Abstract
Background
Glutamate (GLUT) in the lateral hypothalamus (LH) has been suggested to mediate reward behaviors and may promote the ingestion of drugs of abuse. The current study tested the hypothesis that GLUT in the LH stimulates consumption of ethanol and that this effect occurs, in part, via its interaction with local peptides, hypocretin/orexin (OX) and melanin-concentrating hormone (MCH).
Methods
In Experiments 1 and 2, male Sprague-Dawley rats, after being trained to drink 9% ethanol, were microinjected in the LH with N-methyl-D-aspartate (NMDA) or its antagonist, D-AP5, or with alpha-amino-5-methyl-3-hydroxy-4-isoxazole propionic acid (AMPA) or its antagonist, CNQX-ds. Consumption of ethanol, chow, and water was then measured. To provide an anatomical control, a separate set of rats was injected 2 mm dorsal to the LH. In Experiment 3, the effect of LH injection of NMDA and AMPA on the expression of OX and MCH was measured using radiolabeled in situ hybridization (ISH) and also digoxigenin-labeled ISH, to distinguish effects on OX and MCH cells in the LH and the nearby perifornical area (PF) and zona incerta (ZI).
Results
When injected into the LH, NMDA and AMPA both significantly increased ethanol intake while having no effect on chow or water intake. The GLUT receptor antagonists had the opposite effect, significantly reducing ethanol consumption. No effects were observed with injections 2 mm dorsal to the LH. In addition to these behavioral effects, LH injection of NMDA significantly stimulated expression of OX in both the LH and PF while reducing MCH in the ZI, whereas AMPA increased OX only in the LH and had no effect on MCH.
Conclusions
Glutamatergic inputs to the LH, acting through NMDA and AMPA receptors, appear to have a stimulatory effect on ethanol consumption, mediated in part by increased OX in LH and PF and reduced MCH in ZI.
Keywords: Glutamate, Lateral hypothalamus, Ethanol, Orexin, Melanin-concentrating hormone
The neurotransmitter glutamate (GLUT), which exerts its fast actions by binding to the ionotropic receptors N-methyl-D-aspartate (NMDA), alpha-amino-5-methyl-3-hydroxy-4-isoxazole propionic acid (AMPA), and kainate (KA) (Monaghan et al., 1989), has been widely studied in mesolimbic brain regions, where GLUT mediates consummatory and reward-related behaviors. These regions include the nucleus accumbens (NAc), where stimulation of the glutamatergic pathway increases cue-evoked sucrose intake (Stuber et al., 2011). They also include the ventral tegmental area (VTA), where blockade of GLUT receptors decreases drug self-administration (Kenny et al., 2009; You et al., 2007). Together with evidence showing relapse to drug use to be attenuated by intra-VTA infusions of a GLUT antagonist (Sun et al., 2005), the ionotropic GLUT receptors in this region appear to be crucial not only in reinforcement but also in the reinstatement of drug intake.
A third area implicated in GLUT regulation is the lateral hypothalamus (LH), together with the perifornical area (PF) and the zona incerta (ZI) dorsal to the LH. The LH/PF area, which interacts closely with the NAc and VTA, is important in mediating reward (Aston-Jones et al., 2010; Millan et al., 2010). This may be achieved through GLUT transmission, which has extensive afferents throughout the LH/PF and ZI where it also exists in local interneurons (Gao & van den Pol, 2001; van den Pol & Trombley, 1993). The possibility that GLUT in the LH/PF mediates consummatory behavior is supported by feeding studies, showing local injection of GLUT agonists NMDA and AMPA to elicit feeding (Stanley et al., 2011). Whereas there is indirect evidence for the involvement of LH/PF GLUT in drug addiction (Hamlin et al., 2008; Levy et al., 2007), it remains unclear whether GLUT inputs to the area control the consumption of drugs such as alcohol. This is possible since neural mechanisms underlying alcohol intake are similar to those controlling food intake (Leibowitz, 2007).
If GLUT in the LH is involved in controlling alcohol consumption, a neurochemical mechanism through which it acts may include the peptide, hypocretin/orexin (OX), which is expressed in the LH and nearby PF and controls feeding and arousal (de Lecea et al., 1998; Sakurai et al., 1998). This peptide also mediates motivated behaviors such as intake of drugs of abuse (Aston-Jones et al., 2010), including alcohol, as both ethanol exposure and an ethanol-paired cue stimulate LH OX expression (Dayas et al., 2008; Morganstern et al., 2010a) and local injection of OX enhances ethanol intake (Schneider et al., 2007). While evidence has shown OX neurons to express both NMDA and AMPA receptors (Li et al., 2002), it remains to be tested whether GLUT in the LH has effects on OX that, in turn, may influence ethanol intake.
In addition to OX, GLUT in the LH may affect neurons expressing the melanin-concentrating hormone (MCH), which also contain GLUT receptors and are expressed in the LH as well as the ZI (Skofitsch et al., 1985; van den Pol et al., 2004). Being an inhibitory peptide, MCH acts differently from OX in many respects. For example, MCH plays an opposite role from OX in regulating arousal state and sleep cycle (Hassani et al., 2009; Willie et al., 2008), and the administration of MCH into the LH suppresses ethanol drinking (Morganstern et al., 2010b). Thus, if GLUT in the LH influences ethanol intake, it may have differential effects on MCH as compared to OX.
The current study using brain-cannulated rats investigated the GLUT system in the LH/PF in terms of its impact on the consumption of ethanol and also on local peptide-expressing neurons. In Experiments 1 and 2, Sprague-Dawley rats were trained to voluntarily drink 9% ethanol, and the effects of NMDA and AMPA agonists or antagonists in the LH on ethanol, food, and water consumption were examined. To investigate the mechanism underlying the GLUT-induced changes in ethanol drinking, Experiment 3 tested whether LH injections of GLUT agonists could influence local expression of OX and MCH as measured by in situ hybridization.
METHODS
Subjects
Male Sprague-Dawley rats (225 to 250 g) were obtained from Taconic Farms (Germantown, NY). Rats were individually housed in hanging wire cages (Experiments 1 and 2) or plastic shoebox cages (Experiment 3) and maintained on a 12:12-hour reversed light–dark cycle (lights off from 6:00 am). All experiments were conducted during the dark phase. Subjects had ad libitum access to LabDiet rodent chow (St Louis, MO) and water. All animals were allowed 1 week to acclimate to the facility and reversed light-dark cycle before experiments began, which permitted the animals’ behavior and peptide expression to stabilize. Overall, 101 rats were included in the analysis. All procedures were approved by the Princeton University Institutional Animal Care and Use Committee and The Rockefeller University Animal Care Committee, and conformed to the National Institutes of Health guidelines on the ethical use of animals.
Ethanol Training
Subjects were acclimated to unsweetened ethanol by using a variant of a 2-bottle choice procedure (Martinetti et al., 2000). To encourage animals to drink and adapt to the unsweetened ethanol, the concentration of ethanol was gradually increased every 4 days, from 1, 2, 4, 7, to 9% (v/v). Animals had ad libitum access to ethanol solutions in addition to food and water. Tests started after the subjects had at least 10 days of access to 9% ethanol.
Surgery
Subjects were anesthetized using ketamine (80 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.), supplemented with ketamine when necessary. Stainless steel 21-gauge guide shafts (10 mm in length) were implanted bilaterally in the LH (Experiments 1 and 2: A −2.9, L ± 1.9, V 3.9; Experiment 3: A −2.9, L ± 1.9, V 3.5), with reference to bregma, the midsaggital sinus, and the level skull surface. In Experiment 3, the cannulas were implanted dorsal to the target region to avoid tissue damage, which may interfere with analysis of peptide expression. Subjects had 1 week to recover before testing. Stainless steel stylets were left in the guide shafts between injections to prevent occlusion.
