Abstract
Aim:
KCNQ4 channels play an important part in adjusting the function of cochlear outer hair cells. The aim of this study was to investigate the effects of ser/thr phosphatase inhibitors on human KCNQ4 channels expressed in Xenopuslaevis oocytes.
Methods:
Synthetic cRNA encoding human KCNQ4 channels was injected into Xenopus oocytes. We used a two-electrode voltage clamp to measure the ion currents in the oocytes.
Results:
Wild-type KCNQ4 expressed in Xenopus oocytes showed the typical properties of slow activation kinetics and low threshold activation. The outward K+ current was almost completely blocked by a KCNQ4 blocker, linopirdine (0.25 mmol/L). BIMI (a PKC inhibitor) prevented the effects of PMA (a PKC activator) on the KCNQ4 current, indicating that PKC may be involved in the regulation of KCNQ4 expressed in the Xenopus oocyte system. Treatment with the ser/thr phosphatase inhibitors, cyclosporine (2 μmol/L), calyculin A (2 μmol/L) or okadaic acid (1 μmol/L), caused a significant positive shift in V1/2 and a decrease in the conductance of KCNQ4 channels. The V1/2 was shifted from −14.6±0.5 to −6.4±0.4 mV by cyclosporine, −18.8±0.5 to −9.2±0.4 mV by calyculin A, and −14.1±0.5 to −0.7±0.6 mV by okadaic acid. Moreover, the effects of these phosphatase inhibitors (okadaic acid or calyculin A) on the induction of a positive shift of V1/2 were augmented by further addition of PMA.
Conclusion:
These results indicate that ser/thr phosphatase inhibitors can induce a shift to more positive potentials of the activation curve of the KCNQ4 current. It is highly likely that the phosphatase functions to balance the phosphorylated state of substrate protein and thus has an important role in the regulation of human KCNQ4 channels expressed in Xenopus oocytes.
Keywords: KCNQ4 channel, phosphatase inhibitor, calyculin A, okadaic acid, protein kinase C, phosphorylation, electrophysiology, Xenopus oocyte
Introduction
KCNQ4, a novel potassium channel, plays a part in regulating the membrane potential and function of various cell types in the body1. The KCNQ4 current is a low-threshold, slow activating and noninactivating current that is expressed in the outer hair cells of the cochlea, brain, and heart. Mutations in KCNQ4 give rise to an inherited syndrome of deafness2. Therefore, the regulatory pathways of KCNQ4 channels play an important role in adjusting the function of outer hair cells. The KCNQ gene subfamily is composed of five K+ channels, KCNQ1 to KCNQ5. The KCNQ4 channel was included in the Kv nomenclature as Kv 7.4 (voltage-gated potassium channel subunit Kv7.4)3. The heteromers of KCNQ2/KCNQ3 underlie the neuronal M-current, which modulates neuronal excitability. Many intracellular messengers, eg, PIP2, IP3, diacylglycerol (DAG), calmodulin, calcineurin, activators/inhibitors of PKC, tyrosine kinases, and myosin light chain kinase, have been reported to modulate M currents4, 5, 6. Moreover, the A-kinase-anchoring protein AKAP150, which binds PKC, facilitates the inhibition of KCNQ2 current7. Analysis of recombinant KCNQ2 channels suggests that targeting of PKC through association with AKAP150 is important for inhibition. However, the effect of PKC activation on KCNQ channels is still controversial8. The inhibition of the metabolism of DAG by DAG kinase blockers cannot mimic the effect of muscarinic modulation by muscarinic agonist, and it has been suggested that activated PKC and its targets are not essential players in the acute muscarinic modulation of KCNQ channels in mammalian expression systems8. By contrast, the activation of PKC induced a large positive shift in the conductance-voltage curve for KCNQ channels expressed in Xenopus oocytes9. The activation of PKC had a different effect on KCNQ channels expressed in mammalian cells and Xenopus oocytes, which could be due to the different intracellular environment and basal levels of channel phosphorylation.
The electrophysiological properties of KCNQ4 channels are similar to those of heteromers of KCNQ2/KCNQ3, such as the shifting effect of the conductance-voltage response curve induced by M1 receptor stimulation10, but the information about interactions between PKC and KCNQ4 is not clear. PKC, by phosphorylating its target protein and modulating its function, could interact with phosphatases. Protein phosphatases mediate the physiological dephosphorylation of target proteins, an activity that might be expected to reverse the effect induced by PKC. However, the inhibition of phosphatases can sometimes enhance the effect induced by a PKC activator. Therefore, the ser/thr phosphatase inhibitors, cyclosporine, okadaic acid, and calyculin A, were used in this study to investigate the role of phosphatase on the activity of KCNQ4 channels expressed in Xenopus oocytes. Our results demonstrated that phosphatase inhibitors induced a shift in the voltage dependence of KCNQ4 channels to more positive potentials. Furthermore, the PKC activator PMA potentiates the effects of okadaic acid and calyculin A in the modulation of KCNQ4 channels. Thus, we propose that endogenous phosphatases play a role in the regulation of KCNQ4 channels and balance the excess activity of PKC in the Xenopus oocytes expression system.
