Abstract
The majority of chimeric antigen receptor (CAR) T cell research has focused on attacking cancer cells. Here we show that targeting the tumor-promoting, non-transformed stromal cells using CAR T cells may offer several advantages. We developed a retroviral CAR construct specific for the mouse fibroblast activation protein (FAP), comprising a single chain Fv FAP (mAb 73.3) with the CD8α hinge and transmembrane regions, and the human CD3ζ and 4-1BB activation domains. The transduced muFAP-CAR mouse T cells secreted IFNγ and killed FAP-expressing 3T3 target cells specifically. Adoptively transferred 73.3-FAP-CAR mouse T cells selectively reduced FAPhi stromal cells and inhibited the growth of multiple types of subcutaneously transplanted tumors in wild-type, but not FAP-null immune-competent syngeneic mice. The antitumor effects could be augmented by multiple injections of the CAR T cells, by using CAR T cells with a deficiency in diacylglycerol kinase, or by combination with a vaccine. A major mechanism of action of the muFAP-CAR T cells was the augmentation of the endogenous CD8+ T cell antitumor responses. Off-tumor toxicity in our models was minimal following muFAP-CAR T cell therapy. In summary, inhibiting tumor growth by targeting tumor stroma with adoptively transferred CAR T cells directed to FAP can be safe and effective suggesting that further clinical development of anti-human FAP-CAR is warranted.
Keywords: solid tumor, fibroblast activation protein and tumor microenvironment
Introduction
One approach to adoptive T cell therapy has been to transfect patient-derived blood lymphocytes with chimeric antigen receptor (CAR) genes that combine the effector functions of T lymphocytes with the high specificity of single chain antibody fragments (scFv) that recognize predefined surface antigens in a non-MHC-restricted manner (1,2). With recent modifications and improvements, CAR T cell therapy is showing great promise in the clinic (3–5).
To minimize on-target/off-tumor toxicity, target antigens should be expressed at high levels on the surface of tumor components with minimal or no expression on “essential” normal tissues. To date, potential targets have largely been antigens on cancer cells, such as CD19, ErbB2, CEA, or GD2 (3–6). We postulate that non-cancer cell components of the tumor may also be attractive targets for CAR-based immunotherapy. First, the genetic instability of tumor cells contributes to their immune escape whereas stromal cells are more genetically stable. Second, the tumor stroma contributes to tumor growth and resistance to therapy by: i) forming a physical barrier to tumor-targeting agents; ii) supporting tumor cell growth, invasion, and angiogenesis through the production of growth factors, chemokines and matrix (7–11); iii) exerting an immunosuppressive influence by secreting factors that attract immunosuppressive cells, by producing factors that regulate T-cell functions and modulate the phenotype of myeloid cells (7,12), and by expressing inhibitory surface molecules PD-L1 and PD-L2 (13). Finally, the mechanisms by which the cancer stroma supports tumorigenesis are shared among various stromal cell-types, so that therapies targeting such mechanisms are likely to have therapeutic implications across a broad spectrum of cancers.
One attractive stromal cell target is the fibroblast activation protein (FAP), a transmembrane serine protease highly expressed in the cancer-associated stromal cells (CASCs) of virtually all epithelial cancers (14–18). FAP is also expressed during embryonic development, in tissues of healing wounds, and in chronic inflammatory and fibrotic conditions such as liver cirrhosis and idiopathic pulmonary fibrosis (19–22). However, FAP has not been detected by immunohistochemistry in benign tumors nor in most normal quiescent adult stromal cells (23–25). FAP+ cells have been studied using Bac transgenic mice containing the FAP promoter driving the green fluorescent protein (GFP) or the human diphtheria toxin receptor to conditionally eliminate FAP+ cells (26,27). Although the elimination of FAP+ cells caused the decreased growth of immunogenic OVA-expressing Lewis lung carcinoma tumors and augmented the efficacy of an OVA vaccine, the complete ablation of FAP+ cells was associated with anemia and cachexia, thought to result from the loss of FAP+ stromal cells in the bone marrow and muscle. Some FAP expression was also seen in the pancreas.
Therapeutically, FAP+ cells have been successfully targeted using vaccines and immunoconjugate therapy resulting in some tumor growth inhibition without obvious toxicity or effects on wound healing (28–30). However, given the increased efficacy of adoptive T cell transfer, we and others have hypothesized that anti-FAP-CAR T cells may prove more effective. Since the submission of this manuscript, three contrasting papers have been published describing the use of anti-FAP CAR T cells. Tran et al. using anti-FAP CAR mouse T cells (comprising the scFv from the FAP-5 mAb (30) that targets human and mouse FAP) in combination with total body irradiation and IL-2 injections, showed limited antitumor efficacy that was associated with severe bone marrow toxicity and cachexia (31). In contrast, Kakarla et al. showed antitumor efficacy without toxicity using anti-FAP-CAR human T cells (comprising the scFV from the M036 mAb (32) that targets both human and mouse FAP) in an immunodeficient mouse model of human lung cancer (33). Similarly, Schuberth et al. showed a survival advantage in an intraperitoneal mesothelioma xenograft model after administering FAP-CAR human T cells (using a scFv from the human-specific F19 mAb (18)) together with FAP-expressing human mesothelioma cells (34). In this study, the CAR-mediated on-target/off-tumor-toxicity could not be evaluated because the F19 anti-human FAP antibody does not cross-react with the mouse FAP.
Given recent data that FAP-expressing cells may play an important role in regulating antitumor immunity (35), we developed a system to study advanced generation FAP-CAR T cell therapy in wild-type mice with fully intact immune systems. Using a different anti-FAP scFv (mAb 73.3) linked to both human and murine cytoplasmic domains, we generated muFAP-CAR mouse T cells that elicited antitumor efficacy mediated by the activation of endogenous immune responses in multiple tumor models. The anti-FAP CAR T cells demonstrated enhanced antitumor response when combined with a tumor vaccine. Our FAP-CAR constructs induced minimal toxicity with no anemia or weight loss, suggesting that further clinical development of anti-human FAP CAR T cells may be feasible.
Materials and Methods (see Supplemental Methods for more details)
Cell lines
Mouse AE17.ova mesothelioma cells (expressing chicken ovalbumin) were provided by Dr. Delia Nelson, University of Western Australia (36). TC1 lung cancer cells were derived from mouse lung epithelial cells immortalized with human papillomavirus HPV-16 E6 and E7 and transformed with the c-Ha-ras oncogene (37). The mouse LKR cell line was derived from an explant of a pulmonary tumor from an activated K-rasG12D mutant mouse generated in Dr. Tyler Jacks’ lab at M.I.T. (Boston, MA; ref 38). Mouse 4T1 mammary carcinoma, CT26 colon cancer cells and 3T3Balb/C cells, were purchased from the American Type Culture Collection. Mouse FAP expressing 3T3BALB/C (3T3.FAP) cells were created by lentiviral transduction of the FAP- 3T3 parental line with murine FAP (data not shown). All cell lines used were checked for mycoplasma; except for the protein expression of the transgenes no additional authentication was performed.
Antibodies
Specific antibodies used are listed in the Supplemental methods.
Generation of 73.3 hybridoma
FAP-null mice (39) were immunized and twice boosted with FAP-expressing 3T3 cells intraperitoneally (i.p.). Three days after the final boost, splenocytes were harvested and fused to myeloma cells. Hybridoma supernatants were screened for monoclonal antibodies (mAb) that reacted specifically with 3T3.FAP cells and activated primary wild-type fibroblasts, but not the parental 3T3 cells or the activated primary fibroblasts from FAP-null mice. mAb 73.3 with this reactivity profile was purified and characterized by ELISA and by immunoblotting as specific for mouse FAP based on the reactivity with the purified recombinant mouse FAP extracellular domain (rFAP-ECD), its reactivity with a single protein with an apparent molecular weight of approximately 95kD in the protein extracts of FAP+ but not FAP− cells and tissues, and a lack of reactivity with cells expressing human FAP (human FAP transduced 3T3, human foreskin fibroblasts or primary human tumor associated stromal cell (data not shown). Biacore studies (see Supplemental Methods) showed that the affinity of mAb 73.3 for purified rFAP-ECD protein was less than 1 nM and estimated to be between 0.1 and 0.3 nM (data not shown).
