Abstract
Tissue-specific expression of cre recombinase is a well-established genetic tool to analyze gene function, and it is limited only by the efficiency and specificity of available cre mouse strains. Here we report the generation of a transgenic line containing a cre cassette with codon usage optimized for mammalian cells (iCre) under the control of a mouse glycoprotein hormone α-subunit (αGSU) regulatory sequences in a bacterial artificial chromosome genomic clone. Initial analysis of this transgenic line, Tg(αGSU-iCre), with cre reporter strains reveals onset of cre activity in the differentiating cells of the developing anterior pituitary gland at embryonic day 12.5, with a pattern characteristic of endogenous αGSU. In adult mice, αGSU-iCre was active in the anterior lobe of the pituitary gland and in the cells that produce αGSU (gonadotropes and thyrotropes) with high penetrance. Little or no activity was observed in other tissues, including skeletal and cardiac muscle, brain, kidney, lungs, testis, ovary and liver. This αGSU-iCre line is suitable for efficient, specific and developmentally regulated deletion of floxed alleles in anterior pituitary gonadotropes and thyrotropes.
Keywords: transgenic mouse, chorionic gonadotropin alpha subunit, Cga, alpha-GSU, site specific recombination, BAC
Introduction
During the development of the mammalian anterior pituitary gland, the distinct hormone-producing cell types emerge and organize into networks (Davis, Mortensen and Camper 2011, Budry et al. 2011). Initially precursor cells proliferate and differentiate into five different endocrine cell types that are typified by the hormones that they produce: corticotropes produce adrenocorticotropin (ACTH), thyrotropes produce thyroid stimulating hormone (TSH), somatotropes produce growth hormone (GH), lactotropes produce prolactin (PRL), and gonadotropes produce luteinizing hormone (LH) and follicle-stimulating hormone (FSH). In mouse embryonic development, hormone producing cell types become detectable over a period spanning embryonic days 12.5 (e12.5) to e17.5 (Japon, Rubinstein and Low 1994). Genetic and biochemical studies have identified several of the transcription factors that are crucial for cell lineage determination and differentiation and cell-specific hormonal gene expression (Marcil A et al. 2003) (rev in (Zhu and Rosenfeld 2004)). Both gonadotropes and thyrotropes express a common α-glycoprotein subunit (αGSU), which heterodimerizes with separate β-subunits of the glycoprotein hormones, LH, FSH and TSH, to give the biologically active hormones. αGSU is the first pituitary hormone component detected during development. The earliest detectable signal is present at e11.5 in the rostral and ventral most region of Rathke's pouch (Burrows et al. 1996). Deletion of this gene, officially known as chorionic gonadotropin alpha or Cga, revealed that it is necessary after birth for stimulation of thyroid and gonadal growth and function (Kendall et al. 1991).
The cre-loxP system has become a powerful tool to study the effects of gene deletion in a particular cell type or tissue, especially when conventional gene targeting by homologous recombination in embryonic stem cells results in an early lethal phenotype. In order to study the pituitary specific effects of gene deletion, we previously generated transgenic mice that express the cre recombinase under the transcriptional control of the mouse αGSU gene (Cga). A sequence of 4.6 kb of the mouse αGSU gene promoter and enhancer (B6;SJL-Tg(Cga-cre)3Sac/J) targets the gonadotropes and thyrotropes, as well as the other hormone-producing cells of the adult anterior pituitary gland (Cushman et al. 2000). These sequences are sufficient to confer developmentally regulated and hormone-responsive gene expression in the pituitary gland, and they have been used successfully to create pituitary-specific deletions of several genes (Cushman et al. 2000, Kendall et al. 1994, Charles et al. 2006, Bliss et al. 2009). Ectopic cre activity, however, is observed in the ovary and in the skeletal and cardiac muscle of 4.6 kb αGSU transgenic mice, limiting the usefulness of this transgene for some purposes (Uhlenhaut et al. 2009).
To generate αGSU-Cre transgenic mice that more accurately recapitulate endogenous αGSU gene expression, we used homologous recombination in E. coli to introduce improved cre recombinase (iCre) coding sequences into a bacterial artificial chromosome (BAC) containing the mouse αGSU gene. A transgenic line carrying this construct conferred highly penetrant cre mediated excision of floxed reporter genes and had minimal ectopic expression. This suggests that regulatory DNA sequences present in the BAC confer more accurate αGSU gene expression than promoter-proximal sequences alone.
