Abstract
The study examines the extent and frequency of a knockdown-type resistance allele (kdr type) in North American populations of human head lice. Lice were collected from 32 locations in Canada and the United States. DNA was extracted from individual lice and used to determine their zygosity using the serial invasive signal amplification technique to detect the kdr-type T917I (TI) mutation, which is most responsible for nerve insensitivity that results in the kdr phenotype and permethrin resistance. Previously sampled sites were resampled to determine if the frequency of the TI mutation was changing. The TI frequency was also reevaluated using a quantitative sequencing method on pooled DNA samples from selected sites to validate this population genotyping method. Genotyping substantiated that TI occurs at high levels in North American lice (88.4%). Overall, the TI frequency in U.S. lice was 84.4% from 1999 to 2009, increased to 99.6% from 2007 to 2009, and was 97.1% in Canadian lice in 2008. Genotyping results using the serial invasive signal amplification reaction (99.54%) and quantitative sequencing (99.45%) techniques were highly correlated. Thus, the frequencies of TI in North American head louse populations were found to be uniformly high, which may be due to the high selection pressure from the intensive and widespread use of the pyrethrins- or pyrethroid-based pediculicides over many years, and is likely a main cause of increased pediculosis and failure of pyrethrins- or permethrin-based products in Canada and the United States. Alternative approaches to treatment of head lice infestations are critically needed.
Keywords: Pediculus humanus capitis, human head louse, knockdown resistance, serial invasive signal amplification reaction
Pediculosis, caused by the human head louse, Pediculus humanus capitis (De Geer), is one of the most prevalent parasitic infestation of humans (Lee et al. 2000). Pediculicide sales in the United States were estimated at >US$240 million per year in 1997 (Gratz 1997) and increased to >US$350 million per year by 2003 (Jones and English 2003). The overall cost of louse infestations in the United States is estimated at ≈US$1 billion annually. However, this cost estimate is overshadowed by the long-term impact of school absences by ≈1 in every 10 school-aged children and the implementation of ineffective control measures that can lead to multiple trips to the hospital or health clinic (Gratz 1997, Williams et al. 2001, Frankowski and Weiner 2002, Lebwohl et al. 2007). Infestations also often cause intense itching, which can injure skin, allowing secondary infections and self-inoculation with disease-causing bacterial pathogens, such as Staphylococcus aureus. In addition, most people find lice intolerable and repeatedly and prophylactically apply pediculicides, many of which carry the risk of adverse effects, without realizing their potential for harm. Misapplication affects children in particular because of their small size and higher sensitivity.
Currently, the treatment of pediculosis depends primarily on the topical application of insecticides, including the natural pyrethrin esters (pyrethrum), synthetic “pyrethroids” (permethrin, phenothrin), organochlorines (lindane), and the organophosphorus (malathion)- and carbamate (carbaryl)-based formulations (Durand et al. 2012). More recently, clinical demonstration of effectiveness against head lice has allowed the registration of three new treatments in the United States, namely, benzyl alcohol, spinosad, and ivermectin (Stough et al. 2009, Meinking et al. 2010, Pariser et al. 2012). However, the pyrethrins and pyrethroids continue to dominate the head lice treatment market as inexpensive and easily obtainable over-the-counter formulations.
Pyrethrum (natural pyrethrins) formulation for the control of head lice was first introduced in 1945 (Durand et al. 2012) and was then supplemented with the more environmentally stable pyrethroids (e.g., permethrin) during the 1980s (Carson et al. 1988). In the 1990s, permethrin was used in the Nix formulation and marketed worldwide as an over-the-counter product (Durand et al. 2012). This formulation has been used extensively and intensely for >20 yr. The pyrethrins and pyrethroids share a common target site in the nervous system, voltage-sensitive sodium channels (VSSC), and act as agonistic neuroexcitants by prolonging sodium current, leading to nerve depolarization and hyperexcitation, followed by muscle paralysis and death.
Both clinical and parasitological pyrethroid resistance to d-phenothrin was first reported in France in 1994 (Chosidow et al. 1994), with additional reports of clinical failures as follows: permethrin (2001) in the United States (Hipolito et al. 2001), phenothrin (2005) in the United Kingdom (Burgess et al. 2005), and permethrin (2005) in the United Kingdom (Hill et al. 2005). Also, parasitological resistance to pyrethroids has been reported in the Czech Republic (Rupes et al. 1995), the United Kingdom (Downs et al. 1999), Denmark (Kristensen 2005), Israel (Mumcuoglu et al. 1995), the United States (Pollack et al. 1999, Lee et al. 2000), Argentina (Picollo et al. 1998), Japan (Tomita et al. 2003), and Australia (Hunter and Barker 2003).
