Abstract
The hypothesis that ozonated oil has wound healing property was investigated in an excision wound model using Sprague Dawley rats. The animals were divided into four groups, which were treated with sesame oil (vehicle), framycetin (standard), or two doses of ozonated sesame oil (peroxide values 500 and 700 mEq/1000 g, respectively). The formulations were topically applied on the excision wounds once daily for 11 consecutive days and the animals were euthanized on the 12th day. Wound healing was assessed by measuring the wound contracture, tensile strength, collagen content and superoxide dismutase activity of skin of the excised wound area. On the terminal day, areas of the wounds of the group receiving high dose ozonated oil were significantly smaller than those of the group treated with vehicle. Ozonated oil treated wounds had significantly higher tensile strength, collagen content and superoxide dismutase activity than that of the vehicle treated wounds. Histopathological analysis of skin of the excised wound area treated with ozonated oil revealed better healing activity vis-à-vis vehicle-treated wounds. Thus, it can be concluded that ozonated oil can be of potential therapeutic use for healing wounds.
Keywords: Ozonated oil, wound healing, excision wound
Ozone is generally regarded as a toxic gas but medical ozone that can be used clinically is different from atmospheric ozone. It is a mixture of oxygen and ozone with the percentage of the latter ranging from 0.1 to 5%. Ozone can be administered through various routes like intraarterial, subcutaneous, intramuscular, dermal, rectal and vaginal[1].
Ozone therapy dates back to the year 1914 when it was used during World War I for the treatment of gas gangrene. Other medical uses of ozone include for the treatment of chronic colitis and rectal fistulae, ischemic foot ulcers, chronic heart failure, atrophic form of age-related macular degeneration, treatment of low back pain and dental caries[2].
Ozonated water has been used for the treatment of wounds. However, it has a half life of about 10 h at room temperature and about 5 days when stored in the refrigerator. Compared with ozonised water, ozonated oils have a considerably longer shelf-life. Ozone can be stabilised as an ozonide between the double bonds of a monounsaturated fatty acid such as oleic acid. As a consequence, ozonated olive oil remains stable for 2 years at 4°[2].
Prior studies by other researchers have evaluated the potential of ozonated oil in the treatment of cutaneous wounds[3,4,5,6]. Valacchi et al. evaluated the effect of low (949±33 mEq/1000 g), medium (1631±64 mEq/1000 g) and high (3170±101 mEq/1000 g) peroxide values in hairless female SKH1 mice[6]. They studied the wound closure rate, histology of the wounds and the expression of vascular endothelial growth factor (VEGF) and cyclin D1, important regulators of angiogenesis and reepithelialisation, respectively, which are critical for the wound healing process. We evaluated parameters such as wound contracture, tensile strength, histology and collagen content of the skin of the excised wound area to understand the effect of low (500 mEq/1000 g) and high (700 mEq/1000 g) ozonated sesame oil on wound healing. Moreover, reduced oxidative stress has been reported to ameliorate wound healing[7]. Hence, we have also studied the activity of superoxide dismutase (SOD) in the skin of the excised wound. Furthermore, to validate the use of ozonated sesame oil as potential wound healing agent, in addition to employing plain sesame oil as the negative control, we have used framycetin, an antibacterial drug commonly used for treating wounds as our positive control. Hence, the current study investigates the wound-healing potential of ozonated sesame oil in rats and probes into the possible mechanism by which ozonated oils can augment the wound healing process in order to provide proof-of-concept to a traditional drug therapy, which is fraught with apprehensions due to sparse preclinical data.
The ozonated oil was prepared by purging ozone, formed in a water-cooled ozonator (Am Ozonics®) from atmospheric oxygen, through sesame oil. Once the desired peroxide value was reached, ozonation was stopped. Peroxide value is the number that expresses, in milliequivalents of active oxygen, the quantity of peroxide contained in 1000 g of the substance[8]. The peroxide values of the two doses of ozonated oil used in this study were 500 mEq/1000 g (designated as low dose ozonated oil) and 700 mEq/1000 g (designated as high dose ozonated oil). These doses were chosen based on similar work carried out by previous researchers[4,5,6] and the doses used clinically by Ozone Forum of India (providers of ozonated sesame oil) to treat wounds of patients. To calculate the peroxide value, 0.1 g oil was solubilised in glacial acetic acid–chloroform mixture (3:2) and iodometric titration was performed[9]. The peroxide value was calculated as follows: peroxide value (PV)=(sample−blank)×normality of Na2S2O3 ×1000/sample weight.
