Abstract
Development of a functional auditory system in Drosophila requires specification and differentiation of the chordotonal sensilla of Johnston’s organ (JO) in the antenna, correct axonal targeting to the antennal mechanosensory and motor center (AMMC) in the brain, and synaptic connections to neurons in the downstream circuit. Chordotonal development in JO is functionally complicated by structural, molecular and functional diversity that is not yet fully understood, and construction of the auditory neural circuitry is only beginning to unfold. Here we describe our current understanding of developmental and molecular mechanisms that generate the exquisite functions of the Drosophila auditory system, emphasizing recent progress and highlighting important new questions arising from research on this remarkable sensory system.
Introduction
With anatomical locations on the head, thorax, abdomen or limbs, the diversity of insect hearing organs is superficially immense1. However, these organs can be classified into one of two forms; tympanal organs—those that detect pressure acoustic waves that potentially travel over long distances, the acoustic far field—and flagellar organs—those that are activated only close to the sound source by the disturbed air mass near the vibrating sound generator2. Remarkably, the mechanosensitive organs innervating both tympanal and flagellar organs belong to a single subtype of Type I sense organs (monociliated sensory cells with accessory cells), namely chordotonal organs, whose sensory units are called scolopidia. These operate as stretch receptors, arranged with apical attachments to the moving structure, and basal attachments to a relatively stationary reference point, usually another cuticular structure. In the case of auditory organs, the moving part is either the tympanum or the flagellar joint.
Despite the singularity of the sense organ type, and other similarities that clearly distinguish this group, there is also a broad diversity in morphological, developmental, molecular and physiological detail within chordotonal organs3. Chordotonal organs operate as proprioceptors, auditory organs, or sensors for gravity, wind, or temperature. Here we examine the Drosophila Johnston’s organ (JO), an antennal chordotonal organ of about 225 scolopidia that functions in hearing, gravity and wind sensation, that has been the subject of intense study, and that has allowed wondrous revelations about its development and operation. Significantly, key developmental genes and genes encoding structural components are conserved from the Drosophila JO to mammalian ears, making it feasible to use Drosophila for auditory gene discovery. For this reason, Drosophila is also an excellent system in which to test mechanisms of genes known to be important for human hearing, such as crinkled/myosinVIIA4, 5 and diaphanous6.
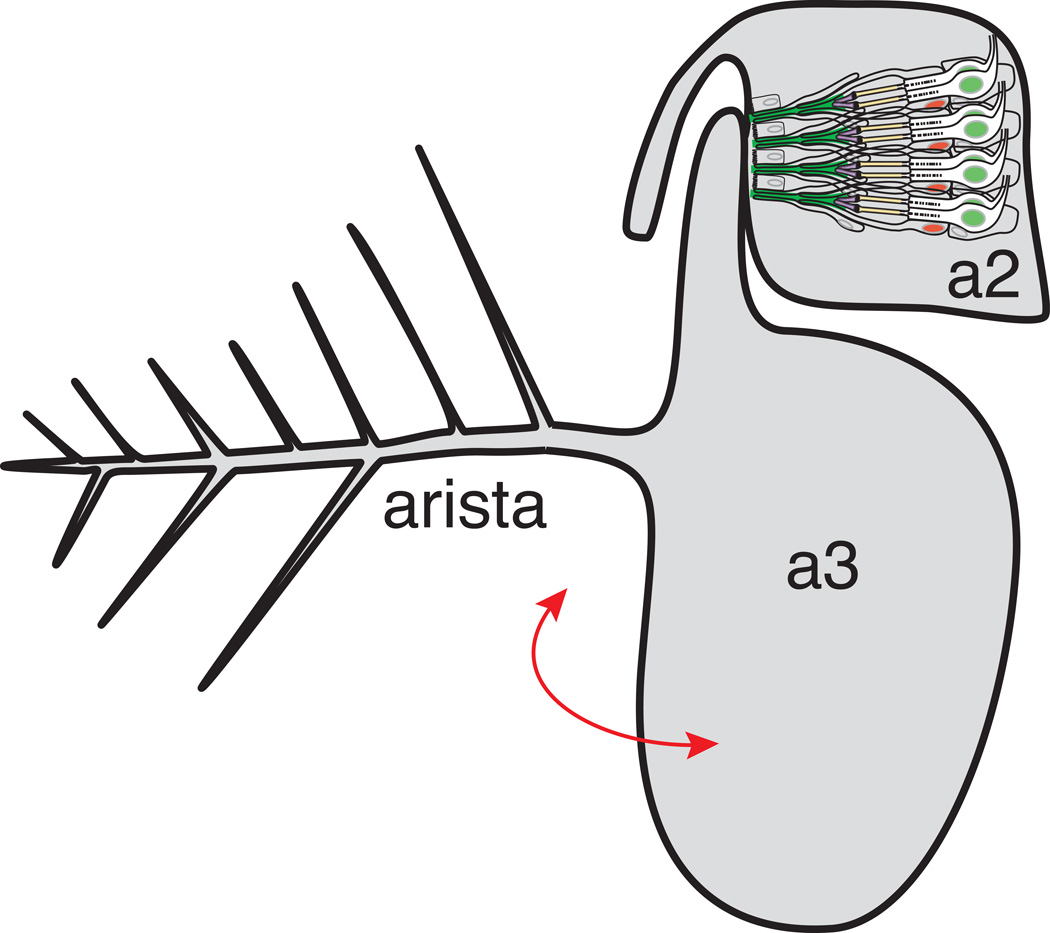
The Drosophila JO resides in the second antennal segment (a2), with scolopidia attached apically to the a2/a3 joint. JO is mechanically stimulated by rotation of a3 and the long branched arista protruding from it (Figure 1A). Movement of the arista by near field sound, wind or gravity results in twisting of the a2/a3 joint, and activation of JO neurons. Two different models have been put forth for how movement at the a2/a3 joint leads to mechanical stimulation of JO neurons. One model puts the axis of rotation at the center of the a3 stalk7. A recent alternative model is that the center of rotation aligns to where the hook of the a3 stalk joins a28. These models ultimately will impact our understanding of the pattern of mechanical stimulation of spatially distinct groups of scolopidia through the cycles of aristal forward- and back-swing. The basic structure and operation of JO are now well understood through genetic, ultrastructural and physiological approaches. Each JO scolopidium is a self-contained sense organ, with two or three sensory neurons associated with a scolopale cell and a cap cell (Figure 1B, C). In addition, ligament cells mediate basal attachment. Cell lineage studies still are needed to determine the origin of the ligament cells and whether there is one-by-one association of ligament cells with scolopidia. Scolopale cells perform three major functions described in more detail below. In brief, these functions are: 1) to contribute to the dendritic cap which mediates connection of the apical sensory dendrite to the joint cuticle; 2) to form a sealed space around the sensory cilia; and 3) to produce and regulate the ionic composition of the endolymph in the scolopale space. The latter two functions are facilitated by the intracellular elaboration of robust cytoskeletal scaffolds termed ‘scolopales’. Scolopales are arrays of thick actin bundles around isolated core microtubules.
Figure 1. Johnston’s organ develops in the second antennal segment.
A. Schematic of the adult Drosophila antenna in which Johnston’s organ resides. Sound displaces the arista, rotating (red arrow) the olfactory third antennal segment (a3). Johnston’s organ in the second antennal segment (a2) serves as the mechanoreceptor for hearing, and also responds to antennal deflections induced by wind or gravity. Only four of more than 225 scolopidia are depicted here, and an individual scolopidium is depicted in greater detail in Figure 1B.