Results and Discussion
Cga, a single gene in the mouse genome, encodes αGSU, and is flanked by Zfp292 and Mobkl2b on mouse chromosome 4. Using recombineering in E. coli, we inserted an improved cre expression cassette (iCre) into a 228 kb mouse αGSU genomic BAC clone in exon 1 at the translation start site (Figure 1). The iCre has reduced CpG content relative to the prokaryotic coding sequence, thereby reducing the chances of epigenetic silencing in mammals (Shimshek et al. 2002). The engineered BAC was injected into the pronucleus of fertilized eggs from a C57BL/6J cross with B6D2F1 mice to generate αGSU-iCre BAC transgenic mice. We identified a founder transgenic mouse containing the complete BAC using polymerase chain reaction (PCR) genotyping with primers for the iCre insertion and the BAC vector backbone. This line Tg(Cga-iCre)Sac961 is referred to here as αGSU-iCre for simplicity.
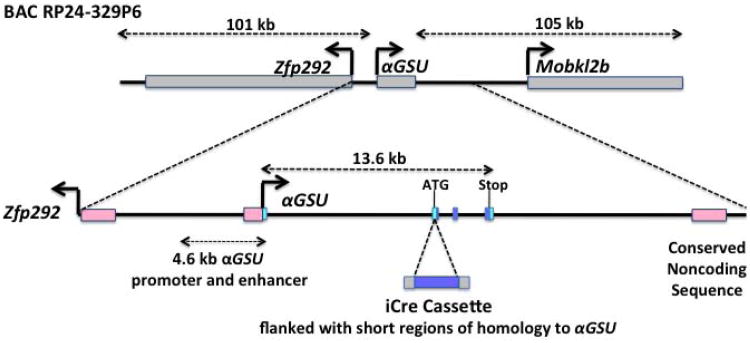
Figure 1. Structure of the BAC Tg(αGSU-iCre) transgene.
The position of the αGSU gene within the RP24-329P6 BAC is indicated, together with Zfp292 (80kb) and a portion of the Mobkl2b gene (60kb/from 208kb). The exon - intron structure of the αGSU gene is shown. The first exon is non-coding (light blue) and 4 exons are coding (blue rectangles). The recombination targeted the second αGSU exon, inserting the iCre coding sequence at the ATG of the αGSU gene and using the αGSU gene polyadenylation sequence. The 4.6 kb promoter used for the previous αGSU-Cre transgenic mouse is indicated.
We determined that the transgene does not affect the expression levels of endogenous αGSU using quantitative PCR (data not shown). To asses the specificity, efficiency and in vivo activity of the αGSU-iCre BAC transgene, transgenic mice were crossed with two different cre-reporter transgenic mice that express a reporter gene only after excision of a floxed stop sequence: Rosa26-LacZ and Rosa26-EYFP (officially B6;129S4-Gt(ROSA)26Sortm1Sor/J and B6.Cg-Gt(ROSA)26Sortm2(CAG-EYFP)Hze/J respectively). Both αGSU-iCre female and male mice were fertile.
The DNA recombination pattern in the developing pituitary and adult tissues was analyzed by crossing αGSU-iCre mice with the Rosa26-LacZ cre reporter line (Soriano 1999, Friedrich and Soriano 1991, Zambrowicz et al. 1997). In transgenic animals carrying both Rosa26-LacZ and αGSU-iCre transgenes, the Cre recombinase catalyzed the removal of the stop sequence, leading to β-galactosidase production. X-Gal stained αGSU-iCre;Rosa26LacZ double transgenic embryos showed that recombination occurred in the developing pituitary gland at embryonic day 11.5 (e11.5), in a few cells (data not shown). This is consistent with the developmental timing of endogenous αGSU expression (Kendall et al. 1991). By e12.5 essentially all of the cells at the ventral aspect of the pouch are stained, with a pattern of expression characteristic of endogenous αGSU expression (Figure 2). Little or no staining was detected in the bodies of whole mount stained embryos, while the 4.6 kb αGSU-cre transgene was active in the somites at e12.5 and other ectopic sites (Cushman et al. 2000). After removing the mandible, transgene activity is obvious in the developing pituitary gland and in other areas that express endogenous αGSU: the mesenchyme adjacent to the eye and the olfactory epithelium (Kendall et al. 1991). Sagittal sections revealed X-gal staining confined to the expected rostral, ventral area of Rathke's pouch where differentiated cells expressing αGSU are appearing. At e11.5 limited β-galactosidase activity was detected (data not shown) (Cushman et al. 2000). This is consistent with the developmental timing of endogenous αGSU expression (Kendall et al. 1991). These results show that the new αGSU-iCre BAC transgenic mouse line mediates recombination much more specifically than the previous line using only 4.6 kb of regulatory sequences.
