Abstract
Insects are infected by a wide array of viruses some of which are insect-restricted and pathogenic, and some of which are transmitted by biting insects to vertebrates. The medical and economic importance of these viruses heightens the need to understand the interaction between the infecting pathogen and the insect immune system in order to develop transmission interventions. The interaction of the virus with the insect host innate immune system plays a critical role in the outcome of infection. The major mechanism of antiviral defense is the siRNA pathway that responds through the detection of virus-derived dsRNA to suppress virus replication. However, other innate antimicrobial pathways such as Imd, Toll, Jak-STAT, and the autophagy pathway have also been shown to play important roles in antiviral immunity. In this review we provide an overview of the current understanding of the main insect antiviral pathways and examine recent findings that further our understanding of the roles of these pathways in facilitating a systemic and specific response to infecting viruses.
Introduction
Viruses are obligate intracellular pathogens with a limited coding capacity mandating the sequestration of cellular resources to promote their replication. Viruses that infect insects have huge consequences economically and medically. Insect transmitted arboviruses, such as the dengue viruses (DENV) and yellow fever virus (YFV), place billions of people across the globe at risk of life-threatening diseases. Obtaining a deeper understanding of the biology of the virus within the insect host and the host response to the virus provides the potential for the development of novel transmission interventions.
In insects, the innate response plays the major role in the control and clearance of pathogens following infection, although there is some evidence for an immune response that resembles the vertebrate adaptive response 1; 2. The innate immune system is characterized by the activation of pattern recognition receptors (PRRs) capable of binding pathogen-associated molecular patterns (PAMPs), molecules present in the pathogen but not found in the host. Binding of PAMPs leads to the activation of signaling pathways resulting in the production of effector molecules capable of suppressing pathogen replication. This system provides the first line of defense against invading pathogens. In insects, the innate response is robust and may function to clear infection in the case of true insect pathogens; however, in the case of arbovirus infection, the insect innate response limits pathogenesis but does not clear the infection allowing transmission of the virus to a vertebrate host. In fact, it can be seen that arbovirus infection of insects in which the innate immune system has been compromised can result in increased viral load, morbidity or mortality suggesting that the innate immune system is engaged and necessary for vector survival 3; 4; 5; 6; 7; 8; 9.
When challenged with viruses, the most robust insect response is through the RNAi pathway that utilizes virus generated double-stranded RNA (dsRNA) to produce small, interfering RNAs (siRNA) that function to target viral RNA for degradation and hence inhibit replication 9; 10; 11; 12. Additionally, signal transduction pathways resulting in changes in cellular gene expression are an important component of the antiviral innate immune system. Nf-κB pathways, Toll and Imd, have been well characterized as essential in the immune response to bacteria and fungi (reviewed in Lemaitre et al. 13). Evidence from studies in Drosophila and mosquitoes indicates that these pathways also play a role in antiviral defenses 14; 15; 16; 17. There is also growing evidence that the Jak-STAT pathway may be functionally analogous to the mammalian interferon system 18. This pathway is typically activated in uninfected bystander cells resulting in alterations in cellular transcription and downstream antiviral activity 4.
In vertebrates, the innate immune system signals for an immediate response to infection that potentiates a systemic and specific adaptive response resulting in immune memory. While insects lack an orthologous adaptive response it is becoming apparent that innate immune pathways are connected and give rise to a systemic antiviral immune response that is specific and has the potential to last beyond the duration of a given viral infection. In this review we will provide an overview of the current understanding of these antiviral pathways, and examine evidence for the connectivity of pathways and a systemic, specific antiviral response.
RNAi Antiviral Response
RNA interference in insects plays a significant role in limiting and controlling virus infection 9; 10; 11; 12. There are currently three well characterized RNAi-related pathways (reviewed in Kim et al. 19): (i) small interfering RNA pathway in which siRNAs are generated from dsRNA derived either from exogenous sources such as virus infection or encoded by the cell genome, (ii) micro-RNA pathway in which miRNAs are generated from cell-encoded transcripts and ultimately function to regulate gene expression generally at the level of translation, and (iii) PIWI-interacting RNA pathway in which piRNAs are transcribed from the cellular genome and do not require processing to function in the epigenetic control of genomic elements in the germ line. The pathway predominantly responsible for antiviral activity in insects is the siRNA pathway and this will be the main focus of the following section.
siRNA Pathway Components
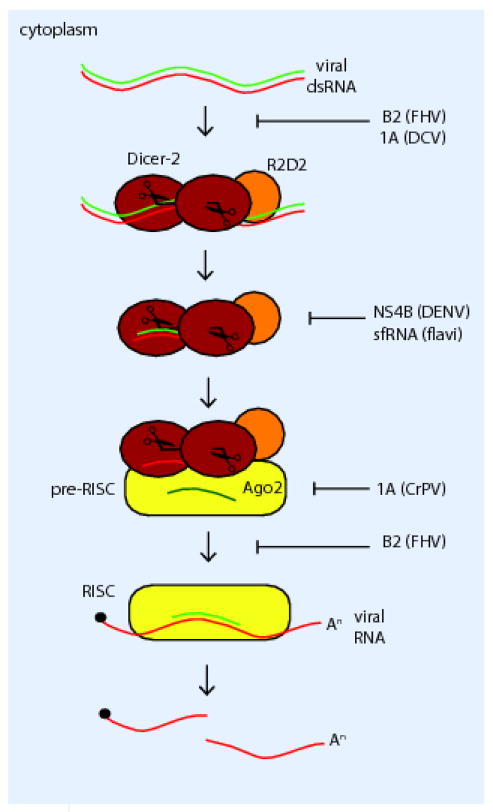
Virus-derived siRNAs are generated through the recognition and processing of double-stranded RNAs (dsRNA) produced during virus infection (reviewed in Ding et al. 20). The dsRNA can occur as a replication intermediate for single-stranded RNA viruses, due to secondary structure in viral RNAs, or as a consequence of base pairing of convergent transcripts. Recognition of the dsRNA by Dicer proteins (members of the RNaseIII family of endoribonucleases) results in the production of siRNA (Figure 1). These are loaded into the pre-RNA induced silencing complex (RISC). Unwinding of the duplex occurs along with guide strand selection that defines target specificity on the basis of complementarity. Targeted RNA is degraded through the RNase activity of Argonaute 21.
Fig. 1. siRNA pathway.
Cytoplasmic, virus-derived dsRNA is recognized by Dicer-2 in complex with R2D2. Dicer-2 cleaves the dsRNA into 21nt siRNAs. The siRNA is loaded into the pre-RISC complex where duplex unwinding and selection of a guide strand occurs. The guide strand functions to direct RISC to the viral RNA target through base-pairing. The Ago2 protein in the RISC cleaves the viral RNA target inhibiting virus replication. Points in the pathway at which viral suppressors of RNAi function are shown.
Most of the characterization of the virus-derived siRNA pathway in insects has been performed in Drosophila. Flies possess two Dicer proteins; Dicer-1 is required for the production of miRNA, whereas Dicer-2 is necessary for processing dsRNA in order to generate siRNA 22; 23. Dicer-2 functions as the activating PRR for the siRNA pathway binding to dsRNA and cleaving it to small 21 nt siRNAs with 2 nt 3′ overhangs. For exogenous dsRNA (such as virus-derived dsRNA) processing by Dicer-2 requires R2D2 and is independent of Loquacious function, whereas Dicer-2 processing of endogenously produced dsRNA requires both of these dsRNA binding proteins24; 25. Dicer-2 also functions as a PRR for the activation of the Jak-STAT pathway in Cx. quinquefasciatus cells 18. Recognition of virus-derived dsRNA by Dicer-2 leads to the secretion from infected cells of a Jak-STAT activating ligand leading to the establishment of an antiviral state in uninfected, responsive cells. This function provides a direct connection between the siRNA pathway and the Jak-STAT pathway implying a deliberate coordination of these responses.
R2D2 and Dicer-2 are the key components of the RISC-loading complex (RLC) that along with Argonaute-2 (Ago-2), the catalytic component of the RISC, selects the guide strand of the siRNA and loads it into the RISC. This guide strand serves to specify the RNA substrate for Ago-2 mediated cleavage resulting in silencing 21; 26.
Flies with null or loss of function mutations of dcr-2 or ago-2 fail to control virus replication and develop disease far more readily than wild-type counterparts 9; 10; 11; 12. Virus-derived siRNAs have been detected in Aedes spp. mosquito cells during infection with arboviruses 7; 27; 28; 29. Inhibition of the RNAi pathway results in greater virus accumulation and increased pathogenesis in infected mosquitoes. These lines of evidence clearly demonstrate the importance of this pathway in the antiviral immune response. Mutations in the pathway do not significantly affect anti-bacterial or anti-fungal immunity, emphasizing the specificity of this response to viruses 20.
Viral Suppressors of RNAi
Perhaps the strongest evidence for the importance of the RNAi pathway in controlling virus infection is the encoding of RNAi suppressor proteins by viral pathogens of insects. The B2 and 1A proteins produced by members of the Nodaviridae and Dicistroviridae respectively have been shown to suppress the RNAi response during infection 12; 20; 30; 31. The absence of these proteins results in reduced virus yield or spread, and clearance of the infection by the insect; however, viruses lacking the RNAi suppressors can productively infect animals deficient in components of the RNAi machinery such as dcr-2 and ago-2.
The Flock House virus (FHV) B2 protein functions as a dimer and inhibits the RNAi pathway by binding to dsRNA thus preventing cleavage of dsRNA by Dicer-2 32; 33 (Figure 1). Additionally, should dsRNA be cleaved by Dicer-2, B2 binding to short dsRNA molecules can block loading into the RISC complex 33. Members of the Dicistroviridae express a suppressor of RNAi, protein 1A. This protein functions differently for different members of the family 31. Drosophila C virus (DCV) 1A is functionally analogous to FHV B2, binding to dsRNA and preventing processing. In contrast the 1A protein of cricket paralysis virus (CrPV) interacts with Ago-2 inhibiting its activity. CrPV 1A is a drastically more potent RNAi suppressor, causing a decrease in insect survivability; whereas, DCV 1A suppresses the RNAi pathway to a lesser extent allowing for survival and the development of a persistent infection 31. Thus closely related viruses have evolved different mechanisms of overcoming the antiviral activity of the RNAi pathway.
While it is apparent that viral pathogens of insects encode suppressors of RNAi, the situation with arboviruses is less clear. Arbovirus infection of insects results in a persistent infection with little associated pathogenesis. It is apparent from in vivo and cell culture systems that, while infection is controlled by the RNAi response, it is not cleared 7; 27; 28. Thus it appears that arboviruses have evolved mechanisms of evading the full effect of the RNAi response in order to maintain a persistent infection. One hypothesis is that the replication intermediates are sequestered in compartments that are inaccessible to the RNAi machinery. In this scenario RNA generated by the replication factories and released into the cytoplasm is susceptible to recognition and silencing by the RNAi machinery; the required templates for generating new virus are compartmentalized and hence protected.
However, Schnettler et al. have provided direct evidence for an arbovirus encoded suppressor of RNAi 34. A subgenomic RNA (sfRNA) corresponding to the 3′UTR of the flavivirus genome functions to counter the RNAi response in both mammalian and insect cells. This RNA inhibits the processing of dsRNAs by human Dicer in vitro, suggesting that it directly disrupts the function of Dicer in cells to suppress the RNAi response. Interestingly while this study found no RNAi suppressor function associated with West Nile virus (WNV) proteins, a subsequent study has reported that the NS4B protein of another flavivirus, DENV-2, functions to suppress miRNA and siRNA pathways in mammalian and insect (Sf21) cells 35.