There are multiple mechanisms that give rise to pyrethrin and pyrethroid resistance in insects, including reduced penetration, enhanced xenobiotic detoxification, and target site insensitivity, also known as knockdown resistance or kdr. The kdr phenotype (recalcitrant to knockdown) is a heritable trait associated with nerve insensitivity to dichlorodiphenyltrichloroethane, the pyrethrins, and pyrethroids and was first discovered in the house fly, Musca domestica L. (Farnham 1977). Point mutations within the α-subunit gene of the VSSC are functionally responsible for the nerve insensitivity to dichlorodiphenyltrichloroethane, the pyrethrins, and pyrethroids and result in the kdr, kdr-type, and super kdr traits (Williamson et al. 1993, Dong and Scott 1994, Knipple et al. 1994). Lee et al. (2000) first reported that head lice from Massachusetts and Florida were resistant to permethrin and exhibited in vivo responses in behavioral bioassays that were consistent with kdr. Subsequently, three point mutations (M815I, T917I, and L920F) in the VSSC α-subunit gene of permethrin-resistant head lice were reported (Lee et al. 2000, 2003) and functionally validated as kdr-type mutations (Yoon et al. 2008). Of the three kdr-type mutations identified in permethrin-resistant head lice, only the T917I (TI) mutation resulted in complete insensitivity of the channel to permethrin when heterologously expressed. Recently, several genotyping techniques based on these mutations have been developed for determining the kdr-type allele frequency using genomic DNA extracted from individual head lice (Lee et al. 2010), and have established that although widespread, kdr-type resistance is not yet uniform worldwide (Hodgdon et al. 2010).
Although the presence of kdr-type mutations alone may not directly predict clinical failure, their increasing frequency in head louse populations coincides with reports of product failures in controlled studies. Early reports of permethrin use from 1984 through 1995 consistently showed 96 to 100% effectiveness (Taplin et al. 1986, Carson et al. 1988, DiNapoli et al. 1988, Bainbridge et al. 1998). In 2001, a report described a reduced effectiveness of only 80%, and subsequent reports of effectiveness have ranged from 28 to 55%, even where treatments have been augmented by nit combing (Burkhart and Burkhart 2000, Hipolito et al. 2001, Stough et al. 2009, Meinking et al. 2010). Therefore, it seems likely that the prevalence of the kdr-type alleles can be an indicator of permethrin resistance and the probable cause of product failure for human head lice.
In this study, we used serial invasive signal amplification reaction (SISAR) to determine the extent and frequency of the TI mutation in individual lice. To date, the TI mutation has always been found in the presence of the other two kdr-type mutations and is the only mutation of the three that produces a VSSC that is completely insensitive to the agonistic action of permethrin (Yoon et al. 2008). An expanded sampling approach that includes 14 locations in Canada and 18 locations in the United States, some of which have been previously sampled, enabled us to analyze whether there were changes in the TI mutation frequency within North American populations of human head lice. The TI mutation frequency was also reevaluated using a quantitative sequencing (QS) method on pooled DNA samples from selected sites to validate this cheaper and faster molecular diagnostic method necessary to create a high resolution kdr-type allele frequency map for use in resistance monitoring and management of head louse populations worldwide. Although QS does not identify the genotype of individual lice as does SISAR, it allows the determination of kdr-type allele frequencies in head louse populations in an effective manner (e.g., cheaper and faster), and then to genotype individual lice by SISAR from only those populations where the kdr-type mutations are not yet at high levels as suggested by Lee et al. (2010).
Materials and Methods
Head Louse Collection
Head lice were collected by volunteers (school nurses and professional lice combers) supporting our research from 12 states within the United States (Table 1). Four of these locations (Arizona, California, Florida, and Texas, see Fig. 1, boxed location) had been previously sampled (Gao et al. 2003), allowing the determination of kdr-type allele frequency changes in those locations over time. Prospective volunteers were contacted by e-mail (contact list was supplied by Topaz Pharmaceuticals LLC, Jenkintown, PA) and provided an information sheet, which explained the study’s objectives, method for louse collection, sample preparation, labeling instructions, storage requirements, a collection key requesting pertinent information, and shipping instructions. Volunteers who enlisted in the study were then sent a package with sufficient materials to collect the required louse samples and an addressed return box with prepaid postage. Each louse population consisted of 2–126 lice collected from 1–30 individuals. Lice were collected from healthy subjects of all races and genders, aged from 2 to 55 yr. The treatment history of subjects from whom lice were collected was unknown. Lice were stored in 70% ethanol upon collection and sent by FedEx to the Pesticide Toxicology Laboratory, University of Massachusetts, Amherst, for genotyping analysis. The protocols for all louse collections were approved by the University of Massachusetts Internal Review Board (# 104-1423 and # 109-1792) and the National Institute of Health (# 06-003).
Table 1.
