Abstract
Aim:
To understand the effects of lithospermic acid (LA), a potent antioxidant from the water-soluble extract of Salvia miltiorrhiza, on the migration and proliferation of rat thoracic aorta vascular smooth muscle cells (VSMCs).
Methods:
VSMC migration, proliferation, DNA synthesis and cell cycle progression were investigated by transwell migration analysis, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, bromodeoxyuridine (BrdU) incorporation assay, and flow cytometric detection, respectively. Intracellular reactive oxygen species (ROS) generation was detected using 2′,7′-dichlorofluorescin diacetate (DCFH-DA). The expression of cyclin D1 protein and matrix metalloproteinase-9 (MMP-9) protein, as well as the phosphorylation state of ERK1/2, were determined using Western blots. The activity of MMP-9 and the expression of MMP-9 mRNA were assessed by gelatin zymography analysis and RT-PCR, respectively.
Results:
LA (25−100 μmol/L) inhibited both lipopolysaccharide (LPS)- and fetal bovine serum (FBS)-induced ROS generation and ERK1/2 phosphorylation. By down-regulating the expression of cyclin D1 and arresting cell cycle progression at the G1 phase, LA inhibited both VSMC proliferation and DNA synthesis as induced by 5% FBS. Furthermore, LA attenuated LPS-induced VSMC migration by inhibiting MMP-9 expression and its enzymatic activity.
Conclusion:
LA is able to inhibit FBS-induced VSMC proliferation and LPS-induced VSMC migration, which suggests that LA may have therapeutic effects in the prevention of atherosclerosis, restenosis and neointimal hyperplasia.
Keywords: lithospermic acid, ERK1/2, matrix metalloproteinase-9, vascular smooth muscle cells, proliferation, migration
Introduction
Abnormal proliferation and migration of vascular smooth muscle cells (VSMCs) play critical roles in the development of atherosclerosis and restenosis1. Several studies have indicated that reactive oxygen species (ROS) and mitogen-activated protein kinases (MAPKs) are involved in vascular remodeling under various pathological conditions2. Overproduction of ROS in VSMCs may promote the migration and proliferation of VSMCs3. ROS generation has been shown to be related to the activation of MAPKs, which are key transducers of extracellular signals that promote cellular growth and movement4, 5. It was reported that ROS levels were increased during restenosis,6 and that antioxidants attenuated neointimal hyperplasia7, 8. Therefore, inhibition of MAPKs by antioxidant treatment may be a therapeutic strategy against atherosclerosis and restenosis.
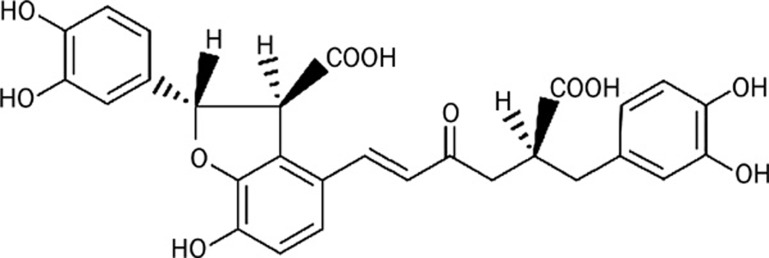
Danshen, the dried root and rhizome of Salvia miltiorrhiza, is a traditional Chinese herbal medicine used for the treatment of cardiovascular diseases, and many of its active components have been purified and analyzed. Previous studies have indicated that S miltiorrhiza prevented restenosis and intimal thickening of air-injured carotid artery in rats9 and inhibited the proliferation of VSMCs in vitro9, 10, 11. In addition, a water-soluble fraction with antioxidant-rich components from S miltiorrhiza was shown to inhibit intimal hyperplasia after balloon injury12 and to reduce atherosclerosis in cholesterol-fed rabbits13, 14. However, the exact active ingredients responsible for these effects and the molecular mechanisms involved remain to be determined. Lithospermic acid (LA), a potent antioxidant in the water-soluble extract of S miltiorrhiza15, possesses anti-inflammatory and hypouricemic activities16, as well as hormone-regulatory effects17. In recent years, LA has attracted considerable interest due to its anti-HIV activity18. However, to our knowledge, no studies on the effects of LA on VSMCs have been reported to date. This study aims to investigate the significant roles that LA play in the protection against oxidative stress as well as in the proliferation and migration of VSMCs in vitro (Figure 1).
Figure 1.
The chemical structure of lithospermic acid.
Materials and methods
Chemicals and drugs
LA was provided by the Department of Phytochemistry, Shanghai Institute of Materia Medica (Shanghai, China). LA is a light brown powder of 99.4% purity. It was dissolved in phosphate buffered saline (PBS) and then diluted with Dulbecco's modified Eagle's medium (DMEM) to a final concentration. Monoclonal antibodies to phospho-ERK1/2, cyclin D1 and polyclonal antibodies to ERK1/2 and MMP-9 were purchased from Santa Cruz Biotechnology (CA, USA). Other reagents, unless specified, were purchased from Sigma.
Cell culture
Primary VSMCs were obtained from the thoracic aorta of SD rats weighing 150–180 g via the tissue explant method, as described previously19. More than 98% of the cells were positive for smooth muscle-specific α-actin, and exhibited the typical hill-and-valley morphology of VSMCs. Cells at passages 3 to 5 were used for experiments. Cells grown to 80%–95% confluence were made quiescent by starvation (0.1% FBS) for 24 h. LA was administered 2 h before treatment with LPS or FBS.
Assessment of intracellular ROS production
ROS production was quantified by the DCFH-DA method20, based on the ROS-dependent oxidation of DCFH-DA to DCF. VSMCs grown in 96-well plates were treated with different concentrations of LA for 2 h prior to LPS (100 ng/mL) or FBS (5%) stimulation for 30 min at 37 °C. The cells were then incubated with DCFH-DA (10 μmol/L) for 30 min. Fluorescence of the samples was monitored at an excitation wavelength of 488 nm and an emission wavelength of 535 nm.
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated using Trizol reagent according to the manufacturer's protocol and quantified by UV absorption at 260 nm using a UV spectrophotometer. Total RNA (4 μg) of each sample was reverse-transcribed into cDNA using the reverse transcription system. The cDNA was amplified with the following specific primers: GAPDH: 5'-GGT CGC TGT GAA CGG ATT T-3' (forward) and 5'-TGG GCG GTT GAA CTT GC-3' (reverse); MMP-9: 5'-CTT AGA TCA TTC TTC AGT GCC-3' (forward) and 5'-GAT CCA CCT TCT GAG ACT TCA-3' (reverse). The PCR products were separated by electrophoresis in a 1% agarose gel and stained with ethidium bromide.
