Abstract
Background and Purpose
The DEFUSE 2 study has shown that the clinical response to endovascular reperfusion differs between patients with and without perfusion-diffusion (PWI-DWI) mismatch: patients with mismatch have a favorable clinical response to reperfusion whereas patients without mismatch do not. This study examined if alternative mismatch criteria can also differentiate patients according to their response to reperfusion.
Methods
Patients from the DEFUSE 2 study were categorized according to vessel occlusion on MRA and DWI lesion volume criteria (MRA-DWI mismatch) and symptom severity and DWI criteria (clinical-DWI mismatch). Favorable clinical response was defined as an improvement of ≥8 points on the National Institutes of Health Stroke Scale (NIHSS) by day 30 or an NIHSS score of ≤1 at day 30. We assessed, for each set of criteria, if the association between reperfusion and favorable clinical response differed according to mismatch status.
Results
A differential response to reperfusion was observed between patients with and without MRA-DWI mismatch defined as an ICA or M1 occlusion and a DWI lesion <50 mL. Reperfusion was associated with good functional outcome in patients who met these MRA-DWI mismatch criteria (OR 8.5; 95% CI 2.3–31.3), whereas no association was observed in patients who did not meet these criteria (OR 0.5; 95% CI 0.08–3.1) (p for difference between the odds = 0.01). No differential response to reperfusion was observed with other variations of the MRA-DWI or clinical-DWI mismatch criteria.
Conclusions
The MRA-DWI mismatch is a promising alternative to DEFUSE 2's PWI-DWI mismatch for patient selection in endovascular stroke trials.
INTRODUCTION
The goal of acute stroke therapy is to restore perfusion to the ischemic penumbra.1, 2 Without timely reperfusion, the penumbra undergoes irreversible injury.2 The rate at which this occurs is variable.3–5 In some patients, the neuronal loss occurs rapidly whereas in others the penumbra survives for hours.3–5 Because of this variability, symptom duration does not closely correlate with the presence of penumbral tissue.5 An assessment of penumbral presence could be used to identify patients who are outside the established time-window for acute stroke treatment but may still benefit from reperfusion therapy.6
Various imaging methods exist to assess the presence of penumbral tissue. The MRI perfusion-diffusion (PWI-DWI) mismatch is one such method.7 It is promising for patient selection based on results of cohort studies, which showed an association between reperfusion and good outcome in patients with a substantial PWI-DWI mismatch, but not in patients without a PWI-DWI mismatch.8–10 However, the few randomized-controlled stroke trials that selected patients based on PWI-DWI mismatch have been negative.11–13 The MRA-DWI mismatch and the clinical-diffusion mismatch are two alternative selection strategies.14, 15 The MRA-DWI mismatch model uses the presence of a vessel occlusion as a surrogate for the volume of the perfusion lesion.11 The clinical-DWI mismatch model uses the severity of a patient’s stroke symptoms as a surrogate for the volume of the perfusion lesion.12 Contemporary clinical trials such as SWIFT-PRIME often use combinations of clinical, angiographic, perfusion, and diffusion criteria for patient selection.16
The aim of this post-hoc analysis of the DEFUSE 2 study is to determine the optimal strategy to select patients for acute stroke trials. We compared the PWI-DWI mismatch model to a variety of alternative mismatch models and assessed which mismatch model most effectively differentiates patients who have a favorable clinical response with reperfusion from patients who do not have a favorable clinical response with reperfusion.