Materials and methods
Preparation of RNA
The molecular biological procedures were performed much as previously described11. The plasmid cDNA encoding the human KCNQ4 channel was a generous gift from Prof Thomas J JENTSCH. The cDNA had been previously subcloned into the expression vector pTLN, which contains the 5′ and 3′ regions of the Xenopus β-globin gene to boost expression in oocytes. Plasmid DNA was linearized with HpaI (Gibco BRL, USA). For synthesis of cRNA, the mMessage mMachine SP6-polymerase kit (Ambion, USA) was used. The nucleotide sequence of the construct was verified by automated DNA sequencing.
Preparation of Xenopus oocytes
Xenopus oocytes were collected from frogs anesthetized in 0.1% sodium bicarbonate solution containing 0.15% tricaine (ethyl 3-aminobenzoate, methanesulfonic acid salt, Sigma-Aldrich). In brief, a lobe of an ovary was surgically removed from the frog's abdominal cavity through a small incision and placed in modified Barth's solution (MBS; in mmol/L: 88 NaCl, 1 KCl, 2.4 NaHCO3, 0.82 MgSO4, 0.41 CaCl2, 0.33 Ca(NO3)2, and 15 HEPES-Tris; adjusted to pH 7.6 with NaOH). Isolated oocytes were defolliculated enzymatically by incubation in collagenase (2 mg/mL, type 1, Gibco, USA) in sterile MBS for 1−2 h followed by several washes in MBS containing 0.1% BSA (Sigma). Stage V–VI oocytes were then incubated and kept overnight at 18 °C. Healthy oocytes were selected, and cRNA (10−15 ng /50 nL) was microinjected into each oocyte using a Nanoject microinjector (Drummond, USA). Injected oocytes were maintained at 18 °C for 2−4 d in MBS supplemented with gentamicin (50 mg/L). The MBS was replaced with fresh medium once a day before electrophysiological recordings. All animal care and experimental procedures were performed according to the guidelines of the Animal Care Committee of the Chung Shan Medical University.
Electrophysiology
The two-electrode voltage clamp (TEVC) technique was used to record whole cell KCNQ4 currents in Xenopus oocytes at room temperature (22−26 °C) using an AxoClamp-2B amplifier (Molecular Devices, San Francisco, USA). One of the electrodes was connected to the HS-2x1L headstage to record voltage, while the other was connected to the HS-2x10 MG headstage to record current. Glass electrodes were made from capillary tubing on a vertical electrode puller (Model PP-830, Narishige Scientific Instrument Lab, Japan) and had a tip resistance of 0.5 to 2.0 MΩ when filled with 3 mol/L KCl. Whole-cell K+ currents were studied on the oocytes 2−4 d after the cRNA injection. During the experiments, oocytes were placed in an RC-3Z recording chamber (Warner Instruments, USA) and immersed in ND 96 solution consisting of (mmol/L) 96 NaCl, 1 KCl, 1 MgCl2, 1 CaCl2, and 5 Hepes; the pH was 7.4. The chamber solution was connected through two 3 mol/L KCl 1% agar-bridged, Ag/AgCl reference electrodes to a virtual ground belonging to a current monitor headstage (VG-2A-x100, Molecular Devices, San Francisco, CA, USA). The condition of each single oocyte was checked before measurements by recording membrane potentials. Only oocytes with membrane potentials below −30 mV were used for current recordings. The data were digitized at 5 kHz and stored using Digidata 1322A (Molecular Devices, San Francisco, USA), and analysis was accomplished with pClamp 9.0 software (Molecular Devices, San Francisco, USA). To determine the conductance-voltage (G–V) relations, a step protocol was employed, whereby the oocytes were clamped at −80 mV for 3 s and depolarized at +60 mV with 20 mV increments to −100 mV, followed by a tail pulse at −30 mV for 2 s.
Data analysis
The tail current protocol was used to generate steady-state activation curves (conductance-voltage curves), which were fitted to a two-state Boltzmann function as follows
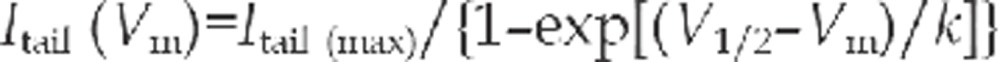 |
where V1/2 is the voltage that produces half-maximal activation of conductance, Vm is the membrane potential, and k is the slope factor. Itail (max) is the maximal tail current amplitude. Data are presented as means±SEM. Statistical significance was determined using Student's t test with one-way analysis of variance (ANOVA), and P values < 0.05 were taken to indicate statistically significant difference.
