Abstract
Background
Galectin 3 (Gal-3) is a potential mediator of cardiac fibrosis, and Gal-3 concentrations predict incident heart failure. The same mechanisms that lead to cardiac fibrosis in heart failure may influence development of atrial fibrosis and atrial fibrillation (AF). We examined the association of Gal-3 and incident AF in the community.
Methods
Plasma Gal-3 concentrations were measured in 3,306 participants of the Framingham Offspring cohort who attended the sixth examination cycle (1995–1998, mean age 58 years, 54% women). Cox proportional hazards regression models were used to assess the association of baseline Gal-3 concentrations and incident AF.
Results
Over a median follow-up period of 10 years, 250 participants developed incident AF. Crude incidence rates of AF by increasing sex-specific Gal-3 quartiles were 3.7%, 5.9%, 9.1%, and 11.5% (log-rank test P < .0001). In age- and sex-adjusted analyses, each 1-SD increase in loge-Gal-3 was associated with a 19% increased hazard of incident AF (hazard ratio 1.19, 95% CI 1.05–1.36, P = .009). This association was not significant after adjustment for traditional clinical AF risk factors (hazard ratio 1.12, 95% CI 0.98–1.28, P = .10).
Conclusion
Higher circulating Gal-3 concentrations were associated with increased risk of developing AF over the subsequent 10 years in age- and sex-adjusted analyses but not after accounting for other traditional clinical AF risk factors. Our results do not support a role for Gal-3 in AF risk prediction. Further studies are needed to evaluate whether Gal-3 plays a role in the development of AF substrate similar to HF.
Background
Atrial fibrillation (AF) affects up to 6.1 million adults in the United States, and the lifetime risk of developing AF is approximately 1 in 4 at age 40 years.1 Atrial fibrillation is closely linked to several cardiovascular sequelae, including mortality, stroke, and heart failure (HF).2 Specifically, AF often precedes or follows the development of HF, and the effects of AF and HF in combination yield a particularly adverse prognosis.3 Pathophysiological mechanisms active in cardiac remodeling and HF also underlie atrial structural remodeling.4,5 In particular, atrial interstitial fibrosis appears to be a key contributor to AF substrate.6,7 Furthermore, the extent of atrial fibrosis appears to predict clinical response to AF ablation.8 The exact pathways leading to atrial fibrosis remain unknown, but some studies have shown involvement of the renin-angiotensin axis9 and transforming growth factor (TGF) β1, a key factor in the development of myocardial fibrosis.10
Galectin 3 (Gal-3) is a β-galactoside–binding lectin that appears to play an important regulatory role in fibrosis and inflammation.11 In experimental studies, Gal-3 was a central component in the development of myocardial and vascular fibrosis,12,13 likely by activating TGF-β–mediated myofibroblast activation and stimulating matrix production.14,15 In human studies, circulating Gal-3 concentrations predicted incident HF in the community16 and increased mortality in individuals with existing HF17 and in the general population.18
The role of Gal-3 in the development of AF has not been studied. Based on the central role of Gal-3 in the development of cardiac fibrosis, we sought to examine the association of circulating Gal-3 concentrations and incident AF in a large well-characterized community-based sample and to estimate reference limits in an apparently healthy sample. We hypothesized that elevated Gal-3 concentrations would predict incident AF events in participants of the Framingham Heart Study accounting for standard AF risk factors. These findings may lend further insights into underlying mechanisms of AF and HF as intertwined clinical entities.
Methods
Participants
The Framingham Offspring cohort includes the children (and their spouses) of the original Framingham Heart Study cohort participants. Since its inception in 1971, the Offspring cohort has undergone serial examinations and medical histories.19 Participants attending the sixth examination cycle (1995–1998) were included in this study. There were 3,450 eligible participants, of whom 3,306 were analyzed: 144 participants were excluded due to missing Gal-3 measurements (n = 2), prevalent AF (n = 106), extreme Gal-3 outliers (>5 loge-SDs above or below the loge-transformed mean, n = 5), missing clinical covariates (n = 22), or no follow-up (n = 9). Participants were followed up until first AF event, death, or last contact up to a maximum of 10 years after baseline examination. The study was approved by the Institutional Review Board of Boston University Medical Center, and all participants provided informed consent.
