Abstract
Monocytes may contribute to tumor progression in part by mediating tumor-induced immunosuppression. Alterations to the monocyte populations and functions in untreated late stage melanoma patients are not fully understood. To characterize these alterations, we compared the frequency, phenotype, and functional capacity of peripheral blood monocytes and other myeloid cells in untreated, newly diagnosed stage IV melanoma patients (n= 18) to those in healthy volunteers. Stage IV untreated melanoma patients exhibited a sizeable decrease in the percentage of monocytes (p<0.0001) that included a drop in the percentage of CD14+CD16− classical monocytes pool (p=0.006). Although there was not a significant difference in the CD14+HLA-DRlow/− monocyte population between the melanoma patients and the healthy volunteers, the HLA-DR levels were considerably lower in the patients’ CD14+CD16+ intermediate (p<0.0001) and CD14lowCD16+ non-classical monocytes populations (p=0.001). Decreased surface expression of CD86 (p=0.0006) and TNFRII (p=0.0001), and increased expression of tissue factor and PD-L1 (p=0.003) were identified on monocytes from melanoma patients. Furthermore, these monocytes had decreased ability to up-regulate CD80 expression and cytokine production following stimulation with agonist of toll-like receptor 3 (TLR3). Peripheral blood dendritic cell subsets were decreased in untreated stage IV melanoma patients. Our study demonstrates that untreated late stage melanoma patients exhibit monocytopenia in addition to phenotypic and functional deficiencies that may negatively affect the patient’s immune function. These findings open new avenues into examining the role of monocyte populations in melanoma development.
Keywords: Monocytes, metastatic melanoma, cytokine production, TLR stimulation and dendritic cells
Introduction
Approximately 10% of the circulating leukocytes in humans consist of monocytes that can differentiate into both macrophages and dendritic cells (DCs)(1). Monocytes can be subdivided into three distinct populations based on the differential expression of CD14 and CD16 (2). Classical monocytes are short-lived cells that are CD14+CD16−, intermediate monocytes are CD14+CD16+, and non-classical monocytes are CD14loCD16+ (1–5). In humans, approximately 80% of the circulating monocytes are of the highly phagocytic classical subset. The non-classical monocytes are considered to be important in both pro-inflammatory and infectious disease states (6, 7). The intermediate monocytes are functionally distinct from the other two subsets, due to their anti-inflammatory properties such as the secretion of IL-10 in response to lipopolysaccharide (LPS) stimulation (8).
Recent evidence has highlighted the importance of monocytes and other myeloid cells in tumor-mediated immunosuppression in metastatic melanoma patients (9–11). In particular, the loss of HLA-DR expression on CD14+ monocytes has been identified as a potential mechanism whereby the melanoma tumors can cause systemic immunosuppresion in the patients (9, 11, 12). However, comparisons between these studies are problematic due to the variations in the types of treatments and the stages of diseases in each cohort. We have reported an increase in CD14+HLA-DRlow/− monocytes (often referred to as monocytic myeloid-derived suppressor cells) in patients with B-cell non-Hodgkin lymphoma, glioblastoma multiforme, and chronic lymphocytic leukemia in which these cells cause or contribute to the systemic immunosuppression and the aggressive disease (13–15).
The immunosuppressive function of other myeloid-derived suppressor cells has also been evaluated although their inhibitory capacity in humans appears to be less than that observed in the murine model (10). Studies of melanoma in humans and in the murine model have demonstrated important species-specific differences in immune responses with the mouse antigen presenting cells (APCs) unable to respond to vascular endothelial growth factor (VEGF) stimulation (16). Studies involving global analysis of cell subsets, gene expression and serum cytokine profiles in stage I to IV melanoma patients have demonstrated that the repolarization of the immune system is partially due to the VEGF-orchestrated chronic inflammation leading to a subsequent Th-2 bias (16, 17). In addition, dendritic cells have a role in the systemic immune dysregulation that are seen in cancer patients (18–20). Dendritic cells are classically divided into myeloid dendritic cells (mDC) and plasmacytoid dendritic cells (pDC). mDC and pDC differ in the expression of toll-like receptors (TLRs) which leads to divergent cytokine production following TLR stimulation (21–23).
In this study, we evaluated the phenotype and functions of monocytes, DC, and other myeloid cells from untreated stage IV melanoma patients. This cohort allows us to measure the tumor’s influence on peripheral blood cells without the complicating effects of treatment. Our results demonstrate that untreated patients with significant tumor burdens have a dysregulated monocyte population. Although the levels of CD14+HLA-DRlow/− monocytes were not elevated in this cohort, there was a dramatic decrease of the HLA-DR levels on the intermediate and non-classical monocytes. Melanoma patient monocytes showed decreased levels of inflammatory markers and increased expression of the inhibitory Programmed Death Ligand 1 (PD-L1/B7-H1/CD274). In addition to a lower frequency in the circulating dendritic cells, monocytes isolated from these patients also failed to properly respond to TLR3 stimulation. These findings demonstrate that substantial changes in the monocyte phenotypes and functions exist in untreated malignant melanoma patients.
