Abstract
Aim:
Resistance to 5-fluorouracil (5-FU) is a major cause of chemotherapy failure in advanced hepatocellular carcinoma (HCC).Rosiglitazone, a peroxisome proliferator-activated receptor γ (PPARγ) agonist, has a crucial role in growth inhibition and induction of apoptosis in several carcinoma cell lines. In this study, we examine rosiglitazone-induced sensitization of HCC cell lines (BEL-7402 and Huh-7 cells) to 5-FU.
Methods:
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay was used to evaluate cell viability. Western blotting analysis was performed to detect the protein expression (PPARγ, PTEN, and COX-2) in BEL-7402 cells. Immunohistochemistry staining was used to examine the expression of PTEN in 100 advanced HCC tissues and paracancerous tissues. In addition, small interfering RNA was used to suppress PPARγ, PTEN, and COX-2 expression.
Results:
Rosiglitazone facilitates the anti-tumor effect of 5-FU in HCC cell lines, which is mediated by the PPARγ signaling pathway. Activation of PPARγ by rosiglitazone increases PTEN expression and decreases COX-2 expression. Since distribution of PTEN in HCC tissues is significantly decreased compared with the paracancerous tissue, over-expression of PTEN by rosiglitazone enhances 5-FU-inhibited cell growth of HCC. Moreover, down-regulation of COX-2 is implicated in the synergistic effect of 5-FU.
Conclusion:
Rosiglitazone sensitizes hepatocellular carcinoma cell lines to 5-FU antitumor activity through the activation of PPARγ. The results suggest potential novel therapies for the treatment of advanced liver cancer.
Keywords: rosiglitazone, hepatocellular carcinoma, PTEN, PPARγ, 5-fluorouracil
Introduction
Rosiglitazone belongs to the hiazolidinedione group of drugs (TZDs) and has been regarded as a synthetic ligand of peroxisome proliferator-activated receptor γ (PPARγ)1. Unlike other members, such as troglitazone, pioglitazone, and ciglitazone, rosiglitazone has the highest affinity for PPARγ2. PPARγ is a ligand-activated nuclear hormone receptor and mediates transcriptional regulation of target genes. PPARγ activation inhibits growth and induces apoptosis of hepatocellular carcinoma cells in vitro3, 4. However, it is not yet known whether rosiglitazone could be used as an antitumor agent.
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer deaths worldwide5. For advanced HCC, especially with portal tumor thrombi, conventional therapies are not generally effective and have potentially significant complications. Inherent or acquired drug resistance remains a major obstacle to chemotherapy. Therefore, exploring the alternative modality of combination chemotherapy for advanced HCC is desirable.
5-Fluorouracil (5-FU) is an inhibitor of deoxynucleoside triphosphate de novo synthesis and is widely used to treat solid tumors, including hepatic, colorectal, gastric and pancreatic cancer in clinical practice. However, the development of drug-resistant phenotypes significantly limits the clinical use of 5-FU. 5-FU alone has been shown to be of limited benefit in enhancing the survival of patients with advanced HCC. Moreover, many patients with HCC have tumors that are inherently resistant to chemotherapy or develop resistance during the course of therapy, leading to failure of HCC chemotherapy6. Therefore, current strategies focus on studying the molecular mechanisms of chemotherapy resistance, searching for effective methods of overcoming this resistance, and developing novel anti-tumor treatment agents.
The aim of this study is to understand the effects of combining 5-FU and rosiglitazone on HCC cell lines and to elucidate the mechanisms underlying the observed synergistic effect.
Materials and methods
Reagents
Rosiglitazone was purchased from Cayman Chemical Company (Ann Arbor, MI, USA). 5-FU was obtained from Sigma Chemical Company (St Louis, MO, USA). These reagents were dissolved in dimethyl sulfoxide (DMSO), and the final concentration of DMSO was maintained at 0.1%. Mouse monoclonal anti-human PPARγ and PTEN, rabbit polyclonal anti-human COX-2 and horseradish peroxidase–conjugated goat anti-mouse/rabbit IgG secondary antibody were provided by Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Cell culture
Human HCC cell lines BEL-7402 and Huh 7 were kindly donated by Prof Xin-yuan GUAN (Hong Kong University, Hong Kong, China). The cells were maintained in Dulbecco's modified Eagle's medium (DMEM, Gibco BRL, Grand Island, NY, USA) containing 10 % (v/v) fetal bovine serum (Bio-Whittaker, Walkersville, MD, USA), penicillin (100 U/mL) and streptomycin (100 mg/L). The cells were maintained at 37 °C in an incubator with a humidified atmosphere of 5 % CO2.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazoliumbromide (MTT) assay for cell viability
Exponentially growing cells were diluted to a concentration of 2.5×104 cells/mL in DMEM, plated in 96-well plates (Corning Inc, Corning, NY, USA) with 200 μL/well. After being treated with the appropriate drug and incubated for 48 h (triplicate wells for each sample), the cells were exposed to 20 μL/well MTT (5 g/L, Amresco, Solon, OH, USA). The medium was incubated for 4 h and then removed, and DMSO (200 μL/well) was added to dissolve the formazan product. Finally, the plate was read in enzyme-linked immunity implement (Bio-Rad 2550, Hercules, CA, USA) at 570 nm.
Western blotting analysis
Cells were rinsed twice with ice-cold PBS buffer and scraped with lysis buffer containing a protease inhibitor cocktail (Boehringer Mannheim, Lewes, UK) for 30 min at 4 °C. The supernatant was isolated by centrifugation at 15 000×g for 20 min. The protein concentration was determined with Coomassie brilliant blue G-250. Cell extracts (50 μg/lane) were separated via sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electrotransferred to polyvinylidine fluoride (PVDF) membranes (Immobilon, Bedford, MA). After being blocked in 20 mmol/L Tris-HCl, pH 7.6 (containing 150 mmol/L NaCl, 0.1% Tween-20, and 5% non-fat dry milk), membranes were incubated with primary antibodies (used as a sample loading control) overnight at 4 °C and then incubated with a horseradish peroxidase-conjugated secondary antibody. Blots were developed using an enhanced chemiluminescence detection system (ECL, Amersham Pharmacia Biotech) according to the manufacturer's instructions.