Female Sprague Dawley rats weighing 180-220 g were procured from the Experimental Animal Centre of Piramal Life Sciences, Mumbai, India. The animals were maintained under standard conditions of temperature (25±5°), relative humidity (55±10 %) and 12/12 h light/dark cycle. They were housed in standard polypropylene cages with wire mesh top and husk bedding and were fed normal diet and water ad libitum. The study protocol was approved by the institutional animal ethics committee (IAEC) of Bombay College of Pharmacy, Mumbai, India.
Excision wound model in rats simulates one of the most commonly encountered wounds in day to day life like abrasions[10]. The hair on the dorsal part of the animals was removed using a clipper and the removal of fine hair was achieved with the help of a depilatory cream. The animals were anaesthetised by intraperitoneal administration of ketamine (80 mg/kg) and xylazine (20 mg/kg) and an open excision-type wound (175 mm2) was created on the dorsal thoracic region to the depth of fascial planes or subcutaneous fat[11]. After recovery from anaesthesia, the animals were randomly assigned to the following four groups having six animals each as follows: vehicle control (plain sesame oil), positive control (framycetin, 1% w/w cream), low dose ozonated oil (500 mEq/1000 g) and high dose ozonated oil (700 mEq/1000 g).
Framycetin sulphate (1 % w/w, Soframycin® cream, Sanofi India Limited) was chosen as a standard drug for comparison because it is one of the most commonly used antibacterial drug for treating wounds[12]. Framycetin was applied to the wound surface using a plastic spatula and the ozonated oils were applied using a dropper. About 0.5 g/day framycetin and 6 drops of oil equivalent to 0.45 g/day were applied to the wound surface of animals in their respective groups. The formulations were applied to the wound surface once daily for 11 consecutive days. After application of the formulations, the wound was covered with cotton gauze held in place with an adhesive tape. Animals were euthanized on the 12th day using solid carbon dioxide and the tensile strength estimations of wounds were carried out on the intact animal. The skin from the excised wound area was then isolated under aseptic conditions and preserved appropriately for biochemical and histopathological examination.
Reduction in the area of the excision wound consequent to the infliction of the wound was measured to represent the efficacy of the healing activity of the formulations tested. The planimetry method was followed for the estimation of wound contraction. A trace of each wound surface was made on a tracing paper on 3rd, 6th, 9th and 12th day postwounding. Wound contracture was expressed as percent reduction in wound area of the original excised wound size[13]. Percentage wound contracture = (wound area on day 1–wound area on day 12/wound area on day 1)×100.
Measurement of tensile strength of the wounds was carried out on the 12th day immediately after sacrificing the animals. The addition of weights to the pan gradually increases the tension on the wound and the weight at which the wound tore apart was noted as the breaking point[14]. Collagen is a component of the extracellular matrix and provides tensile strength to the injured tissue. Hydroxyproline is an amino acid specific to collagen, the extent of collagen deposition in the wound can be indirectly measured and this in turn can be used to estimate the efficacy of the formulations to heal wounds. The procedure used was that of Neuman and Logan and involved the extraction of hydroxyproline from the skin tissue using 6N hydrochloric acid and heat under pressure (120°, 15 psi for 15 min). The pH of the hydrolysate was adjusted to 7.0 followed by oxidation with hydrogen peroxide in the presence of 0.01 M copper sulphate and complexation with p-dimethylaminobanzaldehyde resulting in the formation of a pink colour, the intensity of which was measured at 540 nm. The amount of hydroxyproline in the samples was calculated from a standard plot of L-hydroxyproline; 7.4 parts of hydroxyproline corresponds to 1 part of collagen. Collagen content of the skin of excised wound was expressed in terms of mg/100 mg of tissue[15].
Increased oxidative stress at the wound site impairs wound healing. SOD catalyses the dismutation of superoxide ion into oxygen and hydrogen peroxide and plays an important role as an antioxidant defence. The excised wound surface was homogenised in pH 7.4 phosphate buffer to yield the tissue supernatant. which was further used for the test. The oxidation of epinephrine to adrenochrome is prevented by SOD, thus decreasing the intensity of the colour formed. One unit of SOD activity induced approximately 50% of inhibition of adrenochrome formation[16].
