Abstract
Objective
To compare the long-term effectiveness and cost-effectiveness of 3 approaches to managing elevated blood pressure (BP) in adolescents in the United States: no intervention, “screen-and-treat,” and population-wide strategies to lower the entire BP distribution.
Study design
We used a simulation model to combine several data sources to project the lifetime costs and cardiovascular outcomes for a cohort of 15-year-old U.S. adolescents under different BP approaches and conducted cost-effectiveness analysis. We obtained BP distributions from the National Health and Nutrition Examination Survey 1999–2004 and used childhood-to-adult longitudinal correlation analyses to simulate the tracking of BP. We then used the coronary heart disease policy model to estimate lifetime coronary heart disease events, costs, and quality-adjusted life years (QALY).
Results
Among screen-and-treat strategies, finding and treating the adolescents at highest risk (eg, left ventricular hypertrophy) was most cost-effective ($18 000/QALY [boys] and $47 000/QALY [girls]). However, all screen-and-treat strategies were dominated by population-wide strategies such as salt reduction (cost-saving [boys] and $650/ QALY [girls]) and increasing physical education ($11 000/QALY [boys] and $35 000/QALY [girls]).
Conclusions
Routine adolescents BP screening is moderately effective, but population-based BP interventions with broader reach could potentially be less costly and more effective for early cardiovascular disease prevention and should be implemented in parallel.
Hypertension affects one in 3 adult Americans and is a major risk factor for coronary heart disease (CHD), stroke, and end-stage renal disease.1 Among US adolescents, blood pressure (BP) levels have risen substantially in recent decades, largely because of the obesity epidemic.2 Although end-stage organ damage is rare in children, childhood BP is the strongest predictor of adult hypertension.3,4 The evidence base is well established for the clinical value of diagnosing and treating adults with hypertension, either by medication or lifestyle change.1,5 Whether such “screen-and-treat” approach is equally effective and cost-effective in adolescent hypertension is unknown.
Currently, annual BP screening is recommended for children 3 years of age and older by the American Academy of Pediatrics, the National Heart, Lung, and Blood Institute, the American Medical Association, and the American Heart Association. In contrast, the US Preventive Services Task Force concludes that evidence is insufficient to recommend for or against routine BP screening in children and adolescents. On the other hand, all guidelines endorsed a public health approach to lower the entire pediatric BP distribution, despite limited evidence on its potential long-term impact. Although a decades-long clinical trial would inform the comparative effectiveness of different childhood BP strategies, it is unlikely to occur because of its high cost and time frame. A modeling framework to integrate multiple pieces of clinical and epidemiologic evidence is therefore required to address the effectiveness and cost-effectiveness of adolescent BP screening. In this study, we combined data on BP measurement, tracking, and intervention effect and predicted the lifetime effectiveness on reducing CHD events and associated medical costs under various BP management approaches among US adolescents with the CHD Policy Model.
Methods
We evaluated 3 approaches: (1) no screening/intervention; (2) “screen-and-treat;” and (3) population-wide strategies to lower BP of all adolescents. Under the “screen-and-treat” approach, we examined routine screening for all 15-year-olds, as well as selectively screening only overweight adolescents. Once elevated BP is found, adolescents were treated by individual-based behavioral programs, such as exercise, low-salt diet, and, if overweight, weight reduction. We also evaluated a variation of the screening strategy: treating only those with the highest risk, that is, secondary hypertension or left ventricular hypertrophy (LVH). Finally, we evaluated two hypothetical public health strategies that aim to lower the entire BP distribution, albeit with smaller effect for each individual: policy actions to reduce dietary salt intake and increasing physical education (PE) classes in schools.
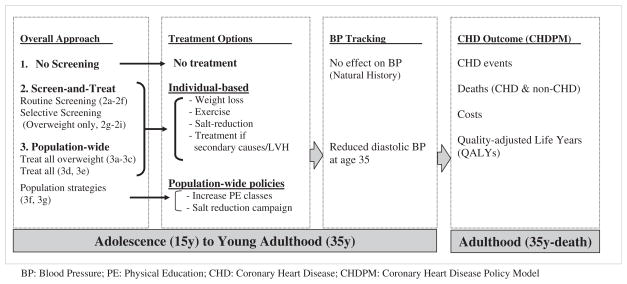
Two-Phased Model Structure (Figure 1; available at www.jpeds.com)
Figure 1.
Schematic diagram of the two-phased BP screening model.
Phase 1: Age 15 to 35 Years
We simulated a cohort of 2 065 127 boys and 1 952 694 girls (2000 US census). We estimated 20-year (age 15 to 35) longitudinal tracking coefficients (1) from combined data of the 8- to 12-year East Boston Study of 337 children and the 16-year follow-up of 822 Framingham Offspring Study subjects (Table I; available at www.jpeds.com). We grouped the baseline 15-year-old cohort into 3 diastolic BP categories: <79, 79–83, and >83 mm Hg (cutoffs correspond to the 90th and 95th percentile for 15-year-olds of median height).6 We used the tracking correlations, the BP distribution at age 15, and intervention effectiveness to predict BP distributions at age 35, the entry point to the CHD Policy Model (Appendix 1; available www.jpeds.com).
Table I.
Effect of longitudinal tracking coefficient values on the distribution of projected diastolic blood pressures at age 35 years, based on diastolic blood pressure at age 15 years
| Sex | DBP at age 15 years* | Longitudinal tracking correlation† | DBP distribution at age 35 years
|
||
|---|---|---|---|---|---|
| <80 mm Hg | 80–90 mm Hg | >90 mm Hg | |||
| Male | >83 mm Hg | 0 | 66.7 | 24.4 | 8.8 |
| 0.19 (lower bound) | 54.8 | 30.5 | 14.7 | ||
| 0.30 (midpoint) | 47.4 | 33.7 | 18.9 | ||
| 0.42 (upper bound) | 39 | 36.7 | 24.2 | ||
| 1 | 1.4 | 28.5 | 70.1 | ||
| 79–83 mm Hg | 0 | 66.7 | 24.4 | 8.8 | |
| 0.19 (lower bound) | 57.7 | 29.2 | 13 | ||
| 0.30 (midpoint) | 52.2 | 32 | 15.8 | ||
| 0.42 (upper bound) | 45.8 | 35.2 | 19 | ||
| 1 | 5.5 | 51 | 43.5 | ||
| <79 mm Hg | 0 | 66.7 | 24.4 | 8.8 | |
| 0.19 (lower bound) | 67.2 | 24.1 | 8.5 | ||
| 0.30 (midpoint) | 68.1 | 23.7 | 8.1 | ||
| 0.42 (upper bound) | 69.4 | 23.1 | 7.4 | ||
| 1 | 72.7 | 22.2 | 5.0 | ||
| Female | >83 mm Hg | 0 | 83 | 14 | 3 |
| 0.32 (lower bound) | 69 | 23.8 | 7.3 | ||
| 0.37 (midpoint) | 66.4 | 25.5 | 8.1 | ||
| 0.43 (upper bound) | 63.1 | 27.6 | 9.2 | ||
| 1 | 15.9 | 60.9 | 23.2 | ||
| 79–83 mm Hg | 0 | 83 | 14 | 3 | |
| 0.32 (lower bound) | 73.9 | 20.6 | 5.4 | ||
| 0.37 (midpoint) | 72.4 | 21.8 | 5.8 | ||
| 0.43 (upper bound) | 70.5 | 23.3 | 6.2 | ||
| 1 | 41.5 | 54.1 | 4.4 | ||
| <79 mm Hg | 0 | 83 | 14 | 3 | |
| 0.32 (lower bound) | 84.2 | 13.2 | 2.7 | ||
| 0.37 (midpoint) | 85.7 | 12.1 | 2.2 | ||
| 0.43 (upper bound) | 87.6 | 10.7 | 1.7 | ||
| 1 | 93.7 | 5.9 | 0.4 | ||
Based on the 90th and the 95th percentile for 15-year-old boys and girls of median height from the Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents.1
Longitudinal tracking correlations of diastolic blood pressure corrected for within-person variance.
