Abstract
INTRODUCTION
Class II malocclusion affects about 15 % of the US population and is characterized by a convex profile and occlusion disharmonies. The specific etiological mechanisms resulting in the range of Class II dento-skeletal combinations observed is not yet understood. Most studies describing the class II phenotypic diversity have utilized moderate sample sizes or have focused on younger individuals that later in life may outgrow their class II discrepancies; such a focus may also preclude the visualization of adult class II features. The majority have utilized simple correlation methods resulting in phenotypes that may not be generalizable to different samples and thus may not be suitable for studies of malocclusion etiology. The purpose of this study is to address these knowledge gaps by capturing the maximum phenotypic variation present in a large Caucasian sample of class II individuals selected with strict eligibility criteria and rigorously standardized multivariate reduction analyses.
METHODS
Sixty-three lateral cephalometric variables were measured from pre-treatment records of 309 Class II Caucasian adults (82 males, 227 females; ages 16–60 years). Principal component analysis (PCA) and cluster analysis were used to generate comprehensive phenotypes in an effort to identify the most homogeneous groups of individuals reducing heterogeneity and improving the power of future malocclusion etiology studies.
RESULTS
PCA resulted in 7 principal components that accounted for 81% of the variation. The first three components represented variation on mandibular rotation, upper incisor angulation and mandibular length, respectively. The cluster analysis identified 5 distinct Class II phenotypes.
CONCLUSIONS
A comprehensive spectrum of Class II phenotypic definitions was obtained that could be generalized to other samples advancing our efforts to the identification of etiological factors underlying Class II malocclusion.
INTRODUCTION
Class II malocclusion is present in about 15% of the US population1 and is often characterized by a deficient mandible leading to a convex profile, unaesthetic facial proportions and occlusion disharmonies. Both environmental and genetic factors and their interactions have been associated with a Class II malocclusion; however, the etiological mechanisms resulting in the array of dento-skeletal combinations observed in Class II patients remain elusive.
Examples of risk factors that can act on the prenatal environment include exposure to alcohol (i.e. fetal alcohol syndrome) and preterm birth, both of which have been associated with retrognathic mandibles and a Class II malocclusion2–4. In addition, post-natal risk factors include low socioeconomic status, caries experience, premature loss of primary teeth, history of prolonged sucking habits and resting tongue habits which may increase susceptibility to or exacerbate an existing Class II malocclusion and reduce treatment effectiveness5–7. Studies of prolonged sucking habits are the most consistent and results indicate associations with a Class II dental relationship, decreased overbite, increased overjet, posterior cross bites and TMJ dysfunction5,8,9. Moreover, anthropological data from remains of Aboriginal Australians and other pre-historic populations point to changes from a hard to a soft diet as an important etiologic factor given the increased prevalence of Class II malocclusion in modern humans. This is presumably due to a decrease in dental attrition and lack of compensatory tooth mesial migration associated with modern soft diets10,11.
Severely retrognathic profiles and Class II malocclusions are common findings in patients with craniofacial anomalies including Pierre Robin sequence, Treacher Collins, Stickler and Turner syndromes, supporting the role of genetics in mandibular retrognathism. The Class II malocclusion has been further subdivided into division1 (div.1) and division 2 (div.2) depending upon the upper incisors proclination or retroclination respectively, although additional skeletal and dental differences exist between the subdivision types beyond upper incisor angulations. Class II div.1 cases occur more frequently (14.9% – 24%) than Class II div.2 cases (3.4% – 5.9%)12,13. Interestingly, patients with Class II div. 2 have a higher incidence of dental anomalies compared to the normal population14,15 suggesting that genetic factors involved in dental development might also be etiological for maxillo-mandibular size discrepancies15.
Human genetic mapping studies of maxillo-mandibular size discrepancies are scarce and so far have focused primarily on the Class III malocclusion16–19. So far, mandibular height and prognathism have been associated with genes GHR, MATRILIN-1, EPB41, TGFB3, LTBP2 and MYO1H19–22 suggesting that molecular pathways implicated in bone (TGFB3, LTBP) and cartilage (GHR, Matrilin-1) development are plausible candidates for mandibular size discrepancies. For the Class II malocclusion, a small study including four Colombian families with mandibular hypoplasia found that all affected individuals were homozygous for the rare allele of the polymorphism rs1348322 within the Noggin gene23 which is essential for mandibular formation in mice24. No etiologic mutations have as yet been identified.
The success of genetic studies aimed at identifying causative genes for malocclusion depends greatly on a well-characterized phenotype to reduce heterogeneity and avoid misclassification of affected individuals25. Multiple studies of cross-sectional and retrospective longitudinal Class II samples utilizing conventional cephalometry or shape analysis methods have attempted to characterized the Class II dento-skeletal morphology over different developmental stages, for varying malocclusion severities, and from across global populations. Overall most studies agree that the dento-skeletal components of the Class II div. 1 malocclusion include an obtuse cranial base angle 26–30 a larger cranial base length31,32, a normal27,28,33 protruded29,32,34,35 or retrusive maxilla36,37, a retrusive mandible which could be both deficient in its overall size26–28,30 and posteriorly located in relation to the cranial base29,38,39. In the vertical dimension, both an anterior upward or downward tipping of the maxilla32,40 and a steep mandibular plane with or without an increased lower face height34,37,41 have been described. Regarding incisor angulations, studies have reported normal26,35, proclined28,32 and retroclined upper incisors30,39 and normal34,35 or proclined lower incisors30,39. In addition, studies utilizing PA cephs and dental models have shown a higher incidence of transverse maxillary deficiencies42.
In contrast, dento-skeletal features most common in patients with Class II div. 2 malocclusion include a larger cranial base length38, a normal14,38 or retrusive maxilla43, mandibular retrusion38, an acute gonial angle, increased posterior facial height with a marked vertical development of the mandibular ramus, decreased anterior facial height and excessive bony chin14,38,43, retroclined upper incisors and normal or retroclined lower incisors14,38. Besides the dento-skeletal differences, particular lip features have been found for both subdivision types. In the Class II div. 1 the proclination of the upper incisors is usually accompanied by a short upper lip and a low lip level with flaccid tone and less lip pressure which is unable to counteract upper incisor protrusion44. Conversely, a high lip level with a thicker lip shape, yet not a hypertonic muscle are associated with the upper incisor retroclination commonly found in the Class II div. 245,46.
Morphometric studies of Class II samples have found mostly large differences in the size and shape of the mandible between Class II and control individuals27,47,48 corroborating previous reports. More sophisticated multivariate methods for cephalometric data analysis such as cluster procedures and principal components analysis have also been utilized providing further insight and facilitating data interpretation beyond univariate methods36,40,49. Moyers et al., 198036 utilized the largest sample (N=697) and applied a clustering procedure that produced 6 horizontal (A–F) and 5 vertical (1–5) overlapping subgroups. Amongst the horizontal subgroups 4 were considered severe (B, C, D, and E) and two others (A and F) were mild including upper dental protrusion in group A and slight mandibular retrognathism in group F. The 5 vertical subgroups were less well defined and some occurred within one particular horizontal subgroup more frequently (Table I). Moyers et al., 36 classified 610 individuals in horizontal subgroups and 495 individuals into both vertical and horizontal subgroups yet failed to classify 87 individuals. Although the methods in the Moyers study were not detailed and a large portion of the variation remained unexplained, important Class II clinical features were visualized.