Western blot analysis
VSMCs were lysed in a lysis buffer containing 50 mmol/L Tris-HCl (pH 7.5), 2 mmol/L ethylenediaminetetraacetic acid (EDTA), 150 mmol/L NaCl, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 1 mmol/L NaF, 1 mmol/L Na3VO4, 1 mmol/L phenylmethylsulfonyl fluoride (PMSF), 1 mmol/L dithiothreitol (DTT), 1 μg/mL leupeptin, 1 μg/mL aprotinin, and 1% Triton. Equal amounts of protein from each sample were subjected to SDS-PAGE and blotted on PVDF membrane, which was incubated for 2 h at room temperature with blocking buffer (5% non-fat milk, 0.1% Tween 20, in TBS, pH 7.6) and then probed with primary antibodies overnight at 4 °C. After incubation with the appropriate secondary antibodies, the immunoreactive band was detected by an ECL Western blotting detection system (GE Healthcare).
Cell proliferation assay
The cell proliferation assay was performed using the MTT method as described previously21. Briefly, growth-arrested VSMCs were incubated with or without LA for 2 h prior to stimulation with 5% FBS for 48 h, and then the cells were incubated with 0.5 mg/mL MTT for 4 h at 37 °C. Finally the culture medium was removed, and the formazan salt crystals were dissolved with 200 μL dimethylsulfoxide (DMSO) and shaken for 10 min. The absorbance was read at a wavelength of 570 nm using Spectramax M2 microplate Reader (Molecular Devices). To validate the MTT assay, trypan blue exclusion was performed in parallel.
BrdU incorporation assay
DNA synthesis in VSMCs was examined using the BrdU incorporation assay according to the method described previously22. Quiescent VSMCs were pretreated with or without LA for 2 h prior to stimulation with 5% FBS for 24 h. Subsequently, 10 μmol/L BrdU was added to the cells and incubated for another 24 h. To immunostain for BrdU, the cells were washed with PBS, fixed in 4% polyformaldehyde and then permeabilized with 0.1% Triton X-100. After DNA denaturation with 4 mol/L HCl, non-specific binding sites were blocked with 5% non-fat milk. The cells were then stained with antibody for BrdU followed by incubation with the Alexa Flour 568 goat-anti-mouse IgG (Invitrogen) secondary antibody. The cell nuclei were stained with Hoechst 33342 and evaluated by fluorescence microscopy with the appropriate fluorescent filters. Results are presented as mitotic index, and defined as the percentage of BrdU-positive nuclei per number of cells.
Flow cytometry analysis of cell cycle
Quiescent VSMCs were pretreated with or without LA for 2 h, followed by 5% FBS treatment for 24 h. Cells were then trypsinized, collected, and washed twice with cold PBS. Cells pellets were fixed in 70% ethanol and stored at 4 °C. Next, the fixed cells were treated with RNase A (10 μg/mL). DNA was stained with propidium iodide (50 μg/mL) for 30 min at 37 °C, and 1×104 cells were analyzed by flow cytometry. The rates of G0/G1, S and G2/M phases were determined using the computer program ModiFit LT.
Cell migration assay
VSMCs migration was examined using transwell cell culture chamber with gelatin-coated polycarbonate membrane as described previously21. Growth-arrested cells were harvested, washed, and resuspended in the media (DMEM+0.1% FBS). Approximately 1×105 cells were placed in the upper chamber of a 24-well transwell that was precoated with 0.1% gelatin. LA was added to both the upper and the lower compartments and was present throughout the duration of the experiment. Migration was induced by the addition of LPS (100 ng/mL) to the lower chamber. After incubation at 37 °C for 24 h, the non-migratory cells were removed from the upper surface of the membrane by scraping them with cotton swabs. The membrane was fixed with 90% ethanol and stained with 0.1% crystal. Migrated cells were counted at 200×magnification in five randomly chosen microscope fields per filter.
Gelatin zymography
Gelatinase activity in the conditioned medium collected from cell cultures was measured with zymography, as described previously23. Conditioned medium were electrophoresed in a polyacrylamide gel containing 0.1% (w/v) gelatin. After being washed at room temperature for 1.5 h with 2.5% Triton X-100, the gel was subsequently incubated at 37 °C for 24 h in a buffer containing 10 mmol/L CaCl2,200 mmol/L NaCl, and 50 mmol/L Tris-HCl (pH 7.5), and then stained with 0.2% coomassie brilliant blue. The gelatinolytic regions were observed as white bands against a blue background and band densities were quantified using Smart View Analysis program.
Statistical analysis
Results are expressed as mean±SEM. Statistical analysis was performed by one-way analysis of variance (ANOVA) or Student's t-test. A P-value of < 0.05 was considered significant.
