Introduction
The prevalence of allergy, whether to venom, aeroallergens or food, continues to increase and results in considerable morbidity for the patient and increasing healthcare costs. While many drugs are available that help ameliorate symptoms, the only intervention that has proven to provide long-term benefit and modulation of disease is immunotherapy. The mainstay of immunotherapy treatment has been subcutaneous immunotherapy (SCIT) that after 3–5 years of high-dose treatment efficacy can be achieved. Newer methods that target the mucosal immune system include sublingual immunotherapy (SLIT) and oral immunotherapy (OIT). With the recent approval of SLIT tablets by the FDA and other new approaches being pursued, it is important to understand the mechanistic action of each of these forms of immunotherapy. Immunotherapy involves a reduction in mast cell and eosinophil numbers, a decrease in basophil activation and a rise in IgG4, and a shift in the cytokine milieu. However, the primary cells that are ultimately responsible for successful immunomodulation are CD4 T cells, specifically peripherally derived T regulatory cells. This review will cover our current knowledge of various immunotherapy methods and how the T cell response is altered to provide lasting benefit.
Methods
Primary literature on this topic was selected based on a PubMed search through October 2013 for articles in English using the keywords “subcutaneous immunotherapy (SCIT),” “sublingual immunotherapy (SLIT),” “oral immunotherapy (OIT)” and “mechanism” or “T cell.” Studies on adults and children with IgE-mediated allergic rhinitis or food allergy were included, with specific emphasis on those describing the T cell response. Pre-clinical trials using mouse models were also included where published clinical trials are not yet available.
Overview of the Regulatory T Lymphocyte Families
Numerous families of T cells have been identified, however it is the regulatory family that has been implicated in the successful response to immunotherapy and therefore will be the main focus of this review. These include the thymic-derived and peripherally derived regulatory T cells 1, 2. Thymus-derived Treg (tTreg; also referred to in the literature as natural (nTreg)) cells are characterized by their constitutive expression of IL-2 receptor α chain (CD25) and the transcription factor Foxp3. Although they can secrete IL-10, membrane TGF-β appears to be primarily responsible for mediating their contact-dependent immune suppression. tTreg cells are produced in the thymus in response to expression of self-antigens and are therefore important in the prevention of autoimmunity. They are unlikely to be involved in tolerance to antigens not presented in the thymus (for example, in tolerance to allergens in healthy subjects or in the immune benefits associated with allergen immunotherapy).
In contrast to the tTreg cells, an additional, less well characterized class of inducible regulatory T cells has been described which can develop in the periphery (pTreg; also referred to as inducible (iTreg)) 2, 3. These pTreg cells are differentiated from preexisting T effector lymphocytes or from circulating naïve Th0 cells and are characterized by their prominent production of IL-10. pTreg expression of Foxp3 and CD25 is controversial, but does appear to occur. For example, it is unclear whether CD25 expression reflects the constitutive expression of this component of the IL-2 receptor, the signature characteristic of tTreg cells or, the derivation of pTreg from activated effector T cells that are transiently expressing CD25. The difficulty in using CD25 as a surface marker or Foxp3 as an intracellulalr marker of pTreg is that high levels of CD25 (IL-2 receptor α) and Foxp3 are transiently expressed by CD4+ and CD8+ effector T cells following T cell receptor (TCR) activation and also in response to IL-2 in a positive feedback loop 4. A sub-group of peripherally derived regulatory cells that are primarily gut-derived and generate mucosal tolerance have been referred to as the Th3 cells. Reflecting their prominent production of TGF-β, in addition to mediating tolerance they are relevant to inducing secretory IgA production. It is the induction of IL-10-producing pTreg cells that plays a key role in reducing allergen-specific T cell responsiveness after immunotherapy 5,6.
Immunology of subcutaneous immunotherapy (SCIT)
Subcutaneous immunotherapy (SCIT) has been performed for over 100 years, yet only in the past few years have we begun to understand the mechanism of efficacy. A prominent role for IL-10–producing activated CD4+ cells was first described in studies involving bee venom SCIT5. The use of either whole bee venom extract or short peptides to phospholipase A2, the major bee venom allergen, resulted in induction of peripheral tolerance. Examination of the T cell response demonstrated that both Th2 (IL-4, IL-5 and IL-13) and Th1 (IFN-γ) cytokine production was decreased while IL-10 levels were increased. These IL-10 producing T cells were able to suppress allergen-specific T cell proliferation and activation and are now recognized as pTregs 7. While the appearance of the IL-10 producing pTregs is rapid, within 7 days of starting bee venom SCIT, full tolerance if it is going to occur requires 3–5 years of treatment.