Biomarker measurement
After an overnight fast, blood samples were collected and immediately centrifuged and stored at −80°C until assayed. Galectin 3 concentrations were measured in plasma using an enzyme-linked immunosorbent assay (BG Medicine, Waltham, MA).20 The assay had a lower detection limit of 1.32 ng/mL with an upper detection limit of 96.6 ng/mL, with within-run and total precision between 2.1% to 5.7% and 4.2% to 12.0% across this measurement range, respectively.20 B-type natriuretic peptide (BNP) and C-reactive protein (CRP) were measured previously.21,22
Clinical assessment
Participants had a comprehensive clinical examination at the study visit. Resting seated blood pressure was obtained manually by a physician and averaged over 2 measurements. Current smoking was defined as smoking 1 or more cigarettes per day in the year before the study visit. Diabetes mellitus was defined as a fasting glucose ≥126 mg/dL, nonfasting glucose ≥200 mg/dL, or the use of insulin or oral hypoglycemic medications. A significant heart murmur was defined as a systolic murmur ≥grade 3/6 or any diastolic murmur. Body mass index was calculated as weight divided by height squared (kilograms per square meter), and obesity was defined as body mass index >30 kg/m2. Heavy alcohol use was defined as ≥14 drinks per week in men and ≥7 drinks per week in women. Estimated glomerular filtration rate (eGFR) was calculated according to the Modification of Diet in Renal Disease equation,23 and chronic kidney disease was defined as an eGFR <60 mL/min per 1.73 m2.
Definition of incident AF
At each follow-up examination and biennial health questionnaire update, interim cardiovascular disease events were identified, and medical records were obtained. Atrial fibrillation was diagnosed by a reviewing cardiologist if either atrial flutter or AF were present on any electrocardiograms after review of all available electrocardiograms performed at the study visit, outpatient or inpatient medical visits, or during Holter monitor studies. All cardiovascular events were adjudicated by a 3-investigator panel after review of medical records; history of HF and myocardial infarction were adjudicated using established criteria.24
Echocardiographic methods
A total of 2,321 participants included in this analysis also had routine M-mode and 2-dimensional echocardiography.25 Left ventricular (LV) end-diastolic dimension (LVDD), LV end-systolic dimension, left atrial end-systolic diameter, and end-diastolic LV septal and posterior wall thicknesses were measured according to American Society of Echocardiography guidelines.26 Left ventricular wall thickness was defined as the sum of end-diastolic LV septal and posterior wall thicknesses. Fractional shortening was calculated as [(LVDD − LV end-systolic dimension)/LVDD] × 100. Analyses were adjusted for height and weight as described below. Detailed echocardiographic protocols and quality metrics have previously been summarized.27
Statistical analysis
Baseline clinical characteristics were summarized for all participants. Because of right-skewed distributions, Gal-3, BNP, and CRP levels were natural log transformed for subsequent analyses. To establish reference limits for Gal-3 concentrations, a subset of healthy participants was selected after exclusion of people with any comorbid conditions (hypertension, cardiovascular disease, obesity, current smoking, diabetes mellitus, chronic kidney disease, abnormal LV function on echocardiography [defined as fractional shortening <0.30 or mild to moderate or greater LV systolic dysfunction], and significant heart murmur). Quantile regression28 was used to obtain age-and sex-specific 90th percentiles of Gal-3 concentrations, the reference limits for this sample.
Crude AF incidence rates were estimated in sex-specific Gal-3 quartiles. Kaplan-Meier estimates were generated, and the log-rank test was used for hypothesis testing. The relation of incident AF with Gal-3 was examined using Cox proportional hazards regression models, first adjusting for age and sex and also adjusting for height, weight, systolic and diastolic blood pressures, antihypertensive medication use, diabetes mellitus, smoking status, history of myocardial infarction, and HF. These covariates were selected based on a prior study, which examined and replicated a risk prediction model for AF using 5 epidemiological cohorts.29 In secondary analyses, we further adjusted for heavy alcohol use, other biomarkers (BNP and CRP), and baseline echocardiographic variables—left atrial dimension, LV fractional shortening, and the sum of LV septal and posterior wall thicknesses.30 Furthermore, interim HF (a time-dependent variable) was included in secondary analyses. We additionally adjusted for history of stroke, transient ischemic attack, and vascular disease including claudication and examined stratified analyses based on eGFR groups (≥90, 60–89, and <60 mL/min per 1.73 m2) in secondary analyses. Sensitivity analyses were performed, excluding participants with prevalent HF and prevalent chronic kidney disease. We tested for age and sex interaction terms. A post hoc power calculation determined the effect size detectable with 80% power and α of .05.