Gene silencing by small interfering RNA
Small interfering RNA and nonspecific control siRNA were purchased from RiboBio (Guangzhou RiboBio Co LTD, China). The cells were plated onto 6-well plates, maintained in antibiotic medium for 24 h, and grown to about 50% confluence. siRNA or control siRNA were transfected with the Lipofectamine™ 2000 reagent (Invitrogen, Madison, WI, USA). In short, oligomer-fectamine reagent was diluted at 1:50 in OptiMEMI reduced serum medium (GIBCO, Palo Alto, CA, USA), mixed gently and incubated for 5 min at room temperature. Subsequently, a mixture of siRNA was added and incubated for 20 min. The mixture was diluted by adding medium to each well, and the final concentration of siRNA in each well was set at 100 nmol/L. The cells were then incubated for 48 hours until they were ready for processing.
Tumor samples for immunohistochemistry
A total of 100 formalin-fixed, paraffin-embedded specimens of human HCC and surrounding non-tumor liver tissues were obtained from the Tumor Tissues Base of the First Affiliated Hospital of Sun Yat-Sen University (Guangzhou, China) from January of 2005 to January of 2007. Consent was obtained before tissue collection, and the study was approved by the Human Ethics Committee of the Sun Yat-Sen University. Of the 100 patients from whom tumor tissue was used, the mean age was 53.5 years, including 82 males and 18 females. Ninety patients were infected with hepatitis B virus and 6 with hepatitis C virus; 4 had other diagnoses. Sections were deparaffinized and rehydrated by routine procedures. Endogenous peroxidase activity was quenched with 1% hydrogen peroxide, and microwave retrieval of antigen was performed. Nonspecific binding was blocked using buffer containing goat serum. The slides were incubated at 4 °C overnight with 1:100 diluted mouse monoclonal anti-human PTEN (Santa Cruz, CA, USA). After being washed three times for 15 min, the slides were incubated with biotin-conjugated secondary antibody (1:200) for 30 min at room temperature. After probing, the ABC complex was added and, finally, 3,3′-diaminobenzidine substrate was used for color development. The slides were then counterstained with hematoxylin and observed under an Advanced Fluorescence Microscope (Nikon 80i, Tokyo, Japan). PTEN expression levels were scored as 0, 1, 2, or 3 according to the immunohistochemical staining intensity, in which 0=no staining; 1=weak staining; 2=moderate staining; and 3=intense staining. The distribution of positive cells was graded as follows: 0≤5%; 1=5%∼25%; 2=26%∼50%; 3=51%∼75%; 4=76%∼100%. A final score was obtained by sum of the two scores above. Samples stained with score 0∼2 were considered as negative, whereas samples with score ≥3 were considered positive7, 8.
Statistical analysis
Data were expressed as mean±SD of at least three separate experiments. Differences between groups were assessed with a one-way ANOVA and Student-Newman-Keuls q test using SPSS 11.0 for windows (SPSS Inc, Chicago, IL, USA). The difference in PTEN expression was analyzed by a χ2-test. P values less than 0.05 were considered statistically significant.
Results
Rosiglitazone facilitates the anti-tumor effect of 5-FU in HCC cell lines
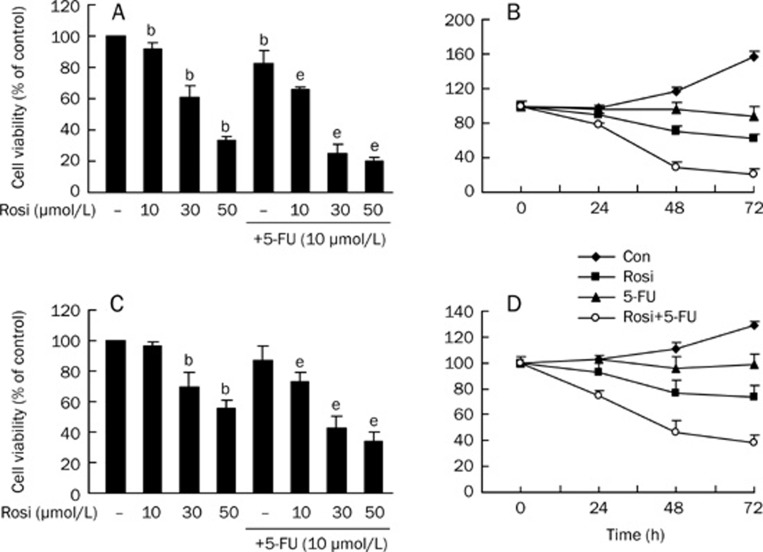
To investigate the effect of rosiglitazone on fluorouracil-inhibited growth of HCC cell lines, we treated BEL-7402 and Huh7 cells with rosiglitazone in the presence or absence of 5-FU. Cell viability was assessed by the MTT assay. As shown in Figure 1, markedly decreased cell viability was observed in both cell lines when they were treated with 5-FU and rosiglitazone, compared with the results of 5-FU alone or rosiglitazone alone. The cell viabilities for BEL-7402 and Huh7 treated with 5-FU alone (10 μmol/L) were (82.56±8.01)% and (87.29±9.38)% at 48 h, respectively. After we co-administered 5-FU with 10, 30, and 50 μmol/L rosiglitazone to both cell lines for 48 h, rosiglitazone significantly enhanced the antitumor activities of 5-FU in a dose- and time-dependent manner. It is noteworthy that the viability of cells with 30 μmol/L rosiglitazone plus 10 μmol/L 5-FU decreased to (25.18±6.00)% and (42.56±7.79)%.These data indicate that a combination of rosiglitazone and 5-FU synergistically inhibits cell growth in HCC cell lines.
