INTRODUCTION
Endothelial progenitor cells (EPC) are essential for neovascularization and tissue repair. Asahara et al1 first described EPCs as cells isolated from human peripheral blood based on cell surface antigen expression and their ability to differentiate in vitro into endothelial cells (EC)1. Prior to this discovery, vasculogenesis was thought to occur only in the fetal environment and angiogenesis, the sprouting of new vessels from existing vasculature, was the only form of neovascularization.
EPCs are cells that have proangiogenic and vasculogenic properties and have an endothelial lineage commitment because they can differentiate into endothelial cells but they lack mature EC markers2. These characteristics differentiate EPCs from ECs and other mononuclear cells.
EPCs are thought to mobilize from the bone marrow and home to sites that require neovascularization following hypoxia, endothelial injury or signaling of certain growth factor and chemokines.3 We hypothesize that EPC mobilization initiates acutely after endothelium damage, which triggers a cascade of events to promote endothelial recovery. In this study, we investigated the early EPC mobilization response to acute wounding by comparing the number of circulating EPCs in the peripheral blood before and after injury (skin surgery). Very few human experimental models of wound repair exist and our goal was to determine if skin surgery could be used to study the early mobilization of EPCs.
MATERIALS AND METHODS
Subjects
Subjects were enrolled from the Dermatologic Surgical practice at the University of Pennsylvania (UPENN). A UPENN IRB approved consent was obtained from each subject. Peripheral blood samples from 20 subjects undergoing Mohs surgery of the face and scalp (mean tumor: 1.3 × 1.4 cm) for basal or squamous cell carcinoma were collected before and 1–2 hours after initial incision (just before closure of the surgical defect). (Table 1)
Table 1.
| Patient Characteristics | (n=20) |
|---|---|
| Age (yrs) | 63.1±2.27 (range 38–86) |
| Age over 55 | 9 (45%) |
| Gender (% male) | 15 (75%) |
| Hypertension (%) | 9 (45%) |
| Diabetes (%) | 4 (20%) |
| Tumor size (mean) | 1.4cm × 1.3 cm |
EPC Characterization
We defined EPCs as mononuclear cells; CD45− and DRAQ5+ with CD34+CD133+, CD34+CD31+, CD34+CXCR4+ or CD34+VEGFR2+. Mononuclear cells were isolated by density centrifugation. Following purification with 3 PBS washings, the collected mononulcear cells were stained with human antibodies for: CD31, CD34, CD45, CD133, VEGFR2, CXCR4 and DRAQ5. Five micro milliliters of antibodies were added to one million cells and incubated for 40 minutes on ice. All quantitative data acquisition was done with polychromatic flow cytometry on a BD FACS Canto instrument measuring 100,000 events per sample. CD45+ cells were gated out as lymphocytes and cell viability was determined by positive DRAQ5. Flow data were then analyzed using FlowJo software.
Statistical Analysis
Statistical analysis was performed with a student’s t-test or Analysis of Variance and results are expressed as mean±SEM. Statistical significance was determined by p<0.05. Results were compared pre and post surgery for each gating type.
RESULTS
EPC Mobilization Begins Shortly after Acute Wound Injury
Table 2 shows the change in circulating EPCs per 100,000 events in the peripheral blood before and 1–2 hour after injury. Peripheral blood cells labeled by CD34+CD133+, CD34+CD31+ and CD34+CXCR4+ showed an increase after acute wound injury. Hypertension or gender did not have a statistically significant effect on the results. No differences were noted by tumor type.
Table 2.
