Abstract
Aim:
To assess whether epinephrine, phenylephrine, and methoxamine act via certain subtypes of adrenoceptors to exert their local anesthetic activity.
Methods:
We investigated cutaneous anesthesia from adrenoceptor agonists and/or antagonists in conscious, unanesthetized Sprague-Dawley male rats (weight 200−250 g). Cutaneous anesthesia was evidenced by a block of the cutaneous trunci muscle reflex, which is characterized by reflex movement of the skin over the back produced by twitches of lateral thoracispinal muscles in response to local dorsal cutaneous noxious pinprick.
Results:
Local infiltration of epinephrine, L-phenylephrine, or methoxamine alone induces cutaneous anesthesia in rats in a dose-dependent way. Epinephrine is found to be 19 and 29 times more potent than those of methoxamine and L-phenylephrine, respectively. The cutaneous anesthesia induced by epinephrine, phenylephrine, or methoxamine can be significantly reduced by α1-adrenoceptor antagonists (eg, prazosin), α1, α2-adrenoceptor antagonist, α1A-adrenoceptor antagonist (eg, 5-methylurapdil), α1B-adrenoceptor antagonist (eg, chloroethylclonidine), or α1D-adrenoceptor antagonist (eg, BMY7873).
Conclusion:
Our results indicate that epinephrine, phenylephrine and methoxamine all act mainly via mixed subtypes of α1-adrenoceptors to induce cutaneous anesthesia in the rat.
Keywords: anesthesia, epinephrine, vasoconstriction, phenylephrine, methoxamine
Introduction
Anesthesiologists often add epinephrine to local anesthetic preparations during peripheral nerve block procedures1, 2. It is generally believed that epinephrine mediates this prolongation of local anesthetic action by its vasconstrictive action3. First, epinephrine may reduce the plasma concentration of local anesthetic and thus minimize the possibility of systemic toxicity4, and second, epinephrine potentiates peripheral nerve block2, 5, 6. Epinephrine may stimulate α-adrenoceptors receptors on the neural vasculature7, which leads to contraction of the vascular smooth muscle8, 9, and reduction of both local blood flow and clearance of local anesthetic from the nerve.
It has been documented that norepinephrine, phenylephrine, or methoxamine injected intradermally may induce thermal hyperalgesia in humans10, and that epinephrine can produce mechanical hyperalgesia in rats11. In contrast, recent findings12, 13 observe that the epinephrine itself induces an unexpected, transient, partial block of the cutaneous trunci muscle reflex, which is characterized by reflex movement of the skin over the back produced by twitches of lateral thoracispinal muscles in response to local dorsal cutaneous noxious pinprick. This raises the possibility that epinephrine, phenylephrine or methoxamine may possess local anesthetic activity in its own right.
To deal with the question, the authors undertook this study to determine a dose-response curve for epinephrine, phenylephrine, or methoxamine on infiltrative anesthesia on conscious, unanesthetized rats. In order to determine whether epinephrine, phenylephrine, and methoxamine act via certain subtypes of α1-adrenoceptors to exert their cutaneous analgesia, local infiltration of different α-adrenoceptor antagonists was made 5 min before injection of epinephrine, phenylephrine, or methoxamine. In addition, we injected the nitric oxide donors (such as nitroglycerin, niroprusside or nifedipine) 5 min before injection of epinephrine, phenylephrine, or methoxamine during cutaneous anesthesia testing to ascertain whether vasoconstriction affected local anesthesia.
Materials and methods
Animals
We investigated local anesthesia from adrenergic agonists and/or antagonist in conscious, unanesthetized Sprague-Dawley male rats (weight 200−250 g). All experiments were performed by using protocols approved by the Chi Mei Medical Center Committee on Animals in accordance with policies of the International Association for the Study of Pain. All rats were housed in groups of three to four for at least one week in a climate-controlled room maintained at 21 °C with approximately 50% humidity. A 12-h light/dark cycle was settled with food and water available ad libitum till the time of investigation.
The experiments were done on handled rats (daily, over 7 days) familiarized with the behavioral experimenter, the laboratorial environment, and the specific procedures of testing. Such familiarization minimized the contamination of animals from stress during experiments and improved experimental performance14. The hairs of the dorsal surface of the thoracolumbar region (6×6 cm2) of rats were mechanically clipped the day before experiments and this small degree of local irritation by clipping disappeared overnight. Six to eight rats in each group were assigned for different treatments.