Figure 1.
The cell death effect of rosiglitazone plus 5-FU on BEL-7402 and Huh7 cell lines. BEL-7402 cells were treated with varying concentrations of rosiglitazone for 48 h in the presence or absence of 5-FU (A) and with the combination of rosiglitazone and 5-FU for the indicated periods of time (B). Huh7 cells were treated with varying concentrations of rosiglitazone for 48 h in the presence or absence of 5-FU (C) and with the combination of rosiglitazone and 5-FU for the indicated periods of time (D). Cell viability was determined by MTT assay. Data are expressed as the mean±SD of three independent experiments. bP<0.05 vs control group; eP<0.05 vs 5-FU alone group. Con, cells treated with 0.1% DMSO.
Rosiglitazone enhances 5-FU-inhibited cell growth in BEL-7402 by the PPARγ pathway
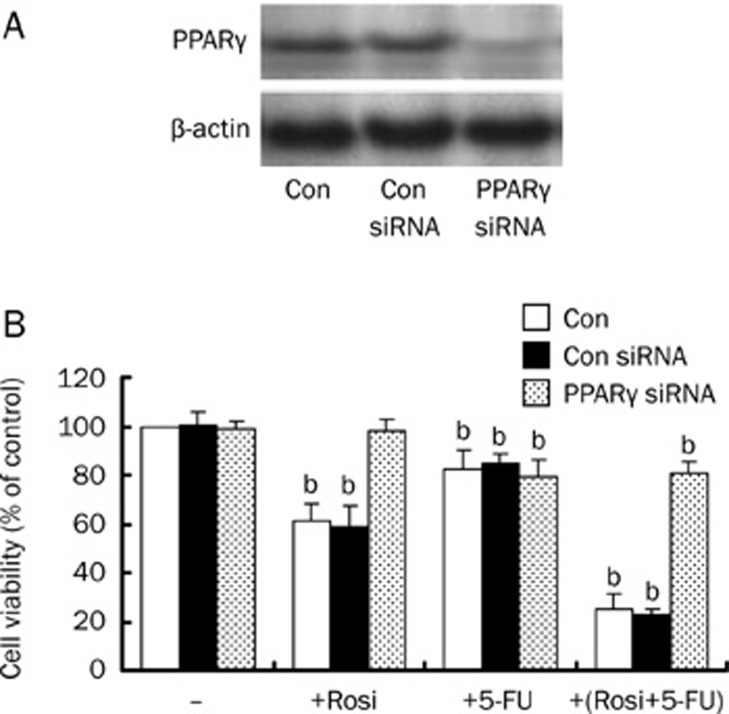
To examine whether the synergistic effect of rosiglitazone plus 5-FU is mediated by the activation of PPARγ, BEL-7402 cells were transfected with PPARγ siRNA or non-specific control siRNA. Cells treated with PBS were used as a control. Unlike control siRNA, PPARγ siRNA almost completely eliminated endogenous PPARγ protein expression (Figure 2A). Subsequently, the cell viability was assessed by MTT assay. We found that 30 μmol/L rosiglitazone did not decrease cell viability after the PPARγ gene was silenced, the cytotoxic effects were exhibited only for 10 μmol/L 5-FU with or without rosiglitazone. This suggests that rosiglitazone increases the growth inhibitive effect of 5-FU in BEL-7402 cells through the activation of the PPARγ signaling pathway. Similar results were obtained in Huh7 cells (data not shown).
Figure 2.
Rosiglitazone's contribution to the anti-tumor effect of 5-FU by the PPARγ signaling pathway. (A) Cellular protein was isolated from BEL-7402 cells transfected with control or PPARγ siRNA for 48 h and was then subjected to Western blotting analysis for PPARγ protein. PPARγ siRNA inhibits PPARγ protein expression. (B) BEL-7402 cells were transfected with control or PPARγ siRNA for 48 h before exposing the cells to rosiglitazone (30 μmol/L) in the presence or absence of 5-FU (10 μmol/L). Afterwards, the cell viabilities were determined by MTT assay up to 48 h. Data are expressed as the mean±SD of three independent experiments. bP<0.05 vs control group; Con, cells treated with 0.1% DMSO; Con siRNA, non-specific siRNA, as a negative control.