Table I.
Horizontal and Vertical types described in the Class II sample by Moyers et al., 1980
| Horizontal Types | Dento-skeletal components | N | % |
|---|---|---|---|
| A | Maxillary dental protrusion | 17 | 2.8 |
| B | Flat cranial base, maxillary protrusion, normal mandible | 104 | 17.0 |
| C | Severe class II with bi-maxillary retrusion with upper and lower incisor proclination | 64 | 10.5 |
| D | Slightly retruded or normal maxilla, small mandible, proclined maxillary incisors | 112 | 18.4 |
| E | Maxillary protrusion with normal or protruded mandible with upper and lower incisor proclination | 63 | 10.3 |
| F | Milder class II skeletal group with mandibular retrusion | 250 | 41.0 |
| Total | 610 | 100.0 | |
|
| |||
| Vertical types | Dento-skeletal components | N | % |
|
| |||
| 1 | Downward inclination of palatal, occlusal and mandibular planes | 132 | 26.7 |
| 2 | Parallel palatal, occlusal and mandibular planes, deep bite tendency | 233 | 47.1 |
| 3 | Palatal plane tipped upward anteriorly and steep mandibular plane, open bite tendency | 80 | 16.2 |
| 4 | Large downward inclination of palatal, occlusal and mandibular planes | 13 | 2.6 |
| 5 | Palatal plane is tipped downward, but occlusal and mandibular planes are normal, skeletal deep bite | 37 | 7.5 |
| Total | 495 | 100.0 | |
|
| |||
| Combinations between horizontal and vertical types | Vertical 1-Horizontal A C D F | ||
| Vertical 2-All Horizontal types | |||
| Vertical 3-Horizontal C D F | |||
| Vertical 4-Horizontal B | |||
| Vertical 5-Horizontal B E | |||
Kasai et al., 1995 utilized a sample of 46 adult Japanese male Class I and Class II crania to investigate the relations between the cranial base and facial morphology and examine differences between both malocclusion groups. A principal component analyses was done separately for linear and angular craniofacial measurements. The study found that 5 linear and 3 angular components explained 73% and 78.6% of the variation respectively. The linear components depicted variation in overall facial size, negative correlations between mandibular size vs. facial height and mandibular width vs. ramus height and lastly variation in cranial base and palatal width. In contrast, the 3 angular principal components represented variation in antero-posterior (AP) jaw relations, the horizontal discrepancy between both jaws and variation in the symphysis and gonial angle respectively. This study also showed that both cranial base size and angulation were accurate predictors of AP and vertical maxillo-mandibular relations40.
Burke et al., 1998 utilized multivariate reduction methods to predict facial and horizontal facial morphology and established correlations with condylar head inclinations. Results showed that patients with horizontal facial morphology displayed anterior condylar head inclination and more superior joint space whereas patients with vertical facial morphology displayed posterior condylar head inclination and less superior condylar space leading to a possible reduction in condylar soft tissue and less growth potential. Thus condylar head inclination is an important aspect in the evaluation of growth potential in Class II patients.
Finally, longitudinal studies in Class II patients indicate that the Class II dento-skeletal characteristics can appear as early as the primary dentition50. Although growth curve profiles are similar between Class II and Class I individuals and catch up growth can occur in some Class II individuals51 growth magnitudes and directions are different48,51 This indicates an overall tendency for the Class II discrepancies not to self-correct and instead be maintained into adulthood28,35,48,51.
In summary, previous studies have shown a large variation in dento-skeletal features for both subdivision types of Class II malocclusion. Yet, the causes of such unfavorable dento-skeletal combinations still remain unknown. To an extent, the most common components observed in Class II individuals have been described leading to improved orthodontic diagnostics and treatment planning. However, most studies to date have limitations in sample sizes, sample selection criteria such as including growing individuals and not excluding other genetic or environmental traits such as missing or impacted teeth, heterogeneity due to race/ethnicity, and lack of or limited standardization of data with respect to key variables such as age and sex before applying the data reduction methods. Therefore, there is uncertainty regarding the extent to which the results from previous work are generalizable to other samples and populations and whether one can identify additional phenotypic variation in other samples.
The goals of this study are to extract phenotypes that could best capture the variation present in a large sample of adult Caucasian Class II individuals utilizing multivariate data reduction methods and to investigate whether identified patterns would replicate those of previous studies in our sample which was restricted to post-pubertal individuals. We also investigated the details of age and gender effects during our attempts to carry out rigorous data standardization prior to the application of data reduction methods to increase the precision of the estimation. Lastly, another goal of this study was to test if we could explain meaningful additional variation in this sample and provide an improvement as far as the assignment of qualitative phenotypic classification for each individual in this sample. Such improvement in phenotypic classification can be important both clinically and for increasing the power of genetic studies. This work will result in a comprehensive set of Class II phenotypes that could be utilized on the unbiased phenotypic characterization of additional Class II individuals to increase the power of future genetic studies by strengthening the phenotype-genotype correlations.
MATERIALS AND METHODS
The study protocol was reviewed and approved by the Institutional Review Board at the University of Iowa. The study sample included unrelated adult Class II patients who were seeking treatment at the University of Iowa Orthodontic Graduate Clinic, University of Iowa Hospital Dentistry Clinic. The sample consisted of 309 healthy Caucasian of European descent post-pubertal subjects (227 females, 82 males; age range 16–60 years) who would have completed 95% of their growth at the time of initial records and met our eligibility criteria (Table II).
Table II.
Eligibility Criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Adult (female ≥ 16 years, male ≥ 18 years) | |
| At least 2 of the following clinical criteria required: | History of severe facial trauma |
| ANB ≥ 4 | Previous orthodontic treatment |
| Overjet ≥ 4 | Presence of facial syndromes |
| Angle CII molar or canine relationship on at least one side | |
| Convex profile | Missing or poor quality records |
| Note: Both Class II division 1 and division 2 subjects were included. | Missing or impacted teeth other than 3rd molars |
| Retained primary teeth |
Cephalometric Procedure
2D pre-treatment lateral cephalometric films of 309 Class II adults were digitized using Dolphin Imaging, version 11.0 (Dolphin Imaging Systems, Chatsworth, Calif). Sixty-three cephalometric measurements were taken representing distance (mm), degree, percentage and difference measures between cephalometric landmarks, which were derived from commonly used lateral cephalometric analyses and described previously52 (Table III). Data were obtained from two different sources (film and digital radiographs). All films taken on conventional/analog cephalometric units from either the College of Dentistry Graduate Orthodontic Clinic or the Hospital Dentistry Clinic were scanned into Dolphin with a 100mm ruler and corrected for magnification by 12% and 13%, respectively. Distance measures for film radiographs were scaled (multiplied by 0.8929 for 12% magnified cephalometric radiographs from the College of Dentistry Graduate Clinic and 0.8850 for 13% magnified cephalometric radiographs from Hospital Dentistry Clinic) to match the digital radiographs which were not corrected for magnification53. In order to reduce landmark identification errors, all scanned analog films were traced twice (by S.H.) and the average value for each variable was used in data analysis54.
Table III.
