (CIA) (Holoshitz et al., 1984). CIA has been widely used as an experimental mouse model for the study of human RA because it closely resembles human RA. A closer look at the phenotype of Sirt1-null mice revealed that Sirt1−/− T cells are hyperproliferative and produce significantly increased amount of cytokines including IL-2 when stimulated in vitro with anti-TCR and anti-CD28 antibodies (Zhang et al., 2009; Kong et al., 2011). This observation is also reproducible in vivo, as T cells isolated from ovalbumin (OVA)-immunized Sirt1−/− mice have significantly higher proliferation upon OVA stimulation and produce more IL-2, IFN-γ and IL-5. OVA-specific antibodies in the sera were also higher in Sirt1−/− OVA-immunized mice (Zhang et al., 2009). Therefore, Sirt1 is a negative regulator of T cell activation both in vitro and in vivo.
Sirt1 inhibits AP-1 transcriptional activity in T cells
One of the underlying molecular mechanisms by which Sirt1 suppresses T cell immunity is through inhibiting the transcription factor AP-1, whose transcriptional activity plays an essential role in the activation of the immune response, particularly in T cells. AP-1 family transcription factors comprises Jun and Fos proteins. The Jun family of proteins has three members including c-Jun, JunB and JunD. Fos family proteins include four members, c-Fos, FosB, Fra1 and Fra2. The promoter-binding activity of AP-1 requires the dimerization of Jun and Fos family proteins. Jun family transcription factors can either form homodimers or heterodimers with other Jun proteins or with Fos family transcription factors. In contrast, Fos family transcription factors bind to promoter DNA only when they form heterodimers with Jun family proteins. In particular, c-Jun and c-Fos heterodimers are up-regulated shortly after T cell activation to induce proliferation, IL-2 production, and differentiation (Foletta et al., 1998). Post-translational modification of AP-1 transcription factors mediated by MAPK phosphorylation has been shown to play important roles in their nuclear translocation and recruitment of transcription machinery for target gene transcription. In addition to phosphorylation, acetylation of c-Jun by the histone acetyltransferase (HAT) has been demonstrated to be required for AP-1 transcriptional activity. We have recently discovered that Sirt1 opposes p300-mediated c-Jun acetylation in T cells to suppress AP-1 transcriptional activity (Zhang et al., 2009). This suppressive activity of Sirt1 requires its interaction with c-Jun and its HDAC activity. More recently, interaction between Sirt1 and AP-1 was also identified in macrophages (Zhang et al., 2010), where Sirt1 can interact with the leucine zipper (LZ) domains of both c-Fos and c-Jun (Gao and Ye, 2008; Zhang et al., 2010), indicating that Sirt1, similar to its functions in T cells, is involved in suppressing macrophage functions. To support this idea, Zhang et al. (2010) discovered that overexpression of Sirt1 reduced the mRNA levels of Cox-2, a target gene of AP-1, in peritoneal macrophages. The reduced production of prostaglandin E in macrophages as a result of this suppression has broad implications in the inflammatory and tumoricidal functions of macrophages. Since AP-1 is critical for T cell activation especially in the induction of IL-2 transcription, Sirt-mediated suppression of AP-1 appears to be a critical molecular mechanism in regulating T cell immune responses.
Sirt1 is a negative regulator of NF-κB
The NF-κB pathway is a central signaling node in inflammatory cytokine stimulation and lymphocyte activation. Upon recognition of specific antigens by the T cell receptor, the NF-κB transcription factor is activated and directly binds to the IL-2 promoter in T cells. NF-κB transcription factors have five family members including p50 (NF-κB1), p52 (NF-κB2), p65/RelA, RelB and c-Rel. The transcriptional activities of two NF-κB family members, including RelA and c-Rel, have been shown to be regulated by acetylation and deacetylation (Yeung et al., 2004). Sirt1 deacetylase was initially shown to deacetylate RelA/p65 at lysine 310 residue (K310), and this deacetylation of p65 leads to reduced NF-κB transcriptional activity (Yeung et al., 2004). However, it should be noted that p65 can be acetylated at multiple residues, and that Sirt1 might regulate p65 activity through deacetylation of multiple lysine residues (Chen et al., 2002). More recently, we have discovered that Sirt1 inhibits T cell proliferation through Bclaf1 (BCL-associated factor 1) by antagonizing NF-κB transcriptional activity at the Bclaf1 promoter. Bclaf1 was initially thought to be a pro-apoptotic protein. A recent study using the gene targeted mutation in mice revealed that Bclaf1 function is required for T cell proliferation. Interestingly, instead of direct deacetylation of NF-κB transcription factors, Sirt1 is recruited to the Bclaf1 promoter through its binding with RelA to deacetylate histone lysine residues, including H3K56 at the promoter region of Bclaf1 (Kong et al., 2011). Similarly, Alvartez et al. (2012) recently discovered that Sirt1 suppresses IL-12 production in human dendritic cells through a direct interaction with the NF-κB transcription factor c-Rel. However, the authors did not obtain evidence of a possible effect of Sirt1 through the deacetylation of c-Rel. Therefore, Sirt1 inhibits the transcriptional activity of NF-κB through two different mechanisms.
Sirt1 targets FoxO family transcription factors
While the effect of acetylation on the transcriptional activity of FoxO family transcription factors is still arguable, it has been clear that Sirt1 functions as a deacetylase of multiple FoxOs including FoxO1, 3 and 4 (Brunet et al., 2004; Motta et al., 2004). It has been shown that IL-2 can signal through the PI3K/Akt pathway to suppress FoxO1, 3 and 4 and to prevent activation-induced cell death (Stahl et al., 2002). Similar to Sirt1 knockout mice, FoxO3-deficiency causes spontaneous lymphoproliferation, associated with inflammation of several organs in mice (Lin et al., 2004). Therefore, it will be interesting to study the crosstalk of Sirt1 with FoxO family transcription factors in immune regulation and inflammation.