Activation of PPARγ by rosiglitazone increases PTEN expression and decreases COX-2 expression
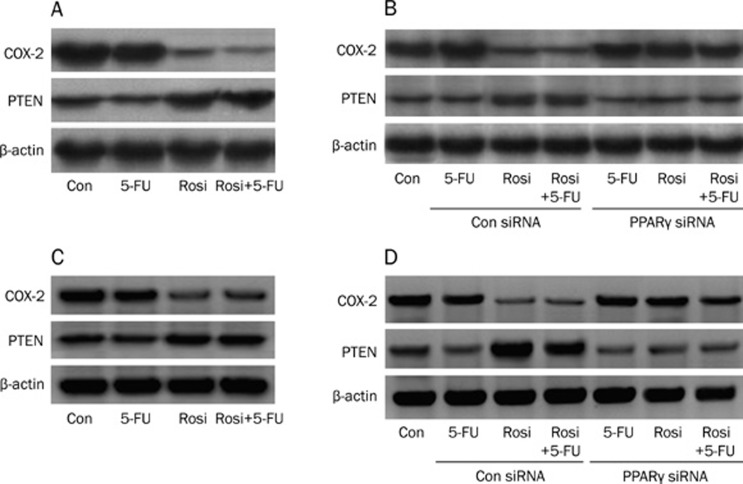
Chemoresistance is an active process of tumor cell survival and a consequence of multiple factors, including reduction of intercellular drug accumulation, repair of tumor cell DNA damage, activation of PI3K/Akt, Ras or MAPK signaling pathways, and dysfunction of the tumor suppression gene9, 10. PTEN is a tumor suppressor gene that inhibits the PI3K/Akt mediated cell survival pathway11. Over-expression of PTEN sensitizes prostate cancer cells to drug-induced apoptosis12. Moreover, previous studies have demonstrated that COX-2 expression is increased in chemo-resistant cancer cells. Clinically, COX-2 inhibitors such as celecoxib are used as cancer treatment drugs13, 14. Thus, we tested the expression of PTEN and COX-2 in BEL-7402 and Huh7 cells by Western blotting analysis. As shown in Figure 3A, compared with the control (0.1% DMSO), rosiglitazone alone or combined with 5-FU increases the expression of PTEN and decreases the expression of COX-2 in BEL-7402 cells, whereas 5-FU alone had little effect on COX-2 and PTEN expression. Furthermore, we found that rosiglitazone alone or combined with 5-FU inactivated the expression of both proteins after the PPARγ gene was silenced by PPARγ siRNA (Figure 3B). These data indicate that the activation of PPARγ by rosiglitazone increases PTEN expression and decreases COX-2 expression. Similar results were obtained in Huh7 cells (Figure 3C and Figure 3D).
Figure 3.
The effect of rosiglitazone on PTEN and COX-2 protein expression. Cellular protein was isolated from BEL-7402 cells (A) or Huh 7 cells (C) that were cultured with rosiglitazone (30 μmol/L) in the presence or absence of 5-FU (10 μmol/L) for 24 h. Then, western blotting analysis was used to detect the PTEN and COX-2 protein expression. (B) The PPARγ signaling pathway was inactivated by PPARγ siRNA method in BEL-7402 cells (B) or Huh 7 cells (D). Western blotting analysis was used to detect the PTEN and COX-2 protein expression of BEL-7402 cells treated with rosiglitazone (30 μmol/L) with or without 5-FU (10 μmol/L) for 24 h. Results are representative of three independent experiments. Con, cells treated with 0.1% DMSO; Con siRNA, non-specific siRNA, as a negative control.
Over-expression of PTEN by PPARγ sensitizes HCC cell lines to 5-FU antitumor activity
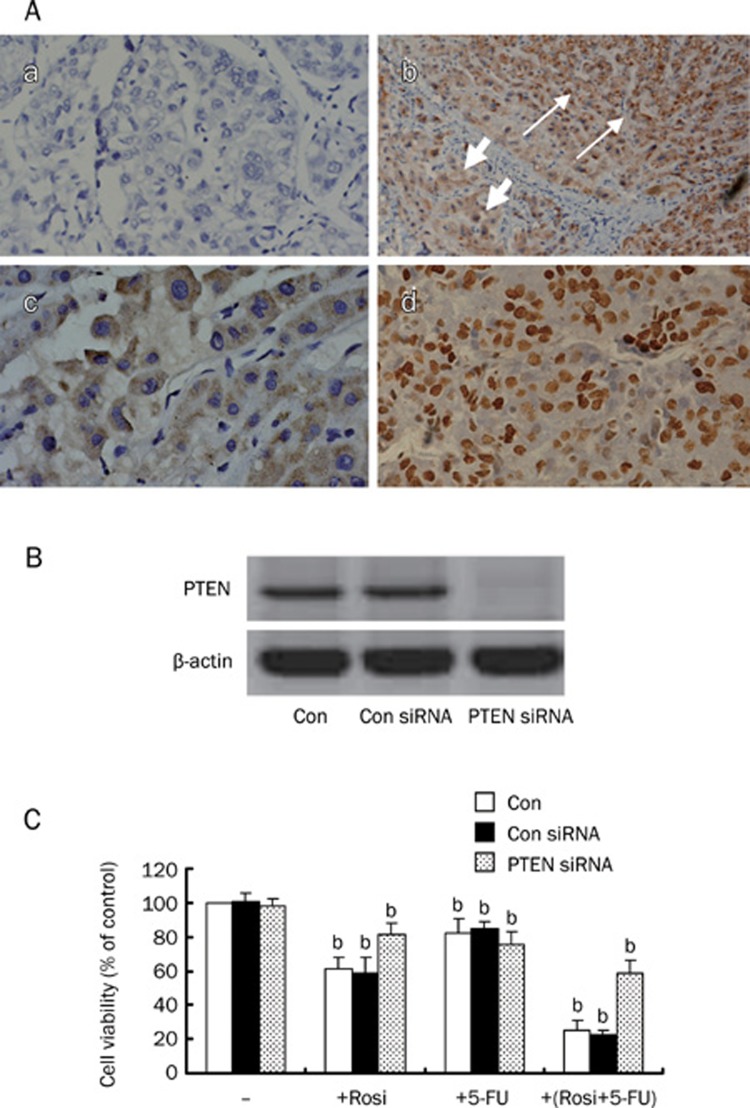
5-FU is widely used to treat advanced HCC, but chemo-resistance remains a major obstacle to its use in the clinical setting. To investigate the relationship between the expression of PTEN and advanced HCC, we examined the expression of PTEN in 100 advanced HCC tissues and paracancerous tissues. Of 100 cases of HCC, 62 cases were positive for PTEN, and 38 cases were negative. Ninety-two cases of adjacent non-tumor tissues were positive as a control. As shown in Figure 4A-b, the staining distribution of PTEN in HCC tissues was significantly decreased as compared with the paracancerous tissue (χ2=25.409, P<0.05). Furthermore, of the 62 positive samples, 45 showed staining in the cytoplasm of cancer cells (Figure 4A-c), and the other 17 exhibited intensive nuclear staining of cancer cells (Figure 4A-d).