Generation of anti-muFAP CAR constructs
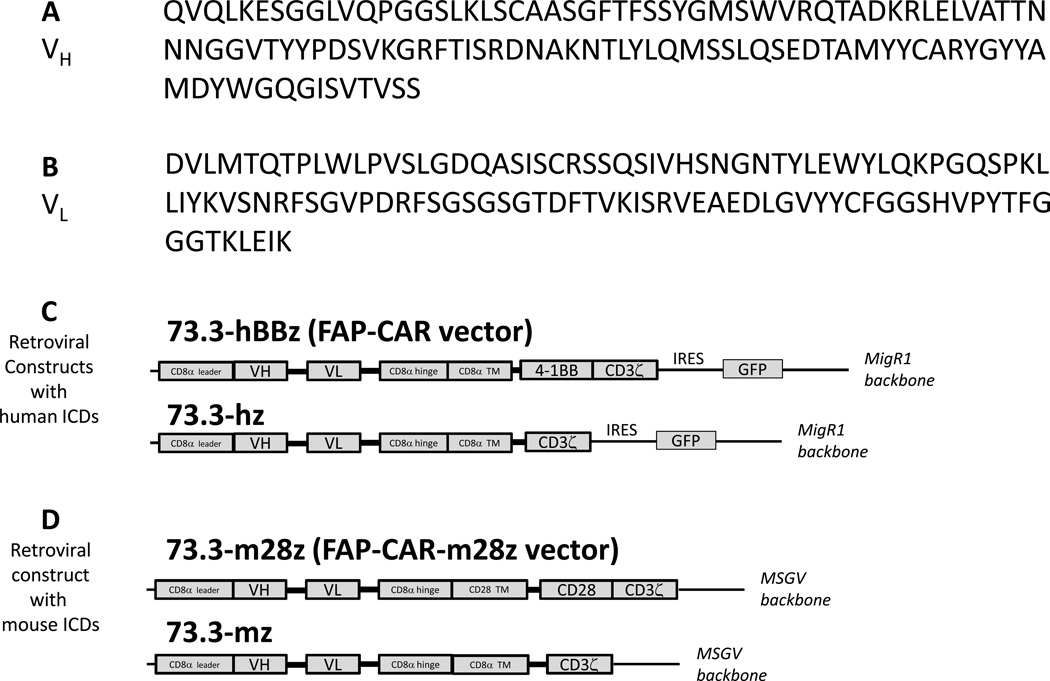
Total RNA from 73.3 hybridoma cells was isolated and reverse transcribed into cDNA. Variable heavy (VH) and light (VL) chains of the 73.3 immunoglobulin were PCR amplified and sequenced (Fig. 1A and 1B). The VH and VL sequences were fused with a CAR construct that is being evaluated in clinical trials (CD8α hinge, CD8α transmembrane domain, and two human intracellular signaling domains (ICDs) derived from 4-1BB and CD3ζ; Ref 40). This CAR was then inserted into an IRES-containing retroviral MigR1 vector (Fig. 1C) that also expresses GFP for tracking purposes (41). A fully mouse construct of FAP-CAR, FAP-CAR-m28ζ, was also created by coupling the same 73.3 scFv with the murine CD3ζ chain and the murine CD28 intracellular signaling domain. This construct was inserted into another retroviral vector MSGV (Fig. 1D; Ref. 42). Infective particles were generated from the supernatants of 293T cells transfected with the retroviral vector plasmid and helper plasmids using Lipofectamine 2000 (Invitrogen), as described (42,43). Two control FAP-CAR constructs, one with human CD3ζ and the other with mouse CD3ζ ICDs, were also generated to evaluate the function of the co-stimulatory domains (see Supplemental Methods).
Figure 1. Structure of FAP-CAR.
Total RNA of 73.3 hybridoma was extracted, reverse transcribed, and the cDNA PCR amplified and inserted into a cloning vector to obtain the sequence of the variable domains of IgG heavy (A) and light (B) chains. The anti-muFAP CAR consists of the anti-muFAP scFv, CD8α hinge and transmembrane (TM) domain, plus 4-1BB and CD3ζ intracellular signaling domains (ICDs), and was cloned into the MigR1 retroviral vector for transduction of primary mouse T cells (C). A fully mouse FAP-CAR construct was also synthesized, which consists of the anti-muFAP scFv, CD8α hinge and CD28 transmembrane domain, plus CD28 and CD3ζ intracellular signaling domains of mouse origin, and was cloned into the MSGV retroviral vector to transduce primary mouse T cells (D). Two first-generation FAP-CAR constructs with a human CD3ζ (C) or a mouse CD3ζ (D) signaling domain were also generated.
Isolation, Transduction and Expansion of Primary Mouse T lymphocytes
Primary murine splenic T cells were isolated and transduced as previously described (43). See Supplemental Methods.
Antigen- or antibody-coated beads
Recombinant FAP-extracellular domain protein (FAP-ECD, see Supplemental Methods), bovine serum albumin (Fisher Scientific) or anti-CD3ε/anti-CD28 antibodies (eBioscience) were chemically crosslinked to tosylactivated 4.5 µm Dynabeads (Invitrogen, #140-13) per manufacturers’ instructions.
Immunoblotting
FAP-CAR transduced T cells were incubated either with BSA or FAP-ECD-coated beads (at 2:1 bead to T cell ratio), or with anti-CD3ε antibody for 10 min. Lysates were then prepared and immunoblotted for phosphorylated ERK, phosphorylated AKT, phosphorylated IKKα/β, or β-actin.
Cytotoxicity and IFNγ ELISA
Parental 3T3 and 3T3.FAP cells were transduced with luciferase as described (44). T cells and target 3T3 cells were co-cultured at the indicated ratios, in triplicate, in 96-well round bottom plates. After 18 hours, the culture supernatants were collected for IFNγ analysis using an ELISA (mouse IFNγ, BD OpEIA). Cytotoxicity of transduced T cells was determined by detecting the remaining luciferase activity from the cell lysate using a previously described assay (43).
CAR T cell transfer into mice bearing established tumors
Mice were injected subcutaneously with 2 × 106 AE17.ova (C57BL/6 mice), 1 × 106 TC1 (C57BL/6 mice), 2 × 106 LKR (C57BL/6 crossed with 129pf/j), 0.5 × 106 4T1 (BALB/c mice), or 1 × 106 CT26 (BALB/c mice) tumor cells into the dorsal-lateral flank. Mice bearing established tumors (100–150 mm3) were randomly assigned to receive either FAP-CAR T cells or MigR1-transduced T cells or remained untreated (minimum, five mice per group, each experiment repeated at least once). 1×107 T cells were administered through the tail vein. Body weight and tumor size were measured by electronic scales and calipers, respectively. At the end of the experiment, tumors and spleens were harvested for flow cytometric analyses.
Flow cytometric analyses
Tumors were harvested 3 and 8 days after adoptive transfer of FAP-CAR T cells to analyze intratumoral cells by flow cytometry as described (42). Cell acquisition was performed on LSR-II using FACSDiva software (BD Bioscience, USA). Data were analyzed using FlowJo (Tree Star).
Statistical Analyses
For flank-tumor studies comparing two groups, the student t test was used. For comparisons of more than two groups, we used one-way ANOVA with appropriate post hoc testing. Differences were considered significant when p <0.05. Data are presented as mean +/− SEM.
Results
In Vitro evaluation of mouse FAP-CAR T cells
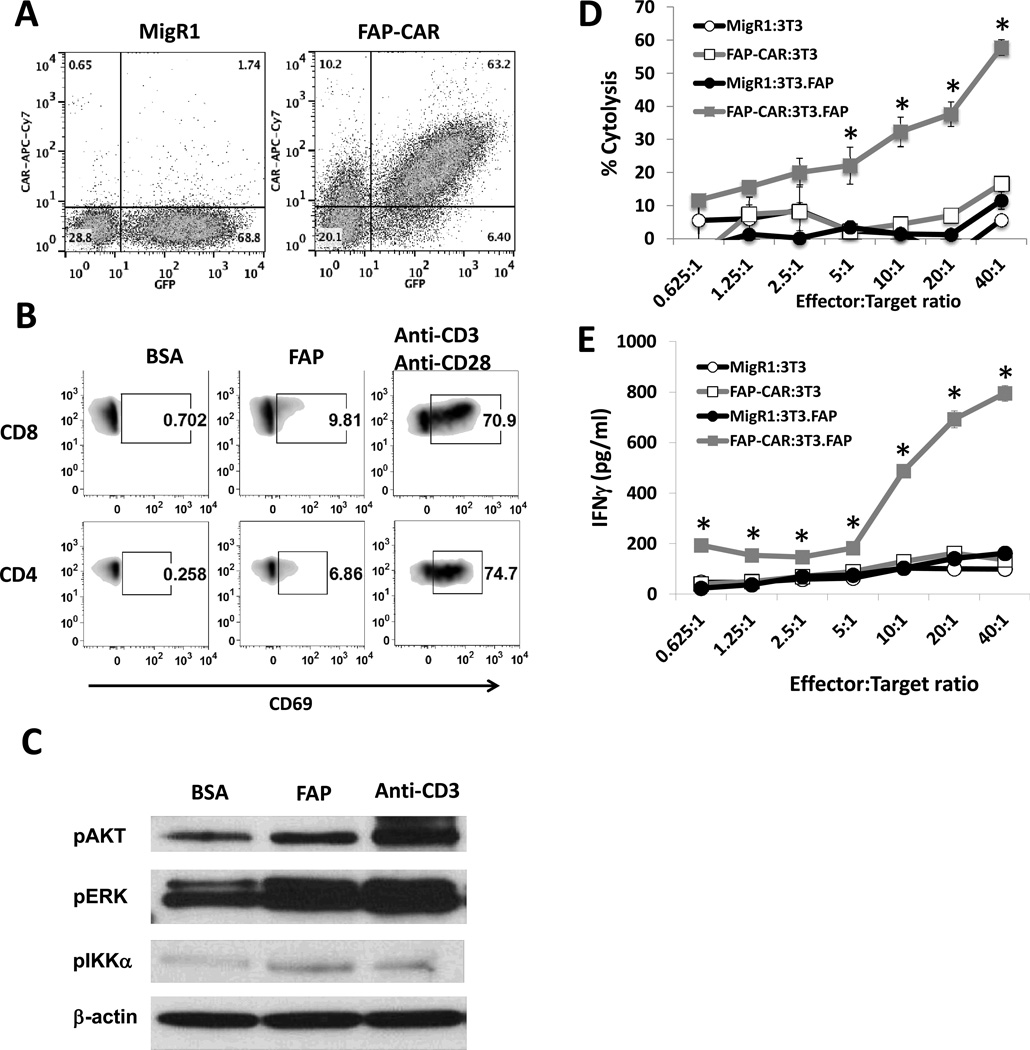
Our primary retroviral CAR construct (containing the scFv from anti-murine FAP antibody 73.3 coupled to the human CD3ζ and 4-1BB cytoplasmic domains that we have used previously in murine models; ref. 42) and a control virus expressing only GFP (Fig. 1) were used to transduce activated mouse T cells resulting in greater than 60% of the T cells expressing GFP (MigR1) or GFP plus FAP-CAR (Fig. 2A).