Subsequent investigations with house dust mite and birch pollen SCIT extended the importance of IL-10 (and TGF-β) production by CD4+CD25+ T cells (pTregs) to inhalant allergy and confirmed that this occurred in parallel to the suppression of Th2 and Th1 proliferative responses and cytokine production 8. In this study, IL-10 responses in non-atopic individuals who had been exposed to allergen were similar to those in the SCIT-treated group, implying the restoration of tolerant T-cell responses in the atopic individual and that the normal immune response is geared toward non-responsiveness or suppression rather than a shift towards a Th1 response. Other studies have also shown IL-10 production by CD4+ cells, but without changes in grass pollen–induced proliferation or Th2 cytokine production 6. Reasons for the differences in the studies could include dose of allergen used and differences related to the allergen itself and the ability to induce an immune response. However, what is consistent is that each of these studies has found cells capable of making high levels of IL-10 (±TGF-β) consistent with the pTreg cell type. A direct role for IL-10 in inducing IgG4 antibody has been demonstrated and, as such, the appearance of allergen-specific IgG4 in association with immunotherapy is consistent with it being a marker for pTreg induction 9,10. The protective effect of pTreg cells is evident from studies demonstrating that following 4 yrs of high dose SCIT, subjects remain non-responsive for at least 3 years after SCIT has been stopped, similar to those who had continued SCIT for the full 7 year period 11.
In order to examine the T cell responses to SCIT in finer detail, MHC class II tetramers and epitope-guided mapping are being utilized. Once the HLA type to be studied is known, a synthetic MHC molecule of that HLA type is designed and labeled with biotin. The biotin labeling allows crosslinking to streptavidin to which four of these synthetic MHC class II molecules will bind. The MHC class II tetramers are then added to pools of peptides derived from the allergen of interest and using flow cytometry, peptides that bind to the tetramers are identified. This is repeated through several rounds of selection until a single peptide is isolated that recognizes that MHC class II molecule 12. CD4+ T cells that bind to the peptide-MHC class II tetramer can subsequently be identified as allergen-specific T cells. The limitation of this approach is that only one epitope of the allergen is being examined so the results obtained are likely an underestimation of the actual response.
Using these methods, the frequency of Fel d 1-specific T cells in allergic individuals was shown to range from 1 in 7,000 to 1 in 30,000 13. Similar frequencies have been found examining other allergen-specific T cells. Surprisingly, allergen-specific T cells have also been identified in non-allergic subjects, however the numbers are lower and the avidity of the T cell receptor is decreased 14. When alder specific T cells were examined, the phenotype of T cells from allergic individuals was characterized by high expression of the cell surface markers CRTh2 (the prostaglanding D2 receptor which is a marker for Th2 cells) and CCR4 with low CXCR3 (chemokine receptors). In contrast the T cells from non-atopic individuals had high CXCR3, moderate CCR4 and low CRTh2 expression 15. When the allergen-specific memory T cell population was phenotyped, two populations could be separated based on the expression of CD27, a member of the tumor necrosis factor (TNF) receptor superfamily which is expressed by naïve and central memory T cells, with loss of expression on effector memory and terminally differentiated, exhausted T cells. The CD27− population had characteristics of classical Th2 cells in that they had high levels of CRTh2 and CCR4 on the cell surface and high intracellular levels of IL-4, IL-5 and IL-13. The CD27+ population had high CXCR3, CCR7 and CD7 on the cell surface with high IFN-γ levels in the intracellular milieu, characteristics of Th1 cells 15. It was the ratio of CD27− to CD27+ cells that distinguished allergic individuals from non-atopic. These techniques were employed to examine what happens to these T cell populations after IT. Successful SCIT was associated with loss of the CD27− population due to apoptosis which allowed the IL-10 secreting pTregs to develop. Previous studies have shown that STAT6 signaling in Th2 cells renders them resistant to Treg suppression 16, so the selective loss of the CD27− population (Th2-like) allows restoration of Treg function.
Mechanisms of slit and efficacy
Sublingual immunotherapy (SLIT) is administered by holding escalating doses of antigen drops or tablets under the tongue for a period of time. This allows for uptake of the antigen by oral antigen presenting cells (APCs) such as Langerhans cells 17. This local presentation of antigen is thought to result in local mucosal induction of IL-10 and TGF-β producing Th3 cells18.