63 Cephalometric Variables
| Cranial Base | Intermaxillary | Dental |
|---|---|---|
| Saddle/Sella Angle (SN-Ar) (°) | ANB (°) | U1 - SN (°) |
| Ant Cranial Base (SN) (mm) | Facial Plane to AB (AB-NPg) (°) | U1 - NA (°) |
| Post Cranial Base (S-Ar) (mm) | Facial Plane to SN (SN-NPg) (°) | U1 - NA (mm) |
| Midface Length (Co-A) (mm) | U1 - FH (°) | |
| Maxilla | P-A Face Ht (S-Go/N-Me) (%) | IMPA (L1-MP) (°) |
| SNA (°) | Y-Axis (N-S-Gn) (°) | L1 - NB (°) |
| Convexity (NA-APg) (°) | Mx/Md Diff (Co-Gn - Co-ANS) (mm) | L1 - NB (mm) |
| N-A || HP (mm) | Wits Appraisal (AO-BO) (mm) | L1 Protrusion (L1-APg) (°) |
| A to N Perp (FH) (mm) | Ant Face Ht (N-Me) (mm) | L1 Protrusion (L1-APg) (mm) |
| Mx Unit Length (Co-ANS) (mm) | Upper Face Ht (N-ANS) (mm) | FMIA (L1-FH) (°) |
| Lower Face Ht (ANS-Me) (mm) | Interincisal Angle (U1-L1) (°) | |
| Mandible | Nasal Ht (N-ANS/N-Me) (%) | UADH (U1-PP) (mm) |
| SNB (°) | PFH:AFH (Co-Go/N-Me) (%) | LADH (L1-MP) (mm) |
| Facial Angle (FH-NPg) (°) | FMA (FH-MP) (°) | UPDH (U6-PP) (mm) |
| Gonial/Jaw Angle (Ar-Go-Me) (°) | SN - GoGn (°) | LPDH (L6 - MP) (mm) |
| Chin Angle (Id-Pg-MP) (°) | Occ Plane to SN (°) | Overjet (mm) |
| Ramus Height (Ar-Go) (mm) | Occ Plane to FH (°) | Overbite (mm) |
| Length of Mn Base (Go-Pg) (mm) | FH - SN (°) | |
| Facial Taper (N-Gn-Go) (°) | Soft Tissue | |
| Articular Angle (S-Ar-Go) (°) | Upper Lip to E-Plane (mm) | |
| N-B || HP (mm) | Lower Lip to E-Plane (mm) | |
| N-Pg || HP (mm) | U Lip to ST N Perp (FH) (mm) | |
| B to N Perp (FH) (mm) | L Lip to ST N Perp (FH) (mm) | |
| Pg to N Perp (FH) (mm) | ST Pg to ST N Perp (FH) (mm) | |
| Mn Unit Length (Co-Gn) (mm) | ||
| Pg - NB (mm) | ||
| Post Facial Ht (mm) (Co-Go) |
Method Error
Inter and intra-rater reliability in landmark location and resulting calculation of craniofacial measurements were determined using the intraclass correlation (ICC)55. For inter-rater reliability a random calibration sample of 15 cephs utilized in a previous study 52 were digitized by both raters (S.H and K.V). For intra-rater reliability a random ceph sample of 15 Class II individuals were digitized twice by the same rater with at least three weeks of time between measurements (S.H). In addition, the possibility of systematic differences between raters or between first and second ratings was assessed using the Wilcoxon Signed Rank procedure. All analyses were performed using SAS for Windows (v9.2, SAS Institute Inc, Cary, NC, USA), and a type I error of 0.05 was specified.
Statistical Analysis
Principal component analyses (PCA) and cluster analysis were used to capture the most significant components of variation and to identify the most homogeneous groups of individuals representing distinct Class II phenotypes to reduce genetic heterogeneity. Data were standardized using a linear model to assess possible effects of age and gender and to consider the possibility of age-by-gender interactions. A separate model was fit for each of the 63 cephalometric measures using standard multiple regression methods. In all, three different configurations of covariate adjustment were used among the 63 models: all included an adjustment for gender, some also required an age adjustment, and others an additional consideration of gender by age interaction, i.e., different age adjustment for each gender. Diagnostic procedures were performed to assure conformance to model assumptions. The studentized (normalized) residuals were extracted from these models and used as the standardized data for the PCA. Standardized PCA scores were the basis for the formation of clusters defining distinct phenotypes within the Class II malocclusion. Cluster analysis was performed via a partitional cluster analysis of extracted principal components using SAS 9.3 statistical software with methods based on the leader 56 and the k-means 57 algorithms using the method of Anderberg 58 called nearest centroid sorting.
The k-means clustering algorithm is sensitive to extreme values as a consequence of the least squares condition; however no subjects in this dataset appeared to represent extreme observations. The clustering algorithm was performed separately for a range of number of clusters, from 2 to 7 clusters. During this process, the iterative reassignment of cluster centroids progressed until no observations changed clusters and convergence was achieved by the cluster algorithm in all configurations. Criterion-based model selection methods, including methods of pseudo F statistic 59 approximate expected over-all R2, and cubic clustering criterion (valid because of the uncorrelated nature of principal components) 60 as well as data visualization techniques were used to determine the appropriate number of clusters61. To visualize the cluster analysis results, a canonical discriminant analysis was performed and scored canonical variables were computed. The scored canonical variables were used to plot pairs or triads of canonical variables in order to aid visual interpretation of cluster differences. R statistical program along with the rgl package were used to produce three-dimensional graphs of the data. Cluster characterization and validation was performed by locating the centroid of each cluster. The cluster centroid is the individual closest to the numerical average of that cluster. The profile of each centroid was drawn to represent the characteristics of that group (Figure IV) and subject’s cephalometric data was examined to ensure that clusters represented distinct clinical phenotypes. A Type I error of 0.05 was specified throughout. All analyses were performed using SAS 9.3 and R statistical software.
Figure IV.
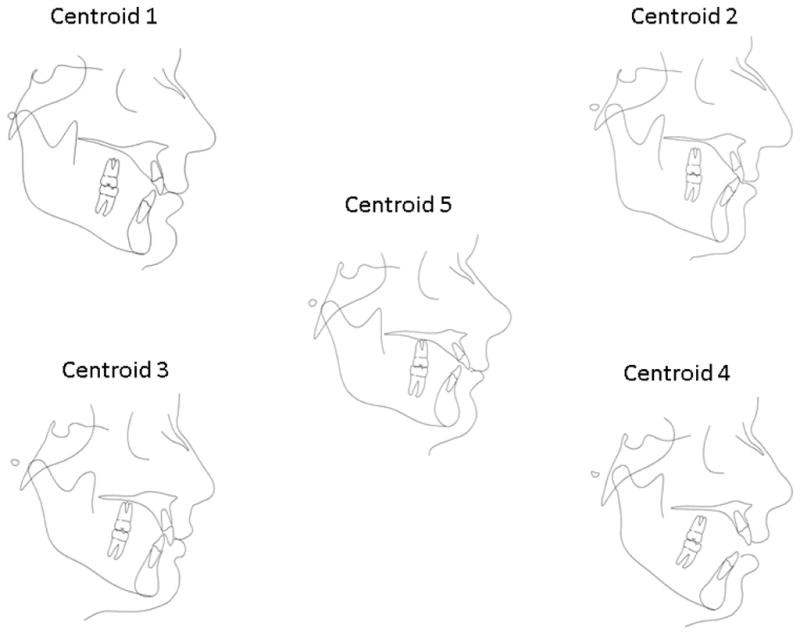
Cluster centroids. Cluster 1 represented the mildest Class II skeletal profile of all groups with individuals presenting a slightly retrusive maxilla, mildly retropositioned mandible and normal vertical dimensions. Cluster 2 represented a moderate Class II malocclusion with a retrusive mandible, dental compensations with retrusive upper incisors and protusive lower incisors and a normal vertical dimension. Cluster 3 represents a more severe Class II skeletal profile in which patients present with both maxillary protrusion and mandibular retrusion and a decrease anterior facial height and deep overbite. Cluster 4 represents patients with a smaller mandible and decreased ramus height, a very steep mandibular plane and a tendency to an anterior open bite. Finally, Cluster 5 represents patients with a very flat mandibular plane angle, short anterior face height, protrusive upper incisors, large overjet and deep overbite.