Methods
Patient population
Blood samples were collected in sodium (Na)-heparin Vacutainer 10-mL blood collection tubes (Becton Dickinson, Franklin Lakes, NJ) from newly diagnosed stage IV patients with no previous treatment and age-matched healthy volunteers. Samples were processed as soon as possible and all within 18 hours of collection. Unfractionated whole blood was used for immunophentotyping and cells from the remaining blood were isolated by density gradient purification. The protocol and informed consent documents for this study were reviewed and approved by the Mayo Clinic Institutional Review Board. The collection, processing and storage of the biospecimens used in this study were performed with adherence to the established standard operating procedures in our laboratory. Patient characteristics are listed in Table 1.
Table 1.
MM patient and HV demographics
| Age (mean, range) | |
| MM patients | 60.2 (45–80) |
| HV controls | 61.2 (48–74) |
| Gender (%) | |
| MM patients | |
| Male | 11 (61%) |
| Female | 7 (39%) |
| HV controls | |
| Male | 9 (50%) |
| Female | 9 (50%) |
| AJCC stage at time of sampling (%) | IV (100%) |
| Metastatic disease with unknown primary at time of diagnosis (%) | 2 (11%) |
| Time to metastatic diseases mean (range) | 3.4 years (0–12.4) |
Peripheral blood immunophenotyping and antibodies
Peripheral blood samples were directly stained with antibodies and analyzed by flow cytometry as described previously (14). Antibodies used for this study are listed in Supplementary Table 1. Samples were run on a BD FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ) calibrated the day of use and the data files were analyzed with Flowjo (Ashland, OR), Cell Quest (BD) and/or Multiset (BD) software.
Monocyte isolation and cytokine analysis
Fresh peripheral blood monocytes (PBMCs) were isolated using density gradient centrifugation (Lymphoprep, MP Biomedicals LLC, Solon, OH). Monocytes were isolated by incubating PBMCs with anti-CD3, anti-CD7, anti-CD16, anti-CD19, anti-CD56, anti-CD123 and glycophorin A (monocyte isolation kit II, Miltenyi Biotec, Auburn, CA) per the manufacturer’s instructions. The magnetically purified monocytes were stimulated with polyinosinic-polycytidylic acid (poly I:C) (Imgenex, San Diego, CA) for 6 hours. Proteins were measured using the Milliplex MAP cytokine/chemokine panel (Millipore, Billerica MA) as per the manufacturer's instructions. Luminex plate reader (Millipore) was used to detect cytokines and chemokines. Protein concentrations were determined using a standard curve generated using multiplex assay analysis software (Millipore).
Statistical analyses
Immunophenotype values from volunteers and patients were tested for statistical significance using the two-tailed non-parametric Mann-Whitney test for unpaired samples. Statistical analyses and graphs were performed using Prism, version 5.0 software (GraphPad Software, San Diego, CA).
Results
Clinical characteristics
Peripheral blood samples from age-matched healthy volunteers (HV; n=18) and untreated stage IV melanoma patients (malignant melanoma; MM; n=18) were collected for analysis. Patient and HV demographics are shown in Table 1. The MM cohort included two patients diagnosed with metastatic melanoma without known primaries; metastatic melanoma was noted in one patient during a routine colonoscopy and in the other patient during the biopsy of an enlarged inguinal lymph node. The remaining patients in the study all had prior biopsies demonstrating melanoma; all cases of ocular melanoma were excluded in this study. There were two patients with a history of additional carcinomas; one patient had breast cancer and was treated with chemotherapy and the second patient had prostate cancer. For the remaining patients the time to development of metastatic disease was between 4 months and 12.4 years.