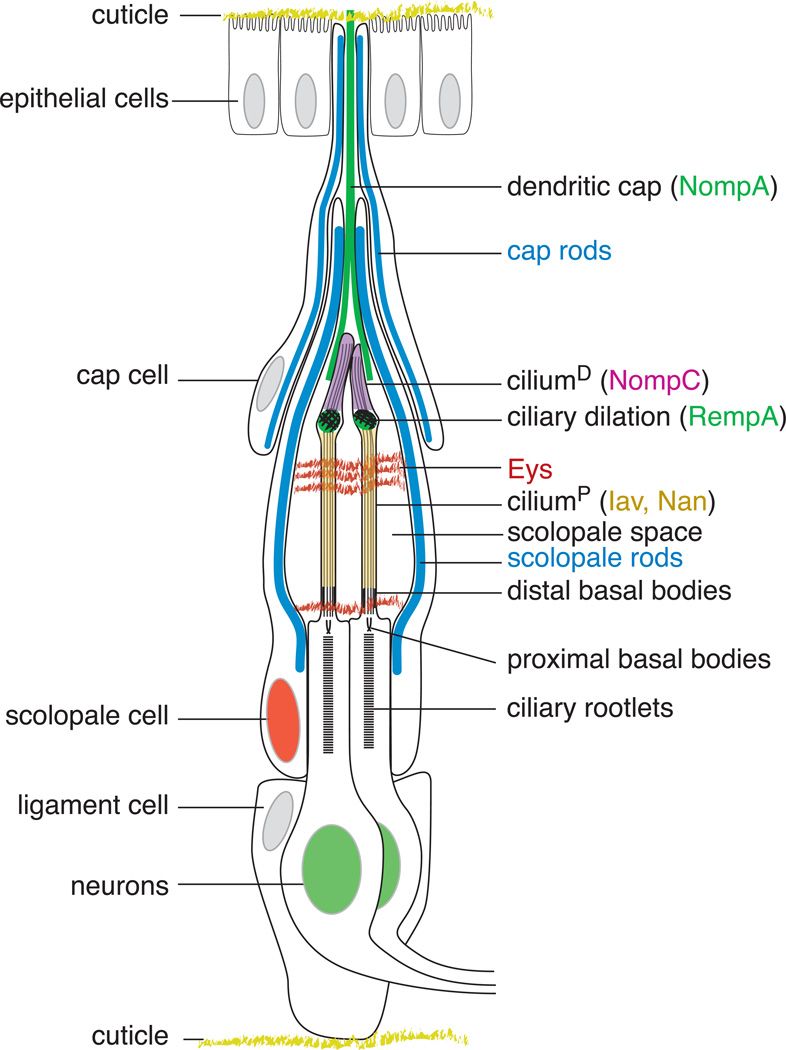
B. Schematic of a typical Drosophila Johnston’s organ scolopidium.
The major structural elements of JO are diagrammed. The scolopidia are suspended between the cuticle attachments at a2/a3 joint (top) and the peripheral cuticle of a2 (bottom), with the dendritic cap (marked by the NompA protein (green)) and ligament cells forming the respective connections. The cap rods and scolopale rods, made of thick actin filaments surrounding nucleating microtubules, are shown in blue. The scolopale cell wraps the sensory dendrites to form the scolopale space, a tightly sealed extracellular cavity thought to contain a specialized receptor lymph. The Eyes shut (Eys) protein recognized by the 21A6 monoclonal Ab (red) forms an extracellular matrix in the scolopale space, protecting against desiccation at higher temperatures. In the sensory dendrite, the ciliary dilation, a feature unique to chordotonal cilia and marked by the RempA protein (green), delimits the distal cilium (ciliumD), where the TRPN channel NompC is localized (magenta), from the proximal cilium (ciliumP), where the TRPV channel Iav/Nan heteromultimer is localized (yellow). The dendritic cap is drawn proportionately shorter to allow detailed depiction of the scolopale space and sensory cilia. Features shown in Figure 1C are depicting here in matching colors.
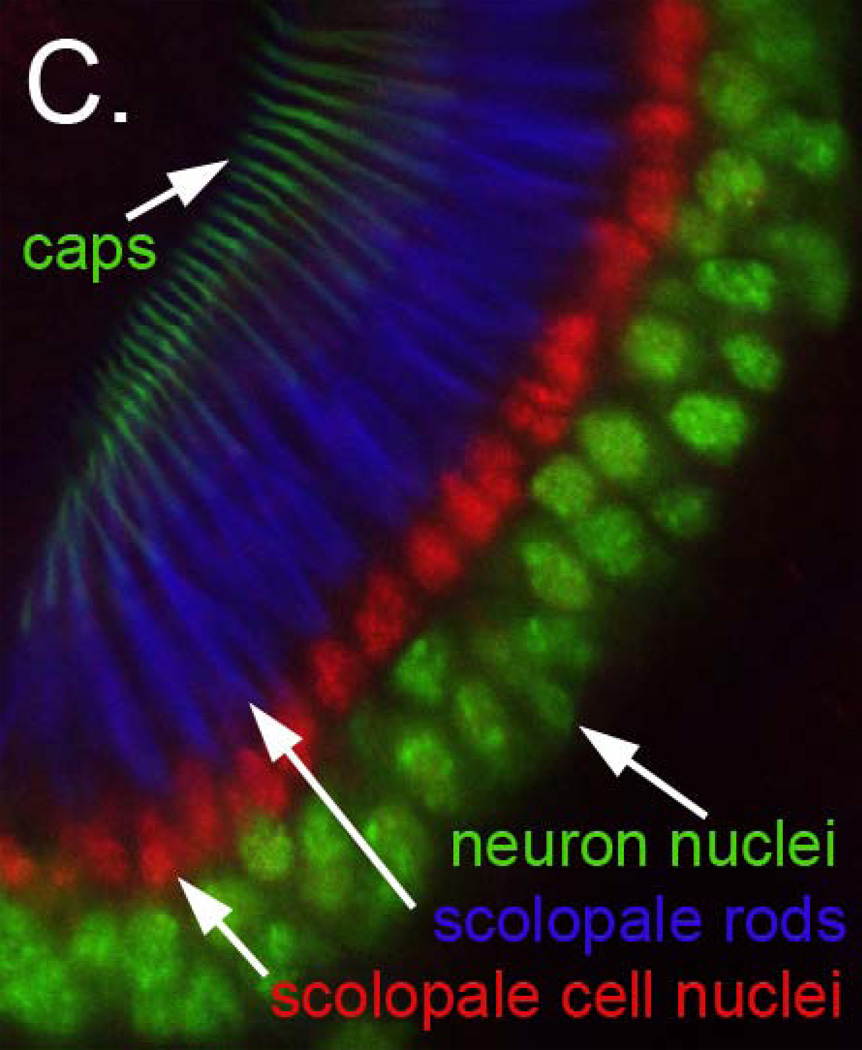
C. Organization of Johnston’s organ scolopidia. Confocal micrograph of a wild type pupal JO in which neuronal nuclei are labeled in green using an antibody to Embryonic lethal, abnormal vision (Elav), scolopale cell nuclei are labeled in red using an antibody to Prospero (Pros), the actin-rich scolopale rods are labeled in blue using Alexa-633 conjugated phalloidin, and the cap structures are labeled using a NompA-GFP transgene. These features are depicted in panel 1C in matching colors.
JO responds to two patterns of mechanical stimuli, vibratory stimuli generated by males with unilateral wing extension during courtship, and slower, more tonic stimuli associated with gravity or wind sensation. These mechanosensory submodalities have been mapped to different subsets of JO neurons. Laser vibrometry studies have significantly advanced our understanding of the relationship between the mechanical properties of the antenna and the physiological and molecular properties of JO7, 9. Using a calcium indicator, static forward deflections of the arista were shown to activate anterior groups of JO neurons and inhibit posterior neurons while rearward deflections activated posterior JO neurons and inhibited anterior ones, suggesting that individual JO neurons are activated only unidirectionally10. In contrast, vibratory stimuli activated both groups of neurons10. More recently, an ablation study focused on ventral JO neurons within the posterior group provided evidence that individual neurons can be activated in both directions by vibratory stimuli8. An initially surprising and fascinating characteristic of the Drosophila JO is the discovery that it does not simply function as a passive sensor; in the absence of acoustic stimulation, the arista shows ~200 Hz low amplitude oscillations11, approximating courtship song frequency components. These oscillations increase the dynamic range of hearing by enhancing sensitivity to low amplitude sounds and are driven by an active physiological mechanism in the JO.
The role of auditory mechanosensation in courtship behavior, and beyond, is becoming better understood (see Sidebar 1). Because Drosophila hearing has been reviewed previously2, 12–21, here we will highlight developmental aspects of JO in light of its exquisite specialization as an auditory organ and a gravity and wind sensor, focusing primarily on more recent contributions to the field of JO biology.
Sidebar 1.
Behavioral Responses to Auditory/Acoustic Stimuli
Reception and processing of sound information plays central roles in regulating Drosophila behavior. In particular, three aspects of fly behavior resulting from auditory stimuli have been studied. First, following the discovery of courtship songs in Drosophila, their effectiveness at stimulating female receptivity was measured as the average latency to copulation among mating pairs (see reviews79, 80). Second, the effect of courtship song on males can be measured by inter-male courtship activity70, 81, 82, and may have a positive feedback effect on the male’s persistence when courting females83. It also may serve in courtship initiation84 and detecting nearby courtship activity of a competing male85. Third, courtship-neutral acoustic stimuli have been reported in two behaviors, an acoustic startle response65, and proboscis-extension reflex conditioning using a sugar reward. The latter reveals an auditory classical conditioning behavior86, analogous to Pavlovian conditioning in dogs. Indeed, airflow augmentation of olfactory behavior is also thought to be integrated in the mushroom bodies87.
Another ancient vibrational communication mode, only recently described for Drosophila, is substrate-borne vibration88. Males display approximately 6 Hz abdominal twitches or quivers. Under conditions that transmit substrate-borne vibrations from these quivers, receptive females reduce their locomotion, evidencing enhanced receptivity and facilitating copulation.
Beyond auditory behaviors, JO mediates other mechanosensory functions, including gravity sensation10, 59, 89, wind sensation38, and air current feedback on flight control90.
Johnston’s organ development
1. Early JO Development
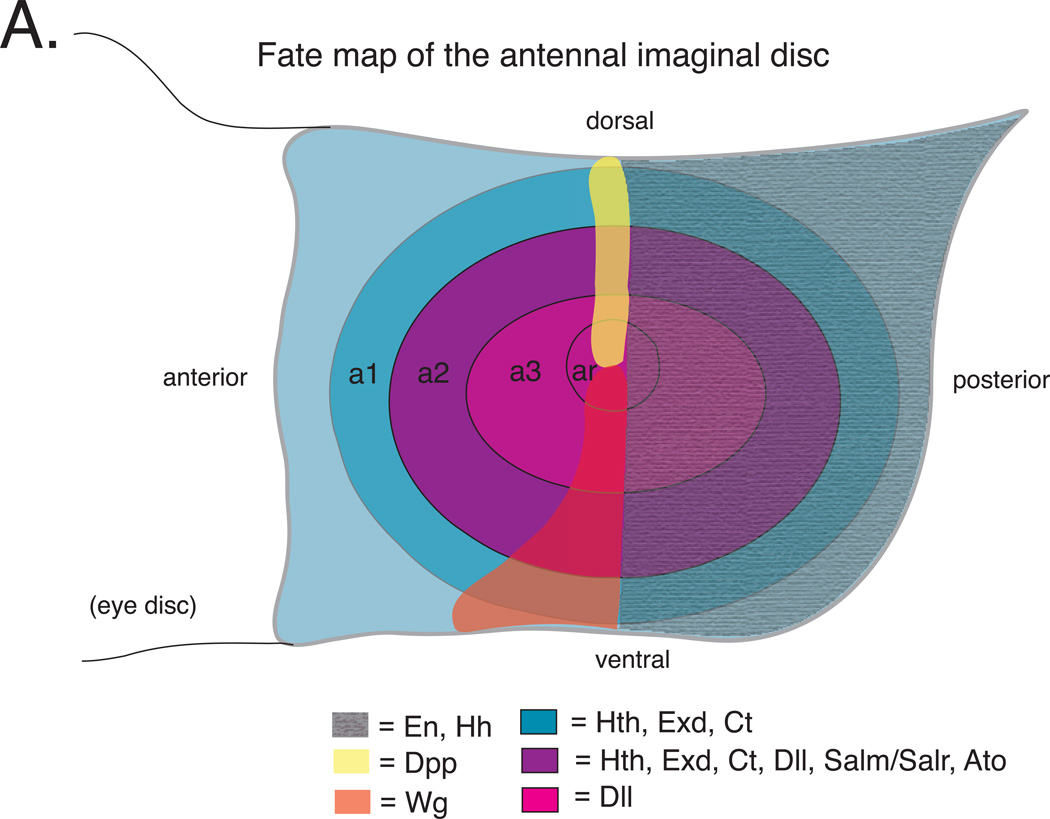
The entire adult antenna, including JO, arises from a structure called the antennal imaginal disc. The antennal imaginal disc is specified during embryogenesis as a small cluster of ~10 epithelial cells (http://www.sdbonline.org/fly/lewheld/imagdisc.htm) 22. These cells proliferate and are patterned throughout the three larval instars reaching a size of ~10,000 cells prior to differentiation during metamorphosis. Patterning occurs via a cascade of signaling molecules and transcription factors that progressively subdivide the roughly circular imaginal disc (Figure 2A)*. Early patterning events include expression of the homeodomain transcription factor Engrailed (En) and the secreted signaling molecule Hedgehog (Hh) in presumptive posterior cells. Hh then activates expression of the secreted Wingless (Wg) signaling molecule ventrally along anterior-posterior compartment boundary and the secreted Decapentaplegic (Dpp) signaling molecule dorsally along the anterior-posterior compartment boundary23, 24. The opposing gradients of Wg and Dpp subdivide the antennal imaginal disc into concentric rings23–26. One of these rings comprises the progenitors of a2, including the JO27.