RESULTS
Reliability testing of landmark location and derived craniofacial measurements showed inter-rater reliability values for the intraclass correlation ranging from 0.844 to 0.996, all ICCs exceeded 0.80, indicating excellent reliability for all measures. There were several variables with significant differences between replicate measures, but all mean differences were <1.87mm. For intra-rater reliability, ICCs ranged from 0.714 to 0.999; only a single variable was associated with an ICC below the value of 0.80 corresponding to excellent intra-rater reliability. Nine variables had significant differences between the first and second measures made by the same rater, and the greatest mean difference was 2.06mm. After examining variables with significant differences, outliers were identified and techniques utilized to improve reliability to acceptable values; discrepancies in cephalometric measurements within 0.5–1mm are generally deemed acceptable in the literature due to the inherent difficulty in landmark location.
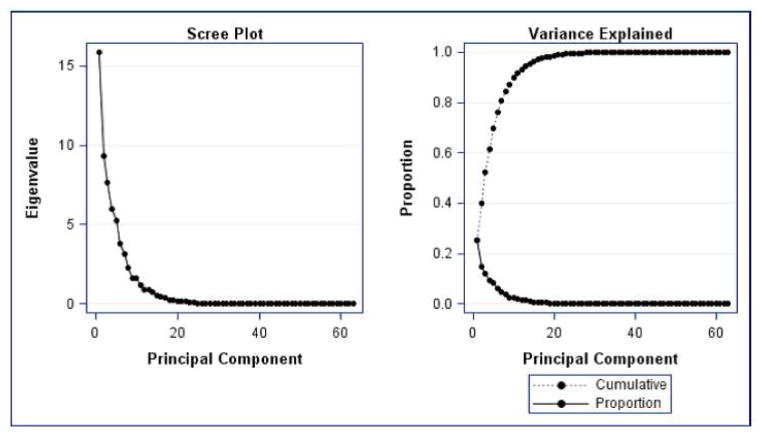
The results of the PCA revealed that 7 principal components accounted for 81% of the total variance in the data (Figure I). The first seven principal components (PCs) were selected because they explained the most variation in the data set and were specific in their anatomic explanation. As shown in Figure I, PCs beyond the 7th component were deemed not informative as the additional variation explained decreased significantly. Table IV contains the variance explained by each component and the set of variables that contributed the most to each PC. To better visualize the variation contain within each principal component, the cephalometric profiles of individuals with extreme PC scores values (i.e. most negative and most positive score) on each component together with the highest loading cephalometric variables are displayed in Figure II. Results showed that about 50% of the variation in this Class II sample is explained by inclination of the mandibular plane, the angulation of the upper incisors and mandibular horizontal and vertical lengths. Interestingly, PC2 which explains 15% of the variation seemed to have captured the incisor variation typically seen between the Class II div. 1 and div. 2 where individuals on the low extreme of this component presented with very retroclined upper incisors (div. 2) and conversely individuals in the high extreme presented with very proclined upper incisors (div. 1).
Figure I.
Principal Component Analyses. Seven principal components accounted for 81% of the variation in the Class II sample.
Table IV.
Principal Component Analysis.
| Principal Component | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Variance Explained | 0.25 | 0.15 | 0.12 | 0.09 | 0.08 | 0.06 | 0.05 |
| Cumulative Variance | 0.40 | 0.52 | 0.62 | 0.70 | 0.76 | 0.81 | |
| *Variables | SNGoGNDeg | U1SNDeg | CoGnmm | U1NaDeg | L1MPDeg | FHSNDeg | AOBOmm (Wits) |
| FHMPDeg | U1L1Deg | CoGomm | U1Namm | NGnGoDeg | ANPerpmm | OJmm | |
| NSGnDeg (Y-axis) | U1FHDeg | NMemm | SNADeg | IdPgMPDeg | ULipSTNPerpmm | ABNPgDeg | |
| PgNhorizmm | LLipSTNPerpmm | U6PPmm | ANHorizmm | SGoNMePerc | LLipSTNPerpmm | ANBDeg |
Variables making the greatest contribution to the respective principal component.
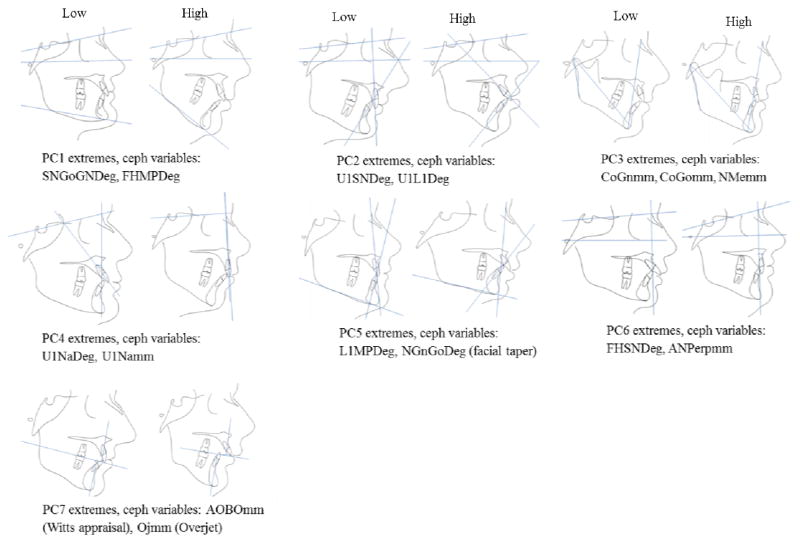
Figure II.
Examples of individuals in opposite extremes of the distribution of principal component scores for each of the seven principal components accounting for 81 % of the variation, together with the highest loading ceph variables on each principal component. PC1 refers to variation in the inclination of the mandibular plane angle and explains 25% of the variation. PC2 explains 15% of the variation and refers to the maxillary incisor angulation. PC3 refers to the mandibular AP and vertical lengths as well as the posterior facial height and explains 12% of the variation. PC4 references the position of the maxilla, especially in regards to the maxillary incisor angulation and accounts for 9% of the variation. PC5 represents mandibular incisor angulation relative to the mandibular plane and the degree of facial taper and explains 8% of the variation. PC6 refers to the angulation of the cranial base and the AP position of the maxilla and explains 6% of the variation. PC7, explains 5%, and refers to variation on the WITS analysis (A–O to B–O) and the amount of overjet.