Abnormal monocyte distribution in untreated stage IV melanoma patients
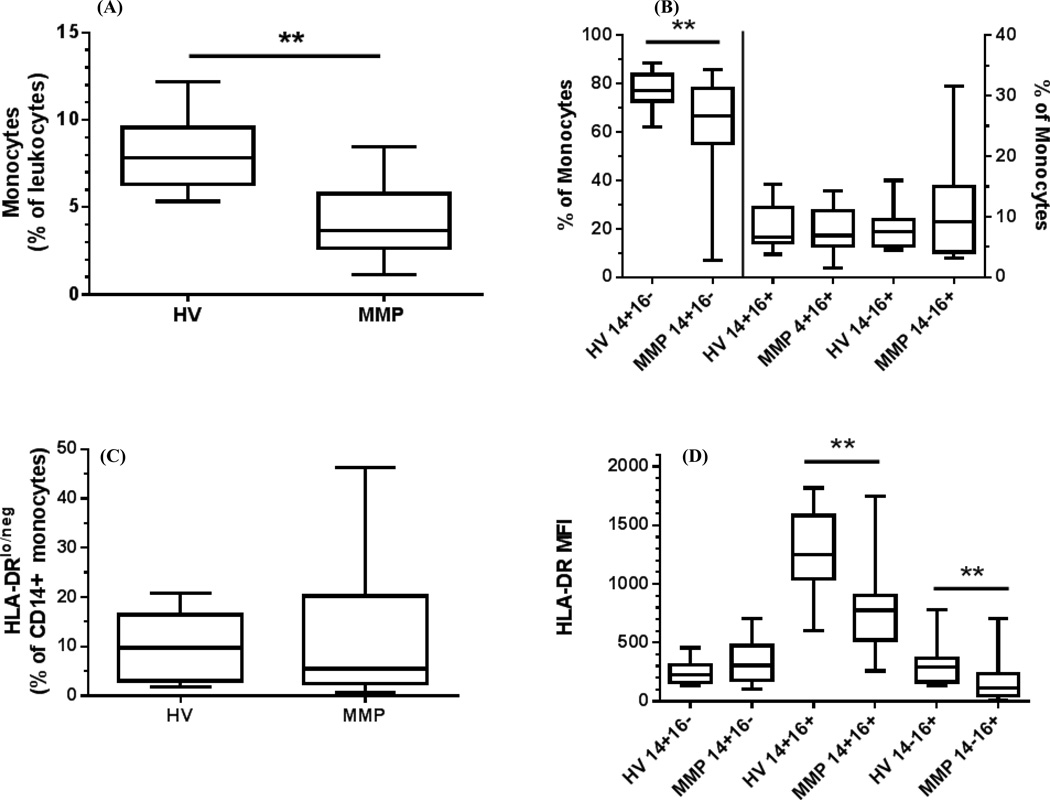
We used a panel of antibodies that was developed and subsequently expanded for this study (Supplemental Table 1 and Gustafson et al (24)). Flow cytometric analysis of unfractionated peripheral blood has become a valuable method to assess the immune status of individuals and our gating strategy is outlined in the supplemental figures. We applied this method to assess the monocyte and DC populations in untreated MM patients. Analysis of the monocytes by forward/side scatter properties demonstrated that stage IV untreated melanoma patients had a significantly lower frequency of circulating monocytes (p<0.0001) (Fig 1A) whereas no differences were observed in the granulocyte and lymphocyte populations compared to HV (Fig S1). Within the monocyte population, we measured the distribution of classical (CD14+CD16−), intermediate (CD14+CD16+), and non-classical monocytes (CD14loCD16+) and found a specific decline of the classical monocytes in MM patients (p=0.006) with no detectable changes in the other compartments (Fig 1B, Fig S2). We did not observe significant differences in the circulating levels of immunosuppressive CD14+HLA-DRlo/neg monocytes between MM patients and HV (Fig 1C, Fig S3). However, when we examined the HLA-DR levels on each of the three subgroups of monocytes, we found lower HLA-DR expression on the intermediate (p<0.0001) and non-classical monocytes (p=0.001) but no measureable differences were observed on the classical monocytes (Fig. 1D). These results reveal changes to the immunophenotypes of peripheral blood monocytes in MM patients that have not been identified previously.
Figure 1. Abnormal monocyte distribution and loss of HLA-DR in stage IV melanoma patients.
Peripheral blood was analyzed by flow cytometry. (A) Frequency of monocytes as measured by forward scatter and side scatter in the leukocyte population of MM patients and HV controls (B). Frequency of CD14+CD16− classical, CD14+CD16+ intermediate, CD14loCD16+ non-classical monocytes subsets as a percent of the monocytes pool in both HV and MM patients. (C). Frequency of CD14+HLA-DRlow/− % of monocytes as a percent of CD14+ monocytes (D). HLA-DR expression as measured by mean fluorescence intensity (MFI) on the monocyte subsets. Box and whisker plots: Horizontal line- mean; box-25th and 75th percentile; whiskers-min and max. *, P<0.05.
Monocytes in MM patients have an inhibitory phenotype
We hypothesized that there may be other markers on the patients’ monocytes that could provide insight into how these monocytes might cause immunosuppression (supplemental table 1). Compared to HV, MM patients had lower frequencies of CD86-positive monocytes (p=0.0006) but not CD80-positive monocytes (Fig. 2A). The tumor necrosis factor receptor 2 (TNFR2) plays an important role in enabling lymphocyte activation and proliferation (25). Expression of this immune marker was significantly lower in MM patients (p=0.0001) (Fig. 2B).
Figure 2. Melanoma patient monocytes have altered expression of inflammatory and surface markers.
Cell surface markers on monocytes were measured by flow cytometry. Expression of cell surface markers on monocytes from MM patients and HV controls for (A) co-stimulatory molecules CD80 and CD86 as a percent of total monocytes; (B), MFI of TNFRII of total monocytes; (C) percentage of tissue factor positive of CD14+ monocytes; and (D) MFI of PD-L1/B7-H1 of total monocytes. A negative correlation is observed in MM patients when measuring surface expression of HLA-DR and PD-LI (E). Box and whisker plots: Horizontal line- mean; box-25th and 75th percentile; whiskers-min and max. *, P<0.05.