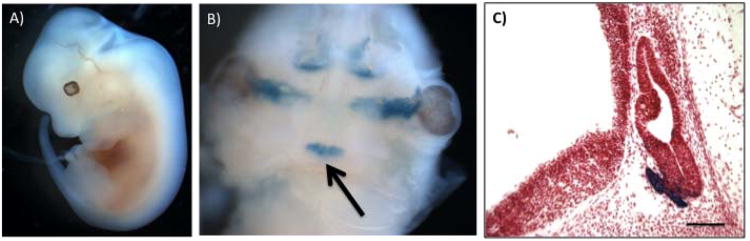
Figure 2.
Expression of the BAC Tg(αGSU-iCre) transgene in the developing pituitary gland at e12.5.
X-Gal stained αGSU-iCre; Rosa26LacZ double transgenic embryos reveal activity of the αGSU-iCre transgene during development. Panel A: Whole X-gal stained double transgenic embryo (e12.5). Panel B: The mandible was removed from the embryo in panel A to visualize the pituitary (arrow). Panel C: Sagittal cryosection of X-gal stained double transgenic embryo showing expression in the forming anterior lobe of the pituitary. Section is counterstained with neutral red. Magnification bar represents 100μm.
The tissue specificity of the αGSU-iCre transgene was analyzed by X-Gal staining of cryosections from adult tissues (2 mo old) from αGSU-iCre; Rosa26LacZ double transgenic mice. Cre transgene activity was detected in the pituitary of 2 mo old adult mice, with no activity in inappropriate tissues such as muscle, kidney, lung, testis and liver (Figure 3). A few rare, positive cells were detected in the brain, and low levels of activity were noted in some follicles of the ovary (Figure 3). Because of the potential for multiple organs in the hypothalamic-pituitary-gonadal axis to be affected in gene deletion studies using the αGSU-icre, we characterized Cre expression in the ovary using a different reporter mouse, Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J, which switches from red fluorescent tdTomato to green fluorescent eGFP upon recombination. Only a few scattered cells in some, but not all ovaries, were detected using either reporter mouse. Although this may affect the results of gene deletion, if the gene of interest is expressed in the ovary, the αGSU-iCre BAC transgenic line greatly improves pituitary specificity over previously available reagents and more accurately reflects the expression of the endogenous αGSU gene.
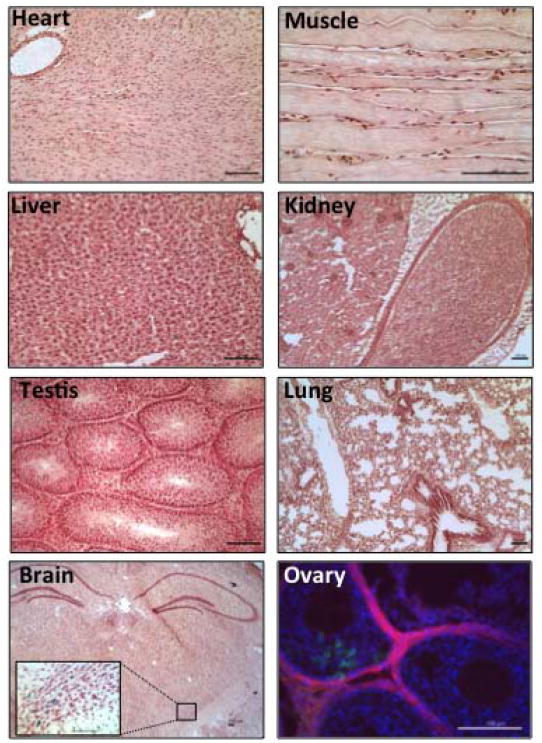
Figure 3.