The cluster analysis identified five sub-groups within the Class II subjects (Figure III). A five cluster model was selected because it yielded the most spatially distinct and clinically meaningful subphenotypes that were statistically acceptable, based on the Pseudo F and Cubic Clustering Criterion results (Table V). While models with 2, 3, or 4 clusters were statistically acceptable, the two and three cluster models were viewed as clinically too simplistic. In addition, upon comparison of the 4 cluster model with the 5 cluster model we realized that the 4 cluster model did not include a separate cluster of individuals with an open-bite tendency and therefore we decided to accept the 5 cluster model to capture this clinically relevant cluster. Though ideally the most well defined clusters would be separate entities, some overlap between groups is expected and was observed (Figure 3). Cluster 2 was the central cluster and contained the most observations (n=85), cluster 4 had the largest standard deviation (spread of observations) and had the fewest observations (n=53) (Table V). Complete dento-skeletal components characterizing cluster centroids are given in Table VI. Cluster 1 represented the mildest Class II skeletal profile of all groups with a slightly retrusive maxilla, mildly retropositioned mandible and normal vertical dimensions. Cluster 2 represented a moderate Class II malocclusion with a retrusive mandible, and normal vertical dimension. Cluster 3 represents a more severe Class II skeletal profile with both maxillary protrusion and mandibular retrusion. Cluster 4 represents patients with a smaller mandible in unit length and ramus height and an open bite tendency. Finally, Cluster 5 represents patients with mild maxillary protrusion, mandibular retrusion, short anterior face height, and deep overbite (Table VI).
Figure III.
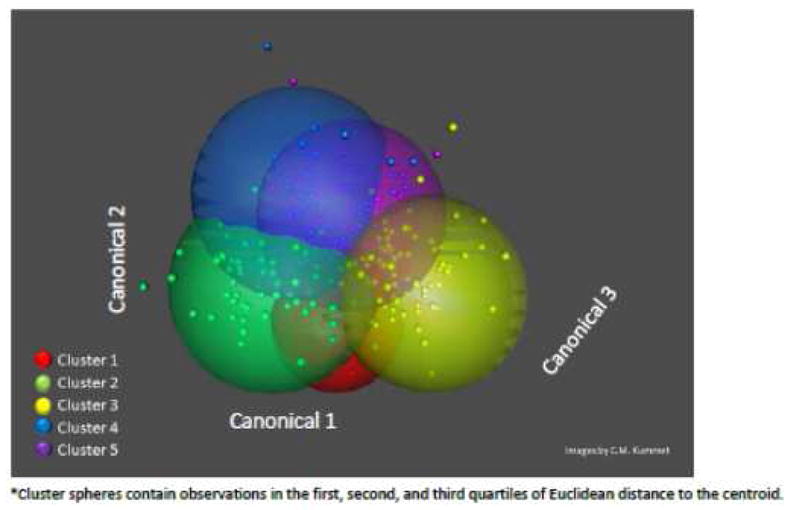
3-D Plot showing 5 spatially distinct clusters of CII malocclusion subjects.
Table V.
Cluster Summary
| Cluster Summary-5 Clusters
| ||||
|---|---|---|---|---|
| Cluster | Frequency (%total) | * Root mean Squares (St.Dev) | Nearest Cluster | Distance Between Centroids |
|
| ||||
| 1 | 56 | 0.75 | 2 | 2.13 |
| 2 | 85 | 0.77 | 5 | 2.10 |
| 3 | 57 | 0.90 | 2 | 2.26 |
| 4 | 53 | 0.94 | 2 | 2.19 |
| 5 | 58 | 0.87 | 2 | 2.10 |
N=309 Cuacasian. Data age, gender adjusted and normalized PCA.
Indicates the average distance between observations in the cluster.
Table VI.
Description of dento-skeletal components on cluster centroids
| Atribute | Cluster 1 (N=56) | Cluster 2 (N=85) | Cluster 3 (N=57) | Cluster 4 (N=53) | Cluster 5 (N=58) |
|---|---|---|---|---|---|
| Cranial Base | Longer anterior cranial base and slightly decreased saddle angle | Normal | Shorter anterior cranial base normal saddle angle | Shorter anterior cranial base and slightly decreased saddle angle | Normal |
| Maxilla | Mildly retrusive | Normal | Mildly protrusive | Mildly protrusive | Mildly protrusive |
| Mandible | Larger unit length yet mildly retropositioned | Moderately retrusive | Smaller unit length, short ramus, retrusive mandible | Retrusive with very short ramus height | Mildly retrusive |
| Vertical | Normal | Mildly decreased mandibular plane angle | Shorter anterior face height, increased overbite, normal mandibular plane | Steep mandibular plane, open bite | Short anterior face height, flat mandibular plane |
| U1 | Upright | Upright | Upright | Normal | Protrusive |
| L1 | Normal | Proclined | Normal | Proclined | Normal |
| Other soft tissue findings | Deep labio mental fold | Slight interlabial gap | Redundant lips | Large interlabial gap | Redundant lips |
MP, Mandibular plane. CB, Cranial base. U1 Upper incisor. L1 Lower incisor.
DISCUSSION
Studies on the etiology of malocclusion have supported the notion of malocclusion being caused by the interplay of multiple craniofacial components whose variation needs to be meticulously interpreted for successful orthodontic diagnosis and treatment planning62. Beyond this natural clinical application a comprehensive dento-skeletal characterization is also invaluable to understand physiologic differences between individuals, to design preventive therapies, to improve treatment outcomes in both nonsyndromic individuals as well as those with craniofacial conditions, to improve prediction of facial growth and to better comprehend the role of genetics so that in the future, genetic approaches could become part of the orthodontic armamentarium.
In the current study, seven PCs of various multivariate traits as well as five clusters within the sample of Class II individuals were identified capturing all Class II components described in previous studies, validating the use of multivariate reduction methods for accurate phenotypic classification and given support to the generalizability of the phenotypes described here to other samples. Given the similarity between our phenotypes and those described by previous studies in younger individuals, results seem to indicate that Class II characteristics can appear early in development and do not always self-correct. Our PCA analysis also resulted in clinically relevant axis of variation with quantitative phenotypic information at the individual level amenable to be included in future genetic and environmental studies of Class II malocclusion etiology.
Comparison of our data with the Moyers et al., study resulted in general agreement as far as vertical and horizontal components and variation in the severity of their expression. The most common horizontal and vertical subgroup described by Moyers et al., was the mild Class II skeletal with normal vertical inclination of the horizontal planes (Horizontal F and Vertical 2). This group is similar to our Clusters 1 and 2, both ranging from more mild to more moderate Class II features respectively. Three of our cluster groups have protrusive maxillas similar to Moyers horizontal groups B and E. Cluster 1 in our study has both maxillary and mandibular retrusion resembling to Moyers horizontal group C. Our Cluster 4 is analogous to Moyers vertical type 3 in that both represent patients with open bite tendencies. Our Clusters 3 and 5 with normal and protrusive upper incisors respectively, approximate Moyers vertical subgroups 2 and 5. None of our clusters were similar to the horizontal group A from Moyers with dental Class II features exclusively. This was not unexpected since our eligibility criteria ensured that all our subjects had a skeletal component to their Class II malocclusion.
Our cephalometric variables are comparable only to those of the angular analysis in Kasai et al., 40 and thus we found that Kasai’s components (C1-C3) approximate our PCs 1, 7 and 5 respectively. The 1st component on both Kasai’s and our study represented variation in the inclination of the mandibular plane with the highest loading observed for the SN-mandibular plane angle in both studies. Kasai’s C2 and PC7 denote variation in maxillo-mandibular horizontal discrepancies as measured by the angle ANB and Witts analysis on Kasai’s and our study respectively. Lastly, Kasai’s C 3 and PC5 characterize variation in the gonial angle, facial taper and the chin angle. Differences in component ranks and percent of variation explained could be attributable to facial differences between Japanese and Caucasian Class II samples.
