Abstract
The purpose of this study was to evaluate the influence of sleep deprivation on emotional expression and subjective emotional experience in a highly controlled, laboratory setting. Twenty-three healthy adult participants watched positive (amusing) and negative (sad) film clips before and after they were randomly assigned to a night of sleep deprivation or a normal sleep control condition. The intensity of their facial expressiveness while viewing the films was coded by human judges and compared to their subjective emotional responses. Relative to the control group, sleep-deprived participants demonstrated less expressiveness, especially in response to positive stimuli. Subjective responses were not significantly different between the sleep-deprived and control groups. These preliminary results suggest that sleep deprivation is associated with attenuated emotional expressiveness in healthy adults.
Inadequate sleep is a pervasive problem in modern society. Not only is sleep often impaired by medical and psychiatric problems (Benca, Obermeyer, Thisted, & Gillin, 1992; Roehrs & Roth, 2005), but artificial lighting and around-the-clock services have facilitated lifestyles that do not allow for adequate levels of sleep. The resulting “sleep debt” is associated with significant daytime performance deficits that carry considerable social, financial, and personal costs.
Experiments dating back to the 1900s have demonstrated that sleep deprivation has deleterious effects on behavior and cognition, including impairments in vigilant and executive attention, working memory, and divergent higher cognitive functions indicative of instability in wakefulness (for recent reviews, see Banks & Dinges, 2007; Durmer & Dinges, 2005). However, surprisingly little research has been conducted to determine the effects of sleep loss on emotions. Hypotheses about relationships between sleep and affect have, therefore, required speculation based on neuroimaging studies of cognitive tasks after sleep deprivation and from clinical populations in which sleep restriction cannot be isolated from other variables.
Perhaps the most influential hypotheses regarding the relationships between sleep and emotion have been proposed by Dahl and Lewin (2002), who suggested that sleep loss leads to deficits in the ability to downregulate emotions via its effects on the prefrontal cortex, a region that has been implicated in inhibitory control of negative emotion in particular (for a review, see Ochsner & Gross, 2005). Only a handful of studies have been conducted with enough scientific rigor to suggest causal links between restricted sleep and emotional functioning, but the available data support the hypothesis that negative emotion, in particular, can become more extreme after sleep loss.
Yoo, Gujar, Hu, Jolesz, and Walker (2007) found increased activation of the amygdala and decreased connectivity between the medial prefrontal cortex (an area involved in inhibitory control) and the amygdala (an area involved in negative emotion) in participants who viewed emotionally negative photographs after sleep deprivation relative to a control group who viewed the same photographs after normal sleep. These results indicate that sleep loss may dysregulate emotion by degrading top-down inhibitory control of emotional responses. Nevertheless, no data on behavioral or subjective emotional responses were reported, so it is unknown how these neural changes were expressed.
Franzen, Siegle, and Buysse (2008) completed a similar study where participants viewed emotionally provocative photographs with and without a night of sleep deprivation, but the primary affective outcome was the change in pupil diameter, a physiological correlate of emotion that has been validated in rested participants (see Bradley, Miccoli, Escrig, & Lang, 2008). They reported increased pupil dilation in response to negative photographs for the sleep-deprived group relative to the control group, suggesting that reduced sleep is associated with greater emotional activation in response to unpleasant stimuli.
In a well-designed field study, Zohar, Tzischinsky, Epstein, and Lavie (2005) used experiential sampling to gather data on sleep and emotion in medical professionals whose sleep fluctuated with the shift they worked. They reported that reduced sleep was associated with a decrease in positive emotion following desirable events and an increase in negative emotion following undesirable events.