Tg(αGSU-iCre) transgene is active primarily in the pituitary gland.
Analysis of cre activity in adult tissues was examined by X-Gal staining of αGSU-iCre;Rosa26LacZ double transgenic mice. Cryo sections were counterstained with neutral red. For the ovary, Tg(αGSU-iCre) mice were crossed with the double-fluorescent cre reporter mTmG line, that expresses membrane targeted tandem dimer tomato (mT) prior to cre mediated excision and membrane targeted green fluorescent protein (mG) after excision (Muzumdar et al. 2007). Ovary sections were counterstained with DAPI (blue). All magnification bars represent 100μm.
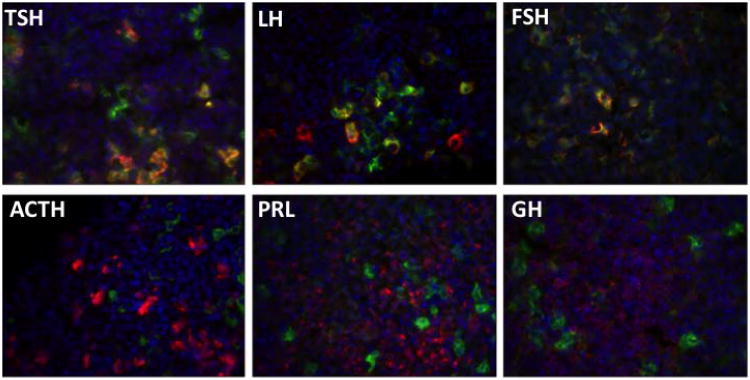
To study the pituitary cell specificity the αGSU-iCre transgenic mice were crossed with Rosa26-EYFP cre reporter line. In this reporter mouse line, cre excision of the loxP-flanked STOP fragment permits EYFP gene expression (Madisen et al. 2010) that can be detected by antibody staining against EGFP. Cell specificity was assessed by following EGFP immunohistochemistry (IHC) with staining for individual antibodies specific to each of the pituitary hormones (αGSU, TSHβ, LHβ, FSHβ, GH, PRL and ACTH). IHC on pituitary cryosections from αGSU-iCre; Rosa26EYFP double transgenic mice reveals high levels of αGSU-iCre activity in the anterior lobe of the pituitary, with no expression in the intermediate and posterior lobe (Figure 4A). Double IHC for eGFP and αGSU reveals that most αGSU positive cells undergo cre recombination (Figure 4B). Quantitation of hormone positive cells for EGFP expression revealed a high level of penetrance in gonadotropes and thyrotropes with a low level of expression in other pituitary cell types (Figure 5, Table 1). About 79% of αGSU producing cells are marked and most thyrotropes and gonadotropes were marked. EYFP IHC was detected in cells positive for TSHβ (96%), LHβ (67%) and FSHβ (91%). Less than 10% of cells positive for other hormones express EYFP: ACTH (8%), GH (6%) or PRL (7%). This may not represent ectopic expression because αGSU is detectable in a variety of pituitary cell types (Childs 1991, Seuntjens et al. 2002, Saeger et al. 2007).
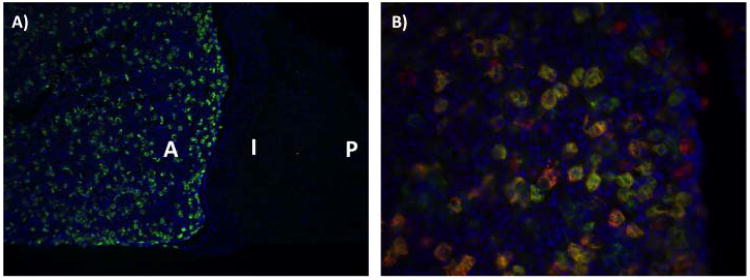
Figure 4.
