Abstract
Background:
TAp63 is a tumour-suppressor protein that is often underexpressed in various types of cancer. It has been shown to activate gene transcription depending on the transcription domain and to be closely related with metastasis. In this study, we demonstrate that TAp63 suppresses metastasis in colon cancer cells through microRNA-133b.
Methods:
We evaluated the correlation of TAp63 and miR-133b with HT-29 and SW-620 cells and investigated the roles of TAp63 in the expression of RhoA, E-cadherin and vimentin. We further investigated the roles of TAp63-mediated invasion and migration of colon cancer cells.
Results:
TAp63 expression is downregulated in colon cancer, and microRNA-133b is a transcriptional target of TAp63. Furthermore, microRNA-133b is essential for the inhibitory effects of TAp63 on RhoA, E-cadherin and vimentin. Moreover, TAp63 inhibits cell migration and invasion through microRNA-133b. Correspondingly, the inhibitory effect of TAp63 on RhoA, E-cadherin, vimentin, migration and invasion can be blocked by the microRNA-133b inhibitor.
Conclusions:
TAp63 and microRNA-133b were able to suppress the metastasis of colon cancer. Both TAp63 and microRNA-133b may be potential biomarkers for diagnosis in colon cancer metastasis and may provide unique therapeutic targets for this common malignancy.
Keywords: TAp63, microRNA-133b, RhoA, colon cancer, metastasis
The major problem of malignancy is cancer metastasis. Epithelial–mesenchymal transition (EMT) is a key step of cancer metastasis (Loboda et al, 2011). P63, a member of the p53 tumour-suppressor gene family, has been shown to have an important role in EMT and metastasis (Zhang et al, 2014), and the expression of epithelial and mesenchymal markers, such as E-cadherin and vimentin, have also been identified as directly correlated with p63 (Choi et al, 2012). Due to the presence of two different promoters, P1 and P2, the protein isoforms of p63 can be divided into two classes: the transactivating TAp63 isoform and the inhibitory ΔNp63 isoform (Ortt and Sinha, 2006). Depending on the transcription domain, TAp63 was able to activate gene transcription and induce cell cycle arrest and apoptosis (Serber et al, 2002; Gressner et al, 2005). Based on these features, TAp63 has been shown as closely related with EMT and metastasis (Anura et al, 2013). Moreover, it has been reported that TAp63 is a transcriptional activator of certain microRNAs (miRNAs) (Knouf et al, 2011; Wu et al, 2012). TAp63 can inhibit EMT and metastasis by the transcriptional regulation of miRNAs (Tran et al, 2012). Indeed, TAp63 is able to regulate the expression of miR-205, which is essential for the inhibitory effects of TAp63 on EMT (Tucci et al, 2012). In agreement with its feature of repressing metastasis, TAp63 is often downmodulated in various types of cancer (Lo Iacono et al, 2011).
RhoA, a protein known to have a critical role in amoeboid invasion, also shows a strong correlation with EMT (Gulhati et al, 2011) and metastasis (Struckhoff et al, 2011). It has been reported that the expression of RhoA is elevated when EMT is actively occurring (Tavares et al, 2006). Further functional studies have demonstrated that inhibiting RhoA mRNA by siRNA can repress both endothelial activation and mesenchymal invasion (Kanlaya et al, 2013). RhoA has become known as an EMT regulator. Recently, some miRNAs have been shown to contribute to EMT by targeting RhoA (Yau et al, 2013). For example, miR-155 can suppress TGF-β-induced EMT through the targeting of RhoA, and it can repress cell migration and invasion (Kong et al, 2008). MiRNA-133b (miR-133b), an important tumour suppressor (Chen et al, 2013), participates in the migration and invasion of certain types of cancer through the post-transcriptional regulation of the target gene (Wen et al, 2013). It has been reported that miR-133b is able to suppress metastasis by targeting RhoA (Qin et al, 2011). And we have previously reported that miR-133b expression is greatly downregulated in human colon cancer cells, inhibiting the growth of colon cancer cells in vitro and in vivo (Hu et al, 2010).
In the present study, we show that TAp63 expression is downregulated in human colon cancer, leading to the transcriptional regulation of the expression of miR-133b. Moreover, we proved that the effects of TAp63 on RhoA, E-cadherin and vimentin are mediated by miR-133b and that TAp63 is able to inhibit cell migration and invasion through miR-133b. Our data suggest that both TAp63 and miR-133b may be potential biomarkers for diagnosis of colon cancer metastasis and provide unique therapeutic targets in this common malignancy.
Materials and methods
Main cell solutions and reagents
Human colon cancer cell lines HT-29 and SW-620 were purchased from the Cell Center of Xiangya School of Medicine, Central South University (Hunan, China). Human normal colon cell line CCD-18Co was purchased from ATCC (American Type Culture Collection, Manassas, VA, USA). Both miR-133b mimics and inhibitor were synthesised by Shanghai GenePharma Co., Ltd (Shanghai, China). The transfection reagent Lipofectamine 2000 was purchased from Invitrogen (Carlsbad, CA, USA). P63 rabbit monoclonal antibodies were purchased from Abcam (Cambridge, UK). RhoA rabbit polyclonal antibody, E-cadherin rabbit polyclonal antibody and vimentin rabbit polyclonal antibody were all purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). GAPDH rabbit polyclonal antibody was purchased from Proteintech Group, Inc. (Chicago, IL, USA). The Alexa Fluor 488 Goat Anti-Rabbit IgG used for immunofluorescence was purchased from Invitrogen. The Chromatin Immunoprecipitation Kit was purchased from EMD Millipore Corporation (Billerica, MA, USA). A transwell system (24 wells, 8 μm pore size with poly-carbonate membrane) was purchased from Corning Costar (Tewksbury, MA, USA). Matrigel was purchased from BD Biosciences (Franklin Lakes, NJ, USA).
Tissue specimens
Fifty cases of colon cancer tissues and paired counterpart normal tissues were collected from patients who underwent a surgical resection of colon cancer at the Third XiangYa Hospital of Central South University (Hunan, China) after informed consent was obtained, and the tumour identity had been verified by a pathologist. All specimens were stored at −80 °C immediately until further processing. The study was approved by the ethics committee of the Third XiangYa Hospital of Central South University. Patient consent was obtained both from the patient and the patient's families.
Plasmid construction and transfection
The pIRES2-ZsGreen1 vector was used to construct a pIRES2-ZsGreen1-TAp63α vector, and the full-length cDNAs for TAp63α were used as the template. Using the pIRES2-ZsGreen1-HK (non-targeting control sequence) plasmid as a control, HT-29 and SW-620 cells were transfected with pIRES2-ZsGreen1-TAp63α or pIRES2-ZsGreen1-HK using Lipofectamine 2000.
RNA isolation and reverse transcription–PCR
Total RNA was isolated using an E.Z.N.A. Total RNA Kit II (Omega Bio-Tek Inc., Norcross, GA, USA). miRNA was isolated using an E.Z.N.A. PF miRNA Isolation Kit (Omega Bio-Tek Inc.). Large RNA reverse transcription was performed using the RevertAid first-strand cDNA synthesis kit (Thermo Fisher Scientific Inc., EU, Lithuania). MiRNA reverse transcription was performed using an All-in-One miRNA qRT–PCR Detection Kit (GeneCopoeia, Rockville, MD, USA). Reverse transcription–PCR was performed using RT–PCR Reagent (Cowin Biotech Co., Ltd., Beijing, China). The primers utilised for cDNA amplification are summarised in Table 1. The primers for TAp63 detected all α, β and γ splice variants except for the ΔN isotypes. Primers for miR-133b and U6-snRNA have been described previously (Hu et al, 2010). The reaction conditions were as follows: an initial 10 min at 95 °C, followed by 40 cycles of 20 s at 95 °C, 15 s at 59 °C, and 30 s at 72 °C. After 40 cycles, the standard PCR reaction mixture underwent a final extension for 5 min at 72 °C, while the quantitative PCR mixture underwent a melt curve to validate the reaction product specificity. For standard PCR, reaction products were detected using 2% (in 1 × TBE) agarose gel electrophoresis. Each sample was amplified in triplicate.