Figure 2. Ex vivo assessment of mouse CAR T cells redirected against FAP and signaling in FAP-CAR T cells.
(A) Retroviral transduced mouse T cells expressed GFP (MigR1) or GFP and anti-mFAP-CAR. (B) Up-regulation of CD69 on CAR (GFP)-positive CD8 and CD4 T cells was determined following stimulation with BSA- or FAP-coated beads for 18 hours. T cells were stimulated with anti-CD3/anti-CD28 beads as positive control. (C) FAP-CAR T cells were exposed to either BSA-or FAP-coated beads for 10 min. Cell lysates were then prepared and immunoblotted for phospho-ERK, phospho-AKT, and phospho-IKKα/β. Anti-CD3ε antibody was used as a positive control for T cell activation, and β-actin was immunoblotted for equal loading.
To determine target-specific cytolytic activity (D) and IFNγ production (E) of FAP-CAR T cells, various Effector:Target ratios of MigR1 and FAP-CAR T cells were reacted with parental 3T3 or 3T3.FAP fibroblasts for 18 hours. * Denotes statistical significance between FAP-CAR-treated 3T3.FAP group versus the other 3 groups, p value < 0.05.
To verify functionality, mouse T cells expressing FAP-CAR were stimulated for 18 hours with beads coated with either bovine serum albumin (BSA; negative control), or recombinant FAP protein, or anti-CD3/anti-CD28 antibodies (positive control). The FAP-coated beads activated FAP-CAR T cells, as shown by increased CD69 expression above that of the negative control (Fig. 2B).
To further evaluate intracellular signaling, lysates from bead-stimulated T cells were electropheresed and immunoblotted. In comparison to BSA-coated beads, FAP-coated beads induced the phosphorylation of AKT, ERK, and IKKα/β in FAP-CAR T cells (Fig. 2C).
To assess effector functions, transduced mouse T cells were co-cultured with 3T3 fibroblasts (which do not express FAP) or with 3T3 fibroblasts transduced to express mouse FAP (3T3.FAP) (data not shown). After 18 hours, T cells expressing the FAP-CAR construct (but not the control GFP construct) effectively killed 3T3.FAP fibroblasts (Fig. 2D) and secreted IFNγ (Fig. 2E) in a dose-dependent manner, but had no effect on parental 3T3 cells.
Injection of mouse FAP-CAR T cells reduces tumor growth in a FAP-specific fashion
We next explored the capability of FAP-CAR mouse T cells to inhibit tumor growth using three different tumor lines which do not express FAP: AE17.ova mesothelioma cells, TC1 and LKR lung cancer cells. Cells were injected into the flanks of syngeneic mice and allowed to form established tumors. The tumors had an easily detectable number of mouse FAP-expressing cells with the majority of the FAP+ cells being CD45−/CD90+ stromal cells (~3% of total tumor cells), and only a small minority being CD45+ hematopoietic cells (~0.2% of total tumor cells) (Table 1, Suppl. Fig. 1).
Table 1.
Depletion of FAP+ cells in flank tumors post FAP-CAR treatment.
| AE17.ova | TC1 | LKR | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Untreated | FAP-CAR | P value | Untreated | FAP-CAR | P value | Untreated | FAP-CAR | P value | |
| CD45-CD90+ | 1.86 | 1.15 | 0.001* | 1.72 | 0.60 | 0.04* | 2.68 | 1.84 | 0.009* |
| CD45+ | 0.12 | 0.08 | 0.157 | 0.10 | 0.04 | 0.066 | 0.28 | 0.15 | 0.035* |
| Average tumor volume (mm3) | 627 | 324 | 0.03* | 379 | 140 | 0.002* | 410 | 260 | 0.02* |
The values above indicate the averages of percent FAP+ cells per total tumor population. Tumors were harvest 7–9 days after T cell infusion, and there were 5 mice in each group. Student paired t test was performed to evaluate FAP+ cell depletion, as well as change in tumor volume, after adoptive T cell therapy.
Denotes statistical significance between untreated and FAP-CAR-treated samples, p value < 0.05.
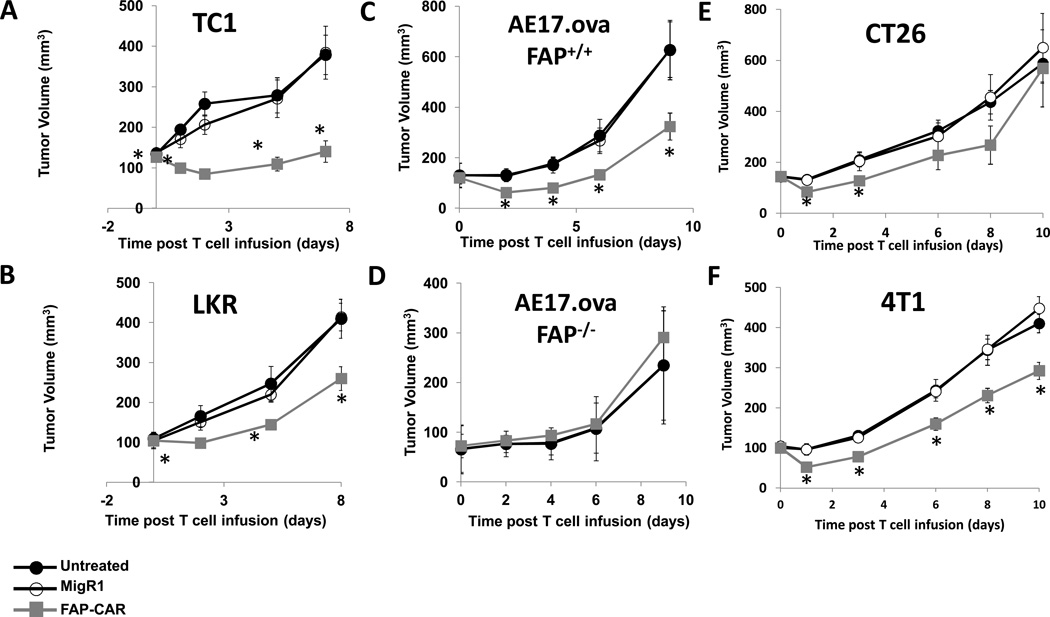
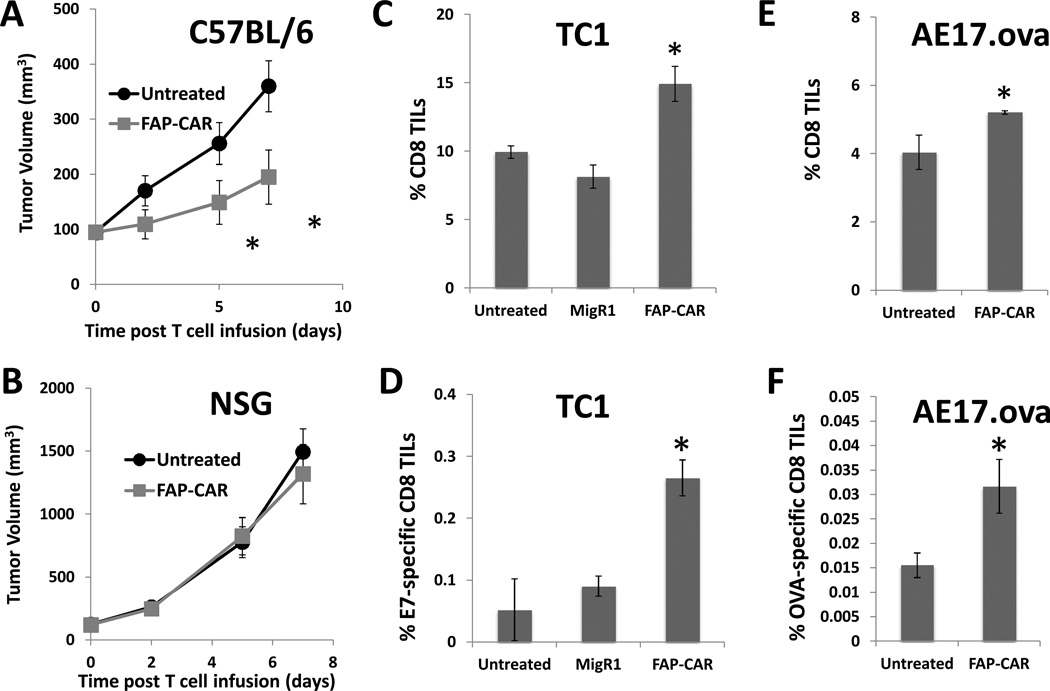
When tumors reached ~100–150 mm3 (7–14 days after tumor cell inoculation), 107 T cells were injected intravenously and the tumors were measured with calipers. FAP-CAR T cells, but not MigR1 T cells, significantly (p<0.05) reduced the growth of TC1 tumors (Fig. 3A), LKR tumors (Fig. 3B) and AE17.ova tumors (Fig. 3C) by 35–50%.