Systemic RNAi response
Over the past decade evidence has accumulated to support the idea of a systemic RNAi response to virus infection in insects (reviewed in detail in Karlikow et al. 36). Initial evidence was obtained from distant silencing of endogenous genes following in vivo dsRNA uptake. This was first observed in Tribolium castaneum (flour beetle) following injection of dsRNA into the haemocoel of adult beetles 37. The effect was transferred to offspring, demonstrating transfer of the signal across cell boundaries. The idea of RNAi spread was further bolstered by observations that infection of Drosophila with Sindbis virus (SINV) expressing a non-viral gene (GFP) could suppress expression of the gene at sites distant from infection 38. These lines of evidence point to a systemic RNAi silencing pathway. The nature of the transmitted molecules, the mechanism by which they are passed from cell to cell and a presumed mechanism by which the signal is amplified are currently unknown; however, evidence related to some of these issues is emerging.
Studies using cultured Drosophila cells indicate that long dsRNAs, but not siRNAs, are internalized when simply added to culture medium 39; 40. This uptake is dependent on the cell surface receptors Sr-CI and Eater, and clathrin-dependent endocytosis 40; however, these receptors do not possess obvious nucleic acid binding motifs. This suggests that the RNA may be associated with other molecules in the form of a vesicle and/or ribonucleoprotein (RNP) complex to protect the dsRNA signal and to promote uptake into naïve cells. Exosomes represent a mechanism of intercellular molecular transport and are an attractive candidate as the mode of dissemination of the dsRNA-based immune signal 41. They are found in numerous extracellular fluids and carry various cargo including components of the RNAi silencing machinery 42. Another hypothesis is that infected cells secrete RNPs that comprised of components necessary for RNA silencing. Evidence of circulating RNPs containing miRNA and Ago in human plasma raises the possibility of secreted RNPs being largely responsible for the transmission of the immune signal 43.
If a dsRNA (or similar) signal is to be passed from an infected to cell to a naïve cell the problem of signal dilution must be overcome. Unlike plants and nematodes, insects do not possess an RNA-dependent RNA polymerase to amplify an incoming dsRNA 20; however, recently Goic et al. have reported a mechanism whereby virus-derived RNA is reverse transcribed into dsDNA and integrated into retrotransposable elements in the genome of Drosophila cells 44. This observation was consistent with the previous finding in mosquito system that sequences from flavivirus genome were present in the genomes of both cultured cells and wild type mosquito strains45. The DNA copies of virus sequence can give rise to dsRNAs that are utilized in siRNA-based immunity. This was reported to occur in cells infected with pathogenic Drosophila viruses as well as SINV and led to virus control and the establishment of a persistent infection. It is tempting to speculate that if dsRNA is released from infected cells and can be taken up by naïve cells then it could in turn be reverse transcribed and stabilized in the cell genome providing a mechanism of resistance to virus infection. Thus a dsRNA signal is effectively amplified through the integration of a DNA copy into the genome followed by continuous transcription of dsRNA.
While evidence for the cell-to-cell transmission of RNAi is mounting in Drosophila, this may not be the case for mosquito species. Arbovirus spread within an infected mosquito is necessary for continued transmission. In addition to spread to salivary glands certain viruses can be transmitted transovarially by mosquitoes. This would indicate that there are differences between insect species in terms of systemic spread of RNAi, or that certain tissues are more capable of participating in such a systemic response 46; 47; 48; 49.
Antiviral effects of other small RNA pathways
The role of miRNAs and piRNAs in insect antiviral immunity is not clear. The role of miRNAs in viral pathogenesis has been more deeply studied in mammalian systems in which virus induction of cellular miRNAs and production of virus encoded miRNAs have been shown to influence the outcome of virus infection and regulation of the cellular response50; 51; 52. The production of an miRNA from a subgenomic RNA corresponding to the 3′ untranslated region of WNV has been demonstrated in Ae. albopictus cells, but not vertebrate cells. This miRNA regulates the synthesis of GATA4 by the cell resulting in a proviral effect 53. It is not yet apparent whether this miRNA-like activity is functionally related to the suppression of RNAi activity also assigned to this subgenomic RNA.
piRNAs of viral origin have been observed in mosquito cells infected with a number of different arboviruses 54; 55. The role of these RNAs remains unresolved, however they may be involved in regulation of the antiviral response as their presence precedes the siRNA response then wanes as the siRNA response increases suggesting a regulatory role. Schnettler et al. have demonstrated an antiviral role for the piRNA pathway in response to Semliki Forest virus (SFV) 56. Knocking down components of the piRNA pathway resulted in increased levels of virus replication and accumulation, particularly in the absence of a Dicer-2 mediated siRNA response57. How directly piRNAs interfere with virus replication is not clear.
Jak-STAT pathway
In addition to the RNAi pathway, several signaling pathways also contribute to the antiviral response in insects. Initially characterized for its role in development and hemocyte proliferation, the Jak-STAT pathway has been shown to also respond to bacterial and viral infections by regulating the production of downstream effector molecules including antimicrobial peptides (AMPs). This pathway is activated in a paracrine fashion through the binding of secreted ligands. Activation appears to be consequent to initial recognition of infection by PRRs that also feed into other pathways. The mechanism by which the Jak-STAT pathway exerts its antiviral effect is not known, but it is apparent that the response is complex, specific and versatile.
Jak-STAT pathway components
The Jak-STAT pathway is a highly conserved insect innate immune signaling pathway that has been characterized primarily in Drosophila. The well-characterized pathway is comprised of one signal transducer and activator of transcription (STAT92E; hereafter referred to as STAT), a Janus kinase (Hopscotch), a receptor (Domeless) and 3 Unpaired-related ligands 58; 59; 60; 61; 62 (Figure 2). Binding of Upd induces dimerization of the receptor Domeless (dome), allowing transphosphorylation of the Hopscotch (hop) kinases. This is followed by the recruitment and trans-phosphorylation of the conserved tyrosine residue in the STAT transcription factors. Phosphorylated STATs dimerize and are transported to the nucleus to regulate the expression of downstream effector genes.
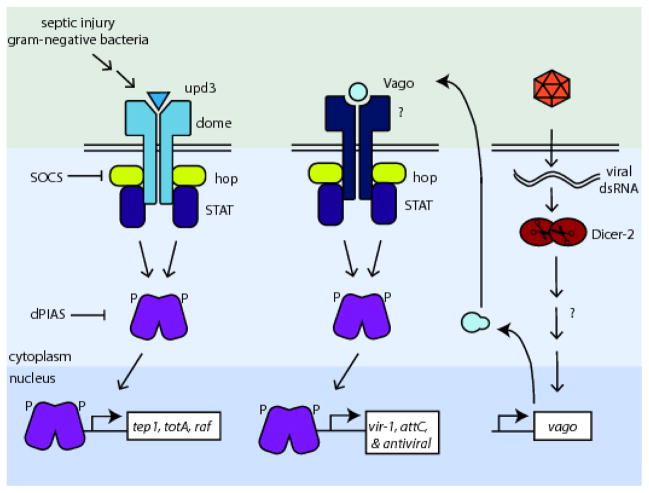
Fig. 2. Insect Jak-STAT pathway.
The pathway is initiated by the binding of a ligand (upd3 or vago) to their receptor (dome or unknown receptor, respectively.) After dimerization of the receptor, the associated Janus kinases (hop) transphosphorlyate each other, recruit and phosphorylate STATs, resulting in translocation to the nucleus and regulation of transcription. SOCS and dPIAS are known to act as inhibitors of hop or STAT, respectively. It was recently found that recognition of viral dsRNA by Dicer2 induces the expression of vago in an RNAi-independent mechanism. Vago is secreted and can activate the Jak-STAT pathway through an as-yet uncharacterized receptor, inducing the transcription of vir-1, attC, and potentially other antiviral molecules.
In addition to these characterized pathway components, several splice variants have been predicted for STAT, hop, and dome in the Drosophila genome and two alternatively spliced STAT isoforms have been identified in Spodoptera frugiperda (army worm) cells 63. In the nucleus, the STAT DNA-binding domain binds the canonical TTCNNNGAA binding site, where C3 and G7 are the critical residues; however, this binding site has been shown to have flexibility in the 5′ Ts, 3′ As, and/or the linker region 62. Both S. frugiperda STAT isoforms show a weak binding affinity to the canonical STAT DNA binding site, but it is unclear whether these isoforms lead to differential expression of downstream effectors 63. Mammalian STATs have additional modifications including serine phosphorylation, acetylation, and glycosylation that alter their transcriptional activity and interactions with co-activators and other transcription factors (reviewed in Schindler et al. 64). The modifications of the arthropod STAT orthologs are not yet known.
A mosquito STAT ortholog has been characterized in An. gambiae and is most similar to Drosophila STAT and mammalian STAT-5 with between 37.6–51.2% protein sequence identity in the conserved central region containing the DNA binding region, the SH2 phosphotyrosine-binding region and a conserved tyrosine known to be phosphorylated by hop 65. In addition, STAT orthologs have also been identified in Ae. albopictus and Cx. tritaeniorhynchus and contain the conserved tyrosine and high levels of protein sequence identity in the DNA-binding and SH2 domains 66. While there is divergence in the C-terminal tail activation region of all mosquito STATs, the Ae.albopictus and Cx.tritaeniorhynchus STATs have significantly longer C-terminal activation domains, although the possible significance of this has not yet been determined. Orthologs of hop, dome, and STAT have been predicted in other mosquito species including Ae. aegypti, Cx. quinquefasciatus, and several Anopheles species.
The An.gambiae, Ae.albopictus, and Cx.tritaeniorhynchus mosquito STAT orthologs respond to bacterial infection through nuclear localization, DNA-binding, and transcriptional activation of a luciferase reporter gene, thereby confirming a conserved Jak-STAT pathway activation and immune function in mosquitoes 65; 66.
Jak-STAT pathway ligands
The Drosophila genome encodes three Upd-like ligands; however, the Upd amino acid sequence is divergent among Drosophila species and has not been found in other insect genomes 67. All three ligands are glycosylated proteins secreted by hemocytes and signal non-autonomously by binding to the dome receptor of fat body cells to activate the Jak-STAT pathway. Of the three Upd-like ligands, Upd and Upd2 are involved in development, segmentation, and hemocyte proliferation and differentiation 68. Although Upd and Upd2 are thought to be semi-redundant, Upd is associated with the extracellular matrix while Upd2 is freely diffusible 59; 69. Upd3 is freely diffusible and plays a role in responding to septic injury and Gram-negative bacterial infection 68; 69. Upd has been shown to be a more potent activator of a STAT-responsive promoter (6×2xDrafLuc) than Upd3 or Upd2, respectively 69; however, as will be discussed, not all STAT-responsive genes are activated equally by a given stimulus.
Recently, a novel ligand of the Jak-STAT pathway has been identified. Deddouche et al. first identified vago mRNA as upregulated by DCV and SINV infection and coding for a protein with a single von Willebrand factor type C motif in Drosophila 70. Paradkar et al. characterized Vago as a secreted protein that activates the Jak-STAT pathway during WNV infection in Cx. quinquefasciatus cells 18 (Figure 2). Interestingly, the protein does not bind the dome receptor. An alternative receptor-ligand pair contributes a new level of complexity to our understanding of the Jak-STAT pathway in insects. Additionally, vago mRNA expression is dependent on Dicer-2 but no other RNAi pathway components 70. This suggests an RNAi-independent signaling mechanism for Dicer-2 and a link between the RNAi and Jak-STAT pathways. Interestingly the Dicer-2-dependent activation of vago expression also appears to be virus specific. In the study by Paradkar et al. only WNV dsRNA was able to stimulate vago production, poly I:C and bluetongue virus dsRNA did not cause vago expression to increase. While the mechanism of discrimination of these RNA species is not clear, it implies a tailoring of the immune response for specific viruses 18.