Despite these preliminary findings, there is reason to suspect that sleep deprivation may have other effects on emotional processes as well. The prefrontal cortex is not the only brain region sensitive to sleep loss. The ascending arousal pathway, which involves the thalamus and hypothalamus as well as cortical regions (for a review, see Saper, Scammell, & Lu, 2005), is also intimately linked to sleep and has been shown to be influenced by sleep deprivation. In fact, evidence that sleep loss results in de-arousal has been extremely well-established over many decades while evidence that executive function tasks (such as inhibitory control) are also sensitive to sleep loss has emerged more recently (see Durmer & Dinges, 2005). It is possible that the de-arousing effects of sleep loss would dampen emotional responding rather than potentiate it.
In this study, our goal was to test the hypothesis of Dahl and Lewin (2002) that sleep loss would be associated with emotional disinhibition—and, thus, more extreme emotional responses—against the alternative hypothesis that de-arousal due to sleep loss would produce less extreme emotional responses. In addition to self-report, we investigated this question by measuring the intensity of facial expressiveness, a component of emotion that has been highly studied since Darwin’s (1871) work in The Expression of the Emotions in Man and Animals, but not yet investigated by sleep researchers. In addition to serving as an objective indicator of emotional functioning, facial expressions are of interest because of their crucial role in communicating private emotional states, regulating social interactions, and even influencing subjective and physiological components of emotion (Levenson, Ekman, & Friesen, 1990).
METHOD
Participants completed the following procedures as part of a larger study. Additional testing, not reported here, included cognitive tasks, reaction time testing, and stress induction using performance demands. Both sleep-deprived and control participants completed identical testing at the same time of day. They entered the laboratory between 9:00 a.m. and 10:00 a.m., and remained in the laboratory for 2 full days and nights. They left the laboratory on the third morning, after 10 hr of recovery sleep opportunity.
Participants
Participants were healthy volunteers between the ages of 22 and 45, recruited from the general community. Fourteen men and nine women (mean age = 30.8 ± 6.8 years) completed a 48-hr laboratory-based protocol approved by the Institutional Review Board of the University of Pennsylvania. All participants provided informed consent and were compensated for their participation. The sample included 12 Caucasian, 8 African American, and 3 participants of other ethnicities.
Volunteers were screened to ensure they had no medical, psychiatric, or sleep-related disorders and that they were non-smokers and were drug free. This was determined by self-reported medical history and psychological questionnaires. Participants reported working neither regular night nor rotating shift work within the past 6 months, and reported not having traveled across time zones in the last 3 months. Sleep schedules were monitored by diary and actigraphy for 7 to 14 days prior to entry into the study. All participants attained 7 to 8 hr of sleep per night and woke between 6 a.m. and 9 a.m. each morning prior to beginning the study. Participants were then removed if they were physically ill, had poor sleep the previous night, or reported Beck Depression Inventory (BDI-II; Beck et al., 1996) scores at or above 14 prior to the first day of testing. Five participants were removed for one or more of these reasons.
Procedures
Sleep deprivation
Participants were randomly assigned to the sleep-deprivation or control conditions. Fifteen participants (9 males, 6 females) completed the sleep-deprivation protocol, and 8 participants (5 males, 3 females) completed the control protocol. More participants were randomized to sleep deprivation to maximize power for within-subjects comparisons. Participants entered the laboratory at 9:00 a.m. and completed consenting procedures. After 1 day of testing, participants either received no sleep opportunity (sleep-deprivation group) or they were given 9 hr of sleep opportunity (control group) from 11:00 p.m. to 8:00 a.m. A second day of testing was then completed, followed by 10 hr of sleep opportunity from 10:00 p.m. to 8:00 a.m. for all participants. They were informed of their condition at 8:00 p.m., after the first day of testing had been completed. At all scheduled wake times, participants were kept awake in the laboratory under continuous behavioral monitoring. During scheduled sleep times, all lights were turned off (less than 1 lux), and participants were monitored by infrared cameras from an adjacent room by trained staff members. Adherence to sleep condition was further validated by actigraphy, an objective method for estimating sleep based on movement (Ancoli-Israel et al., 2003). Participants in both conditions remained in the lab for the full duration of the experiment.