METHODS
Patients
The eligibility criteria for the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution 2 (DEFUSE 2) study were intention to start endovascular stroke therapy within 12 hours of symptom onset, age ≥18 years, baseline NIHSS of ≥5, non-pregnant state, mRS ≤2, and no contraindication for MRI.10 All patients underwent serial MR imaging before and after the endovascular procedure.10 The baseline MRI (GRE, MRA, DWI and PWI sequences) was obtained within 90 minutes prior to the start of the endovascular procedure, the early follow-up scan (GRE, MRA, DWI and PWI) within 12 hours of the end of the endovascular procedure, and the late follow-up MRI (GRE, DWI and FLAIR) on day 5 or at discharge from the hospital, whichever occurred first.10 Patients enrolled in the DEFUSE 2 study were eligible for this sub-study if alternative mismatch status (MRA-DWI mismatch and clinical DWI mismatch) and reperfusion status could be determined.10
Definitions
PWI-DWI Mismatch criteria
In the DEFUSE 2 study, RAPID software was used to post-process the perfusion and diffusion data.10 Segmentation of the DWI lesion was based on an apparent diffusion coefficient (ADC) threshold of <600 s/mm2 and the PWI lesion was segmented based on a Tmax threshold of >6 s.10, 17–19 The PWI-DWI mismatch was defined as a PWI-DWI ratio ≥1.8, an absolute difference between the PWI and DWI lesions of ≥15 mL, an ischemic core volume <70 mL, and <100 mL of tissue with a severe bolus delay (Tmax>10 s). This combination of criteria was termed the Target Mismatch Profile.10
Alternative Mismatch Criteria
MRA-DWI mismatch was primarily defined according to previously reported criteria: an occlusion of the internal carotid artery or the first segment of the middle cerebral artery (M1 segment) and a DWI lesion volume <25 ml; an M2 occlusion with a DWI lesion volume <15 ml; or a non-occlusive narrowing of any intracranial vessel with a DWI lesion volume <15 ml.11 We also examined alternative definitions of the MRA-DWI mismatch.11 The clinical-DWI mismatch was defined according to previously proposed criteria: NIHSS ≥8 and DWI lesion volume <25 ml.12
Reperfusion and Clinical Outcome
Definitions for reperfusion and favorable clinical response (FCR) were adopted from the parent study.10 Reperfusion was defined as a >50% reduction in the volume of the PWI (Tmax >6s) lesion between the baseline and the early follow-up MRI. If the follow-up PWI was not obtained or if it was of insufficient quality, reperfusion was assessed based on dual plane digital subtraction angiography and defined as restoration of blood flow at the completion of the angiographic procedure in >50% of the territory (i.e. TICI 2B) that showed impaired perfusion on the first angiographic run.10 The primary outcome measure was favorable clinical response (FCR) defined as an improvement on the NIHSS of 8 points or more between day 0 and day 30, or an NIHSS score of ≤1 at day 30.