Figure 2. Genetics of Johnston’s organ development.
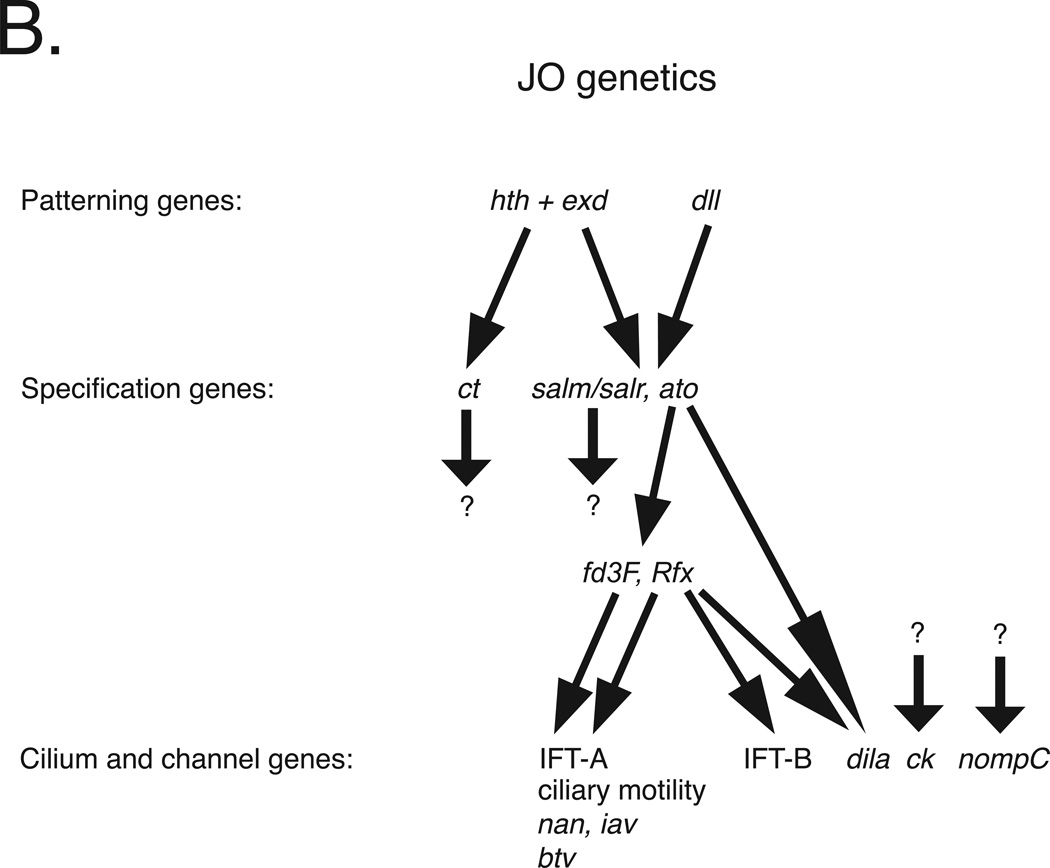
A. Schematic of a third instar larval antennal imaginal disc. a1, a2 and a3 = first, second and third antennal segment precursors, respectively. ar = arista precursors. En and Hh are expressed throughout the posterior compartment of the disc. hth and dll are regulated by Dpp and Wg. Hth and Dll expression overlap in presumptive a2 where they activate salm/salr and ato. ato is required for specification of JO precursors. Based on information in references 23–25, 27, 29, 34.
B. Johnston’s organ development is controlled by a genetic cascade initiated by transcription factors encoded by hth, exd and Dll. Hth and Exd together activate the expression of ct, while Hth, Exd and Dll together activate salm/salr and ato. Both ct and salm/salr mutants are deaf, exhibiting defective JO development followed by JO degeneration. However, genes regulated by the Ct and salm/salr transcription factors are unknown. Ato directly regulates Rfx and dila expression and either directly or indirectly regulates fd3F expression. Together, the Rfx and Fd3F transcription factors activate the expression of a suite of genes required for ciliogenesis, ciliary motility and JO function, including multiple intraflagellar transport A (IFT-A) genes required for retrograde transport, axonemal dyneins required for ciliary motility, the TRPV channels encoded by iav and nan, and the retrograde IFT dynein motor encoded by btv. In addition, Rfx, but not Fd3F, activates a subset of the IFT-B genes required for anterograde transport. Regulators of the myosin VIIA homolog encoded by ck and the TRPN channel encoded by nompC remain unknown. Not indicated here is the restriction of gene expression to subsets of cell types; whereas patterning genes are expressed throughout presumptive a2, the genes at the bottom of the hierarchy tend to be restricted to either neurons or specific subsets support cells. Note that although Rfx and fd3F are expressed in JO, the targets indicated here were identified in larval chordotonal organs. All of the genes shown here have vertebrate homologs, and most of these also are required for vertebrate ear development and/or function. Based on information in references 27, 29, 74, 75.
By late third instar, the a2 progenitors express a unique set of transcription factors, including Distal-less (Dll) and Homothorax (Hth) 27. Dll and Hth, along with the ubiquitously expressed Hth partner Extradenticle (Exd), regulate downstream genes required for the differentiation of JO and associated cuticular structures (Figure 2B)27–29. In particular, the proneural gene atonal (ato) which encodes a basic helix-loop-helix transcription factor is activated by Dll, Exd, and Hth in presumptive a2 in cells that give rise to the JO neurons and supporting cells28, 29. spalt-major (salm) and spalt-related (salr) which encode zinc-finger transcription factors are activated via the activities of Dll, Exd and Hth throughout presumptive a2 in both JO and epidermal precursors27, 29. cut (ct), which encodes a homeodomain transcription factor, is activated throughout presumptive a2, as well as more proximal antennal precursors by Exd and Hth28, 29. Mutations in ato, salm and salr, or ct lead to defects in JO development and deafness29–34.
The lineage of JO cells is thought to resemble that of other chordotonal organs (CHOs) in which a single precursor cell gives rise to both the neurons and support cells that constitute an individual functional unit or scolopidium. As in other CHOs, the supporting cells of each scolopidium include a scolopale cell, a cap cell and a ligament cell. However, unlike other CHOs, most JO scolopidia are doubly innervated, and a small subset of ~10–15% are triply innervated (Figure 3A). In addition, JO differs from other CHOs in that JO precursors are specified simultaneously instead of undergoing sequential recruitment. While it remains unclear how the two or three neurons in each JO scolopidium are generated or how JO neurons are related to one another, it is worth noting that a gene controlling neuron number in a subset of chordotonal organs has been identified. Specifically, mutations in the cousin of atonal (cato) gene lead to neuron duplications in the v’ch1 larval chordotonal organ. The duplicated neurons arise from an extra division of the cell fated to become the neuron35. Based on this, one might expect cato expression to be lacking in wild type JO precursors, thereby permitting additional neurons to form. However, at least some JO precursors express cato36, suggesting that a different mechanism is at work. Other significant developmental differences between JO and other CHOs include the requirements for salm/salr and ct which repress formation of the other CHOs29, 30.
Figure 3. The molecular and structural diversity of Johnston’s organ neurons.
