Abstract
Aim:
To understand the contribution of sphingolipid metabolism and its metabolites to development and aging.
Methods:
A systemic analysis on the changes in activity of sphingolipid metabolic enzymes in kidney, liver and brain tissues during development and aging was conducted. The study was conducted using tissues from 1-day-old to 720-day-old rats.
Results:
Catabolic enzyme activities as well as the level of sphingomyelinase (SMase) and ceramidase (CDase) were higher than that of anabolic enzyme activities, sphingomyelin synthase and ceramide synthase. This suggested an accumulation of ceramide and sphingosine during development and aging. The liver showed the highest neutral-SMase activity among the tested enzymes while the kidney and brain exhibited higher neutral-SMase and ceramidase activities, indicating a high production of ceramide in liver and ceramide/sphingosine in the kidney and brain. The activities of sphingolipid metabolic enzymes were significantly elevated in all tested tissues during development and aging, although the onset of significant increase in activity varied on the tissue and enzyme type. During aging, 18 out of 21 enzyme activities were further increased on day 720 compared to day 180.
Conclusion:
Differential increases in sphingolipid metabolic enzyme activities suggest that sphingolipids including ceramide and sphingosine might play important and dynamic roles in proliferation, differentiation and apoptosis during development and aging.
Keywords: aging, sphingolipid, metabolism, sphingomyelinase, ceramidase, sphingomyelin synthase, ceramide synthase
Introduction
Aging involves a progressive accumulation of diverse and deleterious changes in cells and tissues with time that increase the risk of disease and death1. Several molecular models of aging have evolved, including damage by reactive oxygen species (ROS) generated by metabolism, genome instability, genetically programmed extension mechanisms, cell death, and systemic aging2. Sphingolipid metabolism is considered to be a novel signal transduction pathway that generates multiple lipid messengers, such as ceramide, sphingosine, and sphingosine-1-phosphate3, 4, 5. Oxidative and metabolic stresses as well as other factors that alter the metabolism of sphingolipids increase the risk of development and progression of pathogenesis of several age-related diseases including cancers, diabetes, atherosclerosis, and neurodegenerative disorders6. Recent studies have shown that sphingolipids, including ceramide and sphingosine, accumulate in the liver and brain during aging7, 8. Neutral sphingomyelinase (N-SMase) was also shown to be activated eight fold in senescent fibroblasts9.
The fact that sphingolipid signaling through ceramide and other metabolites can influence the proliferation, differentiation and survival of many different types of cells strongly suggests fundamental roles of bioactive sphingolipid products in development and aging6. Within the last two decades, considerable progress has been made in elucidating the roles of sphingolipids. But till date, the activity changes in sphingolipid metabolic enzymes in developing and aging tissues have not been systematically studied. Thus, in the present study we investigated the changes in activity of sphingolipid metabolic enzymes in kidney, liver and brain tissues from 1-day-old to 720-day-old rats. The investigated enzyme activities include sphingolipid catabolic enzymes (acidic and neutral sphingomyelinases: A-SMase & N-SMase; acidic, neutral, and alkaline ceramidases: Ac-CDase, N-CDase and Al-CDase) and sphingolipid anabolic enzymes (sphingomyelin synthase and ceramide synthase). The activities of the sphingolipid catabolic enzymes (SMase and ceramidases) were significantly higher and changed more robustly than that of the anabolic enzymes studied (SM synthase and ceramide synthase). The activites of the enzymes were elevated with development and aging, and these results differed with the various tissue and enzyme type studied. This indicates a differential accumulation of ceramide and sphingosine and their contribution toward development and aging.
Materials and methods
Study design
To compare sphingolipid metabolic enzyme activities from 1-day-old to 720-day-old rat tissues; rats were mated. Brain, kidney and liver tissues were collected and homogenized as described below. Each enzyme activity was measured as described below using radioactive substrates.