U.S. head louse collections and kdr allele frequencies determined by SISAR analysis
| Geographical locations of louse collections (abbr.) |
Collection month/yr |
No. of subjects/ no. of subject analyzed |
No. of lice collected/ no. of lice analyzed |
kdr genotype frequency (TI mutation) |
||
|---|---|---|---|---|---|---|
| RR | RS | SS | ||||
| Arizona | ||||||
| Pinon (PN-AZ) | May 2007 | 12/12 | 34/30 | 0.50 | 0.30 | 0.20 |
| Phoenix (PX-AZ)a | Dec. 2008 | 8/8 | 30/18 | 1.0 | 0 | 0 |
| California | ||||||
| San Bernardino County (SB-CA) | Sept. 2001 | −/− | 34/34 | 0.35 | 0.35 | 0.30 |
| Daly City (DC-CA)a | Jan. 2008 | 1/1 | 4/4 | 1.0 | 0 | 0 |
| Florida | ||||||
| South Florida (SFL) | July 1999 | −/− | 29/29 | 0.97 | 0 | 0.03 |
| West Palm Beach (WB-FL) | Dec. 2006 | 3/3 | 13/3 | 1.0 | 0 | 0 |
| Ocklawaha (OC-FL) | Dec. 2006 | 1/1 | 19/7 | 1.0 | 0 | 0 |
| Massachusetts | ||||||
| Natick (MA)a | Jan. 2009 | 8/8 | 34/24 | 1.0 | 0 | 0 |
| Michigan | ||||||
| Saginaw (MI) | July 2007 | 2/2 | 33/29 | 0.97 | 0.03 | 0 |
| Minnesota | ||||||
| Tracy (MN) | May 2007 | 1/1 | 2/2 | 1.0 | 0 | 0 |
| New York | ||||||
| Oceanside (NY)a | Nov. 2008 | 10/6 | 39/7 | 1.0 | 0 | 0 |
| Ohio | ||||||
| Willoughby (OH)a | Nov. 2008 | 30/24 | 32/24 | 1.0 | 0 | 0 |
| South Carolina | ||||||
| Summerville (SC) | Jan. 2009 | 3/2 | 30/6 | 1.0 | 0 | 0 |
| Tennessee | ||||||
| Nashville (TN)a | Oct. 2008 | 7/4 | 32/9 | 1.0 | 0 | 0 |
| Texas | ||||||
| Mathis (MA-TX) | Jan. 2001 | 1/1 | 27/27 | 0.11 | 0.52 | 0.37 |
| San Antonio (SA-TX)a | Dec. 2006 | 1/1 | 20/9 | 1.0 | 0 | 0 |
| Missouri City (MC-TX) | Nov. 2008 | 15/3 | 30/7 | 1.0 | 0 | 0 |
| Wisconsin | ||||||
| Reeseville (WI)a | Dec. 2008 | 12/7 | 38/22 | 0.96 | 0.04 | 0 |
Samples analyzed by both SISAR and QS.
Fig. 1.
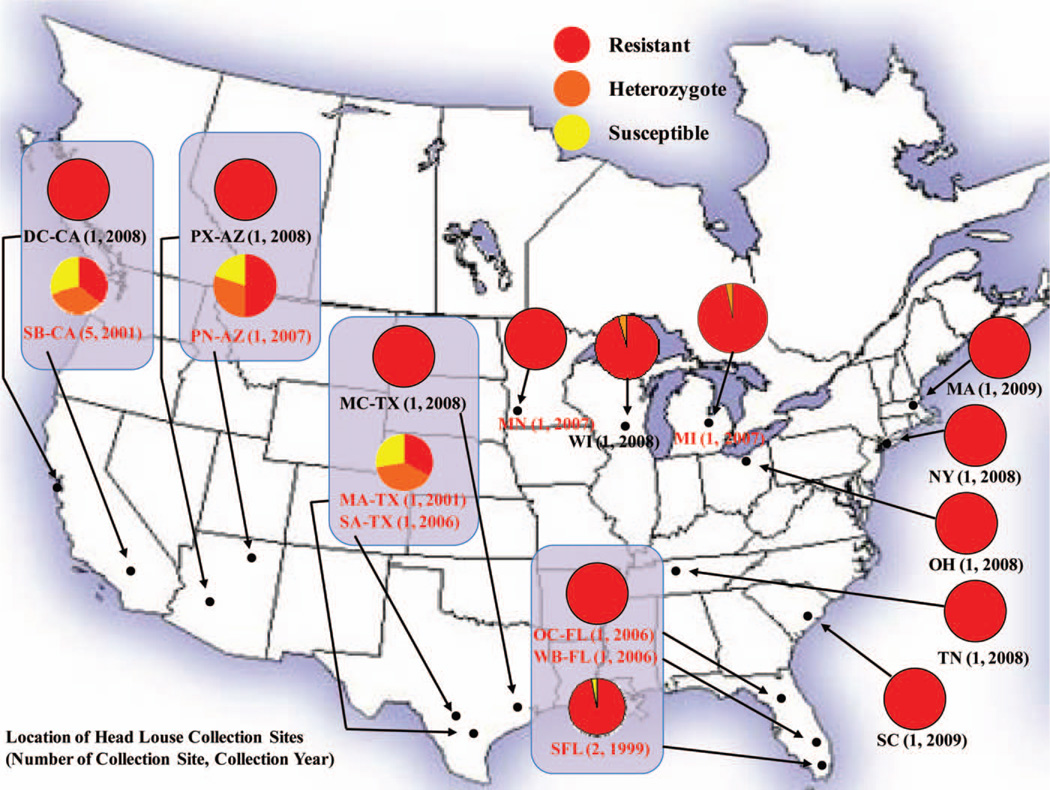
The TI mutation frequency map of head lice collected in the United States. Each pie chart shows zygosity proportions of head lice collected from one or two locations. Proportions of homozygous resistant lice are represented in red, heterozygous lice are orange, and homozygous susceptible lice are yellow. The locations, number of collection sites, and collection year given in red indicate the collections made before 2008. Pie charts that are boxed indicate those states that were sampled at least twice from 1999 to 2008.