Results
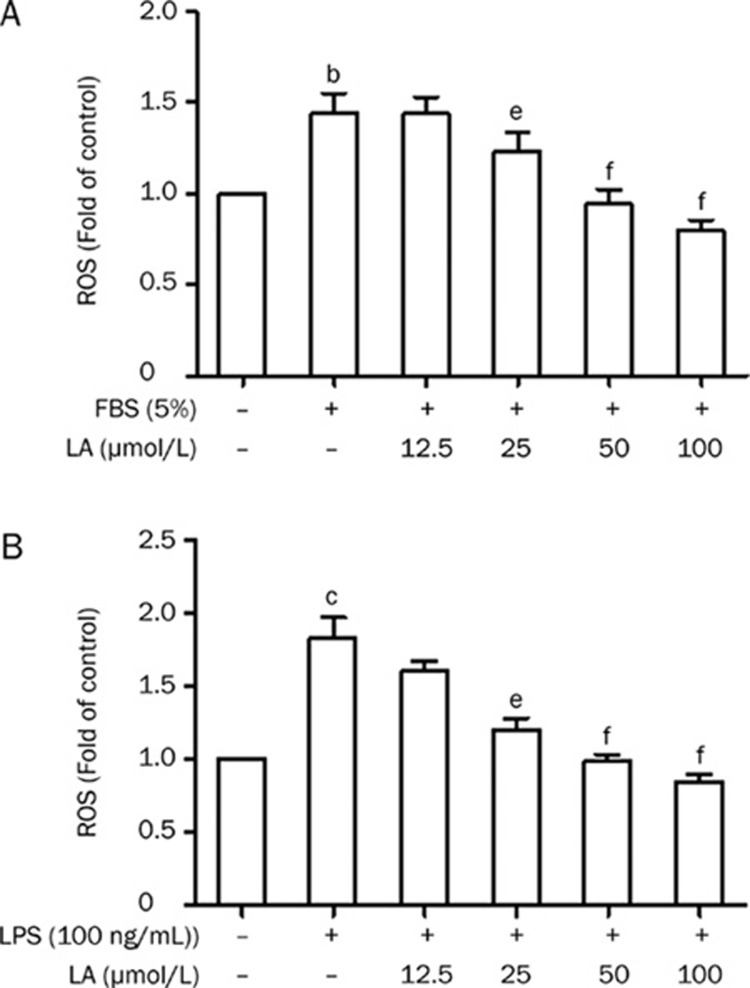
LA inhibits ROS generation in FBS- or LPS-stimulated VSMCs
In this study we investigated the effects of LA on FBS- and LPS-induced ROS generation. Compared with the control group, FBS (5%) or LPS (100 ng/mL) significantly increased ROS generation in VSMCs (Figure 2). However, the increased intracellular ROS generation as induced by FBS or LPS was clearly inhibited by LA in a concentration-dependent manner.
Figure 2.
Effects of LA on (A) FBS (5%) and (B) LPS (100 ng/mL) induced ROS generation in VSMCs. Serum-starved VSMCs were pretreated with LA for 2 h before stimulation with FBS (5%) or LPS (100 ng/mL) for 30 min at 37 °C. The cells were then stained with DCFH-DA (10 μmol/L) and analyzed by a fluorescence plate reader. Relative fluorescence intensities were calculated using untreated control cells as a standard. Results are presented as mean±SEM. n=6. bP<0.05, cP<0.01 vs control. eP<0.05, fP<0.01 vs FBS or LPS.
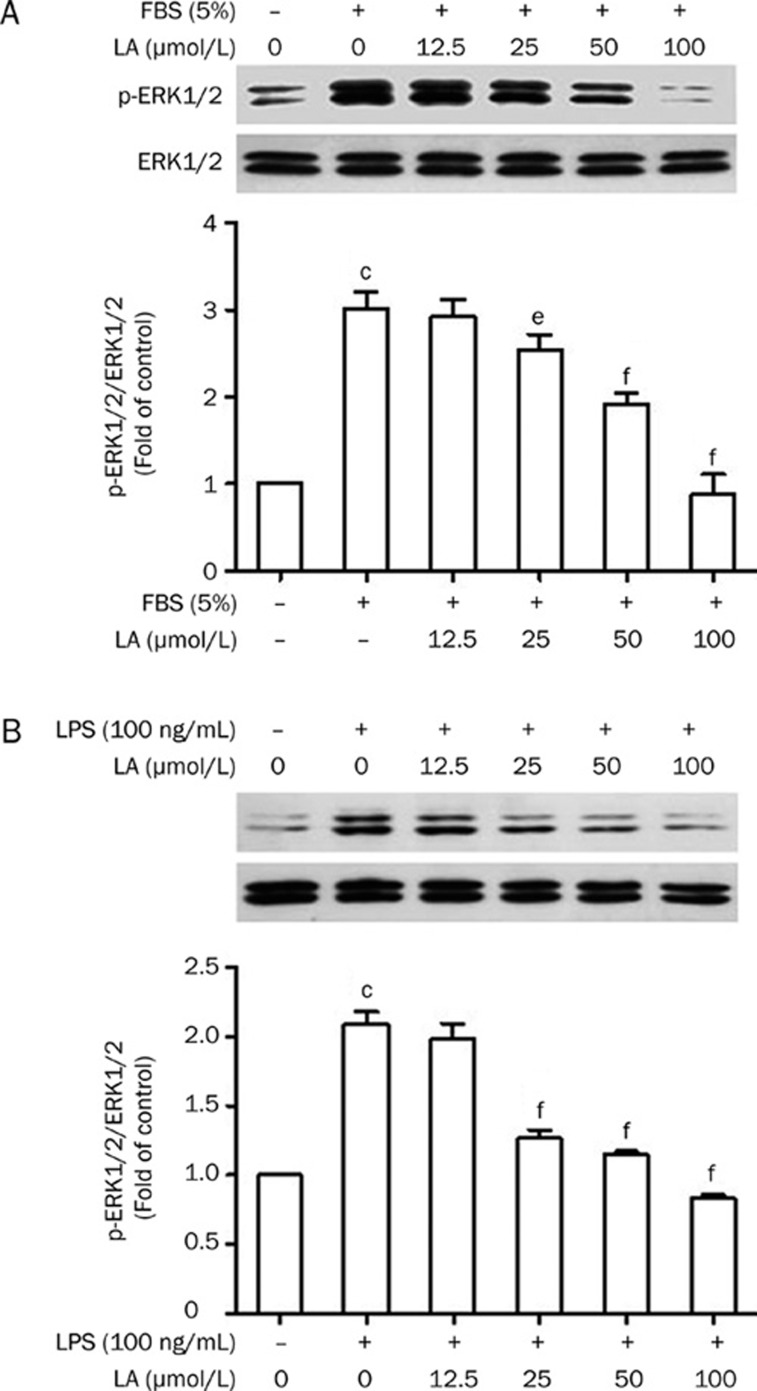
LA inhibits LPS- and FBS-induced ERK1/2 phosphorylation in VSMCs
It has been reported that ERK1/2 activation plays a critical role in the migration and proliferation of VSMCs24. Therefore, we examined the effect of LA on ERK1/2 activation. Our results indicated that exposure of VSMCs to either FBS or LPS for 15 min markedly induced the phosphorylation of ERK1/2. Conversely, LA could completely abrogate ERK1/2 activation in FBS- or LPS-stimulated VSMCs (Figure 3A and 3B).
Figure 3.