Table 1. Primers for quantitative real-time PCR and reverse transcription–PCR.
| Gene detected | Primer | Sequence (5′→3′) | Product size |
|---|---|---|---|
| TAp63 | F | TGTATCCGCATGCAGGACT | 127 |
| |
R |
CTGTGTTATAGGGACTGGTGGAC |
|
| RhoA | F | CGGGAGCTAGCCAAGATGAAG | 158 |
| |
R |
CCTTGCAGAGCAGCTCTCGTA |
|
| E-cadherin | F | ACAGCCCCGCCTTATGATTCTC | 140 |
| |
R |
AAGCGATTGCCCCATTCGTT |
|
| Vimentin | F | CCTTGAACGCAAAGTGGAATC | 106 |
| |
R |
GACATGCTGTTCCTGAATCTGAG |
|
| GAPDH | F | GAAGGTGAAGGTCGGAGT | 155 |
| R | CATGGGTGGAATCATATTGGAA |
Quantitative real time-PCR
Quantitative real time-PCR (qRT–PCR) was performed using Real-Time Quantitative PCR SYBR Green detection reagent (Cowin Biotech Co., Ltd.). miRNA qRT–PCR was performed using an All-in-One miRNA qRT-PCR Detection Kit (GeneCopoeia). The relative expression of TAp63 was normalised using the 2−ΔΔCT method relative to GAPDH. The relative expression of miR-133b was normalised using the 2−ΔΔCT method relative to U6-snRNA. All PCR reactions were run in triplicate.
Western blotting
Cells were lysed in a lysis buffer and centrifuged at 14 000 g at 4 °C for 10 min. The supernatants were collected, and a BCA protein assay was performed. A volume of extract equivalent to 100 μg total protein was separated on a 10% polyacrylamide gel and transferred to PVDF membranes (Invitrogen). The membranes were blocked at 37 °C with 5% dry milk in PBST for 2 h and then incubated with primary antibody at 4 °C overnight. After washing with PBST, the membranes were incubated with HRP-conjugated goat anti-rabbit IgG secondary antibody for 60 min at room temperature. The images were obtained on Kodak film (Shanghai, China) and quantified by densitometry (Beckman, South Pasadena, CA, USA). All experiments were performed in triplicate.
Immunofluorescence
The cultured HT-29 and SW-620 cells were washed three times with PBS and blockaded with 10% rabbit serum at 37 °C for 30 min. After washing with PBS, the cells were incubated with the primary antibody overnight at 4 °C (1 : 100). Then, the cells were incubated with Alexa Fluor 488 Goat Anti-Rabbit IgG (1 : 100) for 30 min at 37 °C and washed with PBS. Observation and photography were performed with an Olympus multifunction microscope (Olympus BX51, Beijing, China).
ChIP
HT-29 and SW-620 cells (5 × 106) were crosslinked for 10 min in a solution containing 1% formaldehyde, and ChIP assays were performed using a Chromatin Immunoprecipitation Kit, referring to the manufacturer's instructions. Cell lysates were associated to obtain chromatin fragments of ∼200–1000 bp. The immunocomplex was immunoprecipitated using a specific antibody anti-TAp63 and non-specific IgG as a technical control. Collected DNA fragments were tested by PCR using three sets of primers located in the miR-133b promoter, amplifying the p53 putative responsive elements (RE) found in the miR-133b promoter. RE-I: forward 5′-TGCAAACACTTGCAGCTGAG-3′ and reverse 5′-TCTACTCTGCCTGCTTGTTG-3′ (198 bp); RE-II forward 5′-CAAACTTGAGCTGCATCGG-3′ and reverse 5′-ATAGCATTGACTAAACTGT-3′ (198 bp); and RE-III: forward 5′-CCAACATGGCAAAACCCCA-3′ and reverse 5′-GGTGACAGAGTGAGACTCC-3′ (198 bp). The p53 RE located on hMDM2, which was used as positive control, was amplified with one set of primers: forward 5′-GGTTGACTCAGCTTTTCCTCTTG-3′ and reverse 5′-GGAAAATGCATGGTTTAAATAGCC-3′ (119 bp) (Antonini et al, 2010).
Promoter cloning
The luciferase reporter plasmid, the expression of which is controlled by the miR-133b promoter sequence, was modified from the pGL3-Basic vector. The miR-133b promoter sequence containing the three p53 elements was amplified using specific oligos (forward 5′-TGCAAACACTTGCAGCTGAG-3′ and reverse 5′-TGACTGCAGCCTCTAGTGTG-3′ 2000 bp), cloned in the PCR 2.1 vector and restricted with NheI and XbaI. This fragment was then cloned in a luciferase vector that had previously been restricted with the same enzymes.
Luciferase assay
HT-29 and SW-620 cells were transiently transfected with miR-133b (miR-133bWT or mutated RE-III) promoters and Renilla luciferase cDNA in the presence or absence of TAp63 using Lipofectamine 2000. SW-620 and HT-29 cells were transiently transfected with wild-type (Wt-RhoA) or mutant (Mut-RhoA) reporter plasmid containing miR-133b potential binding sites in the presence or absence of miR-133b using Lipofectamine 2000. Luciferase assays were performed 36 h post-transfection using the Dual-luciferase assay system, and they were normalised for transfection efficiency with co-transfected Renilla luciferase. Mut-RE-III was obtained by mutating the core binding sequence AGGTTGCA. All experiments were performed in triplicate.
Transwell invasion assay
A transwell invasion assay was performed using a transwell system (24 wells, 8 μm pore size with poly-carbonate membrane) and Matrigel according to the manufacturer's instructions. First, 1 × 105 cells were seeded into the upper chamber with serum-free optiMEM medium. optiMEM with 10% FBS was put into the lower compartment as a chemo-attractant, and the cells were allowed to invade for 48 h. The remaining cells in the upper chamber were scraped out by a cotton swap. Matrigel membranes were fixed with ice-cold methanol and stained with 0.1% crystal violet solution. The number of cells that migrated to the lower side was counted in five randomly selected fields under a light microscope. The cell number was counted and analysed statistically.
Transwell migration assay
For the migration assay, the cells were seeded in upper chambers without coated Matrigel. The rest of assay was performed as the invasion assay. The number of cells that migrated to the lower side was counted in five randomly selected fields under a light microscope. The cell number was counted and analysed statistically.
Statistical analysis
Quantitative data were analysed using SPSS (version 11.0, Armonk, NY, USA) and expressed as the mean±s.d. Statistical differences between groups were compared using ANOVA and two-tailed t-tests. The level of statistical significance was set at P<0.05. All experiments, ANOVA and two-tailed t-tests were repeated three times.