Lice samples were also previously collected from three Canadian provinces, namely, British Columbia, Ontario, and Quebec. Details of this collection and study are given in Table 1 of Marcoux et al. (2010). In this initial study, only the kdr-type allele frequency was reported. In the current study, we now provide the zygosity (homozygous susceptible [SS], heterozygous [RS], and homozygous resistant [RR]) of each sample for comparative purposes. In addition, DNA samples from individual lice that had been previously genotyped were pooled by province and used, in part, to validate the QS method discussed below.
Genomic DNA Extraction and Amplification of 1.1-kb Polymerase Chain Reaction Fragment
Genomic DNA (gDNA) was extracted from individual whole head lice after glass–glass homogenization with the Qiagen DNeasy blood and tissue kit (Qiagen Inc., Valencia, CA) according to the manufacturer’s instructions. A 1.1-kb length fragment of the head louse VSSC α-subunit gene, encompassing the TI mutation and four introns, was amplified using the polymerase chain reaction according to Hodgdon et al. (2010). The concentration of the fragment was quantified using PicoGreen dsDNA quantification kit (Invitrogen, Carlsbad, CA.), diluted to a final concentration of 27 µg/liter, and SISAR (Hodgdon et al. 2010) and QS (Kwon et al. 2008) reactions were performed for the detection of TI mutation.
SISAR Genotyping of the TI kdr-Type Resistance Mutation
SISAR protocols were as initially reported by Kim et al. (2004) and updated by Hodgdon et al. (2010). Net fold over zero (net-FOZ) values for each fluorescent fluorophore (resistant or susceptible) were calculated as determined by Kim et al. (2004), and a SISAR ratio was determined using Equation 1.
| [1] |
QS Genotyping of the TI kdr-Type Resistance Mutation
The QS protocols were as initially reported by Kwonet al. (2008). The nucleotide signal intensities of the resistant and susceptible alleles were determined and signal ratios calculated using Equation 2.
| [2] |
The signal ratios of template DNA mixtures were normalized by multiplying them with the normalization factor (signal ratio of the heterozygous DNA template by signal ratio of the 5:5 standard DNA template). A series of normalized signal ratios were plotted against the corresponding TI mutation frequencies, and standard regression equations together with lower and upper prediction equations were generated for the estimation of TI mutation frequencies of unknown samples. For validation purposes, gDNA samples extracted from individual lice and used in the SISAR analysis above were pooled within a collection location and reanalyzed using QS.
Results
SISAR Analysis of Head Lice Collected in the United States
In total, 115 subjects from 18 geographical locations in 12 U.S. states were examined for the presence of head lice and determined to be positive for an active infestation (Table 1). From these individuals, 480 lice of different developmental stages were collected, but only adults and third instars, which made up the majority of lice collected, were used in the determination of the TI mutation frequency and zygosity. Of the 115 subjects who provided lice for the study, 84 provided lice that were analyzed. The percentage of subjects analyzed per location ranged from 20 to 100% with a mean ± SE value of 83.9 ± 24.4%. Of the 480 lice that were collected, 291 were analyzed. The percentage of collected lice that were analyzed per location ranged from 20 to 100% with a mean ± SE value of 63.0 ± 32.2%. The 189 lice that were not analyzed came from collections where large numbers of lice were collected. In these cases, 20% of the total lice collected were analyzed as a representative subsample. Figure 1 presents the zygosity of head louse populations collected during 23 collections from 18 geographical locations in the United States over an 11-yr interval (1999–2009). Of the 291 lice collected and analyzed over this time interval, 227 were homozygous resistant (78% RR), 37 were heterozygous (12.7% RS), and 27 were homozygous susceptible (9.3% SS), with an overall TI mutation frequency of 84.4% within these 12 states. In louse collections made before May 2007 (MA-TX, OC-FL, PN-AZ, SA-TX, SB-CA, SFL, and WB-FL), the overall TI mutation frequency was 70.4 ± 37.6%. In louse populations collected after May 2007 (DC-CA, OH, MA, MC-TX, MI, MN, NY, PX-AZ, TN, SC, and WI), the overall TI mutation frequency was 99.6 ± 0.9%. In the four states where lice were collected at multiple times (2–3 collections), the overall TI mutation frequency increased from 65% in PN-AZ (2007) to 100% in PX-AZ (2008), from 52.9% in SB-CA(2001) to 100% inDC-CA (2008), from 96.6% in SFL (1999) to 100% in WB-FL and OC-FL (2006) when combined, and from 37% in MA-TX (2001) to 100% in SA-TX (2006) and in MC-TX (2008). The overall increase in the TI mutation frequency in these four states from 1999 to 2009 was 37.1 ± 25.2%.
SISAR Analysis of Head Lice Collected in Canada
In total, 121 subjects from 14 geographical locations in three Canadian providences were determined to be positive for active louse infestations in a previously published study conducted in 2008 (see Table 1 of Marcoux et al. 2010). Of the 421 lice collected from 121 subjects, 137 lice (32.5% of total lice collected) from 92 individuals (76.0% of total subjects providing lice) were analyzed by SISAR, their zygosity was determined, and the TI mutation frequency calculated.