Phase 2: Age 35 Years to Death: CHD Policy Model
The CHD Policy Model is a Markov cohort model of CHD incidence, prevalence, death, and costs in the US population aged 35 years and older (Appendix 2; available at www.jpeds.com). The demographic-epidemiologic submodel predicts incidence of CHD and deaths from other causes, stratified by age, sex, and up to 6 additional categorized risk factors: diastolic blood pressure ([DBP] <80, 80–90, >90 mm Hg), smoking status, high-density lipoprotein (HDL) cholesterol (<40, 40–59, ≥60 mg/dL), low-density lipoprotein (LDL) cholesterol (<100, 100–129.9, ≥130 mg/dL), BMI (<25, 25–29, >30 kg/ m2), and diabetes mellitus. The distributions of risk factor categories derive from the National Health and Nutrition Examination Survey (NHANES) 1999–2004. Each state and event has an annual cost and health-related quality-of-life adjustment.7 All simulated cohorts follow an age-related trend in mean BP, hypertension treatment, and disease incidence based on US data.
BP Screening among US Adolescents
We estimated BP distributions (systolic or diastolic BP ≥95th percentile, 90th–95th percentile, or <90th percentile)6 of U.S. adolescents 13–17 years of age (averaging age 15) from NHANES 1999–2004 (n = 3887).8 We defined overweight as body mass index (BMI) at or above the age- and sex- specific 85th percentile on the basis of the growth standards of the Centers for Disease Control and Prevention.9
We assumed that BMI and BP measurements occur during routine well-child visit and incur no marginal cost, but two additional office visits are required to confirm a diagnosis of hypertension.6 We assumed that 30% of children with high first-visit BP would consistently have hypertension throughout 3 visits.10 In our base case, we assumed that 30% of adolescents with hypertension have LVH.11 We assumed that most adolescents with elevated BP had essential hypertension, and 5% of them have secondary causes.12 We estimated that diagnostic tests to establish causes and comorbidities cost $659 (Table II).13
Table II.
Baseline values for model variables, key assumptions, and sources
| Parameter | Base Case | Source |
|---|---|---|
| Epidemiology of hypertension and screening | ||
| Prevalence of elevated BP at one screen | 26% (M), 7.5% (F) | NHANES |
| %Confirm hypertension given elevated BP at first screen | 30.24% | Sorof et al10 |
| Prevalence of secondary hypertension | 5% | Published data |
| Proportion of secondary hypertension curable | 10% | Prevalence of renal artery stenosis or coarctation of aorta19,32 |
| % Hypertensive adolescents with left ventricular hypertrophy | 30% | Published studies11 |
| Discount rate | 3% | |
| Costs | ||
| Preventive visit to pediatrician’s office | $95 | U.S. Average based on AAP estimates |
| Diagnostic workup for established hypertension for secondary causes or end-organ damage such as left ventricular hypertrophy | $659 | Medicare payment rate for phlebotomy, electrolytes, urea nitrogen, and creatinine, lipid profile, complete blood count, urinalysis, fasting glucose, echocardiography, and renal ultrasound. |
| Treatment of secondary hypertension | $30 000 | Published estimates for coarctation of the aorta and renal artery stenosis20,21 |
| Individual-based weight reduction | $941 | Average between two intervention arms in Goldfield et al33 |
| Individual-based exercise program | $710 | Average between two intervention arms in Sevick et al17 |
| Individual-based dietary counseling on salt reduction | $515 | 4 weekly sessions with dietician18 |
| Pharmacotherapy | $334/year | Published estimates |
| Increase PE classes in high schools | $183/student | Adding 2 classes per week for 3 years (two 18-week semesters per school year), assuming median wage for high school teachers18 and average class size of 40. |
| Policy to promote salt reduction in food & media-based education | $1.70/person | Upper bound of published estimates in upper middle-income countries to achieve a 15% reduction in salt intake.23 |
| Effectiveness of BP Treatments | ||
| Weight reduction program, effect on DBP | −3.57 mm Hg | Published meta-analysis14 |
| Effect of exercise program on DBP | −2.00 mm Hg | Published meta-analysis15 |
| Effect of salt reduction program on DBP | −1.29 mm Hg | Published meta-analysis16 |
| Effect of pharmaceutical treatment on DBP | −8.60 mm Hg | Silverstein et al22 |
| % fail individual-based program to achieve BP goal | 50% | Expert opinion |
| Annual decline in treatment effectiveness | 5% | Assumption |
| Effect of increasing PE classes on DBP | −1.00 mm Hg | Assumed half of exercise program |
| Effect of population-based salt reduction on DBP | −0.35 mm Hg | 27% of dietician-based salt reduction programs23 |
Effectiveness and Costs of Individual-Based Behavioral Interventions
We used estimates from meta-analyses for the effectiveness of interventions on diastolic BP: weight reduction (−3.59 mm Hg),14 exercise (−2.00 mm Hg),15 and salt restriction (−1.29 mm Hg) (Table II).16 In our base case analysis, we assumed that the effects of these interventions decline by 5% every year. This assumption was a conservative estimate on the basis of the reported maintenance of intervention effect among studies reviewed in the 3 meta-analyses.14–16 We subsequently varied this assumption in the sensitivity analysis from 0% to 10%. We also estimated the average cost of a 12-month weight management program to be $941 and an exercise program similar to Project ACTIVE17 to be $710 per participant. Dietary counseling programs focusing on salt reduction were assumed to involve 4 weekly hour-long sessions by nutritionists and cost $515.18
Among the 5% of adolescents with secondary hypertension, we assumed that 10% of them have potentially curable conditions such as renal artery stenosis or aortic coarctation.19 We assumed that treatment cost $30 000 per case,20,21 and once diagnosed, these adolescents would be treated effectively and safely. For the remaining 90% of secondary hypertension cases, we assumed they would receive antihypertensive medications.22
Effectiveness and Costs of Population-Wide Interventions without Screening
Voluntary reduction in salt content by food manufacturers combined with a mass-media campaign could achieve a 15% population-wide reduction in salt intake,23 approximately 27% of the effect size from salt-reduction trials. Proportionally, we assumed that population-wide salt-reduction campaigns would result in 27% of the BP-lowering effect of an individual-based intervention (ie, 0.27 × −1.29 mm Hg = −0.35 mm Hg) and cost $1.70/person.23 On the basis of the median salary of secondary school teachers,18 adding two PE classes per week for 3 academic years would cost ~$183/ student.
Cost-Effectiveness Analysis
For the base case analysis, we calculated the average lifetime cost and health effects, including number of CHD events, life-years, and quality-adjusted life years (QALYs) of all screening and intervention strategies, as well as no screening or intervention. We adopted a societal perspective and discounted all future direct medical costs and health effects at 3% annually.24 We calculated cost-effectiveness ratios for each strategy as costs per QALY gained compared with no screening/intervention, as well as incrementally compared with all other less costly alternatives.24
In a series of sensitivity analyses, we varied each parameter input for cost-effectiveness one at a time to reflect the impact of uncertainty on base case result. In a scenario analysis, we accounted for the potential non-BP cardiovascular benefits from weight reduction, that is, lower LDL cholesterol, higher HDL cholesterol, and lower diabetes risk.25 Finally, we modified the Model to use systolic BP instead of diastolic BP to characterize BP-related cardiovascular risks and the effectiveness of childhood screening and treatment.