Figure 3. Anti-tumor activities of FAP-CAR T cells in mice bearing flank tumors.
Syngeneic mice bearing (A) TC1, (B) LKR, (C) AE17.ova, (E) CT26, and (F) 4T1 tumors were injected intravenously with 10 million FAP-CAR or MigR1 T cells when the tumors reached ~100–150 mm3. Tumor measurements followed. (D) To test the target-specificity of FAP-CAR T cells, AE17.ova tumor cells were also injected into FAP-null C57BL/6 mice. FAP-CAR T cells were given 7 days later. * Denotes statistical significance between untreated, MigR1 and FAP-CAR-treated samples, p value < 0.05.
To confirm specificity, we inoculated AE17.ova cells into FAP-null C57BL/6 mice and treated the tumors as described above. In contrast to the effect on AE17.ova tumors in wild-type C57BL/6 mice (Fig. 3C), FAP-CAR T cells had no effect on the growth of AE17.ova tumors in FAP-null mice (Fig. 3D).
Given the differences between our efficacy data and those of Tran et al (31), we also treated two of the same tumor lines, CT26 and 4T1, they reported. In contrast to their findings, our FAP-CAR construct induced a significant reduction in tumor size (Fig. 3E, 3F), although the changes were smaller than those seen in Figure 3A–3D.
Effect of the injection of mouse FAP-CAR T cells on FAP+ cells
To evaluate the effect of the T cells on the FAP+ stromal cells, we harvested tumors 7 to 9 days post-T cell infusion and analyzed the dissociated cells by flow cytometry. As shown in Table 1, at this time point, the FAP+/CD45−/CD90+ and FAP+/CD45+ populations were decreased by about 50% in comparison with those in the untreated group, while the amount of FAP+ cells remaining in the MigR1 group was similar to that in the untreated controls (data not shown).
We characterized this depletion in more detail using the AE17.ova model, and evaluated the FAP+ cells at 3 days post-T cell transfer. At this earlier time point, we saw larger decreases in FAP+CD90+ stromal cells (a reduction of 82%) and FAP+CD45+ leukocytes (a reduction of 56%) (Suppl. Fig. 2A). Moreover, in control animals, we could identify both low- and high-FAP expressing cells in both the CD45−CD90+ and CD45+ populations (Suppl. Fig. 1). When we gated on these specific populations, we noted that the FAP-CAR T cells selectively depleted the FAP-high expressing cells, with little effect on FAP-low expressing cells (Suppl. Figs. 2B and 2C).
Kinetics of FAP-CAR T cell persistence
We assessed the number of intratumoral FAP-CAR T cells in the AE17.ova model at 3, 7, and 10 days after adoptive transfer and found the number peaked at day 3 after injection and diminished at the 7 and 10 day time points by ~65% (Suppl. Fig. 3A).
To determine if this rapid loss of T cells was a consequence of abnormal function of the human 4-1BB and CD3ζ cytoplasmic domains within the mouse T cells, we engineered a second construct by inserting our scFv anti-FAP 73.3 antibody fragment into a fully murine CAR containing the murine CD28 cytoplasmic domain and the murine CD3ζ chain (73.3m28z) (Figure 1D). In vitro, this construct showed similar cytotoxicity and IFNγ release when reacted with FAP-expressing fibroblasts compared to the human version of the same CAR (73.3-hBBz; Suppl. Figs. 3B and 3C). To confirm the importance of the co-stimulatory cytoplasmic domains, we synthesized two additional FAP-CAR constructs that lack these co-stimulatory domains, i.e. 73.3-human CD3ζ and 73.3-mouseCD3ζ (Figs. 1C and 1D) Neither the mouse nor the human CD3ζ construct showed significant cytolytic activity or IFNγ production when reacted with 3T3mFAP (Suppl. Figs. 3B and 3C).
After being injected into mice bearing AE17.ova tumors, we found that the trafficking and persistence of the two types of second generation FAP-CAR T cells, 73.3-hBBz and 73.3-m28z, were similar (Suppl. Fig. 3D) as were their antitumor efficacy (Suppl. Fig. 3E). These data show that, compared to human CARs injected into immunodeficient mice, mouse CAR T cells injected into syngeneic hosts persisted for a short time, despite the presence of the human or mouse co-stimulatory cytoplasmic domains.
Approaches to enhance FAP-CAR T cell therapy
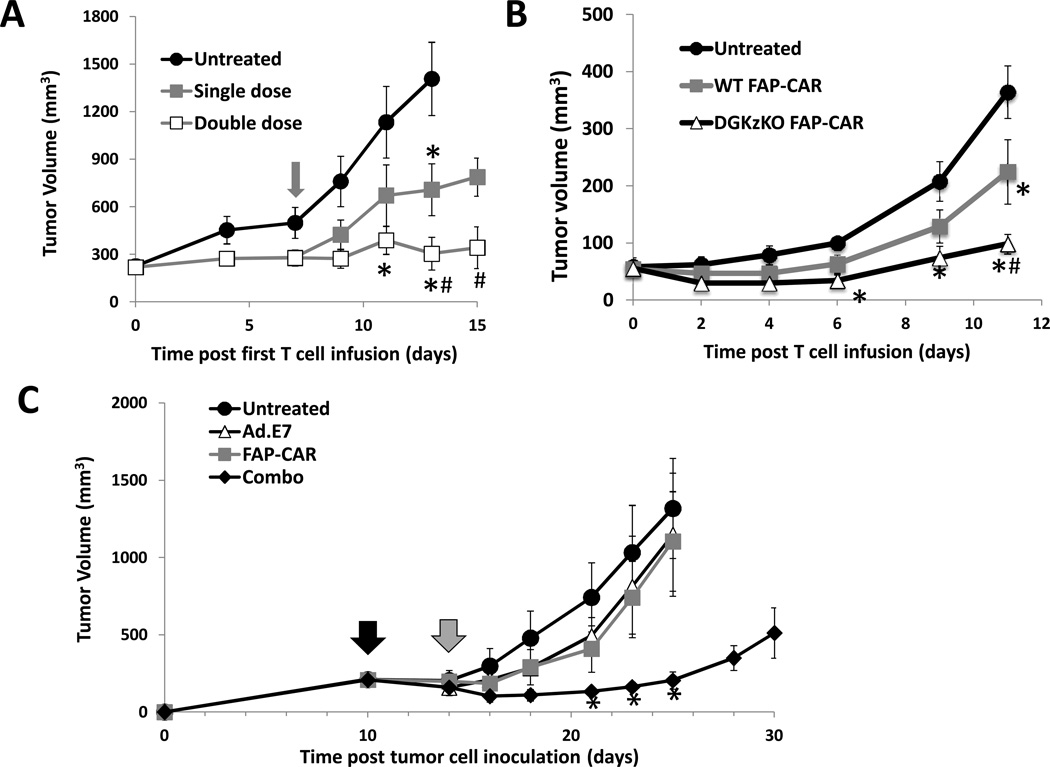
Since our mouse CAR T cells persisted for only a short period of time in vivo, we hypothesized that giving a second infusion of FAP-CAR T cells may enhance therapeutic efficacy. AE17.ova tumor cells were injected into the flanks of C57BL/6 mice. When the tumors reached approximately 100 mm3, a first dose of FAP-CAR T cells was given intravenously. One week later, we randomly divided the FAP-CAR-treated animals into two groups, one treated with an additional dose of control MigR1 T cells (Single dose, Fig. 4A) and one treated with a second dose of FAP-CAR T cells (Double dose, Fig. 4A). At two weeks, tumors in mice given two doses of FAP-CAR T cells were significantly smaller (p<0.05) than those in mice given only one dose of FAP-CAR T cells.
Figure 4. Enhanced therapeutic responses of FAP-CAR T cells.
Mice with AE17.ova flank tumors were injected intravenously with FAP-CAR T cells when tumors were ~100 mm3. A. The overall efficacy of FAP-CAR T cells was enhanced when a second dose of FAP-CAR T cells was given a week later. The grey arrow indicates the injection time of the second dose of FAP-CAR T cells. B. Efficacy could also be enhanced after injection of FAP-CAR T cells lacking the negative intracellular regulator DGKζ. * Denotes statistical significance between untreated and FAP-CAR-treated samples, p value < 0.05. # Denotes statistical significance between single dose FAP-CAR treated group versus double dose group or DGKζ KO FAP-CAR treated group. (C) FAP-CAR T cells enhance efficacy of cancer vaccine. TC1 tumor cells were inoculated into the right flanks of C57BL/6 mice. When tumors reached 200 mm3, one dose of Ad.E7 (109 pfu) was given to the mice contralaterally to their flank tumors (black arrow). FAP-CAR T cells (10 million cells) were given 4 days later (gray arrow). Tumor measurements followed. The values are expressed as the mean ± SEM (n=5). * Denotes significant difference between untreated and the combo groups (p< 0.05).