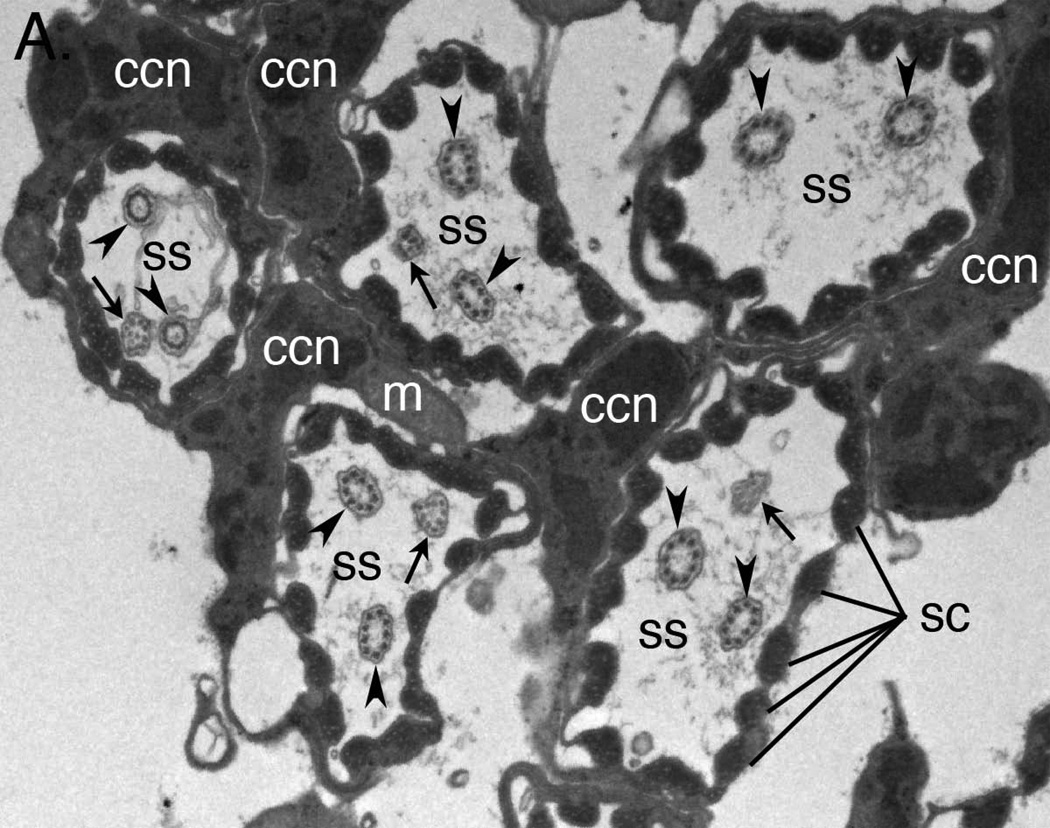
A. Transmission electron micrograph showing cross sections of five JO scolopidia. Each scolopidium possesses two dendrites with typical ciliary 9 × 2+0 axonemes (arrowheads). Four of the five scolopidia in this view show a third dendrite (arrows) with a degenerate axoneme or even disordered microtubules. ss = scolopale space; ccn = cap cell nucleus; sc = scolopales; m = mitochondrion.
B. Functional diversity of JO neurons.
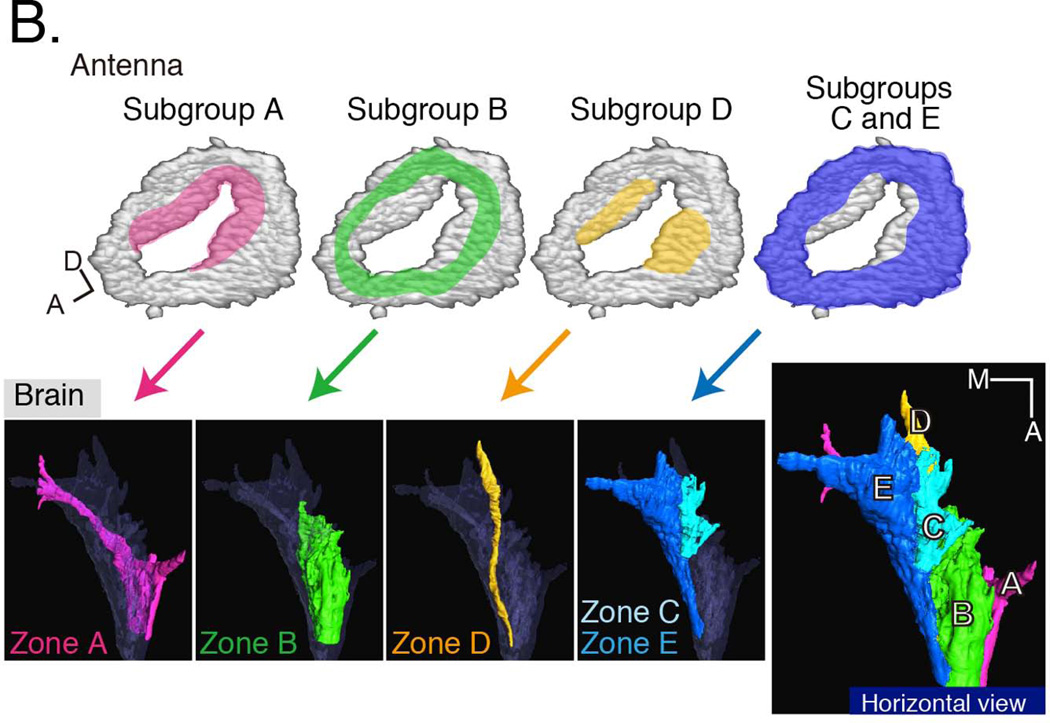
Promoter fusions and enhancer trap lines have been identified that mark subsets of JO neurons. These neurons have been classified into five groups, A–E (upper panels), that innervate distinct zones in the antennal mechanosensory and motor center (AMMC; lower panels)37. The positions of the Type A neuronal cell bodies within JO are highlighted in pink. The locations of the Type B neuronal cell bodies are indicated in green; the locations of the Type D neuronal cell bodies are highlighted in yellow; and the locations of the Types C and E neuronal cell bodies are indicated in blue. Neuronal types A and B are used for sound reception; types C and E are used for gravity and wind reception; the function of type D neurons remains unknown. In addition to the AMMC, auditory information from some Type A neurons is carried to either the subesophageal ganglion (SOG) or the ventrolateral protocerebrum (vlpr). The SOG also receives gustatory information, while the vlpr also receives visual and olfactory information, suggesting that there is convergence of multiple sensory modalities in these brain regions. Reproduced with kind permission from Springer Science and Business Media 92.
2. Differentiation/Later JO Development
JO mediates at least two types of mechanosensory modalities. These functional categories can be grouped as sensing vibratory stimuli (hearing) or non-vibratory stimuli (gravity and wind). Vibratory stimuli evoke fast responses dominated by acceleration of the antenna, with fast adaptation, while non-vibratory stimuli evoke more sustained, slowly adapting responses dominated by velocity or even position. These two categories have been assigned to distinct subgroups of JO scolopidia. Kamikouchi37 defined 5 groups of JO neurons, A–E, based on central projection patterns in flies expressing GFP from different Gal4 lines (Figure 3B). The group A and B neurons have been associated with hearing10, 32, while group C and E neurons appear to mediate gravity and wind sensation32, 38. No function has yet been attributed to the group D neurons that constitute a very small percentage of the total.
Another dimension of scolopidial differences in JO is that in the scolopidia with three, sensory neurons, two neurons always show a clear axonemal arrangement of microtubules in the outer dendritic segment, while the microtubule arrangement in the third neuron often is less organized21 (Figure 3A). The functional significance of this architecture is not known, and to date there are no data that illuminate the relationship between these neurons (or scolopidia that contain them) and the A/B and C/E subgroups. In other words, whether the two or three neurons in each scolopidium are functionally different requires future work. The developmental origin of neurons in triply innervated scolopidia also has not been studied; indeed lineage analysis of the entire JO would advance our understanding of JO biology beyond the extrapolation from CHOs in other locations.
Also unknown are the molecules that distinguish the JO neuron subgroups from each other, either developmentally or physiologically. Enhancer trap lines are so far the primary tool that distinguishes these groups at the morphological level by allowing projection mapping in the brain, and at the functional level by allowing ectopic expression of Ca2+ indicators or toxins10, 32, 37, 39. Many of the 34 Gal4 lines extensively described by Kamikouchi37 have not been associated with particular genes. Importantly, several of the lines show unique expression in zone A, one line uniquely labels zone B. This facilitates functional assessment and manipulation of these isolated neuron groups. In contrast, two lines co-express in groups C and E preventing distinction, and group D neurons are labelled only in combination with group A, requiring subtractive approaches to infer function. Nevertheless, these lines serve the basis for many of the studies reviewed here. On the other hand, the posterior patterning gene en_described above exhibits restricted expression in a subset of JO neurons8. Specifically, it is expressed in some A, some B and some E neurons, and inactivating these neurons results in a loss of ~50% of the auditory sensitivity in the 100–400 Hz range. It is not known whether the neurons that express en_derive exclusively from the posterior compartment cells that express en_earlier. Nor is the function of en_in this subset of neurons known.
As the JO neurons have ciliated dendrites, a salient aspect of differentiation is the localization and assembly of basal bodies and elaboration of the sensory cilium. In contrast to vertebrates, Drosophila has cilia only in Type I sense organs and sperm flagella. Thus, it is possible to recover adult animals lacking cilia, facilitating genetic screens and mechanistic characterization of ciliary mutants. Basal body formation in JO neurons requires the coiled-coil protein Unc40, the pericentrin-like protein D-PLP41, SAK/PLK442, Yuri gagarin43, 44 (extrapolating from sperm and based on expression in JO), Dilatory45 and Chibby46. Intraflagellar transport (IFT) is essential for JO ciliary assembly, including subunits of the anterograde kinesin II motor47, the retrograde dynein motor21, 48, and IFT particle proteins such as IFT88 encoded by nompB49. RempA, the IFT140 protein is essential for formation of the ciliary dilation, a chordotonal-specific structure that subdivides the sensory cilium into distinct functional compartments48 (Figure 1B). The proximal compartment contains inner and outer dynein arms whose assembly or transport depends on the LRRC6 protein encoded by tilB50. DCX-EMAP, a doublecortin domain-containing microtubule associated protein, also localizes to the chordotonal ciliary dilation and is required for hearing51.