Effects of LA on (A) FBS- and (B) LPS-induced ERK1/2 activation in VSMCs. Cells were treated with LA for 2 h prior to FBS (5%) or LPS (100 ng/mL) stimulation for 15 min. Whole cell lysates were prepared and subsequently used for detection of p-ERK1/2 and ERK1/2 by Western blotting. The bar graphs show the ratio of densities of phosphoproteins to total proteins for the blots. n=3. cP<0.01 vs control, eP<0.05, fP<0.01 vs FBS or LPS. Values are presented as mean±SEM.
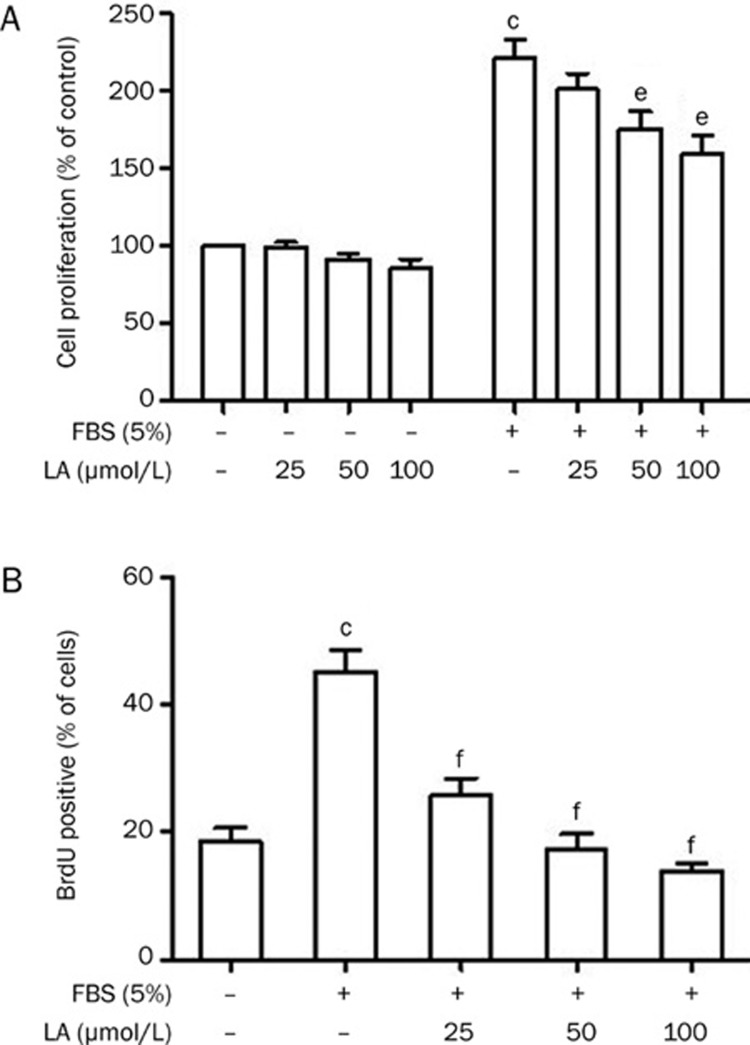
LA inhibits FBS-induced VSMCs proliferation and DNA synthesis
We next studied the effect of LA on the proliferation of VSMCs using the MTT assay. When growth-arrested cells were treated with LA (25, 50, 100 μmol/L) in the presence of 0.1% FBS, no significant difference was observed in cell viability (Figure 4A, left panel), suggesting that LA did not show significant cytotoxicity up to 100 μmol/L. The absence of cytotoxicity was further confirmed with a trypan blue exclusion assay (data not shown). Moreover, we found that FBS induced a 2.21-fold increase in the proliferation of VSMCs. Treatment of cells with LA 2 h before stimulation with FBS reduced cell proliferation in a concentration-dependent manner (Figure 4A, right panel). We further examined the effect of LA on DNA synthesis, as induced by 5% FBS in VSMCs using the BrdU incorporation assay. BrdU incorporation was markedly increased in VSMCs exposed to 5% FBS for 48 h (P<0.01), indicating an increase in DNA synthesis. However, this effect was abolished in VSMCs pretreated with LA (Figure 4B).
Figure 4.
Effects of LA on FBS-induced proliferation and DNA synthesis in VSMCs. (A) Proliferation activities were measured by MTT assay in the absence (left) or presence (right) of 5% FBS. Relative proliferation activities were expressed using untreated control cells as a standard. n=6. (B) DNA synthesis was measured by BrdU incorporation assay. The left part of the diagram shows BrdU incorporation of quiescent and FBS-stimulated VSMCs. On the right side, a concentration-dependent decrease of BrdU incorporation in LA-treated VSMCs is shown. n=6. Values are presented as mean±SEM. cP<0.01 vs control. eP<0.05, fP<0.01 vs FBS.
LA efficiently abrogates cyclin D1 protein expression in VSMCs and arrests FBS-stimulated cells in the G1-phase
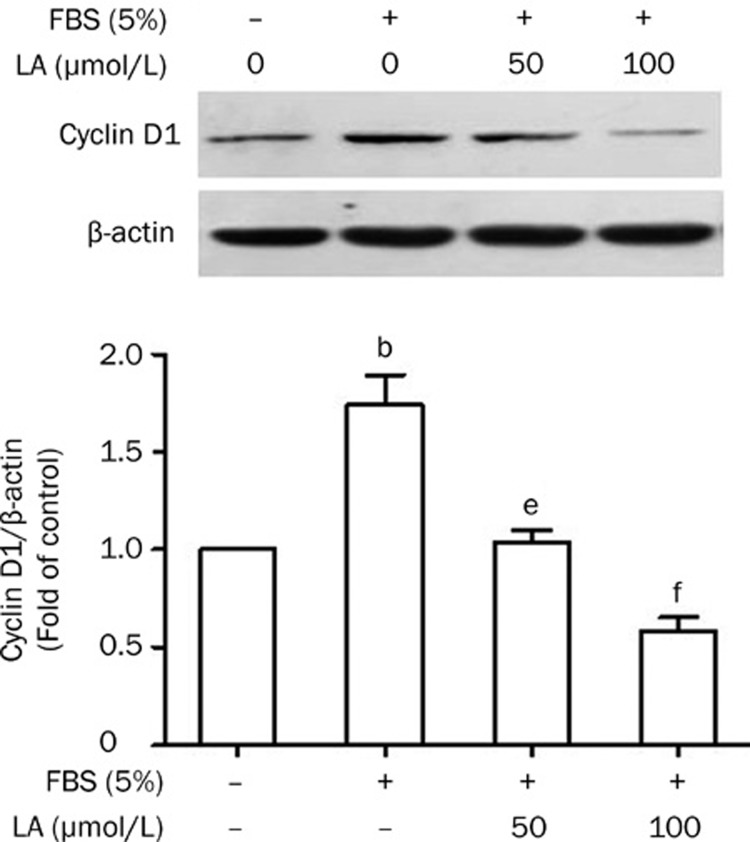
It is known that cyclins, CDKs, and their inhibitors regulate cell cycle progression. Changes in the cyclin D1/CDK complex, for example, can either stop or promote cell proliferation25. We investigated the potential effect of LA on cyclin D1 protein expression. Our results showed that FBS significantly increased the expression of cyclin D1, while LA completely abolished this serum action (Figure 5).