Another, not mutually exclusive, explanation for the lack of tumor eradication might be the suboptimal CAR signaling in mouse T cells and/or functional suppression in the tumor microenvironment. As we recently reported that mesothelin-targeted CAR mouse T cells deficient in the inhibitory enzyme diacylglycerol kinase zeta (DGKζ) had enhanced effector functions in vitro and in vivo and increased persistence (43), we compared the efficacy of comparably transduced FAP-CAR splenic T cells isolated from WT C57BL/6 versus DGKζ-null mice. DGKζ-deficient FAP-CAR T cells were more efficient in lysing 3T3.FAP cells (Suppl. Fig. 4A) and in secreting IFNγ (Suppl. Fig. 4B) with retention of specificity in vitro. The DGKζ-deficient FAP-CAR T cells were also more effective (p<0.05 on day 11) after being injected into AE17.ova bearing mice (Fig. 4B). The increased efficacy was associated with greater persistence of the DGKζ-knockout compared to WT FAP-CAR T cells (GFP+ cells) (Suppl. Fig. 4C). Thus, the enhanced antitumor efficacy was likely due to both increased T cell activity and to increased persistence.
Role of the acquired immune system in the efficacy of FAP-CAR T cells
To evaluate the role of the acquired immune system in the FAP-CAR T cell-mediated antitumor response, we injected AE17.ova tumor cells into the flanks of either wild-type C57BL/6 or immunodeficient NSG mice, followed by one injection of 107 FAP-CAR T cells. AE17.ova tumors grew more rapidly in NSG than in wild-type mice (Fig. 5A vs 5B) reflecting the endogenous antitumor activity in wild-type mice that was lost in the NSG mice. In contrast to their efficacy in wild-type mice (Fig. 5A), the mouse FAP-CAR T cells had no antitumor effects on AE17.ova tumors in the immunodeficient NSG mice, (Fig. 5B). This loss in activity was not due to the loss of FAP expression in the NSG tumor microenvironment, as we confirmed that AE17.ova tumors had a similar level of FAP expression in the NSG mice as in the immune-competent C57BL/6 mice (data not shown).
Figure 5. Adaptive immune response plays a key role in FAP-CAR T cells-induced antitumor response.
AE17.ova tumors were injected into both C57BL/6 (A) and NSG mice (B). When tumors reached ~100–125 mm3, a single dose (10 million) of FAP-CAR T cells were adoptively transferred through tail vein into mice. Tumor measurements followed. * Denotes statistical significance between untreated and FAP-CAR-treated samples, p value < 0.05. FAP-CAR induced infiltration of antigen-specific CD8 T cells into tumors. TC1 (C,D) and AE17.ova (E,F) tumors were harvested 8 days after adoptive transfer of FAP-CAR T cells in mice. Tumors were digested and made into single cell suspensions. Cells were stained with fluorochrome-conjugated tetramer loaded with E7- or SIINFEKEL(ova)-peptide, together with anti-CD8 antibody to determine percent tumor-specific CD8 T cells in tumors. * Denotes statistical significance between untreated, MigR1 and FAP-CAR-treated samples, p value < 0.05.
To further explore this issue, we used TC1 tumor cells expressing the viral oncoprotein HPV-E7 and AE17.ova cells expressing chicken ovalbumin to evaluate the impact of FAP+ cell depletion on the endogenous antitumor immunity by E7- or ova-specific tetramer staining of the infiltrating lymphocytes 8 days after adoptive transfer. We found a statistically significant (p=0.02) increase in the percentage of total CD8+ T cells within the tumors of FAP-CAR-treated mice compared to control- or MigR1-T cell-treated mice (Figs. 5C and 5E). Tumors from the FAP-CAR-treated mice had a significant (p=0.015) increase in the numbers of E7-specific T cells (Fig. 5D) or ova-specific T cells within the TC-1 and AE17.ova tumors, respectively (Fig. 5F).
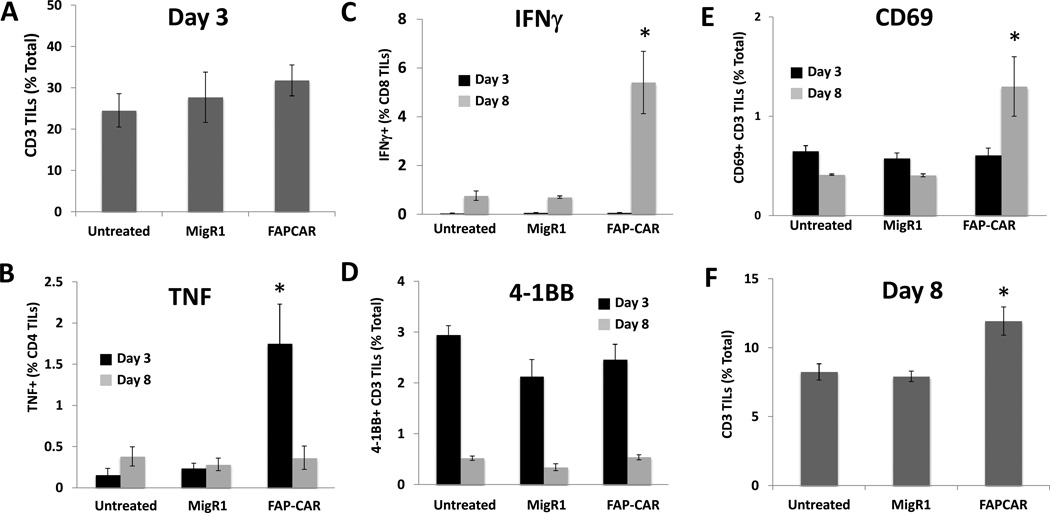
To determine the mechanisms of this immune response, we repeated this experiment in AE17.ova tumor-bearing mice, and analyzed the endogenous (non-GFP-expressing) T cells at 3 and 8 days after T cell injection. Consistent with previous findings by Kraman et al. (26) who used a genetic approach to ablate FAP+ cells, the number of intratumoral T cells was similar between all three groups at 3 days post adoptive transfer (Fig. 6A). However, at this time point, the number of CD4+ T cells producing TNFα was significantly higher in the FAP-CAR T cell group compared to untreated and control T cell-treated groups (Fig. 6B; black bars), while there was no difference in the numbers of activated CD69+ and 4-1BB+ T cells, nor in IFNγ-producing CD8 T cells (Figs. 6C–E; black bars). At 8 days following treatment with FAP-CAR T cells, the number of T cells was higher in tumors treated with FAP-CAR T cells (as above) compared to the two control groups (Fig. 6F). At this time point, the numbers of CD69+ and IFNγ+ CD8+ T cells were increased (Figs. 6C and 6E; gray bars), while the numbers of TNF-producing T cells and 4-1BB+-expressing T cells were similar among the groups (Fig. 6B and 6D; gray bars). Together, these results showed that depletion of FAP+ cells in tumors might enhance antitumor immunity by initially activating endogenous T cells, followed by increasing intratumor T cell infiltration at a later timepoint.
Figure 6. Activation and tumor-infiltration of endogenous T cells following treatment with FAP-CAR T cells.
AE17.ova tumor-bearing mice were injected intravenously with 10 million FAP-CAR or MigR1 T cells when tumors reached ~100 mm3. At 3 and 8 days following adoptive transfer, tumors were harvested and digested to check for the frequencies of CD3+ tumor-infiltrating (A) total T cells 3 days post-transfer; (B) TNF-producing cells; (C) IFNγ-producing cells; (D) 4-1BB+ cells; or (E) CD69+ T cells; (F) total T cells 8 days post-transfer. * Denotes statistical significance between untreated, MigR1 and FAP-CAR-treated samples, p value < 0.05.
Augmentation of the efficacy of FAP-CAR T cells by combination with an antitumor vaccine
Given the effects of FAP+ tumor cell depletion on antitumor immunity, we hypothesized that combining a tumor vaccine with FAP-CAR T cell administration may enhance antitumor efficacy compared to either approach alone. HPV-E7-expressing TC1 tumor cells were injected subcutaneously into C57BL/6 mice; when the tumors reached approximately 200mm3, saline or one subcutaneous dose of a vaccine consisting of 109 pfu of an adenovirus expressing HPV-E7 (Ad.E7) (black arrow) was administered to boost the endogenous T cell response against E7-expressing cells. Four days after injection with saline or the Ad.E7 vaccine, FAP-CAR T cells (107 cells; gray arrow) were given intravenously. Both the Ad.E7 cancer vaccine and the FAP-CAR T cells alone had only modest effects on these large established tumors (Fig. 4C). However, the combination regimen induced tumor regressions and suppressed tumor growth for up to two weeks before further tumors progression.
Toxicity
Since FAP is an endogenous protein and toxicity (especially weight loss and anemia) was recently reported after depletion of FAP+ cells either by genetic ablation (26,27) or FAP-CAR T cell administration (31), we assessed off-tumor/on-target adverse effects after administration of our FAP-CAR T cells. In contrast to these reports, we observed no clinical toxicity or anemia in any of the FAP-CAR T cell studies described above. The body weight of tumor-bearing mice remained constant or increased throughout each experiment (Suppl. Fig. 5).