Increased expression of monocyte tissue factor (TF) has been reported in pancreatic cancer and hepatocellular cancer (26, 27). The expression of TF in the tumor microenvironment is thought to be important for angiogenesis in patients with solid tumors (27, 28). In MM patients, the expression of TF on monocytes was significantly increased (p=0.003) in comparison to the HV (Fig 2C). MM patients also had increased expression of the inhibitory molecule PD-L1 (p=0.003) on their monocytes (Fig 2D). We found a negative correlation between the expression of HLA-DR and PD-L1 (p=0.04) in MM patients (Fig 2E). Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population that play a role in immunosuppression and have been well characterized in the murine model. In our study we did not observe differences in the frequency of myeloid-derived suppressor populations including Lineage−CD33+HLA-DR−, CD15+CD14−, or IL-4Rα/CD124+ monocytes in MM patients and in HV (data not shown). Taken together, these results suggest that monocytes from MM patients exhibit a profile of immunosuppressive characteristics leading to suboptimal antigen presentation/costimulation and the expression of receptors that inhibit T cell proliferation and/or function.
Evaluation of dendritic cell subsets
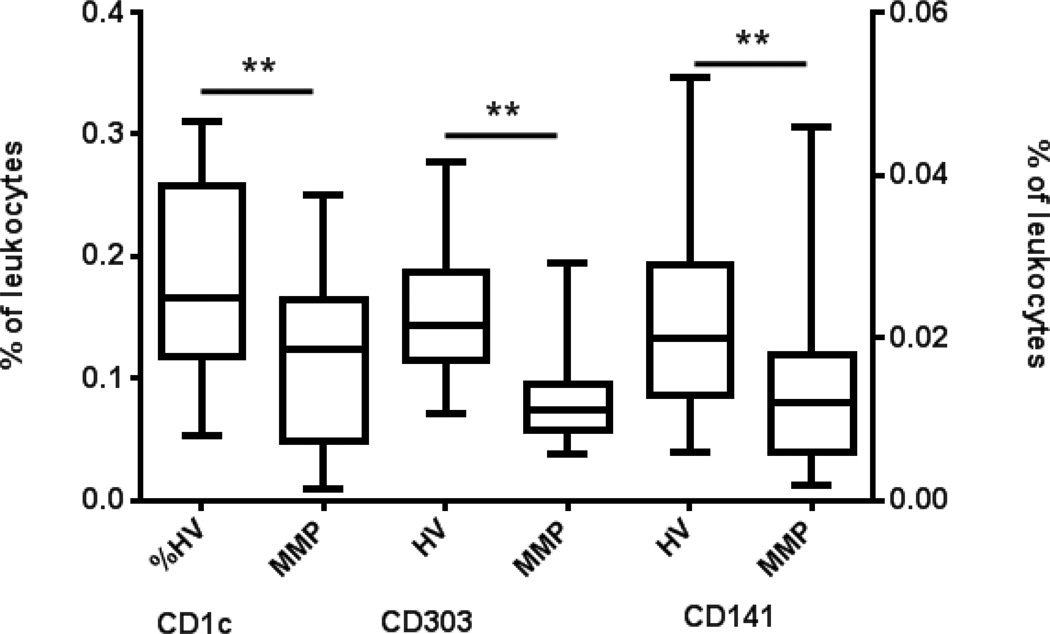
Monocytes are precursor cells of the myeloid CD1c DCs. Given the significant differences noted in the classical monocytes of MM patients we characterized the frequency of the circulating DC subsets. These MM patients had lower populations of circulating CD1c DC (p=0.04), plasmacytoid CD303 DC (p<0.0001), and CD141 DC (p=0.014) (Fig. 3). However, we noted no differences in the expression of CD83, CD86 or HLA-DR on CD1c or CD303 DCs from MM patients (Supplementary figures 4 and 5). These data suggest a frequency deficit of DCs but not necessarily defective activation of DCs in MM patients.
Figure 3. Melanoma patients have fewer circulating peripheral blood dendritic cells.
DC subsets were analyzed by flow cytometry. Lineage negative and HLA-DR positive cells were gated from mononuclear cells and subsequently measured for distinct expression of CD1c+, CD303+ and CD141+ DC and reported as a percent of total leukocytes. Box and whisker plots: Horizontal line- mean; box-25th and 75th percentile; whiskers-min and max. *, P<0.05.