Microinjection Procedures
Drugs were delivered through 26-gauge stainless steel microinjectors with fused-silica tubing inside (74 μm ID, 154 μm OD, Polymicro Technologies, Phoenix AZ) that reached the region of interest (Experiments 1 and 2: V 8.4; Experiment 3: V 8.0). Doses were chosen based on the feeding literature (Hettes et al., 2010; Stanley et al., 1996; Stanley et al., 1993b) and on pilot tests. For Experiment 3, the lower dose of NMDA was used to avoid confounding variables, such as hyperactivity, that may occur in some rats. The 4 drugs and doses used were as follows: (i) N-methyl-D-aspartic acid (NMDA, 5.6 nmol, 2.8 nmol/side); (ii) D,L-aamino-3-hydroxy-5-methyl-isoxazole propionic acid (AMPA, 2.1 nmol, 1.1 nmol/side); (iii) NMDA-receptor antagonist, D-AP5 (33.3 nmol, 16.7 nmol/side); and (iv) AMPA-receptor antagonist, CNQX disodium salt hydrate (CNQX-ds, 15.0 nmol, 7.5 nmol/side). While AMPA and CNQX-ds may act on KA receptors in addition to AMPA receptors (Hettes et al., 2010), the latter predominate in the LH (Eyigor et al., 2001; van den Pol et al., 1994), which leads us to focus on AMPA receptors in the present study. All drugs were purchased from Sigma-Aldrich Co. (St Louis, MO) and dissolved in preservative-free 0.9% NaCl solution (Hospira Inc., Lake Forest, IL) immediately prior to microinjection. For behavioral studies (Experiments 1 and 2), injections were counterbalanced, so each animal received vehicle or drug in counterbalanced order on 2 consecutive days. To minimize stress, animals were handled on an almost daily basis throughout their ethanol training. All injections were given 3 hours into the dark cycle, except for D-AP5 injections, which were given at the onset of the dark period as recommended in a previous study (Stanley et al., 1996). A syringe pump delivered 0.5 μl during 47 seconds with microinjectors left in place for an additional 47 seconds to allow for diffusion. The intake of ethanol, food, and water was measured 1 h, 2 h, and 4 h after injection, by manually weighing the ethanol and water bottles, which had non-drip sipper tubes, and the food hoppers. Ethanol consumption is expressed as g/kg, which was obtained using the following formula: . Tests of each dose included a counterbalanced control injection of saline vehicle. In Experiments 1 and 2, 28 rats received 4 injections, while the others received 2 injections. In Experiment 3, all rats received only 1 injection. For the anatomical control injections, the most effective behavioral dose of each drug was tested.
Ethanol Preference Ratio
The ethanol preference ratio was determined using the following formula: . The average ethanol preference ratio of the vehicle groups during 4 h was 0.33 ± 0.02, which was relatively constant across the sets of injections and consistent with previous studies using Sprague-Dawley rats (Karatayev et al., 2010; Tordoff et al., 2008).
Radiolabeled In Situ Hybridization Histochemistry
In Experiment 3, the mRNA expression of OX and MCH was measured by radiolabeled in situ hybridization (ISH) histochemistry in animals receiving either vehicle, NMDA, or AMPA immediately dorsal to the LH (n = 5–6/group). The animals were sacrificed by rapid decapitation 30 min post-injection. This time period was chosen based on the finding here that glutamatergic agonists stimulated consumption during the first 1 h, post-injection period and on published evidence showing alterations in peptide expression within 30 min after manipulation (Ida et al., 2000). The brains were immediately removed and fixed in 4% paraformaldehyde in phosphate buffer (PB) (0.1 M pH 7.2) for 72 h, cryoprotected in 25% sucrose for 72 h, and then frozen and stored at −80°C. The antisense and sense OX RNA probes were generously donated by Dr. Luis de Lecea, and the MCH RNA probes were donated by Dr. Nicholas A. Tritos. These probes were then labeled with 35S-UTP (Perkin Elmer, Waltham, MA) as previously described (Sakamaki et al., 2005). Free-floating 30 μm coronal sections were processed as follows: 10 min in 0.001% proteinase K, 5 min in 4% paraformaldehyde, and 10 min each in 0.2 N HCl and acetylation solution, with a 10 min wash in PB between each step. After washing, the sections were hybridized with 35S-labeled probe (103 cpm/ml) at 55°C for 18 h. Following hybridization, the sections were washed in 5×SSC, followed by RNase (Sigma, St. Louis, MO) treatment for 30 min at 37°C. Then, sections were run through a series of stringency washes with 0.1 M dithiothreitol (Sigma, St. Louis, MO) in 2×SSC and 1×SSC and 0.1×SSC at 55°C. Finally, sections were mounted, air-dried and exposed to Kodak BioMax MR film for 20 h at −80°C, developed and microscopically analyzed. Gene expression was determined with a computer-assisted microdensitometry of autoradiographic images on the MCID image analysis system (Image Research, Inc., St. Catherines, Canada) as described (Reagan et al., 2004). Microscale 14C standards (Amersham Biosciences, Piscataway, NJ) were exposed on the same Kodak film with the sections and digitized. Gray level/optical density calibrations were performed using a calibrated film strip ladder (Imaging Research, St. Catherines, ON, Canada) for optical density. Optical density was plotted as a function of microscale calibration values. It was determined that all subsequent optical density values of digitized autoradiographic images fell within the linear range of the function. The values obtained represent the average of measurements taken from 10 to 12 sections per animal from Bregma −2.8 to −3.1 mm. The background optical density from a same size area in the thalamus was subtracted from these measurements and the mean density for all the groups was reported as percentage of the vehicle group.
Digoxigenin-Labeled In Situ Hybridization Histochemistry
In Experiment 3, the mRNA expression of OX and MCH was also measured with digoxigenin (DIG)-labeled antisense RNA probes and 30-μm free-floating cryostat sections were used for ISH histochemistry as described previously (Chang et al., 2008). AP-conjugated sheep anti-digoxigenin Fab fragments (1:1000, Roche, Nutley, NJ) and NBT/BCIP (Roche, Nutley, NJ) were used to visualize the signal. Gene expression was measured by semi-quantification with Image-Pro Plus software, version 4.5 (Media Cybernetics, Inc., Silver Spring, MD, USA) and was expressed as number of cells/mm2, reflecting regional density of mRNA-containing cells. In all analyses, the cell number was counted only on one plane in each section, and only those cells containing a nucleus in the plane (>10 μm2) were counted, thereby excluding fractions of cells. This analysis with the DIG-labeled probe allows for more anatomically precise measurements to be made, thus permitting us to analyze separately the sub-populations of OX and MCH neurons in the PF, LH and ZI. The values obtained represent an average of measurements taken from 10 to 12 sections per animal from Bregma −2.8 to −3.1 mm. OX cells lateral to the fornix were considered to be in the LH, and all OX neurons located dorsal and 0.4 mm medial to the fornix were considered to be in the PF. MCH cells lateral to the fornix were considered to be in the LH, while all MCH cells dorsal to the fornix were considered to be in the ZI. The analysis of average cell density was performed by an observer blind to the treatment groups.
Histology and Data Analysis
To verify the sites of microinjections, rats receiving microinjections in Experiments 1 and 2 were sacrificed by rapid decapitation. Their brains were sliced as 40 μm coronal sections and examined microscopically. Rats in Experiment 3 had their cannulation sites examined visually as their brains were cut in 30 μm coronal sections for in situ hybridization, as well as during microscopic analysis. As the injections were made in the LH, a large region that can be clearly identified by anatomical landmarks such as the fornix and optic tract, this microscopic examination permitted us to identify the injection sites. A few animals (1–2 rats/group) with probes 0.5 mm or farther from the LH were included in the corresponding anatomical control group or discarded from the analysis. Data from animals consuming little or no ethanol on measurement days (less than 0.2 g/kg during 4 hours) were also excluded (N = 8; 1–2 rats/group).