Transduction
1. TRP Channels in JO
Analysis of TRP channel expression and function has provided important insights into how the JO functions differentially to sense sound versus gravity or wind. The TRPV channels, encoded by inactive (iav) and nanchung (nan), appear to form heteromeric channel complexes52, 53. These complexes are localized to the proximal ciliary segment up to the ciliary diliation (Figure 1B). In contrast, the TRPN channel encoded by nompC is localized distal to the ciliary dilation54, 55 (Figure 1B). The localization experiments suggest that all three of these TRP channels are expressed in all or almost all scolopidia. Hearing, as measured by sound-evoked potentials (SEPs) in the antennal nerve31, 56, 57, is completely eliminated by loss of the TRPV channels52, 53, but only partially eliminated by loss of the TRPN channel11, 31, 32, 58. Studies of antennal mechanics show distinct effects of TRPV versus TRPN channel loss of function in that spontaneous oscillation of the antenna is essentially lost in TRPN mutants, but enhanced in TRPV mutants11. Furthermore, non-linear amplification of antennal movements under low amplitude stimulation is also lost in TRPN mutants11, 32. Interestingly, the TRPN channel requirement for hearing appears to be localized to the A and B groups of scolopidia32, and TRPN appears to be dispensable for gravity and wind sensation32, 59. In contrast, the TRPA channels encoded by painless and pyrexia appear to be important for gravity sensation59. One persistent question is how to reconcile the expression of NompC in all JO scolopidia with a clear functional requirement only in the A and B subgroups. A potential resolution may lie in functional differences between protein isoforms. Differential isoform expression is suggested by the observation that a commonly used nompC-Gal4 construct drives expression in only a subset of JO neurons59, 60 revealing incomplete reporting of enhancers compared to the antibody staining pattern. However, the long isoform of NompC is sufficient to fully rescue hearing loss in null mutants32, 58, suggesting that the long isoform is sufficient for auditory transduction.
The prevailing model for mechanotransduction in JO, at least for hearing, is that NompC either forms the mechanosensitive transduction channel or the gating spring physically connected to the transduction channel, with the TRPV channels providing both signal propagation along the sensory cilium and feedback modulation of the NompC channel gain11, 17, 32, 58. A direct role of NompC as a mechanosensitive channel is supported by multiple lines of evidence. First, NompC mutants that alter residues in the putative pore region result in loss of transduction in bristle organs61. Second, ectopic expression of NompC in S2 cells or in multidendritic neurons leads to a gain of mechanotransduction62. And third, there is evidence that nompC homologs in worms and fish encode pore-forming mechanotransduction channels63, 64. A perplexing aspect of this model is the long-recognized fact that nompC null mutants are not completely deaf, only partially so31, 32, requiring a second unidentified transduction channel to account for the NompC-independent hearing58. One approach to identifying such a channel would be to screen for completely deaf mutants in a nompC mutant background.
An alternative model, derived from recording currents in voltage-clamped giant fiber neurons that receive inputs from the A/B subgroup of JO neurons, is that the TRPV channels are integral components of the transduction complex, while NompC modulates the strength of mechanical forces that impinge on the transduction complex65. Localization of NompC in the distal cilium puts this protein in series with and before the transduction complex. In this model, the enhanced spontaneous antennal oscillations in TRPV mutants imply that transduction inhibits the active force generation mechanism. Future experiments will be required to reconcile and refine these models.
2. Support Cell Functions
The ability of chordotonal neurons to realize their central role in sensory function depends on support cells, especially the scolopale cell. The scolopale cell has three known functions, each of which is critical for mechanical activation or transduction. One function is to wrap around the sensory dendrites, using septate junctions to seal an extracellular compartment called the scolopale space, isolating the sensory dendrites from the hemolymph. As part of this function, the spindle-shaped actin cytoskeleton constituting the scolopale rods helps to maintain the shape of the scolopale space. The Cbl-associated protein (CAP) localizes to the scolopale cell in a pattern consistent with an integral protein of the scolopale rods, and its loss of function results in partial collapse of the scolopale space and partial loss of hearing66. EB1, a microtubule plus-end tracking protein, is also enriched in scolopale cells and contributes to integrity of chordotonal organs and hearing function67. The second scolopale cell function is to generate the receptor lymph within the scolopale space. The receptor lymph is thought to be rich in K+ 2, 68, resembling the endolymph in the mammalian cochlea, and is required for establishing a strong electrochemical gradient across the membrane of the sensory cilium to drive ion flow through the mechanosensitive ion channels in the ciliary membrane. The Na+/K+ ATPase α subunit ATPα is highly upregulated in the scolopale cell in a manner that depends specifically on the nrv2-encoded β subunit68. Scolopale cell-specific knockdown of either ATPα or nrv2 results in deafness accompanied by extraneous cellular material within the scolopale space, consistent with a central role for this ion pump in generating and maintaining the ionic composition of the scolopale space. The third scolopale cell function is to contribute to the dendritic cap, a tubular extracellular matrix structure connected distally to the cuticle at the a2/a3 and proximally to the sensory cilia21. Thus, the dendritic cap physically transmits movements at the a2/a3 joint to the sensory cilia. The NompA protein expressed in the scolopale cell is secreted and integrated into the dendritic cap69. Elucidating additional specialized contributions of the scolopale cell will help to provide further insight into transduction mechanisms.
Ongoing studies
1. Screens
A well-recognized attribute of the Drosophila model system includes the relative ease with which both forward and reverse genetic screens can be carried out. Auditory mutants have been recovered in forward screens for hearing mutants70, for touch mutants31, 71 and for cilia mutants72, 73.
More recently, several key auditory genes were identified by reverse genetic screens. In a study published in 201174, the Jarman laboratory used fluorescence activated cell sorting (FACS) to isolate green fluorescent protein (GFP) labelled chordotonal organ precursors from dissociated embryonic tissues. RNA was isolated from these precursors and used for microarray expression analysis74. While not specific to JO, this work identified multiple transcription factors central to the development of chordotonal organs as well as a suite of ciliary genes required for the differentiation of chordotonal-specific ciliary features. In a sequel published earlier this year, the transcription factor RFX was shown to cooperate with the Forkhead transcription factor Fd3f to regulate chordotonal-specific ciliary genes (and TRP channel genes), including several already known to be required for hearing75.
In a study published in 201276, the Göpfert laboratory reported on a large-scale, reverse genetic screen that resulted in the identification of 274 genes expressed in the adult JO (see Sidebar 2). Of the first 42 genes tested, 27 are required for normal Drosophila hearing. Mutations in 2 of 27 resulted in hypersensitivity to sound, while mutations in 25 of the 27 resulted in lost or reduced sensitivity to sound. One reason for the success of this screen was due to the setting of rigorous thresholds. First, the transcriptomes of second antennal segments harboring a JO were compared to the transcriptomes of second antennal segments in which the JO had been genetically ablated. An additional layer of stringency was added by subtracting out transcripts equally expressed in the brain, i.e. more general neuronal factors. This screen more than doubled the number of Drosophila genes known to be involved in hearing. However, because the screen was carried out using adult tissue, genes required transiently during auditory organ development were not recovered. These include genes encoding the transcription factors Atonal and Cut, both of which are critical for Drosophila hearing29, 31. In addition, due to the stringency of the screen, at least one gene encoding a structural component of the auditory neurons was not recovered. This category includes Crinkled, which is a Myosin VIIA homolog known to be essential for hearing in both flies and vertebrates4, 5. Thus there probably are other genes expressed and required in the adult JO that are yet to be discovered, and reverse genetic screens at earlier time points are likely to reveal additional genes required for auditory organ development.
Sidebar 2.
Phototransduction genes in hearing?
Perhaps the most surprising finding from the Senthilan et al., 2012 reverse genetic screen76 was the recovery of 26 genes previously thought to be involved primarily in light-sensing. This included 4 of 7 Drosophila rhodopsins. Equally stunning is the demonstration that at least two of these rhodopsins are required for Drosophila hearing. While it has been postulated that sense organs of different modalities share a common evolutionary origin, this study provides some of the most compelling evidence to date in support of a shared origin. The recent discovery of a photomechanical response in Drosophila photoreceptors91 further hints at the notion that sensory modalities may be less distinct than previously thought. From this perspective, it is intriguing that the Senthilan screen also uncovered a variety of genes involved in chemosensation. Future areas of research will undoubtedly focus both on whether these ‘olfactory’ genes are required for Drosophila hearing and whether vertebrate ears also express and require ‘phototransduction genes’.
2. Auditory circuitry
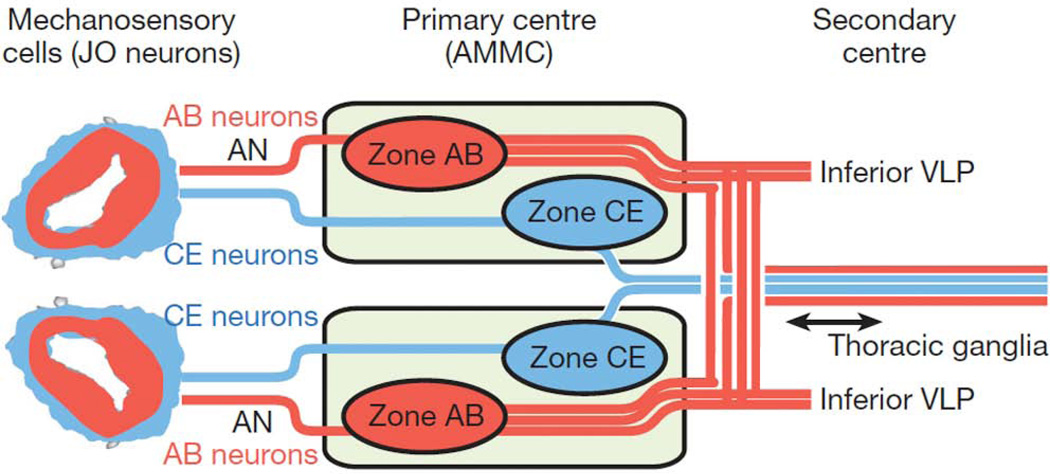
An important emerging area of Drosophila auditory research is mapping the neural circuitry that transmits and processes auditory information. As described briefly above, based on differential gene expression, five groups of JO neurons have been identified37. Two of these groups, A and B, are used for sound reception, while groups C and E are used for gravity and wind sensation. The function of group D neurons remains unknown. Most JO neurons project ipsilaterally to a region of the brain known as the antennal mechanosensory and motor center (AMMC) (Figure 4). Zones of the AMMC are named for the JO neurons that target them. Thus AMMC-A receives input from JO group A neurons and AMMC-B receives input from JO group B neurons. Four types of central neurons innervate AMMC zones A and B and thus may respond to courtship song10. Recent electrophysiological studies of these four neuron types open the way to understanding how auditory sensory information is decoded65, 77. Central neurons that innervate zone A include the giant fiber neuron (1 cell/brain hemisphere), well characterized in the escape behavior pathway, and the AMMC-A1 neurons (2 cells/hemisphere). The giant fiber and AMMC-A1 neurons appear to use neurotransmitters other than acetylcholine and GABA. Zone B is innervated by AMMC-B1 central neurons (about 10 cells/hemisphere) that are cholinergic, and AMMC-B2 neurons (2 cells/hemisphere) that are GABAergic77. Whole-cell patch clamp recordings from all four of these central neuron types revealed that sound pulses elicited graded responses that were well time-locked. Injecting depolarizing current elicited action potentials from the giant fiber neuron but not from AMMC-A1 or AMMC-B2 neurons. These studies suggest that the giant fiber neuron may employ two functional modes: a subthreshold graded response mode used in the auditory circuit, and a full depolarizing action potential mode that drives the escape behavior. How the graded mode reads out to downstream neurons is not yet known. It is thought that for the other AMMC central neurons, operating only in the graded mode may enhance reliability of the signals and may allow more information to be encoded.