Sections of the skin of the excised wound area were embedded in paraffin after fixation in 10% buffered formalin. Serial sections were cut and stained with haematoxylin and eosin for histological evaluation of parameters like epithelial integrity, presence of hair follicles and collagen deposition[17].
All values were expressed as mean±standard error of the mean. Data were analysed using one-way analysis of variance (ANOVA) followed by Dunnett's test. All analysis was done using GraphPad Prism software version 4.0. P<0.05 was considered to be statistically significant.
The wound contracting ability of high dose of ozonated sesame oil (95.70±0.54%) was significantly greater (P<0.05) than that of vehicle control (86.93±0.96%) and also greater than that of framycetin. Low dose ozonated sesame oil did not have a significant effect on wound contracture when compared with vehicle control (Table 1). The tensile strength of both high and low doses of ozonated sesame oil treated wounds (313.0±6.66 and 315.0±8.06 g, respectively) were significantly greater (P<0.05) than that of vehicle control (231.7±18.51 g). The tensile strength of both doses of ozonated sesame oil was greater than that of framycetin treated wounds (273.0±20.44 g) (Table 1).
Table 1.
WOUND HEALING PARAMETERS OF DIFFERENT EXPERIMENTAL GROUPS
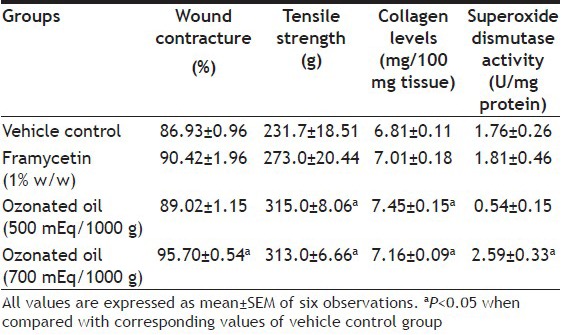
The impact of the different treatments on collagen deposition correlated with the corresponding tensile strengths of the wounds. The collagen content of high (7.16±0.096 mg/100 mg of skin of wound area) and low dose ozonated sesame oil (7.45±0.150 mg/100 mg of skin of wound area) were found to be significantly greater (P<0.05) than that of vehicle control (6.81±0.11 mg/100 mg of skin of wound area) and higher than that of framycetin treated wounds (Table 1).
The activity of the antioxidant enzyme, SOD, in wounds of high dose of ozonated sesame oil (2.594±0.33 U/mg of tissue protein) was significantly greater (P<0.05) than that of vehicle control (1.76±0.26 U/mg of tissue protein). The high dose ozonated oil treated wounds elicited greater SOD activity when compared with framycetin (1.81±0.46 U/mg of tissue protein) (Table 1).
Histolopathological examinations of the skin of the excised wound area from different groups are shown in fig. 1. Wounds of vehicle control rats (fig. 1a) showed thick epithelium with an irregular arrangement of loosely packed collagen bundles, moderate and irregularly deposited collagen fibres, more inflammatory cells and very few regularly shaped hair follicles and glands. Framycetin treated wounds (fig. 1b) showed that the tissue regeneration was much greater than in vehicle control with a thin epithelium, few inflammatory cells, more densely packed and compactly arranged collagen bundles and presence of hair follicles and glands, reflecting a more rapidly healing wound. Low dose ozonated oil treated wounds (fig. 1c) showed on-going epitheliazation with a relatively thin epithelium, few inflammatory cells and no hair follicles as compared with the wounds treated with vehicle. High dose ozonated oil treated wounds (fig. 1d) showed a thin and well organised epithelium, higher collagen deposition, few inflammatory cells and distinct hair follicles and glands.
Fig. 1.