Sirt1 is required for T cell tolerance
Anergy is the state in which T cells are alive but neither are capable of proliferating nor initiate the transcription of IL-2 gene in response to optimal antigenic stimulation provided by APCs (Sloan-Lancaster et al., 1994a, 1994b; Schwartz, 1997,2003). Anergy has been considered to be the mechanism of self-tolerance in vivo (Mondino et al., 1996; Schwartz, 1997). Consequently, reversal of anergy by manipulation of the pathways involved in the induction and maintenance of this state results in autoimmunity, tumor immunity and allograft rejection (Lenschow et al., 1992; Turka et al., 1992; Chen et al., 1993; Guerder et al., 1994; Mondino et al., 1996). Several studies have examined the mechanism by which IL-2 gene transcription is blocked in T cell anergy (Kang et al., 1992; Schwartz, 2003). Anergic cells cannot upregulate protein binding activity and transactivation of AP-1, a critical IL-2 enhancer element (Kang et al., 1992). However, the molecular mechanisms underlying how AP-1 transcriptional activity is inhibited in anergic T cells remain unknown.
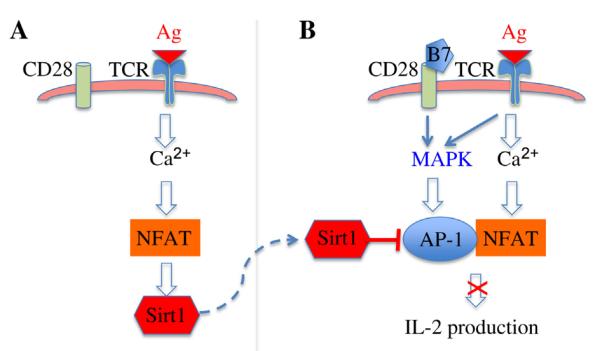
Using an in vitro assay, our laboratory has recently discovered that T cells lacking Sirt1 are hyperproliferative and can be activated without CD28 co-stimulation. In addition, OT-II mice crossed with Sirt1−/− mice could not be tolerized by OVA323-339 peptide. Therefore, Sirt1 proteins appear to be required for T cell immune tolerance as tested by both in vitro and in vivo assays. It is known that T cells that receive a weak TCR signal without CD28 co-stimulation normally undergo tolerance by specifically activating the NFAT pathway without activation of AP-1 and NF-κB transcription factors (Macián et al., 2002). Since Sirt1 protein suppresses the activation of the transcription factor AP-1, which is achieved by interacting with and deacetylating c-Jun, Sirt1-mediated suppression of c-Jun acetylation inhibits AP-1 transcriptional activity to maintain T cell immune tolerance (Fig. 1) (Zhang et al., 2009).
Fig. 1. A model of Sirt1 in T cell tolerance.
A: recognition of self-antigen (Ag) by TCR in the absence of co-stimulation induces Sirt1 gene transcription through NFAT pathway. B: balanced TCR/CD28 signaling activates both AP-1 and NFAT to induce IL-2 production for T cell activation. Up-regulated Sirt1 inhibits AP-1 transcription to block the activation of tolerized T cells when they receive both TCR and CD28 stimuli.
As many other identified anergic genes, such as Itch, Ikaros and Cbl-b (Fang et al., 2001, 2002; Thomas et al., 2007; Venuprasad, 2010; Hoyne, 2011), TCR signaling alone induces Sirt1 gene expression through NFAT pathway and the calcineurin inhibitor cyclosporine A blocks Sirt1 gene transcription during anergy induction (Gao et al., 2012). However, chromatin immunoprecipitation assay could not detect NFAT binding to Sirt1 promoter, implying that factors downstream of NFAT mediate Sirt1 gene transcription. Interestingly, the early growth responsive (EGR) genes 2 and 3 (EGR2 and EGR3), which have been discovered as critical factors of immune tolerance (Droin et al., 2003; Harris et al., 2004; Safford et al., 2005), directly bind to Sirt1 promoter upon TCR stimulation. Since both EGR2 and EGR3 are the downstream target genes of NFAT transcription factors (Rengarajan et al., 2000; Lazarevic et al., 2009), the TCR-NFAT-EGR2/3-Sirt1 pathway appears to be an important signaling pathway for T cell anergy induction (Fig. 1). Interestingly, IL-2 suppresses Sirt1 gene transcription in T cells by sequestering the forkhead transcription factor 3a (FoxO3a) because FoxO3a interacts with EGR2/3 to regulate Sirt1 gene transcription (Gao et al., 2012). Therefore, IL-2 cytokine breaks down T cell tolerance by blocking Sirt1 expression. In addition, Sirt1 functions have been shown to be regulated by post-translational regulation. We have recently shown that Sirt1 protein stability is regulated by the ubiquitin pathway (Lin et al., 2012), and it will be interesting to study whether post-translational modifications are involved in regulating Sirt1 functions during T cell activation and tolerance.
THE ROLE OF Sirt1 IN THE REGULATION OF MACROPHAGE FUNCTIONS
Macrophages are the major source of inflammatory cytokines during RA. Since Sirt1 antagonizes the transcriptional activity of AP-1 and NF-κB, both of which are critical transcription factors in the expression of a variety of inflammatory cytokines, it is not surprising that Sirt1 has a direct regulatory role in macrophage functions during inflammation. In fact, Schug et al. (2010) elegantly showed that myeloid-specific deletion of Sirt1 gene promotes the development of cardiovascular inflammation induced by high fat diet, indicating the anti-inflammatory roles of Sirt1 in myeloid cells. To support this observation, the production of pro-inflammatory cytokines TNF-α, IL-6, and IL-1β by macrophages from the myeloid-specific Sirt1 knockout mice is dramatically increased in response to infection and inflammation. In addition to pro-inflammatory cytokines, Sirt1 is likely involved in the expression of cell surface molecules such as ICAM-1 to facilitate macrophage trafficking during inflammatory response (Yang et al., 2007; Stein et al., 2010). It remains to be characterized the role of AP-1 transcription factors in Sirt-mediated anti-inflammatory activity in macrophages. In contrast, hyperacetylation of NF-κB transcription factor RelA/p65 has been detected in macrophages from myeloid-specific Sirt1 knockout mice, indicating that the anti-inflammatory activity of Sirt1 in macrophages is, at least partially, through NF-κB suppression (Schug et al., 2010).