Evaluation of local anesthesia
Local anesthesia from different adrenoceptor agonists and antagonists were evaluated according to the method reported previously14, 15. In brief, drugs were administered via a 30-gauge needle at a volume of 0.6 mL subcutaneously at a 30° angle into the dorsal surface of the thoracolumbar region. The injections caused a circular raise of the skin, a wheel, approximately two centimeter in diameter that was then marked with ink within 1 min. The effect of the local anesthesia was evaluated using the cutaneous trunci muscle reflex, which was characterized by reflex movement of the skin over the back produced by twitches of the lateral thoracispinal muscles in response to local dorsal subcutaneous stimulation. A von Frey filament (No 15), to which the cut end of an 18-gauge needle was affixed, was used to produce the standardized nociceptive stimulus (19 g). We performed six different pinpricks inside the wheal with a frequency of 0.5−1.0 Hz after observing an animal's normal reaction to pinpricks applied outside the wheal and on the contralateral side and scoring the number to which the rat failed to react. The investigation was applied every 5 min for the first 30 min and then every 10–15 min to 2.5 h until the subcutaneous reflex completely recovered from the blockage. The back was subdivided into four areas on both sides, and each rat was injected 2 times, separated by a washout period of 3 days. For consistency, one experienced investigator, who was unaware of the drugs being injected, was responsible to evaluate cutaneous analgesia effects.
Drugs
All the drugs were freshly prepared. The following drugs were used: α1-agonists (L-phenylephrine HCl; Sigma, methoxamine HCl; Sigma), α2-agonist (clonidine; Sigma, dexmedetomidine; Abbott), β1-agonist (dobutamine; Astra Zeneca), β2-agonist (terbutaline; Sigma), β1β2-agonist (isoproterenol; Sigma), α1α2β1β2 agonist (epinephrine; Sigma), α1-antagonist (prazosin; Sigma), α1α2-antagonist (phentolamine; Sigma), α1A antagonist (5-methylurapdil; Sigma), α1B antagonist (chloroethylclonidine; Sigma), α1D antagonist (BMY7378; Sigma), Ca2+ and α1A antagonist (nifedipine; Sigma) and NO donor vasodilator (sodium nitroprusside; Mulgrave VIC, nitroglycerine; Nippon Kayaku, nifedipine; Sigma). All compounds were dissolved in isotonic saline except prazosin, which was firstly dissolved in polyethylene glycol followed by dilution with isotonic saline.
Experimental procedures
The potencies of drugs on cutaneous analgesia was evaluated. The fitting of dose-response curves of each drug was constructed from percent maximum possible effect (%MPE). In the antagonism studies, we injected the antagonist (at a volume of 0.3 mL) firstly and then the agonist was administered (at a volume of 0.3 mL) 5 min later. After subcutaneous injection (n=6 rats for each dose of each drug), the %MPE of doses of drugs were obtained. The dose-response curves of drugs were then constructed using the %MPE and fitted with a computer-derived SASNLIN analysis (version 9.1, SAS Institute, NC). The values of 50% effective doses (ED50s) of drugs, which were defined as the doses of drugs that caused a 50% blockage of cutaneous trunci muscle reflex, were obtained16.
Statistical analysis
Values were presented as mean±SEM. The differences in ED50s among drugs were evaluated by a one-way analysis of variance (ANOVA), followed by the pariwise Tukey's honest significance difference (HSD) test. A statistical software, SPSS for windows (version 10, 0.7), was used. A P value <0.05 was considered statistically significant.
Results
Epinephrine, phenylephrine, or methoxamine induces infiltrative anesthesia
It can be seen from both Figire 1 and Table 1 that epinephrine, phenylephrine, methoxamine, and norepinephrine induce infiltrative anesthesia in a dose-dependent way in rats. Epinephrine is found to be 19 and 29 times more potent than those of methoxamine and L-phenylephrine, respectively. In addition, the relative potency was found to be epinephrine > lidocaine. On the other hand, clonidine, dexmedetomidine, dobutamine, and terbutaline all exhibit no infiltrative anesthesia.
Figure 1.
(A) Time courses of inhibition of cutaneous trunci muscle reflex by epinephrine, phenylephrine, and methoxamine after subcutaneous injections of drugs in rats (n=6 rats for each drug). Values are mean±SEM. The injected volume was 0.6 mL. Neurological evaluations were done before, and 5, 10, 15, 20, 25, 30, 40, 50, 60, 75, 90, 105, and 120 min after drug injection. (B) The dose-response curves of epinephrine, phenylephrine, and methoxamine on cutaneous analgesia in rats (n=6 rats at each testing point). Values are mean±SEM and were fitted with the SASNLIN analysis.
Table 1. Maximum possible effect of drugs on subcutaneous antinociception in rats.
| Drugs | μmol·L-1/mL | %MPE |
|---|---|---|
| Clonidine | 1 : 1×105 | 0 |
| Dexmedetomidine | 1 : 1×105 | 0 |
| Dobutamine | 1 : 1×104 | 0 |
| Terbutamine | 1 : 1×104 | 0 |
| Isoproterenol | 1 : 1×104 | 0 |
| Norepinephrine | 1 : 1×104 | 81±12 |
| Norepinephrine | 1 : 1×105 | 57±10 |
Alpha-adrenoceptor antagonists reduce infiltrative anesthesia induced by epinephrine, phenylephrine, or methoxamine
In order to determine the effects of antagonism of α1,α2-adrenoceptors on the infiltrative anesthesia induced by epinephrine, phenylephrine, or methoxamine, prazosin or phentolamine was administered 5 min before injection of these adrenoceptor agonists. It can be seen from both Figure 2 and Figure 3 that infiltrative anesthesia induced by epinephrine, phenylephrine, or methoxamine can be significantly reduced by prazosin (Figure 2) or phentolamine (Figure 3).