To assess the incremental benefit of Gal-3 in the prediction of AF, the c statistic was compared between multivariable-adjusted models with and without Gal-3. We also computed the Integrated Discrimination Improvement (IDI) and the category-free Net Reclassification Improvement (NRI) metric for the addition of Gal-3 in models containing traditional clinical risk factors of AF development.31,32 Statistical analyses were conducted using SAS version 9.2 for Windows. Results were considered significant when 2-sided P < .05.
This work was partially supported by the National Heart, Lung, and Blood Institute’s Framingham Heart Study (N01-HC-25195). Dr Ho is supported by a grant from the National Institutes of Health (1 K23-HL116780) and a Department of Medicine Career Investment Award, Boston University School of Medicine. Dr Benjamin is supported by grants from the National Institutes of Health (2R01HL092577, 1R01 HL102214, 1RC1HL101056, 6R01-NS 17950). Dr McManus receives partial salary support provided by National Institutes of Health grants 1U01HL105268-01 and KL2RR031981.
The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the manuscript, and its final contents.
Results
The baseline clinical characteristics of 3,306 study participants are displayed in Table I. The mean age was 58 years, and 54% of participants were women. The median Gal-3 concentration in our sample was 13.7 ng/mL (25th and 75th percentiles 11.6 and 16.3, respectively). Participants who later developed AF had baseline Gal-3 concentrations of 15.0 ng/mL (25th and 75th percentiles 12.6 and 17.2).
Table I.
Baseline characteristics of 3,306 Framingham Heart Study participants
| No AF (n = 3056) | Incident AF (n = 250) | |
|---|---|---|
| Clinical characteristics | ||
| Age, y | 58 (9) | 66 (9) |
| Women, n (%) | 1679 (55) | 102 (41) |
| Height, cm | 167 (9) | 168 (10) |
| Weight, kg | 78 (17) | 82 (19) |
| Systolic blood pressure, mm Hg | 128 (18) | 136 (21) |
| Diastolic blood pressure, mm Hg | 76 (9) | 74 (11) |
| Diabetes mellitus, n (%) | 257 (8) | 52 (21) |
| Prevalent myocardial infarction, n (%) | 92 (3) | 26 (10) |
| Prevalent HF, n (%) | 13 (0.4) | 7 (2.8) |
| Current smoker, n (%) | 470 (15) | 41 (16) |
| Heavy alcohol use, n (%) | 46 (1.5) | 5 (2.0) |
| Chronic kidney disease, n (%) | 239 (8) | 33 (13) |
| Nonskin cancer, n (%) | 215 (7) | 27 (11) |
| Antihypertensive treatment, n (%) | 773 (25) | 132 (53) |
| Lipid-lowering medication, n (%) | 362 (12) | 45 (18) |
| Aspirin use, n (%) | 812 (27) | 122 (49) |
| Vital capacity, L | 3.8 (1.0) | 3.6 (1.0) |
| Laboratory characteristics, median (25th, 75th percentile) | ||
| Gal-3, ng/mL | 13.6 (11.5, 16.2) | 15.0 (12.6, 17.2) |
| BNP, pg/mL | 7.6 (4.0, 16.6) | 22.1 (8.8, 47.5) |
| CRP, mg/L | 2.0 (0.9, 4.6) | 2.9 (1.3, 6.5) |
| eGFR, mL/min per 1.73 m2 | 85 (73, 99) | 81 (68, 93) |
| Risk scores | ||
| CHADS2 score* | 0.54 (0.71) | 1.18 (0.87) |
| CHA2DS-2VASc score† | 1.42 (1.20) | 2.49 (1.59) |
Data shown are means (SD) unless otherwise specified.
CHADS2 score33: 1 point each for history of HF, hypertension, age ≥75 years, diabetes, history of stroke, or transient ischemic attack.
CHA2DS-2VASc score34: 1 point each for history of HF, hypertension, age 65 to 74 years, diabetes, vascular disease, female sex, and 2 points each for age ≥75 years, stroke, or transient ischemic attack.
Reference limits of Gal-3 in a healthy sample
After exclusion of participants with any comorbid conditions, 1,072 healthy participants were retained to derive Gal-3 reference limits. The age- and sex-specific 90th percentiles are displayed in Table II. Median Gal-3 concentrations by quartile varied from 10.7 to 15.5 ng/dL in men and 11.3 to 16.2 ng/dL in women.