Another signaling pathway that has been recently linked to inflammation is the endoplasmic reticulum (ER) response (Hu et al., 2006). ER stress leads to the activation of inositol-requiring enzyme 1 (IRE1), which splices X-box binding protein 1 (Xbp-1) into its functional message, and ultimately leads to suppressed global translation and increased chaperone activity. If the cells fail to reduce the ER load, they will undergo apoptosis (Calfon et al., 2002). Recent studies suggest that IRE1α-Xbp-1 pathway is critical for TLR-induced inflammatory cytokine production by macrophages (Martinon et al., 2010). Xbp-1 is regulated by post-translational acetylation and deacetylation mediated by the acetyltransferase p300 and deacetylase Sirt1, respectively (Wang et al., 2010). Further study is needed to understand the differential effects of Sirt1 on the dynamic regulation of NF-κB, Xbp-1 and AP-1 pathways in macrophages.
THE ROLES OF Sirt1 IN CHONDROCYTES AND SYNOVIAL FIBROBLASTS
Several studies have detected elevated Sirt1 expression levels in human synovial fibroblasts and chondrocytes from patients with RA or mice with CIA. Further in vitro studies suggest that TNF-α is responsible for the elevated Sirt1 expression (Niederer et al., 2011; Huang et al., 2012; Moon et al., 2012). Interestingly, TNF-α also induces Sirt1 protein cleavage through protease cathepsin B activity, leading to the production of a HDAC inactive truncation of Sirt1 with a molecular weight of about 75 kDa instead of the original 110 kDa (Dvir-Ginzberg et al., 2011). This 75 kDa Sirt1 blocks TNF-α-induced apoptosis of chondrocytes through its association with cytochrome c in mitochondria (Oppenheimer et al., 2012), suggesting a feedback regulatory mechanism of TNF-α-induced Sirt1 expression during inflammation. While the suppressive functions of Sirt1 in T cells and macrophages have been relatively well-established, the discoveries of Sirt1 functions in chondrocytes and synovial fibroblasts during inflammatory arthritis are controversial. Some studies suggest that Sirt1 inhibits the productions of inflammatory cytokines, such as TNF-α, IL-1β and IL-6, largely through antagonizing NF-κB transcriptional activity (Huang et al., 2012; Kok et al., 2013; Moon et al., 2013), indicating that Sirt1 activation by its activators like resveratrol has therapeutic roles in inflammatory arthritis treatment. However, Niederer et al. (2011) showed that TNF-α-induced Sirt1 expression has a positive role in the production of inflammatory cytokines including IL-6 and IL-8 by RA synovial fibroblasts as well as by peripheral blood monocytes. Knockdown of Sirt1 expression resulted in a reduction of these inflammatory cytokines analyzed. Further studies are needed to define the roles of Sirt1 in synovial fibroblasts and chondrocytes during inflammatory arthritis.
THE Sirt1 ACTIVATOR RESVERATROL IN RA THERAPY
Resveratrol (trans-3,4,5-trihydroxystillbene) is a polyphenol naturally found in various plant species (Recio et al., 2012). The use of resveratrol in treating various inflammatory diseases has been studied extensively due to its anti-inflammatory and pro-apoptotic properties (Elmali et al., 2007; Imler Jr and Petro, 2009; Sánchez-Fidalgo et al., 2010; Lee et al., 2011; Nakayama et al., 2012; Xuzhu et al., 2012). One of its most well-known pharmacological targets is Sirt1 (Borra et al., 2005; Alcaín and Villalba, 2009). Resveratrol specifically enhances the deacetylase activity of Sirt1 without affecting the activities of any other sirtuin family members including Sirt2–7. Structural studies indicate that resveratrol binding to Sirt1 modulates the conformation of Sirt1 and enhances binding activity to its substrates (Borra et al., 2005). Due to its ability to activate Sirt1 and suppress inflammation, resveratrol has been shown to alleviate inflammatory symptoms in several experimental autoimmune disease models, such as colitis, type I diabetes, encephalomyelitis and RA (Elmali et al., 2007; Imler Jr and Petro, 2009; Sánchez-Fidalgo et al., 2010; Lee et al., 2011; Xuzhu et al., 2012). In both CIA and LPS-induced acute inflammatory arthritis models, resveratrol treatment before or after disease onset was able to reduce synovial hyperplasia, cartilage destruction, leukocyte infiltration, macrophage and T cell activation, and collagen-specific immunoglobulin levels (Elmali et al., 2007;Xuzhu et al., 2012).
A key function of resveratrol is to inhibit the production of inflammatory factors through activation of Sirt1. One of the main substrates of Sirt1 is NF-κB member p65/RelA (Yeung et al., 2004), which is a master regulator of leukocyte activation and inflammatory cytokine signaling and is found to be up-regulated in RA patients (Fujisawa et al., 1996; Bonizzi and Karin, 2004). Sirt1 deacetylation of RelA inhibits its transcriptional activity, resulting in loss of inflammatory cytokine production. The activation of Sirt1 by resveratrol results in the inhibition of RelA acetylation, and a reduction in NF-κB-induced expression of inflammatory factors such as TNF-α, IL-1β, IL-6, metalloproteases MMP1 and MMP3, and cyclooxygenase 2 (Cox-2), all of which have been implicated in the pathogenesis of RA (Fujisawa et al., 1996; Marok et al., 1996; Yamamoto and Gaynor, 2001). In addition, the activation of Sirt1 and inhibition of NF-κB by resveratrol result in reduced responsiveness to inflammatory cytokine signaling. Resveratrol-treated cells are less responsive to TNF-α-induced NF-κB signaling and apoptosis induction, acting as a double block on the NF-κB signaling pathway (Manna et al., 2000). Similarly, resveratrol-treated bone-derived cells showed reduced receptor activator of nuclear factor kappa-B ligand (RANKL)-induced NF-κB acetylation and activation, as well as reduced osteoblastic activity associated with RA (Shakibaei et al., 2011). In addition, Sirt1 mRNA levels were reported to be elevated in resveratrol-treated cells, serving as a positive feedback for its activity (Shakibaei et al., 2011).