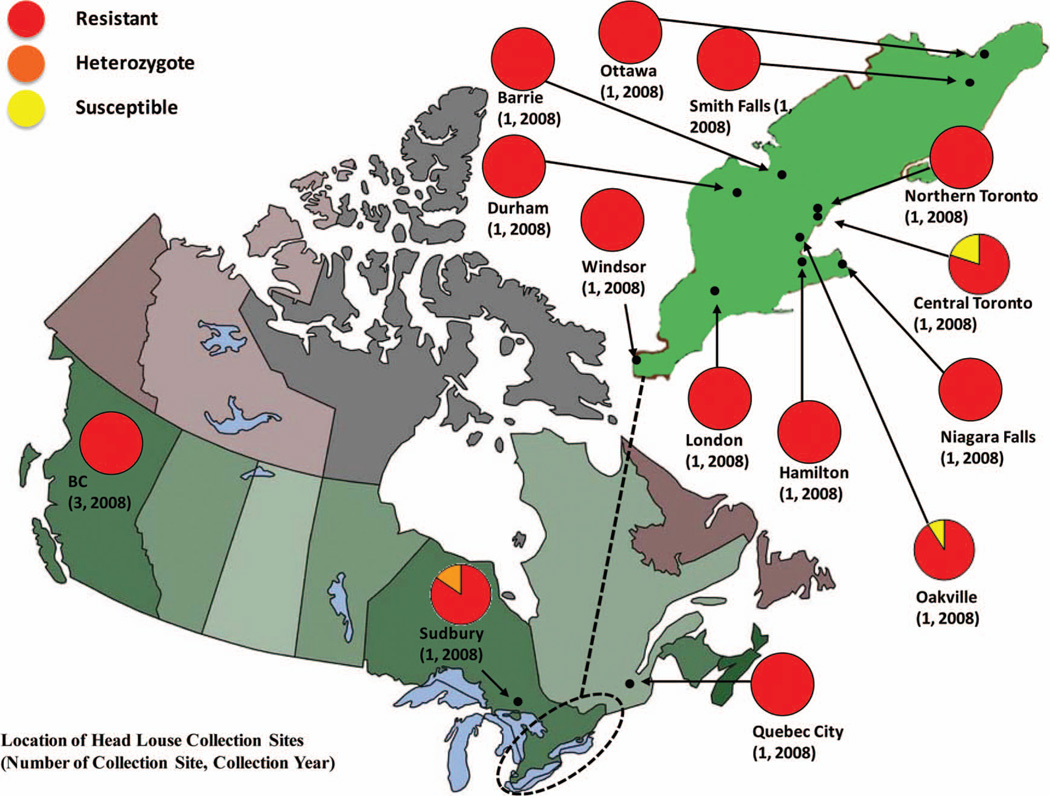
Figure 2 presents the zygosity of the 137 head lice collected in Canada in 2008 (Marcoux et al. 2010). Of these, 132 were homozygous resistant (96.35% RR), 2 were heterozygous (1.46% RS), and 3 were homozygous susceptible (2.19% SS), with an overall TI mutation frequency of 97.1% within these three providences. Of the 14 collection sites, only Central Toronto (2 SS, 20% susceptible allele frequency), Oakville (1 SS, 9.1% susceptible allele frequency), and Sudbury (2 RS, 7.7% susceptible allele frequency) had lice where the susceptible T917 allele was detected.
Fig. 2.
The TI mutation frequency map of head lice collected in Canada. Each pie chart shows zygosity proportions of head lice collected from one or two locations. Proportions of homozygous resistant lice are represented in red, heterozygous lice are orange, and homozygous susceptible lice are yellow.
SISAR Analysis of Head Lice Collected in Canada and the United States
North American lice collected from 32 locations across Canada and the United States from 1999 to 2009 had an overall TI mutation frequency of 88.4% based upon SISAR data. These data included a population from a Navajo reservation, in Arizona (PN-AZ), where pediculicides were not frequently used and had a TI mutation frequency of 65%. SISAR data also included a Mathis, TX, population (MA-TX) that was collected in 2001 and had a TI mutation frequency of 37% and a San Bernardino, CA, population (SB-CA) collected in 2001 that had a TI mutation frequency of 53%. The remaining louse populations collected after 2006 had overall TI mutation frequency of 98.5 ± 4.3%.
Correlation of the TI Mutation Frequencies Determined by SISAR Versus QS
The TI mutation frequency, determined initially by SISAR, of 11 selected populations of North American lice collected after 2006 was redetermined by QS (Table 2). Samples in which both SISAR and QS were performed had TI mutation frequencies of 99.54 and 99.45%, respectively, indicating a high correlation between these two genotyping techniques. QS was not run on the previously mentioned, relatively susceptible, populations (PZ-AZ and MA-TX) or on the populations from Michigan (MI), Minnesota (MN), and Florida (WB-FL and OC-FL), explaining, in part, the difference in the TI mutation frequency between the two methods.
Table 2.