In summary, we have completed the phenotypic characterization of a sample of adult Caucasian Class II individuals into homogenous clusters and also have generated via PCA, various independent dimensions of quantitative phenotypes which can be utilized in the identification of genetic and environmental causes of Class II malocclusion. Despite any differences in statistical methodology, adjustments for gender and age, and populations, the characterization of Class II phenotypes arrived at by the 3 studies compared is very similar indicating an underlying skeletal structure in the Class II patients that could be useful in future studies of malocclusion etiology. For instance, previous studies have found significant correlations between masticatory muscles and lip activity with aspects of craniofacial morphology present in patients with different malocclusions44,63. Moreover, results from a recent study applying complex networks methods to Class II and Class III malocclusion samples 64 suggested a much more complex etiologic model for the Class II than the Class III malocclusion, indicating that aspects such as muscle tone, tongue posture and speech problems appeared to be key starting points for the treatment of Class II patients. Such studies could take advantage of well characterized dento-skeletal phenotypes to reduce heterogeneity and increase the power of their modeling. In addition our cluster structure could shed light on treatment planning if future research shows that particular cluster groups respond better to certain treatment modalities than others. For instance, a randomized clinical trial for Class II div.1 treatment, compared Herbst with fixed appliances vs. Headgear with fixed appliances and results suggested that the former combination induced better tissue changes particularly in individuals with mandibular retrusion and low gonial angles65 which in our study are represented by clusters 2 and 5.
Finally, the study of Class II malocclusion etiology via well characterized Class II phenotypes constitutes the ground work for genetic studies of etiology, growth prediction and treatment responses as these may vary by genetic predisposition. Functional appliances in the early adolescent dentition have an average effect of 1.79 mm of mandibular growth per year in treated individuals compared to untreated controls66. This difference although statistically significant has a small clinical impact. However, if the genetic association between mandibular height with the GHR gene and mandibular prognathism with genes GHR, MATRILIN-1, EPB41, TGFB3, LTBP2 and MYO1H19–22 is confirmed in other populations then it is possible to identify those individuals that carry particular variants for these genes and predict individuals with different growth patterns (i.e. vertical vs. horizontal growth depicted by PC1 and PC3 in our study). Alternatively, perform randomized clinical trials to identify hyper responders to functional appliances. Therefore, once the genetic variants contributing to specific Class II phenotypes are identified and their etiological mechanisms understood, future clinical trials can design and test specific treatments for individuals with different genetic backgrounds and maximize treatment effectiveness.
CONCLUSIONS
An important step towards the identification of genetic and environmental causes implicated in Class II malocclusion is the comprehensive characterization of the phenotypic expression of this condition. Conventional pre-treatment orthodontic records constitute an invaluable resource for the characterization of craniofacial variation since they provide skeletal, soft tissue and 3D dento-alveolar data that can be analyzed to construct comprehensive craniofacial phenotypes. In the current study, seven principal components explained 81% of the observed phenotypic variation, and were used to identify five clusters within the sample of Class II individuals providing a comprehensive spectrum of class II phenotypes. Ongoing studies at the College of Dentistry of the University of Iowa are utilizing these results to target individuals for collection of DNA and environmental data; however, current genetic and environmental studies will necessitate much larger samples and therefore multicenter collaborative projects constitute the ideal scenario for studies of malocclusion etiology. Understanding the genetic and environmental etiology of unbalanced craniofacial growth will have a large impact on orthodontic patient care worldwide via novel and improved therapy and prevention approaches.
Acknowledgments
Funding: AAOF OFDFA_2008–2011 and also supported by the National Center for Advancing Translational Sciences, and the National Institutes of Health (NIH), through Grants 2 UL1 TR000442-06 and T32-DEO14678-09.
We thank Drs. Robert N. Staley, James S. Wefel and George Wehby for their helpful discussions during the preparation of this manuscript. We also thank Chika Takeuchi, Mary E. Hoppens and Patricia Hancock for their assistance with orthodontic record review.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Proffit WR, Fields HW, Sarver DM. In: Contemporary Orthodontics. 4. Proffit William R, Fields Henry W, Jr, Sarver David M., editors. St. Louis, Mo: Mosby Elsevier; 2007. [Google Scholar]
- 2.Sant’Anna LB, Tosello DO. Fetal alcohol syndrome and developing craniofacial and dental structures--a review. Orthod Craniofac Res. 2006;9:172–185. doi: 10.1111/j.1601-6343.2006.00377.x. [DOI] [PubMed] [Google Scholar]
- 3.Naidoo S, Harris A, Swanevelder S, Lombard C. Foetal alcohol syndrome: a cephalometric analysis of patients and controls. Eur J Orthod. 2006;28:254–261. doi: 10.1093/ejo/cji110. [DOI] [PubMed] [Google Scholar]
- 4.Rythen M, Thilander B, Robertson A. Dento-alveolar characteristics in adolescents born extremely preterm. Eur J Orthod. 2012 doi: 10.1093/ejo/cjs034. [DOI] [PubMed] [Google Scholar]
- 5.Warren JJ, Slayton RL, Bishara SE, Levy SM, Yonezu T, Kanellis MJ. Effects of nonnutritive sucking habits on occlusal characteristics in the mixed dentition. Pediatr Dent. 2005;27:445–450. [PubMed] [Google Scholar]
- 6.Hebling SR, Cortellazzi KL, Tagliaferro EP, et al. Relationship between malocclusion and behavioral, demographic and socioeconomic variables: a cross-sectional study of 5-year-olds. J Clin Pediatr Dent. 2008;33:75–79. doi: 10.17796/jcpd.33.1.3457qg88w37h2405. [DOI] [PubMed] [Google Scholar]
- 7.Mtaya M, Brudvik P, Astrom AN. Prevalence of malocclusion and its relationship with socio-demographic factors, dental caries, and oral hygiene in 12- to 14-year-old Tanzanian schoolchildren. Eur J Orthod. 2009;31:467–476. doi: 10.1093/ejo/cjn125. [DOI] [PubMed] [Google Scholar]