Results
With the recommended cut points,6 the prevalence of elevated BP at one screening visit among NHANES adolescents was 26% for boys and 7.5% for girls. After two follow-up visits, we estimated that the prevalence of confirmed hypertension and prehypertension was, respectively, 3.9% and 22.1% in boys and 1.9% and 5.6% in girls. From East Boston Study and the Framingham Offspring Study, the estimated longitudinal tracking coefficients from age 15 to 35 were 0.30 for males and 0.37 for females, consistent with other reported values.26
Cost-Effectiveness of Strategies Compared with No Screening/Intervention
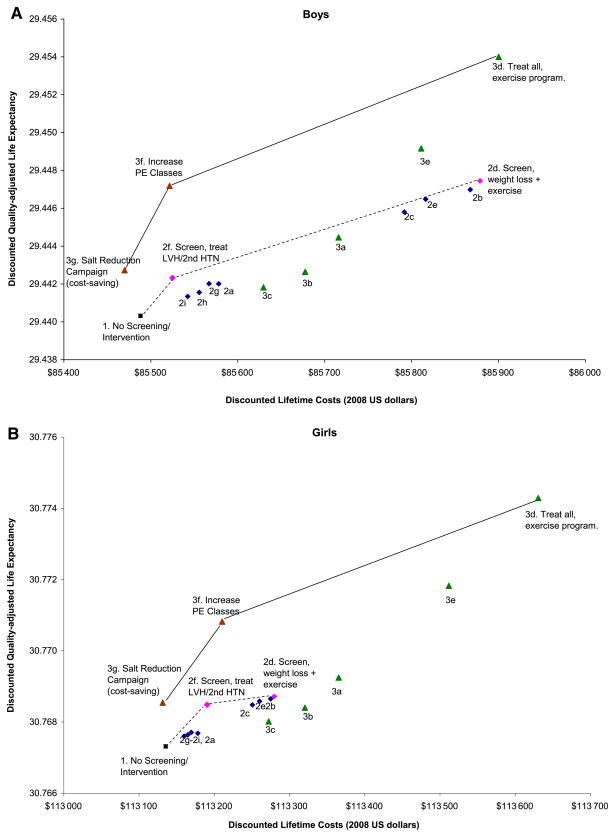
Compared with no screening/treatment, we estimated that routine screening and treating adolescents who have prehypertension or hypertension would increase life expectancy by 2.1 to 8.6 days among 15-year-old boys and by 0.5 to 1.8 days among girls (Table III). The average cost-effectiveness (compared with no screening/intervention) of routine BP screening followed by individual-based weight loss, exercise, or salt reduction interventions, ranging from $61 000 to $67 000 per QALY gained for boys and $116 000 to $135 000 per QALY gained for girls (Figure 2; available at www.jpeds.com). Selectively screening overweight adolescents (strategy 2g) was more cost-effective than routinely screening all adolescents ($54 000/QALY for boys and $103 000/QALY for girls; Table III). Conversely, routine BP screening followed by diagnosis and treatment only for adolescents with LVH or secondary hypertension (strategy 2f), a “high-risk only” approach, resulted in quite attractive average cost-effectiveness ratios: $18 000/QALY for boys and $47 000/QALY for girls.
Table III.
Costs and effects of blood pressure control strategies for adolescents in 2000, relative to no screening or intervention
| Strategy | Life years gained | ΔCost* (×$1000) | Δ QALYs* | Cost per QALY† |
|---|---|---|---|---|
| Boys | ||||
| 1. No Screening or intervention (status quo comparator) | ||||
| 2. Screening BP, followed by interventions for adolescents with elevated BP Universal screening, if high BP: | ||||
| 2a. Weight loss program if obese | 11 324 | $193 642 | 3130 | $61 874 |
| 2b. Exercise program | 44 495 | $814 462 | 12 319 | $66 113 |
| 2c. Salt reduction program | 36 603 | $652 267 | 10 134 | $64 364 |
| 2d. Weight loss program if obese, exercise program otherwise | 47 590 | $839 880 | 13 177 | $63 739 |
| 2e. Weight loss program if obese, salt reduction otherwise | 41 116 | $706 153 | 11 387 | $62 016 |
| 2f. Pharmacologic or surgical treatment if secondary hypertension/LVH | 14 914 | $76 116 | 4125 | $18 450 |
| Selective screening: screen overweight adolescents only, if high BP: | ||||
| 2g. Weight loss program | 11 329 | $170 633 | 3142 | $54 309 |
| 2h. Exercise program | 8238 | $145 216 | 2284 | $63 573 |
| 2i. Salt reduction program | 6773 | $116 770 | 1880 | $62 121 |
| 3. Population-wide approaches to lower entire BP distribution: Individual-based behavioral programs without screening (active) | ||||
| 3a. Weight loss program for all overweight adolescents | 27 658 | $490 272 | 7657 | $64 027 |
| 3b. Exercise program for all overweight adolescents | 15 514 | $402 214 | 4298 | $93 586 |
| 3c. Salt reduction program for all overweight adolescents | 10 013 | $299 330 | 2774 | $107 921 |
| 3d. Exercise program for all adolescents | 91 435 | $915 306 | 25 303 | $36 174 |
| 3e. Salt reduction program for all adolescents Policy or environmental strategies (passive) | 59 077 | $708 388 | 16 350 | $43 325 |
| 3f. Increasing PE classes | 45 861 | $101 434 | 12 694 | $7991 |
| 3g. Salt reduction campaign | 16 041 | ($26 431) | 4442 | ($5950) |
| Girls | ||||
| 1. No Screening or intervention (status quo comparator) | ||||
| 2. Screening BP, followed by interventions for adolescents with elevated BP Universal screening, if high BP: | ||||
| 2a. Weight loss program if obese | 2424 | $85 828 | 633 | $135 654 |
| 2b. Exercise program | 8669 | $281 807 | 2290 | $123 078 |
| 2c. Salt reduction program | 7582 | $233 038 | 2002 | $116 409 |
| 2d. Weight loss program if obese, exercise program otherwise | 9102 | $291 687 | 2405 | $121 285 |
| 2e. Weight loss program if obese, salt reduction otherwise | 8225 | $252 309 | 2174 | $116 066 |
| 2f. Pharmacologic or surgical treatment if secondary hypertension/LVH Selective screening: screen overweight adolescents only, if high BP: | 8683 | $107 508 | 2283 | $47 094 |
| 2g. Weight loss program | 2549 | $69 143 | 673 | $102 692 |
| 2h. Exercise program | 2096 | $59 433 | 555 | $107 088 |
| 2i. Salt reduction program | 1866 | $50 033 | 494 | $101 317 |
| 3. Population-wide approaches to lower entire BP distribution: Individual-based behavioral programs without screening (active) | ||||
| 3a. Weight loss program for all overweight adolescents | 12 578 | $463 147 | 3317 | $139 611 |
| 3b. Exercise program for all overweight adolescents | 7069 | $369 290 | 1865 | $198 063 |
| 3c. Salt reduction program for all overweight adolescents | 4548 | $272 467 | 1202 | $226 728 |
| 3d. Exercise program for all adolescents | 45 438 | $1 017 770 | 11 971 | $85 018 |
| 3e. Salt reduction program for all adolescents Policy or environmental strategies (passive) | 29 380 | $767 923 | 7743 | $99 172 |
| 3f. Increasing PE classes | 22 830 | $172 116 | 6017 | $28 604 |
| 3g. Salt reduction campaign | 7999 | $1364 | 2110 | $647 |
Discounted at 3% per year.
Cost-effectiveness ratio compared to no screening or intervention, also referred to as the average cost-effectiveness ratios.
Figure 2.
Average discounted lifetime costs (in 2008 dollars) and quality-adjusted life expectancy (years) for 15-year-old adolescents under different BP screening and intervention strategies, compared with no screening/intervention. A, Boys; B, Girls. Connecting lines are cost-effectiveness efficiency frontiers linking strategies that are not dominated by their alternatives. Solid lines correspond to the incremental cost-effectiveness analysis that compares all 3 types of strategies (Squares = no screening/ intervention, circles = screen-and-treat, and triangles = population-wide strategies). Dotted lines correspond to the incremental cost-effectiveness analysis that compares screening strategies only.
As a comparison, hypothetically treating all adolescents without BP screening in fact results in lower screening costs but higher treatment and overall costs, as well as greater QALY gains than screen-and-treat strategies. For instance, forgoing BP screening and treating all overweight adolescents with weight-loss programs (strategy 3a) avoided ~649 000 BP screenings but treated ~525 000 more overweight teens. As a result, for the same BP treatment option, (eg, weight loss, the cost-effectiveness for treating all overweight adolescents without screening [3a–3c]) was more attractive than the corresponding screen-and-treat strategy (Table III). Finally, population-wide policy interventions (3f and 3g) appear extremely cost-effective. A population-wide salt-reduction campaign was cost-saving for boys and cost only ~$650/QALY for girls. Adding PE classes in high schools was valued at $8000 per QALY gained for boys and ~$29 000 per QALY gained for girls, relative to no screening or intervention.