Chemicals
PMA (phorbol 12-myristate 13-acetate), cyclosporine, okadaic acid and calyculin A were obtained from LC Laboratories (USA). BIMI (bisindolylmaleimide I) was obtained from Calbiochem (San Diego, CA, USA). Linopirdine was obtained from Sigma (St Louis, MO, USA). PMA, okadaic acid and calyculin A were dissolved as stock solutions in dimethylsulfoxide (DMSO). Linopirdine was dissolved in 0.1 mol/L HCl as a 25 mmol/L stock solution.
Results
Human KCNQ4 currents in Xenopus oocytes
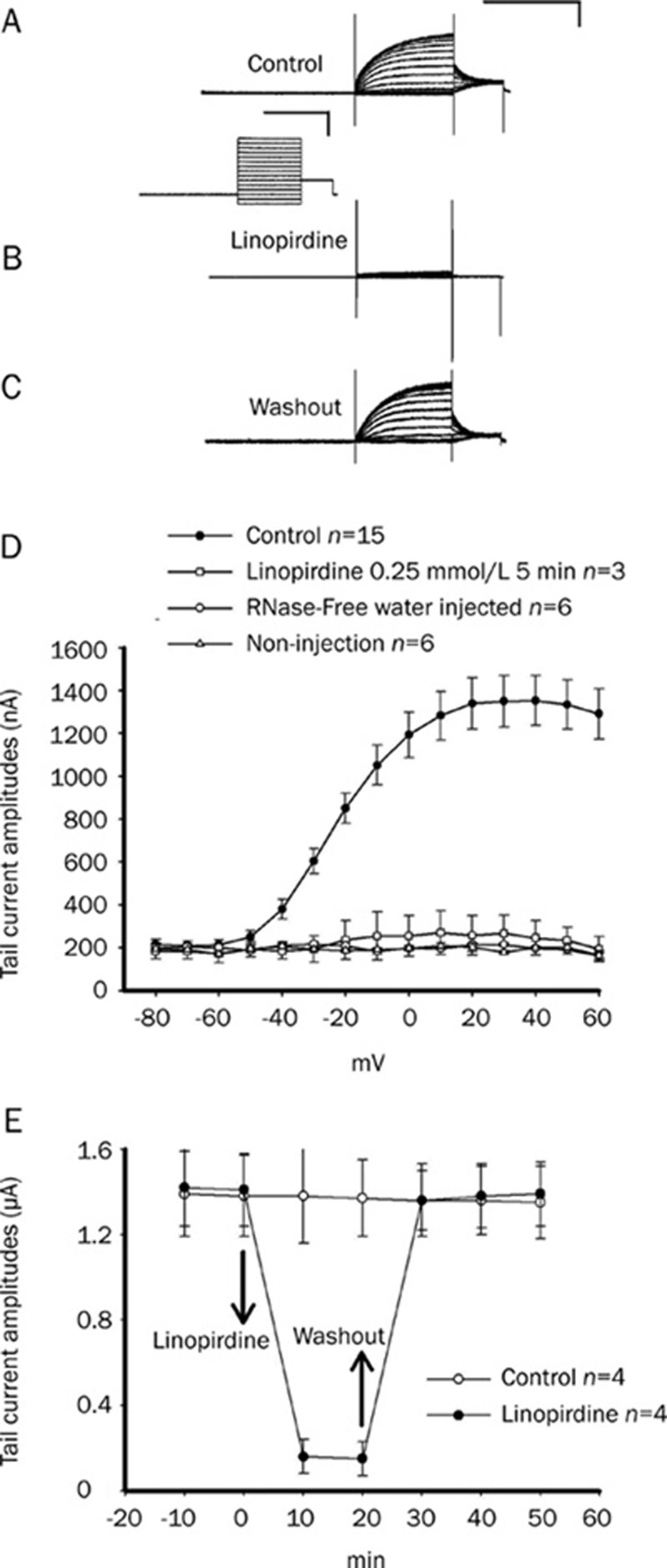
The KCNQ4-expressed oocytes were voltage clamped at −60 mV and stepped to potentials ranging from −80 to +60 mV, which produced slow-activated and low-threshold currents as shown in Figure 1A (upper traces). Application of linopirdine (0.25 mmol/L), a blocker of KCNQ4, to the external bath solution completely abolished the K+ outward current (Figure 1B). The inhibitory effect of linopirdine was reversible by washout (Figure 1C). Figure 1D shows the current-voltage curves of no injection, RNase-free water and KCNQ4 cRNA-injected oocytes. Only the KCNQ4 cRNA group currents show sensitivity to linopirdine. The time course of the peak tail current amplitudes is shown in Figure 1E. The application of linopirdine and washout are indicated by arrows. There was very little run-down of KCNQ4, as indicated by the control curve (without treatment).