Comparison of kdr allele frequency (TI mutation) determined by either SISAR or QS
| Geographical locations of louse collections (abbr.) |
SISAR | QS |
|---|---|---|
| United States | ||
| Daly City, CA (DC-CA) | 100 | 100 |
| Nashville, TN (TN) | 100 | 100 |
| Natick, MA (MA) | 100 | 100 |
| Oceanside, NY (NY) | 100 | 100 |
| Phoenix, AZ (PX-AZ) | 100 | 100 |
| Reeseville, WI (WI) | 98 | 100 |
| San Antonio, TX (SA-TX) | 100 | 100 |
| Willoughby, OH (OH) | 100 | 100 |
| Canada | ||
| Ontario | 97 | 98 |
| Quebec | 100 | 98 |
| British Columbia | 100 | 98 |
Discussion
Our current database of genotyping results obtained from Canada and the United States clearly substantiates the contention that TI mutations are frequently detected at high levels and are commonly found in North American head louse populations. The overall TI mutation frequency in the U.S. populations collected from 1999 to 2008 was 84.4%. When the U.S. populations were examined after 2007, the overall TI mutation frequency increased to 99.6%. In the four states where lice were collected at multiple times, the overall increase in the TI mutation frequency in these states from 1999 to 2008 was 37.1 ± 25.2%. In head louse populations collected in 2008 from three Canadian providences, the overall TI mutation frequency was 97.1%. These results indicate that the TI mutation frequency has rapidly increased to ≈100% within North American head louse populations and is likely a major reason for the treatment failures encountered with pyrethrins- and pyrethroid-based pediculicides in both Canada and the United States. The almost uniform finding of homozygosity of the TI mutation provides further support for believing that the repeated use of pyrethrins and pyrethroid products is selecting for head lice that are refractory to this class of pediculicides.
However, these above results should be interpreted cautiously in that the number of lice collected and analyzed was relatively small, collections were limited to only 12 states in the United States and to 3 Canadian providences, were biased to metropolitan and urban collection sites, and possibly to lice collected from subjects whose infestations had already been treated with permethrin or similar product. Because of these limitations, the actual level of susceptibility may be higher in North American head louse populations (higher occurrence of the susceptible T917 allele) than what we have reported here. Certainly the results obtained from the Indian reservation (PZ-AZ) and the Mathis, Texas (MA-TX) collections, both rural areas, supports the contention that more susceptible alleles may exist in more rural areas. Nevertheless, recent clinical studies on permethrin have suggested its effectiveness is decreasing (Durand et al. 2012), and additional studies should be carried out to sample from more geographical locations in more states and providences, and to sample in suburban and rural areas.
The strong agreement between the genotyping results obtained with QS when compared with those obtained with SISAR will allow us to use the QS technique for population genotyping. Although QS will not allow the determination of zygosity of individual lice, it is an accurate, rapid, easy, and cost-effective population genotyping technique amenable to handling a large number of populations. With this validated method, it should be possible to expand our present kdr-type allele frequency map to other Canadian providences and to states in the northwestern, western, and midwestern United States. The zygosity of those populations that do not have high levels of kdr-type mutations can then be determined efficiently using the SISAR technique.
Regardless of the exact kdr-type allele frequency across Canada and the United States, there is obviously a critical need for a reassessment of an approach to management of head lice infestations that balances effectiveness and safety with treatment expense and the need to use treatments that have novel modes of action, which do not elicit cross-resistance to the widely used pyrethrins- or pyrethroid-based products and organophosphorus- and carbamate-based products. The recent development and commercialization of topically applied pediculicides containing benzyl alcohol (Ulesfia, Sciele Pharma, Atlanta, GA), ivermectin (Sklice, Sanofi Pasteur, Swiftwater, PA), and spinosad (Natroba, ParaPRO LLC, Carmel, IN) will certainly relieve some of the insecticide selection pressure of the pyrethrin- and pyrethroid-based pediculicides on louse populations and provide more effective control of pediculosis (Clark et al. 2013).
Acknowledgments
Canadian head louse collections were provided by Nycomed Canada Inc. Head louse collection from the United States were provided by Topaz Pharmaceuticals, LLC, Jenkintown, PA. This research was supported by the NIH/NIAID (R01 AI045062-04A3 and 1 R56 AI081933-01A2).
References Cited