In Experiments 1 and 2, ethanol, food, and water intake was analyzed for each dose of drug using a two-way repeated measures analysis of variance (ANOVA), with treatment and time as within-subject factors. Pairwise 2-tailed t-tests were used to analyze differences at specific time points (1 h, 2 h, and 4 h), with the p value set at 0.017 after Bonferroni correction for each of the 3 pairwise comparisons. For Experiment 3, changes in peptide expression were analyzed using a one-way ANOVA. Values of p < 0.05 were considered significant for the main effect of ANOVA. In the figures, data are expressed as mean ± standard error of the mean and the cumulative intake at 1 h, 2 h, 4 h is presented. The vehicle intake presented in the figures is the average between the 2 sets of injections, as intake after vehicle, although slightly different in each test, did not significantly differ within each group.
RESULTS
Experiment 1: Effects of NMDA and its antagonist on ethanol intake
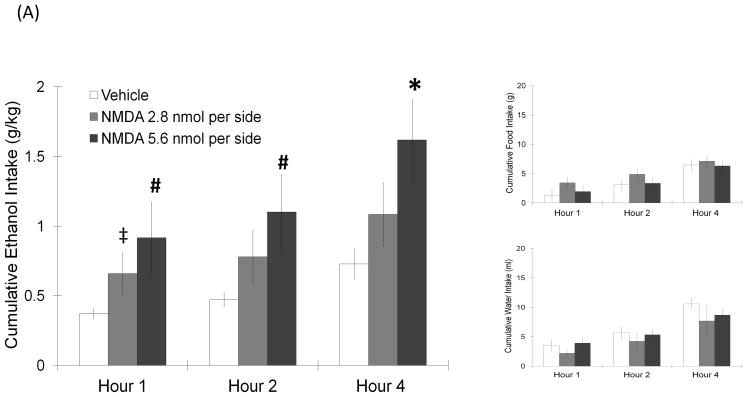
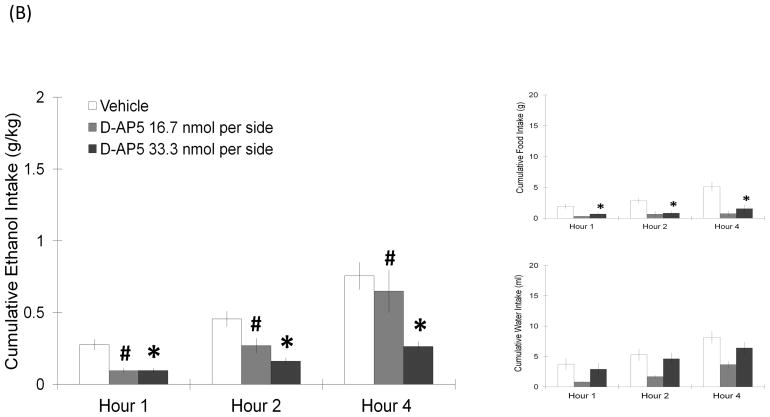
The cannula placements for the injections, which were aimed at the LH, are illustrated in Fig. 1. Rats were injected with NMDA or its antagonist D-AP5, and measurements were taken of ethanol, food, and water consumption. As revealed by two-way repeated measures ANOVA, NMDA injected into the LH significantly increased ethanol intake compared to vehicle (Fig. 2A). At the dose of 5.6 nmol, NMDA significantly stimulated ethanol consumption (F(1,7) = 9.02, p < 0.05), and this effect was observed throughout the full 4 h of testing, when ethanol intake was elevated by 138–235% (4 h: t = 3.7, df = 7, p < 0.017). It also significantly increased ethanol preference (F(1,7) = 14.99, p < 0.05). The lower dose of 2.8 nmol had a similar but smaller effect on ethanol intake (F(1,5) = 7.39, p < 0.05), stimulating ethanol consumption by 69% at 1 h (t = 3.4, df = 5, p = 0.018), although it did not change ethanol preference (F(1,5) = 0.92, p = 0.38). At both doses of NMDA, this stimulatory effect was observed only in the case of ethanol consumption, with food and water intake not affected at any time period. The antagonist of NMDA had the opposite effect, causing a decrease in ethanol drinking (Fig. 2B). Injection of D-AP5 at the dose of 33.3 nmol significantly reduced ethanol intake (F(1,9) = 21.57, p < 0.05), suppresing ethanol drinking by 64% at the 1 h time-point (t = 3.4, df = 9, p < 0.017) and continuing to suppress it over the 4 h post-injection period (t = 4.7, df = 9, p < 0.017). This dose also significantly suppressed ethanol preference (F(1,9) = 6.40, p < 0.05). While it also reduced food intake (F(1,9) = 17.76, p < 0.05), D-AP5 had no effect on intake of water. The lower dose of 16.7 nmol produced a similar reduction of ethanol intake (F(1,5) = 8.12, p < 0.05), without significantly affecting ethanol preference, or consumption of food (F(1,5) = 3.74, p = 0.11) or water (F(1,5) = 4.84, p = 0.08) (Fig. 2B). At the control site 2 mm dorsal to the LH, neither NMDA nor D-AP5 produced any significant change in the intake of ethanol, food, or water (Table 1). These results show that glutamate acts tonically through the NMDA receptor in the LH to stimulate ethanol intake in ethanol-drinking rats.
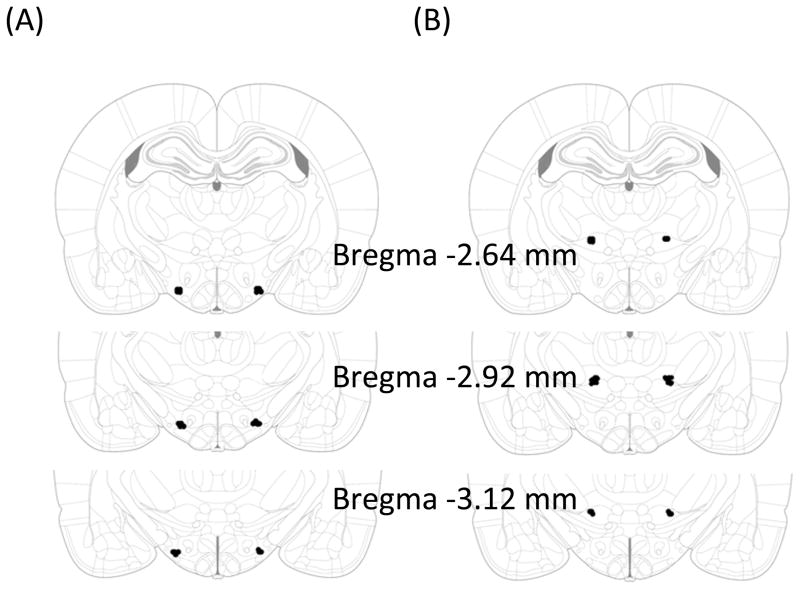
FIGURE 1.
Injection sites for animals included in the analysis. (A) Sites for LH injections, as indicated by black dots. (B) Sites for anatomical control injections. Adapted from The Rat Brain, compact 6th edition, G. Paxinos and C. Watson, Copyright 2007.
FIGURE 2.
(A) NMDA injected in the LH significantly increased ethanol intake but left food and water intake unaffected (5.5 nmol: n = 8/group; 2.8 nmol: n = 6/group). Values are mean ± S.E.M., *p < 0.017, ‡p = 0.018, #p < 0.05, vs. saline injection at specific time point. (B) NMDA receptor antagonist D-AP5 in the LH dose-dependently decreased ethanol consumption (33.3 nmol: n = 10/group; 16.7 nmol: n = 6/group). Values are mean ± S.E.M., *p < 0.017, #p < 0.05 vs. saline injection at specific time point.