Figure 4. Drosophila auditory circuitry.
From JO, auditory information is relayed to a different region of the antennal mechanosensory and motor complex (AMMC) than wind and gravity information. The auditory neurons and their axon tracts are shown in red, while the wind and gravity sensing neurons and their axon tracts are indicated in blue. Reprinted by permission from Macmillan Publishers Ltd: Nature 458: 165–171 (2009).
In an independent study of the auditory circuit using structural connectivity analysis, projection neurons innervating AMMC zones A and B were identified78. Because this study employs different Gal4 drivers than those used in the studies described above, it is not clear whether there is a one-to-one correspondence of the central neuron nomenclature. In this study, frequency tuning analysis using the genetically encoded GCaMP sensor, suggests different tuning properties among the AMMC central neurons. The AMMC-A neurons transmit a broad range of auditory information ranging from 100–700 Hz, while the AMMC-B neurons are specialized for lower frequencies of 100–300 Hz78. Some AMMC-B neurons also respond to pulse songs. From the AMMC, auditory information is relayed both to the contralateral AMMC and to the inferior ventrolateral protocerebrum (IVLP). The contralateral projections may play a role in distinguishing the directionality of the auditory input, but this has yet to be demonstrated. A subset of IVLP neurons are commissural and GABAergic. It is thought that these may mediate ‘gain control’ between bilateral auditory inputs78. From the IVLP, auditory information is relayed to the ventrolateral protocerebrum (VLP), which also receives some gustatory and visual inputs. Because of the multimodal inputs, it is speculated that the VLP may function in integrating different types of sensory information. Together, the AMMC, IVLP and VLP are thought to process and interpret auditory information in order to generate appropriate behavioral responses including mating and escape responses. How this information is relayed and converted into behaviors remains unknown and is a critical area for future research.
Conclusion
The past few years have seen tremendous progress in Drosophila auditory research due to both forward and reverse genetic screens, with more than 50 genes now implicated in auditory development and function. At the same time, significant strides in understanding the development of Johnston’s organ have been made, along with revelations about the physiological operation of the organ as an active sensor. Many of the identified genes can now be assigned to specific structures or functions, either in the sensory neurons or in the support cells. In addition, ~20% of genes whose expression is enriched in Drosophila Johnston’s organ have human homologs associated with deafness76, and a subset function in multiple sensory modalities. With the sophisticated and incisive genetics tools available, Drosophila continues to serve as a powerful system for gene discovery, and auditory genes discovered in flies are likely to be relevant to mammalian auditory biology as well as other sensory modalities. Significant progress in unveiling the nature of the auditory neural circuits in recent years represents an excellent beginning to understanding mechanisms in the development and specificity of auditory neural connectivity, sensory information processing and behavior. Additional breakthroughs in coming years will accelerate and enhance this exciting progress.
Acknowledgments
The authors would like to thank many colleagues for productive discussions, and Alan Kay, Andrew Jarman and Kevin Christie for helpful comments on the manuscript. Work in the Boekhoff-Falk laboratory has been supported by University of Wisconsin Graduate School, and work in the Eberl laboratory was facilitated by the Iowa Center for Molecular Auditory Neuroscience, supported by NIH P30 grant DC010362 to Steven Green.
Footnotes
Grace Boekhoff-Falk, No conflict of interest.
Daniel F. Eberl, No conflict of interest.
Details for all of the genes described in this section can be explored using this link: http://www.sdbonline.org/fly/aimorph/antennaandhearing.htm
Further Reading/Resources
http://www.sdbonline.org/fly/aimorph/antennaandhearing.htm#dafka2
Contributor Information
Grace Boekhoff-Falk, Email: boekhofffalk@wisc.edu, Department of Cell and Regenerative Biology, University of Wisconsin, School of Medicine and Public Health, Madison, WI 53706.
Daniel F. Eberl, Department of Biology, University of Iowa, Iowa City, IA 52242
References
- 1.Hoy RR. Acute as a bug's ear: an informal discussion of hearing in insects. In: Hoy RR, Popper AN, Fay RR, editors. Comparative Hearing: Insects. Vol. 10. New York: Springer; 1998. pp. 1–17. [Google Scholar]
- 2.Eberl DF. Feeling the vibes: chordotonal mechanisms in insect hearing. Curr. Opin. Neurobiol. 1999;9:389–393. doi: 10.1016/S0959-4388(99)80058-0. [DOI] [PubMed] [Google Scholar]
- 3.Field LH, Matheson T. Chordotonal organs of insects. In: Evans PD, editor. Adv. Insect Physiol. Vol. 27. San Diego: Academic Press; 1998. pp. 1–228. [Google Scholar]
- 4.Todi SV, Franke JD, Kiehart DP, Eberl DF. Myosin VIIA defects, which underlie the Usher 1B Syndrome in humans, lead to deafness. Drosophila. Curr. Biol. 2005;15:862–868. doi: 10.1016/j.cub.2005.03.050. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Todi SV, Sivan-Loukianova E, Jacobs JS, Kiehart DP, Eberl DF. Myosin VIIA, important for human auditory function, is necessary for Drosophila auditory organ development. PLoS ONE. 2008;3:e2115. doi: 10.1371/journal.pone.0002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schoen CJ, Emery SB, Thorne MC, Ammana HR, Sliwerska E, Arnett J, Hortsch M, Hannan F, Burmeister M, Lesperance MM. Increased activity of Diaphanous homolog 3 (DIAPH3)/diaphanous causes hearing defects in humans with auditory neuropathy and in Drosophila. Proc. Natl. Acad. Sci. (USA) 2010;107:13396–13401. doi: 10.1073/pnas.1003027107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Göpfert MC, Robert D. Biomechanics: Turning the key on Drosophila audition. Nature. 2001;411:908. doi: 10.1038/35082144. [DOI] [PubMed] [Google Scholar]
- 8.Pézier A, Blagburn JM. Auditory responses of engrailed and invected-expressing Johnston's Organ neurons in Drosophila melanogaster. PLoS ONE. 2013;8:e71419. doi: 10.1371/journal.pone.0071419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Göpfert MC, Robert D. The mechanical basis of Drosophila audition. J. Exp. Biol. 2002;205:1199–1208. doi: 10.1242/jeb.205.9.1199. [DOI] [PubMed] [Google Scholar]
- 10.Kamikouchi A, Inagaki HK, Effertz T, Hendrich O, Fiala A, Göpfert MC, Ito K. The neural basis of Drosophila gravity-sensing and hearing. Nature. 2009;458:165–171. doi: 10.1038/nature07810. [DOI] [PubMed] [Google Scholar]
- 11.Göpfert MC, Albert JT, Nadrowski A, Kamikouchi A. Specification of auditory sensitivity by Drosophila TRP channels. Nature Neurosci. 2006;9:999–1000. doi: 10.1038/nn1735. [DOI] [PubMed] [Google Scholar]
- 12.Bechstedt S, Howard J. Hearing mechanics: a fly in your ear. Curr. Biol. 2008;18:R869–R870. doi: 10.1016/j.cub.2008.07.069. [DOI] [PubMed] [Google Scholar]
- 13.Boekhoff-Falk G. Hearing in Drosophila: development of Johnston's organ and emerging parallels to vertebrate ear development. Dev. Dynam. 2005;232:550–558. doi: 10.1002/dvdy.20207. [DOI] [PubMed] [Google Scholar]
- 14.Caldwell JC, Eberl DF. Towards a molecular understanding of Drosophila hearing. J. Neurobiol. 2002;53:172–189. doi: 10.1002/neu.10126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eberl DF, Boekhoff-Falk G. Development of Johnston's organ in Drosophila. Int. J. Dev. Biol. 2007;51:679–687. doi: 10.1387/ijdb.072364de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jarman AP. Studies of mechanosensation using the fly. Hum. Molec. Genet. 2002;11:1215–1218. doi: 10.1093/hmg/11.10.1215. doi: [DOI] [PubMed] [Google Scholar]
- 17.Kernan MJ. Mechanotransduction and auditory transduction in Drosophila. Pflügers Arch. Eur. J. Physiol. 2007;454:703–720. doi: 10.1007/s00424-007-0263-x. [DOI] [PubMed] [Google Scholar]
- 18.Lu Q, Senthilan PR, Effertz T, Nadrowski B, Gopfert MC. Using Drosophila for studying fundamental processes in hearing. Integr. Comp. Biol. 2009;49:674–680. doi: 10.1093/icb/icp072. [DOI] [PubMed] [Google Scholar]
- 19.Murthy M. Unraveling the auditory system of Drosophila. Curr. Opin. Neurobiol. 2010;20:281–287. doi: 10.1016/j.conb.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 20.Nadrowski B, Effertz T, Senthilan PR, Göpfert MC. Antennal hearing in insects - New findings, new questions. Hearing Res. 2010;273:7–13. doi: 10.1016/j.heares.2010.03.092. [DOI] [PubMed] [Google Scholar]