Incremental Cost-Effectiveness Analyses
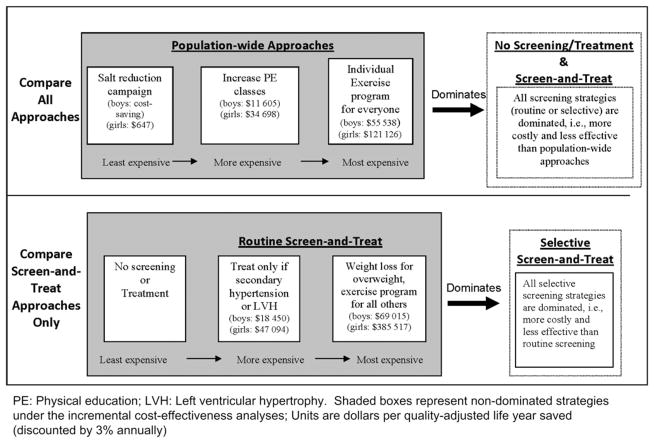
In a separate, incremental analysis, the cost-effectiveness of each strategy was evaluated by comparing its additional costs and its additional effectiveness relative to other less costly alternatives. Considering all strategies altogether, a population-wide salt reduction campaign (3g), increased PE classes (3f), and treating everyone with a structured exercise program (3d) dominated all screening strategies, meaning screen-and-treat approaches are more costly and less effective than their alternatives (Figure 3 and Table IV; available at www.jpeds.com). Increasing PE classes, a “passive” population-based approach to increasing physical activity among adolescents, was more attractive ($11 000/QALY for boys and $35 000/QALY for girls) than individual-based exercise programs for adolescents (~$55 000/QALY for boys and ~$120 000/QALY for girls).
Figure 3.
Results of incremental comparisons of adolescent blood pressure control strategies. Summary of two separate incremental cost-effectiveness analyses.
Table IV.
Incremental cost-effectiveness analyses
| Boys | Girls | |||
|---|---|---|---|---|
| Base Case Results | ||||
| 1. Compare all strategies | Non-dominated Strategies | ICER | Non-dominated Strategies | ICER |
| 3g. Salt reduction campaign | Cost saving | 3g. Salt reduction campaign | — | |
| 3f. Increasing PE classes | $ 11 605 | 3f. Increasing PE classes | $ 34 698 | |
| 3d. Exercise program for all | $ 55 538 | 3d. Exercise program for all | $ 121 126 | |
| Dominated* | 1, 2a–2i, 3a–3c, 3e–3g | 1, 2a–2i, 3a–3c, 3e–3g | ||
| 2. Compare only screening strategies | Non-dominated Strategies | ICER | Non-dominated Strategies | ICER |
| 1. No Screening or intervention | — | 1. No Screening or intervention | ||
| 2f. Screen & treat only secondary hypertension/LVH | $ 18 450 | 2f. Screen & treat only secondary hypertension/LVH | $ 47 094 | |
| 2d. Screen all+ weight loss if overweight, exercise otherwise | $ 69 015 | 2d. Screen all+ weight loss if overweight, exercise otherwise | $ 385 517 | |
| Dominated* | 2a–2c, 2e, 2g–2i | 2a–2c, 2e, 2g–2i | ||
| Alternative Scenario: Assuming Weight Loss Also Lowers LDL and Diabetes and Increases HDL | ||||
| 1. Compare all strategies | Non-dominated Strategies | ICER | Non-dominated Strategies | ICER |
| 3g. Salt reduction campaign | Cost saving | 1. No Screening or intervention | — | |
| 3a. Weight loss program for all | $ 13 648 | 3g. Salt reduction campaign | $ 647 | |
| 3d. Exercise program for all | $ 201 239 | 3a. Weight loss program for all | $ 19 715 | |
| Dominated* | 1, 2a–2i, 3b–3c, 3e–3f | 1, 2a–2i, 3a–3c, 3e–3g | ||
| 2. Compare only screening strategies | Non-dominated Strategies | ICER | Non-dominated Strategies | ICER |
| 1. No Screening or intervention | — | 1. No Screening or intervention | — | |
| 2g. Screen overweight+ weight loss | $ 5181 | 2g. Screen overweight+ weight loss | $ 7116 | |
| 2e. Screen all+ weight loss if overweight, salt reduction otherwise | $ 65 076 | 2e. Screen all+ weight loss if overweight, salt reduction otherwise | $ 76 893 | |
| 2d. Screen all+ weight loss if overweight, exercise otherwise | $ 86 699 | 2d. Screen all+ weight loss if overweight, exercise otherwise | $ 117 297 | |
| Dominated* | 2a–2c, 2e, 2g–2i | 2a–2c, 2e, 2g–2i | ||
A strategy of screening or intervention costs more but was less effective than its alternatives and was therefore dominated.
When we only compared among screening strategies, routine screening followed by treating secondary hypertension and LVH (2f, the “high-risk only” screening strategy) was cost-effective ($18 000/QALY for boys and $47 000/QALY for girls). Compared with such strategy, routine screening followed by treating individuals with hypertension (2d) was not very cost-effective: $69 000/QALY for boys and $386 000/QALY for girls.
Sensitivity Analysis
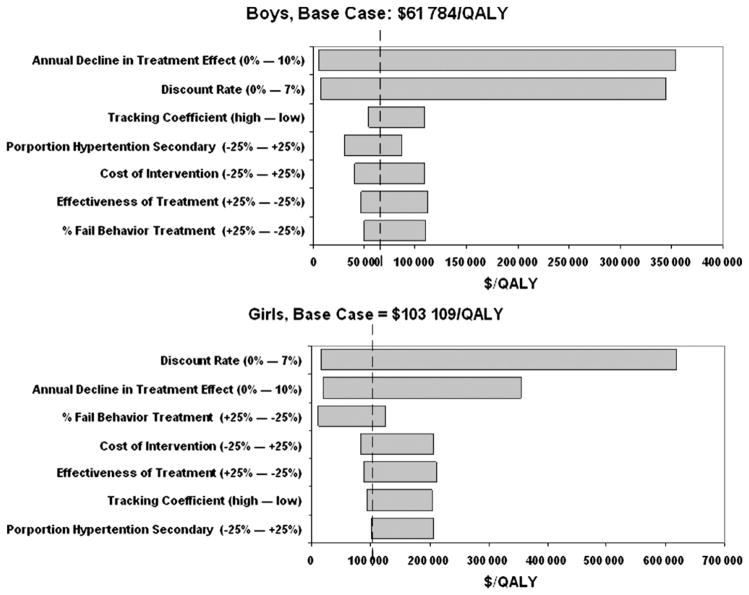
The average cost-effectiveness ratio of routine screening and treatment (strategy 2d) was relatively stable across most parameter inputs. One exception was the assumed discount rate (Figure 4), for example, compared with no screening, the cost-effectiveness of strategy 2d varied from ~$8000 to $336 000 per QALY (base case: $62 000) for boys and from $16 000 to $602 000 (base case: $103 000) for girls when the discount rate varied from 0% to 7%. The rate of decline in treatment effect also substantially affects the cost per QALY estimates (eg, the cost-effectiveness ratio varied from ~$5000 to $349 000 for boys and from $20 000 to $334 000 for girls per QALY as the annual decline changed from 0% to 10%).
Figure 4.
Sensitivity analyses (Tornado diagram): showing the degree to which uncertainty in individual variables affects the average cost-effectiveness ratio of routine BP screening followed by weight loss (if overweight) or exercise (if normal weight), compared with no screening/treatment. Ranges for annual decline in treatment effect and for discount rate are absolute percents. Other ranges are ± changes relative to base case.
When we assumed that weight loss has benefit beyond BP-lowering but also improves cholesterol and diabetes risk, selectively screening and treating overweight adolescents became more cost-effective. The most attractive screen-and-treat strategy was 2g—selective screening of overweight adolescents and treating those found to have hypertension with weight loss programs (~$5000/QALY for boys, ~$7000/ QALY for girls, compared with no screening). The next most attractive strategy was 2e, routine screening followed by weight loss or salt reduction (~$65 000/QALY in boys and ~$77 000/QALY in girls). Routine screening followed by weight loss or exercise (2d) also had a reasonable incremental cost-effectiveness ratio relative 2e (~$87 000/QALY in boys and $117 000/QALY in girls). Despite these ancillary benefits of weight loss boosting the cost-effectiveness of screen-and-treat strategies, population-wide approaches to adolescent BP control still dominated all screening strategies in the incremental cost-effectiveness analysis. A salt reduction campaign remained the most attractive (cost saving in boys and ~$650/QALY in girls). Adding PE classes, however, was dominated by individual-based weight loss interventions for all overweight adolescents without BP screening ($13 000/ QALY for boys and $20 000/QALY for girls).