| Before | After | p value | ||
|---|---|---|---|---|
| All | CD133 | 285(238) | 513(466) | 0.0001 * |
| CD31 | 275(200) | 478(386) | 0.0001 * | |
| CXCR4 | 155(70) | 239(122) | 0.003 * | |
| DM (−) | CD133 | 308(252) | 576(489) | 0.0002 * |
| CD31 | 301(207) | 519(412) | 0.0004 * | |
| CXCR4 | 154(60) | 252((123) | 0.005 * | |
| Age <55 | CD133 | 185(86) | 264(129) | 0.01 * |
| CD31 | 159(81) | 280(158) | 0.006 * | |
| CXCR4 | 151(67) | 225(122) | 0.02 * | |
| DM (+) | CD133 | 178(122) | 212(110) | 0.1 |
| CD31 | 264(117) | 285(63) | 0.7 | |
| CXCR4 | 161(113) | 188(121) | 0.25 | |
| Age ≥55 | CD133 | 195(103) | 356(210) | 0.15 |
| CD31 | 264(117) | 285((63) | 0.7 | |
| CXCR4 | 166(82) | 272(128) | 0.09 |
All cells in the above table are CD34+, CD45−, DRAQ5+
Values reported are: Mean (±SD)
statistically significant at p<0.05
EPC Mobilization Response is Decreased in Diabetic Patients
In patients with diabetes, there was no statistically significant increase in EPCs following acute wound injury. However in non-diabetic patients, CD34+CD133+, CD34+CD31+ and CD34+CXCR4+ cells showed a significant increase.
EPC Mobilization Response is Decreased in Patients Over 55 years old
No statistically significant increase in EPCs following acute wound injury was noted in those more than 55 years of age. However in those younger than 55 years, CD34+CD133+, CD34+CD31+ cells showed a statistically significant increase.
DISCUSSION
New vessel formation occurs by two processes, angiogenesis and vasculogenesis. They are different but not mutually exclusive. They occur concurrently in vivo thus neovascularization requires both the recruitment of EPCs and multiple other cells to orchestrate new vessel formation4.
EPCs represent 0.001–0.1% of all mononuclear cells and are identified by the surface markers CD34+, VEGFR2+ and CD45− or dim. Other endothelial markers such as PECAM1 (CD31), VECadherin (CD144), Tie-2, CXCR4 and stem cell marker CD133 are also often used to describe EPCs. However, the expression levels of these markers vary and change in the course of vasculogenesis because EPCs mature thereby changing surface markers or because EPC cell population changes5,6.
EPCs can be from bone marrow or pre-exist in the peripheral circulation at low levels under normal steady state conditions. It is thought that vascular injury causes signaling to mobilze EPCs from its pool and home to sites of injury7 and acute stress and inflammation without vascular injury does not cause mobilization8. During mobilization, EPCs move from the ”pool” into the circulation due to the release of cytokines or growth factors such as SDF-1, G-CSF, FGF, VEGF or EPO and matrix metalloproteinases such as MMP-2, MMP-9, cathepsin-G and elastase9. Other stimulus for mobilization includes statins, exercise and estrogen.
The goal of our study was to illustrate the early response of EPCs to acute stimuli. Our results showed that shortly after injury, 1–2 hour time frame, there is change in the peripheral EPC pool. This response was blunted in patients with diabetes and by older age. The mechanism for this is not known but may be due to decreased number of stem cells in the bone marrow niche and blunted response to mobilization stimuli along with decreased endothelial turnover and regeneration10.
There is however a limitation to our model in that all subjects had a keratinocyte cancer. While some tumors secrete angiogenic factors we have no reason to believe that this is true for keratinocyte tumors. In fact, basal cell cancers and squamous cell cancers of the skin likely have very different biologies. Furthermore, we noted an increase in EPCs about 2 hours after the tumor was removed not prior to removal.
In summary we have shown that EPC mobilization in humans occurs soon after surgical stress. Because Mohs surgery is a frequent procedure, these subjects may be a good population to study. Further investigations are necessary to examine the signaling sequences for mobilization and to understand the cellular functions and expandability of the EPCs.
Figure 1.