- 21.Todi SV, Sharma Y, Eberl DF. Anatomical and molecular design of the Drosophila antenna as a flagellar auditory organ. Microsc. Res. Tech. 2004;63:388–399. doi: 10.1002/jemt.20053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Held LI., Jr . Imaginal Discs: The genetic and cellular logic of pattern formation. Cambridge: Cambridge University Press; 2005. [Google Scholar]
- 23.Diaz-Benjumea FJ, Cohen B, Cohen SM. Cell interaction between compartments establishes the proximal-distal axis of Drosophila legs. Nature. 1994;372:175–179. doi: 10.1038/372175a0. [DOI] [PubMed] [Google Scholar]
- 24.Lecuit T, Cohen SM. Proximal-distal axis formation in the Drosophila leg. Nature. 1997;388:139–145. doi: 10.1038/40563. [DOI] [PubMed] [Google Scholar]
- 25.Abu-Shaar M, Mann RS. Generation of multiple antagonistic domains along the proximodistal axis during Drosophila leg development. Development. 1998;125:3821–3830. doi: 10.1242/dev.125.19.3821. [DOI] [PubMed] [Google Scholar]
- 26.Dong PD, Chu J, Panganiban G. Proximodistal domain specification and interactions in developing Drosophila appendages. Development. 2001;128:2365–2372. doi: 10.1242/dev.128.12.2365. [DOI] [PubMed] [Google Scholar]
- 27.Dong PDS, Chu J, Panganiban G. Coexpression of the homeobox genes Distal-less and homothorax determines Drosophila antennal identity. Development. 2000;127:209–216. doi: 10.1242/dev.127.2.209. [DOI] [PubMed] [Google Scholar]
- 28.Dong PDS, Scholz Dicks J, Panganiban G. Distal-less and homothorax regulate multiple targets to pattern the Drosophila antenna. Development. 2002;129:1967–1974. doi: 10.1242/dev.129.8.1967. [DOI] [PubMed] [Google Scholar]
- 29.Ebacher DJS, Todi SV, Eberl DF, Boekhoff-Falk G. cut mutant Drosophila auditory organs differentiate abnormally and degenerate. Fly. 2007;1:86–94. doi: 10.4161/fly.4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong PDS, Todi SV, Eberl DF, Boekhoff-Falk G. Drosophila spalt/spalt-related mutants exhibit Townes-Brocks' syndrome phenotypes. Proc. Natl. Acad. Sci. (USA) 2003;100:10293–10298. doi: 10.1073/pnas.1836391100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eberl DF, Hardy RW, Kernan M. Genetically similar transduction mechanisms for touch and hearing in Drosophila. J. Neurosci. 2000;20:5981–5988. doi: 10.1523/JNEUROSCI.20-16-05981.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Effertz T, Wiek R, Göpfert MC. NompC TRP channel is essential for Drosophila sound receptor function. Curr. Biol. 2011;21:592–597. doi: 10.1016/j.cub.2011.02.048. [DOI] [PubMed] [Google Scholar]
- 33.Göpfert MC, Stocker H, Robert D. atonal is required for exoskeletal joint formation in the Drosophila auditory system. Dev. Dynam. 2002;225:106–109. doi: 10.1002/dvdy.10136. [DOI] [PubMed] [Google Scholar]
- 34.Jarman AP, Sun Y, Jan LY, Jan YN. Role of the proneural gene, atonal, in formation of Drosophila chordotonal organs and photoreceptors. Development. 1995;121:2019–2030. doi: 10.1242/dev.121.7.2019. [DOI] [PubMed] [Google Scholar]
- 35.zur Lage PI, Jarman AP. The function and regulation of the bHLH gene, cato, in Drosophila neurogenesis. BMC Dev Biol. 2010;10:34. doi: 10.1186/1471-213X-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goulding SE, White NM, Jarman AP. cato encodes a basic helix-loop-helix transcription factor implicated in the correct differentiation of Drosophila sense organs. Dev. Biol. 2000;221:120–131. doi: 10.1006/dbio.2000.9677. [DOI] [PubMed] [Google Scholar]
- 37.Kamikouchi A, Shimada T, Ito K. Comprehensive classification of auditory sensory projections in the brain of the fruit fly Drosophila melanogaster. J. Comp. Neurol. 2006;499:317–356. doi: 10.1002/cne.21075. [DOI] [PubMed] [Google Scholar]
- 38.Yorozu S, Wong A, Fischer BJ, Dankert H, Kernan MJ, Kamikouchi A, Ito K, Anderson DJ. Distinct sensory representations of wind and near-field sound in the Drosophila brain. Nature. 2009;458:201–205. doi: 10.1038/nature07843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sivan-Loukianova E, Eberl DF. Synaptic ultrastructure of Drosophila Johnston's organ axon terminals as revealed by an enhancer trap. J. Comp. Neurol. 2005;491:46–55. doi: 10.1002/cne.20687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baker JD, Adhikarakunnathu S, Kernan MJ. Mechanosensory-defective, male-sterile unc mutants identify a novel basal body protein required for ciliogenesis in Drosophila. Development. 2004;131:3411–3422. doi: 10.1242/dev.01229. [DOI] [PubMed] [Google Scholar]
- 41.Martinez-Campos M, Basto R, Baker J, Kernan M, Raff JW. The Drosophila pericentrin-like protein is essential for cilia/flagella function, but appears to be dispensible for mitosis. J. Cell Biol. 2004;165:673–683. doi: 10.1083/jcb.200402130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bettencourt-Dias M, Rodriques-Martins A, Carpenter L, Riparbelli M, Lehmann L, Gatt MK, Carmo N, Balloux F, Callaini G, Glover DM. SAK/PLK4 is required for centriole duplication and flagella development. Curr. Biol. 2005;15:2199–2207. doi: 10.1016/j.cub.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 43.Texada MJ, Simonette RA, Deery WJ, Beckingham KM. Tropomyosin is an interaction partner of the Drosophila coiled coil protein yuri gagarin. Exp. Cell Res. 2011;317:474–487. doi: 10.1016/j.yexcr.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Texada MJ, Simonette RA, Johnson CB, Deery WJ, Beckingham KM. yuri gagarin is required for actin, tubulin and basal body functions in Drosophila spermatogenesis. J. Cell Sci. 2008;121:1926–1936. doi: 10.1242/jcs.026559. [DOI] [PubMed] [Google Scholar]
- 45.Ma L, Jarman AP. Dilatory is a Drosophila protein related to AZI1 (CEP131) that is located at the ciliary base and required for cilium formation. J. Cell Sci. 2011;124:2622–2630. doi: 10.1242/jcs.084798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Enjolras C, Thomas J, Chhin B, Cortier E, Duteyrat JL, Soulavie F, Kernan MJ, Laurencon A, Durand B. Drosophila chibby is required for basal body formation and ciliogenesis but not for Wg signaling. J. Cell Biol. 2012;197:313–325. doi: 10.1083/jcb.201109148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sarpal R, Todi SV, Sivan-Loukianova E, Shirolikar S, Subramanian N, Raff EC, Erickson JW, Ray K, Eberl DF. Drosophila KAP interacts with the kinesin II motor subunit KLP64D to assemble chordotonal sensory cilia, but not sperm tails. Curr. Biol. 2003;13:1687–1696. doi: 10.1016/j.cub.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 48.Lee E, Sivan-Loukianova E, Eberl DF, Kernan MJ. An IFT-A protein is required to delimit functionally distinct zones in mechanosensory cilia. Curr. Biol. 2008;18:1899–1906. doi: 10.1016/j.cub.2008.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han Y-G, Kwok BH, Kernan MJ. Intraflagellar transport is required in Drosophila to differentiate sensory cilia but not sperm. Curr. Biol. 2003;13:1679–1686. doi: 10.1016/j.cub.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 50.Kavlie RG, Kernan MJ, Eberl DF. Hearing in Drosophila requires TilB, a conserved protein associated with ciliary motility. Genetics. 2010;185:177–188. doi: 10.1534/genetics.110.114009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bechstedt S, Albert JT, Kreil DP, Muller-Reichert T, Gopfert MC, Howard J. A doublecortin containing microtubule-associated protein is implicated in mechanotransduction in Drosophila sensory cilia. Nat Commun. 2010;1:11. doi: 10.1038/ncomms1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gong Z, Son W, Chung YD, Kim J, Shin DW, McClung CA, Lee Y, Lee HW, Chang D-J, Kaang B-K, et al. Two interdependent TRPV channel subunits, inactive and nanchung, mediate hearing in Drosophila. J. Neurosci. 2004;24:9059–9066. doi: 10.1523/JNEUROSCI.1645-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim J, Chung YD, Park D-y, Choi S, Shin DW, Soh H, Lee HW, Son W, Yim J, Park C-S, et al. A TRPV family ion channel required for hearing in rosophila. Nature. 2003;424:81–84. doi: 10.1038/nature01733. [DOI] [PubMed] [Google Scholar]
- 54.Lee J, Moon S, Cha Y, Chung YD. Drosophila TRPN (=NOMPC) channel localizes to the distal end of mechanosensory cilia. PLoS ONE. 2010;5:e11012. doi: 10.1371/journal.pone.0011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liang X, Madrid J, Saleh HS, Howard J. NOMPC, a member of the TRP channel family, localizes to the tubular body and distal cilium of Drosophila campaniform and chordotonal receptor cells. Cytoskeleton (Hoboken) 2011;68:1–7. doi: 10.1002/cm.20493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eberl DF, Kernan MJ. Measuring sound-evoked potentials from Drosophila Johnston's organ. In: Zhang B, Freeman MR, Waddell S, editors. Drosophila Neurobiology: A Laboratory Manual. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 2010. pp. 237–245. [Google Scholar]