When a systolic BP-based model is used, the cost-effectiveness ratio of routine BP screening and treating obese adolescents with weight loss and nonobese adolescents with salt reduction (strategy 2e) were $68 000/QALY in boys and $90 000/QALY in girls, compared with the base case results of $62 000/QALY in boys and $116 000/QALY in girls. All other screening strategies were still dominated by other alternatives in the incremental analysis. Population-wide salt reduction continued to be much more cost-effective ($6000–18 000/QALY) than routine screening (incremental cost-effectiveness ratio of strategy 2e: $87 000/QALY in boys and $166 000/QALY in girls).
Discussion
In this report, we used a modeling framework to demonstrate the effectiveness and cost tradeoffs between different approaches to early prevention of cardiovascular diseases through lowering BP in adolescence. We found that a routine screen-and-treat strategy could be effective in preventing future CHD burden but is not very cost-effective. Our analysis also suggests that a broad-reaching, policy-based intervention such as salt reduction or increasing PE classes could potentially be more cost-effective than most screen-and-treat strategies. This conclusion rests on the observation that a large number of future cases of CHD occur among youths with normal BP rather than those who have prehypertension or hypertension at age 15.27 However, the study underlines the value of public health approaches to lowering cardiovascular risk in the pediatric population. Of similar reasoning, Cook et al5 estimated that lowering diastolic BP by just 2 mm Hg among all persons 35 to 64 years of age could reduce stroke risk by 14% and CHD risk by 6%. More recently, Bibbins-Domingo et al28 demonstrated that the cardiovascular benefits from reducing a modest 1 g/d of dietary salt at the population level could be comparable with use of medications to lower BP in adults with hypertension.
There are several explanations for the more stark contrast between the effectiveness of screen-and-treat and population approaches in adolescents than in adults, in whom hypertension treatment is much more cost-effective.29 Only a small proportion of adolescents are eligible for pharmacotherapy; behavioral interventions tend to have low compliance and modest effects. As a result, the difference in treatment efficacy between individual-based BP-lowering programs and policy/ environmental approaches is narrower in childhood than in adulthood. Moreover, BP tracks imperfectly over long periods; many adolescents who are diagnosed with hypertension eventually have normal BP at older ages when BP better correlates with short-term cardiovascular risks. Effective treatment targeted at adults with hypertension is therefore more likely to result in immediate clinical benefits. Many adolescents with elevated BP who went through behavioral programs, however, would only accrue small benefit decades later, if at all.
A number of assumptions should be acknowledged which may make our cost-effectiveness analyses of adolescent BP screening unduly pessimistic. We assumed that the BP reduction resulted from behavioral changes earlier in life represent the same cardiovascular benefit as medically-treated hypertension later of the same magnitude. However, drug-treated hypertension in adults does not eliminate cardiovascular risks to the same extent as in patients with naturally-low BP.30 In addition, atherosclerosis starts in youth and may be more amenable to BP-lowering interventions. It is therefore possible that a lower BP achieved by behavioral interventions in childhood is more beneficial than treating adults with hypertension.31 Furthermore, we did not consider any synergy between BP interventions. For example, exercise improves the success of weight management, and weight loss also decreases BP sensitivity to salt.32 On the other hand, several assumptions might have led to optimistic estimates for screen-and-treat approach. We did not include quality of life decrement consequent to screening or intervention. Although obtaining a BP causes minimal discomfort, the disutility of undergoing further evaluation and behavioral interventions could be substantial. We did not include the time costs for making follow-up visits and receiving behavioral treatments, likely making screening and individual-based interventions appear more cost-effective.
The results of our model-based study are further constrained by the assumptions made and the uncertainty with the parameter inputs. Variations on the cost and effectiveness of treatment options can certainly alter the cost-effectiveness estimates. We did not consider the potential differences by race-ethnicity; however, studies have shown little racial-ethnic differences in adolescences than in adulthood.10 Despite these limitations, from a population standpoint, the rank-ordering of strategies appear stable across a wide range of parameter values, and we believe the base case analyses provide reasonable order-of-magnitude valuation of cost-effectiveness.
Because overweight and obesity are strongly linked with multiple cardiovascular risk factors, we were not surprised that our sensitivity analysis that included non-BP beneficial effects of weight loss showed selective screening of overweight adolescents to be more cost-effective than routine screening. Nevertheless, when we considered all approaches, population-wide strategies such as salt reduction and a weight loss program remained both less costly and more effective.
Preventing hypertension and its cardiovascular sequelae therefore should start in youth, especially in the face of the childhood obesity epidemic. Although BP screening and treatment of youths with hypertension are recommended as part of routine pediatric care, our results suggest that a screen-and-treat approach alone to childhood BP may not deliver the best “bang for the buck” at the population level. Policy and environmental interventions that broadly affect BP level of all youths may be more effective and more cost-effective; it is thus of high priority to ensure the implementation of these strategies in parallel to any expansion of clinical services to screen and treat children and adolescents with elevated BP.
Acknowledgments
Supported by National Institutes of Health (grant K24 HL 68041 to M.G.) and Harvard Pilgrim Health Care Foundation. The funding organizations have no role in the design and conduct of the study, collection, management, analysis, and interpretation of the data, and preparation, review, or approval of the manuscript.
We thank Stacy Trent, MD, PhD, and Yu-Ming (Albert) Shen, PhD, for their valuable assistance with the analysis, and Pamela Coxson, PhD, and Milton C. Weinstein, PhD, for their assistance in preparing the manuscript.
Glossary
- BP
Blood pressure
- CHD
Coronary heart disease
- DBP
Diastolic blood pressure
- HDL
High-density lipoprotein
- LDL
Low-density lipoprotein
- LVH
Left ventricular hypertrophy
- NHANES
National Health and Nutrition Examination Survey
- QALY
Quality-adjusted life years
Appendix 1
Calculations of Positive Predictive Values with Longitudinal Tracking Coefficients
We used the positive predictive value (PPV) to calculate the probability that children with observed mean diastolic blood pressure levels (yi0) at or above the 95th percentile (k) after three screens will have an observed mean diastolic blood pressure (yit) greater than the cutoff point z in young adulthood. The positive predictive value can be expressed as follows:
where and and where μpj(x) is the expected mean of the population at time j, ρ0t is the longitudinal tracking correlation from child-hood to young adulthood correlated for within-person variability, is the between-person variance and is the within-person variance at time j, and Φ is the cumulative normal distribution, this computational form for positive predictive value is mathematically equivalent to a more familiar expression: positive predictive value=(sensitivity × prevalence)/[(sensitivity × prevalence)+(1 − specificity) × (1 − prevalence)].
Appendix 2
The CHD Policy Model is a computer-simulated, state-transition (Markov cohort) model of CHD incidence, prevalence, mortality, and costs among persons older than 35 years in the U.S. population.1 In the version we used for this analysis, we apportioned persons age 35 to 85 years in the U.S. population with no history of CHD into 32 400 risk cells, de-fined by 6 modifiable risk factors: diastolic blood pressure (<80, 80 to 89.9, or ≥90 mm Hg), LDL cholesterol level (<2.6, 2.6 to 3.3, or ≥3.4 mmol/L [<100, 100 to 129.9, or ≥130 mg/dL]), HDL cholesterol level (<1.0, 1.0 to 1.5, or ≥1.6 mmol/L [<40, 40 to 59.9, or ≥60 mg/dL]), smoking status (active smoker, nonsmoker with exposure to environmental tobacco smoke, or nonsmoker without environmental exposure), diabetes mellitus (yes or no), and statin use (yes or no), as well as by sex and 10-year age range. We apportioned persons with prevalent CHD into 1300 cells according to their age, sex, and history of myocardial infarction, cardiac arrest, angina, or revascularization.
We determined CHD incidence and non-CHD deaths in persons with no previous CHD by logistic risk functions on the basis of Framingham longitudinal data.2 Transitions in the disease history component of the model were based on age range–specific event and case fatality rates estimated from national data and literature-based relative risks for events among disease history subgroups (such as previous myocardial infarction vs. none). Non-CHD mortality rates among persons with CHD reflected the relative risk of non-CHD death for this sample in the Framingham data. In the absence of evidence of a trend, we assumed all of these rates remained constant. Absolute numbers of events vary with temporal changes in the population, the age range distribution of the population, and in response to user-defined interventions.