Regulation of the Jak-STAT pathway and responsive genes
The pathway can be regulated by a variety of methods including dephosphorylation, nuclear export, or negative feedback from two known inhibitors of hop or STAT, Suppressor of Cytokine Signaling 36E (SOCS36E) and Drosophila Protein Inhibitor of Activated STAT (dPIAS) respectively 71; 72 (Figure 2). Arboviruses can also regulate the pathway by disrupting phosphorylation or nuclear localization of STATs in vertebrates 73; 74; 75; 76; 77; however, the interaction between the viruses and the Jak-STAT pathway is not clear in arthropods. Interestingly, during Japanese encephalitis virus (JEV) infection of mosquito cells (Ae. albopictus, C6/36), there was a decrease in STAT tyrosine phosphorylation, indicating the inhibition of the STAT pathway during JEV infections although no viral inhibitors of the pathway have been identified 66. This could be explained due to the lack of Dicer-2 in the C6/36 cell line, further supporting the Dicer-dependent activation of the pathway through Vago28; 78. The absence of Dicer-2 siRNA associated activity in this cell line may make it of use when trying to determine the connection between the RNAi and Jak-STAT pathways.
Several downstream effector genes have been identified as responsive to the Jak-STAT pathway. Although septic injury and bacterial and viral infections can activate the Jak-STAT pathway, studies using a variety of viruses and model organisms show distinct gene expression profiles associated with virus infections. During septic injury, the Jak-STAT pathway induces the expression of turandot A (totA) in Drosophila 68. The expression of totA is activated in the fat body and dependent on both dome and Upd3 68. Additional members of the tot family, particularly totC and totM, are also thought to be regulated by the Jak-STAT pathway and respond to stress 79, although the specific function of these proteins is not known. Tep1, a thioester-containing protein similar to mammalian complement C3/alpha2 macroglobulin was also shown to be upregulated in response to septic injury and bacterial challenge in An. gambiae. Mutational analysis indicates that Tep1 is involved in promoting phagocytosis 80. Additionally, D-raf, a known component of the Ras/Raf pathway, was shown to be regulated by Jak-STAT in Drosophila during septic injury and involved in hemocyte proliferation; however, its function, if any, during viral infection is unclear. It does, however, suggest a connection between the Jak-STAT and Ras/Raf signaling pathways 81.
Jak-STAT response to virus infection
Many studies have contributed data involving the Jak-STAT response with a variety of viruses using cells, tissues, and whole organism approaches. Jak-STAT is activated by both pathogenic viruses (e.g. DCV, FHV) and arboviruses, which show little pathogenesis (e.g. SINV, DENV). Jak-STAT was first shown to be involved in the antiviral response to a pathogenic DCV infection in flies. Dostert et al. demonstrated that the Jak-STAT pathway was activated and resulted in changes in the transcription profile of DCV infected flies 82. Genes possessing upstream STAT-binding sites such as vir-1, CG9080, and CG12780 were upregulated due to DCV infection. In addition, expression of these genes was significantly decreased in hop mutant flies. The specific functions of these gene products are unknown; however, their expression is specific to virus infection 82. Further studies show that vir-1 is not upregulated by UV-inactivated DCV or viral dsRNA alone, indicating that viral replication is required; although, vir-1 does not appear to directly inhibit virus replication or production 83. Since these initial findings vir-1 expression has often been used as a read-out for Jak-STAT activation.
In regards to other pathogenic Drosophila viruses, Kemp et al. found that hop mutant flies (M38/msv1) were more sensitive to DCV and CrPV than wildtype flies 84. Survival significantly decreased and viral titers significantly increased in the virus-injected hop mutant flies as compared to wildtype. In terms of pathway activation, upd2 and upd3 mRNA levels were significantly higher in DCV and CrPV injected flies. Other pathogenic viruses, such as FHV and invertebrate iridescent virus 6 (IIV-6) did not significantly affect the survival of hop mutant flies 84.
Immune responses to arboviruses have also been studied. In DENV-infected mosquitos, virus titers increased in RNAi knockdowns of STAT pathway components or silenced STAT inhibitors indicating an anti-viral role for the pathway 4. Eighteen genes were identified as Jak-STAT-regulated and DENV-responsive and two of these were characterized as DENV response factors due to their increased expression in response to infection in Ae.aegypti mosquitoes (DVRF1 and DVRF24. In addition, Behura et al. showed increased mRNA expression of genes associated with the Jak-STAT pathway, including jak and stat, in both susceptible and refractory Ae. aegypti strains during DENV infection 85.
Similarly, SINV-replicon mRNA levels also increase in a stat mutant in Drosophila 14. Previously identified targets, vir-1, tep1, and D-raf were not found to be sensitive to SINV-replicon. When examining downstream Jak-STAT effectors, 61 SINV-sensitive genes had STAT binding sites and a subset was confirmed as STAT-responsive through STAT mutant analysis 3. Of these SINV-sensitive, STAT-regulated genes, the gene coding for an antimicrobial peptide, Attacin-C, was further characterized and found to have anti-viral function. Although the Jak-STAT pathway appears to be active during SINV-replicon replication and SINV infection 3; 14, Kemp et al. determined that the survival of hop mutant flies was not significantly different from wildtype flies 84. The survival of hop mutant flies also did not significantly decrease during other virus infections including VSV, FHV, DXV, and IIV-6, although this does not necessarily indicate lack of pathway activity 84. Jak-STAT activation in these experiments was determined by vir-1 mRNA expression.
Activation of the pathway by another alphavirus, SFV, was measured using a 6xdraf reporter in Ae. albopictus U4.4 cell lines 74. The cells did not show up-regulation of the D-raf reporter, but as we come to understand the complexity of the pathway, it is clear that single genes are not representative of activation in every scenario. This is clearly shown by studies that have examined and compared the post-infection transcriptomes of several virus infections. Kemp et al. compared the transcriptomes of DCV, FHV, and SINV at various time points and found that genes that were regulated differently between infections outnumbered those in common 84. Particularly, genes regulated by the Jak-STAT pathway were regulated in a different manner during infections with different viruses. Expression of vir-1 and totM mRNA was examined during DCV, CrPV, FHV, SINV, Vesicular stomatitis virus (VSV), DXV, and IIV-6 infections. vir-1 mRNA was upregulated during DCV, CrPV, and FHV infections, while totM was upregulated in SINV, VSV, DXV. It is intriguing that these two Jak-STAT markers do not overlap in any of the infections; however, it should be taken into account that other pathways may be involved such as MEKK1 activation of totM 84.
Interaction of Jak-STAT with Nf-κB pathways
STATs have been typically characterized as transcriptional activators; however, there is evidence of repressor activity. Drosophila STAT has been shown to form a complex with another transcription factor (dAP-1) and chromatin modifying proteins (Dsp1 and HDAC) to compete for Relish binding sites, thereby regulating Nf-κB immune responses 86.
On a similar note, Souza-Neto et al. found that a Toll ligand (spätzle), four cecropins, and one defensin were down-regulated when the Jak-STAT inhibitor dPIAS was depleted during DENV infection in Ae. aegypti midguts. This finding was confirmed by Colpitts et al. who also found the same four cecropins and defensin were down-regulated when they examined the transcriptome of Ae. aegypti after infection of flaviviruses, DENV, WNV, or YFV. While the mechanism is unclear, this suggests a connection between Jak-STAT activation and inhibition of Toll-regulated genes 4.
Nf-κB Innate Immune Pathways
The Imd and Toll pathways are Nf-κB -related pathways involved in innate immunity in arthropods and are activated upon bacterial and fungal infection in Drosophila. In Drosophila the Imd and Toll pathways activate cellular transcription mediated by two distinct orthologs of the Nf-κB transcription factor. Relish is the terminal transcription factor and Nf-κB ortholog for the Imd pathway, while Dorsal and Dorsal-related immune factor (Dif) function in the Toll pathway. Between the two Toll transcription factors, Dorsal plays a crucial role in early embryogenesis while Dif is involved in antimicrobial immunity 87.
Imd pathway components and signaling
In the Imd pathway, the diaminopimelic-containing peptidolglycan of Gram-negative bacteria and Bacillus spp. is sensed both extra- and intra-cellularly by PRRs, PGRP-LC and PGRP-LE, followed by the activation of the adaptor molecule IMD 88; 89; 90; 91 (Figure 3A). IMD is essential for the activation of Relish via a two-branched signaling pathway. The first branch phosphorylates Relish through the IKK complex 92; 93; 94, and the other branch cleaves the phosphorylated Relish by a caspase, Dredd. After phosphorylation followed by cleavage, the N-terminal DNA binding domain of Relish translocates to the nucleus and thus regulates transcription of effector genes 93; 95; 96; 97.
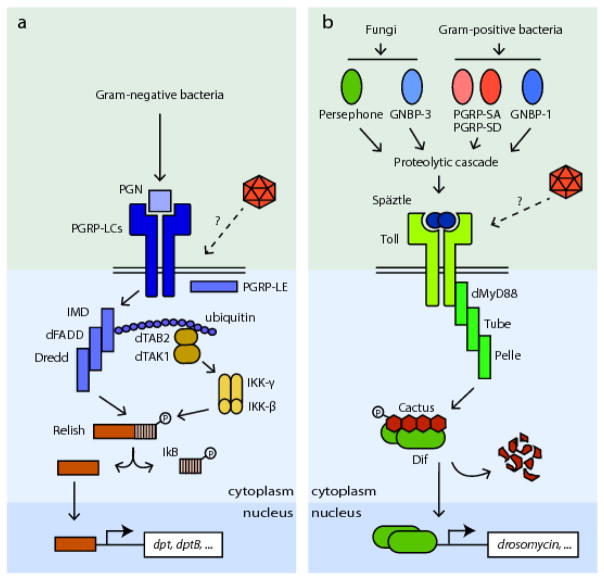
Fig. 3. Insect Nf-κB-related immune pathways.
A) Imd signaling pathway. Transmembrane receptors PGRP-LCs and intracellular receptor PGRP-LE sense the diaminopimelic-containing peptidolglycan of Gram-positive bacteria and Bacillus. The receptor molecules multimerize upon activation and transmit the signal to the adaptor molecule IMD. IMD activation recruits dFADD which recruits a caspase, DREDD. Activation of DREDD leads to polyubiquitination of IMD. TAK1 binds to the polyubiquitin chain and is responsible for the assembly and activation of the IKK complex (IKKβ and IKKγ). The IKK complex mediates the phosphorylation of Relish. Phosphorylated Relish is subsequently cleaved by DREDD, leaving the C-terminal ankyrin repeats in the cytosol. The N-terminal DNA binding domain of Relish translocates to the nucleus and regulates transcription of effector molecules such as diptericin and diptericinB. B) Toll signaling pathway. Toll pathway responds to Gram-positive bacteria and fungi infections. Lys-type peptidoglycan on bacteria cell wall is detected by PGRP-SA, PGRP-SD and GNBP-1. GNBP-3 detects β-glucan on fungal cell wall. Protease Persephone can also sense foreign proteolytic activities result from fungal infection. Both signals from bacteria and fungi are integrated into the same proteolytic cascade and thus lead to the activation of the Toll ligand, Spätzle. Spätzle binds to the Toll receptors and transduce the signals to Cactus-Dif through Toll-induced-signaling complex which contains MyD88, Tube and Pelle. Cactus is phosphorylated and cleaved from Dif, allowing Dif to translocate into nucleus and regulate the transcription of effector genes. The point at which viruses interact with each of these pathways is currently unresolved.