Figure 1.
The expressed currents of KCNQ4 were blocked by the addition of linopirdine on Xenopus oocytes. (A) Typical current traces were recorded from an oocyte cell expressing KCNQ4 channels with a voltage step protocol as follows: the oocyte was clamped at −60 mV for 3 s, and the channel was activated by 2-s command steps from −100 mV to +60 mV in 10-mV increments, followed by a 1-s step to −30 mV. The calibration scale of all current traces is in the upper right corner of (A): 2 s and 1 μA. The calibration scale of the voltage step protocol is shown between (A) and (B): 2 s and 50 mV. (B) The expressed KCNQ4 current was almost completely blocked by the application of linopirdine (0.25 mmol/L) after 5 min. (C) This inhibitory effect of linopirdine on the KCNQ4 current was reversible by washout. (D) The summarized curves of tail current-voltage relationships are shown in the absence (•) and presence (□) of linopirdine. The control curves, with oocytes of RNase-free water injection (○) and without injection (△) are also shown for comparison. (E) Time course effect of linopirdine on KCNQ4 tail-current (•). Treatment with linopirdine and washout are indicated with arrows. The control curve (without chemical treatment; ○) showed little run-down during the 50-min recording. The time course of the tail-current amplitude was measured at a tail potential of −30 mV (from a step command of 30 mV).
Effect of PKC activator on the KCNQ4 current
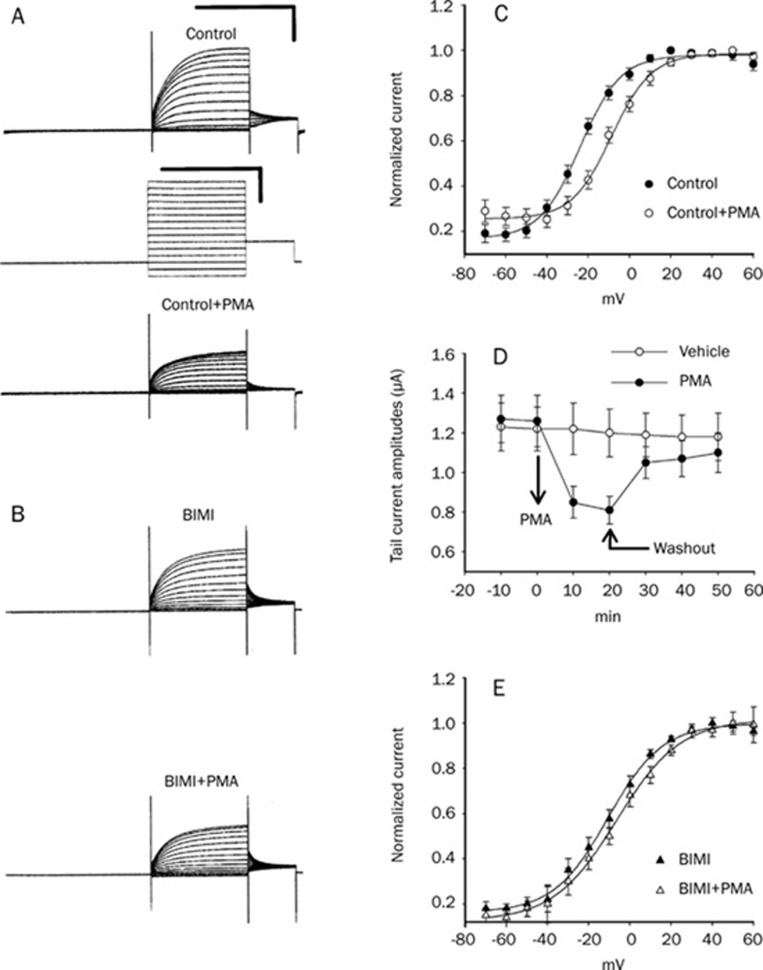
PKC is an important regulator of KCNQ channels, as shown in previous work9. To test whether activation of PKC leads to a functional change of the KCNQ4 current in a Xenopus oocyte model system, PMA (a PKC activator) and BIMI (a PKC inhibitor) were used in this study. Bath application of PMA (2 μmol/L) caused a significant inhibitory effect on the amplitude of the KCNQ4 currents (Figure 2A) and a shift in V1/2 (half-maximum of the conductance-voltage curve) to more positive potentials (Figure 2C). The V1/2 before and after PMA treatment are from −17.7±0.9 mV to −6.5±0.7 mV (P<0.05, n=15). The time course of the tail current amplitudes showed that the effect of PMA is reversible by washout (Figure 2D). An inactive form of PMA, α-PMA, was found to exert an insignificant effect on the V1/2 (-18.3±0.9 mV to −16.4±1.2 mV, n=3). Moreover, pretreatment with the PKC inhibitor BIMI (2 μmol/L) significantly attenuated the positive shift of V1/2 of KCNQ4 induced by PMA. There was no significant difference between the steady-state conductance-voltage curves of BIMI and BIMI plus PMA (Figure 2B and 2E). These results suggested that the effect of PMA on the KCNQ4 channel was through the activation of PKC.