Figure 5.
Inhibitory effect of LA on FBS-stimulated cyclin D1 protein expression. Growth-arrested VSMCs were pretreated with LA (50 or 100 μmol/L) for 2 h and then stimulated with 5% FBS for 24 h. Expression of cyclin D1 protein was examined by Western blotting. β-actin was used as a loading control. Values are presented as mean±SEM. n=3. bP<0.05 vs control. eP<0.05, fP<0.01 vs FBS.
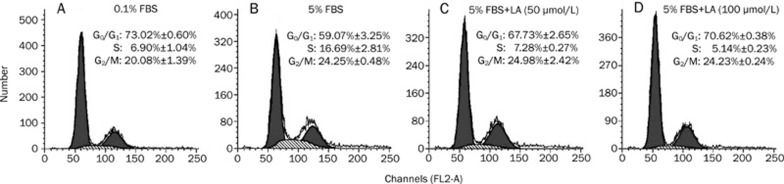
Proliferation cells pass through several cell cycle checkpoints, mainly the G1 to S and G2 to M transitions. The former checkpoint is considered to be the most important step in DNA replication. Accordingly, flow cytometric assessment was performed to determine the effect of LA on cell cycle progression. As shown in Figure 6, the percentage of G0/G1 or S phase cells in the 5% FBS-stimulated group were 59.07%±3.25% and 16.69%±2.81%, respectively. LA at concentrations of 50 to 100 μmol/L effectively increased the proportion of cells in the G0/G1 phase and simultaneously decreased the proportion of cells in the S phase (Figure 6).
Figure 6.
Cell cycle distribution of (A) quiescent and (B) FBS-stimulated VSMCs. (C) and (D) show VSMCs treated with 50 and 100 μmol/L LA, respectively, in the presence of FBS stimulation. It demonstrates LA-induced cell cycle arrest in G0/G1 phase. G0/G1 phase is represented by the first peak, S phase in diagonal and G2/M by the second peak. Results are expressed as the percentage of total cells in G0/G1, S, or G2/M phase of the cell cycle. Values are presented as mean±SEM. n=3.
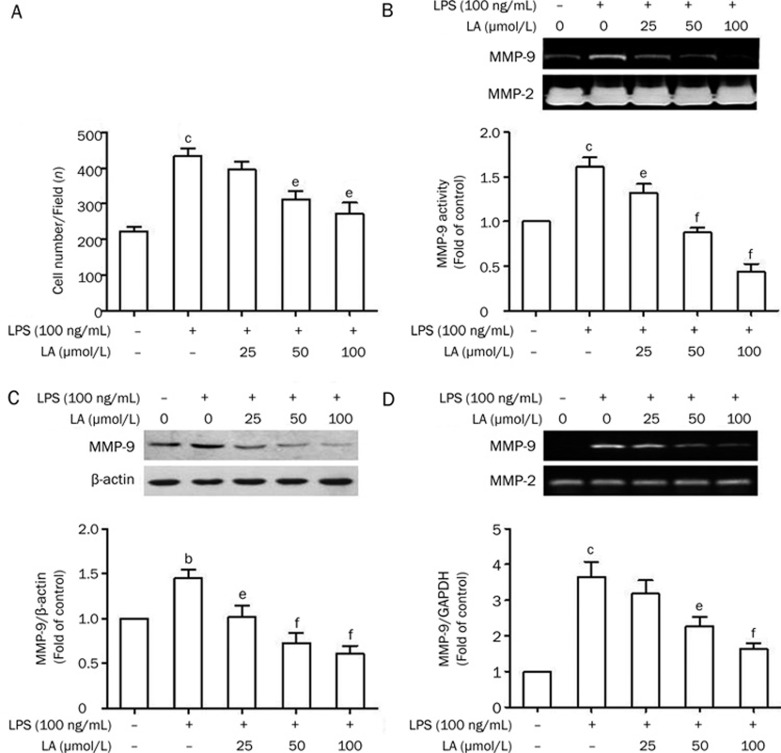
LA inhibits LPS-induced migration of VSMCs, and attenuates the enzymatic activity and expression of MMP-9
Finally, we examined the effect of LA on the migration of VSMCs. VSMC migration was significantly increased from stimulation with LPS (100 ng/mL) for 24 h (P<0.01; Figure 7A). However, LA inhibited the migration of VSMCs in a concentration-dependent manner (Figure 7A). We then investigated the effect of LA on MMP-2 and MMP-9 activity using gelatin zymography. As shown in Figure 7B, the medium from control cells showed very weak proteolytic activity at 92 kDa (corresponding to MMP-9) and high proteolytic activity at 72 kDa (corresponding to MMP-2). LA suppressed LPS-induced MMP-9 activity in a concentration-dependent manner (Figure 7B). However, the level of MMP-2 secretion was not affected by LPS or LA (Figure 7B). Furthermore, we performed Western blots to confirm the decrease of MMP-9 protein. As shown in Figure 7C, LA demonstrated an inhibitory effect on LPS-induced MMP-9 protein expression in VSMCs. Consistent with the results from zymography and Western blotting, we observed that MMP-9 mRNA expression, as induced by LPS, was inhibited by LA (Figure 7D).
Figure 7.