Statistical Analyses
The association between reperfusion and favorable clinical response was compared between patients with and without mismatch. Adjusted odds ratios for favorable clinical response with reperfusion were calculated using multivariate logistic regression models. Variables that were associated with favorable clinical response at an alpha of 0.1 in univariable analyses were included in the multivariable model. Variables that were significant at an alpha of 0.1 in the multivariable analysis were retained in the model. We evaluated the difference in the response to reperfusion between patients with and without mismatch using regression models that included the significant covariates and the interaction between reperfusion and mismatch status as independent variables: covariate I, where C is a constant, R is the presence of reperfusion, and M is the presence of mismatch. All analyses were conducted with SAS 9.3 and Stats Direct (UK).
RESULTS
MRA-DWI mismatch criteria
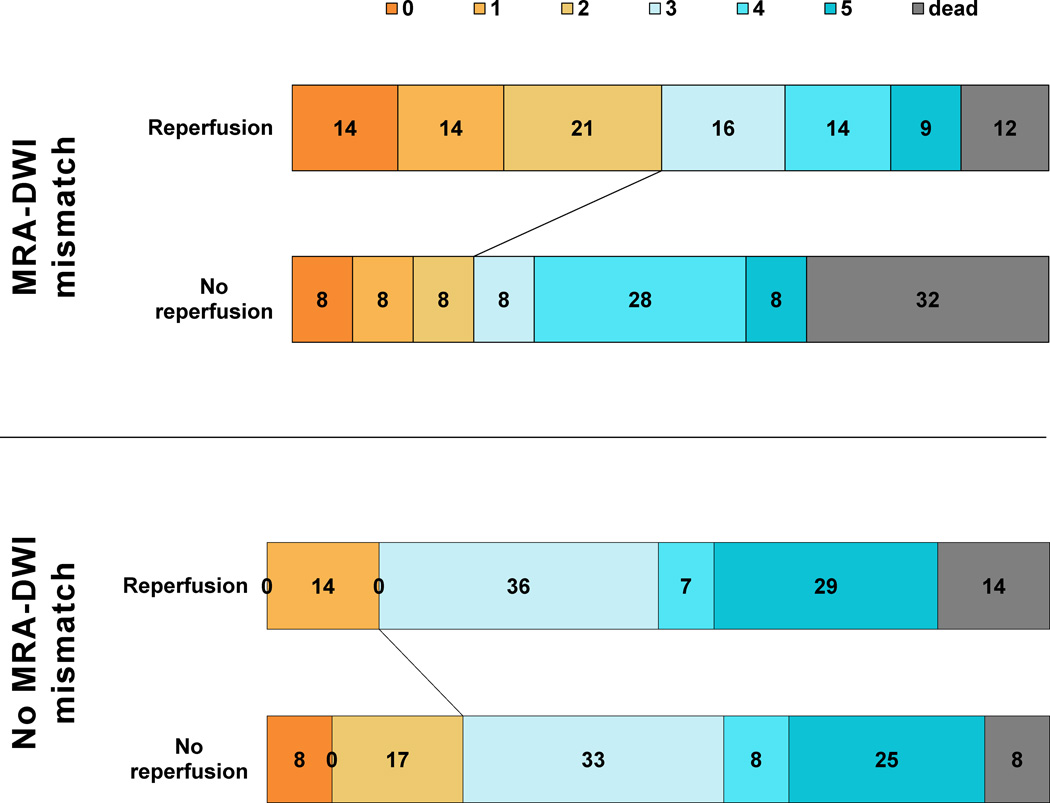
Data on PWI-DWI mismatch status were available for 99 patients from the DEFUSE 2 cohort. Five of these patients did not have an MRA or their MRA was of insufficient quality to assess vessel status. Table 1 shows the baseline characteristics of the 94 patients who had sufficient quality MRAs to assess MRA-DWI mismatch status. 58 (61.7%) of the 94 patients met MRA-DWI mismatch criteria compared to 76 (80.9%) who met PWI-DWI criteria. Agreement between the MRA-DWI and PWI-DWI mismatch models was fair (κ=0.35, 95%CI 0.17–0.54). Twenty-two out of seventy-six patients with a PWI-DWI mismatch did not meet MRA-DWI mismatch criteria. The reperfused (N=14) and non-reperfused (n=8) patients in this subgroup had similar baseline characteristics. The odds ratio for favorable clinical response with reperfusion in this subgroup was 6.8 (95%CI 0.7–70.1).There was no differential response to reperfusion in patients categorized according to the primary MRA-DWI mismatch model (p=0.5). (Table 2) Among the alternative MRA-DWI mismatch models tested (Table 2), only the model with MRA-DWI mismatch defined as an ICA or MCA-M1 occlusion and DWI volume <50 ml (version 3; figure 1), had a differential response to reperfusion (P=0.01). For this model, the agreement with the PWI-DWI mismatch model was good (κ=0.68, 95%CI 0.50–0.86).
Table 1.
Baseline characteristics and 30-day outcome according to mismatch status
| MRA-DWI Mismatch (n=58) | No MRA-DWI Mismatch (n=36) | Clinical-DWI Mismatch (n=60) | No Clinical-DWI Mismatch (n=39) | |||||
|---|---|---|---|---|---|---|---|---|
| Reperfusion | No Reperfusion | Reperfusion | No Reperfusion | Reperfusion | No Reperfusion | Reperfusion | No Reperfusion | |