Decreased activation and cytokine production following poly (I:C) stimulation of monocytes
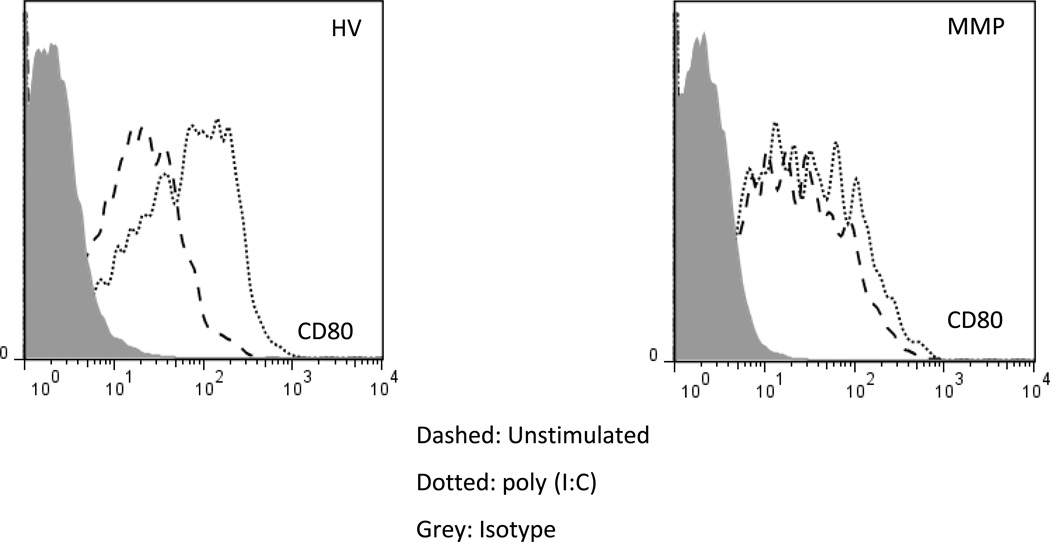
All normal monocytes express TLRs that enable them to initiate immune responses against invading pathogens. We evaluated the ability of monocytes from MM patients to secrete cytokines following TLR3 stimulation. Both positively- and negatively-selected monocytes isolated from density gradient purified peripheral blood mononuclear cells were stimulated with poly (I:C). A minimal response to the stimulation was observed in the positively-selected monocyte populations (data not shown). We also stimulated monocytes in fresh whole blood with poly (I:C) for 6 hours. As shown earlier, the circulating monocytes in MM patients expressed similar levels of the co-stimulatory molecule CD80 in comparison to HV (Fig. 2A). However, MM monocytes failed to properly induce CD80 expression upon poly (I:C) stimulation (Fig 4). Results from these experiments suggest that monocytes from MM patients have a functional deficit in addition to phenotypic changes.
Figure 4. Monocytes from melanoma patients are unresponsive to TLR3 agonists.
HV leukocytes (A) and MM patient leukocytes (B) stimulated with poly (I:C) dotted line and control dashed line. Histogram of CD80 expression following 6 hour stimulation on monocytes. (B). An increase in the expression of CD80 is not observed in MM patients following stimulation with poly (I:C). Representative histograms, from a total of 3 independent experiments.
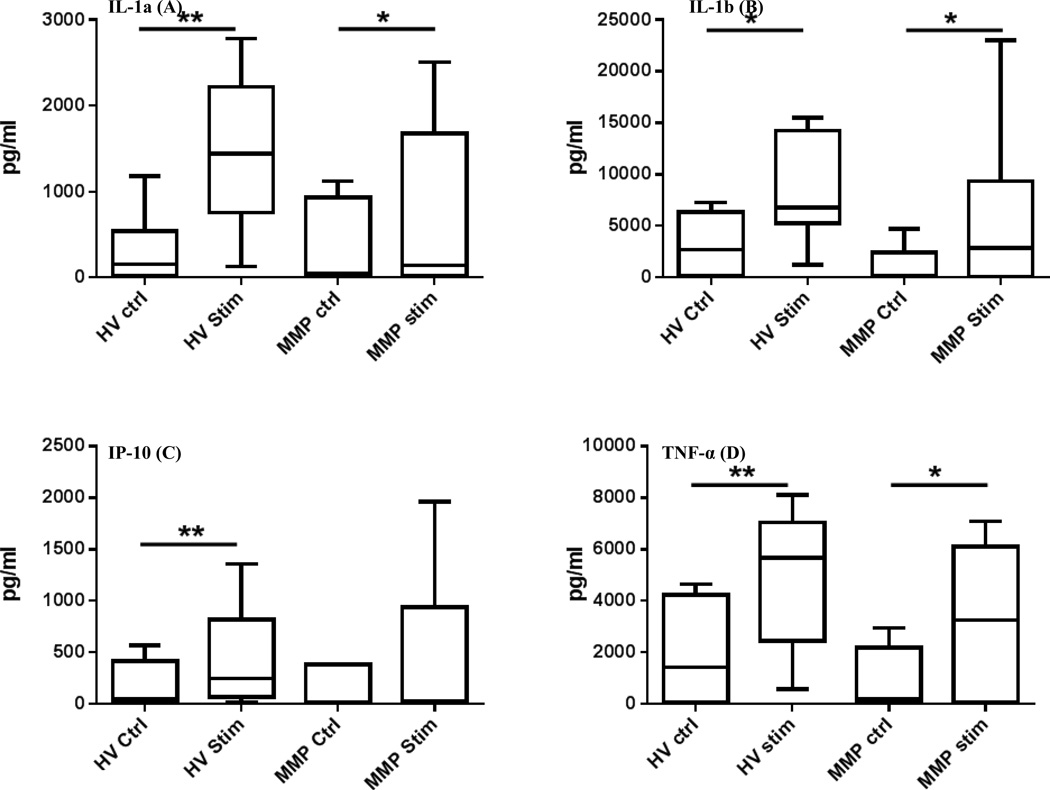
Using the negatively-selected monocytes we measured the concentration of 42 cytokines following stimulation with poly (I:C) and increased production of IL-1α, IL-1β, IP-10 and TNF-α was observed in the samples from MM patients (Fig. 5A–5D). However, the increase in cytokine production was not as robust as that observed for the HV when measuring the concentration of IL-1α (p=0.03 MM patient and p=0.008 HV) and TNF-α (p=0.03 MM patients and p=0.008 HV). A similar increase in the expression of IL-1β was noted in the MM patient (p=0.03) and HV (p=0.02); for IP-10 a significant increase was noted only in the HV population (0.007). These results demonstrate that following TLR3 stimulation with poly (I:C), monocytes in MM patients exhibit impaired inflammatory cytokine production.