Figure 2.
(A) Time courses of inhibition of cutaneous trunci muscle reflex by epinephrine (ED80), phenylephrine (ED80), and methoxamine (ED80) in the presence of prazosine after subcutaneous injections of drugs in rats (n=6 rats for each drug). Values are mean±SEM. Prazosine (0.3 mL) was injected 5 min before the injection of epinephrine (0.3 mL), phenylephrine (0.3 mL), and methoxamine (0.3 mL). Neurological evaluations were done before, and 5, 10, 15, 20, 25, 30, 40, 50, 60, 75, 90, 105, and 120 min after drug injection. (B) The dose-response curves of epinephrine, phenylephrine, and methoxamine in the presence of prazosine on cutaneous analgesia in rats (n=6 rats at each testing point). Values are mean±SEM and were fitted with the SASNLIN.
Figure 3.
(A) Time courses of inhibition of cutaneous trunci muscle reflex by epinephrine, phenylephrine, and methoxamine in the presence of phentolamine after subcutaneous injections of drugs in rats (n=6 rats for each drug). Values are mean±SEM. Phentolamine (0.3 mL) was injected 5 min before the injection of epinephrine (0.3 mL), phenylephrine (0.3 mL), and methoxamine (0.3 mL). Neurological evaluations were done before, and 5, 10, 15, 20, 25, 30, 40, 50, 60, 75, 90, 105, and 120 min after drug injection. (B) The dose-response curves of epinephrine, phenylephrine, and methoxamine in the presence of phentolamine on cutaneous analgesia in rats (n=6 rats at each testing point). Values are mean±SEM and were fitted with the SASNLIN.
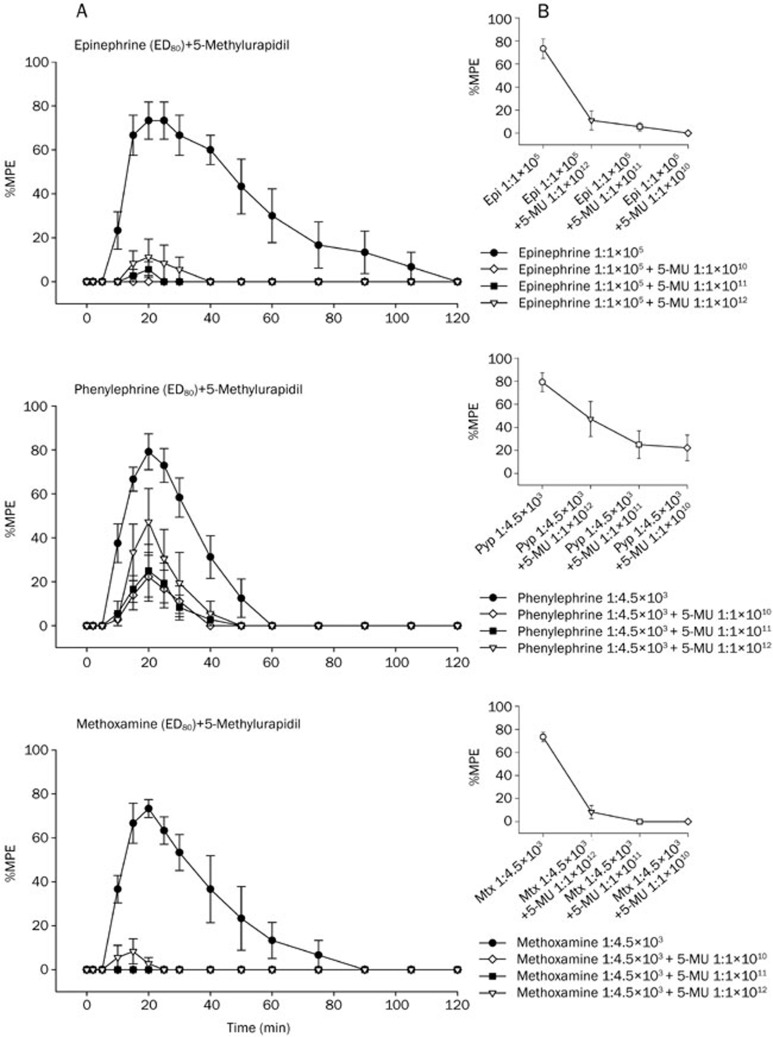
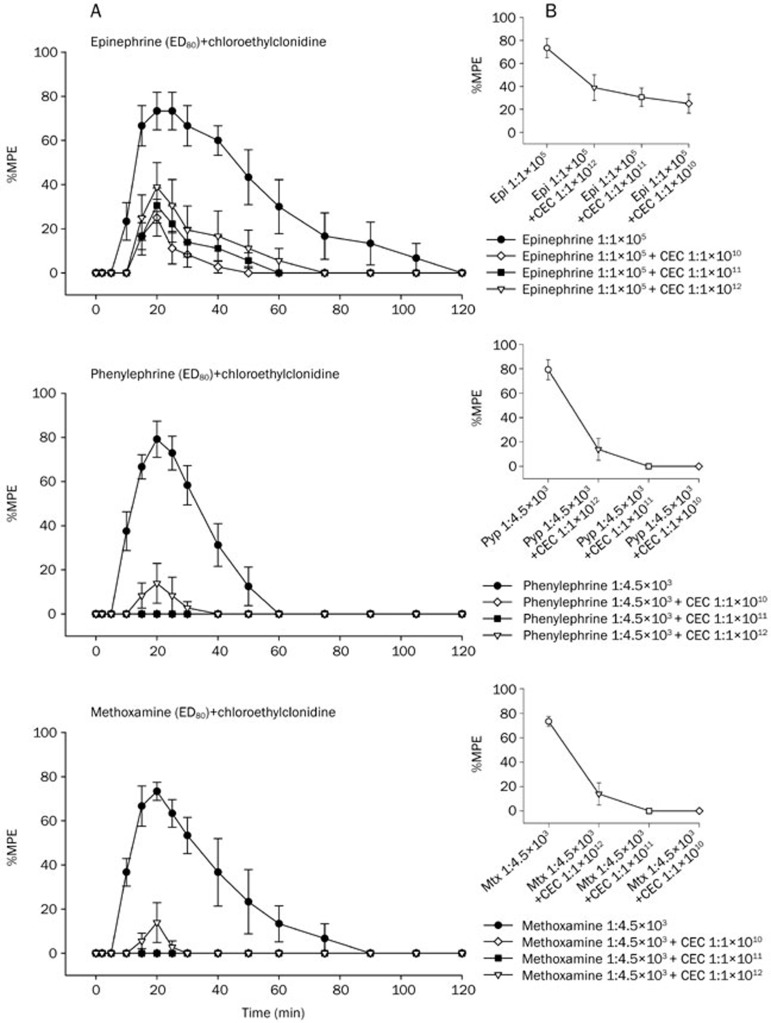
Mixed subtypes of α1-adrenoceptor antagonists reduce infiltrative anesthesia induced by epinephrine, phenylephrine, or methoxamine
It can be seen from Figure 4, Figure 5, and Figure 6 that the infiltrative anesthesia induced by epinephrine, phenylephrine, or methoxamine can be significantly abolished by pretreatment with α1A (5-methylurapdil), α1B (chloroethylclonidine), or α1D (BMY7873) adrenoceptor antagonist 5 min before the injection of epinephrine, phenylephrine, or methoxamine.
Figure 4.