Table II.
Reference limits of Gal-3 in healthy subset of participants (n = 1,072)
| Age group (y) | Gal-3 concentration (ng/mL)
|
|||
|---|---|---|---|---|
| 10th percentile | 50th percentile | 90th percentile | ||
| Men | 40 | 8.15 | 10.74 | 14.55 |
| 50 | 8.83 | 11.77 | 15.58 | |
| 60 | 9.57 | 12.88 | 16.70 | |
| 70 | 10.36 | 14.11 | 17.89 | |
| 80 | 11.23 | 15.45 | 19.16 | |
| Women | 40 | 8.83 | 11.28 | 16.11 |
| 50 | 9.57 | 12.35 | 17.26 | |
| 60 | 10.37 | 13.52 | 18.49 | |
| 70 | 11.23 | 14.80 | 19.81 | |
| 80 | 12.17 | 16.21 | 21.22 | |
Association of incident AF with Gal-3
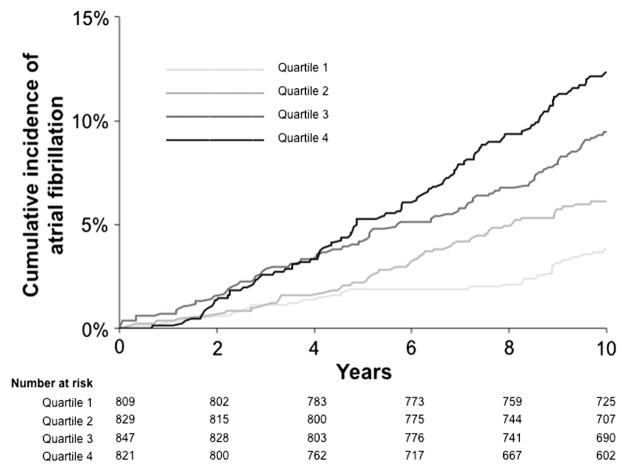
Over a median follow-up time of 10 years, 250 participants (7.6%) developed incident AF. Crude incidence rates of AF by sex-specific Gal-3 quartile were 3.7% in quartile 1, 5.9% in quartile 2, 9.1% in quartile 3, and 11.5% in quartile 4. Cumulative incidence curves in the Figure demonstrate increasing risk of AF with increasing Gal-3 quartiles (P < .0001). In age- and sex-adjusted analyses, each 1-SD increase in loge-Gal-3 was associated with a 19% increased hazard of incident AF (hazard ratio [HR] 1.19, 95% CI 1.05–1.36, P = .009) (Table III). This association was not significant after adjustment for traditional clinical AF risk factors (HR 1.12, 95% CI 0.98–1.28, P = .10).
Figure.
Cumulative incidence of AF by Gal-3 quartiles (log-rank P < .0001). Sex-specific quartile cutoffs are as follows for men: Q1, 3.9 to 11.0 ng/mL; Q2, 11.1 to 12.9 ng/mL; Q3, 13.0 to 15.3 ng/mL; Q4, 15.3 to 54.6 ng/mL; and for women: Q1, 5.0 to 11.9 ng/mL; Q2, 11.9 to 14.2 ng/mL; Q3, 14.3 to 16.8 ng/mL; Q4, 16.9 to 53.0 ng/mL.
Table III.
Association of Gal-3 and incident AF
| Model | HR (95% CI) | P |
|---|---|---|
| Age and sex adjusted | 1.19 (1.05–1.36) | .009 |
| Age, sex, and clinical risk factors* | 1.12 (0.98–1.28) | .10 |
| Age, sex, clinical risk factors, interim HF | 1.12 (0.99–1.28) | .08 |
| Age, sex, clinical risk factors, alcohol, eGFR, BNP, CRP, echocardiographic parameters† | 1.13 (0.95–1.36) | .16 |
Hazard ratio is per 1-SD increase in loge-Gal-3.
Clinical risk factors included height, weight, systolic and diastolic blood pressures, diabetes mellitus, smoking status, history of HF, and history of myocardial infarction.
Echocardiographic parameters included left atrial dimension, fractional shortening, and LV wall thickness.