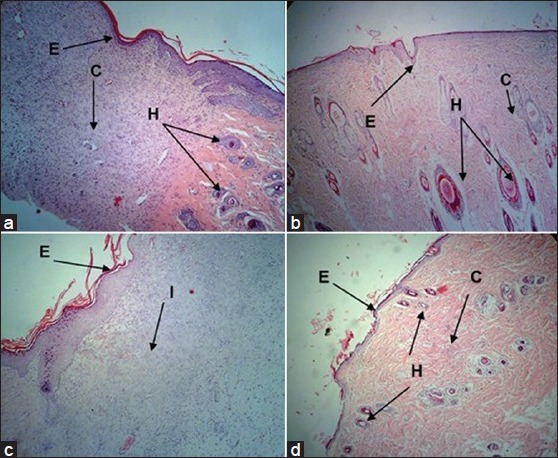
Photomicrographs of sections of the skin of the excised wound area on 12th day postwounding.
E- Epithelium, C- collagen fibres, H- hair follicles, G- granulation, I-inflammatory cells. Representative photomicrographs of haematoxylin and eosin (H and E) stained sections of the skin of the excised wound area on 12th day postwounding, which were topically treated once daily with either sesame oil, Framycetin, low dose and high dose ozonated oil and showing (a) Vehicle control with thick epithelium and irregular arrangement of loosely packed collagen bundles (b) Framycetin treated wounds with thin epithelium, hair follicles, collagen fibres and granulation tissue (c) Low dose ozonated oil treated wounds with on-going epitheliazation and few inflammatory cells (d) High dose ozonated oil treated wounds with a thin epithelium, hair follicles and collagen fibres [H and E, ×100].
Ozonated oil has been used topically for the treatment of various types of skin problems like herpetic infections, trophic ulcers and burns, abscesses, bed sores, vulvovaginitis and gingivitis due to the cleansing, disinfectant and wound healing properties of ozone. However, it is not unanimously accepted among the medical fraternity owing to the fact that ozone is a potent oxidant and that many diseases are associated with increased oxidative stress[2]. The current study was planned, therefore, to explore the potential of ozonated sesame oil in improving excision wound healing and to compare its effects with that of framycetin. The excision wound can be used to study the effect of test drugs on various stages of wound healing such as epithelialisation, contraction, angiogenesis, dermal reconstitution, inflammation, chemotaxis, angiogenesis and functional outcome[10].
Wound contracture is one of the stages in the proliferative phase of wound healing. Greater the wound contracture, faster is the wound healing process. Our study revealed that the topical application of high dose ozonated sesame oil for 11 days on excision wounds leads to significantly faster wound healing process with maximum wound contracture. Our results are in agreement with earlier findings[5,6]. Moreover, the wound contractures of high dose ozonated oil treated wounds were greater than that of framycetin treated wounds. This indicates hastened wound contracture with high dose ozonated oil therapy.
Both high and low doses of ozonated oil showed high collagen levels in the wound tissue indicative of good quality wound healing. Oxygen is implicated in the hydroxylation of proline and lysine residues during collagen formation[18]. Ozone has been reported to increase the oxygen supply by virtue of its interaction with biomolecules as well as its effect on red blood cell metabolism[2]. The collagen content of low dose ozonated oil, however, was greater than that of high dose ozonated oil. This can be probably attributed to the faster transition from type III to type I collagen, which resulted in slightly lower tensile strength than that observed with the lower dose. In the initial stages of wound healing, there is deposition of type III collagen, which is primarily responsible for tensile strength. It reaches its peak levels in 1-3 weeks (depending on wound size) when it is replaced by stronger but less tensile type I[19]. More studies are required that can differentiate between type I and III collagen to get a better insight into this. Ozonated oil upon contact with warm wound exudates form secondary ozonides (Criegee ozonides), which stimulate fibroblasts and hasten wound healing[20]. Tensile strength results of the tissues were in agreement with their collagen levels. These findings reinforce the fact that higher collagen levels in ozonated oil treated animals are responsible for higher tensile strength of the healed skin tissue, a desirable feature of wound healing. Framycetin was seemingly less effective than both the doses of ozonated oil in increasing the collagen content and thus the tensile strength of the wound. Thus ozonated oil may be more beneficial in increasing collagen content and tensile strength of the wounds probably by virtue of its effects on platelet derived growth factor, transforming growth factor β and cyclin D1[6].