| Baseline characteristics | ||||||||
| N | 35 | 23 | 22 | 14 | 34 | 26 | 24 | 15 |
| Age, y, mean (SD) | 66.1(16.3) | 66.1(18.5) | 62.7(14.1) | 62.6(13.0) | 66.6(16.2) | 67.3(18.2) | 62.2(13.9) | 64.5(13.1) |
| Men sex, no. (%) | 14(40%) | 13(56.5%) | 13(59.1%) | 8(57.1%) | 12(35.3%) | 14(53.8%) | 15(62.5%) | 9(60%) |
| Caucasian race, no. (%) | 34(97.1%) | 22(95.7%) | 20(90.9%) | 12(85.7%) | 32(94.1%) | 25(96.2%) | 23(95.8%) | 13(86.7%) |
| NIHSS,median (IQR) | 13(9–17) | 15(11–18) | 19(14–21) | 18(14–20) | 14.5(11–20) | 15(12–18) | 18.5(7.5–20.5) | 18(14–21) |
| mRS, median (IQR) | 0 (0-0) | 0(0–1) | 0(0-0) | 0(0-0) | 0(0-0) | 0(0–1) | 0(0-0) | 0(0-0) |
| SBP, mm Hg, mean (SD) | 143.4(24.7) | 149.5(26.5) | 147.1(24.4) | 147.8(11.5) | 145.1(23.6) | 145.4(23.0) | 145.8(26.5) | 154.6(19.1) |
| DBP, mm Hg, mean (SD) | 79.7(16.8) | 75.3(23.6) | 83.4(21.1) | 82.7(10.9) | 80.4(17.1) | 75(23) | 82.9(20.7) | 84.2(11.2) |
| WBC, ×1000/µl, mean (SD) | 9.2(2.9) | 9.0(2.6) | 8.1(3.1) | 9.7(4.6) | 9.3(3.1) | 9.1(2.8) | 8.3(2.7) | 9.4(4.5) |
| Platelets, ×1000/µl, mean (SD) | 229.7(58.7) | 214.7(74.0) | 234.5(82) | 211.1(54) | 224.1(59.6) | 217.7(67.9) | 245.8(78.6) | 203.1(56.6) |
| Glucose, mg/100ml, mean (SD) | 119.2(25.8)† | 148.3(69.3)† | 146.2(58) | 128.5(37.6) | 121.6(26) | 143.7(63.5) | 140.2(57.6) | 138.3(48.9) |
| Myocardial Infarction, no. (%) | 4(11.4%) | 2(8.7%) | 1(4.5%) | 1(7.7%) | 4(11.8%) | 2(7.7%) | 1(4.2%) | 1(6.7%) |
| Heart Failure, no. (%) | 3(8.6%) | 1(4.3%) | 3(13.6%) | 0(0%) | 2(5.9%) | 2(7.7%) | 4(16.7%) | 1(6.7%) |
| Atrial Fibrillation, no. (%) | 11(31.4%) | 7(30.4%) | 10(45.5%) | 4(30.8%) | 13(38.2%) | 9(34.6%) | 8(33.3%) | 4(26.7%) |
| Previous CABG, no. (%) | 2(5.7%) | 0(0%) | 2(9.1%) | 0(0%) | 2(5.9%) | 0(0%) | 2(8.3%) | 1(6.7%) |
| Hypercholesterolemia, no. (%) | 18(51.4%) | 14(60.9%) | 6(27.3%)‡ | 8(61.5%)‡ | 16(47.1%) | 15(57.7%) | 9(37.5%) | 10(66.7%) |
| Diabetes Mellitus, no. (%) | 4(11.4%) | 5(21.7%) | 6(27.3%) | 2(15.4%) | 4(11.8%) | 6(23.1%) | 6(25%) | 2(13.3%) |
| Hypertension, no. (%) | 22(62.9%) | 18(78.3%) | 13(59.1%) | 9(69.2%) | 22(64.7%) | 20(76.9%) | 1(58.3%) | 11(73.3%) |
| Smoker, no. (%) | 14/34(41.2%) | 10/21(47.6%) | 11(50%) | 10(71.4%) | 14(41.2%) | 10(38.5%) | 12(50%) | 10(66.7%) |
| Prior Stroke, no. (%) | 3(8.6%) | 6(26.1%) | 4(18.2%) | 1(8.6%) | 4(11.8%) | 5(19.2%) | 3(12.5%) | 2(11.8%) |
| Prior TIA, no. (%) | 3(8.6%) | 3(13.0%) | 0(0%) | 0(0%) | 3(8.8%) | 4(15.4%) | 1(4.2%) | 1(6.7%) |
| 30- day outcome | ||||||||
| mRS, median (IQR) | 2(1–4) | 4(2–6) | 3(3–5) | 4(3–5) | 2(1–4) | 4(2–6) | 2(1–4) | 4(2–6) |
| NIHSS, median (IQR) | 2(1–10) | 13(2–42) | 7.5 (4–21) | 8.5(6–19) | 2(1–12) | 10(2–42) | 2(1–12) | 10(2–42) |
Absolute numbers are followed by percentage in brackets, mean values are followed by standard deviations, and median values by inter quartile ranges.