Materials
[N-methyl-14C]-sphingomyelin was purchased from Sigma (St Louis, MO, USA), and [N-palmitoyl-1-14C]-sphingosine from Moravek Biochemicals (Brea, CA, USA). D-erythro, [3-3H]–sphingosine and E3 Enhancer were procured from PerkinElmer Las, Inc (Boston, MA, USA). D-erythro-sphingosine (synthetic) was purchased from Avanti Polar Lipids Inc (Alabaster, AL, USA). Silica gel HPTLC (60 F254, 20×20) was obtained from Merck (Darmstadt, Germany) and Kodak Medical X-ray film from Eastman Kodak Company (NY, USA). All other materials were purchased from Sigma-Aldrich Korea (St Louis, MO, USA).
Tissue collection
Animal care and all experimental procedures were conducted in accordance with the Guide for Animal Experiments edited by the Korean Academy of Medical Sciences. All animals were sacrificed under ether anesthesia between 9∼12 AM on respective days10 and kidney, liver and brain tissues were isolated and used for homogenate preparation as described below. The kidney, liver and brain tissues of 180-day, 510-day, and 720-day-old rats were obtained from the aging tissue bank (Korea national research resource center).
Preparation of tissue homogenates
Rat tissue homogenates were prepared as described by others11 with some modifications. Tissues including kidney, liver and brain were removed from Sprague-Dawley rats and washed in cold phosphate-buffered saline (PBS) separately. The tissues were placed in 20 mmol/L Tris-HCl (pH 7.5) and 2 mmol/L EDTA solution (10 mL, cold), homogenized by Tekmar homogenizer (OH, USA) at 4 °C. The homogenate was centrifuged at 500×g for 10 min to remove unbroken cell debris, and the supernatant was used as an enzyme source for the enzyme analysis that followed.
Sphingomyelinase (SMase) activity
The activity of N- and A-SMases was determined using a method described by Liu and Hannun12 with slight modifications. Briefly, tissue homogenates were centrifuged at 1000×g for 10 min and the supernatant was used for further analysis. The activity of both SMases was measured using radiolabelled substrate, [N-methyl-14C]-sphingomyelin. For N-SMase, the reaction mixture contained 100 nmol of sphingomyelin (1154 dpm/nmol) in 100 mmol/L Tris-HCl (pH 7.4), 5 mmol/L MgCl2, 0.1% Triton X-100 and 5 mmol/L dithiothreitol in a final volume of 0.2 mL, which contained 5 mg of protein. In the case of A-SMase, the assay mixture contained 100 nmol of sphingomyelin (1154 dpm/nmol) in 100 mmol/L sodium acetate (pH 5.0), 0.1% Triton X-100 and 0.1 mmol/L EDTA, which contained 5 mg of protein. After incubation at 37 °C for 1 h the reaction was stopped by adding 1.5 mL of chloroform: methanol (2:1), followed by 0.2 mL of water. A portion of the aqueous phase was transferred to scintillation vials and counted in a liquid scintillation counter for the radioactivity of the reaction product, 14C-choline phosphate.
Ceramidase (CDase) activity
The activity of Al-CDase, N-CDase and Ac-CDase ceramidases was determined using the method of Nikolove-Karakashian and Merrill13 with slight modifications. The activity of the enzymes was measured using radiolabelled substrate [N-palmitoyl-1-14C]-sphingosine. The reaction was initiated by addition of supernatant (5 mg of protein) to the tubes containing 20 μL of substrate mixture (50 nmol of ceramide – 2353 dpm/nmol, 2.5 mg Triton X-100, 1 mg Tween 20, 0.4 mg sodium cholate) and 130 μL of a reaction buffer. The reaction buffer contained 125 mmol/L sucrose, 0.01 mmol/L EDTA and 125 mmol/L sodium acetate (pH 4.5) or 100 mmol/L Tris-HCl (pH 7.2) or 125 mmol/L HEPES (pH 8.0) for Ac-CDase, N-CDase and Al-CDase activity assay, respectively. After incubation at 37 °C for 1 h, the reaction was stopped by adding 2 mL of basic Doyle's solution (isopropanol:heptane:1 mol/L NaOH, 40:10:1, v/v/v), 1.8 mL of heptane and 1.6 mL of water. Samples were then centrifuged and the upper phase was discarded. The lower phase was washed twice with 1.6 mL heptane and then 1 mL of 0.5 mol/L H2SO4 and 2.4 mL of heptane were added. After centrifugation, 1 mL aliquots from the upper phase were transferred to scintillation vials and analyzed for radioactivity of the reaction product, 14C-palmitate.