Effects of LA on LPS-induced VSMC migration as well as MMP-9 enzymatic activity and expression. (A) Cells were treated as indicated and migration assays were performed as described in the Methods section. The diagram shows counted cells on the bottom side of the transwell chamber membrane at different LA con-centrations. n=6. Cells were pretreated with LA for 2 h prior to 100 ng/mL LPS stimulation for 24 h. (B) The conditioned media were prepared and used for gelatin zymography. n=6. (C) The total cellular proteins were prepared and used for Western blotting. n=3. (D) The MMP-9 mRNA levels were measured by RT-PCR. GAPDH was used as an internal control. n=6. Values are presented as mean±SEM. bP<0.05, cP<0.01 vs control. eP<0.05, fP<0.01 vs LPS.
Discussion
LA, a naturally occurring polyphenolic compound, is found in various plants and more abundantly in S miltiorrhiza and Lithospermum. Our study demonstrated that LA inhibited FBS-induced VSMC proliferation and LPS-induced VSMC migration.
ROS play a key role in the proliferation of VSMCs and produce inflammation in the vessel wall. Therefore, ROS are regarded as a risk factor for vascular diseases. It was reported that FBS induced ROS production, leading to cell proliferation via activation of ERK1/2 in smooth muscle cells26, 27, 28. LPS, a bacterial endotoxin that functions as a proinflammatory mediator, is considered to be a strong stimulator of atherosclerosis29. Furthermore, LPS has been shown to induce ROS generation and ERK1/2 phosphorylation in VSMCs30. In our study, LPS and FBS induced ROS generation in VSMCs, which was consistent with previous reports. LA presented inhibitory effects on the generation of ROS in a concentration-dependent manner.
The ROS-ERK1/2 pathway has been implicated in the control of VSMC proliferation and migration3. Addition of exogenous ROS can activate mitogen-activated protein (MAP) kinases, including ERK1/2, p38 MAPkinase, JNK, and ERK5, which are important for cell growth, inflammation, apoptosis, and cell differentiation, respectively31, 32. These findings suggest that ERK1/2 could represent a link between ROS and VSMC proliferation as well as migration in atherosclerosis, restenosis and neointimal hyperplasia. In our study, LA inhibited FBS- and LPS-induced ERK1/2 activation in a concentration-dependent manner.
We replaced a single growth factor with 5% FBS to induce cell proliferation. FBS contains a range of growth factors, including PDGF, FGF, transforming growth factor, serotonin, and thrombin33. Consequently, it was used to mimic the multiple factors present in the environment in vivo. Our results indicated that LA inhibited FBS-induced VSMC proliferation in a concentration-dependent manner. Cell cycle control is a highly regulated process that involves a complex cascade of events. Modulation of the expression and function of cell-cycle regulatory proteins provide an important mechanism for inhibiting cell growth. Cyclin D1 is a primary target for the mitogenic effects of ROS and it plays an important role for G1/S transition in cell cycle progression34. Our results indicated that LA suppressed FBS-induced cyclin D1 protein expression and arrested cells in the G1 phase. Traversing the G1-S phase boundary is coupled to DNA synthesis, followed by entry into G2 and finally mitosis in the M phase. BrdU, a thymidine analog, is widely used as a marker of DNA synthesis, and is able to incorporate into cellular DNA during the S-phase35. In our study, LA (50 μmol/L) exerted strong inhibition on BrdU incorporation into DNA, which indicated that LA significantly inhibited FBS-induced DNA synthesis. However, LA exerted only 30%–40% inhibition on proliferation at the same concentration. The discrepancy may result from the following reason. As the cell cycle comprises a range of highly ordered events that lead to mitosis and the formation of new cells, regulating any of these conserved processes is critical to cell growth and replication. DNA synthesis during S phase is only one step in cell cycle progression. Besides DNA replication, cell proliferation is also affected by mitotic activity, which is regulated by many mitotic kinases and checkpoints36. LA may interfere with the mitotic process directly and result in a loss of coordination between DNA synthesis and subsequent events required for cell division.
It was reported that LPS increased neointimal hyperplasia in balloon-injured rabbit aortas30. When injury occurs, VSMCs migrate from the tunica media to the intima, which leads to neointima formation37. Our results indicated that LA significantly inhibited LPS-induced VSMC migration. It should be noted that the increase in VSMC migration might be a result of the proliferation. However, we can rule out this possibility as LPS (100 ng/mL) did not show any effect on the proliferation of VSMCs (data not shown).
VSMC migration requires the breakdown of the extracellular matrix. One possible mechanism by which VSMCs break down the extracellular matrix is by secreting matrix metalloproteinases (MMPs)38. It was reported that MMPs, specifically MMP-2 and MMP-9, were important for the migration of VSMCs39. LPS has been shown to upregulate MMP-9 via ERK1/2 signaling40, 41. In our study, LPS increased MMP-9 expression and enzymatic activity while LA attenuated these effects in a concentration-dependent manner. Therefore, LA inhibits LPS-induced VSMC migration by attenuating MMP-9 expression and enzymatic activity.
In addition to ERK1/2 signaling, p38 and JNK also play important roles in regulating cell migration42. Accordingly, we cannot rule out the possibility that these two signaling pathways are involved in the anti-migratory effect of LA.
In summary, our results demonstrated that LA inhibited ROS generation and ERK1/2 activation, and exerted inhibitory effects against the proliferation and migration of VSMCs in vitro. The anti-proliferative effect of LA is attributed to its direct down-regulation of cyclin D1 expression and subsequent cell cycle arrest in G1 phase. The anti-migratory effect of LA on VSMCs is attributable to its ability to inhibit MMP-9 expression as well as enzymatic activity. These results suggested that LA, a water-soluble polyphenolic antioxidant, may have therapeutic potential against atherosclerosis, restenosis and neointimal hyperplasia.
Author contribution
Yi-ping WANG, Li CHEN, and Wen-yi WANG designed the research; Li CHEN performed the research and analyzed the data; Li CHEN, Wen-yi WANG, and Yi-ping WANG wrote the manuscript.