Table 1.
The effects of NMDA, AMPA, D-AP5, and CNQX-ds on ethanol were region-specific.
| Nutrient | Hour 1 | Hour 2 | Hour 4 | |
|---|---|---|---|---|
| Ethanol (g/kg) | ||||
| Vehicle | 0.33 ± 0.05 | 0.52 ± 0.07 | 0.69 ± 0.10 | |
| NMDA 5.6 nmol | 0.38 ± 0.05 | 0.55 ± 0.06 | 0.74 ± 0.08 | |
| Food (g) | ||||
| Vehicle | 1.55 ± 0.36 | 2.95 ± 0.56 | 5.99 ± 0.82 | |
| NMDA 5.6 nmol | 1.32 ± 0.63 | 2.42 ± 0.75 | 4.96 ± 1.11 | |
| Water (ml) | ||||
| Vehicle | 3.71 ± 0.45 | 5.31 ± 0.47 | 9.01 ± 0.77 | |
| NMDA 5.6 nmol | 3.54 ± 0.45 | 5.40 ± 0.75 | 8.51 ± 1.15 | |
|
| ||||
| Ethanol (g/kg) | ||||
| Vehicle | 0.31 ± 0.08 | 0.50 ± 0.12 | 0.64 ± 0.17 | |
| AMPA 2.1 nmol | 0.42 ± 0.10 | 0.58 ± 0.14 | 0.74 ± 0.20 | |
| Food (g) | ||||
| Vehicle | 1.47 ± 0.40 | 2.67 ± 0.60 | 5.77 ± 0.80 | |
| AMPA 2.1 nmol | 0.70 ± 0.35 | 1.77 ± 0.67 | 4.27 ± 0.99 | |
| Water (ml) | ||||
| Vehicle | 3.89 ± 0.49 | 5.41 ± 0.49 | 9.28 ± 0.93 | |
| AMPA 2.1 nmol | 3.26 ± 0.47 | 4.70 ± 0.58 | 7.74 ± 1.26 | |
|
| ||||
| Ethanol (g/kg) | ||||
| Vehicle | 0.52 ± 0.14 | 0.76 ± 0.18 | 1.53 ± 0.36 | |
| D-AP5 33.3 nmol | 0.51 ± 0.10 | 0.84 ± 0.18 | 1.38 ± 0.31 | |
| Food (g) | ||||
| Vehicle | 2.46 ± 0.63 | 4.88 ± 0.68 | 8.98 ± 0.89 | |
| D-AP5 33.3 nmol | 3.18 ± 0.21 | 6.43 ± 0.56 | 11.7 ± 2.02 | |
| Water (ml) | ||||
| Vehicle | 5.40 ± 0.99 | 7.76 ± 1.47 | 12.73 ± 2.35 | |
| D-AP5 33.3 nmol | 5.05 ± 0.46 | 7.56 ± 1.55 | 12.11 ± 2.56 | |
|
| ||||
| Ethanol (g/kg) | ||||
| Vehicle | 0.37 ± 0.09 | 0.63 ± 0.12 | 0.97 ± 0.18 | |
| CNQX-ds 15.0 nmol | 0.48 ± 0.12 | 0.69 ± 0.16 | 1.17 ± 0.28 | |
| Food (g) | ||||
| Vehicle | 1.86 ± 0.58 | 5.08 ± 0.78 | 9.65 ± 0.84 | |
| CNQX-ds 15.0 nmol | 2.05 ± 0.73 | 4.56 ± 0.84 | 8.38 ± 1.20 | |
| Water (ml) | ||||
| Vehicle | 4.40 ± 0.16 | 7.63 ± 1.21 | 12.91 ± 1.57 | |
| CNQX-ds 15.0 nmol | 5.23 ± 0.88 | 7.85 ± 0.60 | 12.20 ± 0.94 | |
For NMDA injection, n = 10/group. For AMPA injection, n = 9/group. For D-AP5 and CNQX-ds injections, n = 6/group.
Experiment 2: Effect of AMPA and its antagonist on ethanol intake
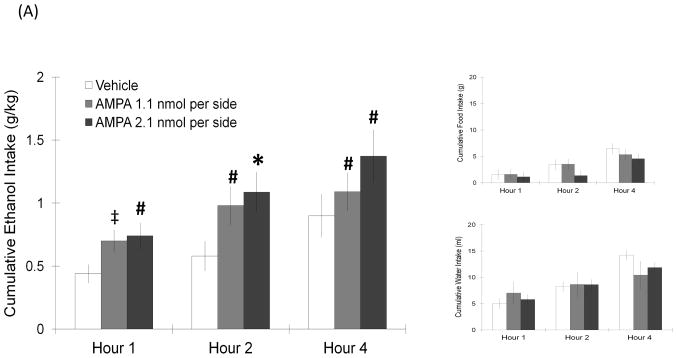
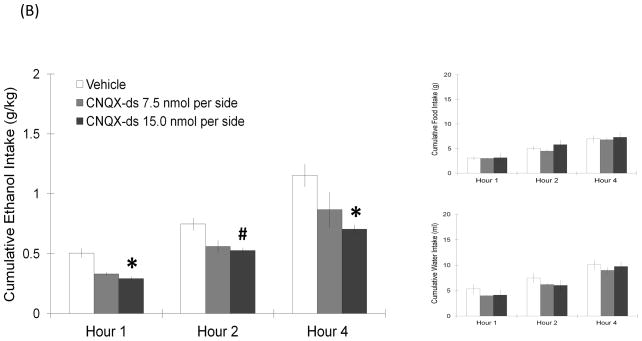
Rats were injected with AMPA or its antagonist CNQX-ds, and measurements were taken of ethanol, food, and water consumption. Injection of AMPA, when compared to vehicle, significantly increased 9% ethanol intake but not food or water consumption (Fig. 3A). A two-way repeated measures ANOVA revealed that, at the higher dose of 2.1 nmol, AMPA significantly increased ethanol drinking (F(1,7) = 8.99, p < 0.05) at 2 h post-injection (t = 2.8, df = 7, p < 0.017) as compared to vehicle. This dose of AMPA also significantly increased the preference for ethanol (F(1,7) = 6.09, p < 0.05). A similar effect was observed in rats receiving the lower, 1.1 nmol dose, which caused ethanol intake to significantly increase (F(1,9) = 8.42, p < 0.05) by 90% at 1 h post-injection (t = 2.9, df = 9, p = 0.019), while not significantly changing ethanol preference. This stimulatory effect was not observed for food or water intake, which remained unchanged at both doses. The opposite effect was observed with injection of the AMPA antagonist, CNQX-ds, which suppressed ethanol intake (Fig. 3B). At the dose of 15.0 nmol, CNQX-ds in the LH significantly reduced ethanol intake (F(1,12) = 10.30, p < 0.05) at 4 h post-injection (t = 3.1, df = 12, p < 0.017). It also marginally reduced preference for ethanol (F(1,12) = 4.33, p = 0.06), while having little effect on food and water consumption at any time point. This suppresion was not observed, however, at the lower dose of 7.5 nmol. At the anatomical control site 2 mm dorsal to the LH, neither AMPA nor CNQX-ds produced any change in the intake of ethanol, food, or water (Table 1). Overall, these results demonstrate that the AMPA receptor is similar to the NMDA receptor in having a stimulatory effect on ethanol drinking.
FIGURE 3.