Results
Decrease of TAp63 expression in human colon cancer
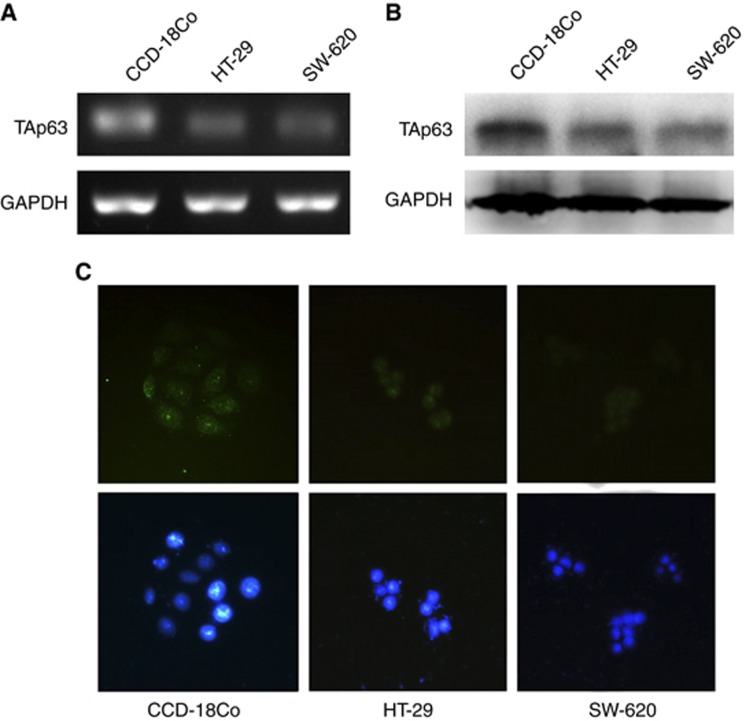
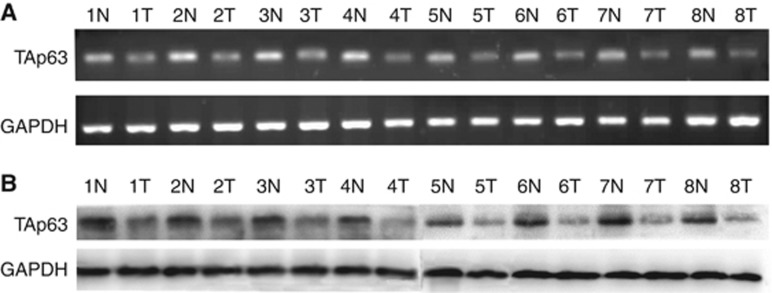
The TAp63 expression in HT-29, SW-620 and CCD-18Co cells was verified by RT–PCR, western blotting and immunofluorescence. The RT–PCR, western blotting and immunofluorescence positive results are shown in Figures 1A–C, respectively. The TAp63 expression level in HT-29 and SW-620 cells was much lower than that in CCD-18Co cells. Further, we analysed the expression of TAp63 in human colon cancer tissue and paired counterpart normal tissues using RT–PCR and western blotting. Lower levels of TAp63 were detected in the tumour tissues compared with the normal tissue (Figure 2).
Figure 1.
Expression of TAp63 in HT-29, SW-620 and CCD-18Co cells. The expression of TAp63 was analysed by RT–PCR, western blot and immunofluorescence. (A) Electrophoresis in 2% (w/v) agarose gel reveals a single 127-bp product following amplification by PCR using TAp63-specific primers. (B) Western blotting analysis of TAp63 on HT-29, SW-620 and CCD-18Co cells (top panel). The GAPDH loading control is shown in the bottom panel. (C) Immunofluorescence assay showed that TAp63 was present in the nuclei of HT-29, SW-620 and CCD-18Co cells. DAPI was used to counterstain the nucleus in blue, and TAp63 staining was in green. The figures show that the TAp63 expression level in HT-29 and SW-620 cells was much lower than that in CCD-18Co cells.
Figure 2.
Expression of TAp63 in colon cancer tissues. The expression of TAp63 was assessed using eight surgical specimens of human colon cancer tissues and adjacent normal tissues. (A) RT–PCR analysis of TAp63. (B) Western blotting analysis of TAp63. As shown in the figure, lower levels of TAp63 were detected in tumour tissues compared with the normal tissue. (N)=Normal, (T)=Tumour.
TAp63 drive the expression of miR-133b
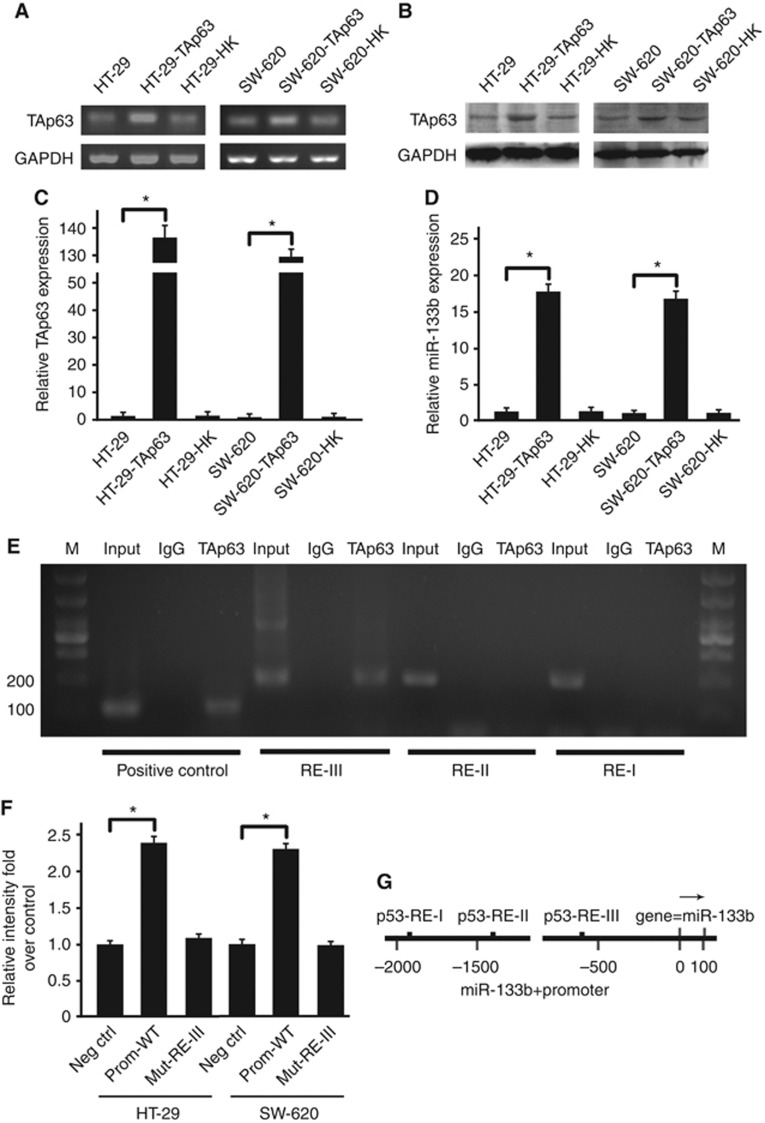
To investigate whether this association was due to a functional relationship between TAp63 and miR-133b, we overexpressed TAp63 by transfecting HT-29 and SW-620 cells with vectors expressing TAp63 and measured the miR-133b expression levels. After the transfection, the expression of TAp63 was detected by RT–PCR and western blotting, which showed a significant increase in TAp63 expression (Figures 3A and B). This result was confirmed by qRT–PCR; HT-29 and SW-620 cells transfected with vectors expressing TAp63 induced a 132-fold increase in TAp63 mRNA expression (Figure 3C). Next, we detected the level of miR-133b by qRT–PCR. Figure 4D shows that the expression of miR-133b was induced roughly 18-fold by TAp63.
Figure 3.