In one of the first mechanistic studies of SLIT in humans, PBMCs from patients treated with house dust mite (HDM) SLIT for 3 years were shown to produce significantly more IL-10 following PHA stimulation when compared to patients with untreated rhinitis 19. This provided indirect evidence for the immunoregulatory role of IL-10 in SLIT. Several years later, a small mechanistic study examining 9 patients undergoing birch SLIT suggested an early and late phase response to therapy 20. By 4 weeks of therapy, there was an increase in circulating pTregs accompanied by an increase in IL-10 and Foxp3 mRNA expression levels and non-specific suppression of antigen-driven lymphocyte proliferation. After 52 weeks of therapy, the suppression of proliferation was specific to Bet v 1 (birch pollen) and the levels of circulating pTregs as well as IL-10 returned to baseline. However, there was a persistent reduction of IL-4 mRNA and an increase in IFN-γ mRNA. This suggests that early in therapy, IL-10 producing pTregs act non-specifically to suppress lymphocyte proliferation in response to antigen, but over time this non-specific response is replaced by an antigen-specific tolerance on the backdrop of a Th1 skewed cytokine milieu 21. This may have important implications in determining the optimal duration of therapy.
Consistent with the transient induction of IL-10+ pTregs seen in the previous study, a randomized double-blind placebo controlled trial of 12 to 24 months of house dust mite (HDM) SLIT also demonstrated a transient increase in the proportion of circulating Tregs, which were confirmed to have the ability to suppress HDM-specific effector T cell proliferation and cytokine production. The study also demonstrated significantly decreased HDM-induced CD4+ T cell proliferation after 6, 12, and 24 months. There was a decrease in IL-5 levels accompanied by an increase in IL-10 and IFN-γ, but no change in IL-4 and no measurement of IL-9 or IL-13 cytokine levels 22.
Several other studies have also confirmed the increase in pTregs following SLIT. In a study of patients treated with grass pollen SLIT for 12–18 months, the number of Foxp3+ cells in the sublingual epithelium was significantly increased in the treatment group compared to the placebo group and non-atopic controls 23. However, the number of IL-10 and TGF-β mRNA positive cells did not change following SLIT, raising the possibility that these Foxp3+ cells were recently activated effector cells rather than true regulatory T cells. Perhaps addressing these technical concerns, another later study of grass pollen SLIT demonstrated increased production of IL-10 and TGF-β by oral Langerhans cells and an increased number of IL-10 and TGF-β–producing Treg cells following therapy 18.
Using the tetramer approach described earlier and a more robust double blind placebo controlled experimental model, Bonvalet et al were unable to detect a significant decrease in grass allergen (Phl1 p 1 and Phl p 5)-specific CD4+ T cells during or after 4 months of SLIT, nor a change in surface phenotype markers. This was despite a significant 41.8% improvement in symptom score after grass pollen challenge in an allergen exposure challenge 24. This raises the possibility that while clinical improvement may be seen as soon as 1 month, parallel immunologic changes involving the shift from a Th2 to a Th1 signature and upregulation of Treg cells require longer treatment courses (up to 12 months).
Although it has been used only on in a limited number of published trials on food allergy, the same increase in pTregs and cytokine shift away from Th2 has been shown in food SLIT. A study of patients receiving 12 months of peanut SLIT found significantly lower levels of serum IL-5 following SLIT than those in the placebo group. No significant difference between the groups was found in IL-10 or IFN-γ levels after treatment. There was a trend towards an increased percentage of Tregs compared with placebo 25.
Mechanisms of OIT and efficacy
Oral immunotherapy (OIT) differs from SLIT in that the antigen is immediately swallowed and delivered to the gut associated lymphoid tissues (GALT). Oral tolerance, or the development of tolerance within the GALT, has been recognized to occur in response to repetitive oral ingestion of antigens since the early 1900s. Oral tolerance is thought to be mediated in large part by pTregs, in particular the Th3 subset which produce primarily TGF-β (+/− IL-10) and promote the production of secretory IgA. This is supported by work demonstrated that tTregs are not required for the induction of oral tolerance 26, while deletion of pTregs in the DEREG mouse model resulted in loss of oral tolerance 27.