Figure 2.
BIMI, a PKC inhibitor, can antagonize the effect of PMA on KCNQ4 channels. (A) The amplitudes of control KCNQ4 currents (upper traces in A) were inhibited by the application of PMA (2 μmol/L) (bottom traces in A). (B) The inhibitory effect of PMA on the KCNQ4 current was attenuated by pretreatment with BIMI (2 μmol/L) (upper traces: BIMI alone; bottom traces: BIMI+PMA). The voltage step protocol of (A) and (B) is as indicated in the middle of (A). Calibration scale of all current traces: 2 s and 1 μA. Calibration scale of the voltage step protocol: 2 s and 50 mV. (C) The midpoint potential of the conductance-voltage curve (V1/2) was shifted significantly to a more positive value after treatment with PMA (before PMA: • after PMA: ○). (D) Representative time courses of KCNQ4 tail-current during application of 2 μmol/L PMA (•) or vehicle (○). The effect of PMA was reversible by washout, as indicated by arrows. (E) The shift effect of V1/2 produced by PMA was attenuated by pretreatment with BIMI (BIMI alone: ▴ BIMI+PMA: △). V1/2 was obtained from the conductance-voltage curves, which were fitted using a two-state Boltzmann equation as described in the Materials and Methods.
Effect of phosphatase inhibitors on the KCNQ4 current
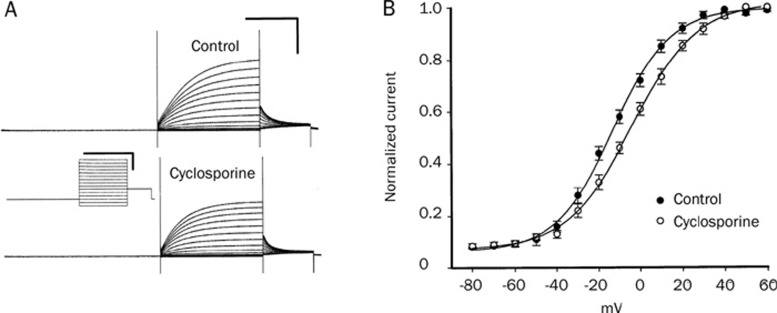
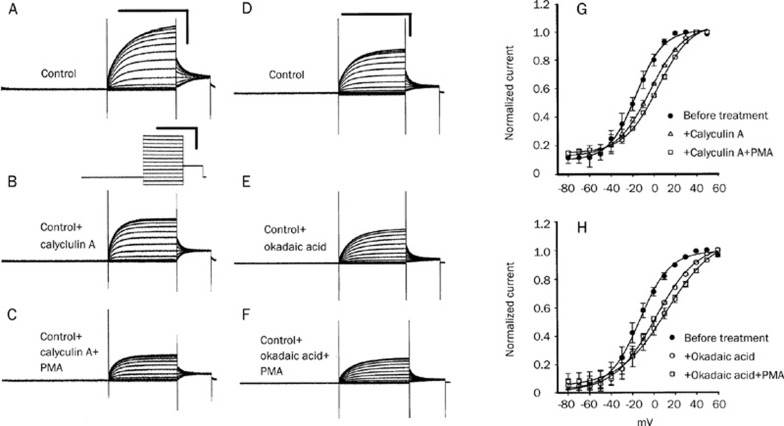
Since the inhibition of phosphatase would maintain a higher level of the phosphorylation of substrate proteins, the effect of ser/thr phosphatase inhibitors might increase the phosphorylation level of KCNQ4 channels. To test whether phosphatase inhibitors (cyclosporine, calyculin A, and okadaic acid) could cause changes in the electrophysiological properties of channels, we applied these phosphatase inhibitors to KCNQ4-expressed oocytes. Bath administration of cyclosporine (2 μmol/L) for 10 min produced a significant inhibitory effect on current amplitude (Figure 3A) and a positive shift of V1/2 (from −14.6±0.5 to −6.4±0.4 mV; n=5) (Figure 3B). Similar results were also obtained from the application of calyculin A (2 μmol/L) and okadaic acid (1 μmol/L). Typical current traces are shown in Figures 4A to C for calyculin A (A, before treatment; B, after calyculin A; C, Calyculin A+PMA) and in Figures 4D to F for okadaic acid (D, before treatment; E, after okadaic acid; F, okadaic acid+PMA). The peak tail current amplitudes of KCNQ4 (at −20 mV) were inhibited by calyculin A (from 2.05±0.08 μA decrease to 1.45±0.13 μA; P<0.05, n=5) and okadaic acid (from 1.64±0.05 μA decrease to 1.2±0.04 μA; P<0.05, n=4). The inhibition effects of calyculin A and okadaic acid were significantly enhanced by the subsequent addition of 2 μmol/L PMA (from 1.45±0.13 μA to 0.76±0.07 μA for calyculin A; from 1.2±0.04 μA to 0.80±0.04 μA for okadaic acid). Detailed information on the half-activation voltage (V1/2) can be obtained through the analysis of conductance-voltage (G–V) curves. The effects of calyculin A and okadaic acid on the conductance-voltage (G–V) curves of KCNQ4 are shown in Figure 4G and 4H, respectively. Both calyculin A and okadaic acid produced a shift in the voltage of half-maximal activation (V1/2) to a more positive potential (calyculin A: from −18.8±0.5 to −9.2±0.4 mV; P<0.05, n=5; okadaic acid: from −14.1±0.5 to −0.7±0.6 mV; P<0.05, n=4). Subsequently, the application of PMA after phosphatase inhibitor (calyculin A or okadaic acid) treatment induced a further positive shift in the V1/2 of KCNQ4 channels. In other words, the change in electrophysiological parameters induced by a combined treatment with PKC activator (PMA) and phosphatase inhibitor on KCNQ4 was greater than that of either alone.