- 57.Eberl DF, Kernan MJ. Recording sound-evoked potentials from the Drosophila antennal nerve. Cold Spring Harb Protoc. 2011;2011 doi: 10.1101/pdb.prot5576. prot5576. [DOI] [PubMed] [Google Scholar]
- 58.Effertz T, Nadrowski B, Piepenbrock D, Albert JT, Gopfert MC. Direct gating and mechanical integrity of Drosophila auditory transducers require TRPN1. Nat Neurosci. 2012;15:1198–1200. doi: 10.1038/nn.3175. [DOI] [PubMed] [Google Scholar]
- 59.Sun Y, Liu L, Ben-Shahar Y, Jacobs JS, Eberl DF, Welsh MJ. TRPA channels distinguish gravity sensing from hearing in Johnston's organ. Proc. Natl. Acad. Sci. (USA) 2009;106:13606–13611. doi: 10.1073/pnas.0906377106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu L, Li Y, Wang R, Yin C, Dong Q, Hing H, Kim C, Welsh MJ. Drosophila hygrosensation requires the TRP channels water witch and nanchung. Nature. 2007;450:294–298. doi: 10.1038/nature06223. [DOI] [PubMed] [Google Scholar]
- 61.Gong J, Wang Q, Wang Z. NOMPC is likely a key component of Drosophila mechanotransduction channels. Eur. J. Neurosci. 2013 doi: 10.1111/ejn.12214. [DOI] [PubMed] [Google Scholar]
- 62.Yan Z, Zhang W, He Y, Gorczyca D, Xiang Y, Cheng LE, Meltzer S, Jan LY, Jan YN. Drosophila NOMPC is a mechanotransduction channel subunit for gentle-touch sensation. Nature. 2013;493:221–225. doi: 10.1038/nature11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kang L, Gao J, Schafer WR, Xie Z, Xu XZS. C. elegans TRP family proteinn TRP-4 is a pore-forming subunit of a native mechanotransduction channel. Neuron. 2010;67:381–391. doi: 10.1016/j.neuron.2010.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sidi S, Friedrich RW, Nicolson T. NompC TRP channel required for vertebrate sensory hair cell mechanotransduction. Science. 2003;301:96–99. doi: 10.1126/science.1084370. [DOI] [PubMed] [Google Scholar]
- 65.Lehnert Brendan P, Baker Allison E, Gaudry Q, Chiang A-S, Wilson Rachel I. Distinct Roles of TRP Channels in Auditory Transduction and Amplification in Drosophila. Neuron. 2013;77:115–128. doi: 10.1016/j.neuron.2012.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bharadwaj R, Roy M, Ohyama T, Sivan-Loukianova E, Delannoy M, Lloyd TE, Zlatic M, Eberl DF, Kolodkin AL. Cbl-associated protein regulates assembly and function of two tension-sensing structures in Drosophila. Development. 2013;140:627–638. doi: 10.1242/dev.085100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Elliott SL, Cullen CF, Wrobel N, Kernan MJ, Ohkura H. EB1 is essential during Drosophila development and plays a crucial role in the integrity of chordotonal mechanosensory organs. Mol. Biol. Cell. 2005;16:891–901. doi: 10.1091/mbc.E04-07-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roy M, Sivan-Loukianova E, Eberl DF. Cell-type–specific roles of Na+/K+ ATPase subunits in Drosophila auditory mechanosensation. Proc. Natl. Acad. Sci. (USA) 2013;110:181–186. doi: 10.1073/pnas.1208866110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chung YD, Zhu J, Han Y-G, Kernan MJ. nompA encodes a PNS-specific, ZP domain protein required to connect mechanosensory dendrites to sensory structures. Neuron. 2001;29:415–428. doi: 10.1016/s0896-6273(01)00215-x. [DOI] [PubMed] [Google Scholar]
- 70.Eberl DF, Duyk GM, Perrimon N. A genetic screen for mutations that disrupt an auditory response in Drosophila melanogaster. Proc. Natl. Acad. Sci. (USA) 1997;94:14837–14842. doi: 10.1073/pnas.94.26.14837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kernan M, Cowan D, Zuker C. Genetic dissection of mechanosensory transduction: mechanoreception-defective mutations of Drosophila. Neuron. 1994;12:1195–1206. doi: 10.1016/0896-6273(94)90437-5. [DOI] [PubMed] [Google Scholar]
- 72.Avidor-Reiss T, Maer AM, Koundakjian E, Polyanovsky A, Keil T, Subramaniam S, Zuker CS. Decoding cilia function: defining specialized genes required for compartmentalized cilia biogenesis. Cell. 2004;117:527–539. doi: 10.1016/s0092-8674(04)00412-x. [DOI] [PubMed] [Google Scholar]
- 73.Laurençon A, Dubruille R, Efimenko E, Grenier G, Bissett R, Cortier E, Rolland V, Swoboda P, Durand B. Identification of novel regulatory factor X (RFX) target genes by comparative genomics in Drosophila species. Genome Biol. 2007;8:R195. doi: 10.1186/gb-2007-8-9-r195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cachero S, Simpson TI, Zur Lage PI, Ma L, Newton FG, Holohan EE, Armstrong JD, Jarman AP. The gene regulatory cascade linking proneural specification with differentiation in Drosophila sensory neurons. PLoS biology. 2011;9:e1000568. doi: 10.1371/journal.pbio.1000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Newton FG, zur Lage PI, Karak S, Moore DJ, Gopfert MC, Jarman AP. Forkhead transcription factor Fd3F cooperates with Rfx to regulate a gene expression program for mechanosensory cilia specialization. Dev. Cell. 2012;22:1221–1233. doi: 10.1016/j.devcel.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Senthilan PR, Piepenbrock D, Ovezmyradov G, Nadrowski B, Bechstedt S, Pauls S, Winkler M, Möbius W, Howard J, Göpfert MC. Drosophila auditory organ genes and genetic hearing defects. Cell. 2012;150:1042–1054. doi: 10.1016/j.cell.2012.06.043. [DOI] [PubMed] [Google Scholar]
- 77.Tootoonian S, Coen P, Kawai R, Murthy M. Neural representations of courtship song in the Drosophila brain. J. Neurosci. 2012;32:787–798. doi: 10.1523/JNEUROSCI.5104-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lai JS, Lo SJ, Dickson BJ, Chiang AS. Auditory circuit in the Drosophila brain. Proc Natl Acad Sci U S A. 2012;109:2607–2612. doi: 10.1073/pnas.1117307109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tauber E, Eberl DF. Acoustic communication in Drosophila. Behav. Proc. 2003;64:197–210. [Google Scholar]
- 80.Ritchie MG, Halsey EJ, Gleason JM. Drosophila song as a species-specific mating signal and the behavioural importance of Kyriacou & Hall cycles in D. melanogaster. Anim. Behav. 1999;58:649–657. doi: 10.1006/anbe.1999.1167. [DOI] [PubMed] [Google Scholar]
- 81.Inagaki HK, Kamikouchi A, Ito K. Protocol for quantifying sound-sensing ability of Drosophila melanogaster. Nature Protocols. 2010;5:26–30. doi: 10.1038/nprot.2009.206. doi: [DOI] [PubMed] [Google Scholar]
- 82.von Schilcher F. The role of auditory stimuli in the courtship of Drosophila melanogaster. Anim. Behav. 1976;24:18–26. [Google Scholar]
- 83.Ewing AW. Arthropod Bioacoustics: Neurobiology and Behaviour. Edinburgh: Edinburgh University Press; 1989. [Google Scholar]
- 84.Ejima A, Griffith LC. Courtship initiation is stimulated by acoustic signals in Drosophila melanogaster. PLoS ONE. 2008;3:e3246. doi: 10.1371/journal.pone.0003246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tauber E, Eberl DF. The effect of male competition on the courtship song of Drosophila melanogaster. J. Insect Behav. 2002;15:109–120. [Google Scholar]
- 86.Menda G, Bar HY, Arthur BJ, Rivlin PK, Wyttenbach RA, Strawderman RL, Hoy RR. Classical conditioning through auditory stimuli in Drosophila: methods and models. J. Exp. Biol. 2011;214:2864–2870. doi: 10.1242/jeb.055202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mamiya A, Beshel J, Xu C, Zhong Y. Neural representations of airflow in Drosophlia mushroom body. PLoS ONE. 2008;3:e4063. doi: 10.1371/journal.pone.0004063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fabre CC, Hedwig B, Conduit G, Lawrence PA, Goodwin SF, Casal J. Substrate-Borne Vibratory Communication during Courtship in Drosophila melanogaster. Curr. Biol. 2012 doi: 10.1016/j.cub.2012.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Armstrong JD, Texada MJ, Munjaal R, Baker DA, Beckingham KM. Gravitaxis in Drosophila melanogaster: a forward genetic screen. Genes Brain Behav. 2006;5:222–239. doi: 10.1111/j.1601-183X.2005.00154.x. [DOI] [PubMed] [Google Scholar]
- 90.Mamiya A, Straw AD, Tomasson E, Dickinson MH. Active and passive antennal movements during visually guided steering in flying Drosophila. J. Neurosci. 2011;31:6900–6914. doi: 10.1523/JNEUROSCI.0498-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hardie RC, Franze K. Photomechanical Responses in Drosophila Photoreceptors. Science. 2012;338:260–263. doi: 10.1126/science.1222376. [DOI] [PubMed] [Google Scholar]
- 92.Matsuo E, Kamikouchi A. Neuronal encoding of sound, gravity, and wind in the fruit fly. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2013;199:253–262. doi: 10.1007/s00359-013-0806-x. [DOI] [PubMed] [Google Scholar]