Most published trials of OIT to date have focused on clinical endpoints such as ability to pass blinded food challenge, skin prick wheal size, or serum level of allergen specific IgE or IgG4. Direct analysis of the cellular immune changes that occur with OIT in humans has been relatively limited to date, and focused primarily on basophil activation, peripheral blood mononuclear cell (PBMC) cytokine production, and changes in the frequency of pTregs 28.
In two studies, peanut OIT was associated with a reduction in Th2 cytokine (IL-4, IL-5 and/or IL-13) production from stimulated PBMCs 29, 30. Other studies have shown increases in IL-5 production however, and cytokine profile does not correlate with clinical outcome in most trials, suggesting there is considerable heterogeneity in the response30, 31
Milk-allergic patients able to tolerate extensively heated milk have higher percentages of milk-specific pTregs than milk-allergic patients unable to tolerate heated milk or patients with outgrown-milk allergy 32 suggesting that the development of oral tolerance may involve expansion of the pTreg compartment just as it does in SLIT. Consistent with this finding, peanut allergic patients who had undergone peanut OIT had an increased frequency of pTregs compared to controls 29, 31. A study of 20 egg-allergic patients treated with egg OIT demonstrated an increase in TGF-β positive dendritic cells following OIT, which were able to induce the conversion of T effector cells to pTregs 33. The combination of decreased numbers of food allergen specific T effector cells and increased allergen specific pTregs is a possible mechanism for the development of tolerance in these patients.
Several recent studies have suggested intriguing possibilities for the future. The use of tetramers to identify peanut-reactive T cells has revealed a significantly higher frequency of Ara h 1-specific T cells in patients with peanut allergy (9 cells per million) compared to healthy controls (1 cell per million) 34. An obvious application of this technique would be to monitor the frequency of these allergen-specific T cells during the course of OIT.
Two published pre-clinical trials have used oral peanut plus a toll-like receptor (TLR)-9 ligand agonist in peanut-sensitized mice and demonstrated increased Th1 and decreased Th2 cytokine production accompanied by reduced IgE levels and anaphylaxis 35, 36. This Th1 skewing and clinical benefit was even more pronounced than in those mice receiving oral peanut alone, suggesting that the use of adjuvants such as TLR agonists may be a powerful tool in increasing the efficacy of OIT.
Comparison between it regimens
There is evidence supporting the use of both SCIT and SLIT for the treatment of rhinitis and asthma, but very few published trials have made a direct head to head comparison of the two routes of IT. A recent meta-analysis performed by Dretzke et al was able to identify only one such well-controlled trial; this trial did not provide conclusive results in favor of either SCIT or SLIT based on symptom score or medication use 37. This meta-analysis as well as another using indirect comparison of trials that used either SCIT or SLIT both demonstrated a trend toward greater efficacy in the SCIT group 37, 38. Caution must be made in interpreting this result however, as the majority of the included studies are heterogeneous in both dose and duration of therapy, rather than direct head-to-head trials which would provide more conclusive evidence favoring one route over another.
As both OIT and SLIT have also demonstrated clinical efficacy in the treatment of food allergy, and have similar profiles in terms of adverse events and ease of administration, one question that arises is which route of delivery is most clinically efficacious, and if this is supported by superior immunologic outcome data. A recently published head-to-head analysis attempted to address this by comparing two previously published double blind placebo controlled trials of 12 months of peanut OIT to SLIT 39. Along with being 3 times more likely to pass a food challenge after treatment, patients in the OIT group had more significant decreases in peanut-specific IgE/IgG4 ratio and basophil activation. Interestingly however there was no significant different in the percentage of Treg cells between the OIT and SLIT groups, nor between those who passed and failed the food challenge.
Future for Clinicans
Many routes of IT are being pursued, however SCIT has the historical record of leading to immune changes that alter the immune response upon subsequent allergen exposure. These changes are mediated by induction of pTregs and only appear to occur following high dose therapy for 3–5 years. Newer methods of SLIT and OIT are currently being investigated, but their efficacy compared to the more traditional method of SCIT is still not known 38, 40. Their mechanism of action is believed to be similar to SCIT, via the induction of pTregs, though this is still an active area of investigation. Obvious questions include the nature of the regulatory response in SLIT and OIT compared to SCIT and the optimal dose and duration of treatment required to achieve lasting immunomodulation. Once these issues are truly understood, allergists will have additional tools at their disposal to alter the morbidity associated with the increasing incidence of allergic disease.