Sphingomyelin synthase activity
The activity of sphingomyelin synthase was determined as reported by Luberto and Hannun14 with slight modifications. In brief, the reaction mixture contained 50 mmol/L Tris-HCl (pH 7.4), 25 mmol/L KCl, 0.5 mmol/L EDTA15 and homogenate containing ∼5 mg of protein. The reaction was initiated by addition of [N-palmitoyl-1-14C]-sphingosine (20 nmol) as an equimolar complex with fatty acid free bovine serum albumin (complex specific activity: ∼9×103 cpm/nmol) and allowed to proceed for 60 min. The reaction was stopped by addition of 3 mL chloroform: methanol (1:2); the mixture was vortexed and kept on ice. Lipids were extracted as indicated by the Bligh and Dyer method16 and resolved by TLC in chloroform: methanol: 15 mmol/L anhydrous CaCl2 (60:35:8). The [N-palmitoyl-1-14C]-SM produced was detected by autoradiography, scraped from the plates and quantitated by liquid scintillation counting. Values for blanks were subtracted from total values of [N-palmitoyl-1-14C]-SM to yield the amount of [N-palmitoyl-1-14C]-SM produced.
Ceramide synthase activity
The assay mixture for sphingosine N-acyltransferase contained 1 μmol/L [3H]sphingosine, 25 mmol/L potassium phosphate buffer (pH 7.4), 0.5 mmol/L dithiothreitol, 200 μmol/L palmitoyl-CoA, and approximately 0.2 mg of microsomal protein in a total volume of 0.1 mL as previously reported17. The reaction was initiated by adding palmitoyl-CoA, and after incubation at 37 °C for 15 min, the products were extracted, resolved by TLC, and quantitated as described above. Background counts were subtracted using data from identical assays that omitted palmitoyl-CoA.
Statistical analysis
The results are expressed as mean±SE of 3–6 determinations. Statistical analysis was performed using GraphPad Prism 3 (GraphPad Software Inc, San Diego, CA, USA). Statistical significance of differences was determined using one-way ANOVA with Tukey test. Significance was accepted at P<0.05.
Results
Increase in SMase activity during development and aging
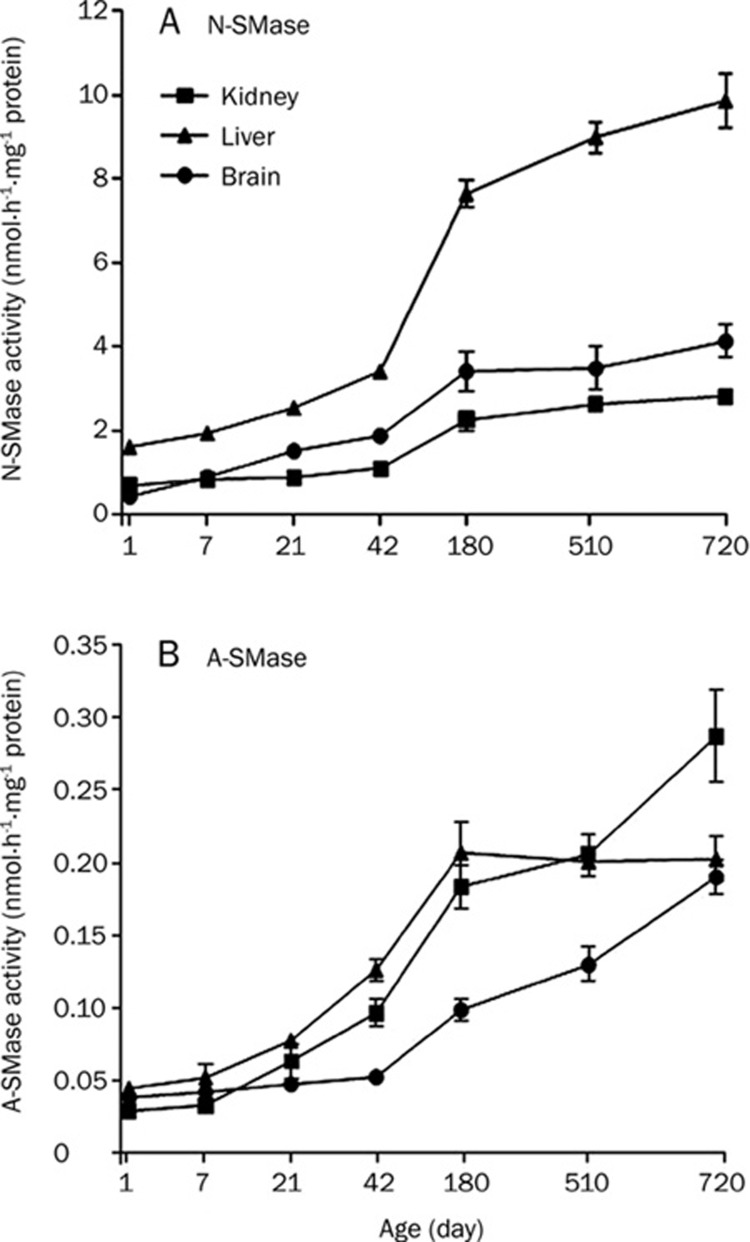