(A) AMPA in the LH produced a dose-dependent stimulatory effect on ethanol drinking similar to NMDA, without affecting food or water consumption (2.1 nmol: n = 8/group; 1.1 nmol: n = 10/group). Values are mean ± S.E.M., *p < 0.017, ‡p = 0.019, #p < 0.05 vs. saline injection at specific time point. (B) AMPA receptor antagonist CNQX-ds significantly suppressed ethanol intake, while having no effect on food and water intake (15.0 nmol: n = 13/group; 7.5 nmol: n = 13/group). Values are mean ± S.E.M., *p < 0.017, #p < 0.05 vs. saline injection at specific time point.
Experiment 3: Effect of glutamate in the LH on expression of OX and MCH
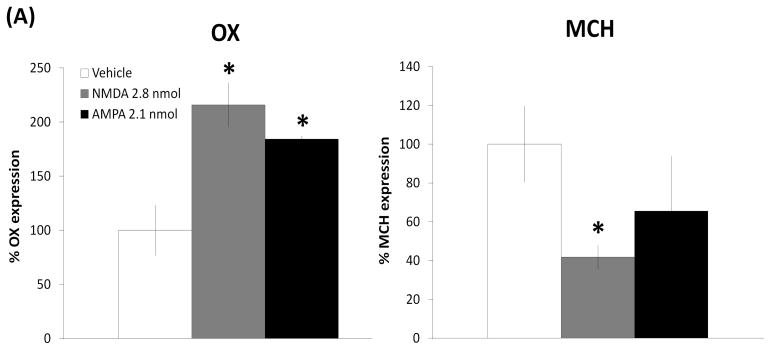
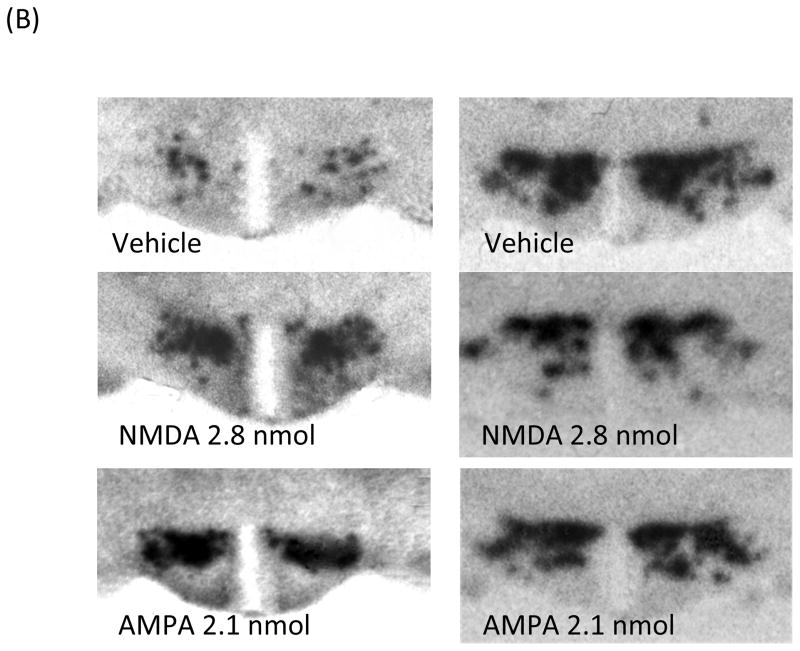
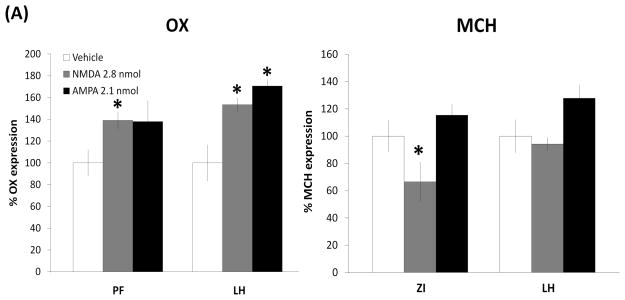
To explore the effect of glutamatergic agonists on local neuropeptides at a dose that stimulates ethanol drinking, the gene expression of OX and MCH in the LH was measured using ISH in animals (n = 5–6/group) given injections of either vehicle, NMDA (2.8 nmol/side), or AMPA (2.1 nmol/side) just dorsal to the LH. The first measurements, performed using a more quantitative radiolabeled probe that does not permit one to clearly distinguish the subregions where the peptides are expressed, are described here for OX in the area of the LH and PF and for MCH in the area of the LH and ZI. As shown in Fig. 4A and illustrated in the photomicrographs of Fig. 4B, NMDA as compared to vehicle produced a significant, 116% increase in OX expression (F(1,11) = 5.95, p < 0.05) while causing a strong suppression of MCH expression (F(1,10) = 7.19, p < 0.05), and AMPA had a similar stimulatory effect on OX expression (F(1,11) = 11.94, p < 0.01) while having no impact on MCH (F(1,11) = 1.27, p = 0.286). The second measurements were performed using a DIG-labeled probe, which permits a more precise anatomical analysis of effects on specific subpopulations of OX and MCH neurons. A one-way ANOVA showed that NMDA as compared to vehicle significantly increased OX expression in both the LH (F(1,11) = 7.66, p < 0.05) and the PF (F(1,11) = 6.81, p < 0.05) (Fig. 5A and 5B) but inhibited MCH expression specifically in the ZI (F(1,11) = 4.91, p = 0.05) and not in the LH area ventral to the ZI (F(1,11) = 0.14, p = 0.72). In contrast, AMPA significantly enhanced OX mRNA in the LH (F(1,11) = 27.41, p < 0.01) but not the PF region (F(1,11) = 1.52, p = 0.24), and it had no effect on MCH mRNA in either the ZI or LH (ZI: F(1,10) = 2.45, p = 0.152; LH: F(1,10) = 2.96, p = 0.12). These results obtained with both radiolabeled and DIG-labeled probes demonstrate that GLUT, acting through both NMDA and AMPA receptors, has a stimulatory effect on the OX system in the LH, while NMDA alone has an inhibitory effect on MCH and only in the ZI.
FIGURE 4.
(A) As measured by radiolabeled in situ hybridization, NMDA in the LH significantly increased the expression of OX while significantly suppressing MCH expression. AMPA in the LH also significantly stimulated the expression of OX while showing a tendency to reduce MCH expression (n = 5–6/group). Values are mean ± S.E.M., *p < 0.05 vs. saline injection. (B) Photomicrographs of radiolabeled in situ hybridization illustrating the stimulatory effect of NMDA and AMPA on OX expression in the LH region, as well as the inhibitory effect of NMDA on MCH expression.
Fig. 5.