Figure 1.
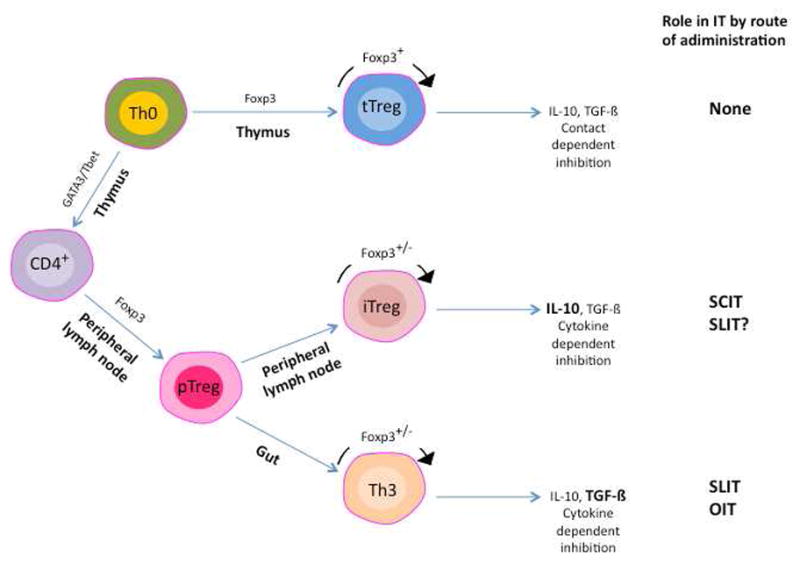
T regulatory cell populations and their involvement in immunotherapy (IT). Naïve cells can develop into T regulatory cells via two pathways. Those that develop in the thymus (tTreg) require and express Foxp3 during development and when mature. The tTregs can secrete inhibitory cytokines but require cell-cell contact for inhibition. As they are generated to inhibit responses to self antigens, they are likely to have little role in IT. Peripheral T regulatory cells (pTreg) develop outside of the thymus and can express Foxp3, though expression is not absolute, and use secreted cytokines to mediate suppression (main inhibitory cytokine in bold). The two main sub-populations of pTregs are shown and their role in IT indicated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 2.Abbas AK, Benoist C, Bluestone JA, et al. Regulatory T cells: recommendations to simplify the nomenclature. Nat Immunol. 2013;14:307–308. doi: 10.1038/ni.2554. [DOI] [PubMed] [Google Scholar]
- 3.Akdis CA, Blaser K. IL-10-induced anergy in peripheral T cell and reactivation by microenviromental cytokines: two key steps in specific immunotherapy. FASEB Journal. 1999;13:603–609. doi: 10.1096/fasebj.13.6.603. [DOI] [PubMed] [Google Scholar]
- 4.Kmieciak M, Gowda M, Graham L, et al. Human T cells express CD25 and Foxp3 upon activation and exhibit effector/memory phenotypes without any regulatory suppressor function. J Trans Med. 2009;7:89. doi: 10.1186/1479-5876-7-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akdis CA, Blesken T, Akdis M, Wuthrich B, Blaser K. Role of interleukin 10 in specific immunotherapy. J Clin Invest. 1998;102:98–106. doi: 10.1172/JCI2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francis JN, Till SJ, Durham SR. Induction of IL-10+CD4+CD25+ T cells by grass pollen immunotherapy. J Allergy Clin Immunol. 2003;111:1255–1261. doi: 10.1067/mai.2003.1570. [DOI] [PubMed] [Google Scholar]
- 7.Muller U, Akdis CA, Fricker M, et al. Successful immunotherapy with T-cell epitope peptides of bee venom phospholipase A2 induces specific T-cell anergy in patients allergic to bee venom. J Allergy Clin Immunol. 1998;101:747–754. doi: 10.1016/S0091-6749(98)70402-6. [DOI] [PubMed] [Google Scholar]
- 8.Jutel M, Akdis M, Budak F, et al. IL-10 and TGF-β cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur J Immunol. 2003;33:1205–1214. doi: 10.1002/eji.200322919. [DOI] [PubMed] [Google Scholar]
- 9.Fellrath J-M, Kettner A, Dufour N, et al. Allergen-specific T-cell tolerance induction with allergen-derived long synthetic peptides: Results of a phase I trial. J Allergy Clin Immunol. 2003;111:854–861. doi: 10.1067/mai.2003.1337. [DOI] [PubMed] [Google Scholar]
- 10.Satoguina JS, Weyand E, Larbi J, Hoerauf A. T regulatory-1 cells induce IgG4 production by B cells: role of IL-10. J Immunol. 2005;174:4718–4726. doi: 10.4049/jimmunol.174.8.4718. [DOI] [PubMed] [Google Scholar]
- 11.Durham SR, Walker SM, Varga EM, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341:468–475. doi: 10.1056/NEJM199908123410702. [DOI] [PubMed] [Google Scholar]
- 12.Nepom GT. MHC class II tetramers. J Immunol. 2012;188:2477–2482. doi: 10.4049/jimmunol.1102398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwok WW, Roti M, Delong JH, et al. Direct ex vivo analysis of allergen-specific CD4+ T cells. J Allergy Clin Immunol. 2010;125:1407–1409. e1. doi: 10.1016/j.jaci.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinnunen T, Nieminen A, Kwok WW, et al. Allergen-specific naive and memory CD4+ T cells exhibit functional and phenotypic differences between individuals with or without allergy. Eur J Immunol. 2010;40:2460–2469. doi: 10.1002/eji.201040328. [DOI] [PubMed] [Google Scholar]