All population distributions, risk factor levels, coefficients, event rates, case-fatality rates, costs, and quality-of-life adjustments can be modified for forecasting simulations. We ran the model on Fortran 95 (Lahey Computer Systems, Incline Village, Nevada).
CHD Prevalence
We estimated the background prevalence of CHD in 2000 from the National Health Interview Survey.3 We estimated the background prevalence of previous revascularization procedures from revascularizations before 2000 and estimated survival after revascularization from the National Hospital Discharge Survey (NHDS)4 and other studies.5,6
CHD Deaths
We obtained data on CHD deaths from the 2000 Vital Statistics Mortality Data.7 We estimated CHD deaths on the basis of International Classification of Diseases, 10th revision, codes I20 to I25, I46, and 2/3 of I49, I50, and I51.8,9 We considered other deaths to be non-CHD deaths.
Cardiac Arrest (Sudden Death) with Resuscitation
The number of persons who survive from cardiac arrest to hospital discharge was estimated from the NHDS4 for 1990 to 1999. Because this number is small in any given year, we averaged the national estimates over the 10-year period.
We estimated prehospital cardiac arrest fatalities on the basis of Vital Statistics Mortality Data for selected causes by place of death.10 For International Classification of Diseases, 10th revision, codes I20 to I25, we assumed all emergency department deaths and those dead on arrival to be deaths from cardiac arrest. We considered all nursing home deaths to be chronic CHD deaths. We estimated that resuscitation was attempted for all in-residence and “other place” deaths on the basis of reported resuscitation rates for witnessed11 or unwitnessed12 cardiac arrest.
Proportion of Cardiac Arrests with No History of CHD
The CHD history is harder to ascertain for patients with cardiac arrest than for those with myocardial infarction because no national registry exists, the numbers are smaller, and fewer studies are available. We estimated the age range–specific proportions of cardiac arrest with and without a history of CHD by a least-squares fit to data from multiple sources.13,14
Myocardial Infarction
We estimated myocardial infarction target incidence as the average annual number of discharges coded as 410 in the NHDS 2000 data set. We eliminated records of myocardial infarction in which hospital stay was fewer than 3 days and no acute revascularization was done in the same hospitalization as probable “rule out myocardial infarction” cases. We reduced remaining counts by the double count fraction reported by Westfall and McGloin15 and applied an additional 3% deduction for miscoding, as reported by Petersen et al.16
Myocardial Infarction Case-Fatality Rates
We obtained mean number of myocardial infarction deaths per adjusted total myocardial infarction from the NHDS, 1996 to 2000, for the older age ranges (65 to 84 years) and used the National Registry of Myocardial Infarction17 for in-hospital case-fatality rates for the younger age ranges (35 to 44 years). Studies of young patients with myocardial infarction estimate an in-hospital mortality rate of 1% to 6%, compared with a rate of 8% to 22% for older patients.18
We estimated in-hospital and 30-day case-fatality rates from hospital discharge records from the State of California Office of Statewide Health Planning and Development for the year 2000.19 The in-hospital case-fatality rate was based on unique person records (duplicate entries eliminated by matching social security numbers). We omitted a small number of records that did not have social security numbers. The overall rate ratio of 30-day case-fatality rate to in-hospital case-fatality rate was 1.28953 (12.07/9.36). We used this ratio to adjust national inhospital case-fatality rates to 30-day mortality rates.
On the basis of the study by Rieves et al,20 we incorporated a mortality odds ratio of 1.6 for patients with previous myocardial infarction and 1.17 for patients with previous angina. Subset case-fatality rates were calculated to reflect these odds ratios and preserve the overall estimated case-fatality rate for myocardial infarction.
Revascularization Rates
We estimated the number of percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG) procedures from the NHDS for 2000. We adjusted the revascularization rate to reflect a repeated revascularization rate for PCI and for CABG in the first year. We estimated a trend in the ratio of PCI to CABG for 2000 to 2004. We assumed that PCI would be included as part of the treatment for myocardial infarction in the same proportion observed in the NHDS data set for 2000, with emergency CABG complicating 2% of these procedures. We included reductions in mortality and reinfarction rates for patients treated with PCI.21,22
Risk Functions for Incident CHD and Non-CHD Death
We determined incident CHD cases (myocardial infarction, cardiac arrest, or angina) and non-CHD deaths in each risk factor cell for the at-risk U.S. population by using risk functions (r) that incorporated age- and sex-specific parameters (α) and risk factor–specific β-coefficients (βk; k = 1, 2, 3, … , 6), which are constant over the time span of a simulation, and cell-specific risk factor means (mk; k = 1, 2, 3, … , 6), which are altered by user-defined intervention:
We determined β-coefficients for non-CHD death from the same examination sets of the Framingham cohort and offspring data that we used for the CHD β-coefficients, but with diastolic blood pressure, smoking, and diabetes as the only statistically significant covariates in the logistic regression analysis.
We estimated overall incidence of CHD and non-CHD death by age range and sex for 2000 by adjusting the Framingham incidence estimates for 1986 to take into account the trends in risk factor means from 1986 to 2000. We estimated the corresponding values of the intercepts by iterative fitting of the risk function to the overall incidence. We estimated incidence of cardiac arrest without previous CHD by using the proportion of cardiac arrest without previous CHD in Olmsted County13 and incidence of myocardial infarction by using the proportion of myocardial infarction without previous CHD in several published studies that analyzed data from the National Registry of Myocardial Infarction 2,23 the Cardiovascular Cooperative Project,24 and the Worcester Heart Attack study.17,25
We assumed all risks and rates to be constant over time, in the absence of evidence of a trend. We incorporated trends as they became apparent, such as that for the use of revascularization between 2000 and the present, but did not project them into the future. The CHD and non-CHD death risk functions are applied to every state in every year of a simulation to accommodate the competing risk for these 2 outcomes naturally over time.
Incident CHD Event Allocation
We assumed that risk factors would affect the incidence of myocardial infarction, cardiac arrest, and angina in proportion to overall incidence, except we assumed smoking had a higher relative risk for infarction and cardiac arrest26 and a proportionately lower coefficient for angina. We assumed that environmental tobacco exposure carried a relative risk of 1.26 for myocardial infarction and cardiac arrest, compared with nonexposed nonsmokers,27 but did not influence angina.
Risk Factor Prevalence and Correlations Between Risk Factors
We estimated the prevalence of each risk factor level and correlations among risk factors (and thus the apportionment of the U.S. population without CHD into the 3240 risk cells) from National Health and Nutrition Examination Study (NHANES), 1999 to 2004.28
Transitions between Risk Factor Levels
We included transfers from 1 risk factor level to another to preserve the NHANES proportions of the population with each risk factor level. For example, the proportion of men 35 to 44 years of age in the lowest DBP category (<80 mm Hg) is 0.628. For men 45 to 54 years of age, the proportion is 0.558. The shift toward higher DBP levels is associated with increasing BP in middle age. In higher age ranges, this trend reverses, so that by age 75 to 84 years, the proportion is 0.874. The change in the upper age ranges is probably caused by a more complex array of factors, such as people with higher risk being more likely to die.
Costs and Quality-of-Life Adjustments
We estimated total health care costs from the perspective of the health care system by using national data.29 We estimated the CHD cost component by using California data,19 deflated by using cost-to-charge ratios30 and the ratio of the U.S. national average costs to the California average31 and then inflated to 2006 dollars by using the Bureau of Labor Statistics Consumer Price Index for Medical Care Costs.32 We based health-related quality-of-life weights on observational data33 and discounted costs and QALYs at a rate of 3% per year.
Quality Control and Validation
The CHD Policy Model was calibrated to reproduce national data on risk factor distributions, total CHD deaths, acute myocardial infarction, witnessed sudden cardiac death and revascularization procedures in the base year. Validation of projections into the future is an ongoing effort in which the model’s results under a broad range of scenarios are compared with data from studies, clinical trials, and surveys, obtained from public sources or by personal communication. Validation required reasonable agreement in outcomes when the conditions that produced the data were incorporated. For example, simulations of persons in the US population age 45 to 64 years that imposed the before and after LDL cholesterol and HDL cholesterol levels recorded in the WOSCOPS (West of Scotland Coronary Prevention Study)34 resulted in similar results for the cumulative percentage of the cohort to have died of CHD or have had a first myocardial infarction, as well as for the ratio of events in participants who were and were not treated with statins.