Acknowledgments
This work was supported by National Basic Research Program of China (No 2009CB930300)
References
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–9. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Kyaw M, Yoshizumi M, Tsuchiya K, Izawa Y, Kanematsu Y, Tamaki T. Atheroprotective effects of antioxidants through inhibition of mitogen-activated protein kinases. Acta Pharmacol Sin. 2004;25:977–85. [PubMed] [Google Scholar]
- Lee MY, Griendling KK. Redox signaling, vascular function, and hypertension. Antioxid Redox Signal. 2008;10:1045–59. doi: 10.1089/ars.2007.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han MJ, Kim BY, Yoon SO, Chung AS. Cell proliferation induced by reactive oxygen species is mediated via mitogen-activated protein kinase in Chinese hamster lung fibroblast (V79) cells. Mol Cells. 2003;15:94–101. [PubMed] [Google Scholar]
- Lo IC, Shih JM, Jiang MJ. Reactive oxygen species and ERK 1/2 mediate monocyte chemotactic protein-1-stimulated smooth muscle cell migration. J Biomed Sci. 2005;12:377–88. doi: 10.1007/s11373-005-1703-2. [DOI] [PubMed] [Google Scholar]
- Szocs K, Lassegue B, Sorescu D, Hilenski LL, Valppu L, Couse TL, et al. Upregulation of Nox-based NAD(P)H oxidases in restenosis after carotid injury. Arterioscler Thromb Vasc Biol. 2002;22:21–7. doi: 10.1161/hq0102.102189. [DOI] [PubMed] [Google Scholar]
- Ghigliotti G, Mereto E, Eisenberg PR, Martelli A, Orsi P, Sini D, et al. N-acetyl-cysteine reduces neointimal thickening and procoagulant activity after balloon-induced injury in abdominal aortae of New Zealand white rabbits. Thromb Haemost. 2001;85:724–9. [PubMed] [Google Scholar]
- Kappert K, Sparwel J, Sandin A, Seiler A, Siebolts U, Leppanen O, et al. Antioxidants relieve phosphatase inhibition and reduce PDGF signaling in cultured VSMCs and in restenosis. Arterioscler Thromb Vasc Biol. 2006;26:2644–51. doi: 10.1161/01.ATV.0000246777.30819.85. [DOI] [PubMed] [Google Scholar]
- Zhou XM, Lu ZY, Wang DW.Experimental study of Salvia miltiorrhiza on prevention of restenosis after angioplasty Zhongguo Zhong Xi Yi Jie He Za Zhi 199616480–2.Chinese. [PubMed] [Google Scholar]
- Sun B, Yuan Y, Zhang W, Che D. Effects of hypoxic endothelial cell conditioned medium on proliferation and collagen synthesis of smooth muscle cells and inhibitory effects of radix Salviae miltiorrhizae. Chin Med J (Engl) 1995;108:855–8. [PubMed] [Google Scholar]
- Du X, Yang F, Wang W, Gong Y.The mechanism of Radix Salviae Miltiorrhizae Injection on the inhibition of smooth muscle cell proliferation induced by H2O2 Zhong Yao Cai 200326647–9.Chinese. [PubMed] [Google Scholar]
- Chen YL, Yang SP, Shiao MS, Chen JW, Lin SJ. Salvia miltiorrhiza inhibits intimal hyperplasia and monocyte chemotactic protein-1 expression after balloon injury in cholesterol-fed rabbits. J Cell Biochem. 2001;83:484–93. doi: 10.1002/jcb.1233. [DOI] [PubMed] [Google Scholar]
- Wu YJ, Hong CY, Lin SJ, Wu P, Shiao MS. Increase of vitamin E content in LDL and reduction of atherosclerosis in cholesterol-fed rabbits by a water-soluble antioxidant-rich fraction of Salvia miltiorrhiza. Arterioscler Thromb Vasc Biol. 1998;18:481–6. doi: 10.1161/01.atv.18.3.481. [DOI] [PubMed] [Google Scholar]
- Li S, Wan L. Experimental study on the preventive mechanism of Salviae miltiorrhizae against atherosclerosis in rabbits models. J Huazhong Univ Sci Technolog Med Sci. 2004;24:233–5. doi: 10.1007/BF02831998. [DOI] [PubMed] [Google Scholar]
- Chen J, Wang F, Lee FS, Wang X, Xie M. Separation and identification of water-soluble salvianolic acids from Salvia miltiorrhiza Bunge by high-speed counter-current chromatography and ESI-MS analysis. Talanta. 2006;69:172–9. doi: 10.1016/j.talanta.2005.09.041. [DOI] [PubMed] [Google Scholar]
- Liu X, Chen R, Shang Y, Jiao B, Huang C. Lithospermic acid as a novel xanthine oxidase inhibitor has anti-inflammatory and hypouricemic effects in rats. Chem Biol Interact. 2008;176:137–42. doi: 10.1016/j.cbi.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Wagner H, Horhammer L, Frank U.Lithospermic acid, the antihormonally active principle of Lycopus europaeus L. and Symphytum officinale. 3. Ingredients of medicinal plants with hormonal and antihormonal-like effect Arzneimittelforschung 197020705–13.German. [PubMed] [Google Scholar]
- Zhang ZF, Peng ZG, Gao L, Dong B, Li JR, Li ZY, et al. Three new derivatives of anti-HIV-1 polyphenols isolated from Salvia yunnanensis. J Asian Nat Prod Res. 2008;10:391–6. doi: 10.1080/10286020801966591. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Fortuno A, Gomez-Ambrosi J, Zalba G, Diez J, Fruhbeck G. The inhibitory effect of leptin on angiotensin II-induced vasoconstriction in vascular smooth muscle cells is mediated via a nitric oxide-dependent mechanism. Endocrinology. 2007;148:324–31. doi: 10.1210/en.2006-0940. [DOI] [PubMed] [Google Scholar]
- Kim SH, Kang KA, Zhang R, Piao MJ, Ko DO, Wang ZH, et al. Protective effect of esculetin against oxidative stress-induced cell damage via scavenging reactive oxygen species. Acta Pharmacol Sin. 2008;29:1319–26. doi: 10.1111/j.1745-7254.2008.00878.x. [DOI] [PubMed] [Google Scholar]
- Ishizawa K, Izawa-Ishizawa Y, Ohnishi S, Motobayashi Y, Kawazoe K, Hamano S, et al. Quercetin glucuronide inhibits cell migration and proliferation by platelet-derived growth factor in vascular smooth muscle cells. J Pharmacol Sci. 2009;109:257–64. doi: 10.1254/jphs.08236fp. [DOI] [PubMed] [Google Scholar]