Toll pathway components and signaling
The antimicrobial responses of the Toll pathway are initiated by two detection methods (Figure 3B). The first method involves the PRRs, PGRP-SA, PGRP-SD and GNBP-1, which detect cell wall components of Gram-positive bacteria, as well as GNBP-3 that detects β-glucan on fungal cell wall 88; 98; 99; 100; 101; 102. The second method involves the protease Persephone (Psh) that senses foreign proteolytic activity including proteases secreted by fungi 102; 103. The signals from PRRs are integrated into a modular serine protease ModSP, which leads to the activation of another set of serine proteases including Grass, spirit, Spheroide, and Sphinx1/2 103; 104; 105. Prior to this, the Toll pathway ligand, Spätzle, is inactive with its Toll recognition site masked by a prodomain. Signals from the serine proteases and Psh can activate the Spätzle-processing enzyme. Processing of Spätzle removes the prodomain allowing binding to Toll receptors 106. Signal transduction through the pathway degrades a homolog of the mammalian IκB, Cactus, allowing the nuclear translocation of Dif and subsequent regulation of effector genes 107.
Nf-κB effectors in anti-bacterial and –fungal immunity
Both Relish and Dif regulate the transcription of a number of AMPs upon translocation. Relish has been shown to bind well to upstream sequences of AMPs, GGGGATCCCC and GGGGATTCCC, while Dif binds well to GGGAAAACCC. However, these binding sites are not highly conserved and may have great variations 108. The AMPs are secreted into the hemolymph by fat body cells, a counterpart of human liver. Knocking down AMP production leads to enhanced pathogenesis in flies during bacterial and fungal infection 109. Pathway specific AMPs like Drosomycin (Toll) and Diptericin (Imd) also serve as indicators of pathway activation.
Nf-κB pathways in mosquitoes
Genome sequences of An. gambiae, Ae. aegypti and Cx. quinquefasciatus indicate that mosquito species share a conserved antimicrobial immune pathway with Drosophila. Mosquitoes have two Nf-κB orthologs: Rel1 (An. gambiae) or Rel1A and Rel1B (Ae. aegypti) orthologous to Dorsal, and Rel2 (An. gambiae and Ae. aegypti) orthologous to Relish. No Dif orthologs have been identified in mosquitoes 110; 111; 112. Both the Dorsal orthologs and the Relish ortholog synergistically mediate the anti-bacterial response in An. gambiae and anti-fungal responses in Ae. aegypti 113; 114. The intracellular signaling module of the Toll and Imd pathways in mosquitoes is very similar to that in Drosophila, although genes involved in pathogen detection and effector molecules show some diversification, which is expected due to different selection pressures 112.
Imd and Toll pathways in antiviral immunity: pathogenic viruses
Both pathways have been shown to play a role in the antiviral immunity against arthropod pathogens. Knocking down Imd signaling components in Drosophila increased their mortality due to CrPV infection 115. Likewise, there were increased transcription levels of Imd components PGRP-SB1 and PGRP-SD, as well as downstream antimicrobial peptides in flies infected with a vertically transmitted arthropod pathogen, Sigma virus (SIGMAV) 116. Contradictory results finding no induction of Imd- or Toll- regulated genes were seen in another study examining SIGMAV infection using a different strain of flies 117. Recently, microarray analysis of Drosophila persistently infected with Nora virus, showed significant alteration of a gene involved in JNK signaling, an alternative Imd signaling branch 118; 119 Significantly less is known about the Toll pathway responses to pathogenic viruses in arthropods; although, Zambon et al. showed that flies with reduced levels of Dif, are more sensitive to infection with DXV as measured by survival 120. It should be noted that Dicer-2 mutations in Drosophila have little effect on both Nora virus and DXV infections implying that other pathways such as Nf-κB play a significant role in the control of these viruses 11; 121.
Imd and Toll pathways in antiviral immunity: arboviruses
The Imd and Toll pathways have also been shown to play a role in antiviral immunity against arboviruses. Flies harboring a SINV replicon demonstrated increased levels of genomic viral RNA replication when components of the Imd signaling pathway were mutated (Rel, FADD, DREDD, IMD, Ird5, Key) 122. A follow-up study showed that knocking down Imd-regulated AMP, DptB, resulted in developmental defects in flies harboring SINV replicon as well as enhanced viral replication and production when mutant flies were injected with SINV 3. SFV infection did not activate either Imd or Toll pathway as measured by reporter constructs of attA and drosomycin, respectively; however, pretreating U4.4 mosquito cells with heat-inactivated E. coli, thus activating the Imd pathway prior to SFV infection showed a decrease in the expression of both viral genome and subgenome suggesting that the Imd pathway has an antiviral effect early in SFV infection 123. Additionally, flies homozygous negative for rel demonstrated increased levels of virus replication when infected with SINV, SFV and Ross River virus (Avadhanula and Hardy, unpublished data).
In recent years, global transcriptome analysis has facilitated the studies with vectored viruses in mosquitoes, which lack the abundant genetic tools available in Drosophila. Microarray analysis of DENV infected Ae. aegypti showed increased expression of Toll pathway associated genes. Likewise, activating the Toll pathway in Ae. aegypti cells by silencing its negative regulator, cactus, resulted in lower DENV titers, while inactivating the pathway led to high viral titers 124. This antiviral effect of the Toll pathway was consistent with different DENV serotypes in both lab and field mosquitoes 125. In another study, the midgut transcription profiles of Ae. aegypti mosquitoes fed with SINV blood meal showed an induction of dif mRNA early in infection; this returned to uninfected levels later in infection 126. In An. gambiae infected with O’nyong-nyong virus, the Imd positive regulator Ikkb was inhibited while its downstream effector molecules CEC3 and dpt were upregulated; however, the antiviral role of the Imd pathway is still unclear as knocking down rel2 did not affect viral load. 127
Autophagy
In recent years, autophagy has been proposed as an alternative antiviral mechanism in insects that is independent of the Imd, Toll or JAK-STAT pathways 128; 129. Autophagy is the process by which de novo synthesized membranes encompass large cytoplasmic components including damaged organelles or protein aggregates, elongate and form closed double membrane vesicles named autophagosomes. These vesicles then fuse with lysosomes and degrade the engulfed materials. Under nutrient-deprived conditions, autophagy helps recycle nutrients and maintain cellular homeostasis 130; 131. The signaling of autophagy is mediated through the phosphoinositide 3-kinase (PI3K)-Akt pathway. Activation of the PI3K-Akt pathway increases the level of TOR, the negative regulator of autophagy, and thus inhibits this process 132.
Autophagy in innate immunity
Autophagy also plays a role in innate immunity and targets intracellular parasites or bacteria that enter the cytosol 129; 133; 134; 135. The antiviral effect of autophagy has been previously demonstrated in plants and mammals 136; 137; 138. Using a Drosophila model of human degenerative disease, Nezis et al. demonstrated that ref(2)P was associated with protein aggregates involved in autophagy 139; 140. Several studies have also found that ref(2)P determines the permissivity for SIGMA virus (Rhabdoviridae) infection in Drosophila141; 142; 143.
More direct studies on the antiviral role of autophagy were conducted in a Drosophila model successfully infected with VSV, another member of the Rhabdoviridae. Shelly et al. found that dsRNA knockdown of autophagy-related genes resulted in higher VSV production both in cultured S2 cells and in flies. Depleting one of these autophagy-related genes, Atg5, prevented the formation of virus-induced autophagosomes. The induction of autophagy has been shown to occur during virus entry as both UV-inactivated virus and replication-competent virus led to similar number of autophagosomes. Moreover, VSV glycoprotein (VSV-G) was sufficient for this induction. Therefore, rather than the viral replication complex or RNA replication intermediates, the PAMP triggering autophagy in this case is the VSV glycoprotein 144. Later, the same group proposed that one of the Drosophila TLR orthologs, Toll-7, was responsible for sensing VSV on the cell surface. Toll-7 signaling was activated upon VSV infection and dsRNA knockdown of Toll-7 led to higher viral protein level in vitro and greater pathogenesis in vivo 128. The signaling of this antiviral response is also through the PI3K-Akt pathway. Overexpression of a phosphatase blocking PI3K signaling, PTEN, led to lower viral protein production. Similarly, loss of Akt by dsRNA silencing in flies resulted in decreased viral protein level 144.
Interestingly, in other virus-host systems, viruses subvert autophagy to play a proviral role. In mammals, autophagy facilitates viral RNA replication and virion maturation of HCV, DENV, and poliovirus 145; 146; 147. In Ae. albopictus cells and Drosophila SINV replication complex activated PI3K-Akt signaling and enhanced cap-dependent translation including viral RNA translation148. One explanation for the contrast in the role for components of the autophagy pathway during VSV and SINV infection is that natural virus-host pairs have co-evolved and the virus has developed certain mechanisms of antagonizing or masking host defense responses 149. More studies with other viruses in both Drosophila and mosquitoes would be helpful to elucidate a universal or virus-specific role for autophagy in antiviral immunity.
Concluding remarks
Over the past two decades amazing progress has been made in understanding the innate immune system of insects. The discovery of Toll as an innate immune response receptor and the recognition of the importance of RNAi in response to virus infection have been major leaps forward 9; 10; 11; 12; 87. In recent years, the picture of the antiviral response in insects has become richer and more complex. Moving from a view of signaling pathways as isolated, independent responses it is now apparent that these pathways are integrated and responsive to one another, providing a pathogen-specific response.
The importance of dsRNA as a major PAMP triggering the innate immune response has been clearly demonstrated. Dicer proteins, particularly Dicer-2 in Drosophila and mosquitoes, are recognized as the main PRR involved in recognition of virus-derived dsRNA and initiating not only the RNAi response, but also the Jak-STAT response 18; 20; 70. This discovery is particularly significant in that the recognition of the dsRNA led to a specific response; only WNV dsRNA triggered the Dicer-2 dependent production and secretion of the Vago ligand by infected cells. This implies the ability for Dicer-2 to differentiate between dsRNA of different sources and consequently initiate an appropriate response. The question of how this is achieved warrants further attention, as does the question of whether Dicer-2 functions as a proximal PRR for other signaling pathways.
The activation of the Jak-STAT pathway by a previously unrecognized ligand also opens up new lines of investigation to identify the cellular receptor for Vago, and examine the possibility that other ligand-receptor pairs feed into the Jak-STAT pathway. This increased level of complexity may help explain the different STAT-dependent responses seen for different viruses and allow a greater level of versatility in the Jak-STAT response 84. In addition to the obvious connection between the siRNA pathway and Jak-STAT, it is apparent that the Jak-STAT pathway regulates the activity of the Nf-κB pathways at the level of transcriptional repression of Nf-κB-responsive genes 4; 86. These observations highlight the need for greater understanding of the connectivity of all the innate immune response pathways in order to elucidate the mechanisms by which pathogen-specific responses are elicited.
While the mechanism by which the siRNA response exerts its antiviral effect is well understood, far less is known about the effector molecules produced by the Jak-STAT and Nf-κB pathways. AMPs have been identified as being up-regulated during viral infections 3. AMPs have been traditionally associated with antibacterial and antifungal activity, however a human AMP, alpha defensin 5, has been found to stabilize the viral capsid, preventing uncoating and thus inhibiting viral infection 150; 151; 152. No antiviral molecular mechanism has yet been uncovered for arthropod AMPs, though Diptericin B and Attacin C have been implicated to have antiviral effect 3.