Besides Sirt1, resveratrol has been proposed to work through other targets, all of which similarly result in the reduction of autoimmune symptoms. One of the mechanisms of resveratrol for the alleviation of RA is the induction of apoptosis of synovial cells. Human RA synovial cell lines treated with resveratrol underwent rapid apoptosis, which required the activation of caspases 3, 8 and 9, but not Sirt1-target p53 (Byun et al., 2008; Nakayama et al., 2012). Resveratrol treatment has also been discovered to inhibit p300 expression and promote IkBa degradation, but it is not clear whether this process is through Sirt1 activation (Shakibaei et al., 2011). In addition, other known substrates of resveratrol include AMPK, an essential energy sensor in cells which incidentally regulates the activity of Sirt1 by regulating the cellular levels of available NAD+ (Price et al., 2012). Nevertheless, the function of resveratrol is definitely mediated in part by Sirt1, as the anti-inflammatory properties of resveratrol are abrogated by the genetic deletion of Sirt1, or the addition of Sirt1 inhibitors such as Sirtinol (Nakayama et al., 2012;Price et al., 2012). Similarly, small molecule activators of Sirt1 are currently used in clinical trials toward the treatment of autoimmune diseases such as RA by Sirtris, a GSK company. In view of the recent successes of specific Sirt1 activators and resveratrol in experimental and clinical autoimmune disease settings, there is definitely potential for the development of resveratrol and similar Sirt1 activators as therapeutic targets against inflammation-related diseases including RA.
CLOSING REMARKS
In addition to T cells, macrophages, chondrocytes and synovial fibroblasts, Sirt1 may play important roles in other immune cells, such as B cells and neutrophils, both of which are involved in RA. In particular, the destructive capacity of neutrophils has long been appreciated, and the presence of extraordinary numbers of neutrophils in the synovial fluid of patients with RA supports a role for these cells in the pathogenesis of joint destruction. Several transcription factors, including NF-κB, STAT3 and IRF3, which are involved in the expression of chemotaxins produced by neutrophils, have been discovered as Sirt1 substrates in other cell types. It is possible that Sirt1 may regulate the production of chemotaxins, such as IL-8 and IFN-γ, by neutrophils during RA. It will be also interesting to study whether Sirt1 participates in regulating the phagocytosis activity of neutrophils. In addition, Sirt1 is a ubiquitously expressed protein in mammals. The investigation on how Sirt1 affects the activation of the immune system might also shed light on novel genes and pathways which could contribute to the proliferation and differentiation of various cell types. Importantly but not surprisingly, Sirt1 activators, such as SRT1720, which have significantly improved specificity and activity in activating Sirt1, have been found to hold great therapeutic efficacy in autoimmune therapy and are currently used in clinical tails (www.sirtris.com). However, further studies are needed to characterize whether SRT1720 and its orthologs achieve their anti-inflammatory activity in a Sirt1-dependent manner using experimental arthritis models as well as in patients.
While we discussed the anti-inflammatory roles of Sirt1 in RA, some studies have implied that Sirt1 may have inflammatory roles during inflammation. In particular, it has been shown that administration of HDAC inhibitors, including those more specific to Sirt1 deacetylase activity, such as sirtinol, protects mice from different types of inflammatory diseases including arthritis (Grabiec et al., 2011; Villalba and Alcain, 2012). Therefore, while the specificity of small molecular inhibitors is always a reasonable concern, the inflammatory roles of Sirt1 may exist and further efforts in the study are needed.
In summary, while there are still many questions to be answered, much progress has been made to explore and establish the role of Sirt1 in regulating the immune response. Further investigation, along with the development of new models and techniques for probing the role of Sirt1, will move a step forward to the clinical application of Sirt1 targeting in treating RA.
ACKNOWLEDGEMENTS
This work was supported by the grants (Nos. R01AI079056 and R56AI79056) and a “Type I Diabetes Pathfinder Award” (No. DK083050) to D.F. from the National Institute of Health.
REFERENCES
- Afshar G, Murnane JP. Characterization of a human gene with sequence homology to Saccharomyces cerevisiae SIR2. Gene. 1999;234:161–168. doi: 10.1016/s0378-1119(99)00162-6. [DOI] [PubMed] [Google Scholar]
- Alcaín FJ, Villalba JM. Sirtuin activators. Expert Opin. Ther. Pat. 2009;19:403–414. doi: 10.1517/13543770902762893. [DOI] [PubMed] [Google Scholar]
- Bach FH, Bach ML, Sondel PM. Differential function of major histocompatibility complex antigens in T-lymphocyte activation. Nature. 1976;259:273–281. doi: 10.1038/259273a0. [DOI] [PubMed] [Google Scholar]
- Bartok B, Firestein GS. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol. Rev. 2011;233:233–255. doi: 10.1111/j.0105-2896.2009.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonizzi G, Karin M. The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Borra MT, Smith BC, Denu JM. Mechanism of human SIRT1 activation by resveratrol. J. Biol. Chem. 2005;280:17187–17195. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- Byun HS, Song JK, Kim Y-R, Piao L, Won M, Park KA, Choi BL, Lee H, Hong JH, Park J, Seok JH, Lee YJ, Kang SW, Hur GM. Caspase-8 has an essential role in resveratrol-induced apoptosis of rheumatoid fibroblast-like synoviocytes. Rheumatology. 2008;47:301–308. doi: 10.1093/rheumatology/kem368. [DOI] [PubMed] [Google Scholar]
- Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–96. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- Chen L.-f., Mu Y, Greene WC. Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-κB. EMBO J. 2002;21:6539–6548. doi: 10.1093/emboj/cdf660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Mohapatra S, Mohapatra SS, Sehon AH. Cytokine gene expression of CD8+ suppressor T cells induced by tolerogenic conjugates of antigen and mPEG. Cell. Immunol. 1993;149:409–421. doi: 10.1006/cimm.1993.1166. [DOI] [PubMed] [Google Scholar]
- Cheng HL, Mostoslavsky R, Saito S, Manis JP, Gu Y, Patel P, Bronson R, Appella E, Alt FW, Chua KF. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc. Natl. Acad. Sci. USA. 2003;100:10794–10799. doi: 10.1073/pnas.1934713100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droin NM, Pinkoski MJ, Dejardin E, Green DR. Egr family members regulate nonlymphoid expression of Fas ligand, TRAIL, and tumor necrosis factor during immune responses. Mol. Cell. Biol. 2003;23:7638–7647. doi: 10.1128/MCB.23.21.7638-7647.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvir-Ginzberg M, Gagarina V, Lee EJ, Booth R, Gabay O, Hall DJ. Tumor necrosis factor a-mediated cleavage and inactivation of SirT1 in human osteoarthritic chondrocytes. Arthritis Rheum. 2011;63:2363–2373. doi: 10.1002/art.30279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmali N, Baysal O, Harma A, Esenkaya I, Mizrak B. Effects of eesveratrol in inflammatory arthritis. Inflammation. 2007;30:1–6. doi: 10.1007/s10753-006-9012-0. [DOI] [PubMed] [Google Scholar]
- Fang D, Elly C, Gao B, Fang N, Altman Y, Joazeiro C, Hunter T, Copeland N, Jenkins N, Liu YC. Dysregulation of T lymphocyte function in itchy mice: a role for Itch in TH2 differentiation. Nat. Immunol. 2002;3:281–287. doi: 10.1038/ni763. [DOI] [PubMed] [Google Scholar]
- Fang D, Wang HY, Fang N, Altman Y, Elly C, Liu YC. Cbl-b, a RING-type E3 ubiquitin ligase, targets phosphatidylinositol 3-kinase for ubiquitination in T cells. J. Biol. Chem. 2001;276:4872–4878. doi: 10.1074/jbc.M008901200. [DOI] [PubMed] [Google Scholar]
- Fiocco U, Sfriso P, Lunardi F, Pagnin E, Oliviero F, Scagliori E, Cozzi L, Vezzu M, Molena B, Scanu A, Panziera C, Nardacchione R, Rubaltelli L, Dayer JM, Calabrese F, Punzi L. Molecular pathways involved in synovial cell inflammation and tumoral proliferation in diffuse pigmented villonodular synovitis. Autoimmun. Rev. 2011;9:780–784. doi: 10.1016/j.autrev.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Foletta V, Segal D, Cohen D. Transcriptional regulation in the immune system: all roads lead to AP-1. J. Leukoc. Biol. 1998;63:139–152. doi: 10.1002/jlb.63.2.139. [DOI] [PubMed] [Google Scholar]
- Fujisawa K, Aono H, Hasunuma T, Yamamoto K, Mita S, Nishioka K. Activation of transcription factor NF-κB in human synovial cells in response to tumor necrosis factor a. Arthritis Rheum. 1996;39:197–203. doi: 10.1002/art.1780390205. [DOI] [PubMed] [Google Scholar]
- Gao B, Kong Q, Kemp K, Zhao YS, Fang D. Analysis of sirtuin 1 expression reveals a molecular explanation of IL-2-mediated reversal of T-cell tolerance. Proc. Natl. Acad. Sci. USA. 2012;109:899–904. doi: 10.1073/pnas.1118462109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Ye J. Inhibition of transcriptional activity of c-JUN by SIRT1. Biochem. Biophys. Res. Commun. 2008;376:793–796. doi: 10.1016/j.bbrc.2008.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabiec AM, Tak PP, Reedquist KA. Function of histone deacetylase inhibitors in inflammation. Crit. Rev. Immunol. 2011;31:233–263. doi: 10.1615/critrevimmunol.v31.i3.40. [DOI] [PubMed] [Google Scholar]
- Guerder S, Picarella DE, Linsley PS, Flavell RA. Costimulator B7-1 confers antigen-presenting-cell function to parenchymal tissue and in conjunction with tumor necrosis factor a leads to autoimmunity in transgenic mice. Proc. Natl. Acad. Sci. USA. 1994;91:5138–5142. doi: 10.1073/pnas.91.11.5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JE, Bishop KD, Phillips NE, Mordes JP, Greiner DL, Rossini AA, Czech MP. Early growth response gene-2, a zinc-finger transcription factor, is required for full induction of clonal anergy in CD4+ T cells. J. Immunol. 2004;173:7331–7338. doi: 10.4049/jimmunol.173.12.7331. [DOI] [PubMed] [Google Scholar]
- Holoshitz J, Matitiau A, Cohen IR. Arthritis induced in rats by cloned T lymphocytes responsive to mycobacteria but not to collagen type II. J. Clin. Invest. 1984;73:211–215. doi: 10.1172/JCI111193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyne GF. Mechanisms that regulate peripheral immune responses to control organ-specific autoimmunity. Clin. Dev. Immunol. 2011;2011:294968. doi: 10.1155/2011/294968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P, Han Z, Couvillon AD, Kaufman RJ, Exton JH. Autocrine tumor necrosis factor a links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1α-mediated NF-κB activation and down-Regulation of TRAF2 expression. Mol. Cell. Biol. 2006;26:3071–3084. doi: 10.1128/MCB.26.8.3071-3084.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Chakravarty SD, Ivashkiv LB. Regulation of interferon and Toll-like receptor signaling during macrophage activation by opposing feedforward and feedback inhibition mechanisms. Immunol. Rev. 2008;226:41–56. doi: 10.1111/j.1600-065X.2008.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang QQ, Sobkoviak R, Jockheck-Clark AR, Shi B, Mandelin AM, 2nd, Tak PP, Haines GK, 3rd, Nicchitta CV, Pope RM. Heat shock protein 96 is elevated in rheumatoid arthritis and activates macrophages primarily via TLR2 signaling. J. Immunol. 2009;182:4965–4973. doi: 10.4049/jimmunol.0801563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Shang WL, Wang HD, Wu WW, Hou SX. Sirt1 overexpression protects murine osteoblasts against TNF-alpha-induced injury in vitro by suppressing the NF-κB signaling pathway. Acta Pharmacol. Sin. 2012;33:668–674. doi: 10.1038/aps.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- Imler TJ, Jr, Petro TM. Decreased severity of experimental autoimmune encephalomyelitis during resveratrol administration is associated with increased IL-17+IL-10+ T cells, CD4− IFN-γ+ cells, and decreased macrophage IL-6 expression. Int. Immunopharmacol. 2009;9:134–143. doi: 10.1016/j.intimp.2008.10.015. [DOI] [PubMed] [Google Scholar]