TAp63 modulates the expression of miR-133b. HT-29 and SW-620 cells were transfected with either an empty vector or with TAp63 for 72 h. (A) Endogenous TAp63 levels were assessed by RT–PCR. (B) The expression level of TAp63 is shown in the western blotting. (C). Endogenous TAp63 levels were again confirmed by qRT–PCR. As the figure shows, HT-29 and SW-620 cells transfected with TAp63 expressing vector induced a significant increase in TAp63 expression. (D) Endogenous miR-133b levels were assessed by qRT–PCR. After transfection with a TAp63-expressing vector, HT-29 and SW-620 cells showed a significant increase in miR-133b expression. (E) Chromatin immunoprecipitation (ChIP) experiment showed that p63 is able to bind the p53 RE-III site but not p53 RE-I and p53 RE-II. (F) Insertion of miR-133b promoter region in a luciferase reporter gene leads to increased luciferase activity in the presence of TAp63 in HT-29 and SW-620 cells. Mutation of the RE-III p53-binding site abolished TAp63-mediated luciferase activity. (G) The miR-133b promoter region containing the three p53 consensus sites (p53RE). All qRT–PCR results were relative to U6-snRNA or GAPDH and were normalised to the expression of miR-133b or TAp63 in HT-29 cells. Data represent the means±s.d. of three different experiments analysed in triplicate. *P<0.05.
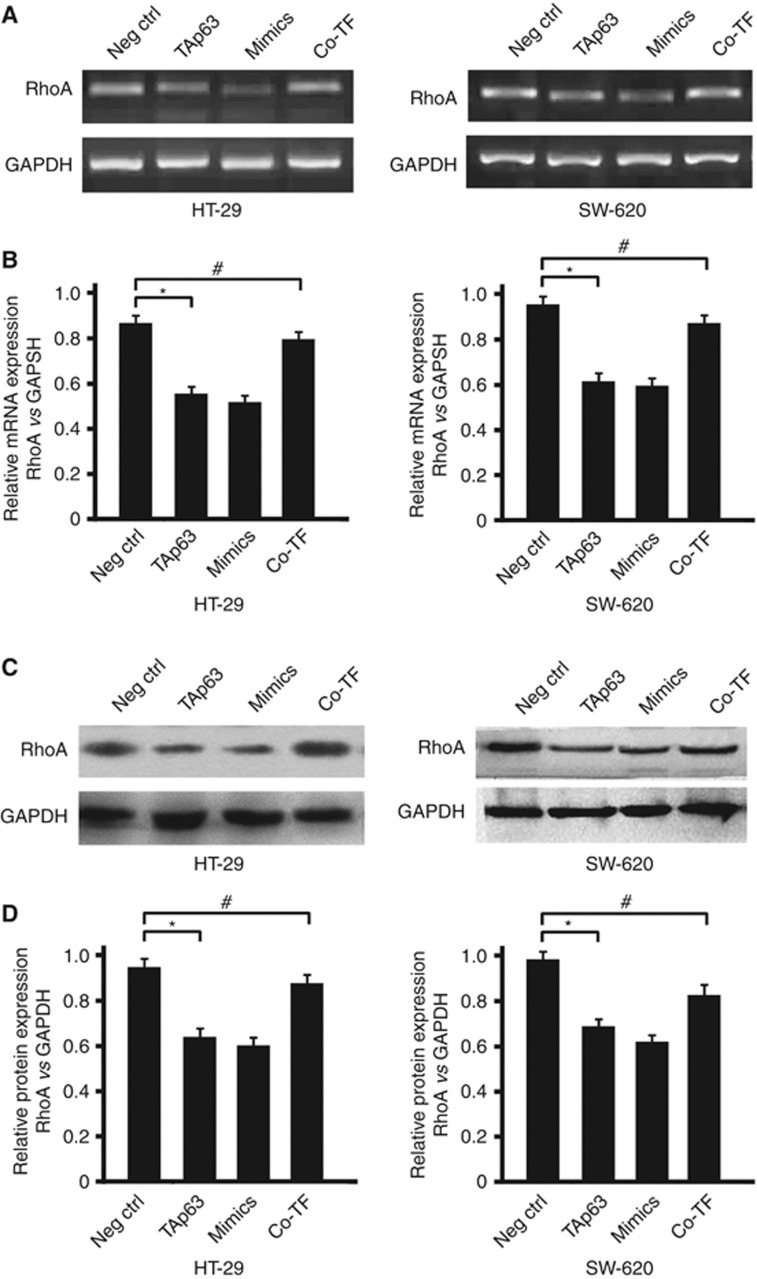
Figure 4.
TAp63 targets RhoA through miR-133b. HT-29 and SW-620 cells were either transfected with negative control (Neg Ctrl), TAp63 or miR-133b mimics or co-transfected with TAp63-expressing vector with miR-133b inhibitor (co-TF) for 72 h. (A) RhoA induction levels were detected by RT–PCR. (B) Percentage of RhoA mRNA is expressed relative to GAPDH. (C) Western blotting of RhoA levels in HT-29- and SW-620-transfected cells. (D) Percentage of RhoA protein is expressed relative to GAPDH. All data represent the means±s.d. of three different experiments analysed in triplicate. *P<0.05, #P>0.05.
To assess whether miR-133b is a transcriptional target of TAp63, we performed ChIP analysis for a site matching the p63 consensus binding site. Because p63 can bind to p53 consensus sequences (Antonini et al, 2010), we screened a region of ∼2000 bp upstream of the miR-133b transcriptional start site for consensus p53 response elements (p53RE) by TRANSFAC. Three putative p53-binding sites were chosen as follows (Figure 3G): RE-I located at −1904/1894, RE-II at −1376/1366, and RE-III at −584/−576. To determine which of the p53REs is bound by TAp63, we performed both a ChIP experiment and a luciferase assay with p53RE. TAp63 bound to the p53RE-III site (Figure 3E) but not to p53RE-I or p53RE-II. The entire 2 000-bp region was cloned upstream of a luciferase reporter and co-transfected with TAp63 or empty vector in HT-29 and SW-620 cells. TAp63 resulted in an approximately 2.5-fold enhancement of promoter activity with the p53-binding site, and a loss of promoter activity was observed using a mutated p53RE-III (Figure 3F), thus demonstrating that p63 directly drives the miR-133b promoter via the binding site.
TAp63 modulate RhoA expression via miR-133b
Based on the fact that TAp63 drives the expression of miR-133b and participates in the process of EMT, we asked whether the cellular levels of a miR-133b target, RhoA, would also be affected by the expression of TAp63. The RhoA expression in HT-29, SW-620 and CCD-18CO cells was assessed by RT–PCR, western blotting and immunofluorescence. The RhoA expression levels in HT-29 and SW-620 cells were both much higher than that in CCD-18CO cells (Supplementary Figures S3 A–C). RhoA had been reported as a target for miR-133b in cervical carcinoma (Qin et al, 2011). Here we also identified a 3′-untranslated region (3′-UTR) of RhoA-mRNA as a potential miR-133b target in colon cancer cells (Supplementary Figure S1A, *P<0.05). To confirm that TAp63 regulates the expression of RhoA, we transiently transfected HT-29 and SW-620 cells with vectors expressing TAp63, resulting in a reduction of RhoA levels around 40%, as detected by RT–PCR and western blotting. Similarly, transiently transfecting HT-29 and SW-620 cells with miR-133b mimics also resulted in a reduction of about 45% of RhoA at both the mRNA and protein levels, as detected by RT–PCR and western blotting. We then tested whether the modulation of RhoA expression by TAp63 required miR-133b; a vector expressing TAp63 was co-transfected with miR-133b inhibitor in HT-29 and SW-620 cells. The levels of RhoA were detected by RT–PCR and western blotting. We found that the RhoA levels remained unchanged in the presence of the miR-133b inhibitor. Therefore, TAp63 represses RhoA expression through the direct activation of its target miR-133b (Figures 4A–D).