Figure 3.
Effect of phosphatase inhibitor cyclosporine (2 μmol/L) on the KCNQ4 current expressed in Xenopus oocytes. (A) The control KCNQ4 currents (upper current traces) were significantly inhibited by the addition of cyclosporine (2 μmol/L; lower current traces). Calibration scale of current traces: 2 s and 1 μA (upper right corner). Calibration scale of voltage step protocol: 2 s and 50 mV (between two current traces). (B) The V1/2 was shifted significantly to a more positive value after the treatment with cyclosporine (control: • cyclosporine treatment: ○).
Figure 4.
Effects of phosphatase inhibitors (calyculin A and okadaic acid) and the combination of phosphatase inhibitor with PMA on the KCNQ4 currents. Typical recording traces of KCNQ4 are shown in figures (A)(F). The traces in Figures (A)–(C) and Figures (D)–(F) are recorded from the same oocyte, respectively. Tail current amplitudes of control KCNQ4 (A) were significantly inhibited by the addition of 2 μmol/L calyculin A (B) and, subsequently, addition of PMA (2 mol/L) caused a further decrease in the current amplitudes (C). The effect of okadaic acid (1 μmol/L) on the KCNQ4 current amplitudes was similar to that of calyculin A (D: before the treatment with okadaic acid; E: after the treatment with okadaic acid; F: okadaic acid plus 2 mol/L PMA). All static values of current amplitudes are shown in the results section. Calibration scales of all current traces are shown in the upper right corners of (A) and (D): 2 s and 2 μA. The voltage-step protocol is shown below (A). Figures (G) and (H) show conductance-voltage (G–V) curves of KCNQ4. Both calyculin A and okadaic acid can produce a positive shift of the half voltage of the GV curve (•: before the treatment in Figs G and H; △: after treatment with calyculin A in Figure G; ○: after treatment with okadaic acid in Figure H). Subsequently, the further addition of PMA after the phosphatase inhibitor can produce a more positive shift of V1/2 (□: calyculin A plus PMA and okadaic acid plus PMA in Figures G and H, respectively).
Discussion
In a previous study, Gamper et al12 found that the inhibition of tyrosine phosphatase reduces the conductance of KCNQ channels expressed in transfected Chinese hamster ovary cells. In the present study, we demonstrate that ser/thr phosphatase inhibitors can inhibit the conductance of KCNQ4 channels and shift the V1/2 (midpoint of conductance-voltage curve) to a more positive potential in a Xenopus oocyte expression system. This implies that the endogenous phosphatase is required for maintaining KCNQ4 channel activities in the Xenopus expression system. Moreover, the combination treatment of PMA (a PKC activator) and a phosphatase inhibitor showed a greater inhibitory effect than that of each alone, indicating that the most important inhibition in channel activity was due to the enhanced phosphorylation of KCNQ4. Thus, we propose that KCNQ4 channel activity is regulated by a balance between phosphorylation and dephosphorylation by the protein kinase and phosphatase.