Secondary analyses
The effect estimate of Gal-3 remained stable after additional adjustment for heavy alcohol use, eGFR, biomarkers (BNP and CRP), and echocardiographic variables—left atrial end-systolic diameter, LV wall thickness, fractional shortening—(HR 1.14, 95% CI 0.95–1.36, P = .16) and accounting for interim HF (HR 1.12, 95% CI 0.99–1.28, P = .08). Results were not materially different after additional adjustment for the use of lipid-lowering medication or aspirin at baseline (online Appendix Supplementary Table I). Furthermore, adjustment for history of stroke, transient ischemic attack, and vascular disease did not appreciably change results (online Appendix Supplementary Table I). The association of Gal-3 and AF was not significant within eGFR subgroups (≥90, 60–89, and <60 mL/min per 1.73 m2) (online Appendix Supplementary Table II). After excluding participants with prevalent HF and chronic kidney disease, there was no significant association of Gal-3 and incident AF (HR 1.09, 95% CI 0.94–1.28, P = .26). We had 80% power to detect an multivariable-adjusted HR of ≥1.21 per 1-SD increase in log-Gal-3 at significance level 0.05.
Performance of Gal-3 as a biomarker
When added to the clinical model for AF, Gal-3 did not substantially increase the c statistic (0.781–0.782), and there were negligible changes in the IDI, relative IDI, category-free NRI, and category-based NRI (online Appendix Supplementary Table III).
Discussion
We found that higher circulating Gal-3 concentrations were associated with increased risk of developing AF over the subsequent 10 years in age- and sex-adjusted analyses. However, this association was no longer significant after adjusting for clinical risk factors that previously have been demonstrated to predict AF risk.29 Our study may have had limited power to detect a modest association of Gal-3 and AF, but our results do not support a useful role for Gal-3 in AF risk prediction. However, the association of Gal-3 and incident AF in age- and sex-adjusted analyses is interesting nonetheless and suggests that common mechanisms may be at play in the development of AF and HF.
Atrial fibrosis appears to be a key contributor in the development of AF and can occur in overall cardiac remodeling seen in HF.6 The exact mechanisms of atrial interstitial fibrosis are unclear but may involve activation of fibrotic pathways via the renin-angiotensin system9 and TGF-β110 as well as inflammatory and oxidative stress pathways.35 Data on circulating fibrosis markers and AF are limited; however, markers of collagen turnover are associated with type and duration of AF36 and were associated with postsurgical AF37 and AF recurrence after ablation.38
Galectin 3 is a β-galactoside–binding lectin that appears to play an important role in a number of fibrotic conditions, including cardiac fibrosis.12 In experimental studies, Gal-3 expression is up-regulated in HF-prone hearts, where it induces fibroblast proliferation and type I fibrillar collagen production.12 In clinical studies, elevated Gal-3 concentrations have been associated with cardiac remodeling and adverse prognosis in individuals with existing HF17 and predict incident HF in ostensibly healthy community-dwelling adults.16 Moreover, pharmacologic inhibition of Gal-3 has been shown to attenuate cardiac fibrosis and remodeling and prevented the development of HF in animal studies.39 Taken together, these data suggest that Gal-3 may be a potential pathway that could be targeted pharmacologically, to alter mechanisms driving cardiac fibrosis.
Although our results do not suggest utility for Gal-3 as a biomarker in AF risk prediction based on performance metrics, we believe that the association of Gal-3 and AF found in age- and sex-adjusted analyses is still notable. Galectin 3 was previously associated with hypertension, age, body mass index, renal function, and prior cardiovascular disease in our population.16 We speculate that circulating Gal-3 concentrations may reflect an underlying fibrotic process that might lead to a number of cardiovascular effects, and thus, it would be expected that the association of Gal-3 and AF might be attenuated after adjustment for other clinical variables. In this case, we believe that biomarkers that lend biological insights can be useful beyond risk prediction, particularly if a potential therapeutic target can be identified, as is the case with Gal-3. In this regard, future studies are needed to further elucidate the potential role of Gal-3 in AF. It may be that serial measurements of Gal-3 could be more informative in predicting AF risk over the long term.