Figure 5. Melanoma patient monocytes have impaired cytokine secretion.
IL-1α (A), IL-1β (B), IP-10 (C) and TNF-α (D) production following stimulation of purified monocytes with poly (I:C). An increase in the production of IP-10 is not observed in MM patients. Box and whisker plots: Horizontal line- mean; box-25th and 75th percentile; whiskers-min and max. *, P<0.05.
Discussion
We hypothesized that monocytes from untreated stage IV melanoma patients would show evidence of phenotypic and functional changes resulting from metastatic disease. To test this, we analyzed and compared the immunophenotypes and functional responses to TLR stimulation of monocytes from MM patients and from healthy volunteers. In our small cohort (n=18), MM patients had not received any prior chemotherapy treatments that could have affected the frequency, phenotype and/or function of the monocytes. As such, this cohort allows us to assess the differences in the frequency of monocyte subsets associated with malignant melanoma without the confounding factors of previous treatment and different stages of disease. The changes in monocyte immunophenotypes in melanoma patients may provide insights into as yet unidentified mechanisms of how melanoma tumors cause immunosuppression. We found that in untreated late stage melanoma patients, monocytes are present in much lower frequencies when compared to healthy volunteers. Even within the smaller monocyte compartment the classical monocytes were lower in frequency than that in HV. As recent evidence highlights the immunosuppressive role of CD14+HLA-DRlow/− monocytes in melanoma (9–11), we found that the CD14+HLA-DRlow/− monocytes were not different in our cohort although 4 patients exhibited high levels of these immunosuppressive monocytes (>2 standard deviations above the HV mean: 24.1%). However, when we examined the HLA-DR levels on each of the 3 monocytic subgroups, we found a significant decrease of HLA-DR surface expression on the intermediate and non-classical monocytes. To our knowledge, this is the first observation of differential down-regulation of HLA-DR on monocyte subgroups in cancer patients. One potential reason that we did not see a difference in CD14+HLA-DRlow/− monocytes between the patient and the HV control groups is that the levels of HLA-DR on classical monocytes were not different between MM patients and HVs. Since the classical monocytes are the largest monocyte subgroup, it may mask the HLA-DR drop when monocytes are analyzed as a whole. Therefore, we would suggest that future studies continue to analyze the three monocytes compartments separately. The role of each of the three monocyte subgroups remains unclear in melanoma pathology, however, studies have shown that these subsets are functionally different and do not have the same cytokine production profile.
To gain further insight into the differences in the frequency of monocyte subsets associated with malignant melanoma, we evaluated the expression of a variety of immune markers involved in the activation of monocytes and signaling to T cells. The expression of co-stimulatory molecules CD80 and CD86 are required for T cell activation and they play an important role in modulating immune responses including antitumor responses (29). Our study demonstrated no measureable changes in the expression of CD80 in MM patients in comparison to the decrease noted in CD86 expression. Both CD80 and CD86 are required for efficient activation of T cells and these results, along with the loss of HLA-DR, suggest that monocytes have an impaired ability to present antigen to T cells in patients with late stage melanoma. Monocytes can also negatively affect T cell proliferation and function by expressing inhibitory ligands like PD-L1. Clinical trials evaluating antibodies that block the PD-1/PD-L1 interaction in melanoma are ongoing and the initial results have been favorable (30). Not only was PD-L1 expression increased in MM monocytes, the expressions of PD-L1 and HLA-DR were inversely correlated. The expression of TNFR2 was decreased in MM monocytes suggesting that these monocytes may not be able to fully respond to TNFα signals.