- 1.The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(Suppl 4th Report):555–76. [PubMed] [Google Scholar]
- 2.Framingham Heart Study [CD-ROM] Washington, D.C: Department of Health and Human Services; 2005. [Google Scholar]
- 3.Centers for Disease Control and Prevention. [Accessed 25 August 2008];National Health Interview Survey. 2008 www.cdc.gov/nchs/nhis.htm.
- 4.Centers for Disease Control and Prevention. [Accessed 25 August 2008];National Hospital Discharge Survey. 2006 www.cdc.gov/nchs/about/major/hdasd/nhds.htm.
- 5.van Domburg RT, Foley DP, Breeman A, van Herwerden LA, Serruys PW. Coronary artery bypass graft surgery and percutaneous transluminal coronary angioplasty. Twenty-year clinical outcome. Eur Heart J. 2002;23:543–9. doi: 10.1053/euhj.2001.2821. [DOI] [PubMed] [Google Scholar]
- 6.Yusuf S, Zucker D, Passamani E, et al. Effect of coronary artery bypass graft surgery on survival: overview of 10-year results from randomised trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration. Lancet. 1994;344:563–70. doi: 10.1016/s0140-6736(94)91963-1. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. [Accessed 25 August 2008];Year 2000 Vital Statistics Mortality Data: Population Death by Cause. 2002 www.cdc.gov/nchs/data/dvs/wktbli.pdf.
- 8.Centers for Disease Control and Prevention. [Accessed 25 August 2008];Deaths for 358 selected causes, by 5-year age groups, race and sex: United States, 1999–2000. 2002 www.cdc.gov/nchs/data/statab/mortfinal2000_work292.pdf.
- 9.Consensus recommendations for the management of chronic heart failure. On behalf of the membership of the advisory council to improve outcomes nationwide in heart failure. Am J Cardiol. 1999;83:1A–38. [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. [Accessed 25 August 2008];GMWK307: Deaths from 39 selected causes by place of death, status of decedent when death occurred in hospital or medical center, and age: United States, 1999–2004. 2006 www.cdc.gov/nchs/datawh/statab/unpubd/mortabs/gmwk307.htm.
- 11.de Vreede-Swagemakers JJM, Gorgels APM, Dubois-Arbouw WI, et al. Out-of-hospital cardiac arrest in the 1990s: a population-based study in the Maastricht area on incidence, characteristics and survival. J Am Coll Cardiol. 1997;30:1500–5. doi: 10.1016/s0735-1097(97)00355-0. [DOI] [PubMed] [Google Scholar]
- 12.Kuisma M, Jaara K. Unwitnessed out-of-hospital cardiac arrest: is resuscitation worthwhile? Ann Emerg Med. 1997;30:69–75. doi: 10.1016/s0196-0644(97)70114-8. [DOI] [PubMed] [Google Scholar]
- 13.Goraya TY, Jacobsen SJ, Kottke TE, Frye RL, Weston SA, Roger VL. Coronary heart disease death and sudden cardiac death: a 20-year population-based study. Am J Epidemiol. 2003;157:763–70. doi: 10.1093/aje/kwg057. [DOI] [PubMed] [Google Scholar]
- 14.Albert CM, Chae CU, Grodstein F, et al. Prospective study of sudden cardiac death among women in the United States. Circulation. 2003;107:2096–101. doi: 10.1161/01.CIR.0000065223.21530.11. [DOI] [PubMed] [Google Scholar]
- 15.Westfall JM, McGloin J. Impact of double counting and transfer bias on estimated rates and outcomes of acute myocardial infarction. Med Care. 2001;39:459–68. doi: 10.1097/00005650-200105000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Petersen L, Wright S, Normand S-L, Daley J. Positive predictive value of the diagnosis of acute myocardial infarction in an administrative database. J Gen Intern Med. 1999;14:555–8. doi: 10.1046/j.1525-1497.1999.10198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaccarino V, Krumholz HM, Yarzebski J, Gore JM, Goldberg RJ. Sex differences in 2-year mortality after hospital discharge for myocardial infarction. Ann Intern Med. 2001;134:173–81. doi: 10.7326/0003-4819-134-3-200102060-00007. [DOI] [PubMed] [Google Scholar]
- 18.Choudhury L, Marsh J. Myocardial infarction in young patients. Am J Med. 1999;107:254–61. doi: 10.1016/s0002-9343(99)00218-1. [DOI] [PubMed] [Google Scholar]
- 19.California Patient Discharge Data January 1-December 31 2000, Public Version A-24 [CD-ROM] Sacramento: California Office of Statewide Health Planning and Development; 2001. [Google Scholar]
- 20.Rieves D, Wright G, Gupta G, Shacter E. Clinical trial (GUSTO-1 and INJECT) evidence of earlier death for men than women after acute myo-cardial infarction. Am J Cardiol. 2000;85:147–53. doi: 10.1016/s0002-9149(99)00652-9. [DOI] [PubMed] [Google Scholar]
- 21.Lagerqvist Bo, Husted S, Kontny F, et al. A long-term perspective on the protective effects of an early invasive strategy in unstable coronary artery disease: Two-year follow-up of the FRISC-II invasive study. J Am Coll Cardiol. 2002;40:1902–14. doi: 10.1016/s0735-1097(02)02572-x. [DOI] [PubMed] [Google Scholar]
- 22.Zijlstra F, Hoorntje JCA, de Boer M-J, et al. Long-term benefit of primary angioplasty as compared with thrombolytic therapy for acute myocardial infarction. N Engl J Med. 1999;341:1413–9. doi: 10.1056/NEJM199911043411901. [DOI] [PubMed] [Google Scholar]
- 23.Rogers WJ, Canto JG, Barron HV, et al. Treatment and outcome of myocardial infarction in hospitals with and without invasive capability. J Am Coll Cardiol. 2000;35:371–9. doi: 10.1016/s0735-1097(99)00505-7. [DOI] [PubMed] [Google Scholar]
- 24.Krumholz HM, Chen J, Chen Y-T, Wang Y, Radford MJ. Predicting one-year mortality among elderly survivors of hospitalization for an acute myocardial infarction: results from the Cooperative Cardiovascular Project. J Am Coll Cardiol. 2001;38:453–9. doi: 10.1016/s0735-1097(01)01395-x. [DOI] [PubMed] [Google Scholar]
- 25.Goldberg RJ, McCormick D, Gurwitz JH, Yarzebski J, Lessard D, Gore JM. Age-related trends in short- and long-term survival after acute myocardial infarction: a 20-year population-based perspective (1975–1995) Am J Cardiol. 1998;82:1311–7. doi: 10.1016/s0002-9149(98)00633-x. [DOI] [PubMed] [Google Scholar]
- 26.Parish S, Collins R, Peto R, et al. Cigarette smoking, tar yields, and non-fatal myocardial infarction: 14000 cases and 32000 controls in the United Kingdom. BMJ. 1995;311:471–7. doi: 10.1136/bmj.311.7003.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Law MR, Morris JK, Wald NJ. Environmental tobacco smoke exposure and ischaemic heart disease: an evaluation of the evidence. BMJ. 1997;315:973–80. doi: 10.1136/bmj.315.7114.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. [Accessed 25 August 2008];National Health and Nutrition Examination Survey. 2008 www.cdc.gov/nchs/nhanes.htm.
- 29.Centers for Medicare & Medicaid Services. [Accessed 14 Feburary 2008];Personal health care spending by type of spending, age group, and source of payment distribution, calendar year 1999. 2000 www.cms.hhs.gov/NationalHealthExpendData/downloads/agetables.pdf.
- 30.Office of Statewide Health Planning and Development. [Accessed 15 December 2008];Hospital finan-cial data for cost to charge ratio, CA inpatient discharge data hospital annual financial data, pivot profiles 1999–2000. 2008 www.oshpd.ca.gov/HID/Products/Hospitals/AnnFinanData/PivotProfles/default.asp.