- Bainbridge CV, Klein GL, Neibart SI, Hassman H, Ellis K, Manring D, Goodyear R, Newman J, Micik S, Hoehler F, et al. Comparative study of the clinical effectiveness of a pyrethrin-based pediculicide with combing versus a permethrin-based pediculicide with combing. Clin. Pediatr. (Phila) 1998;37:17–22. doi: 10.1177/000992289803700103. [DOI] [PubMed] [Google Scholar]
- Burgess IF, Brown CM, Lee PN. Treatment of head louse infestation with 4% dimeticone lotion: randomised controlled equivalence trial. BMJ. 2005;330:1423. doi: 10.1136/bmj.38497.506481.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhart CG, Burkhart CN. Clinical evidence of lice resistance to over-the-counter products. J. Cutan. Med. Surg. 2000;4:199–201. doi: 10.1177/120347540000400405. [DOI] [PubMed] [Google Scholar]
- Carson DS, Tribble PW, Weart CW. Pyrethrins combined with piperonyl butoxide (RID) vs 1% permethrin (NIX) in the treatment of head lice. Am. J. Dis. Child. 1988;142:768–769. doi: 10.1001/archpedi.1988.02150070082031. [DOI] [PubMed] [Google Scholar]
- Chosidow O, Chastang C, Brue C, Bouvet E, Izri M, Monteny N, Bastuji-Garin S, Rousset J-J, Revuz J. Controlled study of malathion and d-phenothrin lotions for Pediculus humanus var capitis-infested schoolchildren. Lancet. 1994;344:1724–1727. doi: 10.1016/s0140-6736(94)92884-3. [DOI] [PubMed] [Google Scholar]
- Clark JM, Yoon KS, Lee SH, Pittendrigh BR. Human lice: Past, present and future control. Pestic. Biochem. Physiol. 2013;106:162–171. [Google Scholar]
- DiNapoli JB, Austin RD, Englender SJ, Gomez MP, Barrett JF. Eradication of head lice with a single treatment. Am. J. Public Health. 1988;78:978–980. doi: 10.2105/ajph.78.8.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong K, Scott JG. Linkage of kdr-type resistance and the para-homologous sodium channel gene in German cockroaches (Blattella germanica) Insect Biochem. Mol. Biol. 1994;24:647–654. doi: 10.1016/0965-1748(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Downs AMR, Stafford KA, Coles GC. Head lice: prevalence in schoolchildren and insecticide resistance. Parasitol. Today. 1999;15:1–4. doi: 10.1016/s0169-4758(98)01361-1. [DOI] [PubMed] [Google Scholar]
- Durand R, Bouvresse S, Berdjane Z, Izri A, Chosidow O, Clark JM. Insecticide resistance in head lice: clinical, parasitological and genetic aspects. Clin. Microbiol. Infect. 2012;18:338–344. doi: 10.1111/j.1469-0691.2012.03806.x. [DOI] [PubMed] [Google Scholar]
- Farnham AW. Genetics of resistance of house fly (Musca domestica) to pyrethroids. I. Knock-down resistance. Pestic. Sci. 1977;8:631–636. [Google Scholar]
- Frankowski BL, Weiner LB. Head lice. Pediatrics. 2002;110:638–643. [PubMed] [Google Scholar]
- Gao J-R, Yoon KS, Lee SH, Takano-Lee M, Edman JD, Meinking TL, Taplin D, Clark JM. Increased frequency of the T929I and L932F mutations associated with knockdown resistance in permethrin-resistant populations of the human head louse, Pediculus capitis, from California, Florida, and Texas. Pestic. Biochem. Physiol. 2003;77:115–124. [Google Scholar]
- Gratz NG. Switzerland: World Health Organization; 1997. Humanlice: their prevalence, control and resistance to insecticides. [Google Scholar]
- Hill N, Moor G, Cameron MM, Butlin A, Preston S, Williamson MS, Bass C. Single blind, randomised, comparative study of the Bug Buster kit and over the counter pediculicide treatments against head lice in the United Kingdom. BMJ. 2005;331:384–387. doi: 10.1136/bmj.38537.468623.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipolito RB, Mallorca FG, Zuniga-Macaraig ZO, Apolinario PC, Wheeler-Sherman J. Head lice infestation: single drug versus combination therapy with one percent permethrin and trimethoprim/sulfamethoxazole. Pediatrics. 2001;107:E30. doi: 10.1542/peds.107.3.e30. [DOI] [PubMed] [Google Scholar]
- Hodgdon HE, Yoon KS, Previte DJ, Kim HJ, Aboelghar GE, Lee SH, Clark JM. Determination of knockdown resistance allele frequencies in global human head louse populations using the serial invasive signal amplification reaction. Pest Manag. Sci. 2010;66:1031–1040. doi: 10.1002/ps.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter JA, Barker SC. Susceptibility of head lice (Pediculus humanus capitis) to pediculicides in Australia. Parasitol. Res. 2003;90:476–478. doi: 10.1007/s00436-003-0881-y. [DOI] [PubMed] [Google Scholar]
- Jones KN, English JC., 3rd Review of common therapeutic options in the United States for the treatment of pediculosis capitis. Clin. Infect. Dis. 2003;36:1355–1361. doi: 10.1086/374840. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Symington SB, Lee SH, Clark JM. Serial invasive signal amplification reaction for genotyping permethrin-resistant (kdr-like) human head lice, Pediculus capitis. Pestic. Biochem. Physiol. 2004;80:173–182. [Google Scholar]
- Knipple DC, Doyle KE, Marsella-Herrick PA, Soderlund DM. Tight genetic linkage between the kdr insecticide resistance trait and a voltage-sensitive sodium channel gene in the house fly. Proc. Natl. Acad. Sci. U.S.A. 1994;91:2483–2487. doi: 10.1073/pnas.91.7.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen M. Identification of sodium channel mutations in human head louse (Anoplura: Pediculidae) from Denmark. J. Med. Entomol. 2005;42:826–829. doi: 10.1093/jmedent/42.5.826. [DOI] [PubMed] [Google Scholar]