- 8.Melink S, Vagner MV, Hocevar-Boltezar I, Ovsenik M. Posterior crossbite in the deciduous dentition period, its relation with sucking habits, irregular orofacial functions, and otolaryngological findings. Am J Orthod Dentofacial Orthop. 2010;138:32–40. doi: 10.1016/j.ajodo.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 9.Montaldo L, Montaldo P, Cuccaro P, Caramico N, Minervini G. Effects of feeding on non-nutritive sucking habits and implications on occlusion in mixed dentition. Int J Paediatr Dent. 2011;21:68–73. doi: 10.1111/j.1365-263X.2010.01092.x. [DOI] [PubMed] [Google Scholar]
- 10.Begg PR. Stone Age man’s dentition: With reference to anatomically correct occlusion, the etiology of malocclusion, and a technique for its treatment. Am J Orthod C V Mosby Co. 1954;40:373–383. Available from: http://linkinghub.elsevier.com/retrieve/pii/0002941654900354?showall=true. [Google Scholar]
- 11.Evensen JP, Ogaard B. Are malocclusions more prevalent and severe now? A comparative study of medieval skulls from Norway. Am J Orthod Dentofacial Orthop. 2007;131:710–716. doi: 10.1016/j.ajodo.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 12.Thilander B, Pena L, Infante C, Parada SS, de Mayorga C. Prevalence of malocclusion and orthodontic treatment need in children and adolescents in Bogota, Colombia. An epidemiological study related to different stages of dental development. Eur J Orthod. 2001;23:153–167. doi: 10.1093/ejo/23.2.153. [DOI] [PubMed] [Google Scholar]
- 13.Borzabadi-Farahani A, Borzabadi-Farahani A, Eslamipour F. Malocclusion and occlusal traits in an urban Iranian population. An epidemiological study of 11- to 14-year-old children. Eur J Orthod. 2009;31:477–484. doi: 10.1093/ejo/cjp031. [DOI] [PubMed] [Google Scholar]
- 14.Peck S, Peck L, Kataja M. Class II Division 2 malocclusion: a heritable pattern of small teeth in well-developed jaws. Angle Orthod. 1998;68:9–20. doi: 10.1043/0003-3219(1998)068<0009:CIDMAH>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 15.Basdra EK, Kiokpasoglou M, Stellzig A. The Class II Division 2 craniofacial type is associated with numerous congenital tooth anomalies. Eur J Orthod. 2000;22:529–535. doi: 10.1093/ejo/22.5.529. [DOI] [PubMed] [Google Scholar]
- 16.Yamaguchi T, Park SB, Narita A, Maki K, Inoue I. Genome-wide linkage analysis of mandibular prognathism in Korean and Japanese patients. J Dent Res. 2005;84:255–259. doi: 10.1177/154405910508400309. [DOI] [PubMed] [Google Scholar]
- 17.Frazier-Bowers S, Rincon-Rodriguez R, Zhou J, Alexander K, Lange E. Evidence of linkage in a Hispanic cohort with a Class III dentofacial phenotype. J Dent Res. 2009;88:56–60. doi: 10.1177/0022034508327817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Q, Zhang F, Li X, Chen F. Genome scan for locus involved in mandibular prognathism in pedigrees from China. PLoS One. 2010;5:e12678. doi: 10.1371/journal.pone.0012678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Q, Li X, Zhang F, Chen F. The identification of a novel locus for mandibular prognathism in the Han Chinese population. J Dent Res. 2011;90:53–57. doi: 10.1177/0022034510382546. [DOI] [PubMed] [Google Scholar]
- 20.Zhou J, Lu Y, Gao XH, et al. The growth hormone receptor gene is associated with mandibular height in a Chinese population. J Dent Res. 2005;84:1052–1056. doi: 10.1177/154405910508401116. [DOI] [PubMed] [Google Scholar]
- 21.Xue F, Wong R, Rabie AB. Identification of SNP markers on 1p36 and association analysis of EPB41 with mandibular prognathism in a Chinese population. Arch Oral Biol. 2010;55:867–872. doi: 10.1016/j.archoralbio.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 22.Tassopoulou-Fishell M, Deeley K, Harvey EM, Sciote J, Vieira AR. Genetic variation in myosin 1H contributes to mandibular prognathism. Am J Orthod Dentofacial Orthop. 2012;141:51–59. doi: 10.1016/j.ajodo.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gutierrez SJ, Gomez M, Rey JA, Ochoa M, Gutierrez SM, Prieto JC. Polymorphisms of the noggin gene and mandibular micrognathia: a first approximation. Acta Odontol Latinoam. 2010;23:13–19. [PubMed] [Google Scholar]
- 24.Stottmann RW, Anderson RM, Klingensmith J. The BMP antagonists Chordin and Noggin have essential but redundant roles in mouse mandibular outgrowth. Dev Biol. 2001;240:457–473. doi: 10.1006/dbio.2001.0479. [DOI] [PubMed] [Google Scholar]
- 25.Wilcox MA, Wyszynski DF, Panhuysen CI, et al. Empirically derived phenotypic subgroups - qualitative and quantitative trait analyses. BMC Genet. 2003;4 (Suppl 1):S15. doi: 10.1186/1471-2156-4-S1-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sayin MO, Turkkahraman H. Cephalometric evaluation of nongrowing females with skeletal and dental Class II, division 1 malocclusion. Angle Orthod. 2005;75:656–660. doi: 10.1043/0003-3219(2005)75[656:CEONFW]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 27.Franchi L, Baccetti T, Stahl F, McNamara JA., Jr Thin-plate spline analysis of craniofacial growth in Class I and Class II subjects. Angle Orthod. 2007;77:595–601. doi: 10.2319/070506-275. [DOI] [PubMed] [Google Scholar]
- 28.Stahl F, Baccetti T, Franchi L, McNamara JA., Jr Longitudinal growth changes in untreated subjects with Class II Division 1 malocclusion. Am J Orthod Dentofacial Orthop. 2008;134:125–137. doi: 10.1016/j.ajodo.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 29.Al-Khateeb EA, Al-Khateeb SN. Anteroposterior and vertical components of class II division 1 and division 2 malocclusion. Angle Orthod. 2009;79:859–866. doi: 10.2319/062208-325.1. [DOI] [PubMed] [Google Scholar]
- 30.Perillo L, Padricelli G, Isola G, Femiano F, Chiodini P, Matarese G. Class II malocclusion division 1: a new classification method by cephalometric analysis. Eur J Paediatr Dent. 2012;13:192–196. [PubMed] [Google Scholar]
- 31.Kerr WJ, Hirst D. Craniofacial characteristics of subjects with normal and postnormal occlusions--a longitudinal study. Am J Orthod Dentofacial Orthop. 1987;92:207–212. doi: 10.1016/0889-5406(87)90413-6. [DOI] [PubMed] [Google Scholar]
- 32.Rothstein T, Yoon-Tarlie C. Dental and facial skeletal characteristics and growth of males and females with class II, division 1 malocclusion between the ages of 10 and 14 (revisited)-part I: characteristics of size, form, and position. Am J Orthod Dentofacial Orthop. 2000;117:320–332. doi: 10.1016/s0889-5406(00)70237-x. [DOI] [PubMed] [Google Scholar]
- 33.Proff P, Will F, Bokan I, Fanghanel J, Gedrange T. Cranial base features in skeletal Class III patients. Angle Orthod. 2008;78:433–9. doi: 10.2319/013007-48.1. [DOI] [PubMed] [Google Scholar]
- 34.Ishii N, Deguchi T, Hunt NP. Craniofacial morphology of Japanese girls with Class II division 1 malocclusion. J Orthod. 2001;28:211–215. doi: 10.1093/ortho/28.3.211. [DOI] [PubMed] [Google Scholar]
- 35.Vasquez MJ, Baccetti T, Franchi L, McNamara JA., Jr Dentofacial features of Class II malocclusion associated with maxillary skeletal protrusion: a longitudinal study at the circumpubertal growth period. Am J Orthod Dentofacial Orthop. 2009;135:568, e1–7. doi: 10.1016/j.ajodo.2007.05.026. discussion 568–9. [DOI] [PubMed] [Google Scholar]