- Sasu S, LaVerda D, Qureshi N, Golenbock DT, Beasley D. Chlamydia pneumoniae and chlamydial heat shock protein 60 stimulate proliferation of human vascular smooth muscle cells via toll-like receptor 4 and p44/p42 mitogen-activated protein kinase activation. Circ Res. 2001;89:244–50. doi: 10.1161/hh1501.094184. [DOI] [PubMed] [Google Scholar]
- Gurjar MV, DeLeon J, Sharma RV, Bhalla RC. Mechanism of inhibition of matrix metalloproteinase-9 induction by NO in vascular smooth muscle cells. J Appl Physiol. 2001;91:1380–6. doi: 10.1152/jappl.2001.91.3.1380. [DOI] [PubMed] [Google Scholar]
- Nelson PR, Yamamura S, Mureebe L, Itoh H, Kent KC. Smooth muscle cell migration and proliferation are mediated by distinct phases of activation of the intracellular messenger mitogen-activated protein kinase. J Vasc Surg. 1998;27:117–25. doi: 10.1016/s0741-5214(98)70298-8. [DOI] [PubMed] [Google Scholar]
- Li JM, Brooks G. Cell cycle regulatory molecules (cyclins, cyclin-dependent kinases and cyclin-dependent kinase inhibitors) and the cardiovascular system; potential targets for therapy. Eur Heart J. 1999;20:406–20. doi: 10.1053/euhj.1998.1308. [DOI] [PubMed] [Google Scholar]
- Pandya HC, Snetkov VA, Twort CH, Ward JP, Hirst SJ. Oxygen regulates mitogen-stimulated proliferation of fetal human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1220–30. doi: 10.1152/ajplung.00268.2001. [DOI] [PubMed] [Google Scholar]
- Taille C, Almolki A, Benhamed M, Zedda C, Megret J, Berger P, et al. Heme oxygenase inhibits human airway smooth muscle proliferation via a bilirubin-dependent modulation of ERK1/2 phosphorylation. J Biol Chem. 2003;278:27160–8. doi: 10.1074/jbc.M300364200. [DOI] [PubMed] [Google Scholar]
- Brar SS, Kennedy TP, Whorton AR, Murphy TM, Chitano P, Hoidal JR. Requirement for reactive oxygen species in serum-induced and platelet-derived growth factor-induced growth of airway smooth muscle. J Biol Chem. 1999;274:20017–26. doi: 10.1074/jbc.274.28.20017. [DOI] [PubMed] [Google Scholar]
- Stoll LL, Denning GM, Weintraub NL. Potential role of endotoxin as a proinflammatory mediator of atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:2227–36. doi: 10.1161/01.ATV.0000147534.69062.dc. [DOI] [PubMed] [Google Scholar]
- Lin FY, Chen YH, Tasi JS, Chen JW, Yang TL, Wang HJ, et al. Endotoxin induces toll-like receptor 4 expression in vascular smooth muscle cells via NADPH oxidase activation and mitogen-activated protein kinase signaling pathways. Arterioscler Thromb Vasc Biol. 2006;26:2630–7. doi: 10.1161/01.ATV.0000247259.01257.b3. [DOI] [PubMed] [Google Scholar]
- Torres M. Mitogen-activated protein kinase pathways in redox signaling. Front Biosci. 2003;8:d369–91. doi: 10.2741/999. [DOI] [PubMed] [Google Scholar]
- Mehdi MZ, Azar ZM, Srivastava AK. Role of receptor and nonreceptor protein tyrosine kinases in H2O2-induced PKB and ERK1/2 signaling. Cell Biochem Biophys. 2007;47:1–10. doi: 10.1385/cbb:47:1:1. [DOI] [PubMed] [Google Scholar]
- Peng CY, Pan SL, Huang YW, Guh JH, Chang YL, Teng CM. Baicalein attenuates intimal hyperplasia after rat carotid balloon injury through arresting cell-cycle progression and inhibiting ERK, Akt, and NF-kappaB activity in vascular smooth-muscle cells. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:579–88. doi: 10.1007/s00210-008-0328-1. [DOI] [PubMed] [Google Scholar]
- Burch PM, Heintz NH. Redox regulation of cell-cycle re-entry: cyclin D1 as a primary target for the mitogenic effects of reactive oxygen and nitrogen species. Antioxid Redox Signal. 2005;7:741–51. doi: 10.1089/ars.2005.7.741. [DOI] [PubMed] [Google Scholar]
- Taupin P. BrdU immunohistochemistry for studying adult neurogenesis: paradigms, pitfalls, limitations, and validation. Brain Res Rev. 2007;53:198–214. doi: 10.1016/j.brainresrev.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Nigg EA. Mitotic kinases as regulators of cell division and its checkpoints. Nat Rev Mol Cell Biol. 2001;2:21–32. doi: 10.1038/35048096. [DOI] [PubMed] [Google Scholar]
- Ross R. Cell biology of atherosclerosis. Annu Rev Physiol. 1995;57:791–804. doi: 10.1146/annurev.ph.57.030195.004043. [DOI] [PubMed] [Google Scholar]
- Dollery CM, McEwan JR, Henney AM. Matrix metalloproteinases and cardiovascular disease. Circ Res. 1995;77:863–8. doi: 10.1161/01.res.77.5.863. [DOI] [PubMed] [Google Scholar]
- Johnson C, Galis ZS. Matrix metalloproteinase-2 and -9 differentially regulate smooth muscle cell migration and cell-mediated collagen organization. Arterioscler Thromb Vasc Biol. 2004;24:54–60. doi: 10.1161/01.ATV.0000100402.69997.C3. [DOI] [PubMed] [Google Scholar]
- Lee WJ, Shin CY, Yoo BK, Ryu JR, Choi EY, Cheong JH, et al. Induction of matrix metalloproteinase-9 (MMP-9) in lipopolysaccharide-stimulated primary astrocytes is mediated by extracellular signal-regulated protein kinase 1/2 (Erk1/2) Glia. 2003;41:15–24. doi: 10.1002/glia.10131. [DOI] [PubMed] [Google Scholar]
- Lai WC, Zhou M, Shankavaram U, Peng G, Wahl LM. Differential regulation of lipopolysaccharide-induced monocyte matrix metalloproteinase (MMP)-1 and MMP-9 by p38 and extracellular signal-regulated kinase 1/2 mitogen-activated protein kinases. J Immunol. 2003;170:6244–9. doi: 10.4049/jimmunol.170.12.6244. [DOI] [PubMed] [Google Scholar]
- Huang C, Jacobson K, Schaller MD. MAP kinases and cell migration. J Cell Sci. 2004;117:4619–28. doi: 10.1242/jcs.01481. [DOI] [PubMed] [Google Scholar]