TAp63 affects the expression of epithelial and mesenchymal markers
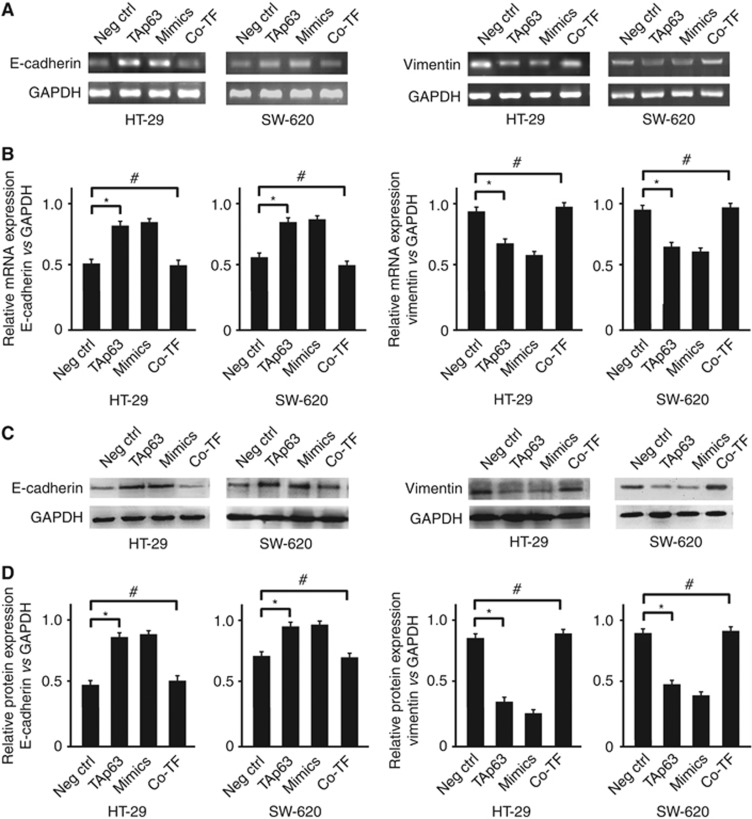
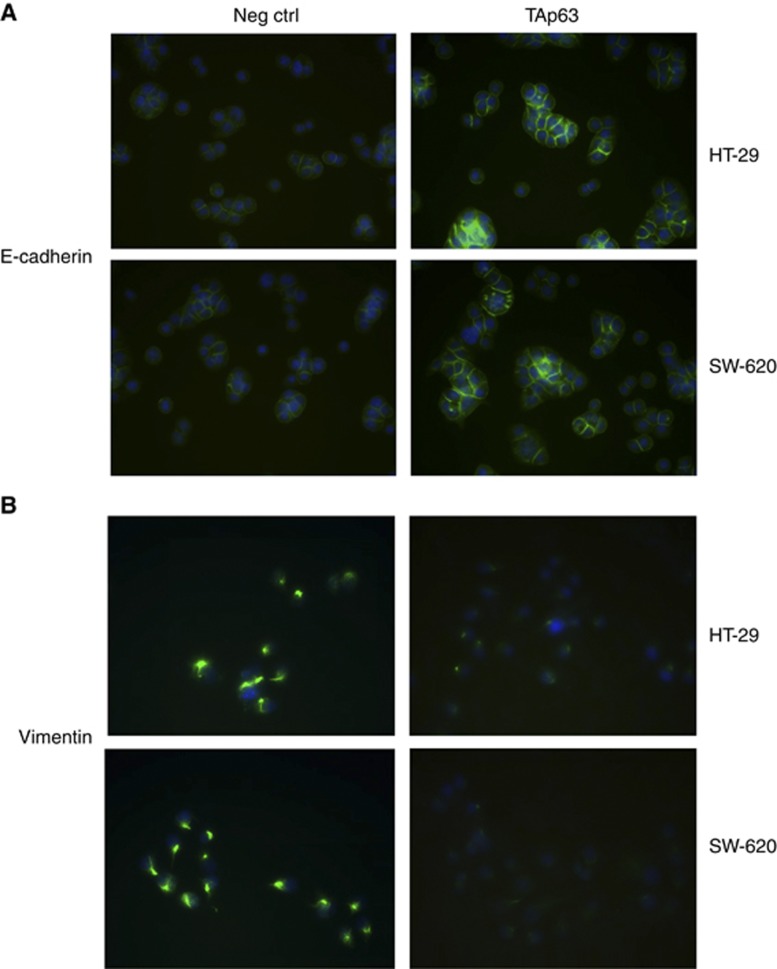
Blocking the expression of RhoA could inhibit the upregulation of the expression of epithelial and mesenchymal markers, and its repression is independent of its Rho-kinase activity (Hutchison et al, 2009). Here, we wanted to assess whether TAp63 can affect the expression of E-cadherin and vimentin through miR-133b. In HT-29 and SW-620 cells transfected with vectors expressing TAp63, the expression of E-cadherin was increased at both the mRNA and protein levels, whereas the expression of vimentin was reduced. Similarly, the transfection of miR-133b mimics also produced an increase in E-cadherin mRNA and protein, with a reduction in vimentin expression. Co-transfection of TAp63-expressing vectors with the miR-133b inhibitor rescued the expression of E-cadherin and vimentin (Figures 5A–D). The increase in E-cadherin expression, after transfection with vectors expressing TAp63, in adherens junctions and cell–cell contacts was confirmed by immunofluorescence (Figure 6A). The reduction in vimentin expression, after transfection with vectors expressing TAp63, was also confirmed by immunofluorescence (Figure 6B).
Figure 5.
TAp63 affects the expression of epithelial and mesenchymal markers. HT-29 and SW-620 cells were either transfected with negative control (Neg Ctrl), TAp63 or miR-133b mimics or co-transfected with TAp63-expressing vector with miR-133b inhibitor (co-TF) for 72 h. (A) E-cadherin and vimentin induction levels were detected by RT–PCR. (B) Percentage of E-cadherin and vimentin mRNA is expressed relative to GAPDH. (C) Western blots of E-cadherin and vimentin levels in HT-29- and SW-620-transfected cells. (D) Percentage of E-cadherin and vimentin protein is expressed relative to GAPDH. All of the data represent the means±s.d. of three different experiments analysed in triplicate. *P<0.05, #P>0.05.
Figure 6.
Immunofluorescence staining of HT-29 and SW-620 cells for E-cadherin and vimentin. HT-29 and SW-620 cells were transfected with negative control (Neg Ctrl) or TAp63-expressing vector for 72 h. Then, the nuclei were counterstained with DAPI (blue), and E-cadherin and vimentin were stained in green. (A) Immunofluorescent images of HT-29 and SW-620 cells transfected with Neg Ctrl or TAp63 stained with antibodies against E-cadherin. (B) Immunofluorescent images of HT-29 and SW-620 cells transfected with Neg Ctrl or TAp63 stained with antibodies against vimentin.