Expression of the BAC Tg(αGSU-iCre) transgene in the adult pituitary gland.
αGSU-iCre; Rosa26-EYFP double transgenic mouse reveals a high level of the αGSU-iCre activity in the anterior lobe of the pituitary, with no expression in the intermediate and posterior lobe. Panel A: Pituitary cryo section of a αGSU-iCre; Rosa26-EYFP double transgenic mouse stained with EGFP antibody. Panel B: Double immunohistochemistry analysis with antibody against EGFP (green) and αGSU (red) in pituitary cryosections from αGSU-iCre; Rosa26-EYFP mice. Cell nuclei were stained with DAPI (blue). A, anterior lobe; I, intermediate lobe; P, posterior lobe.
Figure 5.
Tg(αGSU-iCre) is specific for thyrotropes and gonadotropes.
Adult pituitary cryo sections from mice doubly transgenic for Tg(αGSU-iCre) and Rosa26EYFP reporter strain were stained by double immunohistochemistry with antibodies against EYFP (green) and each pituitary hormone (red). EYFP expression co-localizes with LHβ, FSHβ and TSHβ in pituitaries from αGSU-iCre; Rosa26-EYFP double transgenic mice; and not with ACTH, PRL, and GH. Cell nuclei were stained with DAPI (blue).
Table 1. Anterior pituitary cell specificity of Tg:αGSU-iCre activity.
| Hormone1 | Hormone producing cells that express EYFP (%)2 |
|---|---|
| αGSU | 79 ± 5* |
| TSHβ | 96 ± 3* |
| LHβ | 67 ± 6* |
| FSHβ | 91 ± 4* |
| ACTH | 8 ± 0.2# |
| PRL | 6 ± 0.9# |
| GH | 5 ± 0.5# |
Immunohistochemistry performed with antibodies specific to the indicated hormone
The data shown represent the mean ± SEM of three mice per hormone, counting approximately 500 cells for each pituitary hormone tested.
different from
, p<0.05.
In summary, we have generated an improved αGSU-iCre transgenic mouse using BAC recombineering. Compared to the original αGSU-cre transgenic mouse, the new line mediates recombination in the pituitary thyrrotropes and gonadotropes, without broad expression in other pituitary cell types and without ectopic recombination in the heart, skeletal muscle, and other ectopic sites characteristic of the original line. It is tempting to speculate that the evolutionarily conserved noncoding sequence located 3′ of the αGSU gene could have a role in generating appropriate tissue specific, developmentally regulated and cell specific expression, similar to 3′ regulatory elements that have been identified for many genes (Antoniou et al. 1988). BAC recombineered transgenes can be an effective and efficient approach to developing cre strains because identification of all the regulatory elements for accurate gene expression can be tedious, and because knock-in alleles can create haploinsufficiency for the endogenous gene and influence the phenotype.
Methods
BAC Transgene Construction
BAC clone RP24-329P6 containing mouse Cga was obtained from the BACPAC Resource Center (Oakland, California). The iCre clone was a kind gift of R. Sprengel (Shimshek et al. 2002). To place iCre under the control of Cga, the loxP recombinase site in the pTARBAC1 BAC cloning vector (Zeng et al. 2001) was replaced with an amp resistance cassette from the pTAMP plasmid as described (Lee et al. 2001). A plasmid with an R6K origin of replication containing iCre (nucleotides 1-1056) and an FRT-gb2-kan-FRT cassette was assembled in Pir1 bacteria. To introduce iCre, a 100 bp oligonucleotide was synthesized with 20 bp of homology to the iCre cassette and 80 bp homology immediately downstream of Cga exon 2 (ENSMUSE00000177972) nucleotide 97. This primer replaced the Cga initiator methionine with the initiator methionine of iCre. A second 100 bp oligo was synthesized that contained 80 bp homology that began with intron 2 nucleotide 10 and 20 bp of homology with the iCre cassette. The oligos were used to PCR amplify the iCre-FRT-gb2-kan-FRT cassette from the donor plasmid. The PCR product was used to introduce the iCre-FRT-gb2-kan-FRT cassette into Cga exon 2 of the BAC as described above. FLPe was used to excise the FRT flanked drug selection cassette from the BAC (Lee et al. 2001). Recombineered BACs were identified by pulsed field restriction enzyme mapping as described (Van Keuren et al. 2009). The critical region of the BAC was completely sequenced after PCR amplifying the Cga exon 2 iCre knock-in region. Primers used to amplify the iCre-FRT-gb2-kan-FRT cassette were: Primer 1, which aligns to vector backbone and Cga: 5′ TGAAAGACGGTA GAAAGGAACTAA TCATTATTGATG TGCATCTAGAAA CTTGTTTCCCTT TTCATGGCAAGC CTGAGCTTATAT ACGAAGTTATAA GCTT 3′ and Primer 2, which aligns to iCre and Cga: 5′ ACAGCTGGCTTG GGTTATGACTGG TAAGCTAAGATT ACACTGTTATTA TTTTTTTTTTCA TGTGCAGCTTGC AGAAGAGCTATG GTGCCCAAGAAG AAGA 3′, with a product size of 2330 bp.