Several limitations deserve mention. Circulating Gal-3 concentrations are not specific to the cardiovascular system and could potentially reflect other fibrotic conditions, which may have limited our ability to detect an association. As such, it may be possible that Gal-3 is increased in noncardiovascular fibrotic conditions, which, in turn, may increase risk of AF. Given that ours was an observational study, such causal effects cannot be inferred from this analysis. Our study may not have been powered to detect a modest effect size as demonstrated by our post hoc power calculation, given an ambulatory community-based sample with very few prevalent HF cases. Furthermore, our study may be subject to ascertainment bias, in that paroxysmal AF was probably less well assessed compared with permanent AF. Echocardiographic measures were obtained using M-mode, and volumetric data were not available. Our study was done using a single Gal-3 measurement, and it may be that Gal-3 trajectory or repeat measures may be more informative. Lastly, generalizability to other populations is limited given a predominantly white middle-aged to older study sample.
In summary, we found that higher circulating Gal-3 concentrations were not associated with increased risk of developing AF over the subsequent 10 years after accounting for traditional clinical risk factors for AF development. These results do not support a useful role for Gal-3 in AF risk prediction. Future studies are needed to further clarify the role of Gal-3 in AF, particularly in populations with existing HF.
Supplementary Material
Footnotes
Disclosures
Galectin 3 assays were provided by BG Medicine, Inc (Waltham, MA). This company did not have access to study data and had no input into the data analyses, interpretation, or preparation of the manuscript for submission.
References
- 1.Lloyd-Jones DM, Larson MG, Leip EP, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106(24):3068–72. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 2.Magnani JW, Rienstra M, Lin H, et al. Atrial fibrillation: current knowledge and future directions in epidemiology and genomics. Circulation. 2011;124(18):1982–93. doi: 10.1161/CIRCULATIONAHA.111.039677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang TJ, Larson MG, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107(23):2920–5. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 4.Li D, Fareh S, Leung TK, et al. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. 1999;100(1):87–95. doi: 10.1161/01.cir.100.1.87. [DOI] [PubMed] [Google Scholar]
- 5.Maisel WH, Stevenson LW. Atrial fibrillation in heart failure: epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol. 2003;91(6A):2D–8. doi: 10.1016/s0002-9149(02)03373-8. [DOI] [PubMed] [Google Scholar]
- 6.Everett TH, Olgin JE. Atrial fibrosis and the mechanisms of atrial fibrillation. Heart Rhythm. 2007;4(3 Suppl):S24–7. doi: 10.1016/j.hrthm.2006.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol. 2008;1(1):62–73. doi: 10.1161/CIRCEP.107.754564. [DOI] [PubMed] [Google Scholar]
- 8.Oakes RS, Badger TJ, Kholmovski EG, et al. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. 2009;119(13):1758–67. doi: 10.1161/CIRCULATIONAHA.108.811877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goette A, Staack T, Rocken C, et al. Increased expression of extracellular signal-regulated kinase and angiotensin-converting enzyme in human atria during atrial fibrillation. J Am Coll Cardiol. 2000;35(6):1669–77. doi: 10.1016/s0735-1097(00)00611-2. [DOI] [PubMed] [Google Scholar]
- 10.Verheule S, Sato T, Everett TT, et al. Increased vulnerability to atrial fibrillation in transgenic mice with selective atrial fibrosis caused by overexpression of TGF-beta1. Circ Res. 2004;94(11):1458–65. doi: 10.1161/01.RES.0000129579.59664.9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Boer RA, Yu L, van Veldhuisen DJ. Galectin-3 in cardiac remodeling and heart failure. Curr Heart Fail Rep. 2010;7(1):1–8. doi: 10.1007/s11897-010-0004-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma UC, Pokharel S, van Brakel TJ, et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation. 2004;110(19):3121–8. doi: 10.1161/01.CIR.0000147181.65298.4D. [DOI] [PubMed] [Google Scholar]
- 13.Calvier L, Miana M, Reboul P, et al. Galectin-3 mediates aldosterone-induced vascular fibrosis. Arterioscler Thromb Vasc Biol. 2013;33(1):67–75. doi: 10.1161/ATVBAHA.112.300569. [DOI] [PubMed] [Google Scholar]