Figure 4.
Representative sections showing PTEN protein expression by immunohistochemical staining. (A-a) a negative control section of HCC (primary antibody substituted with PBS, original magnification, ×200); (A-b) the positive staining of PTEN was mainly observed in the cytoplasm of cells. The strong staining of PTEN protein was shown in paracancerous tissue (the long arrow), and the weak staining was shown in tumor tissue (the short arrow, original magnification, ×100); (A-c) PTEN protein was expressed in the cytoplasm of carcinoma cells (original magnification, ×200); (A-d) PTEN protein was expressed in the nuclei of carcinoma cells (original magnification, ×200); (B) Cellular protein was isolated from BEL-7402 cells transfected with control or PTEN siRNA for 48 h and was then subjected to Western blotting analysis for PTEN protein. PTEN siRNA inhibits PTEN protein expression. (C) BEL-7402 cells were transfected with control or PTEN siRNA for 48 h before exposing the cells to rosiglitazone (30 μmol/L) in the presence or absence of 5-FU (10 μmol/L). Afterwards, the cell viabilities were determined by MTT assay up to 48 h. Data are expressed as the mean±SD of three independent experiments. bP<0.05 vs control group; Con, cells treated with 0.1% DMSO; Con siRNA, non-specific siRNA, as a negative control.
Over-expression of PTEN was detected in the process of rosiglitazone-induced cell growth arrest of HCC cell lines (Figure 1 and Figure 3A). To further confirm the role of PTEN in the rosiglitazone-mediated cell growth inhibition, BEL-7402 cells were transfected with PTEN siRNA or control siRNA, and endogenous PTEN protein expression was completely eliminated (Figure 4B). Then, cells were treated with rosiglitazone, 5-FU, or rosiglitazone plus 5-FU for 48 h. As shown in Figure 4C, the cell viabilities of rosiglitazone alone and rosiglitazone plus 5-FU were (81.46±6.44)% and (58.91±7.68)%, respectively, whereas no changes were observed in the control siRNA group. These results suggest that the over-expression of PTEN is involved in the inhibitory effect of rosiglitazone and rosiglitazone plus 5-FU. Similar results were obtained in Huh7 cells (data not shown).
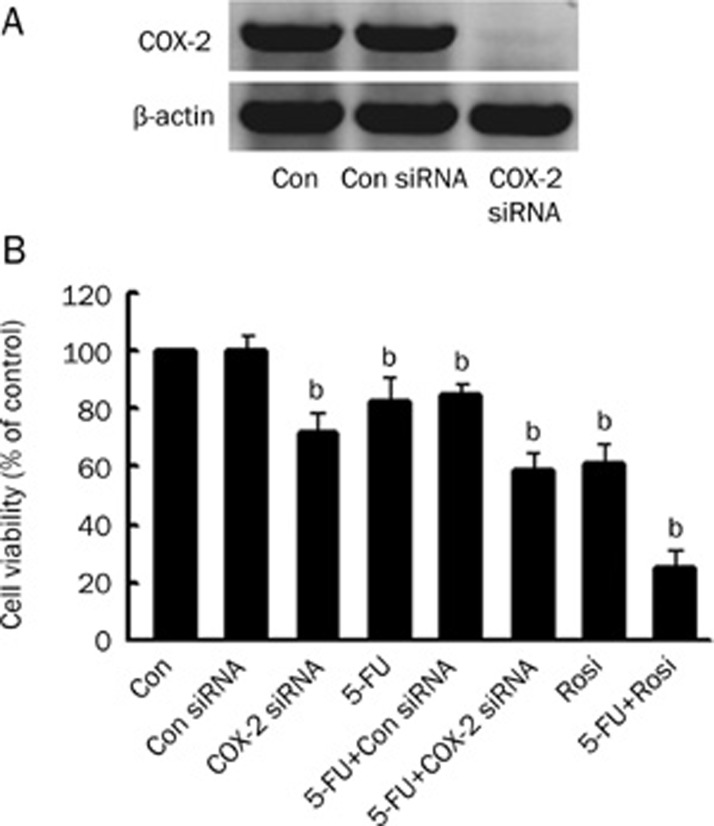
Down-regulation of Cox-2 increased 5-FU antitumor activity in HCC cell lines
COX-2 expression is associated with tumor cell proliferation and tumorigenesis15, 16. Significantly increased COX-2 protein expression was observed in HCC tissues and cell lines17, 18, which coincides with our results (Figure 3A). To evaluate the significance of down-regulation of COX-2 expression in BEL-7402 cells, we investigated cell viability by MTT assay after BEL-7402 cells were treated with rosiglitazone or COX-2 siRNA (Figure 5A). We found that the knockdown of COX-2 by specific siRNA reduced cell viability (71.49±6.62)%. Moreover, the cell growth inhibitive effect is dramatically increased in the combination of COX-2 siRNA and 5-FU group, in which the cell viability was (59.02±6.05)% (Figure 5B). Unlike the control siRNA group, this reduction in COX-2 expression by COX-2 siRNA or rosiglitazone correlates with growth inhibition of BEL-7402 cells, indicating that down-regulation of COX-2 is responsible for growth arrest of BEL-7402 cells. Similar results were obtained in Huh7 cells (data not shown).
Figure 5.