- Jain J, McCaffrey PG, Valge-Archer VE, Rao A. Nuclear factor of activated T cells contains Fos and Jun. Nature. 1992a;356:801–804. doi: 10.1038/356801a0. [DOI] [PubMed] [Google Scholar]
- Jain J, Valge-Archer VE, Rao A. Analysis of the AP-1 sites in the IL-2 promoter. J. Immunol. 1992b;148:1240–1250. [PubMed] [Google Scholar]
- Jain J, Valge-Archer VE, Sinskey AJ, Rao A. The AP-1 site at −150 bp, but not the NF-κB site, is likely to represent the major target of protein kinase C in the interleukin 2 promoter. J. Exp. Med. 1992c;175:853–862. doi: 10.1084/jem.175.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Yaron A, Alkalay I, Hatzubai A, Avraham A, Ben-Neriah Y. Costimulation requirement for AP-1 and NF-κB transcription factor activation in T cells. Ann. N. Y. Acad. Sci. 1995;766:245–252. doi: 10.1111/j.1749-6632.1995.tb26672.x. [DOI] [PubMed] [Google Scholar]
- Kang SM, Beverly B, Tran AC, Brorson K, Schwartz RH, Lenardo MJ. Transactivation by AP-1 is a molecular target of T cell clonal anergy. Science. 1992;257:1134–1138. doi: 10.1126/science.257.5073.1134. [DOI] [PubMed] [Google Scholar]
- Kok SH, Lin LD, Hou KL, Hong CY, Chang CC, Hsiao M, Wang JH, Lai EH, Lin SK. Simvastatin inhibits Cyr61 expression in rheumatoid arthritis synovial fibroblasts through the regulation of SIRT1/FoxO3a signaling. Arthritis Rheum. 2013;65:639–649. doi: 10.1002/art.37807. [DOI] [PubMed] [Google Scholar]
- Kong S, Kim S-J, Sandal B, Lee S-M, Gao B, Zhang DD, Fang D. The type III histone deacetylase Sirt1 protein suppresses p300-mediated histone H3 lysine 56 acetylation at Bclaf1 promoter to inhibit T cell activation. J. Biol. Chem. 2011;286:16967–16975. doi: 10.1074/jbc.M111.218206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarevic V, Zullo AJ, Schweitzer MN, Staton TL, Gallo EM, Crabtree GR, Glimcher LH. The gene encoding early growth response 2, a target of the transcription factor NFAT, is required for the development and maturation of natural killer T cells. Nat. Immunol. 2009;10:306–313. doi: 10.1038/ni.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SM, Yang H, Tartar D, Gao B, Luo X, Ye S, Zaghouani H, Fang D. Prevention and treatment of diabetes with resveratrol in a non-obese mouse model of type 1 diabetes. Diabetologia. 2011;54:1136–1146. doi: 10.1007/s00125-011-2064-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenschow DJ, Zeng Y, Thistlethwaite JR, Montag A, Brady W, Gibson MG, Linsley PS, Bluestone JA. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4lg. Science. 1992;257:789–792. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- Lin L, Hron JD, Peng SL. Regulation of NF-κB, Th activation, and autoinflammation by the forkhead transcription factor Foxo3a. Immunity. 2004;21:203–213. doi: 10.1016/j.immuni.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Lin Z, Yang H, Kong Q, Li J, Lee SM, Gao B, Dong H, Wei J, Song J, Zhang DD, Fang D. USP22 antagonizes p53 transcriptional activation by deubiquitinating Sirt1 to suppress cell apoptosis and is required for mouse embryonic development. Mol. Cell. 2012;46:484–494. doi: 10.1016/j.molcel.2012.03.024. [DOI] [PubMed] [Google Scholar]
- Liszt G, Ford E, Kurtev M, Guarente L. Mouse Sir2 homolog SIRT6 is a nuclear ADP-ribosyltransferase. J. Biol. Chem. 2005;280:21313–21320. doi: 10.1074/jbc.M413296200. [DOI] [PubMed] [Google Scholar]
- Macián F, GarcIa-Cózar F, Im S-H, Horton HF, Byrne MC, Rao A. Transcriptional mechanisms underlying lymphocyte tolerance. Cell. 2002;109:719–731. doi: 10.1016/s0092-8674(02)00767-5. [DOI] [PubMed] [Google Scholar]
- Manna SK, Mukhopadhyay A, Aggarwal BB. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-κB, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J. Immunol. 2000;164:6509–6519. doi: 10.4049/jimmunol.164.12.6509. [DOI] [PubMed] [Google Scholar]
- Marok R, Winyard PG, Coumbe A, Kus ML, Gaffney K, Blades S, Mapp PI, Morris CJ, Blake DR, Kaltschmidt C, Baeuerle PA. Activation of the transcription factor nuclear factor-kB in human inflamed synovial tissue. Arthritis Rheum. 1996;39:583–591. doi: 10.1002/art.1780390407. [DOI] [PubMed] [Google Scholar]
- Martinon F, Chen X, Lee AH, Glimcher LH. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat. Immunol. 2010;11:411–418. doi: 10.1038/ni.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBurney MW, Yang X, Jardine K, Bieman M, Th’ng J, Lemieux M. The absence of SIR2alpha protein has no effect on global gene silencing in mouse embryonic stem cells. Mol. Cancer Res. 2003;1:402–409. [PubMed] [Google Scholar]
- McInnes IB, Liew FY, Gracie JA. Interleukin-18: a therapeutic target in rheumatoid arthritis? Arthritis Res. Ther. 2005;7:38–41. doi: 10.1186/ar1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondino A, Whaley CD, DeSilva DR, Li W, Jenkins MK, Mueller DL. Defective transcription of the IL-2 gene is associated with impaired expression of c-Fos, FosB, and JunB in anergic T helper 1 cells. J. Immunol. 1996;157:2048–2057. [PubMed] [Google Scholar]