(A) Time courses of inhibition of cutaneous trunci muscle reflex by epinephrine, phenylephrine, and methoxamine in the presence of 5-methylurapidil after subcutaneous injections of drugs in rats (n=6 rats for each drug). Values are mean±SEM. 5-Methylurapidil (0.3 mL) was injected 5 min before the injection of epinephrine (0.3 mL), phenylephrine (0.3 mL), and methoxamine (0.3 mL). Neurological evaluations were done before, and 5, 10, 15, 20, 25, 30, 40 , 50, 60, 75, 90, 105, and 120 min after drug injection. (B) The dose-response curves of epinephrine, phenylephrine, and methoxamine in the presence of 5-methylurapidil on cutaneous analgesia in rats (n=6 rats at each testing point). Values are mean±SEM and were fitted with the SASNLIN.
Figure 5.
(A) Time courses of inhibition of cutaneous trunci muscle reflex by epinephrine, phenylephrine, and methoxamine in the presence of chloroethylclonidine (CEC) after subcutaneous injections of drugs in rats (n=6 rats for each drug). Values are mean±SEM. CEC (0.3 mL) was injected 5 min before the injection of epinephrine (0.3 mL), phenylephrine (0.3 mL), and methoxamine (0.3 mL). Neurological evaluations were done before, and 5, 10, 15, 20, 25, 30, 40, 50, 60, 75, 90, 105, and 120 min after drug injection (B). The dose-response curves of epinephrine, phenylephrine, and methoxamine in the presence of CEC on cutaneous analgesia in rats (n=6 rats at each testing point). Values are mean±SEM and were fitted with the SASNLIN.
Figure 6.
(A) Time courses of inhibition of cutaneous trunci muscle reflex by epinephrine, phenylephrine, and methoxamine in the presence of BMY7378 after subcutaneous injections of drugs in rats (n=6 rats for each drug). Values are mean±SEM. BMY7378 (0.3 mL) was injected 5 min before the injection of epinephrine (0.3 mL), phenylephrine (0.3 mL), and methoxamine (0.3 mL). Neurological evaluations were done before, and 5, 10, 15, 20, 25, 30, 40, 50, 60, 75, 90, 105, and 120 min after drug injection. (B) The dose-response curves of epinephrine, phenylephrine, and methoxamine in the presence of BMY7378 on cutaneous analgesia in rats (n=6 rats at each testing point). Values are mean±SEM and were fitted with the SASNLIN.