(A) Cellular protein was isolated from BEL-7402 cells transfected with control or COX-2 siRNA for 48 h and was then subjected to Western blotting analysis for COX-2 protein. COX-2 siRNA inhibits COX-2 protein expression. (B) After BEL-7402 cells were transfected with control or COX-2 siRNA for 48 h the cells were exposed to rosiglitazone (30 μmol/L) in the presence or absence of 5-FU (10 μmol/L). Then, the cell viabilities were determined by MTT assay for 48 h. Data are expressed as the mean±SD of three independent experiments. bP<0.05 vs control group; Con, cells treated with 0.1% DMSO; Con siRNA, non-specific siRNA, as a negative control.
Discussion
Multiple factors are involved in increasing resistance to chemotherapeutic agents, reduction of intracellular drug accumulation, and DNA damage repair by the modulation of proliferative or anti-apoptotic proteins, etc19. 5-FU causes cell injury by inhibiting thymidylate synthesis. It has become a mainstay of treatment for advanced HCC. Unfortunately, many advanced-stage cancers are inherently resistant to 5-FU or develop resistance during the course of therapy. This is also the major reason for the treatment failure of advanced HCC chemotherapy20. There is so far no effective single agent or polychemotherapeutic regimen for the treatment of resistant HCC21, 22, 23. Therefore, a new drug combined with 5-FU for anticancer therapy in advanced HCC is needed.
PPARγ is a nuclear receptor with multiple biologic effects. Recent evidence has demonstrated that PPARγ activation by TZDs inhibits cell growth and induces cell apoptosis in liver cancer cell lines24, 25. Moreover, rosiglitazone, as a PPARγ synthetic ligand, has been shown to enhance the antitumor activity of some chemotherapeautic drugs by the modulation of cancer cell agents26, 27. However, the effects of rosiglitazone on hepatoma cells have been poorly understood until now.
Our results showed that rosiglitazone suppressed cell growth in a dose-dependent manner and facilitated the anti-tumor effect of 5-FU in HCC cell lines. Cell viabilities of BEL-7402 and Huh7 cell lines were slightly inhibited by 5-FU alone, and the inhibitive effects for both HCC cell lines were dramatically increased by combining rosiglitazone with 5-FU, indicating that the combination of both drugs synergistically induces cell growth arrest in HCC cell lines. Subsequently, we found that rosiglitazone enhanced 5-FU-inhibited cell growth of HCC via PPARγ activation, because the effect was completely abrogated by the inactivation of PPARγ through PPARγ siRNA. Han et al have demonstrated that rosiglitazone inhibits non-small cell lung cancer (NSCLC) cell growth by both PPARγ-dependent and PPARγ-independent signaling pathways28. Our results show that rosiglitazone increases the antitumor effect of 5-FU via the PPARγ-dependent signaling pathway.
PTEN is a phosphase with dual-specificity possessing both lipid and protein phosphatases. It decreases the phosphorylation level of Akt and negatively affects the PI3K/Akt pathway. In addition, it plays a critical part in control of normal cell growth29. The deletion and low expression of PTEN in tumor tissues results in decreased sensitivity of mutant cells to apoptotic stimuli and promotes cellular over-growth and tumorigenesis30. Our results show that 62% of our HCC tissues were positive for PTEN. Compared with the control (92%), the difference was significant (P<0.05), indicating that HCC tumor tissues have lower expression of PTEN. Interestingly, PTEN protein was localized in the cytoplasm of cells in paracancerous tissues, while 17% of positive cases for PTEN showed nuclear intensive staining of cancer cells. PTEN protein in the cytoplasm of normal cells regulates cell differentiation, proliferation, and apoptosis. The role of PTEN protein in the nuclei of some tumor cells is not clearly understood, which we pursued in further study.
Decreased expression of PTEN might decrease the sensitivity of cancer cells to chemotherapeutic drugs. Over-expression of PTEN sensitizes human ovarian cancer cells to cisplatin-induced apoptosis31. However, the effect has not been elucidated in HCC cell lines. We found upregulation of PTEN in HCC cell lines with the groups treated with rosiglitazone and rosiglitazone plus 5-FU, which could be one mechanism by which rosiglitazone increases the 5-FU anticancer effect.
COX-2, one isoform of COX, is an enzyme inducible by a number of factors, including cytokines, growth factors, and tumor promoters32. It is known that over-expression of COX-2 is associated with tumor cell proliferation, escape from apoptosis and tumor angiogenesis33. Higher levels of COX-2 are identified in hepatocellular carcinoma cells, which increases PGE2 production and growth rate17. Whereas COX-2 inhibitor enhances cell apoptosis in human carcinoma cell lines34, non-steroidal anti-inflammatory drugs (NSAIDs), the inhibitors of COX-1 and COX-2, are also shown to reduce the risk of sporadic colorectal, breast, prostate, and lung cancers35, 36. In our study, rosiglitazone reduced COX-2 expression via the PPARγ signaling pathway. The down-regulation of COX-2 has a role in rosiglitazone-induced cell growth inhibition, suggesting that rosiglitazone increases 5-FU's antitumor effect and reduces HCC cell resistance, possibly through the COX-2 pathway.
In summary, the present study demonstrates that a combination of rosiglitazone and 5-FU strongly induces growth inhibition in HCC cell lines. Additionally, our results suggest that rosiglitazone increases PTEN expression and decreases COX-2 expression through the PPARγ signaling pathway, which may explain the underlying mechanisms by which rosiglitazone facilitates 5-FU-inhibited HCC growth. These observations suggest potential novel therapies for the treatment of advanced liver cancer.
Author contribution
Qian WANG designed research; Liang-qi CAO and Xiao-li WANG performed research; Ping XUE, Xing-yuan JIAO, He-ping PENG, Hai-wu LU, and Qiang ZHENG contributed new analytical tools and reagents; Xi-lin CHEN, Xiao-hui HUANG, Xin-hui FU, and Jing-song CHEN analyzed data; Liang-qi CAO wrote the paper.