PMA, a PKC activator, has been shown to produce a positive shift in the voltage dependence of KCNQ2 channels, while chelerythrine, a PKC inhibitor, attenuated the shift induced by muscarinic stimulation in the Xenopus oocytes expression system9. In results consistent with the previous studies, we also found that the effect of PMA on the KCNQ4 channels was antagonized by pretreatment with a PKC inhibitor, BIMI. Interestingly, PKA can reduce the effects of elevated Ca2+ on run-down of expressed KCNQ4 channels13. The different modulating effect by PKA and PKC may be due to the PKC phosphorylation sites that do not overlap with those phosphorylated by PKA.
A detailed characterization of the sites of phosphorylation of KCNQ2/KCNQ3 channels has been identified by mass spectrometry14. The phosphorylation sites are the S4-S5 intracellular loop and the domain for tetramerization in the C terminus15, 16. Taken together, these studies suggest that the cytoplasmic phosphorylation sites of KCNQ channels may play an important role in channel inhibition through the activation by PKC. However, it has been reported that the stimulation of the diacylglycerol-PKC pathway does not play an essential role in the acute modulation of the KCNQ channel in a mammalian expression system8. One possible explanation for the different results between Xenopus oocytes and mammalian expression systems is that a DAG-insensitive PKC (atypical PKC) exists in mammalian cells; another possibility is that the amount of active PKC or/and auxiliary protein differs depending on the intracellular environment. A recent study concerning the adaptor/auxiliary protein AKAP150 has found that it can interact simultaneously with both KCNQ2 and PKC, promoting the PKC-induced serine phosphorylation of KCNQ2 in a mammalian expression system7. A mutant form of AKAP150 that is unable to bind PKC attenuates the current inhibition by M1- receptor activation. These observations support the hypothesis that the auxiliary protein AKAP150 is required for PKC-induced inhibition of KCNQ channels by M1-receptor activation.
Another possible way to modulate the activity of KCNQ channels is with phosphatases. The dephosphorylation of phosphorylated KCNQ proteins may be due to an initial stimulation of the membrane phosphatases, which would be expected to antagonize the inhibitory effect induced by PKC stimulation on KCNQ channels. In this case, the inhibition of phosphatase activity accounts for the altered balance between kinases and phosphatases, which in turn probably contributes to increasing the phosphorylated state of KCNQ4. Phosphatase inhibitors would be expected to cause a positive shift in V1/2 potentials if the endogenous phosphatase activity is present in this model system. Among the phosphatases, protein phosphatases 1 and 2A are major ser/thr protein phosphatases involved in many cellular events, including regulation of the cell cycle of Xenopus laevis oocytes17, 18, 19. Cyclosporine, okadaic acid, and calyculin A are potent inhibitors of protein phosphatase. In this study, we show that treatment of Xenopus oocytes expressing human KCNQ4 with cyclosporine, okadaic acid, or calyculin A produced a positive shift of V1/2. These results indicate that endogenous phosphatase may modulate the human KCNQ4 channels expressed in Xenopus oocytes. A combination treatment of PMA plus phosphatase inhibitor produced a more positive shift of V1/2 than that of phosphatase inhibitor alone, indicating that the maintenance of a higher level of the phosphorylated proteins would result in a more dramatic positive shift in the V1/2 of KCNQ4 channels. Although our studies did not reveal the phosphorylation or dephosphorylation sites of KCNQ4, the inhibition of the dephosphorylation of phosphorylated proteins by phosphatase inhibitors did cause a significant change in the channel properties of KCNQ4. In summary, we demonstrated that phosphatase inhibitors induce a positive shift of the activation curve and an inhibition of channel conductance in human KCNQ4 expressed in Xenopus oocytes. Phosphatase may interact with substrates such as KCNQ4 or other auxiliary proteins to balance the phosphorylation level induced by protein kinase and thus play an important role in the regulation of KCNQ4 channel activity in a Xenopus oocyte expression system.
Author contribution
Tzu-rong SU performed research. Cay-huyen CHEN analyzed data and preparations. Shih-jen HUANG analyzed data and reagents. Chun-yi LEE did part of preparations. Mao-chang SU performed TEVC recording. Gwan-hong CHEN performed oocytes isolation and TEVC recording. Shuan-yow LI, Jiannjou YANG wrote part of introduction. Min-jon LIN wrote the paper and performed part of research.
Acknowledgments
Prof Thomas J JENTSCH is acknowledged for kindly supplying human KCNQ4 cDNA. These studies were supported by grants from the National Science Council, Taiwan (NSC 96-2320-B-040-013), CSMU-TSMH-097-007, and TCRD-TPE-95-39.