We also examined other myeloid subsets in MM patients. A decrease in the frequency was noted in the circulating DC subsets yet there was little difference in the expression of co-stimulatory molecules CD83, CD86 or HLA-DR. These results suggest that the deficiency may be a result of the numbers of DCs and not the baseline functional status of DCs. Further mechanistic studies will be required to establish the relationship between the decline in monocytes and in circulating DCs in MM patients. We did not detect differences in Lineage−CD33+HLA-DR−, CD15+CD14−, or IL-4Rα/CD124+ myeloid-derived suppressor cells. In agreement with other groups, we also did not find differences in other myeloid-derived suppressor cells and thus their role in melanoma development remains in question.
To evaluate the functional capacity of the monocytes, we stimulated the isolated monocytes with poly (I:C) and using a multiplex ELISA cytokine concentrations were measured 12 hours post-stimulation. Monocytes from MM patients were not able to fully recapitulate the induction of cytokines observed in HV and unable to upregulate CD80 expression. Although we did not directly test the ability of the MM monocytes to respond to GM-CSF and IL-4, our previous work has shown that monocytes with reduced expression of HLA-DR and TNFR2 are unable to fully differentiate into mature DCs (14, 15). Taken together these results suggest that monocytes from MM patients have a decreased functional capacity. Further studies will be necessary to test patient monocytes on T cell mixed lymphocytes reactions as our study had insufficient material for such analyses.
Since these analyses were performed in untreated patients, the changes that we observed are likely a direct effect of the tumor. Other factors like age and gender are likely not contributing to the monocytes changes as we did not see age- or gender-related differences in our melanoma cohort. We hypothesize that monocyte interactions with melanoma cells and/or exposure to tumor-derived factors are the probable mechanisms for the monocyte defects observed in this cohort. The data presented here suggest that, in addition to the deficit in the circulation, monocytes in untreated MM patients express inhibitory signals that negatively affect T cells, are unable to fully present antigens with an adequate co-stimulatory signal, and unable to respond to inflammatory signals like TNFα or TLR agonists. These factors therefore may contribute to an environment of systemic immunosuppression in the MM patients through a mechanism that has yet to be defined. The MM patients in our study were placed into various clinical trials at our institution and consequently we were unable to correlate our current findings with survival studies. However, our data provide the impetus to begin to dissect the functions of the monocyte subgroups during tumor development in future clinical trials. In addition, these findings have considerable implications for the development of novel immunotherapeutic approaches for treating melanoma.
In conclusion, our results demonstrated significant changes in the monocyte populations in this relatively small cohort of untreated late stage melanoma patients. Future studies with larger cohorts will be required to further define the contributions of each of the monocyte subgroups in melanoma development and tumor-mediated immunosuppression. Results from our study indicate that the development of better treatment strategies must take into consideration the immunosuppressive characteristics of the monocytes in melanoma patients.
Supplementary Material
References
- 1.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 2.Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–e80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 3.Skrzeczynska-Moncznik J, Bzowska M, Loseke S, Grage-Griebenow E, Zembala M, Pryjma J. Peripheral blood CD14high CD16+ monocytes are main producers of IL-10. Scand J Immunol. 2008;67:152–159. doi: 10.1111/j.1365-3083.2007.02051.x. [DOI] [PubMed] [Google Scholar]
- 4.Wong KL, Yeap WH, Tai JJ, Ong SM, Dang TM, Wong SC. The three human monocyte subsets: implications for health and disease. Immunol Res. 2012;53:41–57. doi: 10.1007/s12026-012-8297-3. [DOI] [PubMed] [Google Scholar]
- 5.Ziegler-Heitbrock HW, Fingerle G, Strobel M, Schraut W, Stelter F, Schutt C, et al. The novel subset of CD14+/CD16+ blood monocytes exhibits features of tissue macrophages. Eur J Immunol. 1993;23:2053–2058. doi: 10.1002/eji.1830230902. [DOI] [PubMed] [Google Scholar]
- 6.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 7.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 8.Skrzeczyńska-Moncznik J, Bzowska M, Lo˝seke S, Grage-Griebenow E, Zembala M, Pryjma J. Peripheral Blood CD14high CD16+ Monocytes are Main Producers of IL-10. Scandinavian Journal of Immunology. 2008;67:152–159. doi: 10.1111/j.1365-3083.2007.02051.x. [DOI] [PubMed] [Google Scholar]