- 15.Wambre E, DeLong JH, James EA, LaFond RE, Robinson D, Kwok WW. Differentiation stage determines pathologic and protective allergen-specific CD4+ T-cell outcomes during specific immunotherapy. J Allergy Clin Immunol. 2012;129:544–551. e1–7. doi: 10.1016/j.jaci.2011.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pillemer BB, Qi Z, Melgert B, Oriss TB, Ray P, Ray A. STAT6 activation confers upon T helper cells resistance to suppression by regulatory T cells. J Immunol. 2009;183:155–163. doi: 10.4049/jimmunol.0803733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smarr CB, Bryce PJ, Miller SD. Antigen-specific tolerance in immunotherapy of th2-associated allergic diseases. Crit Rev Immunol. 2013;33:389–414. doi: 10.1615/critrevimmunol.2013007046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allam JP, Wurtzen PA, Reinartz M, et al. Phl p 5 resorption in human oral mucosa leads to dose-dependent and time-dependent allergen binding by oral mucosal Langerhans cells, attenuates their maturation, and enhances their migratory and TGF-beta1 and IL-10-producing properties. J Allergy Clin Immunol. 2010;126:638–645. e1. doi: 10.1016/j.jaci.2010.04.039. [DOI] [PubMed] [Google Scholar]
- 19.Ciprandi G, Fenoglio D, Cirillo I, et al. Induction of interleukin 10 by sublingual immunotherapy for house dust mites: a preliminary report. Ann Allergy Asthma Immunol. 2005;95:38–44. doi: 10.1016/S1081-1206(10)61186-6. [DOI] [PubMed] [Google Scholar]
- 20.Bohle B, Kinaciyan T, Gerstmayr M, Radakovics A, Jahn-Schmid B, Ebner C. Sublingual immunotherapy induces IL-10-producing T regulatory cells, allergen-specific T-cell tolerance, and immune deviation. J Allergy Clin Immunol. 2007;120:707–713. doi: 10.1016/j.jaci.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 21.Frew AJ. How does sublingual immunotherapy work? J Allergy Clin Immunol. 2007;120:533–536. doi: 10.1016/j.jaci.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 22.O'Hehir RE, Gardner LM, de Leon MP, et al. House dust mite sublingual immunotherapy: the role for transforming growth factor-beta and functional regulatory T cells. Am J Respir Crit Care Med. 2009;180:936–947. doi: 10.1164/rccm.200905-0686OC. [DOI] [PubMed] [Google Scholar]
- 23.Scadding GW, Shamji MH, Jacobson MR, et al. Sublingual grass pollen immunotherapy is associated with increases in sublingual Foxp3-expressing cells and elevated allergen-specific immunoglobulin G4, immunoglobulin A and serum inhibitory activity for immunoglobulin E-facilitated allergen binding to B cells. Clin Exp Allergy. 2010;40:598–606. doi: 10.1111/j.1365-2222.2010.03462.x. [DOI] [PubMed] [Google Scholar]
- 24.Bonvalet M, Moussu H, Wambre E, et al. Allergen-specific CD4+ T cell responses in peripheral blood do not predict the early onset of clinical efficacy during grass pollen sublingual immunotherapy. Clin Exp Allergy. 2012;42:1745–1755. doi: 10.1111/cea.12015. [DOI] [PubMed] [Google Scholar]
- 25.Kim EH, Bird JA, Kulis M, et al. Sublingual immunotherapy for peanut allergy: clinical and immunologic evidence of desensitization. J Allergy Clin Immunol. 2011;127:640–646. e1. doi: 10.1016/j.jaci.2010.12.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ, Curotto de Lafaille MA. Oral tolerance in the absence of naturally occurring Tregs. J Clin Invest. 2005;115:1923–1933. doi: 10.1172/JCI24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hadis U, Wahl B, Schulz O, et al. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity. 2011;34:237–246. doi: 10.1016/j.immuni.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 28.Tang ML, Martino DJ. Oral immunotherapy and tolerance induction in childhood. Pediatr Allergy Immunol. 2013;24:512–520. doi: 10.1111/pai.12100. [DOI] [PubMed] [Google Scholar]