Generation and genotyping of transgenic mice
All experiments using mice were approved by the University of Michigan Committee on Use and Care of Animals. The engineered BAC (αGSU-iCre) was injected into the pronuclei of fertilized eggs from a C57BL/6J cross with B6D2F1 mice and the fertilized eggs were transferred into pseudopregnant foster mothers. Transgene positive progeny were genotyped using PCR amplification of genomic DNA with primers that flank the junction of the αGSU promoter and the inserted iCre coding sequence: 5′-TGAAGCCATCTCTCTGAGCAA-3′ and 5′- CACAGTCAGCAGGTTGGAGA-3′, under the following conditions: 92°C for 2 minutes, followed by 30 cycles of 92°C for 10 seconds, 57°C for 30 seconds and 72°C for 30 seconds, and a final 10 minute extension at 72°C. Transgene positive progeny were also genotyped using primers specific for the chloramphenicol resistance gene located in the BAC backbone to identify mice that harbor the complete BAC: 5′-TCACTACCGGGCGTATTTTT-3′ and 5′-GCCGGATAAAACTTGTGCTT-3′ and the same PCR cycling conditions.
B6;129S4-Gt(ROSA)26Sortm1Sor/J, B6.Cg-Gt(ROSA)26Sortm2(CAG-EYFP)Hze/J, and Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J reporter mice are all available from The Jackson Laboratory, and were maintained as homozygotes.
In all the experiments animals carried one allele of the cre transgene and one allele of the reporter gene, while controls were negative for the cre transgene but positive for a reporter gene.
Histology and Immunohistochemistry
Adult tissues and embryos were collected and fixed for 1 hour in 4% formaldehyde in PBS. For embryos, noon of the day of the vaginal plug is designated as embryonic day 0.5. X-gal staining was performed as previously described (Brinkmeier et al. 1998). After staining, embryos and adult organs were embedded in OCT (Sakura Finetek Co., Torrance, CA), and cryosectioned at 16μm thickness. Cryosections were counterstained for 2 minutes with 1% neutral red stain plus 4% sodium acetate: glacial acetic acid. Sections were mounted with xylene:permount 1:2 (Fisher) mounting media.
Immunohistochemistry for GFP and hormones was performed on 16μm pituitary cryosections. Frozen pituitary sections were fixed for 5 min in 4% formaldehyde and rinsed in 1× PBS. Immunostaining was performed using a goat polyclonal anti-GFP (Abcam) antibody overnight at 4°C, diluted 1:1000 in a blocking solution comprised of 3% normal donkey serum, 1% BSA, and 0.5% Triton-×100 in 1× PBS. Slides were washed 3 times for 5 minutes in 1× PBS, followed by 1 hour incubation with biotinylated anti-goat secondary antibody (Jackson Immunoresearch). Streptavidin-Alexa 488 (Invitrogen) was added for 1 hour at 1:100 dilution in 1×PBS. Immunostaining for the different hormones was carried out with antibodies against each of the pituitary hormones: rabbit anti-αGSU (1:50), guinea pig anti-LHβ (1:100), rabbit anti-FSHβ (1:50), rabbit anti-TSHβ (1:200), rabbit anti recombinant PRL (1:200), monkey anti-GH (1:500), and rabbit anti-ACTH (1:150) (National Hormone and Pituitary Program, NIDKK). Secondary detection was performed as described above using biotinylated anti-rabbit or anti-guinea antibody (Vector Laboratories) and streptavidin-Cy3 (Jackson Immunoresearch), then washed in PBS and mounted with fluorescent mounting media and images were captured using a Retiga 2000R camera mounted on a Leica DMRB fluorescent microscope.