Nitric oxide donors attenuate infiltrative anesthesia caused by epinephrine, phenylephrine, or methoxamine
As summarized in Table 2, the infiltrative anesthesia caused by epinephrine, phenylephrine, or methoxamine can be completely abolished by pretreatment with nitroglycerin, nitroprusside, or nifedipine 5 min before the injection of epinephrine, phenylephrine or methoxamine.
Table 2. Maximum possible effect of α-1 adrenoceptor agonist combined with NO donor on subcutaneous antinociception in rats.
| Vasodilator | %MPE±SEM | ||
|---|---|---|---|
| Epinephrine (ED80) | + | Nitroglycerin (1 : 1×107) | 0 |
| Nitroglycerin (1 : 1×108) | 0 | ||
| Nitroglycerin (1 : 1×109) | 0 | ||
| Phenylephrine (ED80) | + | Nitroglycerin (1 : 1×107) | 0 |
| Nitroglycerin (1 : 1×108) | 0 | ||
| Nitroglycerin (1 : 1×109) | 0 | ||
| Methoxamine (ED80) | + | Nitroglycerin (1 : 1×107) | 0 |
| Nitroglycerin (1 : 1×108) | 0 | ||
| Nitroglycerin (1 : 1×109) | 0 | ||
| Epinephrine (ED80) | + | Nitroprusside (1 : 1×107) | 0 |
| Nitroprusside (1 : 1×108) | 0 | ||
| Nitroprusside (1 : 1×109) | 0 | ||
| Phenylephrine (ED80) | + | Nitroprusside (1 : 1×107) | 0 |
| Nitroprusside (1 : 1×108) | 0 | ||
| Nitroprusside (1 : 1×109) | 0 | ||
| Methoxamine (ED80) | + | Nitroprusside (1 : 1×107) | 0 |
| Nitroprusside (1 : 1×108) | 0 | ||
| Nitroprusside (1 : 1×109) | 0 | ||
| Epinephrine (ED80) | + | Nifedipine (1 : 1×107) | 0 |
| Nifedipine (1 : 1×108) | 7±4 | ||
| Nifedipine (1 : 1×109) | 20±12 | ||
| Phenylephrine (ED80) | + | Nifedipine (1 : 1×107) | 0 |
| Nifedipine (1 : 1×108) | 0 | ||
| Nifedipine (1 : 1×109) | 4±4 | ||
| Methoxamine (ED80) | + | Nifedipine (1 : 1×107) | 0 |
| Nifedipine (1 : 1×108) | 0 | ||
| Nifedipine (1 : 1×109) | 8±5 |
Discussion
Both clinical and preclinical studies have indicated that the sympathetic nervous system contributes to pain following nerve injury17, 18. It is generally believed that sympathetic-afferent coupling occurs at three distinct sites; at the site of injury, at the sensory terminal, and within dorsal root ganglia. Sympathectomy can relieve the different manifestations of hyperalgesia and allodynia in various nerve injury models to varying degrees19, 20, 21. In addition, both behavioral and electrophysiological studies suggest that α2-adrenoceptors are primarily mediators of sympathetic-afferent coupling following nerve injury22, 23, 24, 25. Both α2-18, 26 and α1-adrenoceptors24, 27 are related to afferent excitation following nerve injury. Clonidine, an α2-adrenoceptor agonist commonly used in the treatment of hypertension, has been used to relieve hyperalgesia in some patients with sympathetically maintained pain due to a localized action28. The efficacy of local clonidine in sympathetically maintained pain may result from presynaptic inhibition of norepinephrine released from sympathetic nerves as well as actions directly on primary afferent nerve terminals.
Probably, the most striking findings of the present study are that α1-adrenoceptor agonists (eg, epinephrine, phenylephrine, and methoxamine) but not α2-adreneceptor agonists (eg, clonidine and dexmedetomidine), β1-adrenoceptor agonist (eg, dobutamine), β2-adrenoceptor agonist (eg, terbutaline), or β1β2-adrenoceptor agonist (eg, isoproterenol) induce infiltrative anesthesia in a dose-related manner after subcutaneous infiltration in the rat. Epinephrine is found to be more potent than that of lidocaine (present results) or bupivacaine13. The infiltrative anesthesia induced by epinephrine, phenylephrine, or methoxamine can be significantly reduced by α1-antagonist (eg prazosin), α1α2-antagonist (eg, phentolamine), α1A-adrenoceptor antagonist (eg, 5-methylurapidil), α1B-adrenoceptor antagonist (eg, chloroethylclonidine), or α1D-adrenoceptor antagonist (eg, BMY7873). These results indicate that epinephrine, phenylephrine, or methoxamine can act mainly via mixed subtypes of α1-adrenoceptors to induce cutaneous analgesia in the rat. In fact, the contention is not consistent with a more recent report showing that α-2 adrenoceptor agonists enhance the local anesthetic action of lidocaine, and suggest that dexmedetomidine (which has more than eight times the affinity for α-2 adrenoceptors of clonidine) acts via α-2A adrenoceptors in guinea pigs29. It should be noted that they showed that all α-2 adrenoceptor agonists enhanced the degree of local anesthesia of lidocaine in a dose-dependent manner but did not demonstrate the effects of clonidine or dexmedetomidine itself on local anesthesia. In addition, the discrepancy between their and our results may be due to species difference.