Emotion induction
Positive and negative emotions (amusement and sadness) were induced between 4:00 p.m. and 6:00 p.m. on 2 consecutive days of testing. Three clips were chosen from a library of previously investigated film clips found to elicit target emotions (Gross & Levenson, 1995), and one clip (Steve Martin from Saturday Night Live) was chosen based on the recommendation of an expert in the field (M. Shiota, personal communication, December 12, 2005). On Day 1, prior to the sleep manipulation, all participants watched a clip from Bambi (duration = 146 sec) to induce sadness, followed by a clip of Steve Martin from Saturday Night Live (duration = 186 sec) to induce amusement. On Day 2, after the sleep manipulation, all participants watched a clip from The Champ (duration = 181 sec) to induce sadness, followed by a clip from When Harry Met Sally (duration = 156 sec) to produce amusement.
Measures
Facial expressiveness
Participants were videotaped while they watched the emotionally provocative film clips. These videos were later scored without sound in a randomized order. Two raters, one of whom was blind to all conditions, scored all of these videotapes. The videos were scored for global level of expressiveness based on the FACES scoring system (Kring & Sloan, 2007). This is a single rating of overall expressiveness for the entire film segment rated on a scale ranging from 1 to 5. To improve reliability, the following anchors were added to the existing 5-point scale: 1 (no emotion), 2 (one or two small displays), 3 (either one big display or several smaller displays), 4 (many small displays or one or more large displays), and 5 (several large displays or near-constant small displays). The averages of raters’ scores were used for statistical analyses. The frequency, intensity, and duration of individual expressions were not rated because we found the reliability of these items to be unacceptably low during pilot testing.
Reliability was calculated using the intraclass correlation, a ratio of the variance of interest over the sum of the variance of interest plus error (Bartko, 1966). Intraclass correlation coefficients (ICCs) were calculated according to the recommendations of Shrout and Fleiss (1979). Participants were treated as a random factor, and the raters were treated as fixed factors. Measures of absolute agreement, rather than consistency, were calculated because systematic differences among levels of ratings were determined to be relevant. This is the more conservative statistical choice. ICCs of .70 or greater were considered adequate. When measurements failed to reach this criterion, additional raters were added until the ICC increased to an acceptable value. All ratings were used in analyses by using the average of judges’ ratings.
Ratings of participants’ responses to three film clips were determined to be above the reliability threshold (ICCs > .82), but responses to the clip from Bambi (duration = 146 sec; sadness-inducing on Day 1) did not meet our reliability threshold (ICC = .60). An additional rater, blind to sleep condition, rated participants’ responses to this film clip. When these ratings were added, reliability for this clip increased above threshold (ICC = .75). The average ratings from all three raters were used in all subsequent analyses.
Subjective emotional responses
Immediately after viewing each film clip, participants completed a post-film questionnaire (Rottenberg, Ray, & Gross, 2007). The instructions were to indicate, on a 9-point scale, the greatest amount of each of 18 specific emotions that they experienced while watching the film clip. The participants’ responses to the item “amusement” were used in all analyses related to the amusing film clips. Participants’ responses to the item “sadness” were used in all analyses related to the sad film clips.
Statistical Procedures
The effects of sleep loss on subjective emotional responses and facial expressiveness were analyzed using repeated measures analysis of variance to test for a main effect of sleep deprivation, as well as interactions between sleep condition and emotional valence of film clips viewed (amusing and sad). Where possible, Cohen’s d effect sizes (Cohen, 1988) were calculated using the mean difference between sleep-deprived and control participants divided by the pooled variance of both groups.
RESULTS
Each participant was videotaped while viewing 4 film clips, producing 92 video segments for scoring. Five of these could not be scored due to equipment malfunction or experimenter error. After each clip, participants completed a post-film questionnaire, producing 92 questionnaires. Nine of these could not be used in analysis due to participant error in completing the forms.