The MRA-DWI mismatch patients who reperfused had lower blood glucose levels (p=0.03).
Patients without MRA-DWI mismatch who did not reperfuse more often had a history of hypercholesterolemia (p=0.05).
mRS indicates modified Rankin scale score; SBP, systolic blood pressure; DBP, diastolic blood pressure; CABG, coronary artery bypass grafting; WBC, white blood cells; NIHSS, National Institutes of Health Stroke Scale; and TIA, transient ischemic attack.
Table 2.
Association between reperfusion and favorable clinical response according to mismatch status
| Selection Criteria | Penumbral Criteria | Core Criteria | Mismatch Absent | Mismatch Present | |||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | n | OR | 95%CI | n | P* | |||
| DEFUSE 2 PWI-DWI mismatch criteria | |||||||||
| PWI-DWI | PWI/DWI ≥1.8 and PWI-DWI ≥15 ml | DWI <70 ml | 7.1 | 2.0 – 25.0 | 76 | 0.4 | 0.02 – 5.7 | 18 | 0.04 |
| MRA-DWI mismatch criteria | |||||||||
| MRA-DWI v1 | ICA or M1 occlusion | DWI <25 ml | 7.6 | 1.4 – 39.7 | 58 | 1.9 | 0.4 – 8.5 | 36 | 0.3 |
| ICA or M1 reduced flow or M2, ACA, or PCA occlusion | DWI <15 ml | ||||||||
| MRA-DWI v2 | ICA or M1 occlusion | DWI <25 ml | 12.9 | 1.9 – 88.8 | 50 | 1.6 | 0.4 – 6.0 | 44 | 0.16 |
| MRA-DWI v3 | ICA or M1 occlusion | DWI <50 ml | 8.5 | 2.3 – 31.3 | 68 | 0.5 | 0.1 – 3.0 | 26 | 0.01 |
| MRA-DWI v4 | ICA or M1 occlusion | none | 4.6 | 1.5 – 14.1 | 80 | 2.2 | 0.05 – 88.9 | 14 | 0.3 |
| Clinical-DWI mismatch criteria | |||||||||
| Clinical-DWI | NIHSS ≥ 8 | DWI < 25ml | 4.3 | 1.1 – 17.3 | 60 | 3 | 0.7 – 12.4 | 39 | 0.9 |
p-value for the interaction term Reperfusion × Mismatch and Odds Ratio (OR) adjusted for age, natural log of DWI, and prior stroke.
Figure 1.
Distribution of 30-day modified Rankin Scale scores amongst patients with and without MRA-DWI mismatch defined as ICA or M1 occlusion and DWI volume <50 ml (v3).
Clinical-DWI mismatch criteria
The correlation between PWI lesion volumes (Tmax >6 s) and the NIHSS scores was fair (r2= 0.18, 95%CI 0.06–0.32, p<0.0001). Data on clinical-DWI mismatch status were available for all 99 patients from the DEFUSE 2 cohort. Table 1 lists the baseline characteristics for patients with and without the clinical-DWI mismatch. Sixty (61%) of the 99 patients met clinical-DWI mismatch criteria compared to 81 (82%) who met PWI-DWI criteria. Agreement between the clinical-DWI and PWI-DWI mismatch models was fair (kappa=0.23, 95%CI 0.05–0.41). Twenty-seven out of the eighty-one patients with PWI-DWI mismatch did not meet the clinical-DWI mismatch criteria; twenty patients did not have a DWI volume <25, four did not have an NIHSS ≥8, and three had neither. For patients, who met PWI-DWI mismatch criteria but did not meet clinical-DWI mismatch criteria, the odds ratio for favorable clinical response with reperfusion was 9.6 (95%CI 1.2–77.6). There was no differential response to reperfusion in patients categorized according to the clinical-DWI mismatch model (p for the reperfusion × mismatch interaction term = 0.9). (Table 2)
DISCUSSION
This study suggests that the MRA-DWI mismatch, defined as the presence of an ICA or MCA-M1 occlusion and a DWI lesion volume <50 ml at baseline, differentiates patients according to their response to reperfusion. Differentiating patients based on these MRA-DWI mismatch criteria appears to be comparable to differentiating patients based on PWI-DWI mismatch criteria.10, 20 Other MRA-DWI mismatch criteria and Clinical-DWI mismatch criteria did not differentiate patients according to their response to reperfusion.