Apparently, data from our results give cause to question the conventional wisdom described in the former section. Adrenoreceptor activation may affect various factors that regulate excitability, such as K+ channel30, Cl− channel, or the Na+-K+ pump31. Epinephrine binding to α1 and α2 adrenoceptors of vascular smooth muscle causes vessel vasoconstriction, whereas epinephrine binding to β2 receptors causes vasodilatation8. Percutaneously injected epinephrine will reach and vasoconstrict the vessels in the superficial epineural space first, and then penetrate into the nerve and the muscle. Vasoconstriction by epinephrine could result in a transient neural ischemia that directly induces nerve block32. Such an ischemia-induced nerve block may account for the local analgesia so apparent after injection of epinephrine, phenylephrine, or methoxamine12, 13 (present results). The latency for this action, 15–20 min (Figure 1), was distinctly longer than that for almost immediate-acting, consistent with an accumulating reaction to ischemia or to a receptor-second messenger-mediated effect in neurons, rather than a direct local anesthetic action33. Our current data show that epinephrine and other α1-adrenoceptor agonists cause local anesthesia, which can be blocked by treatment with nitric oxide donors (eg, nitroglycerin, nifedipine, and sodium nitroprusside) or Ca2+-channel blocker (eg, nifedipine). This suggests that the local anesthetic activity of alpha-1 adrenoceptor agonists is due to nerve block resultant from neural ischemia mainly since it is expected that the effects of nitric oxide and nifedipine would oppose the effects of alpha-1 adrenoceptor agonists in both the nerve and vascular smooth. The contention is supported by many investigators. For example, epinephrine, clinically added to preparations of local anesthetics, prolonged the duration of action by reducing skin blood flow34. Adding epinephrine to lidocaine solutions increases the intensity and duration of sciatic nerve block in the rat35. By stimulating alpha-1 adrenoceptors on the neural vasculature7, epinephrine mediates contraction of the vascular smooth muscle8, 9, induces vasoconstriction, and thereby slows clearance of lidocaine from the nerve. Although systemic toxicity has not been reported to occur after subcutaneous infiltration of epinephrine, potential local toxicity such as delayed wound healing36, increased wound infection rate37, increased myocutaneous flap loss38, and toxicity to skin39 exists.
In summary, the current study provides the evidence to show that epinephrine and other α1-adrenoceptor agonists can mainly act via mixed subtypes of α1-adrenoceptor to induce local anesthetic activity.
Author contribution
Ja-ping SHIEH and Mao-tsun LIN designed research; Ja-ping SHIEH and Chin-chen CHU performed research; Ja-ping SHIEH and Jhi-joung WANG contributed new analytical tools and reagents; Ja-ping SHIEH and Chin-chen CHU analyzed data; Mao-tsun LIN wrote the paper.
Acknowledgments
This work was support in part by the National Science Council (Taipei, Taiwan, China) NSC 96-2314-B-384-002 and NSC 96-2314-B-384-003-MY3.
References
- Braid DP, Scott DB. Effect of adrenaline on the systemic absorption of local anaesthetic drugs. Acta Anaesthesiol Scand Suppl. 1966;23:334–46. doi: 10.1111/j.1399-6576.1966.tb01031.x. [DOI] [PubMed] [Google Scholar]
- Bernards CM, Kopacz DJ. Effect of epinephrine on lidocaine clearance in vivo: a microdialysis study in humans. Anesthesiology. 1999;91:962–8. doi: 10.1097/00000542-199910000-00015. [DOI] [PubMed] [Google Scholar]
- Tucker GT, Mather LE. Properties, absorption, and distribution of local anesthetic agents, neural blockade in clinical anesthesia and management of pain, 3rd edition. In: Cousins MJ, Bridenbaugh PO, editors. Philadelphia, Lippincott-Raven. 1998. pp. p73–74.
- Berde CB, Stricharz GR.Local anesthetics, anesthesia5th edition. In: Miller RE, editor. Philadelphia: Churchill-Living-stone 2000p491–521.