To further evaluate toxicity, necropsies were performed and visceral organs (heart, lungs, pancreas, liver, spleen, kidneys, skeletal muscle, and bone marrow) were harvested, sectioned, stained and analyzed in a blinded fashion eight days after T cell injection in mice treated with one dose of WT FAP-CAR T cells and eight days after a second dose of WT-FAP CAR T cells from the mice from the experiment shown in Figure 4A. When compared to control tumor-bearing mice, no abnormalities were observed in the mice given WT FAP-CAR T cells. Specifically, this included a lack of bone marrow hypoplasia (Suppl. Fig. 6A–6C) as reported by Tran et al. (31), or any change in skeletal muscle (data not shown).
We also preformed necropsies on mice 8 days after injection of the hyperactive DGKζ–deficient FAP-CAR T cells from the experiment depicted in Figure 4B. No abnormalities were noted, except in the pancreatic sections that showed some mild focal peri-vascular and peri-islet lymphocytic infiltration (Suppl. Fig. 6F). These changes were not seen in mice injected with wild-type FAP-CAR T cells (Suppl. Fig. 6E).
Discussion
In this study, we investigated the antitumor efficacy and safety of CAR-transduced T cells targeted to cells expressing FAP, which is highly up-regulated in the tumor stroma. Given that cancer-associated stromal cells may have a major immune modulating effect on both innate and acquired immunity (13,26), we used fully immune-competent mice, without bone marrow-ablation, so that we could evaluate the role of the acquired immune system in FAP-CAR T cell-mediated antitumor response.
We demonstrate in multiple mouse models with established mesothelioma and lung cancer that our mouse FAP-CAR T cells exhibited antigen-specific cytotoxicity against FAP+ stromal cells and markedly reduced the rare subset of FAP+/CD45+/F4/80+ myeloid cells and the more prevalent FAP+/CD90+ stromal cells (Table 1). A single treatment with the FAP-CAR T cells resulted in ~80% depletion of the FAPhi stromal cells at 3 days following treatment of FAP-CAR T cells (Suppl. Fig. 2A), leaving white blood cells and FAPlo cells relatively unaffected (Suppl. Fig. 2B and 2C). The depletion of FAP+ cells was associated with a significant inhibition (35–50%) of tumor growth compared to untreated and vector control-transduced CAR T cell (MigR1)-treated tumors (Fig. 3A–3C, 3E, 3F). Importantly, the antitumor activity of the FAP-CAR T cells was lost in the FAP-null mice (Fig. 3D) indicating that the antitumor activity is dependent on FAP expression on host-derived cells.
The antitumor efficacy of the FAP-CAR T cells was also lost in immunodeficient mice (Fig. 5B) indicating the importance of the acquired immune system in these tumor models. To delineate this effect, we evaluated the endogenous T cells within the tumors at 3 and 8 days after CAR T cell infusion. At the earlier time-point, we did not see an increase in T cell infiltration or CD8 T cell activation. However, there was an increase in the number of TNF-α–producing CD4+ T cells (Fig. 6B). Similarly, the Fearon group showed that there was no difference in the numbers of CD4+ or CD8+ cells in the tumors 48 hours after genetically ablating FAP+ cells with DT (26), but they did note an increase in the levels of TNF-α mRNA. In contrast, at the later time-point, we observed an increase in total CD8+ T cells as well as antigen-specific CD8+ T cells in both the AE17.ova and E7-positive TC1 tumors. More IFNγ-producing CD8+ T cells and CD69+ T cells were also found at this time point (Fig. 6C and 6E). We postulate that the FAP-CAR T cells enter the tumors, deplete the FAP+ cells, and by an as yet unknown mechanism activate the endogenous CD4+ T cells to produce TNF-α. The high levels of TNF-α may induce tumor cell apoptosis, and a temporary tumor vasculature shut down that may limit early infiltration by endogenous T cells. It appears that by 8 days after FAP-CAR T cell treatment activated CD8+ cells enter the tumor and function to further limit tumor growth. It should be noted that the tumors in our mouse models are relatively immunogenic with few fibroblasts, and therefore the contribution of the immune-mediated mechanisms may be more prominent than the contribution of the non-immune mediated mechanisms (i.e. alterations in matrix and/or angiogenesis) in non-immunogenic and fibroblast-rich tumors. Preliminary studies using more desmoplastic, non-immunogenic mouse tumor models and human xenografts support this idea.
In our tumor models, the FAP-CAR T cells elicited significant but temporary inhibition of tumor growth. One explanation for the transient effect is the short persistence of the murine CAR T cells; the number of intra-tumoral murine FAP-CAR T cells rapidly decreased with time (Suppl. Fig. 3A). This is likely due to a number of well-known intrinsic differences between mouse and human T cells. After expanding human CAR T cells, the lymphocytes are “rested down” before injection and are less sensitive to immediate activation-induced cell death (AICD). In the mouse system, the injected cells are highly activated (as required for retroviral transduction). Whereas the human T cells persist and proliferate for weeks in tumors in immunodeficient mice (3,44), the transduced mouse T cells have short lifespan and undergo AICD (Suppl. Fig. 7). There is relatively little information about the persistence of mouse CAR T cells, but data from this study and our previous work with mesothelin-CAR mouse T cells (43) are consistent with the recent paper from the Hwu group (45) using in vitro expanded pmel-1 T cells that showed similar short persistence of mouse T cells in mice. In their study, pmel-1 T cells were undetectable in the peripheral blood by Day 9 after adoptive transfer and by Day 13 at the tumor site. To ensure that the short lifespan was not caused by the human CD3ζ and human 4-1BB activation domains, we constructed a fully mouse FAP-CAR comprised the 73.3 scFv coupled with the mouse CD3ζ chain and mouse CD28 domain (Fig. 1D). We found virtually equal efficacy in killing, cytokine production, persistence, and antitumor activity between the T cells expressing both constructs (Suppl. Figs. 3B to 3E). We also found that both CARs were equally susceptible to AICD (Suppl. Fig. 7B). We determined whether our FAP-CAR mouse T cells might be enhanced if we pre-conditioned the host by inducing lymphodepletion (46, 47), but no increase in efficacy was observed in mice irradiated prior to injection of our FAP-CAR T cells (data not shown). We have not yet tested whether the administration of IL2 might enhance their efficacy.
Given the short persistence of our FAP-CAR T cells (Suppl. Fig. 3A), a second dose of T cells was administered one week after the first injection and showed added efficacy (Fig. 4A), which clearly indicated that enhanced persistence would augment efficacy. Our recent observation (43) indicates that blocking a T cell-intrinsic negative regulatory mechanism (up-regulation of the enzyme DGK) augments the killing ability and persistence of murine CAR T cells. Similar to our observations with mouse CAR-T cells targeted to mesothelin (42), FAP-CAR T cells deficient in DGKζ had enhanced ability to kill FAP-expressing cells in vitro and were clearly more efficacious in vivo (Fig. 4B). Our data suggest that it will be advantageous to optimize both the persistence and potency of the T cells.
As in any cancer therapy where the target is not completely tumor-specific, potential toxicity is a major question. In previous studies using a vaccine against FAP and with immunoconjugate therapy (28–30) murine tumor growth was inhibited without obvious toxicity. Some of these studies also analyzed and showed that there was no significant inhibition of wound-healing (28,29). In a Phase I human trial, trace levels of131I-labeled humanized anti-FAP antibody (sibrotuzamab) showed excellent tumor-targeting with no detectable uptake in normal organs and no major toxicity at any dose level (48). However, as described above, two recent papers reported that ablation of FAP+ cells (using a genetic approach in ref. 27 or using FAP-CAR T cells in ref. 31) led to significant cachexia (weight loss) and anemia. We used flow cytometry to assess the percentage of FAP+ cells in the pancreas, lungs, tumors and bone marrows collected from mice bearing different flank tumors. Despite different mouse strains used in each mouse tumor model, we found less than 0.1% of FAP+ stromal cells in the lungs and bone marrows (data not shown). In contrast and consistent with that found by Roberts et al. (27), 3–5% of the dissociated cells from the pancreas expressed FAP (data not shown). However, when we compared FAP expression on those pancreatic stromal cells with that on the tumor-associated stromal cells isolated from the same hosts, we found that the cancer-associated stromal cells expressed higher levels of FAP (Suppl. Fig. 8).
In contrast to these reports (27,31) and consistent with the findings of Kakarla et al. (33) and Schuberth et al.(34), administration of one dose or even two doses of our FAP-CAR T cells did not cause any weight loss (Suppl. Fig. 5), nor did they cause any decrease in hematocrit or increase in serum amylase (data not shown). Furthermore, detailed histologic analyses of necropsy samples showed no microscopic abnormalities, and specifically no damage to the muscle, the pancreas (Suppl. Fig. 6D–6E), or the bone marrow (Suppl. Fig. 6A–6C).