The failure of the Clinical-DWI mismatch model to differentiate patients according to their response to reperfusion is explained by the limited agreement in patient selection between this model and the PWI-DWI mismatch criteria from the DEFUSE 2 study. One-third of the patients who met these PWI-DWI mismatch criteria did not meet the Clinical-DWI mismatch criteria; these patients had an increased likelihood of favorable clinical response with reperfusion (OR 9.6) thus obscuring a differential response to reperfusion between patients with and without Clinical-DWI mismatch. For similar reasons the primary MRA-DWI mismatch criteria did not differentiate patients according to their response to reperfusion.
We explored alternatives to the primary MRA-DWI mismatch criteria. Selection based on stricter angiographic criteria that limit inclusion to patients with ICA or M1 occlusions (Table 2) improves the specificity for identifying patients in whom reperfusion is associated with a favorable clinical response. For example, patients who meet strict MRA-DWI mismatch criteria (i.e. ICA or M1 occlusions and a DWI volume <25mL) show a very strong association between reperfusion and favorable clinical outcome (OR 12.9). Randomized controlled trials that use such strict MRA-DWI criteria to select patients are thus most likely to demonstrate benefit of acute stroke treatments aimed at restoring perfusion. However, this comes at a cost of reducing the proportion of patients with mismatch from approximately 80% with the PWI-DWI criteria from the DEFUSE 2 study to 50% with strict MRA-DWI criteria. This leads to the exclusion of some patients who may benefit from reperfusion such as patients with DWI lesion volumes in the 25–50 ml range.
Less strict MRA-DWI mismatch selection criteria, defined as the presence of an ICA or MCA-M1 branch occlusion and a DWI lesion volume <50 ml select approximately 75% of the population, agree more closely with selection based on the PWI-DWI mismatch criteria from the DEFUSE 2 study, and were associated with a differential response to reperfusion. Given the good agreement between this MRA-DWI mismatch model and the PWI-DWI mismatch criteria from DEFUSE 2, and given the previously reported DEFUSE 2 results showing a differential response to reperfusion according to PWI-DWI mismatch criteria, it is not surprising that these MRA-DWI mismatch criteria are also associated with a differential response to reperfusion. These MRA-DWI mismatch criteria therefore present a suitable alternative to the PWI-DWI mismatch.
The post-hoc nature of the analyses is a limitation of this study. The results therefore require validation in independent cohorts. Another limitation is that despite the positive association between reperfusion and favorable outcome in patients with mismatch shown in this study and in previous cohort studies,10, 21 patient selection based on perfusion imaging is not supported by results from randomized controlled trials (RCTs). DIAS II, an RCT of intravenous desmoteplase, selected patients based on a visual, qualitative assessment of mismatch on CTP or PWI-DWI maps and failed to show a benefit from treatment in this population.12 Recently, the MR Rescue trial failed to show a benefit from endovascular therapy in patients with a “penumbral profile” based on MR or CT perfusion imaging.11 Several factors may have contributed to the negative results of these studies. This includes the introduction of heterogeneity in the patient population because both CT perfusion and MRI-based criteria were used, limited effectiveness of the intervention, and inclusion of patients who may not benefit from reperfusion. Patients who do not benefit from reperfusion may include those with mild deficits due to distal MCA branch occlusions22, patients with large stroke cores who have a poor prognosis regardless of treatment23, and patients who have no or little penumbral tissue because the volume of critically hypoperfused tissue is overestimated.24 Ultimate proof of the utility of image-based patient selection therefore will need to come from randomized controlled trials that demonstrate a benefit of treatment in patients with mismatch and no benefit in patients without mismatch.
In conclusion, the response to reperfusion varies markedly among patients. The PWI-DWI mismatch criteria of the DEFUSE 2 study and MRA-DWI mismatch criteria that require an ICA or MCA-M1 occlusion and a DWI lesion volume under 50 ml show good agreement in terms of patient selection and perform optimally in terms of differentiating patients according to their response to reperfusion. Randomized controlled trials that use these criteria to select patients are therefore warranted.