- Swerdlow M, Jones R. The duration of action of bupivacaine, prilocaine and lignocaine. Br J Anaesth. 1970;42:335–9. doi: 10.1093/bja/42.4.335. [DOI] [PubMed] [Google Scholar]
- Albert J, Lofstrom B.Bilateral ulnar nerve blocks for the evaluation of local anesthetic agents3. Tests with a new agent, prilocaine, and with lidocaine in solutions with and without epinephrine. Acta Anaesthesiol Scand 1965 9203–11. [DOI] [PubMed] [Google Scholar]
- Appenzeller O, Dhital KK, Cowen T, Burnstock G. The nerves to blood vessels supplying blood to nerves: the innervation of vasa nervorum. Brain Res. 1984;304:383–6. doi: 10.1016/0006-8993(84)90344-5. [DOI] [PubMed] [Google Scholar]
- Bevan JA, Bevan RD, Duckles SP. In: Bohr DF, Somlyo AP, Sparks AB, editors. Maryland: American Physiological Society; 1980. Adrenergic regulation of vascular smooth muscle. Hand book of Physiology. Section 2, vol. II; pp. p515–566. [Google Scholar]
- Selander D, Mansson LG, Karlsson L, Svanvik J. Adrenergic vasoconstriction in peripheral nerves of the rabbit. Anesthesiology. 1985;62:6–10. doi: 10.1097/00000542-198501000-00002. [DOI] [PubMed] [Google Scholar]
- Raja SN. Peripheral modulatory effects of catecholamines in inflammatory and neuropathic pain. Adv Pharmacol. 1998;42:567–71. doi: 10.1016/s1054-3589(08)60814-5. [DOI] [PubMed] [Google Scholar]
- Khasar SG, McCarter G, Levine JD. Epinephrine produces a beta-adrenergic receptor-mediated mechanical hyperalgesia and in vitro sensitization of rat nociceptors. J Neurophysiol. 1999;81:1104–12. doi: 10.1152/jn.1999.81.3.1104. [DOI] [PubMed] [Google Scholar]
- Khodorova AB, Strichartz GR. The addition of dilute epinephrine produces equieffectiveness of bupivacaine enantiomers for cutaneous analgesia in the rat. Anesth Analg. 2000;91:410–6. doi: 10.1097/00000539-200008000-00034. [DOI] [PubMed] [Google Scholar]
- Chen YW, Liu KS, Wang JJ, Chou W, Hung CH. Isobolographic analysis of epinephrine with bupivacaine, dextromethorphan, 3-methoxymorphinan, or dextror phan on infiltrative anesthesia in rats: dose-response studies. Reg Anesth Pain Med. 2008;33:115–21. doi: 10.1016/j.rapm.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Khan MA, Gerner P, Kuo Wang G. Amitriptyline for prolonged cutaneous analgesia in the rat. Anesthesiology. 2002;96:109–16. doi: 10.1097/00000542-200201000-00023. [DOI] [PubMed] [Google Scholar]
- Tzeng JI, Cheng KI, Huang KL, Chen YW, Chu KS, Chu CC.et al. The cutaneous analgesic effect of class I antiarrhythmic drugs Anesth Analg 2007104955–8. [DOI] [PubMed] [Google Scholar]
- Minkin S, Kundhal K. Likelihood-based experimentla design for estimation of ED50. Biometrics. 1999;55:1030–7. doi: 10.1111/j.0006-341x.1999.01030.x. [DOI] [PubMed] [Google Scholar]
- Janig W, Levine JD, Michaelis M. Interactions of sympathetic and primary afferent neurons following nerve injury and tissue trauma. Prog Brain Res. 1996;113:161–84. doi: 10.1016/s0079-6123(08)61087-0. [DOI] [PubMed] [Google Scholar]
- Perl ER. Causalgia, pathological pain, and adrenergic receptors. Proc Natl Acad Sci USA. 1999;96:7664–7. doi: 10.1073/pnas.96.14.7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KJ, Yoon YW, Chung JM. Comparison of three rodent neuropathic pain models. Exp Brain Res. 1997;113:200–6. doi: 10.1007/BF02450318. [DOI] [PubMed] [Google Scholar]
- Lee BH, Yoon YW, Chung K, Chung JM. Comparison of sympathetic sprouting in sensory ganglia in three animal models of neuropathic pain. Exp Brain Res. 1998;120:432–8. doi: 10.1007/s002210050416. [DOI] [PubMed] [Google Scholar]
- Ramer MS, Bisby MA. Adrenergic innervation of rat sensory ganglia following proximal or distal painful sciatic neuropathy: distinct mechanisms revealed by anti-NGF treatment. Eur J Neurosci. 1999;11:837–46. doi: 10.1046/j.1460-9568.1999.00491.x. [DOI] [PubMed] [Google Scholar]
- Sato J, Perl ER. Adrenergic excitation of cutaneous pain receptors induced by peripheral nerve injury. Science (Wash DC) 1991;251:1608–10. doi: 10.1126/science.2011742. [DOI] [PubMed] [Google Scholar]