The reasons for these differences in toxicity are not completely understood. It is possible that the total body irradiation prior to adoptive T cell transfer used by Tran et al. (31) somehow contributed to the side effects. Although our main construct had the 4-1BB co-stimulatory molecule rather than CD28, in vitro studies showed no differences in their killing ability or IFN-production (Suppl. Figs. 3B and 3C) or in vivo activity (Suppl. Fig. 3E). A likely explanation relates to the different properties of the scFv antibodies used. The affinities of the scFv antibodies for mouse FAP are similar. The affinity of the FAP-5 antibody for mouse FAP was reported to be 0.6 nM (31). Our biacore measurement showed that the affinity of the 73.3 mAb for mouse FAP protein was less than 1 nM (0.1–0.3 nM). However, we found that FAP-5 and 73.3 reacted with distinct epitopes on FAP in antibody competition experiments (Suppl. Fig. 9), consistent with the specificity of the 73.3 mAb for murine FAP compared to that of FAP-5 for an epitope shared by murine and human FAP. The distinct epitope specificity appears to allow the FAP-CAR T cells expressing the 73.3 scFv to efficiently eliminate the FAPhi cells, while sparing cells expressing lower levels of FAP (Suppl. Fig. 2), such as cells in the pancreas, and presumably cells in the bone marrow and muscle. Data from the Roberts and Tran studies suggest that complete ablation of FAP+ cells may cause side effects like fatigue and anemia (27,31). However, our data show that it is possible to partially deplete tumor-associated FAP+ cells while retaining antitumor efficacy, but without eliciting severe side effects. The only instance in which we observed any histologic abnormalities was in the pancreas (Suppl. Fig. 6F) but only when we used the hyperactive DGKζ-deleted cells. The Kakarla data support this idea (33).
Further studies will be needed to determine why we and two other groups (33,34) induced significant antitumor efficacy while Tran et al. (31) did not. The constructs are all slightly different, however we showed antitumor activity with CARs containing either human or mouse cytoplasmic domains (Suppl. Fig. 3E). Our data suggest that endogenous immune responses may be important for the activity of our CARs. Since Tran et al (31) used total body irradiation, they may have lost some of that effect. Alternatively irradiation might have impacted the tumor stroma, thus limiting the effect or anti-stromal therapy. Furthermore, the amount of stroma was very low in the small mouse tumors studied by Tran et al /31) (data not shown). In any case, we were able to show clear antitumor efficacy albeit to a lesser extent in two of the tumor cell lines used by Tran et al (31) (Fig. 3A–3C and 3E, 3F).
The optimal utility of FAP-CAR T cells may be in combination with other therapies, including immunotherapy and chemotherapy where stromal disruption could enhance drug delivery. Kakarla et al (33) showed augmented efficacy by combining FAP-CAR T cells with CAR T cells targeting EphA2 on tumor cells. In this study, we combined FAP-CAR T cells with an Ad.E7 cancer vaccine that elicits E7-specific adaptive immune response against TC1 cells. As expected, neither the cancer vaccine nor the FAP-CAR T cells alone worked well on large, established tumors (Fig. 4C); however, in combination, there was an additive or synergistic effect against TC1 tumors.
In summary, we showed that FAP-CAR T cells reduce tumor growth in vivo in an antigen-specific manner. Targeting tumor stromal cells with CAR T cells augmented antitumor immunity. Although we did not eradicate tumors completely in our animal models, we believe that the efficacy of FAP-CAR T cells could be enhanced by improving the persistence of T cells, generating more highly active T cells, administering multiple doses of FAP-CAR-T cells, and by combining with other types of immunotherapy or chemotherapy. We did not observe toxicity under the dosing conditions tested and in otherwise healthy tumor-bearing animals.
We believe that data from Karkala et al.(33), Schuberth et al. (34), and in this report support the development of anti-human FAP-CAR for potential translation to the clinic. Potential toxicities, such as anemia and/or cachexia, should they occur, are tolerable and similar to those seen in most conventional chemotherapy treatments. Importantly, our current clinical approach using biodegradable mRNA-transduced CAR T cells (49,50) will allow us to determine if these toxicities may only be temporary. It may well be that the FAP-elimination approach will best be used in combination with other therapies and may only need to be given for short periods of time.
Supplementary Material
Acknowledgements
The authors would like to thank Jennifer Whealdon, James Schecter, Geetha Muthukumaran and Danielle Qing for their technical support. We would also like to thank Dr. Gary Koretzky for providing DGKζ knockout mice, Dr. Wayne Hancock for providing Thy1.1 congenic C57BL/6 mice, Boehringer Ingleheim for providing FAPLacZ knock-in mice and Jonathan Cheng for providing FAP-ECD-producing HEK293 cells. This study made use of the Wistar Institute Cancer Center Flow Cytometry Core facility, the Protein Production, Libraries & Molecular Screening Facility (thanks to Dr. David C. Schultz, Director), and infrastructure support to the Wistar Institute from the Commonwealth of Pennsylvania.
Grant Support
This work was supported by NCI (National Cancer Institute) grants P01 CA 66726-07 (to SMA and CHJ), R01 CA 141144 and RO1 CA 172921 (to SMA and EP) and a “Clinic and Laboratory Integration Program” Grant from the Cancer Research Institute (EP and SMA). LCSW was supported by the MARF (Mesothelioma Applied Research Foundation) award, and AL was sponsored by a START fellowship from the Cancer Research Institute.
Footnotes
All authors declare that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
References
- 1.June CH. Principles of adoptive T cell cancer therapy. J Clin Invest. 2007;117:1204–1212. doi: 10.1172/JCI31446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.June CH. Adoptive T cell therapy for cancer in the clinic. J Clin Invest. 2007;117:1466–1476. doi: 10.1172/JCI32446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porter D, Levine B, Kalos M, Bagg A, June CH. Delayed tumor lysis and CLL remission from chimeric antigen receptor-modified T cells. New Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalos M, Levine B, Porter D, Katz S, Grupp SA, Bagg A, June CH. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Trans. Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter D, Rheingold S, et al. Complete remissions of ALL by chimeric antigen receptor-expressing T cells. New Engl J Med. 2013 doi: 10.1056/NEJMoa1215134. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jena B, Dotti G, Cooper LJ. Redirecting T-cell specificity by introducing a tumor-specific chimeric antigen receptor. Blood. 2010;116:1035–1044. doi: 10.1182/blood-2010-01-043737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 9.Santos AM, Jung J, Aziz N, Kissil JL, Pure E. Targeting fibroblast activation protein inhibits tumor stromagenesis and growth in mice. J Clin Invest. 2009;119:3613–3625. doi: 10.1172/JCI38988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito TK, Ishii G, Chiba H, Ochiai A. The VEGF angiogenic switch of fibroblasts is regulated by MMP-7 from cancer cells. Oncogene. 2007;26:7194–7203. doi: 10.1038/sj.onc.1210535. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Tang H, Cai J, Zhang T, Guo J, Feng D, et al. Ovarian cancer-associated fibroblasts contribute to epithelial ovarian carcinoma metastasis by promoting angiogenesis, lymphangiogenesis and tumor cell invasion. Cancer Lett. 2011;303:47–55. doi: 10.1016/j.canlet.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Fridlender ZV, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta:"N190022; versus"N2" TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nazareth MR, Broderick L, Simpson-Abelson MR, Kelleher RJ, Jr, Yokota SJ, Bankert RB. Characterization of human lung tumor-associated fibroblasts and their ability to modulate the activation of tumor-associated T cells. J Immunol. 2007;178:5552–5562. doi: 10.4049/jimmunol.178.9.5552. [DOI] [PubMed] [Google Scholar]
- 14.Cohen SJ, Alpaugh RK, Palazzo I, Meropol NJ, Rogatko A, Xu Z, et al. Fibroblast activation protein and its relationship to clinical outcome in pancreatic adenocarcinoma. Pancreas. 2008;37:154–158. doi: 10.1097/MPA.0b013e31816618ce. [DOI] [PubMed] [Google Scholar]
- 15.Goscinsky MA, Suo Z, Florenes VA, Vlatkovic L, Nesland JM, Giercksky KE. FAP-alpha and uPA show different expression patterns in premalignant and malignant esophageal lesions. Ultrastruct Pathol. 2008;32:89–96. doi: 10.1080/01913120802034934. [DOI] [PubMed] [Google Scholar]
- 16.Henry LR, Lee HO, Lee JS, Klein-Szanto A, Watts P, Ross EA, et al. Clinical implications of fibroblast activation protein in patients with colon cancer. Clin Cancer Res. 2007;15(13):1736–1741. doi: 10.1158/1078-0432.CCR-06-1746. [DOI] [PubMed] [Google Scholar]