TAp63 inhibits cell invasion and migration through miR-133b
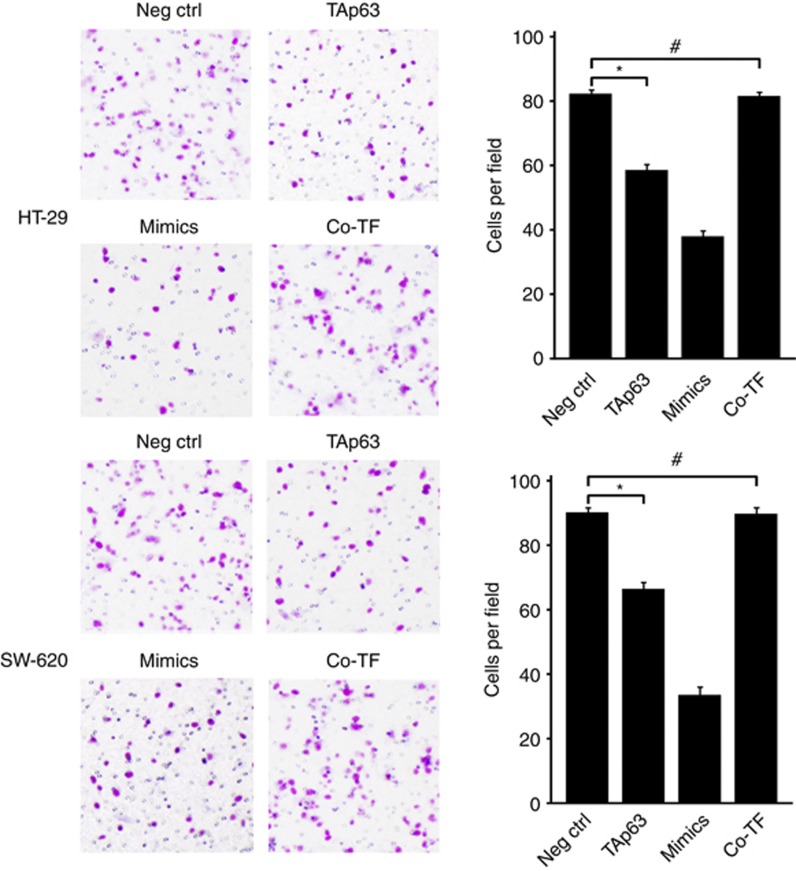
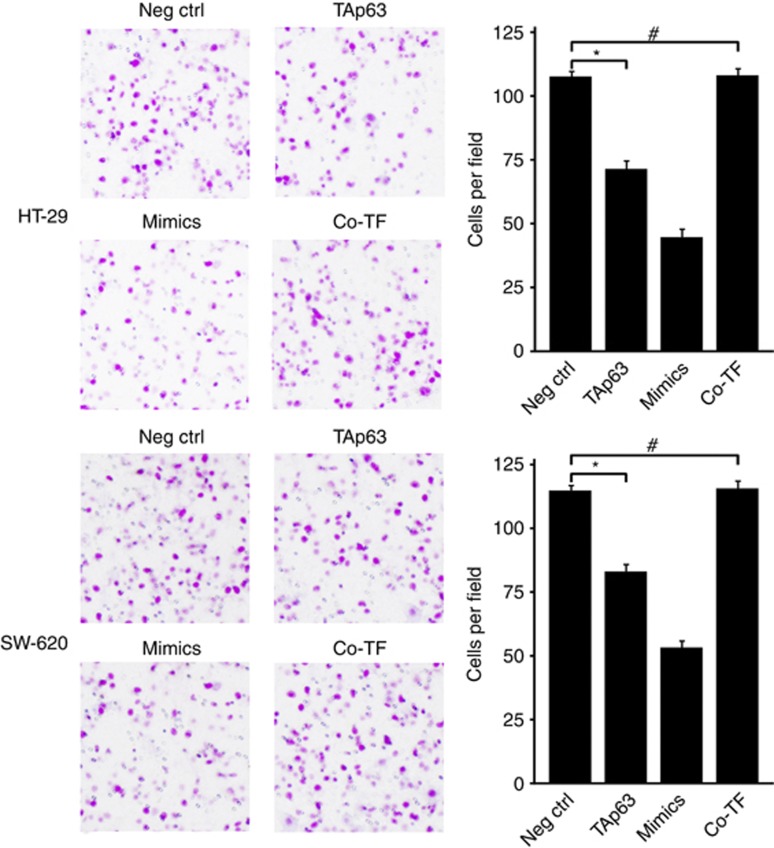
The repression of RhoA and its E-cadherin and vimentin targets suggests that TAp63 may have an important role in cell invasion and migration through miR-133b. To assess this, we performed transwell invasion assay and transwell migration assay. The transwell invasion assay was implemented to investigate the effect of TAp63 on the invasion of HT-29 and SW-620 cells. The transwell migration assay was implemented to investigate the effect of TAp63 on the migration of HT-29 and SW-620 cells. As shown in Figure 7, the invasion of HT-29 and SW-620 cells was restrained by overexpressing TAp63, compared with those transfected with the empty vector (*P<0.05). Similar results were obtained following the transfection of cells with miR-133b mimics. Conversely, cell invasion remained unchanged in cells co-transfecting the TAp63-expressing vector with the miR-133b inhibitor (#P>0.05). To further test the influence of TAp63 on cell migration, we performed a transwell migration assay. As shown in Figure 8, the migration of HT-29 and SW-620 cells was restrained by overexpressing TAp63, compared with those transfected with the empty vector (*P<0.05). Conversely, cell migration remained unchanged in cells co-transfecting the TAp63-expressing vector with the miR-133b inhibitor (#P>0.05).
Figure 7.
TAp63 inhibits cell invasion. Transwell invasion assays of HT-29 and SW-620 cells either transfected with negative control (Neg Ctrl), TAp63 or miR-133b mimics or co-transfected TAp63 expressing vector with miR-133b inhibitor (co-TF). After being transfected for 24 h, the cells were collected and seeded into the upper chamber. After 48 h, invasive cells were counted in five random high-power fields. All of the data represent the means±s.d. of three different experiments analysed in triplicate. *P<0.05, #P>0.05.
Figure 8.
TAp63 inhibits cell migration. Transwell migration assays of HT-29 and SW-620 cells either transfected with negative control (Neg Ctrl), TAp63 or miR-133b mimics or co-transfected with TAp63-expressing vector with miR-133b inhibitor (co-TF). After transfection for 24 h, the cells were collected and seeded into the upper chamber. The cells were allowed to migrate for 48 h and counted in five random high-power fields. All of the data represent the means±s.d. of three different experiments analysed in triplicate. *P<0.05, #P>0.05.
Effects of TAp63 overexpression on cell proliferation and apoptosis
To investigate whether overexpression of TAp63 has an influence on cells' proliferation in vitro, proliferation assay was performed to detect the proliferation of HT-29 and SW-620 cells. We did not observe a significant difference in in vitro cell proliferation between the TAp63-overexpressing group and the control (Supplementary Figures S2A and B, #P>0.05). To evaluate accurately the effect of overexpression of TAp63 on the apoptotic rate of HT-29 and SW-620 cells, the flow cytometry analysis was performed to measure the amount of apoptotic cells. Our data revealed that apoptosis rate inTAp63-overexpressing group was similar with the control (Supplementary Figures S2E and F, #P>0.05).