Sleep Manipulation
Participants were monitored continuously by trained staff members either in person or by closed-circuit, infrared cameras. All participants in the control group were in bed with lights out from 11:00 p.m. to 8:00 a.m., and all participants in the sleep-deprivation condition were awake from the time of their arrival at the laboratory until the second night when recovery sleep was allowed. Actigraphy confirmed that all participants in the sleep-deprivation group were continuously active and received no sleep opportunity, whereas participants in the control condition had low enough activity levels to allow an average of 8.99 hr (± 0.26 hr) of sleep. Actigraphy data were not available on 1 participant in the control condition due to equipment malfunction.
Subjective Responses to Film Clips
A manipulation check revealed that the film clips elicited high levels of the intended emotions (based on self-reports on a scale ranging from 0–8). The average reported intensity of amusement in response to the amusing clips was 6.1 (± 1.4) and 6.0 (± 1.8) on Days 1 and 2, respectively. The average reported sadness in response to the sad clips was 6.2 (± 1.8) and 6.0 (± 2.0) on Days 1 and 2, respectively. A paired-samples t test found no differences in subjective responses between the two amusing clips, t(16) = 0.68, p = .51, or between the two sad clips, t(19) = 0.34, p = .74, indicating that, although the clips were different, they elicited comparable intensities of the target emotions.
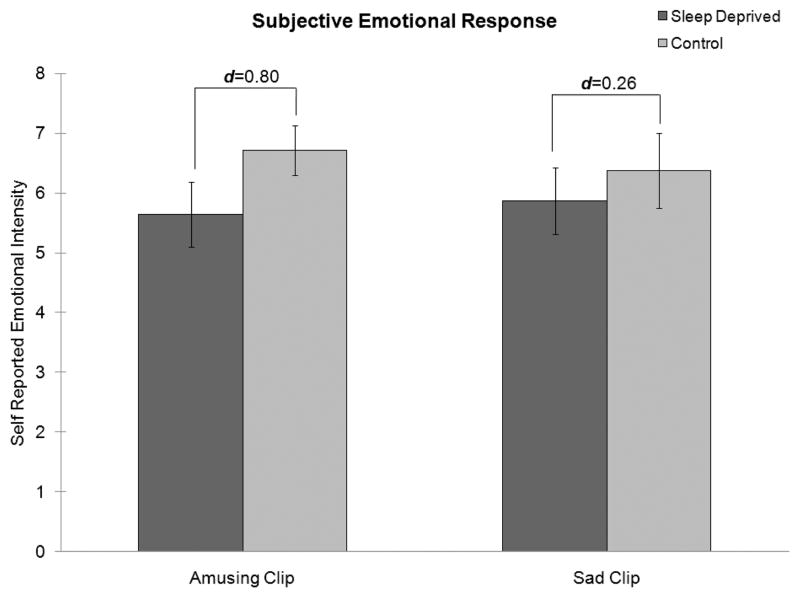
Subjective responses to film clips were analyzed with a repeated measures analysis of variance with group (sleep-deprived or control) as a between-subject factor and response to video as a within-subjects factor. The effect of sleep deprivation on subjective responses to films failed to meet criteria for statistical significance, F(1, 15) = 2.03, p = .17. Sleep-deprived participants reported relatively less amusement than controls in response to the amusing film clip (d = 0.80) and relatively less sadness than controls in response to the sadness-inducing clip (d = 0.26). These results are shown in Figure 1.
FIGURE 1.
Mean subjective emotional responses to an amusing and a sad film clip for sleep-deprived participants (dark gray columns) and rested control participants (light gray lines). Note. The effect of sleep deprivation failed to meet criteria for statistical significance (p = .17). The sleep-deprived participants reported less intense reactions to both the amusing clip (d = 0.80) and the sad clip (d = 0.26) than the rested control participants. Error bars represent standard errors.