- 29.Varshney P, Jones SM, Scurlock AM, et al. A randomized controlled study of peanut oral immunotherapy: clinical desensitization and modulation of the allergic response. J Allergy Clin Immunol. 2011;127:654–660. doi: 10.1016/j.jaci.2010.12.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blumchen K, Ulbricht H, Staden U, et al. Oral peanut immunotherapy in children with peanut anaphylaxis. J Allergy Clin Immunol. 2010;126:83–91. e1. doi: 10.1016/j.jaci.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 31.Jones SM, Pons L, Roberts JL, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009;124:292–300. e1–97. doi: 10.1016/j.jaci.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shreffler WG, Wanich N, Moloney M, Nowak-Wegrzyn A, Sampson HA. Association of allergen-specific regulatory T cells with the onset of clinical tolerance to milk protein. J Allergy Clin Immunol. 2009;123:43–52. e7. doi: 10.1016/j.jaci.2008.09.051. [DOI] [PubMed] [Google Scholar]
- 33.Wu Z. Antigen specific immunotherapy generates CD27(+) CD35(+) tolerogenic dendritic cells. Cell Immunol. 2013;283:75–80. doi: 10.1016/j.cellimm.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 34.DeLong JH, Simpson KH, Wambre E, James EA, Robinson D, Kwok WW. Ara h 1-reactive T cells in individuals with peanut allergy. J Allergy Clin Immunol. 2011;127:1211–1218. e3. doi: 10.1016/j.jaci.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu FG, Kandimalla ER, Yu D, Agrawal S. Oral administration of a synthetic agonist of Toll-like receptor 9 potently modulates peanut-induced allergy in mice. J Allergy Clin Immunol. 2007;120:631–637. doi: 10.1016/j.jaci.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 36.Bashir ME, Louie S, Shi HN, Nagler-Anderson C. Toll-like receptor 4 signaling by intestinal microbes influences susceptibility to food allergy. J Immunol. 2004;172:6978–6987. doi: 10.4049/jimmunol.172.11.6978. [DOI] [PubMed] [Google Scholar]
- 37.Dretzke J, Meadows A, Novielli N, Huissoon A, Fry-Smith A, Meads C. Subcutaneous and sublingual immunotherapy for seasonal allergic rhinitis: a systematic review and indirect comparison. J Allergy Clin Immunol. 2013;131:1361–1366. doi: 10.1016/j.jaci.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 38.Di Bona D, Plaia A, Leto-Barone MS, La Piana S, Di Lorenzo G. Efficacy of subcutaneous and sublingual immunotherapy with grass allergens for seasonal allergic rhinitis: a meta-analysis-based comparison. J Allergy Clin Immunol. 2012;130:1097–1107. e2. doi: 10.1016/j.jaci.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 39.Chin SJ, Vickery BP, Kulis MD, et al. Sublingual versus oral immunotherapy for peanut-allergic children: a retrospective comparison. J Allergy Clin Immunol. 2013;132:476–478. e2. doi: 10.1016/j.jaci.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khinchi MS, Poulsen LK, Carat F, Andre C, Hansen AB, Malling HJ. Clinical efficacy of sublingual and subcutaneous birch pollen allergen-specific immunotherapy: a randomized, placebo-controlled, double-blind, double-dummy study. Allergy. 2004;59:45–53. doi: 10.1046/j.1398-9995.2003.00387.x. [DOI] [PubMed] [Google Scholar]