- 36.Moyers RE, Riolo ML, Guire KE, Wainright RL, Bookstein FL. Differential diagnosis of class II malocclusions. Part 1. Facial types associated with class II malocclusions. Am J Orthod. 1980;78:477–494. doi: 10.1016/0002-9416(80)90299-7. [DOI] [PubMed] [Google Scholar]
- 37.McNamara JA., Jr Components of class II malocclusion in children 8–10 years of age. Angle Orthod. 1981;51:177–202. doi: 10.1043/0003-3219(1981)051<0177:COCIMI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 38.Brezniak N, Arad A, Heller M, Dinbar A, Dinte A, Wasserstein A. Pathognomonic cephalometric characteristics of Angle Class II Division 2 malocclusion. Angle Orthod. 2002;72:251–257. doi: 10.1043/0003-3219(2002)072<0251:PCCOCI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 39.Saltaji H, Flores-Mir C, Major PW, Youssef M. The relationship between vertical facial morphology and overjet in untreated Class II subjects. Angle Orthod. 2012;82:432–440. doi: 10.2319/050711-322.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kasai K, Moro T, Kanazawa E, Iwasawa T. Relationship between cranial base and maxillofacial morphology. Eur J Orthod. 1995;17:403–410. doi: 10.1093/ejo/17.5.403. [DOI] [PubMed] [Google Scholar]
- 41.Lau JW, Hagg U. Cephalometric morphology of Chinese with Class II division 1 malocclusion. Br Dent J. 1999;186:188–190. doi: 10.1038/sj.bdj.4800059. [DOI] [PubMed] [Google Scholar]
- 42.Franchi L, Baccetti T. Transverse maxillary deficiency in Class II and Class III malocclusions: a cephalometric and morphometric study on postero-anterior films. Orthod Craniofac Res. 2005;8:21–28. doi: 10.1111/j.1601-6343.2004.00312.x. [DOI] [PubMed] [Google Scholar]
- 43.Pancherz H, Zieber K, Hoyer B. Cephalometric characteristics of Class II division 1 and Class II division 2 malocclusions: A comparative study in children. Angle Orthod. 1997;67:111–120. doi: 10.1043/0003-3219(1997)067<0111:CCOCID>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 44.Lambrechts H, De Baets E, Fieuws S, Willems G. Lip and tongue pressure in orthodontic patients. Eur J Orthod. 2010;32:466–471. doi: 10.1093/ejo/cjp137. [DOI] [PubMed] [Google Scholar]
- 45.Lapatki BG, Mager AS, Schulte-Moenting J, Jonas IE. The importance of the level of the lip line and resting lip pressure in Class II, Division 2 malocclusion. J Dent Res. 2002;81:323–328. doi: 10.1177/154405910208100507. [DOI] [PubMed] [Google Scholar]
- 46.McIntyre GT, Millett DT. Lip shape and position in Class II division 2 malocclusion. Angle Orthod. 2006;76:739–744. doi: 10.1043/0003-3219(2006)076[0739:LSAPIC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 47.Lowe BF, Jr, Phillips C, Lestrel PE, Fields HW., Jr Skeletal jaw relationships: a quantitative assessment using elliptical Fourier functions. Angle Orthod. 1994;64:299–308. doi: 10.1043/0003-3219(1994)064<0299:SJRAQA>2.0.CO;2. discussion 309–10. [DOI] [PubMed] [Google Scholar]
- 48.Ngan PW, Byczek E, Scheick J. Longitudinal evaluation of growth changes in Class II division 1 subjects. Semin Orthod. 1997;3:222–231. doi: 10.1016/s1073-8746(97)80055-2. [DOI] [PubMed] [Google Scholar]
- 49.Burke G, Major P, Glover K, Prasad N. Correlations between condylar characteristics and facial morphology in Class II preadolescent patients. Am J Orthod Dentofacial Orthop. 1998;114:328–336. doi: 10.1016/s0889-5406(98)70216-1. [DOI] [PubMed] [Google Scholar]
- 50.Baccetti T, Franchi L, McNamara JA, Jr, Tollaro I. Early dentofacial features of Class II malocclusion: a longitudinal study from the deciduous through the mixed dentition. Am J Orthod Dentofacial Orthop. 1997;111:502–509. doi: 10.1016/s0889-5406(97)70287-7. [DOI] [PubMed] [Google Scholar]
- 51.Bishara SE, Jakobsen JR, Vorhies B, Bayati P. Changes in dentofacial structures in untreated Class II division 1 and normal subjects: a longitudinal study. Angle Orthod. 1997;67:55–66. doi: 10.1043/0003-3219(1997)067<0055:CIDSIU>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 52.Moreno Uribe LM, Vela KC, Kummet C, Dawson DV, Southard TE. Phenotypic diversity in white adults with moderate to severe Class III malocclusion. Am J Orthod Dentofacial Orthop. 2013;144:32–42. doi: 10.1016/j.ajodo.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cohen JM. Comparing digital and conventional cephalometric radiographs. Am J Orthod Dentofacial Orthop. 2005;128:157–160. doi: 10.1016/j.ajodo.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 54.Baumrind S, Frantz RC. The reliability of head film measurements. 1. Landmark identification. Am J Orthod. 1971;60:111–27. doi: 10.1016/0002-9416(71)90028-5. [DOI] [PubMed] [Google Scholar]
- 55.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 56.Hartigan JA. Clustering Algorithms [by] John A Hartigan. New York: Wiley; 1975. [Google Scholar]
- 57.Macqueen JB. Some Methods for classification and analysis of multivariate observations. Vol. 1. University of California Press; 1967. pp. 281–297. [Google Scholar]
- 58.Anderberg M. Cluster Analysis for Applications. New York, NY: Academic Press, Inc; 1973. [Google Scholar]
- 59.Caliński T, Harabasz J. A dendrite method for cluster analysis. Communications in Statistics. 1974;3:1–27. [Google Scholar]
- 60.Sarle WS. The Cubic Clustering Criterion. Cary, NC, USA: SAS Institute; 1983. p. A–108. [Google Scholar]
- 61.Cooper MC, Milligan GW. In: Data, expert knowledge and decisions. Gaul W, Schader M, editors. London, UK, UK: Springer-Verlag; 1988. pp. 319–328. [Google Scholar]
- 62.Sassouni V. A classification of skeletal facial types. Am J Orthod. 1969;55:109–123. doi: 10.1016/0002-9416(69)90122-5. [DOI] [PubMed] [Google Scholar]
- 63.Lowe AA, Takada K. Associations between anterior temporal, masseter, and orbicularis oris muscle activity and craniofacial morphology in children. Am J Orthod. 1984;86:319–330. doi: 10.1016/0002-9416(84)90143-x. [DOI] [PubMed] [Google Scholar]
- 64.Auconi P, Caldarelli G, Scala A, Ierardo G, Polimeni A. A network approach to orthodontic diagnosis. Orthod Craniofac Res. 2011;14:189–197. doi: 10.1111/j.1601-6343.2011.01523.x. [DOI] [PubMed] [Google Scholar]
- 65.Baccetti T, Franchi L, Stahl F. Comparison of 2 comprehensive Class II treatment protocols including the bonded Herbst and headgear appliances: a double-blind study of consecutively treated patients at puberty. Am J Orthod Dentofacial Orthop. 2009;135:698, e1–10. doi: 10.1016/j.ajodo.2008.03.015. discussion 698–9. [DOI] [PubMed] [Google Scholar]
- 66.Marsico E, Gatto E, Burrascano M, Matarese G, Cordasco G. Effectiveness of orthodontic treatment with functional appliances on mandibular growth in the short term. Am J Orthod Dentofacial Orthop. 2011;139:24–36. doi: 10.1016/j.ajodo.2010.04.028. [DOI] [PubMed] [Google Scholar]