References
- Kharkovets T, Hardelin JP, Safieddine S, Schweizer M, El-Amraoui A, Petit C, et al. KCNQ4, a K+ channel mutated in a form of dominant deafness, is expressed in the inner ear and the central auditory pathway. Proc Natl Acad Sci USA. 2000;97:43338. doi: 10.1073/pnas.97.8.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubisch C, Schroeder BC, Friedrich T, Lutjohann B, El-Amraoui A, Marlin S, et al. KCNQ4, a novel potassium channel expressed in sensory outer hair cells is, mutated in dominant deafness. Cell. 1999;96:437–46. doi: 10.1016/s0092-8674(00)80556-5. [DOI] [PubMed] [Google Scholar]
- Gutman GA, Chandy KG, Adelman JP, Aiyar J, Bayliss DA, Clapham DE, et al. International Union of Pharmacology. XLI. Compendium of voltage-gated ion channels: potassium channels. Pharmacol Rev. 2003;55:583–6. doi: 10.1124/pr.55.4.9. [DOI] [PubMed] [Google Scholar]
- Marrion NV. Control of M-current. Annu Rev Physiol. 1997;59:483–504. doi: 10.1146/annurev.physiol.59.1.483. [DOI] [PubMed] [Google Scholar]
- Loussouarn G, Park KH, Bellocq C, Baro I, Charpentier F, Escande D. Phosphatidylinositol-4,5-bisphosphate, PIP2, controls KCNQ1/KCNE1 voltage-gated potassium channels: a functional homology between voltage-gated and inward rectifier K+ channels. EMBO J. 2003;22:541221. doi: 10.1093/emboj/cdg526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Craciun LC, Mirshahi T, Rohacs T, Lopes CM, Jin T, et al. PIP2 activates KCNQ channels, and its hydrolysis underlies receptor-mediated inhibition of M currents. Neuron. 2003;37:963–75. doi: 10.1016/s0896-6273(03)00125-9. [DOI] [PubMed] [Google Scholar]
- Hoshi N, Zhang JS, Omaki M, Takeuchi T, Yokoyama S, Wanaverbecq N, et al. AKAP150 signaling complex promotes suppression of the M-current by muscarinic agonists. Nat Neurosci. 2003;6:564571. doi: 10.1038/nn1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh BC, Hille B. Does diacylglycerol regulate KCNQ channels. Pflugers Archive Eur J Physiol. 2006;453:293–301. doi: 10.1007/s00424-006-0092-3. [DOI] [PubMed] [Google Scholar]
- Nakajo K, Kubo Y. Protein kinase C shifts the voltage dependence of KCNQ/M channels expressed in Xenopus oocytes. J Physiol. 2005;569:59–74. doi: 10.1113/jphysiol.2005.094995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas P, Brown DA. Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat Rev Neurosci. 2005;6:85062. doi: 10.1038/nrn1785. [DOI] [PubMed] [Google Scholar]
- Su CC, Li SY, Yang JJ, Su MC, Lin MJ. Studies of the effect of ionomycin on the KCNQ4 channel expressed in Xenopus oocytes. Biochem Biophys Res Commun. 2006;348:295300. doi: 10.1016/j.bbrc.2006.07.053. [DOI] [PubMed] [Google Scholar]
- Gamper N, Stockland JD, Shapiro MS. Subunit-specific modulation of KCNQ potassium channels by Src tyrosine kinase. J Neurosci. 2003;23:84–95. doi: 10.1523/JNEUROSCI.23-01-00084.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambard JM, Ashmore JF. Regulation of the voltage-gated potassium channel KCNQ4 in the auditory pathway. Pfluger Arch Eur J Physiol. 2005;450:34–44. doi: 10.1007/s00424-004-1366-2. [DOI] [PubMed] [Google Scholar]
- Surti TS, Huang L, Jan YN, Jan LY, Cooper EC. Identification by mass spectrometry and functional characterization of two phosphorylation sites of KCNQ2/KCNQ3 channels. Proc Natl Acad Sci USA. 2005;102:17828–33. doi: 10.1073/pnas.0509122102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwake M, Jentsch TJ, Friedrich T. A carboxy-terminal domain determines the subunit specificity of KCNQ K+ channel assembly. EMBO Rep. 2003;4:76–81. doi: 10.1038/sj.embor.embor715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard RJ, Clark KA, Holton JM, Minor DL Jr. Structural insight into KCNQ (Kv7) channel assembly and channelopathy. Neuron. 2007;53:66375. doi: 10.1016/j.neuron.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DH, DePaoli-Roach AA, Maller JL. Multiple roles for protein phosphatase 1 in regulating the Xenopus early embryonic cell cycle. Mol Biol Cell. 1992;3:68798. doi: 10.1091/mbc.3.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TH. The role of protein phosphatase type-2A in the Xenopus cell cycle: initiation of the G2/M transition. Semin Cancer Biol. 1995;6:2039. doi: 10.1006/scbi.1995.0027. [DOI] [PubMed] [Google Scholar]
- Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signaling. Biochem J. 2001;353:41739. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]