Acknowledgments
This work was supported by research grants from the National Natural Science Foundation of China (No 30672053), Guangdong Natural Science Foundation (No 9451018201003643), and the Doctoral start-up funds of Guangzhou Medical College (No 2008C35). The authors appreciate the Surgical Laboratory Department of the First Affiliated Hospital of Sun Yat-Sen University for providing experimental instruments and equipment.
References
- Vamecq J, Latruffe N. Medical significance of peroxisome proliferator-activated receptors. Lancet. 1999;354:141–8. doi: 10.1016/S0140-6736(98)10364-1. [DOI] [PubMed] [Google Scholar]
- Young PW, Buckle DR, Cantello BC, Chapman H, Clapham JC, Coyle PJ, et al. Identification of high-affinity binding sites for the insulin sensitizer rosiglitazone (BRL-49653) in rodent and human adipocytes using a radioiodinated ligand for peroxisomal proliferator activated receptor gamma. J Pharmacol Exp Ther. 1998;284:751–9. [PubMed] [Google Scholar]
- Rumi MA, Sato H, Ishihara S, Kawashima K, Hamamoto S, Kazumori H, et al. Peroxisome proliferator-activated receptor gamma ligand-induced growth inhibition of human hepatocellular carcinoma. Br J Cancer. 2001;84:1640–7. doi: 10.1054/bjoc.2001.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern MA, Schubert D, Sahi D, Schöneweiss MM, Moll I, Haugg AM, et al. Proapoptotic and antiproliferative potential of selective cyclooxygenase-2 inhibitors in human liver tumor cells. Hepatology. 2002;36:885–94. doi: 10.1053/jhep.2002.36125. [DOI] [PubMed] [Google Scholar]
- Hall AJ, Wild CP. Liver cancer in low and middle income countries. BMJ. 2003;326:994–5. doi: 10.1136/bmj.326.7397.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CT, Seol JY, Lee SY, Park KH, Han SJ, Yoo CG, et al. The effect of adenovirus-IkappaBalpha transduction on the chemosensitivity of lung cancer cell line with resistance to cis-diamminedichloroplatinum(II)(cisplatin) and doxorubicin(adriamycin) Lung Cancer. 2003;41:199–206. doi: 10.1016/s0169-5002(03)00227-7. [DOI] [PubMed] [Google Scholar]
- Rahman MA, Dhar DK, Yamaguchi E, Maruyama S, Sato T, Hayashi H, et al. Coexpression of inducible nitric-oxide synthase and COX-2 in hepatocellular carcinoma and surrounding liver: possible involvement of COX-2 in the angiogenesis of hepatitis C virus-positive cases. Clin Cancer Res. 2001;7:1325–32. [PubMed] [Google Scholar]
- Xue LY, Hu N, Song YM, Zou SM, Shou JZ, Qian LX, et al. Tissue microarray analysis reveals a tight correlation between protein expression pattern and progression of esophageal squamous cell carcinoma. BMC Cancer. 2006;6:296. doi: 10.1186/1471-2407-6-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan HC, Sun M, Kaneko S, Feldman RI, Nicosia SV, Wang HG, et al. Akt phosphorylation and stabilization of X-linked inhibitor of apoptosis protein (XIAP) J Biol Chem. 2004;279:5405–12. doi: 10.1074/jbc.M312044200. [DOI] [PubMed] [Google Scholar]
- Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–79. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- Leslie NR, Bennett D, Lindsay YE, Stewart H, Gray A, Downes CP, et al. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J. 2003;22:5501–10. doi: 10.1093/emboj/cdg513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan XJ, Whang YE. PTEN sensitizes prostate cancer cells to death receptor-mediated and drug-induced apoptosis through a FADD-dependent pathway. Oncogene. 2002;21:319–27. doi: 10.1038/sj.onc.1205054. [DOI] [PubMed] [Google Scholar]
- Mathieu A, Remmelink M, D'Haene N, Penant S, Gaussin JF, Van Ginckel R, et al. Development of a chemoresistant orthotopic human nonsmall cell lung carcinoma model in nude mice: analyses of tumor heterogenity in relation to the immunohistochemical levels of expression of cyclooxygenase-2, ornithine decarboxylase, lung-related resistance protein, prostaglandin E synthetase, and glutathione-S-transferase-alpha (GST)-alpha, GST-mu, and GST-pi. Cancer. 2004;101:1908–18. doi: 10.1002/cncr.20571. [DOI] [PubMed] [Google Scholar]
- Tuynman JB, Vermeulen L, Boon EM, Kemper K, Zwinderman AH, Peppelenbosch MP, et al. Cyclooxygenase-2 inhibition inhibits c-Met kinase activity and Wnt activity in colon cancer. Cancer Res. 2008;68:1213–20. doi: 10.1158/0008-5472.CAN-07-5172. [DOI] [PubMed] [Google Scholar]
- Fosslien E. Molecular pathology of cyclooxygenase-2 in neoplasia. Ann Clin Lab Sci. 2000;30:3–21. [PubMed] [Google Scholar]
- Kase S, Osaki M, Honjo S, Takeda A, Adachi K, Araki K, et al. A selective cyclooxygenase-2 inhibitor, NS398, inhibits cell growth and induces cell cycle arrest in the G2/M phase in human esophageal squamous cell carcinoma cells. J Exp Clin Cancer Res. 2004;23:301–7. [PubMed] [Google Scholar]