- 14.Henderson NC, Mackinnon AC, Farnworth SL, et al. Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc Natl Acad Sci U S A. 2006;103(13):5060–5. doi: 10.1073/pnas.0511167103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henderson NC, Mackinnon AC, Farnworth SL, et al. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am J Pathol. 2008;172(2):288–98. doi: 10.2353/ajpath.2008.070726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho JE, Liu C, Lyass A, et al. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J Am Coll Cardiol. 2012;60(14):1249–56. doi: 10.1016/j.jacc.2012.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah RV, Chen-Tournoux AA, Picard MH, et al. Galectin-3, cardiac structure and function, and long-term mortality in patients with acutely decompensated heart failure. Eur J Heart Fail. 2010;12:826–32. doi: 10.1093/eurjhf/hfq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Boer RA, van Veldhuisen DJ, Gansevoort RT, et al. The fibrosis marker galectin-3 and outcome in the general population: data from PREVEND. J Intern Med. 2011;272:55–64. doi: 10.1111/j.1365-2796.2011.02476.x. [DOI] [PubMed] [Google Scholar]
- 19.Kannel WB, Feinleib M, McNamara PM, et al. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110(3):281–90. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 20.Christenson RH, Duh SH, Wu AH, et al. Multi-center determination of galectin-3 assay performance characteristics: anatomy of a novel assay for use in heart failure. Clin Biochem. 2010;43(7–8):683–90. doi: 10.1016/j.clinbiochem.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Wang TJ, Larson MG, Levy D, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350(7):655–63. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 22.Schnabel R, Larson MG, Dupuis J, et al. Relations of inflammatory biomarkers and common genetic variants with arterial stiffness and wave reflection. Hypertension. 2008;51(6):1651–7. doi: 10.1161/HYPERTENSIONAHA.107.105668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 24.McKee PA, Castelli WP, McNamara PM, et al. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285(26):1441–6. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 25.Vasan RS, Benjamin EJ, Larson MG, et al. Plasma natriuretic peptides for community screening for left ventricular hypertrophy and systolic dysfunction: the Framingham heart study. JAMA. 2002;288(10):1252–9. doi: 10.1001/jama.288.10.1252. [DOI] [PubMed] [Google Scholar]
- 26.Sahn DJ, DeMaria A, Kisslo J, et al. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58(6):1072–83. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 27. [accessed on 01/21/14.]; http://www.framinghamheartstudy.org/share/protocols/echo1_6s_protocol.pdf.
- 28.Koenker R, Bassett G. Regression quantiles. Econometrica. 1978;46 (1):33–50. [Google Scholar]
- 29.Alonso A, Krijthe BP, Aspelund T, et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc. 2013;2 (2):e000102. doi: 10.1161/JAHA.112.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vaziri SM, Larson MG, Benjamin EJ, et al. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation. 1994;89(2):724–30. doi: 10.1161/01.cir.89.2.724. [DOI] [PubMed] [Google Scholar]
- 31.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, et al. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–72. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 32.Pencina MJ, D’Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30(1):11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285(22):2864–70. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 34.Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263–72. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 35.Saba S, Janczewski AM, Baker LC, et al. Atrial contractile dysfunction, fibrosis, and arrhythmias in a mouse model of cardiomyopathy secondary to cardiac-specific overexpression of tumor necrosis factor-{alpha} Am J Physiol Heart Circ Physiol. 2005;289(4):H1456–67. doi: 10.1152/ajpheart.00733.2004. [DOI] [PubMed] [Google Scholar]
- 36.Tziakas DN, Chalikias GK, Papanas N, et al. Circulating levels of collagen type I degradation marker depend on the type of atrial fibrillation. Europace. 2007;9(8):589–96. doi: 10.1093/europace/eum072. [DOI] [PubMed] [Google Scholar]
- 37.Swartz MF, Fink GW, Sarwar MF, et al. Elevated pre-operative serum peptides for collagen I and III synthesis result in post-surgical atrial fibrillation. J Am Coll Cardiol. 2012;60(18):1799–806. doi: 10.1016/j.jacc.2012.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okumura Y, Watanabe I, Nakai T, et al. Impact of biomarkers of inflammation and extracellular matrix turnover on the outcome of atrial fibrillation ablation: importance of matrix metalloproteinase-2 as a predictor of atrial fibrillation recurrence. J Cardiovasc Electrophysiol. 2011;22(9):987–93. doi: 10.1111/j.1540-8167.2011.02059.x. [DOI] [PubMed] [Google Scholar]
- 39.Yu L, Ruifrok WP, Meissner M, et al. Genetic and pharmacological inhibition of galectin-3 prevents cardiac remodeling by interfering with myocardial fibrogenesis. Circ Heart Fail. 2013;6(1):107–17. doi: 10.1161/CIRCHEARTFAILURE.112.971168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.