- Kwon DH, Yoon KS, Strycharz JP, Clark JM, Lee SH. Determination of permethrin resistance allele frequency of human head louse population by quantitative sequencing. J. Med. Entomol. 2008;45:912–920. doi: 10.1603/0022-2585(2008)45[912:dopraf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Lebwohl M, Clark L, Levitt J. Therapy for head lice based on life cycle, resistance, and safety considerations. Pediatrics. 2007;119:965–974. doi: 10.1542/peds.2006-3087. [DOI] [PubMed] [Google Scholar]
- Lee SH, Yoon KS, Williamson MS, Goodson SJ, Takano-Lee M, Edman JD, Devonshire AL, Clark JM. Molecular analysis of kdr-like resistance in permethrin-resistant strains of head lice, Pediculus capitis. Pestic. Biochem. Physiol. 2000;66:130–143. [Google Scholar]
- Lee SH, Gao J-R, Yoon KS, Mumcuoglu KY, Taplin D, Edman JD, Takano-Lee M, Clark JM. Sodium channel mutations associated with knockdown resistance in the human head louse, Pediculus capitis (De Geer) Pestic. Biochem. Physiol. 2003;75:79–91. [Google Scholar]
- Lee SH, Clark JM, Ahn YJ, Lee W-J, Yoon KS, Kwon DH, Seong KM. Molecular mechanisms and monitoring of permethrin resistance in human head lice. Pestic. Biochem. Physiol. 2010;97:109–114. [Google Scholar]
- Marcoux D, Palma KG, Kaul N, Hodgdon H, Van Geest A, Previte DJ, Abou-Elghar GE, Yoon KS, Clark JM. Pyrethroid pediculicide resistance of head lice in Canada evaluated by serial invasive signal amplification reaction. J. Cutan. Med. Surg. 2010;14:115–118. doi: 10.2310/7750.2010.09032. [DOI] [PubMed] [Google Scholar]
- Meinking TL, Villar ME, Vicaria M, Eyerdam DH, Paquet D, Mertz-Rivera K, Rivera HF, Hiriart J, Reyna S. The clinical trials supporting benzyl alcohol lotion 5% (Ulesfia): a safe and effective topical treatment for head lice (pediculosis humanus capitis) Pediatr. Dermatol. 2010;27:19–24. doi: 10.1111/j.1525-1470.2009.01059.x. [DOI] [PubMed] [Google Scholar]
- Mumcuoglu KY, Hemingway J, Miller J, Ioffe-Uspensky I, Klaus S, Ben-Ishai F, Galun R. Permethrin resistance in the head louse Pediculus capitis from Israel. Med. Vet. Entomol. 1995;9:427–432. doi: 10.1111/j.1365-2915.1995.tb00018.x. [DOI] [PubMed] [Google Scholar]
- Pariser DM, Meinking TL, Bell M, Ryan WG. Topical 0.5% ivermectin lotion for treatment of head lice. N. Engl. J. Med. 2012;367:1687–1693. doi: 10.1056/NEJMoa1200107. [DOI] [PubMed] [Google Scholar]
- Picollo MI, Vassena CV, Casadio AA, Massimo J, Zerba EN. Laboratory studies of susceptibility and resistance to insecticides in Pediculus capitis (Anoplura; Pediculidae) J. Med. Entomol. 1998;35:814–817. doi: 10.1093/jmedent/35.5.814. [DOI] [PubMed] [Google Scholar]
- Pollack RJ, Kiszewski A, Armstrong P, Hahn C, Wolfe N, Rahman HA, Laserson K, Telford SR, III, Spielman A. Differential permethrin susceptibility of head lice sampled in the United States and Borneo. Arch. Pediatr. Adolesc. Med. 1999;153:969–973. doi: 10.1001/archpedi.153.9.969. [DOI] [PubMed] [Google Scholar]
- Rupes V, Moravec J, Chmela J, Ledvinka J, Zelenkova J. A resistance of head lice (Pediculus capitis) to permethrin in Czech Republic. Cent. Eur. J. Public Health. 1995;3:30–32. [PubMed] [Google Scholar]
- Stough D, Shellabarger S, Quiring J, Gabrielsen AA., Jr Efficacy and safety of spinosad and permethrin creme rinses for pediculosis capitis (head lice) Pediatrics. 2009;124:e389–e395. doi: 10.1542/peds.2008-3762. [DOI] [PubMed] [Google Scholar]
- Taplin D, Meinking TL, Castillero PM, Sanchez R. Permethrin 1% creme rinse for the treatment of Pediculus humanus var capitis infestation. Pediatr. Dermatol. 1986;3:344–348. doi: 10.1111/j.1525-1470.1986.tb00538.x. [DOI] [PubMed] [Google Scholar]
- Tomita T, Yaguchi N, Mihara M, Takahashi M, Agui N, Kasai S. Molecular analysis of a para sodium channel gene from pyrethroid-resistant head lice, Pediculus humanus capitis (Anoplura: Pediculidae) J. Med. Entomol. 2003;40:468–474. doi: 10.1603/0022-2585-40.4.468. [DOI] [PubMed] [Google Scholar]
- Williams LK, Reichert A, MacKenzie WR, High-tower AW, Blake PA. Lice, nits, and school policy. Pediatrics. 2001;107:1011–1015. doi: 10.1542/peds.107.5.1011. [DOI] [PubMed] [Google Scholar]
- Williamson MS, Denholm I, Bell CA, Devonshire AL. Knockdown resistance (kdr) to DDT and pyrethroid insecticides maps to a sodium channel gene locus in the housefly (Musca domestica) Mol. Gen. Genet. 1993;240:17–22. doi: 10.1007/BF00276878. [DOI] [PubMed] [Google Scholar]
- Yoon KS, Symington SB, Lee SH, Soderlund DM, Clark JM. Three mutations identified in the voltage-sensitive sodium channel α-subunit gene of permethrin-resistant human head lice reduce the permethrin sensitivity of house fly Vssc1 sodium channel expressed in Xenopus oocytes. Insect Biochem. Mol. Biol. 2008;38:296–306. doi: 10.1016/j.ibmb.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]