- 31.U.S. Census Bureau. Average cost to community hospitals per patient, by state (Table 204) Washington: Government Printing Office; 1998. Statistical Abstract of the United States. [Google Scholar]
- 32.Bureau of Labor Statistics. [Accessed 25 August 2008];Consumer Price Index. 2008 www.bls.gov/CPI/
- 33.Fryback DG, Dasbach EJ, Klein R, et al. The Beaver Dam Health Outcomes Study: initial catalog of health-state quality factors. Med Decis Making. 1993;13:89–102. doi: 10.1177/0272989X9301300202. [DOI] [PubMed] [Google Scholar]
- 34.Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med. 1995;333:1301–8. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
Footnotes
The authors declare no conflicts of interest.
An earlier version of this analysis was presented in the 30th Annual Meeting of Society for Medical Decision Making (Philadelphia, PA) in October 2008.
References
- 1.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 2.Muntner P, He J, Cutler JA, Wildman RP, Whelton PK. Trends in blood pressure among children and adolescents. JAMA. 2004;291:2107–13. doi: 10.1001/jama.291.17.2107. [DOI] [PubMed] [Google Scholar]
- 3.Berenson G, Srinivansan S, Webber L, et al. Current Concepts. Kalamazoo, MI: Upjohn; 1991. Cardiovascular risk in early life: the Bogalusa Heart Study. [Google Scholar]
- 4.Gillman MW, Cook NR, Rosner B, Beckett LA, Evans DA, Keough ME, et al. Childhood blood pressure tracking correlations corrected for within-person variability. Stat Med. 1992;11:1187–94. doi: 10.1002/sim.4780110905. [DOI] [PubMed] [Google Scholar]
- 5.Cook NR, Cohen J, Hebert PR, Taylor JO, Hennekens CH. Implications of small reductions in diastolic blood pressure for primary prevention. Arch Intern Med. 1995;155:701–9. [PubMed] [Google Scholar]
- 6.The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–76. [PubMed] [Google Scholar]
- 7.Fryback DG, Dasbach EJ, Klein R, Klein BE, Dorn N, Peterson K, et al. The Beaver Dam Health Outcomes Study: initial catalog of health-state quality factors. Med Decis Making. 1993;13:89–102. doi: 10.1177/0272989X9301300202. [DOI] [PubMed] [Google Scholar]
- 8.National Center for Health Statistics. National Health and Nutrition Examination Survey (NHANES) http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm.
- 9.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002;11:1–190. [PubMed] [Google Scholar]
- 10.Sorof JM, Lai D, Turner J, Poffenbarger T, Portman RJ. Overweight, ethnicity, and the prevalence of hypertension in school-aged children. Pediatrics. 2004;113:475–82. doi: 10.1542/peds.113.3.475. [DOI] [PubMed] [Google Scholar]
- 11.Hanevold C, Waller J, Daniels S, Portman R, Sorof J. The effects of obesity, gender, and ethnic group on left ventricular hypertrophy and geometry in hypertensive children: a collaborative study of the International Pediatric Hypertension Association. Pediatrics. 2004;113:328–33. doi: 10.1542/peds.113.2.328. [DOI] [PubMed] [Google Scholar]
- 12.Chiong JR, Aronow WS, Khan IA, Nair CK, Vijayaraghavan K, Dart RA, et al. Secondary hypertension: current diagnosis and treatment. Int J Cardiol. 2008;124:6–21. doi: 10.1016/j.ijcard.2007.01.119. [DOI] [PubMed] [Google Scholar]
- 13.American Medical Association. Current Procedural Terminology. 4. 2008. [Google Scholar]
- 14.Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM. Influence of weight reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension. 2003;42:878–84. doi: 10.1161/01.HYP.0000094221.86888.AE. [DOI] [PubMed] [Google Scholar]
- 15.Kelley GA, Kelley KS, Tran ZV. The effects of exercise on resting blood pressure in children and adolescents: a meta-analysis of randomized controlled trials. Prev Cardiol. 2003;6:8–16. doi: 10.1111/j.1520-037x.2003.01224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He FJ, MacGregor GA. Importance of salt in determining blood pressure in children: meta-analysis of controlled trials. Hypertension. 2006;48:861–9. doi: 10.1161/01.HYP.0000245672.27270.4a. [DOI] [PubMed] [Google Scholar]
- 17.Sevick MA, Dunn AL, Morrow MS, Marcus BH, Chen GJ, Blair SN. Cost-effectiveness of lifestyle and structured exercise interventions in sedentary adults: results of project ACTIVE. Am J Prev Med. 2000;19:1–8. doi: 10.1016/s0749-3797(00)00154-9. [DOI] [PubMed] [Google Scholar]
- 18.Bureau of Labor Statistics, U.S. Department of Labor. Occupational Outlook Handbook, 2008–09 Edition. http://www.bls.gov/oco/
- 19.Rocchini AP. Cardiovascular causes of systemic hypertension. Pediatr Clin North Am. 1993;40:141–7. doi: 10.1016/s0031-3955(16)38486-3. [DOI] [PubMed] [Google Scholar]
- 20.Blaufox MD, Middleton ML, Bongiovanni J, Davis BR. Cost efficacy of the diagnosis and therapy of renovascular hypertension. J Nucl Med. 1996;37:171–7. [PubMed] [Google Scholar]
- 21.Garson A, Jr, Allen HD, Gersony WM, Gillette PC, Hohn AR, Pinsky WW, et al. The cost of congenital heart disease in children and adults. A model for multicenter assessment of price and practice variation. Arch Pediatr Adolesc Med. 1994;148:1039–45. doi: 10.1001/archpedi.1994.02170100037008. [DOI] [PubMed] [Google Scholar]
- 22.Silverstein DM, Champoux E, Aviles DH, Vehaskari VM. Treatment of primary and secondary hypertension in children. Pediatr Nephrol. 2006;21:820–7. doi: 10.1007/s00467-006-0087-5. [DOI] [PubMed] [Google Scholar]
- 23.Asaria P, Chisholm D, Mathers C, Ezzati M, Beaglehole R. Chronic disease prevention: health effects and financial costs of strategies to reduce salt intake and control tobacco use. Lancet. 2007;370:2044–53. doi: 10.1016/S0140-6736(07)61698-5. [DOI] [PubMed] [Google Scholar]
- 24.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276:1253–8. [PubMed] [Google Scholar]
- 25.Bibbins-Domingo K, Coxson P, Pletcher MJ, Lightwood J, Goldman L. Adolescent overweight and future adult coronary heart disease. N Engl J Med. 2007;357:2371–9. doi: 10.1056/NEJMsa073166. [DOI] [PubMed] [Google Scholar]
- 26.Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117:3171–80. doi: 10.1161/CIRCULATIONAHA.107.730366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis BR, Oberman A, Blaufox MD, Wassertheil-Smoller S, Zimbaldi N, Kirchner K, et al. Lack of effectiveness of a low-sodium/high-potassium diet in reducing antihypertensive medication requirements in overweight persons with mild hypertension. TAIM Research Group. Trial of Antihypertensive Interventions and Management. Am J Hypertens. 1994;7:926–32. doi: 10.1093/ajh/7.10.926. [DOI] [PubMed] [Google Scholar]
- 28.Bibbins-Domingo K, Chertow GM, Coxson PG, Moran A, Lightwood JM, Pletcher MJ, et al. Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med. 2010;362:590–9. doi: 10.1056/NEJMoa0907355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edelson JT, Weinstein MC, Tosteson AN, Williams L, Lee TH, Goldman L. Long-term cost-effectiveness of various initial monothera-pies for mild to moderate hypertension. JAMA. 1990;263:407–13. [PubMed] [Google Scholar]
- 30.Law MR, Wald NJ, Morris JK, Jordan RE. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 rando-mised trials. BMJ. 2003;326:1427. doi: 10.1136/bmj.326.7404.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collins R, Peto R, MacMahon S, Hebert P, Fiebach NH, Eberlein KA, et al. Blood pressure, stroke, and coronary heart disease. Part 2, Short-term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet. 1990;335:827–38. doi: 10.1016/0140-6736(90)90944-z. [DOI] [PubMed] [Google Scholar]
- 32.Rocchini AP, Key J, Bondie D, Chico R, Moorehead C, Katch V, et al. The effect of weight loss on the sensitivity of blood pressure to sodium in obese adolescents. N Engl J Med. 1989;321:580–5. doi: 10.1056/NEJM198908313210905. [DOI] [PubMed] [Google Scholar]