The intriguing idea that insects can mount a systemic immune response using the spread of an RNA-based signal to facilitate siRNA antiviral immunity is gaining more traction as evidence mounts in its support. Distant dsRNA-mediated gene silencing in conjunction with the remarkable discovery that reverse transcribed DNA copies of viral RNA are incorporated into the genome of the host cell and transcribed to participate in an siRNA response, changes the way we must think about this type of immunity 37; 38; 44. These findings imply a long-lasting, widespread and specific response to virus infection, capable of modulating pathogenesis, and possibly required for the establishment of persistent infection necessary for efficient arbovirus transmission. Further studies defining the nature of the transmitted signal and the mechanisms by which it is shared will allow a much deeper understanding of this aspect of insect antiviral immunity.
Finally, it is important to note that much of the current picture of the insect immune response has been elucidated in Drosophila. While Drosophila has proven an invaluable tool for understanding the virus-insect host interaction, it is important to keep in mind when looking at the vector-arbovirus interaction that the virus and mosquito immune pathways have co-evolved. Hence, as mosquito species become genetically more accessible it will be important to confirm findings from Drosophila studies in the natural vector species.
Highlights.
Innate immunity plays a critical role in the outcome of virus infection of insects
RNAi is a major mechanism of antiviral defense and is a systemic response
Multiple pathways are activated in response to virus infection
Connections between these pathways have been uncovered
Pathway interactions lead to a tailored immune response to viruses
Acknowledgments
We thank Pranav Danthi for critical reading of the manuscript. This work was supported by R01 AI AI090077 to R.W.H.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dong Y, Taylor HE, Dimopoulos G. AgDscam, a hypervariable immunoglobulin domain-containing receptor of the Anopheles gambiae innate immune system. PLoS Biology. 2006;4:e229. doi: 10.1371/journal.pbio.0040229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watson FL, Puttmann-Holgado R, Thomas F, Lamar DL, Hughes M, Kondo M, Rebel VI, Schmucker D. Extensive diversity of the Ig-superfamily proteins of the immune system of insects. Science. 2005;309:1874–1878. doi: 10.1126/science.1116887. [DOI] [PubMed] [Google Scholar]
- 3.Huang Z, Kingsolver MB, Avadhanula V, Hardy RW. An antiviral role for antimicrobial peptides during the arthropod response to alphavirus replication. J Virol. 2013;87:4272–80. doi: 10.1128/JVI.03360-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Souza-Neto JA, Sim S, Dimopoulos G. An evolutionary conserved function of the JAK-STAT pathway in anti-dengue defense. Proc Natl Acad Sci U S A. 2009;106:17841–6. doi: 10.1073/pnas.0905006106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keene KM, Foy BD, Sanchez-Vargas I, Beaty BJ, Blair CD, Olson KE. RNA interference acts as a natural antiviral response to O’nyong-nyong virus (Alphavirus; Togaviridae) infection of Anopheles gambiae. Proc Natl Acad Sci U S A. 2004;101:17240–5. doi: 10.1073/pnas.0406983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell CL, Keene KM, Brackney DE, Olson KE, Blair CD, Wilusz J, Foy BD. Aedes aegypti uses RNA interference in defense against Sindbis virus infection. BMC Microbiol. 2008;8:47. doi: 10.1186/1471-2180-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanchez-Vargas I, Scott JC, Poole-Smith BK, Franz AW, Barbosa-Solomieu V, Wilusz J, Olson KE, Blair CD. Dengue virus type 2 infections of Aedes aegypti are modulated by the mosquito’s RNA interference pathway. PLoS Pathog. 2009;5:e1000299. doi: 10.1371/journal.ppat.1000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shelly S, Lukinova N, Bambina S, Berman A, Cherry S. Autophagy is an essential component of Drosophila immunity against vesicular stomatitis virus. Immunity. 2009;30:588–598. doi: 10.1016/j.immuni.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galiana-Arnoux D, Dostert C, Schneemann A, AHJ, Imler JL. Essential function in vivo for Dicer-2 in host defense against RNA viruses in drosophila. Nature Immunology. 2006;7:590–597. doi: 10.1038/ni1335. [DOI] [PubMed] [Google Scholar]
- 10.van Rij RP, Saleh MC, Berry B, Foo C, Houk A, Antoniewski C, Andino R. The RNA silencing endonuclease Argonaute 2 mediates specific antiviral immunity in Drosophila melanogaster. Genes Dev. 2006;20:2985–95. doi: 10.1101/gad.1482006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zambon RA, Vakharia VN, Wu LP. RNAi is an antiviral immune response against a dsRNA virus in Drosophila melanogaster. Cell Microbiol. 2006;8:880–9. doi: 10.1111/j.1462-5822.2006.00688.x. [DOI] [PubMed] [Google Scholar]
- 12.Wang XH, AR, Li WX, Li HW, Kim K, et al. RNA Interference Directs Innate Immunity Against Viruses in Adult Drosophila. 2006;312:452–454. doi: 10.1126/science.1125694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lemaitre B, Hoffmann J. Host defense in Drosophila melanogaster. Annual Review of Immunology. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- 14.Avadhanula V, Weasner BP, Hardy GG, Kumar JP, Hardy RW. A novel system for the launch of alphavirus RNA synthesis reveals a role for the Imd pathway in arthropod antiviral response. PLoS Pathog. 2009;5:e1000582. doi: 10.1371/journal.ppat.1000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zambon RA, Nandakumar M, Vakharia VN, Wu LP. The Toll pathway is important for an antiviral response in Drosophila. Proc Natl Acad Sci U S A. 2005;102:7257–62. doi: 10.1073/pnas.0409181102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costa A, Jan E, Sarnow P, Schneider D. The Imd pathway is involved in antiviral immune responses in Drosophila. PLoS One. 2009;4:e7436. doi: 10.1371/journal.pone.0007436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xi Z, Ramirez JL, Dimopoulos G. The Aedes aegypti toll pathway controls dengue virus infection. PLoS Pathog. 2008;4:e1000098. doi: 10.1371/journal.ppat.1000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paradkar PN, Trinidad L, Voysey R, Duchemin JB, Walker PJ. Secreted Vago restricts West Nile virus infection in Culex mosquito cells by activating the Jak-STAT pathway. Proc Natl Acad Sci U S A. 2012;109:18915–20. doi: 10.1073/pnas.1205231109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol. 2009;10:126–139. doi: 10.1038/nrm2632. [DOI] [PubMed] [Google Scholar]
- 20.Ding SW. RNA-based antiviral immunity. Nat Rev Immunol. 2010;10:632–644. doi: 10.1038/nri2824. [DOI] [PubMed] [Google Scholar]
- 21.Rand TA, Ginalski K, Grishin NV, Wang X. Biochemical identification of Argonaute 2 as the sole protein required for RNA-induced silencing complex activity. Proc Natl Acad Sci U S A. 2004:101. doi: 10.1073/pnas.0405913101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role of a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- 23.Lee YS, Nakahara K, Pham JW, Kim K, He Z, Sontheimer EJ, Carthew RW. Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell. 2004;117:69–81. doi: 10.1016/s0092-8674(04)00261-2. [DOI] [PubMed] [Google Scholar]
- 24.Liu Q, Rand TA, Kalidas S, Du F, Kim HE, Smith DP, Wang X. R2D2, a bridge between the initiation and effector stes of the Drosophila RNAi pathway. Science. 2003;301:1921–1925. doi: 10.1126/science.1088710. [DOI] [PubMed] [Google Scholar]
- 25.Okamura K, Chung WJ, Ruby JG, Guo H, Bartel DP, Lai EC. The Drosophila hairpin RNA pathway generates endogenous short interfering RNAs. Nature. 2008:453. doi: 10.1038/nature07015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okamura K, Ishizuka A, Siomi H, Siomi MC. Distinct roles for Argonaute proteins in small RNA-directed RNA cleavage pathways. Genes Dev. 2004;18:1655–1666. doi: 10.1101/gad.1210204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Myles KM, Wiley MR, Morazzani EM, Adelman ZN. Alphavirus-derived small RNAs modulate pathogenesis in disease vector mosquitoes. Proc Natl Acad Sci U S A. 2008;105:19938–19943. doi: 10.1073/pnas.0803408105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott JC, Brackney DE, Campbell CL, Bondu-Hawkins V, Hjelle B, Ebel GD, Olson KE, Blair CD. Comparison of dengue virus type 2-specific small RNAs from RNA interference-competent and -incompetent mosquito cells. PLoS Negl Trop Dis. 2010;4:e848. doi: 10.1371/journal.pntd.0000848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sui RWC, Fragkoudis R, Simmonds P, Donald CL, Chase-Topping ME, Barry G, Attarzadeh-Yazdi G, Rodriguez-Andres J, Nash A, Merits A, Fazakerley JK, Kohl A. Antiviral RNA interference responses induced by Semliki Forest virus infection of mosquito cells: characterization, origin, and frequency-dependent functions of virus-deriveed small interfering RNAs. J Virol. 2011;85:2907–2917. doi: 10.1128/JVI.02052-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aliyari R, Wu Q, Li HW, Wang XH, Li F, Green LD, Han CS, Li WX, Ding S-W. Mechanism of induction and suppression of antiviral immunity directed by virus-derived small RNAs in Drosophila. Cell Host Microbe. 2008;4:387–397. doi: 10.1016/j.chom.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nayak A, Berry B, Tassetto M, Kunitomi M, Acevedo A, Deng C, Krutchinsky A, Gross J, Antoniewski C, Andino R. Cricket paralysis virus antagonizes Argonaute 2 to modulate antiviral defense in Drosophila. Nat Struct Mol Biol. 2010;17:547–554. doi: 10.1038/nsmb.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lingel A, Simon B, Izaurralde E, Sattler M. The structure of the Flock House virus B2 protein, a viral suppressor of RNA interference, shows a novel mode of double-stranded RNA recognition. EMBO Rep. 2005;6:1149–1155. doi: 10.1038/sj.embor.7400583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chao JA, Lee JH, Chapados BR, Debler EW, Schneemann A, Williamson JR. Dual modes of RNA-silencing by Flock House virus protein B2. Nat Struct Mol Biol. 2005;12:952–957. doi: 10.1038/nsmb1005. [DOI] [PubMed] [Google Scholar]