Discussion
P63, a p53 homologue participating in the transcriptional regulatory mechanisms, is composed of two classes of isoforms: TA and ΔN isoforms (Suh et al, 2006; Gallegos et al, 2008). ΔNp63 isoforms enhance cell proliferation and repress apoptosis (Yang et al, 1998), whereas TAp63 isoforms can repress cell cycle progression (Wu et al, 2003) and induce cell apoptosis (Hsueh et al, 2013). As a consequence of its involvement in cancer formation and progression, TAp63 is usually reported to be deregulated in some types of carcinomas. We have previously reported that miR-133b expression was greatly downregulated in human colon cancer cells. The studies of both Akçakaya and Navon et al showed similar results (Navon et al, 2009; Akçakaya et al, 2011). However, to our knowledge, there has been no exhaustive characterisation exploring the cause of miR-133b downregulation in colon cancer. Recently, some studies have shown a close relationship between miRNAs and TAp63. In particular, TAp63 has been shown to suppress metastasis through the transcriptional regulation of miR-34a and miR-130b (Su et al, 2010). Although many studies have demonstrated that TAp63 is clearly a transcriptional activator and showed the possibility of miRNA involvement in its regulation, the potential relationship between the transcription factor function of TAp63 and miR-133b expression in colon cancer is not clear. During our study, we found that the expression of TAp63 is downregulated in colon cancer, similar to miR-133b. Moreover, when TAp63 was upregulated, we observed a rescue of miR-133b processing. Interestingly, the data showed a large discrepancy between the effects of TAp63 overexpression on TAp63 mRNA and protein levels: HT-29 and SW-620 cells transfected with vectors expressing TAp63 induced a 132-fold increase in TAp63 mRNA expression, whereas protein levels induced only about two-fold increases. In accordance with the study by Carroll et al (2006), the discrepancy between mRNA levels and protein expression indicates a post-transcriptional regulation analogous to that seen in p53 (Carroll et al, 2006; Gu et al, 2006). This remarkable level of correlation between the expression of TAp63 and miR-133b strongly suggests that miR-133b may be a transcriptional target of TAp63. To assess this hypothesis, we performed a Chip analysis and found a significant level of p63 binding at the miR-133b promoter. Luciferase assays proved that TAp63 transactivated the miR-133b reporter. Our study offers a framework for understanding the basis of miR-133b downregulation in colon cancer.
In addition to regulating the expression of miR-133b, we proved TAp63 to be an important regulator in the process of metastasis via miR-133b. TAp63 has been implicated in the process of tumour metastasis formation through the downregulation of E-cadherin and upregulation of vimentin. RhoA, a type of GTPase, is strongly correlated with the metastasis through the activation of RhoA/ROCK signalling and the modulation of the expression of epithelial and mesenchymal markers (Cheng et al, 2012). In the past, most of the researchers studying the relationship between RhoA and metastasis focused on the activity of RhoA. However, recently, some studies have shown that miRNA can promote cell migration by overexpressing RhoA, which is related to the activation of the metastasis (Yau et al, 2013). Moreover, blocking the expression of RhoA could inhibit the upregulation of the expression of epithelial and mesenchymal markers; its repression is independent of its Rho-kinase activity (Hutchison et al, 2009). Our results demonstrate that TAp63-overexpressing cells have lower expression levels of RhoA through the direct activation of miR-133b. And the expression of E-cadherin was increased when colon cancer cells were transfected with vectors expressing TAp63, whereas the expression of vimentin was reduced. In a word, our study suggests a role for TAp63 in the modulation of RhoA and epithelial–mesenchymal markers via miR-133b. According to the effects of the expression of RhoA and epithelial–mesenchymal markers, TAp63 has an important role in cell migration and therefore in metastasis, rather than directly affecting the proliferation and apoptosis of colon cancer cells. This is the first study reporting the role of TAp63's modulation of RhoA and epithelial–mesenchymal markers via miR-133b; this result suggests a potential strategy to inhibit metastasis in colon cancer by targeting TAp63 and miR-133b.
In conclusion, the present study demonstrated that TAp63 directly regulates miR-133b transcription and modulates the expression of RhoA and epithelial–mesenchymal markers in colon cancer. These findings suggest transcriptional mechanisms that govern miR-133b expression in colon cancer and indicate novel activators of miR-133b that are implicated in metastasis. In addition, the TAp63/miR-133b axis may be a useful clinical predictor of metastatic behaviour in colon cancer, revealing a new therapeutic target to prevent the spread of this cancer.
Acknowledgments
This work was supported by the Key Program for International Cooperation Projects of Hunan Province (no. 2011WK2011) and the Chinese National Science Foundation (No. 81172298).
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Antonini D, Russo MT, De Rosa L, Gorrese M, Del Vecchio L, Missero C. Transcriptional repression of miR-34 family contributes to p63-mediated cell cycle progression in epidermal cells. J Invest Dermatol. 2010;130:1249–1257. doi: 10.1038/jid.2009.438. [DOI] [PubMed] [Google Scholar]
- Akçakaya P, Ekelund S, Kolosenko I, Caramuta S, Ozata DM, Xie H, Lindforss U, Olivecrona H, Lui WO. miR-185 and miR-133b deregulation is associated with overall survival and metastasis in colorectal cancer. Int J Oncol. 2011;39:311–318. doi: 10.3892/ijo.2011.1043. [DOI] [PubMed] [Google Scholar]
- Anura A, Das RK, Pal M, Bag S, Conjeti S, Sheet D, Paul RR, Sengupta S, Ray AK, Chakraborty C, Chatterjee J. Pro-emt and neo-angiogenic attributes of oral sub-mucous fibrosis and visualization of stage progression panorama through automated image stitching. J Cell Commun Signal. 2013;7 (1:94. [Google Scholar]
- Carroll DK, Carroll JS, Leong CO, Cheng F, Brown M, Mills AA, Brugge JS, Ellisen LW. p63 regulates an adhesion programme and cell survival in epithelial cells. Nat Cell Biol. 2006;8:551–561. doi: 10.1038/ncb1420. [DOI] [PubMed] [Google Scholar]
- Chen Z, Saad R, Jia P, Peng D, Zhu S, Washington MK, Zhao Z, Xu Z, El-Rifai W. Gastric adenocarcinoma has a unique microRNA signature not present in esophageal adenocarcinoma. Cancer. 2013;119:1985–1993. doi: 10.1002/cncr.28002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng IK, Tsang BC, Lai KP, Ching AK, Chan AW, To KF, Lai PB, Wong N. GEF-H1 over-expression in hepatocellular carcinoma promotes cell motility via activation of RhoA signalling. J Pathol. 2012;228 (4:575–585. doi: 10.1002/path.4084. [DOI] [PubMed] [Google Scholar]
- Choi W, Shah JB, Tran M, Svatek R, Marquis L, Lee IL, Yu D, Adam L, Wen S, Shen Y, Dinney C, McConkey DJ, Siefker-Radtke A. p63 expression defines a lethal subset of muscle-invasive bladder cancers. PLoS One. 2012;7 (1:e30206. doi: 10.1371/journal.pone.0030206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallegos JR, Litersky J, Lee H, Sun Y, Nakayama K, Nakayama K, Lu H. SCF TrCP1 activates and ubiquitylates TAp63gamma. J Biol Chem. 2008;283:66–75. doi: 10.1074/jbc.M704686200. [DOI] [PubMed] [Google Scholar]