Acknowledgments
Sources of Funding:
The work was supported by the National Institute for Neurological Disorders and Stroke (R01 NS03932505 and R01 NS075209)
Footnotes
Contributions:
Nishant K Mishra (NKM): study design, analyses, and drafting of the manuscript.
Gregory W. Albers (GAW): study design, supervision, data acquisition, editing, and funding.
Soren Christensen (SC): data analysis, image analyses, supervision, and editing.
Michael Marks (MM): data acquisition.
Scott Hamilton (SH): data acquisition and analysis.
Matus Straka (MS): data acquisition and image analysis.
John Liggins (JL): writing.
Stephanie Kemp (SK): data acquisition and supervision.
Michael Mlynash (MM): data analysis, image analyses, and supervision.
Roland Bammer (RB): image analysis and supervision.
Maarten Lansberg (ML): study design, drafted manuscript, supervision, and funding.
Disclosures:
NKM -- None
GAW -- IschemaView: equity interest; Advisory Board: Covidien, Codman, and Lundbeck
SC -- Consultant for IschemaView Inc and Toshiba
MM -- None
MS -- Consultant for IschemaView Inc
JL -- None
SK -- None
M Mlynash -- none
RB -- Owner/stockholder of iSchemaView, Inc
ML -- None
References
- 1.Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, et al. Time to treatment with intravenous alteplase and outcome in stroke: An updated pooled analysis of ecass, atlantis, ninds, and epithet trials. Lancet. 2010;375:1695–1703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- 2.Brott T, Bogousslavsky J. Treatment of acute ischemic stroke. The New England journal of medicine. 2000;343:710–722. doi: 10.1056/NEJM200009073431007. [DOI] [PubMed] [Google Scholar]
- 3.Hakimelahi R, Gonzalez RG. Neuroimaging of ischemic stroke with ct and mri: Advancing towards physiology-based diagnosis and therapy. Expert review of cardiovascular therapy. 2009;7:29–48. doi: 10.1586/14779072.7.1.29. [DOI] [PubMed] [Google Scholar]
- 4.Abou-Chebl A. Endovascular treatment of acute ischemic stroke may be safely performed with no time window limit in appropriately selected patients. Stroke. 2010;41:1996–2000. doi: 10.1161/STROKEAHA.110.578997. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez RG. Clinical mri of acute ischemic stroke. J Magn Reson Imaging. 2012;36:259–271. doi: 10.1002/jmri.23595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mishra NK, Albers GW, Davis SM, Donnan GA, Furlan AJ, Hacke W, et al. Mismatch-based delayed thrombolysis: A meta-analysis. Stroke. 2010;41:e25–e33. doi: 10.1161/STROKEAHA.109.566869. [DOI] [PubMed] [Google Scholar]
- 7.Albers GW. Expanding the window for thrombolytic therapy in acute stroke. The potential role of acute mri for patient selection. Stroke. 1999;30:2230–2237. doi: 10.1161/01.str.30.10.2230. [DOI] [PubMed] [Google Scholar]
- 8.Furlan AJ, Eyding D, Albers GW, Al-Rawi Y, Lees KR, Rowley HA, et al. Dose escalation of desmoteplase for acute ischemic stroke (dedas): Evidence of safety and efficacy 3 to 9 hours after stroke onset. Stroke. 2006;37:1227–1231. doi: 10.1161/01.STR.0000217403.66996.6d. [DOI] [PubMed] [Google Scholar]
- 9.Hacke W, Albers G, Al-Rawi Y, Bogousslavsky J, Davalos A, Eliasziw M, et al. The desmoteplase in acute ischemic stroke trial (dias): A phase ii mri-based 9-hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke. 2005;36:66–73. doi: 10.1161/01.STR.0000149938.08731.2c. [DOI] [PubMed] [Google Scholar]