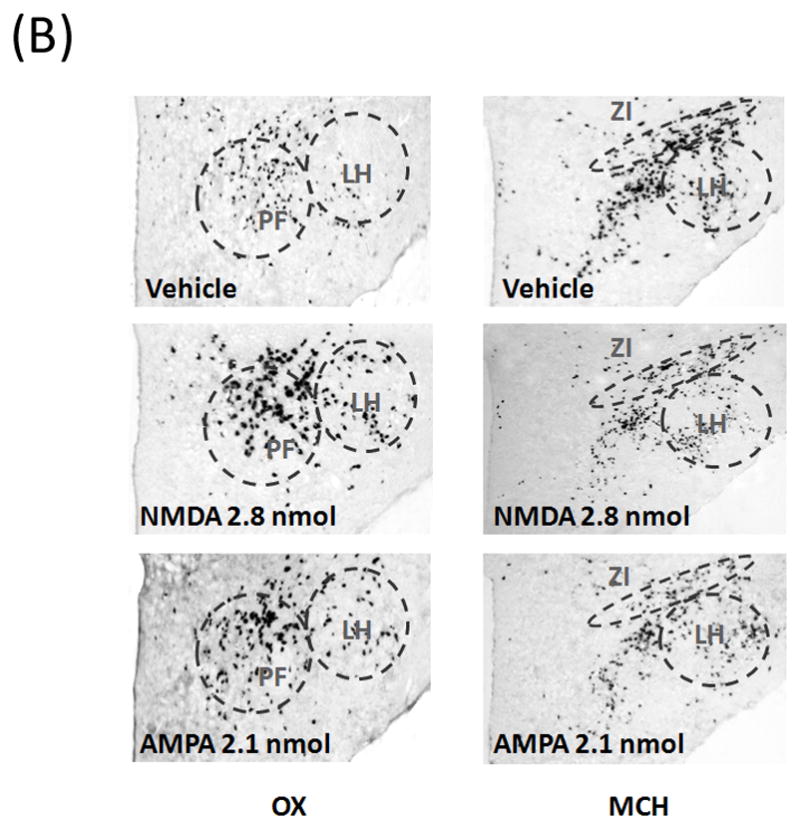
(A) The stimulatory effect of glutamate on OX expression was confirmed using in situ hybridization with a digoxigenin-labeled probe (n = 5–6/group). In the LH, NMDA significantly increased the expression of OX in both PF and LH region, while AMPA significantly stimulated the OX in the LH but not the PF. Conversely, NMDA decreased the expression of MCH in ZI region, an effect not observed in the LH, and AMPA had no effect on MCH in any area. Values are mean ± S.E.M., *p < 0.05 vs. saline injection. (B) Photomicrographs of digoxigenin-labeled in situ hybridization illustrating the stimulatory effect of NMDA and AMPA on OX expression and the inhibitory effect of NMDA on MCH expression in the ZI.
DISCUSSION
The present study assessed the role of GLUT in the LH in affecting consummatory behavior in ethanol-drinking rats and also endogenous expression of local neuropeptides, OX and MCH. We found that GLUT, acting through both NMDA and AMPA receptors, significantly stimulates the drinking of and preference for ethanol. It also increases local expression of OX in the LH/PF area, while reducing MCH expression in the ZI. These results provide evidence that, in animals trained to drink ethanol, GLUT inputs to the LH increase the consumption of ethanol rather than food or water, possibly through their impact on local orexigenic peptide systems.
Glutamatergic signaling in the hypothalamus mediates motivated behaviors
Glutamatergic transmission is important in regulating consummatory behaviors through reward-related regions, such as the NAc or VTA (Kenny et al., 2009; Maldonado-Irizarry et al., 1995). The LH, which has extensive interactions with the NAc and VTA (Aston-Jones et al., 2010; Millan et al., 2010), contains GLUT inputs and interneurons that have also been implicated in motivated behaviors, such as feeding and cocaine addiction (Levy et al., 2007; Rada et al., 2003; Stanley et al., 1993a). The present findings are in agreement with these results, showing that GLUT acts on both NMDA and AMPA receptors in the LH to mediate ethanol drinking. Whereas the NMDA antagonist D-AP5 has also been shown to suppress chow intake (Stanley et al., 1996), the results here revealed no effects of NMDA or AMPA and its antagonist on food or water intake in rats trained to consume ethanol. This indicates that the presence of ethanol overrides the role of these LH GLUT receptors in controlling feeding behavior and causes them instead to stimulate the intake of ethanol.
NMDA and AMPA increase ethanol drinking possibly by stimulating local OX
With the evidence indicating that GLUT acts in the LH to stimulate ethanol intake, we next examined the mechanism that may mediate this effect. Results from our in situ hybridization analysis showed that LH injection of NMDA and AMPA, at a dose that stimulates ethanol drinking, also increases the expression of OX in the LH. Consistent with a recent study showing AMPA to stimulate c-Fos immunoreactivity in OX neurons (Li et al., 2011), our results demonstrate that GLUT acts through both NMDA and AMPA receptors to enhance OX expression and that this effect when induced by NMDA occurs in both the LH and PF but only in the LH when induced by AMPA. The possibility that this peptide underlies the GLUT-induced ethanol drinking is consistent with evidence that OX administration in the LH facilitates ethanol intake (Schneider et al., 2007) and that the OX system in the LH, in turn, is activated by acute ethanol exposure (Morganstern et al., 2010a). The differential effects of NMDA and AMPA on the two subpopulations of OX neurons suggest that these GLUT receptors may potentiate ethanol drinking through different behavioral processes. The OX neurons in the two areas are found to have dichotomous functions, with those in the LH enhanced primarily by reward and those in the PF preferentially activated by arousal (Harris & Aston-Jones, 2006). Thus, NMDA may act in these areas to increase both reward salience and arousal state, whereas AMPA may act only in the LH to stimulate the process of reward. These differential effects of the two receptors may be related to the finding that blockade of LH AMPA receptors has no impact on feeding during the light period of the circadian cycle, whereas NMDA acts in the light as well as dark period (Hettes et al., 2010), suggesting its additional stimulatory effect on arousal. Further tests using OX antagonists could more precisely identify the mechanism and brain regions involved in the process. For example, antagonism of OX in the VTA attenuates drug-seeking behaviors (Espana et al., 2011; James et al., 2011; Mahler et al., 2012), suggesting that GLUT-stimulated ethanol drinking may occur, in part, through a stimulation of OX release in the VTA. It is important to note that these changes in OX expression were demonstrated in the dark phase, and the magnitude of changes may differ depending on the specific time of day since OX activity is found differ across the circadian cycle (Hassani et al., 2009; McGregor et al., 2011; Stutz et al., 2007). Overall, our results demonstrate that the GLUT system in the LH, via both NMDA and AMPA receptors, may facilitate ethanol drinking by enhancing OX expression.
Significance of glutamate-OX interaction in the LH
The interaction between GLUT and OX has been found in other brain regions, such as the VTA, where these neurochemical systems appear to be crucial for potentiating drug abuse (Harris et al., 2004). In the LH, the GLUT-OX interaction may be especially important in integrating the diverse functions of this region. The OX cell bodies in the LH receive multiple inputs from areas related to stress, emotion, and reward, many of which are glutamatergic in nature (Henny & Jones, 2006), suggesting that the stimulatory effect of GLUT on OX is important in transmitting information and generating an allostatic state (Boutrel et al., 2010). The reciprocal, stimulatory effect of OX on GLUT activity (Doane et al., 2007; van den Pol et al., 1998), providing positive feedback, may be involved in sustaining behavioral outputs over a long period, possibly explaining the persistent stimulatory effect of GLUT on ethanol intake observed in the present study. This GLUT-OX interaction in the LH may also have a specific function in initiating ethanol drinking. This is supported by evidence revealing a surge of LH GLUT release during the initiation of feeding (Rada et al., 2003) and is consistent with the involvement of GLUT in relapse to alcohol drinking (Knackstedt & Kalivas, 2009) and the importance of OX in reinstatement of addiction (Aston-Jones et al., 2010; Lawrence et al., 2006). The present findings elucidate the nature of the GLUT-OX interaction in the LH, indicating its crucial role not only in integrating diverse information from extra-hypothalamic regions but also in initiating and prolonging ethanol drinking behavior.
Glutamate interacts with the MCH system
Our results clearly dissociate MCH from OX in controlling ethanol intake, with both NMDA and AMPA having little effect on MCH expression in the LH. The only effect seen with measurements of MCH was an inhibitory effect of NMDA on its expression in the ZI. This change in MCH expression was demonstrated in the dark phase, and with evidence showing that MCH is especially active during sleep (Hassani et al., 2009; Stutz et al., 2007), the magnitude of a GLUT-induced alteration may differ, and further changes may be revealed, with a different time for the tests. Although the functional role of this change is not yet clear, this reduction in ZI MCH may be related to alcohol drinking behavior, with studies showing acute ethanol to suppress MCH expression in the ZI but not LH (Morganstern et al., 2010b). The ZI, in addition to the neuronally inhibitory peptide MCH, contains glutamatergic neurons that promote locomotion (Heise & Mitrofanis, 2004; Milner & Mogenson, 1988). Together, this suggests that ethanol may activate the NMDA receptor to suppress MCH neurons in the ZI and disinhibit this region, consistent with our hypothesis that NMDA facilitates ethanol drinking, in part, by promoting arousal.