Facial Expressiveness in Response to Film Clips
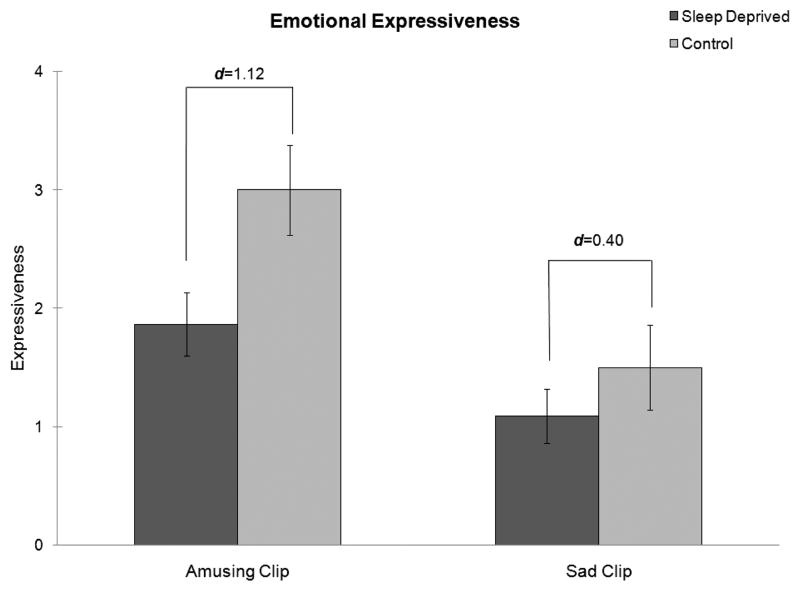
There were no significant differences between groups in facial expressiveness prior to sleep deprivation, F(1, 19) = 0.59, p = .45. Differences between sleep-deprived and control participants after the sleep manipulation were investigated using repeated measures analysis of variance. There was a significant effect of condition, demonstrating that sleep-deprived participants were significantly less expressive than control participants overall, F(1, 20) = 4.57, p = .045. The Emotion × Group interaction failed to meet criteria for statistical significance, F(1, 20) = 1.81, p = .19, indicating that the effect was not due exclusively to differences in response to the positive or negative film clip. There was a larger effect of sleep deprivation on expressiveness in response to the amusing film clip (d = 1.12) than on expressiveness in response to the sad clip (d = 0.40). These results are summarized in Figure 2.
FIGURE 2.
Mean facial expressiveness in response to an amusing and a sad film clip for sleep-deprived participants (dark gray columns) and rested control participants (light gray lines). Note. There was a significant main effect of sleep deprivation on expressiveness (p = .045). The interaction of Sleep Condition × Type of Clip failed to reach statistical significance (p = .19). Sleep deprivation was associated with a larger effect (d = 1.12) for the amusing clip than for the sad clip (d = 0.40). Error bars represent standard errors.
DISCUSSION
It is widely believed that inadequate sleep has deleterious effects on emotional functioning, but only a handful of studies have directly investigated this hypothesis in an experimental context. This study is one of the first to examine how sleep deprivation influences basic emotional processes in healthy adults. We tested the hypothesis that sleep loss would result in disinhibited emotional processes against the hypothesis that de-arousal due to sleep loss would result in flattened emotional responses. We found that facial expressiveness, one of the primary ways in which emotion is communicated, was lower in sleep-deprived participants than in rested control participants. These results favored the de-arousal hypothesis, highlighting the importance of areas other than the prefrontal cortex in emotional functioning after sleep loss. Nevertheless, it is clear that inhibitory control is compromised by sleep deprivation (Chua, Venkatraman, Dinges, & Chee, 2006), and neural areas necessary for emotion regulation are altered (Yoo et al., 2007). Future theoretical models of sleep and emotion will need to address both components to adequately represent the complexities of emotional responses in a sleep-deprived state.