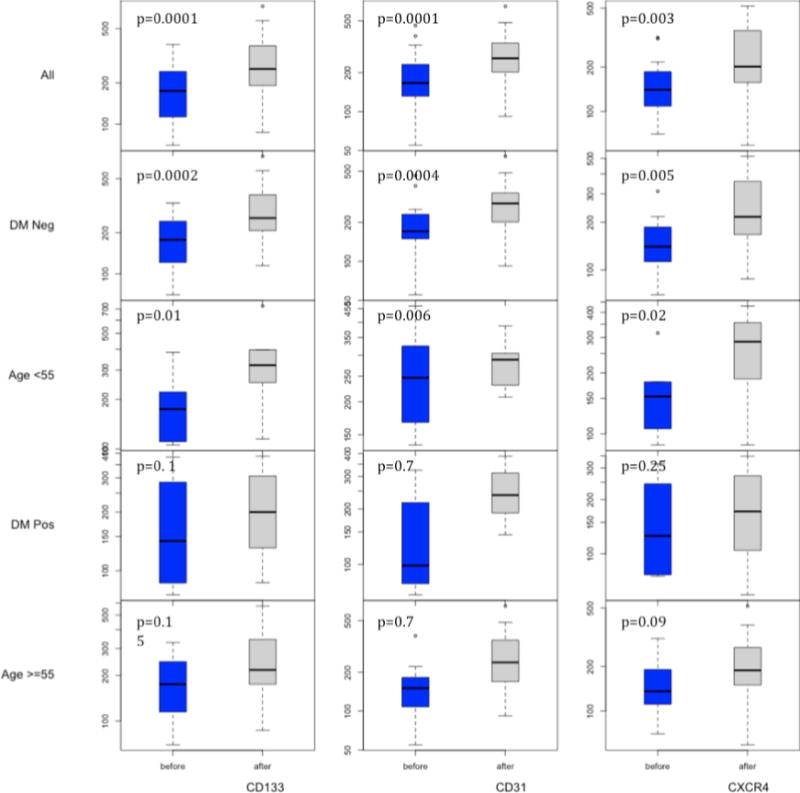
All cells in the above figure are CD34+, CD45−, DRAQ5+
Values reported are: Mean (±SD)
*statistically significant at p<0.05
Acknowledgments
This work was supported by grant R01-DK094260 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; to S.R.T.).
Footnotes
None of the authors have any financial interest in or a financial conflict with the subject matter or materials discussed in this manuscript
References
- 1.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 2.Asahara T, Kawamoto A, Masuda H. Concise review: circulating endothelial progenitor cells for vascular medicine. Stem Cells. 2011;29(11):1650–1655. doi: 10.1002/stem.745. [DOI] [PubMed] [Google Scholar]
- 3.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95(4):343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 4.George AL, Bangalore-Prakash P, Rajoria S, et al. Endothelial progenitor cell biology in disease and tissue regeneration. J Hematol Oncol. 2011;4:24. doi: 10.1186/1756-8722-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prater DN, Case J, Ingram DA, Yoder MC. Working hypothesis to redefine endothelial progenitor cells. Leukemia. 2007;21(6):1141–1149. doi: 10.1038/sj.leu.2404676. [DOI] [PubMed] [Google Scholar]
- 6.Estes ML, Mund JA, Mead LE, Prater DN, Cai S, Wang H, et al. Application of polychromatic flow cytometry to identify novel subsets of circulating cells with angiogenic potential. Cytometry A. 2010;77(9):831–839. doi: 10.1002/cyto.a.20921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gill M, Dias S, Hattori K, Rivera ML, Hicklin D, Witte L, et al. Vascular trauma induces rapid but transient mobilization of VEGFR2(+)AC133(+) endothelial precursor cells. Circ Res. 2001;88(2):167–174. doi: 10.1161/01.res.88.2.167. [DOI] [PubMed] [Google Scholar]
- 8.Padfield GJ, Tura O, Haeck ML, Short A, Freyer E, Barclay GR, et al. Circulating endothelial progenitor cells are not affected by acute systemic inflammation. Am J Physiol Heart circ Physiol. 2010;298(6):H2054–2061. doi: 10.1152/ajpheart.00921.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laird DJ, von Andrian UH, Wagers AJ. Stem cell trafficking in tissue development, growth, and disease. Cell. 2008;132(4):612–630. doi: 10.1016/j.cell.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 10.Heiss C, Keymel S, Niesler U, Ziemann J, Kelm M, Kalka C. Impaired progenitor cell activity in age-related endothelial dysfunction. J Am Coll Cardiol. 2005;45(9):1441–1448. doi: 10.1016/j.jacc.2004.12.074. [DOI] [PubMed] [Google Scholar]