- 9.Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25:2546–2553. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 10.Gros A, Turcotte S, Wunderlich JR, Ahmadzadeh M, Dudley ME, Rosenberg SA. Myeloid cells obtained from the blood but not from the tumor can suppress T-cell proliferation in patients with melanoma. Clin Cancer Res. 2012;18:5212–5223. doi: 10.1158/1078-0432.CCR-12-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poschke I, Mougiakakos D, Hansson J, Masucci GV, Kiessling R. Immature immunosuppressive CD14+HLA-DR-/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer Res. 2010;70:4335–4345. doi: 10.1158/0008-5472.CAN-09-3767. [DOI] [PubMed] [Google Scholar]
- 12.Ugurel S, Uhlig D, Pfohler C, Tilgen W, Schadendorf D, Reinhold U. Down-regulation of HLA class II and costimulatory CD86/B7-2 on circulating monocytes from melanoma patients. Cancer Immunol Immunother. 2004;53:551–559. doi: 10.1007/s00262-003-0489-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gustafson MP, Abraham RS, Lin Y, Wu W, Gastineau DA, Zent CS, et al. Association of an increased frequency of CD14+ HLA-DR lo/neg monocytes with decreased time to progression in chronic lymphocytic leukaemia (CLL) Br J Haematol. 2012;156:674–676. doi: 10.1111/j.1365-2141.2011.08902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gustafson MP, Lin Y, New KC, Bulur PA, O'Neill BP, Gastineau DA, et al. Systemic immune suppression in glioblastoma: the interplay between CD14+HLA-DRlo/neg monocytes, tumor factors, and dexamethasone. Neuro Oncol. 2010;12:631–644. doi: 10.1093/neuonc/noq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin Y, Gustafson MP, Bulur PA, Gastineau DA, Witzig TE, Dietz AB. Immunosuppressive CD14+HLA-DRlow)/- monocytes in B-cell non-Hodgkin lymphoma. Blood. 2011;117:872–881. doi: 10.1182/blood-2010-05-283820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Block MS, Nevala WK, Leontovich AA, Markovic SN. Differential response of human and mouse dendritic cells to VEGF determines interspecies discrepancies in tumor-mediated TH1/TH2 polarity shift. Clin Cancer Res. 2011;17:1776–1783. doi: 10.1158/1078-0432.CCR-10-2836. [DOI] [PubMed] [Google Scholar]
- 17.Nevala WK, Vachon CM, Leontovich AA, Scott CG, Thompson MA, Markovic SN. Evidence of systemic Th2-driven chronic inflammation in patients with metastatic melanoma. Clin Cancer Res. 2009;15:1931–1939. doi: 10.1158/1078-0432.CCR-08-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kushwah R, Hu J. Complexity of dendritic cell subsets and their function in the host immune system. Immunology. 2011;133:409–419. doi: 10.1111/j.1365-2567.2011.03457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 20.Paglia P, Chiodoni C, Rodolfo M, Colombo MP. Murine dendritic cells loaded in vitro with soluble protein prime cytotoxic T lymphocytes against tumor antigen in vivo. J Exp Med. 1996;183:317–322. doi: 10.1084/jem.183.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jarrossay D, Napolitani G, Colonna M, Sallusto F, Lanzavecchia A. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur J Immunol. 2001;31:3388–3393. doi: 10.1002/1521-4141(200111)31:11<3388::aid-immu3388>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 22.Schreibelt G, Tel J, Sliepen KH, Benitez-Ribas D, Figdor CG, Adema GJ, et al. Toll-like receptor expression and function in human dendritic cell subsets: implications for dendritic cell-based anti-cancer immunotherapy. Cancer Immunol Immunother. 2010;59:1573–1582. doi: 10.1007/s00262-010-0833-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tel J, van der Leun AM, Figdor CG, Torensma R, de Vries IJ. Harnessing human plasmacytoid dendritic cells as professional APCs. Cancer Immunol Immunother. 2012;61:1279–1288. doi: 10.1007/s00262-012-1210-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gustafson MP, Lin Y, LaPlant B, Liwski CJ, Maas ML, League SC, et al. Immune monitoring using the predictive power of immune profiles. Journal for ImmunoTherapy of Cancer. 2013;1 doi: 10.1186/2051-1426-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grell M, Becke FM, Wajant H, Mannel DN, Scheurich P. TNF receptor type 2 mediates thymocyte proliferation independently of TNF receptor type 1. Eur J Immunol. 1998;28:257–263. doi: 10.1002/(SICI)1521-4141(199801)28:01<257::AID-IMMU257>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 26.Khorana AA, Ahrendt SA, Ryan CK, Francis CW, Hruban RH, Hu YC, et al. Tissue factor expression, angiogenesis, and thrombosis in pancreatic cancer. Clin Cancer Res. 2007;13:2870–2875. doi: 10.1158/1078-0432.CCR-06-2351. [DOI] [PubMed] [Google Scholar]
- 27.Poon RT, Lau CP, Ho JW, Yu WC, Fan ST, Wong J. Tissue factor expression correlates with tumor angiogenesis and invasiveness in human hepatocellular carcinoma. Clin Cancer Res. 2003;9:5339–5345. [PubMed] [Google Scholar]
- 28.Nakasaki T, Wada H, Shigemori C, Miki C, Gabazza EC, Nobori T, et al. Expression of tissue factor and vascular endothelial growth factor is associated with angiogenesis in colorectal cancer. Am J Hematol. 2002;69:247–254. doi: 10.1002/ajh.10061. [DOI] [PubMed] [Google Scholar]
- 29.Driessens G, Kline J, Gajewski TF. Costimulatory and coinhibitory receptors in anti-tumor immunity. Immunol Rev. 2009;229:126–144. doi: 10.1111/j.1600-065X.2009.00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and Tumor Responses with Lambrolizumab (Anti-PD-1) in Melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.