The αGSU-iCre mice will be available upon request. We recommend using them as hemizygotes since we evaluated a total of 45 mice borne from a cross of two hemizygous carriers, and none of them were homozygous; suggesting that homozygosity for the transgene is lethal.
Acknowledgments
NICHD (R0130428 and R3734283 to SAC), International Endocrine Scholar Program (MIPM), and the Transgenic Animal Model Core (NIH grant numbers CA46592, AR20557, and DK34933). We acknowledge Wanda Filipiak and the late Maggie Van Keuren of the University of Michigan Transgenic Animal Model Core for preparation of the transgenic mice. We would also like to acknowledge the many contributions that Dr. Van Keuren made to our lives and research.
References
- Antoniou M, deBoer E, Habets G, Grosveld F. The human beta-globin gene contains multiple regulatory regions: identification of one promoter and two downstream enhancers. EMBO J. 988;7(2):377–384. doi: 10.1002/j.1460-2075.1988.tb02824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss SP, Miller A, Navratil AM, Xie J, McDonough SP, Fisher PJ, Landreth GE, Roberson MS. ERK signaling in the pituitary is required for female but not male fertility. Mol Endocrinol. 2009;23:1092–1101. doi: 10.1210/me.2009-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmeier ML, Gordon DF, Dowding JM, Saunders TL, Kendall SK, Sarapura VD, Wood WM, Ridgway EC, Camper SA. Cell-specific expression of the mouse glycoprotein hormone alpha-subunit gene requires multiple interacting DNA elements in transgenic mice and cultured cells. Mol Endocrinol. 1998;12:622–633. doi: 10.1210/mend.12.5.0103. [DOI] [PubMed] [Google Scholar]
- Budry L, Lafont C, El Yandouzi T, Chauvet N, Conejero G, Drouin J, Mollard P. Related pituitary cell lineages develop into interdigitated 3D cell networks. Proc Natl Acad Sci USA. 2011;108:12515–12520. doi: 10.1073/pnas.1105929108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows HL, Birkmeier TS, Seasholtz AF, Camper SA. Targeted ablation of cells in the pituitary primordia of transgenic mice. Mol Endocrinol. 1996;10:1467–1677. doi: 10.1210/mend.10.11.8923471. [DOI] [PubMed] [Google Scholar]
- Charles MA, Saunders TL, Wood WM, Owens K, Parlow AF, Camper SA, Ridgway EC, Gordon DF. Pituitary-specific Gata2 knockout: effects on gonadotrope and thyrotrope function. Mol Endocrinol. 2006;20:1366–1377. doi: 10.1210/me.2005-0378. [DOI] [PubMed] [Google Scholar]
- Childs GV. Multipotential pituitary cells that contain ACTH and other pituitary hormones. Trends Endocrinol Metab. 1991;2:112–117. doi: 10.1016/s1043-2760(05)80007-4. [DOI] [PubMed] [Google Scholar]
- Cushman LJ, Burrows HL, Seasholtz AF, Lewandoski M, Muzyczka N, Camper SA. Cre-mediated recombination in the pituitary gland. Genesis. 2000;28:167–174. doi: 10.1002/1526-968x(200011/12)28:3/4<167::aid-gene120>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Davis SW, Mortensen AH, Camper SA. Birthdating studies reshape models for pituitary gland cell specification. Dev Biol. 2011;352:215–227. doi: 10.1016/j.ydbio.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich G, Soriano P. Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev. 1991;5:1513–1523. doi: 10.1101/gad.5.9.1513. [DOI] [PubMed] [Google Scholar]
- Japon MA, Rubinstein M, Low MJ. In situ hybridization analysis of anterior pituitary hormone gene expression during fetal mouse development. J Histochem Cytochem. 1994;42:1117–1125. doi: 10.1177/42.8.8027530. [DOI] [PubMed] [Google Scholar]