- 34.Schnettler E, Sterken MG, Leung JY, Metz SW, Geertsema C, Goldbach RW, Vlak JM, Kohl A, Khromykh AA, Pijlman GP. Noncoding flavivirus RNA displays RNA interference suppressor activity in insect and mammalian cells. J Virol. 2012;86:13486–13500. doi: 10.1128/JVI.01104-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kakumani PK, Ponia SS, Rajgokul KS, Sood V, Chinnappan M, Benerjea AC, Medigeshi GR, Malhotra P, Mukherjee SK, Bhatnagar RK. Role of RNA interference (RNAi) in dengue virus replication and identification of NS4B as an RNAi suppressor. J Virol. 2013;87:8870–8883. doi: 10.1128/JVI.02774-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karlikow M, Goic B, Saleh MC. RNAi and antiviral defense in Drosophila: Setting up a systemic immune response. Dev Comp Immunol. 2013 doi: 10.1016/j.dci.2013.05.004. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 37.Bucher G, Scholten J, Klingler M. Parental RNAi in Tribolium (Coleoptera) Curr Biol. 2002;12:R85–86. doi: 10.1016/s0960-9822(02)00666-8. [DOI] [PubMed] [Google Scholar]
- 38.Saleh MC, Tassetto M, van Rij RP, Goic B, Gausson V, Berry B, Jacquier C, Antoniewski C, Andino R. Antiviral immunity in Drosophila requires systemic RNA interference spread. Nature. 2009;458:346–50. doi: 10.1038/nature07712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saleh MC, van Rij RP, Hekele A, Gillis A, Foley E, O’Farrell PH, Andino R. The endocytic pathway mediates cell entry of dsRNA to induce RNAi silencing. Nat Cell Biol. 2006;8:793–802. doi: 10.1038/ncbl439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ulvila J, Prikka M, Kleino A, Sormunen R, Ezekowitz RA, Kocks C, Ramet M. Double-stranded RNA is internalized by scavenger receptor-mediated endocytosis in Drosophila S2 cells. J Biol Chem. 2006;281:14370–14375. doi: 10.1074/jbc.M513868200. [DOI] [PubMed] [Google Scholar]
- 41.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007:9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 42.Gibbings DJ, Ciauda C, Erhardt M, Voinnet O. Multivesicular bodies associate with components of the miRNA effector complexes and modulate miRNA activity. Nat Cell Biol. 2009:11. doi: 10.1038/ncb1929. [DOI] [PubMed] [Google Scholar]
- 43.Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL, Tait JF, Tewari M. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011:108. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goic B, Vodavar N, Mondotte JA, Monot C, Frangeul L, Blanc H, Gausson V, Vera-Otarola J, Cristofari G, Saleh MC. RNA-mediated interference and reverse transcription control the persistence of RNA viruses in the insect model Drosophila. Nat Immunol. 2013;14:396–403. doi: 10.1038/ni.2542. [DOI] [PubMed] [Google Scholar]
- 45.Crochu S, Cook S, Attoui H, Charrel RN, De Chesse R, Belhouchet M, Lemasson JJ, de Micco P, de Lamballerie X. Sequences of flavivirus-related RNA viruses persist in DNA form integrated in the genome of Aedes spp. mosquitoes. J Gen Virol. 2004;85:1971–80. doi: 10.1099/vir.0.79850-0. [DOI] [PubMed] [Google Scholar]
- 46.Rosen L, Shroyer DA, Tesh RB, Freier JE, Lien JC. Transovarial transmission of dengue viruses by mosquitoes: Aedes albopictus and Aedes aegypti. Am J Trop Med Hyg. 1983;32:1108–19. doi: 10.4269/ajtmh.1983.32.1108. [DOI] [PubMed] [Google Scholar]
- 47.Joshi V, Mourya DT, Sharma RC. Persistence of dengue-3 virus through transovarial transmission passage in successive generations of Aedes aegypti mosquitoes. Am J Trop Med Hyg. 2002;67:158–61. doi: 10.4269/ajtmh.2002.67.158. [DOI] [PubMed] [Google Scholar]
- 48.Tesh RB, Gubler DJ. Laboratory studies of transovarial transmission of La Crosse and other arboviruses by Aedes albopictus and Culex fatigans. Am J Trop Med Hyg. 1975;24:876–80. doi: 10.4269/ajtmh.1975.24.876. [DOI] [PubMed] [Google Scholar]
- 49.Thompson WH, Beaty BJ. Venereal transmission of La Crosse (California encephalitis) arbovirus in Aedes triseriatus mosquitoes. Science. 1977;196:530–1. doi: 10.1126/science.850794. [DOI] [PubMed] [Google Scholar]
- 50.Cullen BR. Viruses and microRNAs: RISCy interactions with serious consequences. Genes Dev. 2011;25:1881–94. doi: 10.1101/gad.17352611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pfeffer S, Zavolan M, Grasser FA, Chien M, Russo JJ, Ju J, John B, Enright AJ, Marks D, Sander C, Tuschl T. Identification of virus-encoded microRNAs. Science. 2004;304:734–6. doi: 10.1126/science.1096781. [DOI] [PubMed] [Google Scholar]
- 52.Jopling CL, Norman KL, Sarnow P. Positive and negative modulation of viral and cellular mRNAs by liver-specific microRNA miR-122. Cold Spring Harb Symp Quant Biol. 2006;71:369–76. doi: 10.1101/sqb.2006.71.022. [DOI] [PubMed] [Google Scholar]
- 53.Hussain M, Torres S, Schnettler E, Funk A, Grundhoff A, Pijlman GP, Khromykh AA, Asgari S. West Nile virus encodes a microRNA-like small RNA in the 3′ untranslated region which up-regulates GATA4 mRNA and facilitates virus replication in mosquito cells. Nucl Acids Res. 2012;40:2210–2223. doi: 10.1093/nar/gkr848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hess AM, Prasad AN, Ptitsyn A, Ebel GD, Olson KE, Barbacioru C, Monighetti C, Campbell CL. Small RNA profiling of dengue virus-mosquito interactions implicates the PIWI RNA pathway in anti-viral defense. BMC Microbiol. 2011;11:45. doi: 10.1186/1471-2180-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vodavar N, Bronkhorst AW, van Cleef KW, Miesen P, Blanc H, van Rij RP, Saleh MC. Arbovirus-derived piRNAs exhibit a ping-pong signature in mosquito cells. PLoS One. 2012;7:e30861. doi: 10.1371/journal.pone.0030861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schnettler E, Donald CL, Human S, Watson M, Siu RW, McFarlane M, Fazakerley JK, Kohl A, Fragkoudis R. Knockdown of piRNA pathway proteins results in enhanced Semliki Forest virus production in mosquito cells. J Gen Virol. 2013;94:1680–1689. doi: 10.1099/vir.0.053850-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morazzani EM, Wiley MR, Murreddu MG, Adelman ZN, Myles KM. Production of virus-derived ping-pong-dependent piRNA-like small RNAs in the mosquito soma. PLoS Pathog. 2012;8:e1002470. doi: 10.1371/journal.ppat.1002470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brown S, Hu N, Hombria JC. Identification of the first invertebrate interleukin JAK/STAT receptor, the Drosophila gene domeless. Curr Biol. 2001;11:1700–5. doi: 10.1016/s0960-9822(01)00524-3. [DOI] [PubMed] [Google Scholar]
- 59.Hombria JC, Brown S, Hader S, Zeidler MP. Characterisation of Upd2, a Drosophila JAK/STAT pathway ligand. Dev Biol. 2005;288:420–33. doi: 10.1016/j.ydbio.2005.09.040. [DOI] [PubMed] [Google Scholar]
- 60.Harrison DA, McCoon PE, Binari R, Gilman M, Perrimon N. Drosophila unpaired encodes a secreted protein that activates the JAK signaling pathway. Genes Dev. 1998;12:3252–63. doi: 10.1101/gad.12.20.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Binari R, Perrimon N. Stripe-specific regulation of pair-rule genes by hopscotch, a putative Jak family tyrosine kinase in Drosophila. Genes Dev. 1994;8:300–12. doi: 10.1101/gad.8.3.300. [DOI] [PubMed] [Google Scholar]
- 62.Yan R, Small S, Desplan C, Dearolf CR, Darnell JE., Jr Identification of a Stat gene that functions in Drosophila development. Cell. 1996;84:421–30. doi: 10.1016/s0092-8674(00)81287-8. [DOI] [PubMed] [Google Scholar]
- 63.Yeh MS, Cheng CH, Chou CM, Hsu YL, Chu CY, Chen GD, Chen ST, Chen GC, Huang CJ. Expression and characterization of two STAT isoforms from Sf9 cells. Dev Comp Immunol. 2008;32:814–24. doi: 10.1016/j.dci.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 64.Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines. J Biol Chem. 2007;282:20059–63. doi: 10.1074/jbc.R700016200. [DOI] [PubMed] [Google Scholar]
- 65.Barillas-Mury C, Han YS, Seeley D, Kafatos FC. Anopheles gambiae Ag-STAT, a new insect member of the STAT family, is activated in response to bacterial infection. EMBO J. 1999;18:959–67. doi: 10.1093/emboj/18.4.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin CC, Chou CM, Hsu YL, Lien JC, Wang YM, Chen ST, Tsai SC, Hsiao PW, Huang CJ. Characterization of two mosquito STATs, AaSTAT and CtSTAT. Differential regulation of tyrosine phosphorylation and DNA binding activity by lipopolysaccharide treatment and by Japanese encephalitis virus infection. J Biol Chem. 2004;279:3308–17. doi: 10.1074/jbc.M309749200. [DOI] [PubMed] [Google Scholar]
- 67.Waterhouse RM, Kriventseva EV, Meister S, Xi Z, Alvarez KS, Bartholomay LC, Barillas-Mury C, Bian G, Blandin S, Christensen BM, Dong Y, Jiang H, Kanost MR, Koutsos AC, Levashina EA, Li J, Ligoxygakis P, Maccallum RM, Mayhew GF, Mendes A, Michel K, Osta MA, Paskewitz S, Shin SW, Vlachou D, Wang L, Wei W, Zheng L, Zou Z, Severson DW, Raikhel AS, Kafatos FC, Dimopoulos G, Zdobnov EM, Christophides GK. Evolutionary dynamics of immune-related genes and pathways in disease-vector mosquitoes. Science. 2007;316:1738–43. doi: 10.1126/science.1139862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Agaisse H, Petersen UM, Boutros M, Mathey-Prevot B, Perrimon N. Signaling role of hemocytes in Drosophila JAK/STAT-dependent response to septic injury. Dev Cell. 2003;5:441–50. doi: 10.1016/s1534-5807(03)00244-2. [DOI] [PubMed] [Google Scholar]
- 69.Wright VM, Vogt KL, Smythe E, Zeidler MP. Differential activities of the Drosophila JAK/STAT pathway ligands Upd, Upd2 and Upd3. Cell Signal. 2011;23:920–7. doi: 10.1016/j.cellsig.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 70.Deddouche S, Matt N, Budd A, Mueller S, Kemp C, Galiana-Arnoux D, Dostert C, Antoniewski C, Hoffmann JA, Imler JL. The DExD/H-box helicase Dicer-2 mediates the induction of antiviral activity in drosophila. Nat Immunol. 2008;9:1425–32. doi: 10.1038/ni.1664. [DOI] [PubMed] [Google Scholar]
- 71.Callus BA, Mathey-Prevot B. SOCS36E, a novel Drosophila SOCS protein, suppresses JAK/STAT and EGF-R signalling in the imaginal wing disc. Oncogene. 2002;21:4812–21. doi: 10.1038/sj.onc.1205618. [DOI] [PubMed] [Google Scholar]