- Tracey DJ, Cunningham JE, Romm MA. Peripheral hyperalgesia in experimental neuropathy: mediation by alpha 2-adrenoreceptors on post-ganglionic sympathetic terminals. Pain. 1995;60:317–27. doi: 10.1016/0304-3959(94)00141-z. [DOI] [PubMed] [Google Scholar]
- Chen Y, Michaelis M, Janig W, Devor M. Adrenoreceptor subtype mediating sympathetic-sensory coupling in injured sensory neurons. J Neurophysiol. 1996;76:3721–30. doi: 10.1152/jn.1996.76.6.3721. [DOI] [PubMed] [Google Scholar]
- Moon DE, Lee DH, Han HC, Xie J, Coggeshall RE, Chung JM. Adrenergic sensitivity of the sensory receptors modulating mechanical allodynia in a rat neuropathic pain model. Pain. 1999;80:589–95. doi: 10.1016/S0304-3959(98)00252-8. [DOI] [PubMed] [Google Scholar]
- Kingery WS, Guo TZ, Davies MF, Limbird L, Maze M. The alpha (2A) adrenoceptor and the sympathetic postganglionic neuron contribute to the development of neuropathic heat hyperalgesia in mice. Pain. 2000;85:345–58. doi: 10.1016/S0304-3959(99)00286-9. [DOI] [PubMed] [Google Scholar]
- Lee DH, Liu X, Kim HT, Chung K, Chung JM. Receptor subtype mediating the adrenergic sensitivity of pain behavior and ectopic discharges in neuropathic Lewis rats. J Neurophysiol. 1999;81:2226–33. doi: 10.1152/jn.1999.81.5.2226. [DOI] [PubMed] [Google Scholar]
- Davis KD, Treede RD, Raja SN, Meyer RA, Campbell JN. Topical application of clonidine relieves hyperalgesia in patients with sympathetically maintained pain. Pain. 1991;47:309–17. doi: 10.1016/0304-3959(91)90221-I. [DOI] [PubMed] [Google Scholar]
- Yoshitomi T, Kohjitani A, Maeda S, Higuchi H, Shimada M, Miyawaki T. Dexmedetomidine enhances the local anesthetic action of lidocaine via an alpha-2A adrenoceptor. Anesth Analg. 2008;107:96–101. doi: 10.1213/ane.0b013e318176be73. [DOI] [PubMed] [Google Scholar]
- Strong JA, Kaczmarek LK. In: Kaczmarek LK, Leuitan IB, editors. New York: Oxford: Oxford University Press; 1987. Potassium currents that regulate action potentials and repetitive firing, neuromodulation; pp. p119–137. [Google Scholar]
- Fink BR, Aasheim GM, Levy BA. Neural pharmacokinetics of epinephrine. Anesthesiology. 1978;48:263–6. doi: 10.1097/00000542-197804000-00008. [DOI] [PubMed] [Google Scholar]
- Nau C, Wang SY, Strichartz GR, Wang GK. Point mutations at N434 in D1-S6 of mu1 Na+ channels modulate binding affinity and stereoselectivity of local anesthetic enantiomers. Mol Pharmacol. 1999;56:404–13. doi: 10.1124/mol.56.2.404. [DOI] [PubMed] [Google Scholar]
- Voeikov VL, Lefkowitz RJ. Effects of local anesthetics on guanyl nucleotide modulation of the catecholamine-sensitive adenylate cyclase system and on beta-adrenergic receptors. Biochim Biophys Acta. 1980;629:266–81. doi: 10.1016/0304-4165(80)90100-2. [DOI] [PubMed] [Google Scholar]
- Newton DJ, Burke D, Khan F, McLeod GA, Belch JJ, McKenzie M, Bannister J. Skin blood flow changes in response to intradermal injection of bupivacaine and levobupivacaine, assessed by laser Doppler imaging. Reg Anesth Pain Med. 2000;25:626–31. doi: 10.1053/rapm.2000.9853. [DOI] [PubMed] [Google Scholar]
- Sinnott CJ, Cogswell III LP, Johnson A, Strichartz GR. On the mechanism by which epinephrine potentiates lidocaine's peripheral nerve block. Anesthesiology. 2003;98:181–8. doi: 10.1097/00000542-200301000-00028. [DOI] [PubMed] [Google Scholar]
- Bodvall B, Rais O. Effects of infiltration anesthesia on the healing of incision in traumatized and non-traumatized tissues. Acta Chir Scand. 1962;123:83–91. [Google Scholar]
- Magee C, Rodeheaver GT, Edgerton MT, Golden GT, Haury B, Edlich RF. Studies of the mechanisms by which epinephrine damages tissue defenses. J Surg Res. 1977;23:126–31. doi: 10.1016/0022-4804(77)90200-1. [DOI] [PubMed] [Google Scholar]
- Wu G, Calamel PM, Shedd DP. The hazards of injecting local anesthetic solutions with epinephrine into flaps: experimental study. Plast Reconstr Surg. 1978;62:396–403. doi: 10.1097/00006534-197809000-00010. [DOI] [PubMed] [Google Scholar]
- Burk RW, III, Serafin D, Klitzman B. Toxic effects of catecholamines on skin. Plast Reconstr Surg. 1990;85:92–9. doi: 10.1097/00006534-199001000-00016. [DOI] [PubMed] [Google Scholar]