- Kendall SK, Gordon DF, Birkmeier TS, Petrey D, Sarapura VD, O'Shea KS, Wood WM, Lloyd RV, Ridgway EC, Camper SA. Enhancer-mediated high level expression of mouse pituitary glycoprotein hormone alpha-subunit transgene in thyrotropes, gonadotropes, and developing pituitary gland. Mol Endocrinol. 1994;8:1420–1433. doi: 10.1210/mend.8.10.7531821. [DOI] [PubMed] [Google Scholar]
- Kendall SK, Saunders TL, Jin L, Lloyd RV, Glode LM, Nett TM, Keri RA, Nilson JH, Camper SA. Targeted ablation of pituitary gonadotropes in transgenic mice. Mol Endocrinol. 1991;5:2025–2036. doi: 10.1210/mend-5-12-2025. [DOI] [PubMed] [Google Scholar]
- Lee EC, Yu D, Martinez de Velasco J, Tessarollo L, Swing DA, Court DL, Jenkins NA, Copeland NG. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics. 2001;73:56–65. doi: 10.1006/geno.2000.6451. [DOI] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcil A, Dumontier E, Chamberland M, Camper SA, Drouin J. Pitx1 and Pitx2 are required for development of hindlimb buds. Development. 2003;130:45–55. doi: 10.1242/dev.00192. [DOI] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- Saeger W, Ludecke DK, Buchfelder M, Fahlbusch R, Quabbe HJ, Petersenn S. Pathohistological classification of pituitary tumors: 10 years of experience with the German Pituitary Tumor Registry. Eur J Endocrinol. 2007;156:203–216. doi: 10.1530/eje.1.02326. [DOI] [PubMed] [Google Scholar]
- Seuntjens E, Hauspie A, Vankelecom H, Denef C. Ontogeny of plurihormonal cells in the anterior pituitary of the mouse, as studied by means of hormone mRNA detection in single cells. J Neuroendocrinol. 2002;14:611–619. doi: 10.1046/j.1365-2826.2002.00808.x. [DOI] [PubMed] [Google Scholar]
- Shimshek DR, Kim J, Hubner MR, Spergel DJ, Buchholz F, Casanova E, Stewart AF, Seeburg PH, Sprengel R. Codon-improved Cre recombinase (iCre) expression in the mouse. Genesis. 2002;32:19–26. doi: 10.1002/gene.10023. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Uhlenhaut NH, Jakob S, Anlag K, Eisenberger T, Sekido R, Kress J, Treier AC, Klugmann C, Klasen C, Holter NI, Riethmacher D, Schutz G, Cooney AJ, Lovell-Badge R, Treier M. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell. 2009;139:1130–1142. doi: 10.1016/j.cell.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Van Keuren ML, Gavrilina GB, Filipiak WE, Zeidler MG, Saunders TL. Generating transgenic mice from bacterial artificial chromosomes: transgenesis efficiency, integration and expression outcomes. Transgenic Res. 2009;18:769–785. doi: 10.1007/s11248-009-9271-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrowicz BP, Imamoto A, Fiering S, Herzenberg LA, Kerr WG, Soriano P. Disruption of overlapping transcripts in the ROSA beta geo 26 gene trap strain leads to widespread expression of beta-galactosidase in mouse embryos and hematopoietic cells. Proc Natl Acad Sci USA. 1997;94:3789–3794. doi: 10.1073/pnas.94.8.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C, Kouprina N, Zhu B, Cairo A, Hoek M, Cross G, Osoegawa K, Larionov V, de Jong P. Large-insert BAC/YAC libraries for selective re-isolation of genomic regions by homologous recombination in yeast. Genomics. 2001;77:27–34. doi: 10.1006/geno.2001.6616. [DOI] [PubMed] [Google Scholar]
- Zhu X, Rosenfeld MG. Transcriptional control of precursor proliferation in the early phases of pituitary development. Curr Opin Genet Dev. 2004;14:567–574. doi: 10.1016/j.gde.2004.08.006. [DOI] [PubMed] [Google Scholar]