- 72.Betz A, Lampen N, Martinek S, Young MW, Darnell JE., Jr A Drosophila PIAS homologue negatively regulates stat92E. Proc Natl Acad Sci U S A. 2001;98:9563–8. doi: 10.1073/pnas.171302098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Breakwell L, Dosenovic P, Karlsson Hedestam GB, D’Amato M, Liljestrom P, Fazakerley J, McInerney GM. Semliki Forest virus nonstructural protein 2 is involved in suppression of the type I interferon response. J Virol. 2007;81:8677–84. doi: 10.1128/JVI.02411-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fragkoudis R, Chi Y, Siu RW, Barry G, Attarzadeh-Yazdi G, Merits A, Nash AA, Fazakerley JK, Kohl A. Semliki Forest virus strongly reduces mosquito host defence signaling. Insect Mol Biol. 2008;17:647–56. doi: 10.1111/j.1365-2583.2008.00834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fros JJ, Liu WJ, Prow NA, Geertsema C, Ligtenberg M, Vanlandingham DL, Schnettler E, Vlak JM, Suhrbier A, Khromykh AA, Pijlman GP. Chikungunya virus nonstructural protein 2 inhibits type I/II interferon-stimulated JAK-STAT signaling. J Virol. 2010;84:10877–87. doi: 10.1128/JVI.00949-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Simmons JD, White LJ, Morrison TE, Montgomery SA, Whitmore AC, Johnston RE, Heise MT. Venezuelan equine encephalitis virus disrupts STAT1 signaling by distinct mechanisms independent of host shutoff. J Virol. 2009;83:10571–81. doi: 10.1128/JVI.01041-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Simmons JD, Wollish AC, Heise MT. A determinant of Sindbis virus neurovirulence enables efficient disruption of Jak/STAT signaling. J Virol. 2010;84:11429–39. doi: 10.1128/JVI.00577-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brackney DE, Scott JC, Sagawa F, Woodward JE, Miller NA, Schilkey FD, Mudge J, Wilusz J, Olson KE, Blair CD, Ebel GD. C6/36 Aedes albopictus cells have a dysfunctional antiviral RNA interference response. PLoS Negl Trop Dis. 2010;4:e856. doi: 10.1371/journal.pntd.0000856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Agaisse H, Perrimon N. The roles of JAK/STAT signaling in Drosophila immune responses. Immunol Rev. 2004;198:72–82. doi: 10.1111/j.0105-2896.2004.0133.x. [DOI] [PubMed] [Google Scholar]
- 80.Levashina EA, Moita LF, Blandin S, Vriend G, Lagueux M, Kafatos FC. Conserved role of a complement-like protein in phagocytosis revealed by dsRNA knockout in cultured cells of the mosquito, Anopheles gambiae. Cell. 2001;104:709–18. doi: 10.1016/s0092-8674(01)00267-7. [DOI] [PubMed] [Google Scholar]
- 81.Kwon EJ, Park HS, Kim YS, Oh EJ, Nishida Y, Matsukage A, Yoo MA, Yamaguchi M. Transcriptional regulation of the Drosophila raf proto-oncogene by Drosophila STAT during development and in immune response. J Biol Chem. 2000;275:19824–30. doi: 10.1074/jbc.M001114200. [DOI] [PubMed] [Google Scholar]
- 82.Dostert C, Jouanguy E, Irving P, Troxler L, Galiana-Arnoux D, Hetru C, Hoffmann JA, Imler JL. The Jak-STAT signaling pathway is required but not sufficient for the antiviral response of drosophila. Nat Immunol. 2005;6:946–53. doi: 10.1038/ni1237. [DOI] [PubMed] [Google Scholar]
- 83.Hedges LM, Johnson KN. Induction of host defence responses by Drosophila C virus. J Gen Virol. 2008;89:1497–501. doi: 10.1099/vir.0.83684-0. [DOI] [PubMed] [Google Scholar]
- 84.Kemp C, Mueller S, Goto A, Barbier V, Paro S, Bonnay F, Dostert C, Troxler L, Hetru C, Meignin C, Pfeffer S, Hoffmann JA, Imler JL. Broad RNA interference-mediated antiviral immunity and virus-specific inducible responses in Drosophila. J Immunol. 2013;190:650–8. doi: 10.4049/jimmunol.1102486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Behura SK, Gomez-Machorro C, Harker BW, deBruyn B, Lovin DD, Hemme RR, Mori A, Romero-Severson J, Severson DW. Global cross-talk of genes of the mosquito Aedes aegypti in response to dengue virus infection. PLoS Negl Trop Dis. 2011;5:e1385. doi: 10.1371/journal.pntd.0001385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim LK, Choi UY, Cho HS, Lee JS, Lee WB, Kim J, Jeong K, Shim J, Kim-Ha J, Kim YJ. Down-regulation of NF-kappaB target genes by the AP-1 and STAT complex during the innate immune response in Drosophila. PLoS Biol. 2007;5:e238. doi: 10.1371/journal.pbio.0050238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–83. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 88.Gottar M, Gobert V, Michel T, Belvin M, Duyk G, Hoffmann JA, Ferrandon D, Royet J. The Drosophila immune response against Gram-negative bacteria is mediated by a peptidoglycan recognition protein. Nature. 2002;416:4. doi: 10.1038/nature734. [DOI] [PubMed] [Google Scholar]
- 89.Choe KM, Werner T, Stoven S, Hultmark D, Anderson KV. Requirement for a peptidoglycan recognition protein (PGRP) in Relish activation and antibacterial immune responses in Drosophila. Science. 2002;296:3. doi: 10.1126/science.1070216. [DOI] [PubMed] [Google Scholar]
- 90.Ramet M, Manfruelli P, Pearson A, Mathey-Prevot B, Ezekowitz RA. Functional genomic analysis of phagocytosis and identification of a Drosophila receptor for E. coli. Nature. 2002;416:4. doi: 10.1038/nature735. [DOI] [PubMed] [Google Scholar]
- 91.Kaneko T, Yano T, Aggarwal K, Lim J-H, Ueda K, Oshima Y, Peach C, Erturk-Hasdemir D, Goldman WE, Oh B-H, Kurata S, Silverman N. PGRP-LC and PGRP-LE have essential yet distinct functions in the drosophila immune response to monomeric DAP-type peptidoglycan. 2006;7:715–723. doi: 10.1038/ni1356. [DOI] [PubMed] [Google Scholar]
- 92.Hedengren M, Asling B, Dushay MS, Ando I, Ekengren S, Wihlborg M, Hultmark D. Relish, a central factor in the control of humoral but not cellular immunity in Drosophila. Mol Cell. 1999;4:10. doi: 10.1016/s1097-2765(00)80392-5. [DOI] [PubMed] [Google Scholar]
- 93.Silverman N, Zhou R, Stöven S, Pandey N, Hultmark D, Maniatis T. A Drosophila IkappaB kinase complex required for Relish cleavage and antibacterial immunity. Genes Dev. 2000;14:10. doi: 10.1101/gad.817800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stöven S, Silverman N, Junell A, Hedengren-Olcott M, Erturk D, Engstrom Y, Maniatis T, Hultmark D. Caspase-mediated processing of the Drosophila NF-kappaB factor Relish. Proc Natl Acad Sci U S A. 2003;100:5991–6. doi: 10.1073/pnas.1035902100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Leulier F, Rodriguez A, Khush RS, Abrams JM, Lemaitre B. The Drosophila caspase Dredd is required to resist gram-negative bacterial infection. EMBO Rep. 2000;1:5. doi: 10.1093/embo-reports/kvd073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Leulier F, Vidal S, Saigo K, Ueda R, Lemaitre B. Inducible expression of double-stranded RNA reveals a role for dFADD in the regulation of the antibacterial response in Drosophila adults. Curr Biol. 2002;25:4. doi: 10.1016/s0960-9822(02)00873-4. [DOI] [PubMed] [Google Scholar]
- 97.Naitza S, Rossé C, Kappler C, Georgel P, Belvin M, Gubb D, Camonis J, Hoffmann JA, Reichhart JM. The Drosophila immune defense against gram-negative infection requires the death protein dFADD. Immunity. 2002;17:6. doi: 10.1016/s1074-7613(02)00454-5. [DOI] [PubMed] [Google Scholar]
- 98.Takahasi K, Ochiai M, Horiuchi M, Kumeta H, Ogura K, Ashida M, Inagaki F. Solution structure of the silkworm betaGRP/GNBP3 N-terminal domain reveals the mechanism for beta-1,3-glucan-specific recognition. Proc Natl Acad Sci U S A. 2009;106:5. doi: 10.1073/pnas.0901671106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Michel T, Reichhart JM, Hoffmann JA, Royet J. Drosophila Toll is activated by Gram-positive bacteria through a circulating peptidoglycan recognition protein. Nature. 2001;414:3. doi: 10.1038/414756a. [DOI] [PubMed] [Google Scholar]
- 100.Bischoff V, Vignal C, Boneca IG, Michel T, Hoffmann JA, Royet J. Function of the drosophila pattern-recognition receptor PGRP-SD in the detection of Gram-positive bacteria. Nat Immunol. 2004;5:5. doi: 10.1038/ni1123. [DOI] [PubMed] [Google Scholar]
- 101.Gobert V, Gottar M, Matskevich AA, Rutschmann S, Royet J, Belvin M, Hoffmann JA, Ferrandon D. Dual activation of the Drosophila toll pathway by two pattern recognition receptors. Science. 2003;302:4. doi: 10.1126/science.1085432. [DOI] [PubMed] [Google Scholar]
- 102.Gottar M, Gobert V, Matskevich AA, Reichhart JM, Wang C, Butt TM, Belvin M, Hoffmann JA, Ferrandon D. Dual detection of fungal infections in Drosophila via recognition of glucans and sensing of virulence factors. Cell. 2006;127:12. doi: 10.1016/j.cell.2006.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.El Chamy L, Leclerc V, Caldelari I, Reichhart JM. Sensing of ‘danger signals’ and pathogen-associated molecular patterns defines binary signaling pathways ‘upstream’ of Toll. Nat Immunol. 2008;9:5. doi: 10.1038/ni.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Buchon N, Poidevin M, Kwon HM, Guillou A, Sottas V, Lee BL, Lemaitre B. A single modular serine protease integrates signals from pattern-recognition receptors upstream of the Drosophila Toll pathway. Proc Natl Acad Sci U S A. 2009;106:5. doi: 10.1073/pnas.0901924106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kambris Z, Brun S, Jang IH, Nam HJ, Romeo Y, Takahashi K, Lee WJ, Ueda R, Lemaitre B. Drosophila immunity: a large-scale in vivo RNAi screen identifies five serine proteases required for Toll activation. Curr Biol. 2006;16:5. doi: 10.1016/j.cub.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 106.Arnot CJ, GN, Gangloff M. Molecular mechanism that induces activation of Spätzle, the ligand for the Drosophila Toll receptor. J Biol Chem. 2010;285:7. doi: 10.1074/jbc.M109.098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Meng X, Khanuja BS, Ip YT. Toll receptor-mediated Drosophila immune response requires Dif, an NF-kappaB factor. Genes Dev. 1999;13:5. doi: 10.1101/gad.13.7.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Busse MS, Arnold CP, Towb P, Katrivesis J, Wasserman SA. A kappaB sequence code for pathway-specific innate immune responses. EMBO J. 2007;26:3826–35. doi: 10.1038/sj.emboj.7601798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tzou P, Reichhart JM, Lemaitre B. Constitutive expression of a single antimicrobial peptide can restore wild-type resistance to infection in immunodeficient Drosophila mutants. Proc Natl Acad Sci U S A. 2002;99:5. doi: 10.1073/pnas.042411999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bartholomay LC, WR, Mayhew GF, Campbell CL, Michel K, Zou Z, Ramirez JL, Das S, Alvarez K, Arensburger P, Bryant B, Chapman SB, Dong Y, Erickson SM, Karunaratne SH, Kokoza V, Kodira CD, Pignatelli P, Shin SW, Vanlandingham DL, Atkinson PW, Birren B, Christophides GK, Clem RJ, Hemingway J, Higgs S, Megy K, Ranson H, Zdobnov EM, Raikhel AS, Christensen BM, Dimopoulos G, Muskavitch MA. Pathogenomics of Culex quinquefasciatus and meta-analysis of infection responses to diverse pathogens. Science. 2010;330:2. doi: 10.1126/science.1193162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Waterhouse RM, Kriventseva EV, Meister S, Xi Z, Alvarez KS, Bartholomay LC, Barillas-Mury C, Bian G, Blandin S, Christensen BM, Dong Y, Jiang H, Kanost MR, Koutsos AC, Levashina EA, Li J, Ligoxygakis P, MacCallum RM, Mayhew GF, Mendes A, Michel K, Osta MA, Paskewitz S, Shin SW, Vlachou D, Wang L, Wei W, Zheng L, Zou Z, Severson DW, Raikhel AS, Kafatos FC, Dimopoulos G, Zdobnov EM, Christophides GK. Evolutionary Dynamics of Immune-Related Genes and Pathways in Disease-Vector Mosquitoes. 2007;316:1738–1743. doi: 10.1126/science.1139862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Christophides GK, ZE, Barillas-Mury C, Birney E, Blandin S, Blass C, Brey PT, Collins FH, Danielli A, Dimopoulos G, Hetru C, Hoa NT, Hoffmann JA, Kanzok SM, Letunic I, Levashina EA, Loukeris TG, Lycett G, Meister S, Michel K, Moita LF, Müller HM, Osta MA, Paskewitz SM, Reichhart JM, Rzhetsky A, Troxler L, Vernick KD, Vlachou D, Volz J, vonMering C, Xu J, Zheng L, Bork P, Kafatos FC. Immunity-related genes and gene families in Anopheles gambiae. Science. 2002;298:6. doi: 10.1126/science.1077136. [DOI] [PubMed] [Google Scholar]