- Moon MH, Jeong JK, Lee YJ, Seol JW, Jackson CJ, Park SY. SIRT1, a class III histone deacetylase, regulates TNF-α-induced inflammation in human chondrocytes. Osteoarthritis Cartilage. 2013;21:470–480. doi: 10.1016/j.joca.2012.11.017. [DOI] [PubMed] [Google Scholar]
- Morand EF. New therapeutic target in inflammatory disease: macrophage migration inhibitory factor. Intern. Med. J. 2005;35:419–426. doi: 10.1111/j.1445-5994.2005.00853.x. [DOI] [PubMed] [Google Scholar]
- Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L. Mammalian SIRT1 re-presses forkhead transcription factors. Cell. 2004;116:551–563. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Yaguchi T, Yoshiya S, Nishizaki T. Resveratrol induces apoptosis MH7A human rheumatoid arthritis synovial cells in a sirtuin 1-dependent manner. Rheumatol. Int. 2012;32:151–157. doi: 10.1007/s00296-010-1598-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederer F, Ospelt C, Brentano F, Hottiger MO, Gay RE, Gay S, Detmar M, Kyburz D. SIRT1 overexpression in the rheumatoid arthritis synovium contributes to proinflammatory cytokine production and apoptosis resistance. Ann. Rheum. Dis. 2011;70:1866–1873. doi: 10.1136/ard.2010.148957. [DOI] [PubMed] [Google Scholar]
- Ofosu-Appiah WA, Warrington RJ, Wilkins JA. Interleukin 2 responsive T cell clones from rheumatoid and normal subjects: proliferative responses to connective tissue elements. Clin. Immunol. Immunopathol. 1989;50:264–271. doi: 10.1016/0090-1229(89)90134-7. [DOI] [PubMed] [Google Scholar]
- Ohori M. ERK inhibitors as a potential new therapy for rheumatoid arthritis. Drug News Perspect. 2008;21:245–250. doi: 10.1358/DNP.2008.21.5.1219006. [DOI] [PubMed] [Google Scholar]
- Oppenheimer H, Gabay O, Meir H, Haze A, Kandel L, Liebergall M, Gagarina V, Lee EJ, Dvir-Ginzberg M. 75-kd sirtuin 1 blocks tumor necrosis factor α-mediated apoptosis in human osteoarthritic chondrocytes. Arthritis Rheum. 2012;64:718–728. doi: 10.1002/art.33407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell JD, Lerner CG, Ewoldt GR, Schwartz RH. The −180 site of the IL-2 promoter is the target of CREB/CREM binding in T cell anergy. J. Immunol. 1999;163:6631–6639. [PubMed] [Google Scholar]
- Price Nathan L., Gomes Ana P., Ling Alvin J.Y., Duarte Filipe V., Martin-Montalvo A, North Brian J., Agarwal B, Ye L, Ramadori G, Teodoro Joao S., Hubbard Basil P., Varela Ana T., Davis James G., Varamini B, Hafner A, Moaddel R, Rolo Anabela P., Coppari R, Palmeira Carlos M., de Cabo R, Baur Joseph A., Sinclair David A. SIRT1 is required for AMPK activation and the beneficial effects of Resveratrol on mitochondrial function. Cell Metab. 2012;15:675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recio MC, Andujar I, Rios JL. Anti-inflammatory agents from plants: progress and potential. Curr. Med. Chem. 2012;19:2088–2103. doi: 10.2174/092986712800229069. [DOI] [PubMed] [Google Scholar]
- Rengarajan J, Mittelstadt PR, Mages HW, Gerth AJ, Kroczek RA, Ashwell JD, Glimcher LH. Sequential involvement of NFAT and Egr transcription factors in FasL regulation. Immunity. 2000;12:293–300. doi: 10.1016/s1074-7613(00)80182-x. [DOI] [PubMed] [Google Scholar]
- Rincon M, Flavell RA. AP-1 transcriptional activity requires both T-cell receptor-mediated and co-stimulatory signals in primary T lymphocytes. EMBO J. 1994;13:4370–4381. doi: 10.1002/j.1460-2075.1994.tb06757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelofs MF, Boelens WC, Joosten LA, Abdollahi-Roodsaz S, Geurts J, Wunderink LU, Schreurs BW, van den Berg WB, Radstake TR. Identification of small heat shock protein B8 (HSP22) as a novel TLR4 ligand and potential involvement in the pathogenesis of rheumatoid arthritis. J. Immunol. 2006;176:7021–7027. doi: 10.4049/jimmunol.176.11.7021. [DOI] [PubMed] [Google Scholar]
- Safford M, Collins S, Lutz MA, Allen A, Huang CT, Kowalski J, Blackford A, Horton MR, Drake C, Schwartz RH, Powell JD. Egr-2 and Egr-3 are negative regulators of T cell activation. Nat. Immunol. 2005;6:472–480. doi: 10.1038/ni1193. [DOI] [PubMed] [Google Scholar]
- Sánchez-Fidalgo S, Cárdeno A, Villegas I, Talero E, de la Lastra CA. Dietary supplementation of resveratrol attenuates chronic colonic inflammation in mice. Eur. J. Pharmacol. 2010;633:78–84. doi: 10.1016/j.ejphar.2010.01.025. [DOI] [PubMed] [Google Scholar]
- Schug TT, Xu Q, Gao H, Peres-da-Silva A, Draper DW, Fessler MB, Purushotham A, Li X. Myeloid deletion of SIRT1 induces inflammatory signaling in response to environmental stress. Mol. Cell. Biol. 2010;30:4712–4721. doi: 10.1128/MCB.00657-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RH. T cell clonal anergy. Curr. Opin. Immunol. 1997;9:351–357. doi: 10.1016/s0952-7915(97)80081-7. [DOI] [PubMed] [Google Scholar]
- Schwartz RH. T cell anergy. Annu. Rev. Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- Sen M. Wnt signalling in rheumatoid arthritis. Rheumatology (Oxford) 2005;44:708–713. doi: 10.1093/rheumatology/keh553. [DOI] [PubMed] [Google Scholar]
- Shakibaei M, Buhrmann C, Mobasheri A. Resveratrol-mediated SIRT-1 interactions with p300 modulate receptor activator of NF-κB ligand (RANKL) activation of NF-κB signaling and inhibit osteoclasto-genesis in bone-derived cells. J. Biol. Chem. 2011;286:11492–11505. doi: 10.1074/jbc.M110.198713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds RE, Foxwell BM. Signalling, inflammation and arthritis: NF-κB and its relevance to arthritis and inflammation. Rheumatology (Oxford) 2008;47:584–590. doi: 10.1093/rheumatology/kem298. [DOI] [PubMed] [Google Scholar]