During wound healing, granulocytes produce reactive oxygen species (ROS) in the milieu of the wound, which impair wound healing. Increased levels of SOD in wound tissues is an indication of good quality wound healing[7]. Upon contact with the wound surface, ozone immediately reacts with the polyunsaturated fatty acids (PUFAs) to form ROS like hydrogen peroxide. Hydrogen peroxide is considered to be toxic but a brief exposure to an agent that is toxic at a higher dose stimulates the body's defence responses. This phenomenon is known as hormesis. Thus the small amounts of ROS and lipid peroxidation products produced by ozone serve to boost the intrinsic antioxidant system of the body[21]. In our studies, high dose of ozonated oil showed maximum SOD activity in the skin of the excised wound. Our results are in agreement with the findings of other researchers[22]. In our study, framycetin demonstrated higher SOD activity than low dose ozonated oil but lower than that of high dose ozonated oil. This implies that high dose of ozonated sesame oil renders the skin with a greater ability to quench toxic superoxide radicals and that it has a greater antioxidant activity than the antibacterial, framycetin. Higher activity in control group than low dose of ozonated oil could be possibly attributed to the interaction of ozonides with the vehicle sesame oil.
Histopathological studies revealed that the quality of wound healing in ozonated oil treated groups was better than the vehicle treated group. Low dose ozonated oil was less effective than framycetin in accelerating the rate of wound healing as evidenced by on-going epithelisation and absence of hair follicles. The histopathological features of high dose ozonated oil were similar to framycetin treated wounds. There appears to be a dose–response relationship between the two doses of ozonated oil and higher degree of ozonation (peroxide value=700 mEq/1000 g) results in better wound healing properties. More studies are required to establish this dose-dependence.
Taking cognisance of the benefits of topical application of ozonated sesame oil to excision wound in this study, ozone could be a part of the wound care armamentarium not only by virtue of its antibacterial activity, but also due to its antioxidant potential, which is not imparted by conventional antimicrobials used in wound healing. With the emergence of resistant microorganisms to the currently used antibiotics, especially in chronic wounds[23], ozone therapy may be looked upon as a potential modality for the treatment of wounds. However, the dose of ozone used is critical in order to prevent oxidative stress to the host. Previous studies have revealed that ozone concentrations in 10-40 μg/ml resulted in reepithelisation and granulation, whereas concentrations greater than 80 μg/ml were found to be cytotoxic[24]. Thus it is necessary to titrate the dose of ozone in order to gain the maximum benefit of a traditional wound care agent.
ACKNOWLEDGEMENTS
The authors wish to thank Pharminfo.net for providing financial aid for carrying out this work and Ozone Forum of India for providing gift samples of ozonated oils.
Footnotes
Pai, et al.: Wound Healing Activity of Ozonated Sesame Oil
REFERENCES
- 1.Bocci V. Is it true that ozone is always toxic? The end of a dogma. Toxicol Appl Pharmacol. 2006;216:493–504. doi: 10.1016/j.taap.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Bocci VA. Scientific and medical aspects of ozone therapy. State of the art. Arch Med Res. 2006;37:425–35. doi: 10.1016/j.arcmed.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Patel PV, Kumar S, Vidya GD, Patel A, Holmes JC, Kumar V. Cytological assessment of healing palatal donor site wounds and grafted gingival wounds after application of ozonated oil: An eighteen month randomised controlled trial. Acta Cytol. 2012;56:277–84. doi: 10.1159/000336889. [DOI] [PubMed] [Google Scholar]
- 4.Sakazaki F, Kataoka H, Okuno T, Ueno H, Semma M, Ichikawa A, et al. Ozonated olive oil enhances the growth of granulation tissue in mouse model of pressure ulcer. Ozone Sci Eng. 2007;29:503–7. [Google Scholar]