Conclusions
Our results show that glutamatergic inputs to the LH elicit ethanol drinking through both NMDA and AMPA receptors and that this occurs, in part, by stimulating OX while inhibiting MCH. Whereas NMDA and AMPA receptors may affect ethanol drinking primarily by enhancing reward processing, the NMDA receptor may also mediate this behavior by facilitating arousal. This analysis of the functional role of GLUT and its interaction with local peptides underscores the importance of these neurochemical systems in the LH, and also demonstrates their potential as targets for future medications for treating alcohol abuse.
Acknowledgments
This research was supported by USPHS Grant AA12882 and the E. H. Lane Foundation. We thank Dr. de Lecea (Stanford University) and Dr. Nicholas A. Tritos (Joslin Diabetes Center, Harvard University) for kindly providing the RNA probes used in this study.
References
- Aston-Jones G, Smith RJ, Sartor GC, Moorman DE, Massi L, Tahsili-Fahadan P, Richardson KA. Lateral hypothalamic orexin/hypocretin neurons: A role in reward-seeking and addiction. Brain Res. 2010;1314:74–90. doi: 10.1016/j.brainres.2009.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutrel B, Cannella N, de Lecea L. The role of hypocretin in driving arousal and goal-oriented behaviors. Brain Res. 2010;1314:103–111. doi: 10.1016/j.brainres.2009.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Gaysinskaya V, Karatayev O, Leibowitz SF. Maternal high-fat diet and fetal programming: increased proliferation of hypothalamic peptide-producing neurons that increase risk for overeating and obesity. J Neurosci. 2008;28:12107–12119. doi: 10.1523/JNEUROSCI.2642-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayas CV, McGranahan TM, Martin-Fardon R, Weiss F. Stimuli linked to ethanol availability activate hypothalamic CART and orexin neurons in a reinstatement model of relapse. Biol Psychiatry. 2008;63:152–157. doi: 10.1016/j.biopsych.2007.02.002. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doane DF, Lawson MA, Meade JR, Kotz CM, Beverly JL. Orexin-induced feeding requires NMDA receptor activation in the perifornical region of the lateral hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1022–1026. doi: 10.1152/ajpregu.00282.2007. [DOI] [PubMed] [Google Scholar]
- Espana RA, Melchior JR, Roberts DC, Jones SR. Hypocretin 1/orexin A in the ventral tegmental area enhances dopamine responses to cocaine and promotes cocaine self-administration. Psychopharmacology (Berl) 2011;214:415–426. doi: 10.1007/s00213-010-2048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyigor O, Centers A, Jennes L. Distribution of ionotropic glutamate receptor subunit mRNAs in the rat hypothalamus. J Comp Neurol. 2001;434:101–124. doi: 10.1002/cne.1167. [DOI] [PubMed] [Google Scholar]
- Gao XB, van den Pol AN. Melanin concentrating hormone depresses synaptic activity of glutamate and GABA neurons from rat lateral hypothalamus. J Physiol. 2001;533:237–252. doi: 10.1111/j.1469-7793.2001.0237b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin AS, Clemens KJ, McNally GP. Renewal of extinguished cocaine-seeking. Neuroscience. 2008;151:659–670. doi: 10.1016/j.neuroscience.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Byrne R, Aston-Jones G. Glutamate-associated plasticity in the ventral tegmental area is necessary for conditioning environmental stimuli with morphine. Neuroscience. 2004;129:841–847. doi: 10.1016/j.neuroscience.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Hassani OK, Lee MG, Jones BE. Melanin-concentrating hormone neurons discharge in a reciprocal manner to orexin neurons across the sleep-wake cycle. Proc Natl Acad Sci U S A. 2009;106:2418–2422. doi: 10.1073/pnas.0811400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise CE, Mitrofanis J. Evidence for a glutamatergic projection from the zona incerta to the basal ganglia of rats. J Comp Neurol. 2004;468:482–495. doi: 10.1002/cne.10971. [DOI] [PubMed] [Google Scholar]
- Henny P, Jones BE. Innervation of orexin/hypocretin neurons by GABAergic, glutamatergic or cholinergic basal forebrain terminals evidenced by immunostaining for presynaptic vesicular transporter and postsynaptic scaffolding proteins. J Comp Neurol. 2006;499:645–661. doi: 10.1002/cne.21131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettes SR, Gonzaga WJ, Heyming TW, Nguyen JK, Perez S, Stanley BG. Stimulation of lateral hypothalamic AMPA receptors may induce feeding in rats. Brain Res. 2010;1346:112–120. doi: 10.1016/j.brainres.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Ida T, Nakahara K, Murakami T, Hanada R, Nakazato M, Murakami N. Possible involvement of orexin in the stress reaction in rats. Biochem Biophys Res Commun. 2000;270:318–323. doi: 10.1006/bbrc.2000.2412. [DOI] [PubMed] [Google Scholar]
- James MH, Charnley JL, Levi EM, Jones E, Yeoh JW, Smith DW, Dayas CV. Orexin-1 receptor signalling within the ventral tegmental area, but not the paraventricular thalamus, is critical to regulating cue-induced reinstatement of cocaine-seeking. Int J Neuropsychopharmacol. 2011;14:684–690. doi: 10.1017/S1461145711000423. [DOI] [PubMed] [Google Scholar]
- Karatayev O, Barson JR, Carr AJ, Baylan J, Chen YW, Leibowitz SF. Predictors of ethanol consumption in adult Sprague-Dawley rats: relation to hypothalamic peptides that stimulate ethanol intake. Alcohol. 2010;44:323–334. doi: 10.1016/j.alcohol.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Chartoff E, Roberto M, Carlezon WA, Jr, Markou A. NMDA receptors regulate nicotine-enhanced brain reward function and intravenous nicotine self-administration: role of the ventral tegmental area and central nucleus of the amygdala. Neuropsychopharmacology. 2009;34:266–281. doi: 10.1038/npp.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Kalivas PW. Glutamate and reinstatement. Curr Opin Pharmacol. 2009;9:59–64. doi: 10.1016/j.coph.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. Br J Pharmacol. 2006;148:752–759. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz SF. Overconsumption of dietary fat and alcohol: mechanisms involving lipids and hypothalamic peptides. Physiol Behav. 2007;91:513–521. doi: 10.1016/j.physbeh.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, Shabat-Simon M, Shalev U, Barnea-Ygael N, Cooper A, Zangen A. Repeated electrical stimulation of reward-related brain regions affects cocaine but not “natural” reinforcement. J Neurosci. 2007;27:14179–14189. doi: 10.1523/JNEUROSCI.4477-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li FW, Deurveilher S, Semba K. Behavioural and neuronal activation after microinjections of AMPA and NMDA into the perifornical lateral hypothalamus in rats. Behav Brain Res. 2011;224:376–386. doi: 10.1016/j.bbr.2011.06.021. [DOI] [PubMed] [Google Scholar]