- 113.Shin SW, KV, Bian G, Cheon HM, Kim YJ, Raikhel AS. REL1, a homologue of Drosophila dorsal, regulates toll antifungal immune pathway in the female mosquito Aedes aegypti. J Biol Chem. 2005;280:8. doi: 10.1074/jbc.M500711200. [DOI] [PubMed] [Google Scholar]
- 114.Meister S, KS, Zheng XL, Luna C, Li TR, Hoa NT, Clayton JR, White KP, Kafatos FC, Christophides GK, Zheng L. Immune signaling pathways regulating bacterial and malaria parasite infection of the mosquito Anopheles gambiae. Proc Natl Acad Sci U S A. 2005;102:5. doi: 10.1073/pnas.0504950102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Costa A, Jan E, Sarnow P, Schneider D. The Imd Pathway Is Involved in Antiviral Immune Responses in Drosophila. 2009;4:e7436. doi: 10.1371/journal.pone.0007436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tsai CW, ME, Ammar ED, Dietzgen RG, Hogenhout SA. Drosophila melanogaster mounts a unique immune response to the Rhabdovirus sigma virus. Appl Environ Microbiol. 2008;74:5. doi: 10.1128/AEM.02248-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Carpenter J, Hutter S, Baines JF, Roller J, Saminadin-Peter SS, Parsch J, Jiggins FM. The transcriptional response of Drosophila melanogaster to infection with the sigma virus (Rhabdoviridae) 2009;4:e6838. doi: 10.1371/journal.pone.0006838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cordes EJ, LMK, Carlson KA. Differential gene expression related to Nora virus infection of Drosophila melanogaster. Virus Res. 2013;175:5. doi: 10.1016/j.virusres.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Boutros M, AH, Perrimon N. Sequential activation of signaling pathways during innate immune responses in Drosophila. Dev Cell. 2002;3:11. doi: 10.1016/s1534-5807(02)00325-8. [DOI] [PubMed] [Google Scholar]
- 120.Zambon RA, Nandakumar M, Vakharia VN, Wu LP. The Toll pathway is important for an antiviral response in Drosophila. 2005;102:7257–7262. doi: 10.1073/pnas.0409181102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Habayeb MS, Ekstrom JO, Hultmark D. Nora virus persistent infections are not affected by the RNAi machinery. PLoS One. 2009;4:e5731. doi: 10.1371/journal.pone.0005731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Avadhanula V, Weasner BP, Hardy GG, Kumar JP, Hardy RW. A Novel System for the Launch of Alphavirus RNA Synthesis Reveals a Role for the Imd Pathway in Arthropod Antiviral Response. 2009;5:e1000582. doi: 10.1371/journal.ppat.1000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fragkoudis R, Chi Y, Siu RWC, Barry G, Attarzadeh-Yazdi G, Merits A, Nash AA, Fazakerley JK, Kohl A. Semliki Forest virus strongly reduces mosquito host defence signaling. 2008;17:647–656. doi: 10.1111/j.1365-2583.2008.00834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xi Z, Ramirez JL, Dimopoulos G. The Aedes aegypti toll pathway controls dengue virus infection. 2008;4:e1000098. doi: 10.1371/journal.ppat.1000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ramirez JL, DG The Toll immune signaling pathway control conserved anti-dengue defenses across diverse Ae. aegypti strains and against multiple dengue virus serotypes. Dev Comp. 2010;34:4. doi: 10.1016/j.dci.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sanders HR, Foy BD, Evans AM, Ross LS, Beaty BJ, Olson KE, Gill SS. Sindbis virus induces transport processes and alters expression of innate immunity pathway genes in the midgut of the disease vector, Aedes aegypti. 2005;35:1293–1307. doi: 10.1016/j.ibmb.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 127.Waldock J, OK, Christophides GK. Anopheles gambiae antiviral immune response to systemic O’nyong-nyong infection. PLoS Negl Trop Dis. 2012:6. doi: 10.1371/journal.pntd.0001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nakamoto M, MR, Xu J, Bambina S, Yasunaga A, Shelly SS, Gold B, Cherry S. Virus recognition by Toll-7 activates antiviral autophagy in Drosophila. Immunity. 2012;36:9. doi: 10.1016/j.immuni.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yano T, MS, Ohmori H, Oshima Y, Fujimoto Y, Ueda R, Takada H, Goldman WE, Fukase K, Silverman N, Yoshimori T, Kurata S. Autophagic control of listeria through intracellular innate immune recognition in drosophila. Nat Immunol. 2008;9:8. doi: 10.1038/ni.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kuma A, HM, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T, Mizushima N. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:4. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- 131.Mizushima N, YA, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Cell Biol. 2004;15:10. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Stephan JS, HP The regulation of autophagy in eukaryotic cells: do all roads pass through Atg1? Autophagy. 2006;2:2. doi: 10.4161/auto.2.2.2485. [DOI] [PubMed] [Google Scholar]
- 133.Voronin D, CD, Steven A, Taylor MJ. Autophagy regulates Wolbachia populations across diverse symbiotic associations. Proc Natl Acad Sci U S A. 2012;109:8. doi: 10.1073/pnas.1203519109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kim JJ, LH, Shin DM, Kim W, Yuk JM, Jin HS, Lee SH, Cha GH, Kim JM, Lee ZW, Shin SJ, Yoo H, Park YK, Park JB, Chung J, Yoshimori T, Jo EK. Host cell autophagy activated by antibiotics is required for their effective antimycobacterial drug action. Cell Host Microbe. 2012;11:11. doi: 10.1016/j.chom.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 135.Wu J, RK, Wu LP. ird1 is a Vps15 homologue important for antibacterial immune responses in Drosophila. Cell Microbiol. 2007;9:12. doi: 10.1111/j.1462-5822.2006.00853.x. [DOI] [PubMed] [Google Scholar]
- 136.Liang XH, KL, Jiang HH, Gordon G, Goldman JE, Berry G, Herman B, Levine B. Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. J Virol. 1998;72:10. doi: 10.1128/jvi.72.11.8586-8596.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liu Y, SM, Czymmek K, Tallóczy Z, Levine B, Dinesh-Kumar SP. Autophagy regulates programmed cell death during the plant innate immune response. Cell. 2005;121:10. doi: 10.1016/j.cell.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 138.Orvedahl A, MS, Sumpter R, Jr, Tallóczy Z, Zou Z, Levine B. Autophagy protects against Sindbis virus infection of the central nervous system. Cell Host Microbe. 2010;7:12. doi: 10.1016/j.chom.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bartlett BJ, IP, Lewerenz J, Sanchez H, Kotzebue RW, Cumming RC, Harris GL, Nezis IP, Schubert DR, Simonsen A, Finley KD. p62, Ref(2)P and ubiquitinated proteins are conserved markers of neuronal aging, aggregate formation and progressive autophagic defects. Autophagy. 2011;7:11. doi: 10.4161/auto.7.6.14943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nezis IP, SA, Sagona AP, Finley K, Gaumer S, Contamine D, Rusten TE, Stenmark H, Brech A. Ref(2)P, the Drosophila melanogaster homologue of mammalian p62, is required for the formation of protein aggregates in adult brain. J Cell Biol. 2008;180:6. doi: 10.1083/jcb.200711108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fleuriet A. Polymorphism of the Hereditary Sigma Virus in Natural Populations of DROSOPHILA MELANOGASTER. Genetics. 1980;95:6. doi: 10.1093/genetics/95.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dru P, BF, Dezélée S, Gay P, Petitjean AM, Pierre-Deneubourg A, Teninges D, Contamine D. Unusual variability of the Drosophila melanogaster ref(2)P protein which controls the multiplication of sigma rhabdovirus. Genetics. 1993;133:11. doi: 10.1093/genetics/133.4.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Contamine D. Role of the Drosophila genome in Sigma virus multiplication. I. Role of the ret(2)P gene; selection of host-adapted mutants at the nonpermissive allele Pp. Virology. 1981;114:14. doi: 10.1016/0042-6822(81)90227-0. [DOI] [PubMed] [Google Scholar]
- 144.Shelly S, LN, Bambina S, Berman A, Cherry S. Autophagy is an essential component of Drosophila immunity against vesicular stomatitis virus. Immunity. 2009;30:588–98. doi: 10.1016/j.immuni.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dreux M, Chisari FV. Autophagy proteins promote hepatitis C virus replication. Autophagy. 2009;5:1224–5. doi: 10.4161/auto.5.8.10219. [DOI] [PubMed] [Google Scholar]
- 146.Jackson WT, Giddings TH, Jr, Taylor MP, Mulinyawe S, Rabinovitch M, Kopito RR, Kirkegaard K. Subversion of cellular autophagosomal machinery by RNA viruses. PLoS Biol. 2005;3:e156. doi: 10.1371/journal.pbio.0030156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mateo R, Nagamine CM, Spagnolo J, Méndez E, Rahe M, Gale M, Jr, Yuan J, Kirkegaard K. Inhibition of cellular autophagy deranges dengue virion maturation. J Virol. 2013;87:9. doi: 10.1128/JVI.02177-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Patel RK, Hardy RW. Role for the phosphatidylinositol 3-kinase-Akt-TOR pathway during sindbis virus replication in arthropods. 2012;86:3595–3604. doi: 10.1128/JVI.06625-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cherry S. VSV infection is sensed by Drosophila, attenuates nutrient signaling, and thereby activates antiviral autophagy. Autophagy. 2009;5:1. doi: 10.4161/auto.5.7.9730. [DOI] [PubMed] [Google Scholar]
- 150.Flatt JW, Kim R, Smith JG, Nemerow GR, Stewart PL. An intrinsically disordered region of the adenovirus capsid is implicated in neutralization by human alpha defensin 5. PLoS One. 2013;8:e61571. doi: 10.1371/journal.pone.0061571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Smith JG, Silvestry M, Lindert S, Lu W, Nemerow GR, Stewart PL. Insight into the mechanisms of adenovirus capsid disassembly from studies of defensin neutralization. PLoS Pathog. 2010;6:e1000959. doi: 10.1371/journal.ppat.1000959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Snijder J, Reddy VS, May ER, Roos WH, Nemerow GR, Wuite GJ. Integrin and defensin modulate the mechanical properties of adenovirus. J Virol. 2013;87:2756–66. doi: 10.1128/JVI.02516-12. [DOI] [PMC free article] [PubMed] [Google Scholar]