- 10.Lansberg MG, Straka M, Kemp S, Mlynash M, Wechsler LR, Jovin TG, et al. Mri profile and response to endovascular reperfusion after stroke (defuse 2): A prospective cohort study. Lancet Neurol. 2012;11:860–867. doi: 10.1016/S1474-4422(12)70203-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kidwell CS, Jahan R, Gornbein J, Alger JR, Nenov V, Ajani Z, et al. A trial of imaging selection and endovascular treatment for ischemic stroke. The New England journal of medicine. 2013;368:914–923. doi: 10.1056/NEJMoa1212793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hacke W, Furlan AJ, Al-Rawi Y, Davalos A, Fiebach JB, Gruber F, et al. Intravenous desmoteplase in patients with acute ischaemic stroke selected by mri perfusion-diffusion weighted imaging or perfusion ct (dias-2): A prospective, randomised, double-blind, placebo-controlled study. Lancet Neurol. 2009;8:141–150. doi: 10.1016/S1474-4422(08)70267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis SM, Donnan GA, Parsons MW, Levi C, Butcher KS, Peeters A, et al. Effects of alteplase beyond 3 h after stroke in the echoplanar imaging thrombolytic evaluation trial (epithet): A placebo-controlled randomised trial. Lancet Neurol. 2008;7:299–309. doi: 10.1016/S1474-4422(08)70044-9. [DOI] [PubMed] [Google Scholar]
- 14.Lansberg MG, Thijs VN, Bammer R, Olivot JM, Marks MP, Wechsler LR, et al. The mra-dwi mismatch identifies patients with stroke who are likely to benefit from reperfusion. Stroke. 2008;39:2491–2496. doi: 10.1161/STROKEAHA.107.508572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davalos A, Blanco M, Pedraza S, Leira R, Castellanos M, Pumar JM, et al. The clinical-dwi mismatch: A new diagnostic approach to the brain tissue at risk of infarction. Neurology. 2004;62:2187–2192. doi: 10.1212/01.wnl.0000130570.41127.ea. [DOI] [PubMed] [Google Scholar]
- 16.Solitaire™ FR as Primary Treatment for Acute Ischemic Stroke (SWIFT PRIME). the U.S. National Institutes of Health ClinicalTrials.gov. [accessed on January 9 2014]; website http://clinicaltrials.gov/show/NCT01657461.
- 17.Straka M, Albers GW, Bammer R. Real-time diffusion-perfusion mismatch analysis in acute stroke. J Magn Reson Imaging. 2010;32:1024–1037. doi: 10.1002/jmri.22338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calamante F, Christensen S, Desmond PM, Ostergaard L, Davis SM, Connelly A. The physiological significance of the time-to-maximum (tmax) parameter in perfusion mri. Stroke. 2010;41:1169–1174. doi: 10.1161/STROKEAHA.110.580670. [DOI] [PubMed] [Google Scholar]
- 19.Kakuda W, Lansberg MG, Thijs VN, Kemp SM, Bammer R, Wechsler LR, et al. Optimal definition for pwi/dwi mismatch in acute ischemic stroke patients. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2008;28:887–891. doi: 10.1038/sj.jcbfm.9600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lansberg MG, Lee J, Christensen S, Straka M, De Silva DA, Mlynash M, et al. Rapid automated patient selection for reperfusion therapy: A pooled analysis of the echoplanar imaging thrombolytic evaluation trial (epithet) and the diffusion and perfusion imaging evaluation for understanding stroke evolution (defuse) study. Stroke. 2011;42:1608–1614. doi: 10.1161/STROKEAHA.110.609008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: The diffusion and perfusion imaging evaluation for understanding stroke evolution (defuse) study. Ann Neurol. 2006;60:508–517. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]
- 22.Lemmens R, Christensen S, Straka M, De Silva DA, Mlynash M, Campbell BC, et al. Patients with single distal mca perfusion lesions have a high rate of good outcome with or without reperfusion. Int J Stroke. 2013;9:156–159. doi: 10.1111/ijs.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoo AJ, Verduzco LA, Schaefer PW, Hirsch JA, Rabinov JD, Gonzalez RG. Mri-based selection for intra-arterial stroke therapy: Value of pretreatment diffusion-weighted imaging lesion volume in selecting patients with acute stroke who will benefit from early recanalization. Stroke. 2009;40:2046–2054. doi: 10.1161/STROKEAHA.108.541656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kidwell CS, Alger JR, Saver JL. Beyond mismatch: Evolving paradigms in imaging the ischemic penumbra with multimodal magnetic resonance imaging. Stroke. 2003;34:2729–2735. doi: 10.1161/01.STR.0000097608.38779.CC. [DOI] [PubMed] [Google Scholar]