- Leng J, Han C, Demetris AJ, Michalopoulos GK, Wu T. Cyclooxygenase-2 promotes hepatocellular carcinoma cell growth through Akt activation: evidence for Akt inhibition in celecoxib-induced apoptosis. Hepatology. 2003;38:756–68. doi: 10.1053/jhep.2003.50380. [DOI] [PubMed] [Google Scholar]
- Yu J, Qiao L, Zimmermann L, Ebert MP, Zhang H, Lin W, et al. Troglitazone inhibits tumor growth in hepatocellular carcinoma in vitro and in vivo. Hepatology. 2006;43:134–43. doi: 10.1002/hep.20994. [DOI] [PubMed] [Google Scholar]
- Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–79. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- Shi X, Liu S, Kleeff J, Friess H, Büchler MW. Acquired resistance of pancreatic cancer cells towards 5-fluorouracil and gemcitabine is associated with altered expression of apoptosis-regulating genes. Oncology. 2002;62:354–62. doi: 10.1159/000065068. [DOI] [PubMed] [Google Scholar]
- Mader RM, Müller M, Steger GG. Resistance to 5-fluorouracil. Gen Pharmacol. 1998;31:661–6. doi: 10.1016/s0306-3623(98)00191-8. [DOI] [PubMed] [Google Scholar]
- Sakon M, Nagano H, Dono K, Nakamori S, Umeshita K, Yamada A, et al. Combined intraarterial 5-fluorouracil and subcutaneous interferon-alpha therapy for advanced hepatocellular carcinoma with tumor thrombi in the major portal branches. Cancer. 2002;94:435–42. doi: 10.1002/cncr.10246. [DOI] [PubMed] [Google Scholar]
- Kondo M, Nagano H, Wada H, Damdinsuren B, Yamamoto H, Hiraoka N, et al. Combination of IFN-alpha and 5-fluorouracil induces apoptosis through IFN-alpha/beta receptor in human hepatocellular carcinoma cells. Clin Cancer Res. 2005;11:1277–86. [PubMed] [Google Scholar]
- Date M, Fukuchi K, Morita S, Takahashi H, Ohura K. 15-Deoxy-delta12,14-prostaglandin J2, a ligand for peroxisome proliferators-activated receptor-gamma, induces apoptosis in human hepatoma cells. Liver Int. 2003;23:460–6. doi: 10.1111/j.1478-3231.2003.00877.x. [DOI] [PubMed] [Google Scholar]
- Cao LQ, Chen XL, Wang Q, Huang XH, Zhen MC, Zhang LJ, et al. Upregulation of PTEN involved in rosiglitazone-induced apoptosis in human hepatocellular carcinoma cells. Acta Pharmacol Sin. 2007;28:879–87. doi: 10.1111/j.1745-7254.2007.00571.x. [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Tang XQ, Sun L, Dong L, Qin Y, Liu HQ, et al. Rosiglitazone enhances fluorouracil-induced apoptosis of HT-29 cells by activating peroxisome proliferator-activated receptor gamma. World J Gastroenterol. 2007;13:1534–40. doi: 10.3748/wjg.v13.i10.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Hur GY, Jung KH, Jung HC, Lee SY, Kim JH, et al. PPAR-gamma agonist increase gefitinib's antitumor activity through PTEN expression. Lung Cancer. 2006;51:297–301. doi: 10.1016/j.lungcan.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Han S, Roman J. Rosiglitazone suppresses human lung carcinoma cell growth through PPARgamma-dependent and PPARgamma-independent signal pathways. Mol Cancer Ther. 2006;5:430–7. doi: 10.1158/1535-7163.MCT-05-0347. [DOI] [PubMed] [Google Scholar]
- Maehama T, Taylor GS, Dixon JE. PTEN and myotubularin: novel phosphoinositide phosphatases. Annu Rev Biochem. 2001;70:247–79. doi: 10.1146/annurev.biochem.70.1.247. [DOI] [PubMed] [Google Scholar]
- Tamura M, Gu J, Matsumoto K, Aota S, Parsons R, Yamada KM. Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science. 1998;280:1614–7. doi: 10.1126/science.280.5369.1614. [DOI] [PubMed] [Google Scholar]
- Yan X, Fraser M, Qiu Q, Tsang BK. Over-expression of PTEN sensitizes human ovarian cancer cells to cisplatin-induced apoptosis in a p53-dependent manner. Gynecol Oncol. 2006;102:348–55. doi: 10.1016/j.ygyno.2005.12.033. [DOI] [PubMed] [Google Scholar]
- Harris RE. Cyclooxygenase-2 (cox-2) and the inflammogenesis of cancer. Subcell Biochem. 2007;42:93–126. doi: 10.1007/1-4020-5688-5_4. [DOI] [PubMed] [Google Scholar]
- Kase S, Osaki M, Honjo S, Adachi H, Tsujitani S, Kaibara N, et al. Expression of cyclo-oxygenase-2 is correlated with high intratumoral microvessel density and low apoptotic index in human esophageal squamous cell carcinomas. Virchows Arch. 2003;442:129–35. doi: 10.1007/s00428-002-0706-x. [DOI] [PubMed] [Google Scholar]
- Honjo S, Osaki M, Ardyanto TD, Hiramatsu T, Maeta N, Ito H, et al. COX-2 inhibitor, NS398, enhances Fas-mediated apoptosis via modulation of the PTEN-Akt pathway in human gastric carcinoma cell lines. DNA Cell Biol. 2005;24:141–7. doi: 10.1089/dna.2005.24.141. [DOI] [PubMed] [Google Scholar]
- Thun MJ, Namboodiri MM, Heath CW., Jr Aspirin use and reduced risk of fatal colon cancer. N Engl J Med. 1991;325:1593–6. doi: 10.1056/NEJM199112053252301. [DOI] [PubMed] [Google Scholar]
- Harris RE, Beebe-Donk J, Doss H, Burr Doss D. Aspirin, ibuprofen, and other non-steroidal anti-inflammatory drugs in cancer prevention: a critical review of non-selective COX-2 blockade (review) Oncol Rep. 2005;13:559–83. [PubMed] [Google Scholar]