- Li Y, Gao XB, Sakurai T, van den Pol AN. Hypocretin/Orexin excites hypocretin neurons via a local glutamate neuron-A potential mechanism for orchestrating the hypothalamic arousal system. Neuron. 2002;36:1169–1181. doi: 10.1016/s0896-6273(02)01132-7. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Aston-Jones G. Interactions between VTA orexin and glutamate in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2012 doi: 10.1007/s00213-012-2681-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Irizarry CS, Swanson CJ, Kelley AE. Glutamate receptors in the nucleus accumbens shell control feeding behavior via the lateral hypothalamus. J Neurosci. 1995;15:6779–6788. doi: 10.1523/JNEUROSCI.15-10-06779.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinetti MP, Andrzejewski ME, Hineline PN, Lewis MJ. Ethanol consumption and the matching law: a choice analysis using a limited-access paradigm. Exp Clin Psychopharmacol. 2000;8:395–403. doi: 10.1037//1064-1297.8.3.395. [DOI] [PubMed] [Google Scholar]
- McGregor R, Wu MF, Barber G, Ramanathan L, Siegel JM. Highly specific role of hypocretin (orexin) neurons: differential activation as a function of diurnal phase, operant reinforcement versus operant avoidance and light level. J Neurosci. 2011;31:15455–15467. doi: 10.1523/JNEUROSCI.4017-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan EZ, Furlong TM, McNally GP. Accumbens shell-hypothalamus interactions mediate extinction of alcohol seeking. J Neurosci. 2010;30:4626–4635. doi: 10.1523/JNEUROSCI.4933-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner KL, Mogenson GJ. Electrical and chemical activation of the mesencephalic and subthalamic locomotor regions in freely moving rats. Brain Res. 1988;452:273–285. doi: 10.1016/0006-8993(88)90031-5. [DOI] [PubMed] [Google Scholar]
- Monaghan DT, Bridges RJ, Cotman CW. The excitatory amino acid receptors: their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu Rev Pharmacol Toxicol. 1989;29:365–402. doi: 10.1146/annurev.pa.29.040189.002053. [DOI] [PubMed] [Google Scholar]
- Morganstern I, Chang GQ, Barson JR, Ye Z, Karatayev O, Leibowitz SF. Differential effects of acute and chronic ethanol exposure on orexin expression in the perifornical lateral hypothalamus. Alcohol Clin Exp Res. 2010a;34:886–896. doi: 10.1111/j.1530-0277.2010.01161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganstern I, Chang GQ, Chen YW, Barson JR, Zhiyu Y, Hoebel BG, Leibowitz SF. Role of melanin-concentrating hormone in the control of ethanol consumption: Region-specific effects revealed by expression and injection studies. Physiol Behav. 2010b;101:428–437. doi: 10.1016/j.physbeh.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada P, Mendialdua A, Hernandez L, Hoebel BG. Extracellular glutamate increases in the lateral hypothalamus during meal initiation, and GABA peaks during satiation: microdialysis measurements every 30 s. Behav Neurosci. 2003;117:222–227. doi: 10.1037/0735-7044.117.2.222. [DOI] [PubMed] [Google Scholar]
- Reagan LP, Rosell DR, Wood GE, Spedding M, Munoz C, Rothstein J, McEwen BS. Chronic restraint stress up-regulates GLT-1 mRNA and protein expression in the rat hippocampus: reversal by tianeptine. Proc Natl Acad Sci U S A. 2004;101:2179–2184. doi: 10.1073/pnas.0307294101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamaki R, Uemoto M, Inui A, Asakawa A, Ueno N, Ishibashi C, Hirono S, Yukioka H, Kato A, Shinfuku N, Kasuga M, Katsuura G. Melanin-concentrating hormone enhances sucrose intake. Int J Mol Med. 2005;15:1033–1039. [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Schneider ER, Rada P, Darby RD, Leibowitz SF, Hoebel BG. Orexigenic peptides and alcohol intake: differential effects of orexin, galanin, and ghrelin. Alcohol Clin Exp Res. 2007;31:1858–1865. doi: 10.1111/j.1530-0277.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- Skofitsch G, Jacobowitz DM, Zamir N. Immunohistochemical localization of a melanin concentrating hormone-like peptide in the rat brain. Brain Res Bull. 1985;15:635–649. doi: 10.1016/0361-9230(85)90213-8. [DOI] [PubMed] [Google Scholar]
- Stanley BG, Ha LH, Spears LC, Dee MG., 2nd Lateral hypothalamic injections of glutamate, kainic acid, D,L-alpha-amino-3-hydroxy-5-methyl-isoxazole propionic acid or N-methyl-D-aspartic acid rapidly elicit intense transient eating in rats. Brain Res. 1993a;613:88–95. doi: 10.1016/0006-8993(93)90458-y. [DOI] [PubMed] [Google Scholar]
- Stanley BG, Urstadt KR, Charles JR, Kee T. Glutamate and GABA in lateral hypothalamic mechanisms controlling food intake. Physiol Behav. 2011;104:40–46. doi: 10.1016/j.physbeh.2011.04.046. [DOI] [PubMed] [Google Scholar]
- Stanley BG, Willett VL, 3rd, Donias HW, Dee MG, 2nd, Duva MA. Lateral hypothalamic NMDA receptors and glutamate as physiological mediators of eating and weight control. Am J Physiol. 1996;270:R443–449. doi: 10.1152/ajpregu.1996.270.2.R443. [DOI] [PubMed] [Google Scholar]
- Stanley BG, Willett VL, 3rd, Donias HW, Ha LH, Spears LC. The lateral hypothalamus: a primary site mediating excitatory amino acid-elicited eating. Brain Res. 1993b;630:41–49. doi: 10.1016/0006-8993(93)90640-9. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, Bonci A. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutz AM, Staszkiewicz J, Ptitsyn A, Argyropoulos G. Circadian expression of genes regulating food intake. Obesity (Silver Spring) 2007;15:607–615. doi: 10.1038/oby.2007.564. [DOI] [PubMed] [Google Scholar]
- Sun W, Akins CK, Mattingly AE, Rebec GV. Ionotropic glutamate receptors in the ventral tegmental area regulate cocaine-seeking behavior in rats. Neuropsychopharmacology. 2005;30:2073–2081. doi: 10.1038/sj.npp.1300744. [DOI] [PubMed] [Google Scholar]
- Tordoff MG, Alarcon LK, Lawler MP. Preferences of 14 rat strains for 17 taste compounds. Physiol Behav. 2008;95:308–332. doi: 10.1016/j.physbeh.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Acuna-Goycolea C, Clark KR, Ghosh PK. Physiological properties of hypothalamic MCH neurons identified with selective expression of reporter gene after recombinant virus infection. Neuron. 2004;42:635–652. doi: 10.1016/s0896-6273(04)00251-x. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Gao XB, Obrietan K, Kilduff TS, Belousov AB. Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. J Neurosci. 1998;18:7962–7971. doi: 10.1523/JNEUROSCI.18-19-07962.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Hermans-Borgmeyer I, Hofer M, Ghosh P, Heinemann S. Ionotropic glutamate-receptor gene expression in hypothalamus: localization of AMPA, kainate, and NMDA receptor RNA with in situ hybridization. J Comp Neurol. 1994;343:428–444. doi: 10.1002/cne.903430307. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Trombley PQ. Glutamate neurons in hypothalamus regulate excitatory transmission. J Neurosci. 1993;13:2829–2836. doi: 10.1523/JNEUROSCI.13-07-02829.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willie JT, Sinton CM, Maratos-Flier E, Yanagisawa M. Abnormal response of melanin-concentrating hormone deficient mice to fasting: hyperactivity and rapid eye movement sleep suppression. Neuroscience. 2008;156:819–829. doi: 10.1016/j.neuroscience.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You ZB, Wang B, Zitzman D, Azari S, Wise RA. A role for conditioned ventral tegmental glutamate release in cocaine seeking. J Neurosci. 2007;27:10546–10555. doi: 10.1523/JNEUROSCI.2967-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]