- 17.Scanlan MJ, Mohan Raj BK, Calvo B, Garin-Chesa P, Sanz-Moncasi MP, Healey JH, et al. Molecular cloning of fibroblast activtion proteinα, a member of the serine protease family selectively expressed in stromal fibroblasts of epithelial cancers. Proc Natl Acad Sci USA. 1994;91:5657–5661. doi: 10.1073/pnas.91.12.5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garin-Chesa P, Old LJ, Rettig WJ. Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Proc Natl Acad Sci USA. 1990;87:7235–7239. doi: 10.1073/pnas.87.18.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niedermeyer J, Garin-Chesa P, Kriz M, Hilberg F, Mueller E, Bamberger U, et al. Expression of the fibroblast activation protein during mouse embryo development. Int J Dev Biol. 2001;45:445–447. [PubMed] [Google Scholar]
- 20.Mathew S, Scanlan MJ, Mohan Raj BK, Murty VV, Garin-Chesa P, Old LJ, et al. The gene for fibroblast activation protein [alpha] (FAP), a putative cell surface-bound serine protease expressed in cancer stroma and wound healing, maps to chromosome band 2q23. Genomics. 1995;25:335–337. doi: 10.1016/0888-7543(95)80157-h. [DOI] [PubMed] [Google Scholar]
- 21.Wang XM, Yao TW, Nadvi NA, Osborne B, McCaughan GW, Gorrell MD. Fibroblast activation protein and chronic liver disease. Front Biosci. 2008;13:3168–3180. doi: 10.2741/2918. [DOI] [PubMed] [Google Scholar]
- 22.Acharya PS, Zukas A, Chandan V, Katzenstein AL, Puré E. Fibroblast activation protein: a serine protease expressed at the remodeling interface in idiopathic pulmonary fibrosis. Hum Pathol. 2006;37:352–360. doi: 10.1016/j.humpath.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 23.Rettig WJ, Garin-Chesa P, Beresford HR, Oettgen HF, Melamed MR, Old LJ. Cell-surface glycoproteins of human sarcomas: differential expression in normal and malignant tissues and cultured cells. Proc Natl Acad Sci U S A. 1988;85:3110–3114. doi: 10.1073/pnas.85.9.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huber MA, Kraut N, Park JE, Schubert RD, Rettig WJ, Peter RU, et al. Fibroblast activation protein: differential expression and serine protease activity in reactive stromal fibroblasts of melanocytic skin tumors. J Invest Dermatol. 2003;120:182–188. doi: 10.1046/j.1523-1747.2003.12035.x. [DOI] [PubMed] [Google Scholar]
- 25.Aertgeerts K, Levin I, Shi L, Snell GP, Jennings A, Prasad GS, et al. Structural and kinetic analysis of the substrate specificity of human fibroblast activation protein alpha. J Biol Chem. 2005;280:19441–19444. doi: 10.1074/jbc.C500092200. [DOI] [PubMed] [Google Scholar]
- 26.Kraman M, Bambrough PJ, Arnold JN, Roberts EW, Magiera L, Jones JO, et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science. 2010;330:827–830. doi: 10.1126/science.1195300. [DOI] [PubMed] [Google Scholar]
- 27.Roberts EW, Deonarine A, Jones JO, Denton AE, Feig C, Lyons SK, et al. Depletion of stromal cells expressing fibroblast activation protein-a from skeletal muscle and bone marrow results in cachexia and anemia. J Exp Med. 2013;210:1137–1151. doi: 10.1084/jem.20122344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loeffler M, Kruger JA, Niethammer AG, Reisfeld RA. Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. J Clin Invest. 2006;116:1955–1962. doi: 10.1172/JCI26532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee J, Fassnacht M, Nair S, Boczkowski D, Gilboa E. Tumor immunotherapy targeting fibroblast activation protein, a product expressed in tumor-associated fibroblasts. Cancer Res. 2005;65:11156–11163. doi: 10.1158/0008-5472.CAN-05-2805. [DOI] [PubMed] [Google Scholar]
- 30.Ostermann E, Garin-Chesa P, Heider KH, Kalat M, Lamche H, Puri C, et al. Effective immunoconjugate therapy in cancer models targeting a serine protease of tumor fibroblasts. Clin Cancer Res. 2008;14:4584–4592. doi: 10.1158/1078-0432.CCR-07-5211. [DOI] [PubMed] [Google Scholar]
- 31.Tran E, Chinnasamy D, Yu Z, Morgan RA, Lee CC, Restifo NP, et al. Immune targeting of fibroblast activation protein triggers recognition of multipotent bone marrow stromal cells and cachexia. J Exp Med. 2013;210:1125–1135. doi: 10.1084/jem.20130110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee PP, Yee C, Savage PA, Fong L, Brockstedt D, et al. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5:677–685. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 33.Kakarla S, Chow KKh, Mata M, Shaffer DR, Song XT, Wu MF, et al. Antitumor effects of chimeric receptor engineered human T cells directed to tumor stroma. Mol Ther. 2013;21:1611–1620. doi: 10.1038/mt.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schuberth PC, Hagedorn C, Jenesen SM, Gulati P, van den Broek M, Mischo A, et al. Treatment of malignant pleural mesothelioma by fibroblast activation protein-specific re-directed T cells. J Transl Med. 2013;11:187. doi: 10.1186/1479-5876-11-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu R, Li H, Liu L, Yu J, Ren X. Fibroblast activation protein: A potential therapeutic target in cancer. Cancer Biol Ther. 2012;13:123–129. doi: 10.4161/cbt.13.3.18696. [DOI] [PubMed] [Google Scholar]
- 36.Jackaman C, Bundell CS, Kinnear BF, Smith AM, Fillion P, van Hagen D, Robinson BWS, Nelson DJ. IL-2 intratumoral immunotherapy enhances CD8+ T cells that mediate destruction of tumor cells and tumor-associated vasculature: a novel mechanism for IL-2. J Immunol. 2003;171:5051–5063. doi: 10.4049/jimmunol.171.10.5051. [DOI] [PubMed] [Google Scholar]
- 37.Lin KY, Guarnieri FG, Staveley-O'Carroll KF, Levitsky HI, August JT, Pardoll DM, et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56:21–26. [PubMed] [Google Scholar]
- 38.Johnson L, Mercer K, Greenbaum D, Bronson RT, Crowley D, Tuveson DA, et al. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature. 2001;410:1111–1116. doi: 10.1038/35074129. [DOI] [PubMed] [Google Scholar]
- 39.Niedermeyer J, Kriz M, Garin-Chesa P, Bamberger U, Lenter MC, Park J, et al. Targeted disruption of mouse fibroblast activation protein. Mol Cell Biol. 2000;20:1089–1094. doi: 10.1128/mcb.20.3.1089-1094.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Milone MC, Fish JD, Carpenito C, Carroll RG, Binder GK, Teachey D, et al. Chimeric Receptors Containing CD137 Signal Transduction Domains Mediate Enhanced Survival of T Cells and Increased Antileukemic Efficacy In Vivo. Mol Ther. 2009;17:1453–1464. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pear WS, Miller JP, Xu L, Pui JC, Soffer B, Quackenbush RC, et al. Efficient and rapid induction of a chronic myelogenous leukemia-like myeloproliferative disease in mice receiving P210 bcr/abl-transduced bone marrow. Blood. 1998;92(10):3780–3792. [PubMed] [Google Scholar]
- 42.Morgan RA, Johnson LA, Davis JL, Zheng Z, Woolard KD, Reap EA, et al. Recognition of glioma stem cells by genetically modified T cells targeting EGFRvIII and development of adoptive cell therapy for glioma. Hum Gene Ther. 2012;23:1043–1053. doi: 10.1089/hum.2012.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riese MJ, Wang L-CS, Moon EK, Ranganathan A, Joshi RP, June CH, et al. Enhanced effector responses in activated CD8+ T cells deficient in diacylglycerol kinases. Cancer Res. 2013;73:3566–3577. doi: 10.1158/0008-5472.CAN-12-3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moon EK, Carpenito C, Sun J, Wang L-CS, Kapoor V, Predina J, et al. Expression of a functional CCR2 receptor enhances tumor localization and tumor eradication by retargeted human T cells expressing a mesothelin-specific chimeric antibody receptor. Clin. Cancer Res. 2011;17:4719–4730. doi: 10.1158/1078-0432.CCR-11-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peng W, Liu C, Xu C, Lou Y, Chen J, Yang Y, et al. PD-1 blockade enhances T-cell migration to tumors by elevating IFN-γ inducible chemokines. Cancer Res. 2012;72:5209–5218. doi: 10.1158/0008-5472.CAN-12-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chinnasamy D, Tran E, Yu Z, Morgan RA, Restifo NP, Rosenberg SA. Simultaneous targeting of tumor antigens and the tumor vasculature using T lymphocyte transfer synergize to induce regression of established tumors in mice. Cancer Res. 2013;73:3371–3380. doi: 10.1158/0008-5472.CAN-12-3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muranski P, Boni A, Wrzesinski C, Citrin DE, Rosenberg SA, Childs R, et al. Increased intensity lymphodepletion and adoptive immunotherapy-how far can we go? Nat Clin Pract Oncol. 2006;3:668–681. doi: 10.1038/ncponc0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scott AM, Wiseman G, Welt S, Adjei A, Lee FT, Hopkins W, et al. A Phase I dose-escalation study of sibrotuzumab in patients with advanced or metastatic fibroblast activation protein-positive cancer. Clin Cancer Res. 2003;9:1639–1647. [PubMed] [Google Scholar]
- 49.Zhao Y, Moon E, Carpenito C, Paulos CM, Liu X, Brennan AL, et al. Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer Res. 2010;70:9053–9061. doi: 10.1158/0008-5472.CAN-10-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barrett DM, Liu X, Jiang S, June CH, Grupp SA, Zhao Y. Regimen-specific effects of RNA-modified chimeric antigen receptor T cells in mice with advanced leukemia. Human Gene Ther. 2013;24:717–727. doi: 10.1089/hum.2013.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.