Although sleep-deprived participants demonstrated a reduction in facial expressiveness, they did not report equivalent reductions in subjective emotional experiences. The lower self-reported emotional responses in the sleep-deprived group relative to the control group failed to meet criteria for statistical significance, but the relatively small sample size prevents strong conclusions from null findings. Nevertheless, effect size estimates suggested that sleep deprivation had a moderate to large effect on subjective responses, which was in the same direction as the effects on facial expressiveness. Additional studies with larger samples are needed to get a more accurate estimate of these effects; but, based on our findings, we would predict that sleep deprivation has a larger effect on behavioral indicators of emotion than on the subjective experience of emotion.
The larger response of objective indicators of emotion relative to subjective responses may provoke a question of what the “real” influence of sleep deprivation was on emotion. This question is perhaps best addressed by remembering that emotions are multifaceted constructs involving dissociable components of subjective feeling states and emotional expression (Ekman & Oster, 1979). Both are real aspects of emotion, but the former is the private experience of the emotion, and the latter is a public display of it. Previous studies, especially in clinical populations, have clearly demonstrated the dissociability of these two features of emotion (for an example in schizophrenia, see Kring, Kerr, Smith, & Neale, 1993). Our findings suggest that sleep deprivation may degrade the normal relationship between public and private emotional responses, causing people to appear less emotional than they feel. This hypothesis should be investigated in future studies using objective measures (e.g., psychophysiology, neuroimaging, behavioral, etc.), as well as self-report measures of emotion.
People who are chronically unable to attain sufficient sleep, whether due to busy lifestyles or medical conditions, may experience significant consequences from dampened emotional expressiveness. Several decades of research have established many important functions for facial expressions of emotion including eliciting help, primarily through negative emotional displays; and maintaining relationships and reinforcing desired behaviors in others, primarily through positive emotional displays (for a detailed review, see Keltner, Ekman, Gonzaga, & Beer, 2003). A reduction in emotional expressiveness could, therefore, have serious consequences for long-term social functioning and well-being. Additional studies are needed to determine whether or not reduced facial expressiveness is a clinically relevant symptom of inadequate sleep in real-world settings.
Limitations and Future Directions
This study has a number of limitations, the most important of which is the relatively small sample size. Several between-group differences failed to meet criteria for statistical significance, despite moderate effect size estimates. Although the high costs of laboratory-based studies may prevent using very large samples, future studies should seek to maximize statistical power by using larger samples and experimental designs that allow for more powerful statistical procedures.
In addition to increasing statistical power, we recommend that future studies quantify sleep using polysomnography (PSG) if possible, rather than actigraphy as we did. PSG offers a better estimate of total sleep time and allows sleep stages to be measured. Larger studies using PSG could investigate relationships between sleep variables and emotional functioning; but, given our small sample size, it was unlikely that we would have detected any such relationships.
Finally, we recommend that future studies analyze additional variables related to facial expressiveness. This study focused on a global measure of overall expressiveness that relied on human raters. Using other methods for quantifying expressiveness, such as electromyography and computerized analysis, may allow for a more detailed description of the influence of sleep deprivation on emotional expressions.
Acknowledgments
This research was supported by the National Space and Biomedical Research Institute through NASA NCC 9-58 and the National Institutes of Health through National Research Service Award F31 MH079604.
We thank Amaka Anekwe, Cara Bergamo, Danielle Brinckman, Michele Carlin, Hilary Caruso, and Adrian Ecker.
Contributor Information
Jared Minkel, Department of Psychology and the School of Medicine, University of Pennsylvania.
Oo Htaik, School of Medicine, University of Pennsylvania.
Siobhan Banks, School of Medicine, University of Pennsylvania, Centre for Sleep Research, University of South Australia.
David Dinges, School of Medicine, University of Pennsylvania.