- Sloan-Lancaster J, Evavold BD, Allen PM. Th2 cell clonal anergy as a consequence of partial activation. J. Exp. Med. 1994a;180:1195–1205. doi: 10.1084/jem.180.4.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan-Lancaster J, Shaw AS, Rothbard JB, Allen PM. Partial T cell signaling: altered phospho-zeta and lack of zap70 recruitment in APL-induced T cell anergy. Cell. 1994b;79:913–922. doi: 10.1016/0092-8674(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Solomon JM, Pasupuleti R, Xu L, McDonagh T, Curtis R, DiStefano PS, Huber LJ. Inhibition of SIRT1 catalytic activity increases p53 acetylation but does not alter cell survival following DNA damage. Mol. Cell. Biol. 2006;26:28–38. doi: 10.1128/MCB.26.1.28-38.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl M, Dijkers PF, Kops GJ, Lens SM, Coffer PJ, Burgering BM, Medema RH. The forkhead transcription factor FoxO regulates transcription of p27Kip1 and Bim in response to IL-2. J. Immunol. 2002;168:5024–5031. doi: 10.4049/jimmunol.168.10.5024. [DOI] [PubMed] [Google Scholar]
- Stamp LK, James MJ, Cleland LG. Interleukin-17: the missing link between T-cell accumulation and effector cell actions in rheumatoid arthritis? Immunol. Cell Biol. 2004;82:1–9. doi: 10.1111/j.1440-1711.2004.01212.x. [DOI] [PubMed] [Google Scholar]
- Stein S, Schafer N, Breitenstein A, Besler C, Winnik S, Lohmann C, Heinrich K, Brokopp CE, Handschin C, Landmesser U, Tanner FC, Luscher TF, Matter CM. SIRT1 reduces endothelial activation without affecting vascular function in ApoE−/− mice. Aging (Albany NY) 2010;2:353–360. doi: 10.18632/aging.100162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutmuller R, Garritsen A, Adema GJ. Regulatory T cells and toll-like receptors: regulating the regulators. Ann. Rheum. Dis. 2007;66(Suppl. 3):iii91–iii95. doi: 10.1136/ard.2007.078535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tak PP. Chemokine inhibition in inflammatory arthritis. Best Pract. Res. Clin. Rheumatol. 2006;20:929–939. doi: 10.1016/j.berh.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Thomas RM, Chunder N, Chen C, Umetsu SE, Winandy S, Wells AD. Ikaros enforces the costimulatory requirement for IL2 gene expression and is required for anergy induction in CD4+ T lymphocytes. J. Immunol. 2007;179:7305–7315. doi: 10.4049/jimmunol.179.11.7305. [DOI] [PubMed] [Google Scholar]
- Turka LA, Linsley PS, Lin H, Brady W, Leiden JM, Wei RQ, Gibson ML, Zheng XG, Myrdal S, Gordon D. T-cell activation by the CD28 ligand B7 is required for cardiac allograft rejection in vivo. Proc. Natl. Acad. Sci. USA. 1992;89:11102–11105. doi: 10.1073/pnas.89.22.11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman KS, Northrop JP, Admon A, Crabtree GR. Jun family members are controlled by a calcium-regulated, cyclosporin A-sensitive signaling pathway in activated T lymphocytes. Genes Dev. 1993;7:188–196. doi: 10.1101/gad.7.2.188. [DOI] [PubMed] [Google Scholar]
- Venuprasad K. Cbl-b and itch: key regulators of peripheral T-cell tolerance. Cancer Res. 2010;70:3009–3012. doi: 10.1158/0008-5472.CAN-09-4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba JM, Alcain FJ. Sirtuin activators and inhibitors. Biofactors. 2012;38:349–359. doi: 10.1002/biof.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang FM, Chen YJ, Ouyang HJ. Regulation of unfolded protein response modulator XBP1s by acetylation and deacetylation. Biochem. J. 2010;433:245–252. doi: 10.1042/BJ20101293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuzhu G, Komai-Koma M, Leung BP, Howe HS, McSharry C, McInnes IB, Xu D. Resveratrol modulates murine collagen-induced arthritis by inhibiting Th17 and B-cell function. Ann. Rheum. Dis. 2012;71:129–135. doi: 10.1136/ard.2011.149831. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-κB pathway in the treatment of inflammation and cancer. J. Clin. Invest. 2001;107:135–142. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S-R, Wright J, Bauter M, Seweryniak K, Kode A, Rahman I. Sirtuin regulates cigarette smoke-induced proinflammatory mediator release via RelA/p65 NF-κB in macrophages in vitro and in rat lungs in vivo: implications for chronic inflammation and aging. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;292:L567–L576. doi: 10.1152/ajplung.00308.2006. [DOI] [PubMed] [Google Scholar]
- Yavuz S, Elbir Y, Tulunay A, Eksioglu-Demiralp E, Direskeneli H. Differential expression of toll-like receptor 6 on granulocytes and monocytes implicates the role of microorganisms in Behcet’s disease etiopathogenesis. Rheumatol. Int. 2008;28:401–406. doi: 10.1007/s00296-007-0470-y. [DOI] [PubMed] [Google Scholar]
- Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Minter-Dykhouse K, Lou Z. A c-MyceSIRT1 feedback loop regulates cell growth and transformation. J. Cell Biol. 2009;185:203–211. doi: 10.1083/jcb.200809167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z, Zhang X, Sengupta N, Lane WS, Seto E. SIRT1 regulates the function of the Nijmegen breakage syndrome protein. Mol. Cell. 2007;27:149–162. doi: 10.1016/j.molcel.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Lee SM, Shannon S, Gao B, Chen W, Chen A, Divekar R, McBurney MW, Braley-Mullen H, Zaghouani H, Fang D. The type III histone deacetylase Sirt1 is essential for maintenance of T cell tolerance in mice. J. Clin. Invest. 2009;119:3048–3058. doi: 10.1172/JCI38902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Chen HZ, Liu JJ, Jia YY, Zhang ZQ, Yang RF, Zhang Y, Xu J, Wei YS, Liu DP, Liang CC. SIRT1 suppresses activator protein-1 transcriptional activity and cyclooxygenase-2 expression in macrophages. J. Biol. Chem. 2010;285:7097–7110. doi: 10.1074/jbc.M109.038604. [DOI] [PMC free article] [PubMed] [Google Scholar]