- 5.Kim HS, Noh SU, Han YW, Kim KM, Kang H, Kim HO, et al. Therapeutic effects of topical application of ozone on acute cutaneous wound healing. J Korean Med Sci. 2009;24:368–74. doi: 10.3346/jkms.2009.24.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valacchi G, Lim Y, Belmonte G, Miracco C, Zanardi I, Bocci V, et al. Ozonated sesame oil enhances cutaneous wound healing in SKH1 mice. Wound Repair Regen. 2011;19:107–15. doi: 10.1111/j.1524-475X.2010.00649.x. [DOI] [PubMed] [Google Scholar]
- 7.Schafer M, Werner S. Oxidative stress in normal and impaired wound repair. Pharmacol Res. 2003;58:165–71. doi: 10.1016/j.phrs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Vol. 2. London: British Pharmacopoeia Commission Secretariat; 1993. British Pharmacopoeia; p. A147. [Google Scholar]
- 9.Tellez GM, Lozano OL, Gómez MF. Measurement of peroxidic species in ozonized sunflower oil. Ozone: Sci Eng. 2006;28:181–5. [Google Scholar]
- 10.Gottrup F, Agren MS, Karlsmark T. Models for use in wound healing research: A survey focusing on in vitro and in vivo adult soft tissue. Wound Repair Reg. 2000;8:83–96. doi: 10.1046/j.1524-475x.2000.00083.x. [DOI] [PubMed] [Google Scholar]
- 11.Tang T, Yin L, Yang J, Shan G. Emodin, an anthraquinone derivative from Rheum officinale Baill, enhances cutaneous wound healing in rats. Eur J Pharmacol. 2007;567:177–85. doi: 10.1016/j.ejphar.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 12.Kumara Swamy HM, Krishna V, Shankarmurthy K, Abdul Rahiman B, Mankani KL, Mahadevan KM, et al. Wound healing activity of embelin isolated from the ethanol extract of leaves of Embelia ribes Burm. J Ethnopharmacol. 2007;109:529–34. doi: 10.1016/j.jep.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Sadaf F, Saleem R, Ahmed M, Ahmad SI, Navaid-ul-Zafar Healing potential of cream containing extract of Sphaeranthus indicus on dermal wounds in Guinea pigs. J Ethnopharmacol. 2006;107:161–3. doi: 10.1016/j.jep.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 14.Saha K, Mukherjee PK, Das J, Pal M, Saha BP. Wound healing activity of Leucas lavandulaefolia Rees. J Ethnopharmacol. 1997;56:139–44. doi: 10.1016/s0378-8741(97)01522-5. [DOI] [PubMed] [Google Scholar]
- 15.Neuman RE, Logan MA. The determination of collagen and elastin in tissues. J Biol Chem. 1950;186:549–56. [PubMed] [Google Scholar]
- 16.Misra HP, Fridovich I. The generation of superoxide radical during the auto oxidation of hemoglobin. J Biol Chem. 1972;247:6960–2. [PubMed] [Google Scholar]
- 17.Yaduvanshi B, Mathur R, Mathur SR, Velpandian T. Evaluation of wound healing potential of topical formulation of leaf juice of Tridax procumbens L. In mice. Indian J Pharm Sci. 2011;73:303–6. doi: 10.4103/0250-474X.93523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhutani S, Vishwanath G. Hyperbaric oxygen and wound healing. Indian J Plast Surg. 2012;45:316–24. doi: 10.4103/0970-0358.101309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhn K. The classical collagens: Type I, II and III. In: Burgeson MR, editor. Structure and function of collagen types. Orlando: Academic Press; 1987. pp. 1–42. [Google Scholar]
- 20.Travagli V, Zanardi I, Valacchi G, Bocci V. Ozone and ozonated oils in skin diseases: A review. Mediators Inflamm 2010. 2010 doi: 10.1155/2010/610418. 610418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calabrese EJ. Hormesis and medicine. Br J Clin Pharmacol. 2008;66:594–617. doi: 10.1111/j.1365-2125.2008.03243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zamora Z, Gonzalez R, Guanche D, Merino N, Menendez S, Hernandez F, et al. Ozonized sunflower oil reduces oxidative damage induced by indomethacin in rat gastric mucosa. Inflamm Res. 2008;57:39–43. doi: 10.1007/s00011-007-7034-1. [DOI] [PubMed] [Google Scholar]
- 23.Howell-Jones RS, Wilson MJ, Hill KE, Howard AJ, Price PE, Thomas DW. A review of the microbiology, antibiotic usage and resistance in chronic skin wounds. J Antimicrob Chemother. 2005;55:143–9. doi: 10.1093/jac/dkh513. [DOI] [PubMed] [Google Scholar]
- 24.Bocci V. Ozone as a bioregulator. Pharmacology and toxicology of ozone therapy today. J Biol Regul Homeost Agents. 1996;10:31–53. [PubMed] [Google Scholar]