References
- Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. Journal of Clinical Sleep Medicine. 2007;3:519–528. [PMC free article] [PubMed] [Google Scholar]
- Bartko JJ. The intraclass correlation coefficient as a measure of reliability. Psychological Reports. 1966;19:3–11. doi: 10.2466/pr0.1966.19.1.3. [DOI] [PubMed] [Google Scholar]
- Beck A, Steer RA, Brown GK. Manual for the Beck Depression Inventory–II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders. A meta-analysis. Archives of General Psychiatry. 1992;49:651–668. doi: 10.1001/archpsyc.1992.01820080059010. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Miccoli L, Escrig MA, Lang PJ. The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology. 2008;45:602–607. doi: 10.1111/j.1469-8986.2008.00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuah YML, Venkatraman V, Dinges DF, Chee MWL. The neural basis of interindividual variability in inhibitory efficiency after sleep deprivation. Journal of Neuroscience. 2006;26:7156–7162. doi: 10.1523/JNEUROSCI.0906-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1988. [Google Scholar]
- Dahl RE, Lewin DS. Pathways to adolescent health sleep regulation and behavior. Journal of Adolescent Health. 2002;31(Suppl 6):175–184. doi: 10.1016/s1054-139x(02)00506-2. [DOI] [PubMed] [Google Scholar]
- Darwin C. The expression of the emotions in man and animals. New York: Appleton; 1871. [Google Scholar]
- Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Seminars in Neurology. 2005;25:117–129. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- Ekman P, Oster H. Facial expressions of emotion. Annual Review of Psychology. 1979;30:527–554. [Google Scholar]
- Franzen PL, Siegle GJ, Buysse DJ. Relationships between affect, vigilance, and sleepiness following sleep deprivation. Journal of Sleep Research. 2008;17:34–41. doi: 10.1111/j.1365-2869.2008.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ, Levenson RW. Emotion elicitation using films. Cognition and Emotion. 1995;9:87–108. [Google Scholar]
- Keltner D, Ekman P, Gonzaga G, Beer J. Facial expression of emotion. In: Davidson RJ, Scherer KR, Goldsmith HH, editors. Handbook of affective sciences. New York: Oxford University Press; 2003. pp. 415–432. [Google Scholar]
- Kring AM, Kerr SL, Smith DA, Neale JM. Flat affect in schizophrenia does not reflect diminished subjective experience of emotion. Journal of Abnormal Psychology. 1993;102:507–517. doi: 10.1037//0021-843x.102.4.507. [DOI] [PubMed] [Google Scholar]
- Kring AM, Sloan DM. The facial expression coding system (FACES): Development, validation, and utility. Psychological Assessment. 2007;19:210–224. doi: 10.1037/1040-3590.19.2.210. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Ekman P, Friesen WV. Voluntary facial action generates emotion specific autonomic nervous system activity. Psychophysiology. 1990;27:363–384. doi: 10.1111/j.1469-8986.1990.tb02330.x. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Science. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Roehrs T, Roth T. Sleep and pain: Interaction of two vital functions. Seminars in Neurology. 2005;25:106–116. doi: 10.1055/s-2005-867079. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Ray RD, Gross JJ. Emotion elicitation using films. In: Coan JA, Allen JJB, editors. Handbook of emotion elicitation and assessment. New York: Oxford University Press; 2007. pp. 9–28. [Google Scholar]
- Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing rater reliability. Psychological Bulletin. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Yoo SS, Gujar N, Hu P, Jolesz FA, Walker MP. The human emotional brain without sleep—A prefrontal amygdala disconnect. Current Biology. 2007;17:R877–R878. doi: 10.1016/j.cub.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Zohar D, Tzischinsky O, Epstein R, Lavie P. The effects of sleep loss on medical residents’ emotional reactions to work events: A cognitive-energy model. Sleep. 2005;28:47–54. doi: 10.1093/sleep/28.1.47. [DOI] [PubMed] [Google Scholar]