- Gressner O, Schilling T, Lorenz K, Schulze Schleithoff E, Koch A, Schulze-Bergkamen H, Lena AM, Candi E, Terrinoni A, Catani MV, Oren M, Melino G, Krammer PH, Stremmel W, Müller M. TAp63alpha induces apoptosis by activating signaling via death receptors and mitochondria. EMBO J. 2005;24:2458–2471. doi: 10.1038/sj.emboj.7600708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Lundqvist EN, Coates PJ, Thurfjell N, Wettersand E, Nylander K. Dysregulation of TAp63 mRNA and protein levels in psoriasis. J Invest Dermatol. 2006;126:137–141. doi: 10.1038/sj.jid.5700010. [DOI] [PubMed] [Google Scholar]
- Gulhati P, Bowen KA, Liu J, Stevens PD, Rychahou PG, Chen M, Lee EY, Weiss HL, O'Connor KL, Gao T, Evers BM. mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res. 2011;71:3246–3256. doi: 10.1158/0008-5472.CAN-10-4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh YJ, Kuo PC, Chen JK. Transcriptional regulators of the ΔNp63: their role in limbal epithelial cell proliferation. J Cell Physiol. 2013;228:536–546. doi: 10.1002/jcp.24160. [DOI] [PubMed] [Google Scholar]
- Hu G, Chen D, Li X, Yang K, Wang H, Wu W. miR-133b regulates the MET proto-oncogene and inhibits the growth of colorectal cancer cells in vitro and in vivo. Cancer Biol Ther. 2010;10:190–197. doi: 10.4161/cbt.10.2.12186. [DOI] [PubMed] [Google Scholar]
- Hutchison N, Hendry BM, Sharpe CC. Rho isoforms have distinct and specific functions in the process of epithelial to mesenchymal transition in renal proximal tubular cells. Cell Signal. 2009;21:1522–1531. doi: 10.1016/j.cellsig.2009.05.012. [DOI] [PubMed] [Google Scholar]
- Kanlaya R, Sintiprungrat K, Thongboonkerd V. Secreted products of macrophages exposed to calcium oxalate crystals induce epithelial mesenchymal transition of renal tubular cells via RhoA-dependent TGF-β1 pathway. Cell Biochem Biophys. 2013;67:1207–1215. doi: 10.1007/s12013-013-9639-z. [DOI] [PubMed] [Google Scholar]
- Knouf EC, Garg K, Arroyo JD, Correa Y, Sarkar D, Parkin RK, Wurz K, O'Briant KC, Godwin AK, Urban ND, Ruzzo WL, Gentleman R, Drescher CW, Swisher EM, Tewari M. An integrative genomic approach identifies p73 and p63 as activators of miR-200 microRNA family transcription. Nucleic Acids Res. 2011;40:499–510. doi: 10.1093/nar/gkr731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong W, Yang H, He L, Zhao JJ, Coppola D, Dalton WS, Cheng JQ. MicroRNA-155 is regulated by the transforming growth factor beta/Smad pathway and contributes to epithelial cell plasticity by targeting RhoA. Mol Cell Biol. 2008;28:6773–6784. doi: 10.1128/MCB.00941-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Iacono M, Monica V, Saviozzi S, Ceppi P, Bracco E, Papotti M, Scagliotti GV. p63 and p73 isoform expression in non-small cell lung cancer and corresponding morphological normal lung tissue. J Thorac Oncol. 2011;6:473–481. doi: 10.1097/JTO.0b013e31820b86b0. [DOI] [PubMed] [Google Scholar]
- Loboda A, Nebozhyn MV, Watters JW, Buser CA, Shaw PM, Huang PS, Van't Veer L, Tollenaar RA, Jackson DB, Agrawal D, Dai H, Yeatman TJ. EMT is the dominant program in human colon cancer. BMC Med Genomics. 2011;4:9. doi: 10.1186/1755-8794-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navon R, Wang H, Steinfeld I, Tsalenko A, Ben-Dor A, Yakhini Z. Novel rank-based statistical methods reveal microRNAs with differential expression in multiple cancer types. PLoS One. 2009;4:e8003. doi: 10.1371/journal.pone.0008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortt K, Sinha S. Derivation of the consensus DNA-binding sequence for p63reveals unique requirements that are distinct from p53. FEBS Lett. 2006;580:4544–4550. doi: 10.1016/j.febslet.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Qin W, Dong P, Ma C, Mitchelson K, Deng T, Zhang L, Sun Y, Feng X, Ding Y, Lu X, He J, Wen H, Cheng J. MicroRNA-133b is a key promoter of cervical carcinoma development through the activation of the ERK and AKT1 pathways. Oncogene. 2011;31:4067–4075. doi: 10.1038/onc.2011.561. [DOI] [PubMed] [Google Scholar]
- Serber Z, Lai HC, Yang A, Ou HD, Sigal MS, Kelly AE, Darimont BD, Duijf PH, Van Bokhoven H, McKeon F, Dötsch V. A C-terminal inhibitory domain controls the activity of p63 by an intramolecular mechanism. Mol Cell Biol. 2002;22:8601–8611. doi: 10.1128/MCB.22.24.8601-8611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struckhoff AP, Rana MK, Worthylake RA. RhoA can lead the way in tumor cell invasion and metastasis. Front Biosci. 2011;16:1915–1926. doi: 10.2741/3830. [DOI] [PubMed] [Google Scholar]
- Suh EK, Yang A, Kettenbach A, Bamberger C, Michaelis AH, Zhu Z, Elvin JA, Bronson RT, Crum CP, McKeon F. p63 protects the female germ line during meiotic arrest. Nature. 2006;444:624–628. doi: 10.1038/nature05337. [DOI] [PubMed] [Google Scholar]
- Su X, Chakravarti D, Cho MS, Liu L, Gi YJ, Lin YL, Leung ML, El-Naggar A, Creighton CJ, Suraokar MB, Wistuba I, Flores ER. p63 suppresses metastasis through coordinate regulation of Dicer and miRNAs. Nature. 2010;467:986–990. doi: 10.1038/nature09459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares AL, Mercado-Pimentel ME, Runyan RB, Kitten GT. TGF beta-mediated RhoA expression is necessary for epithelial-mesenchymal transition in the embryonic chick heart. Dev Dyn. 2006;235:1589–1598. doi: 10.1002/dvdy.20771. [DOI] [PubMed] [Google Scholar]
- Tran MN, Choi W, Navai N, Wszolek MF, Lee I-LC, Siefker-Radtke AO, McConkey DJ. P63 inhibits epithelial-mesenchymal transition by promoting the expression of miR205 in human bladder cancer cells. Cancer Res. 2012;72 (8 Supplement 1:LB–472. [Google Scholar]
- Tucci P, Agostini M, Grespi F, Markert EK, Terrinoni A, Vousden KH, Muller PA, Dötsch V, Kehrloesser S, Sayan BS, Giaccone G, Lowe SW, Takahashi N, Vandenabeele P, Knight RA, Levine AJ, Melino G. Loss of p63 and its microRNA-205 target results in enhanced cell migration and metastasis in prostate cancer. Proc Natl Acad Sci USA. 2012;109:15312–15317. doi: 10.1073/pnas.1110977109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen D, Li S, Ji F, Cao H, Jiang W, Zhu J, Fang X. miR-133b acts as a tumor suppressor and negatively regulates FGFR1 in gastric cancer. Tumour Biol. 2013;34:793–803. doi: 10.1007/s13277-012-0609-7. [DOI] [PubMed] [Google Scholar]
- Wu G, Nomoto S, Hoque MO, Dracheva T, Osada M, Lee CC, Dong SM, Guo Z, Benoit N, Cohen Y, Rechthand P, Califano J, Moon CS, Ratovitski E, Jen J, Sidransky D, Trink B. DeltaNp63alpha and TAp63alpha regulate transcription of genes with distinct biological functions in cancer and development. Cancer Res. 2003;63:2351–2357. [PubMed] [Google Scholar]
- Wu N, Sulpice E, Obeid P, Benzina S, Kermarrec F, Combe S, Gidrol X. The miR-17 family links p63 protein to MAPK signaling to promote the onset of human keratinocyte differentiation. PLoS One. 2012;7:e45761. doi: 10.1371/journal.pone.0045761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dötsch V, Andrews NC, Caput D, McKeon F. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998;2:305–316. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- Yau WL, Lam CS, Ng L, Chow AK, Chan ST, Chan JY, Wo JY, Ng KT, Man K, Poon RT, Pang RW. Over-expression of miR-106b promotes cell migration and metastasis in hepatocellular carcinoma by activating epithelial-mesenchymal transition process. PLoS One. 2013;8:e57882. doi: 10.1371/journal.pone.0057882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yan W, Chen X. P63 regulates tubular formation via epithelial-to-mesenchymal transition. Oncogene. 2014;33 (12:1548–1557. doi: 10.1038/onc.2013.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.