Since 180-day-old tissue was used as the representative young adult tissue in the present study, activity changes in each enzyme from 1-day to 180-day-old tissues were analyzed as developmental changes, while the changes from 180-day to 720-day-old-tissues were categorized as aging-dependent changes. N-SMase and A-SMase activities were measured in rat kidney, liver and brain tissue homogenates. In the liver, both activities increased significantly from day 42, in comparison to the 1-day-old tissue (Figure 1 and Table 1). N-SMase was further increased in the 720-day-old tissue compared to 180-day but no changes were observed in A-SMase from day 180 (Figure 1 and Table 2). N-SMase activity in liver was higher than that of the kidney and brain (Figure 1A). In brain, N-SMase increased significantly from day 42, while A-SMase from day 180 (Figure 1 and Table 1). In the kidney, both activities increased from day 180. A-SMase activities in the brain and kidney tissues from day 720 increased significantly, compared to the respective 180-day- and 520-day-old tissues (Figure 1B and Table 2). N-SMase activities in the 710-day-old kidney tissue increased compared to the 180-day-old tissue but not in the brain (Figure 1A and Table 2). However, A-SMase activity was relatively lower than N-SMase in all the tested tissues. For example, N-SMase activities of kidney, liver and brain tissues at 710-days were about 9-fold, 49-fold and 20-fold higher than that of A-SMase activities from each respective tissue. Nevertheless, the increase in activity of A-SMase appeared to be dependent on aging after 180 days in the kidney and brain tissues. This implied practical roles of A-SMase during aging in the kidney and brain. In the liver, however, N-SMase might play a more significant role during aging based on its relatively higher activity among the different tissues and its further increase during aging.
Figure 1.
SMase activity in rat kidney, liver and brain tissues. N-SMase (A) and A-SMase (B) activities were measured from 7-day, 21-day, 42-day, 180-day, 510-day, and 720-day-old rat kidney, liver and brain tissues. Rat tissue homogenates were incubated with [N-methyl-14C]–sphingomyelin. Lipids were extracted and radioactivity of reaction product 14C-choline phosphate counted in a liquid scintillation counter. Acidic and neutral sphingomyelinases: A-SMase & N-SMase.
Table 1. The earliest day of significant increase observed for each enzyme activity compared to respective 1-day-old tissue during development.
| A-SMase | N-SMase | Ac-CDase | N-CDase | Al-CDase | SM synthase | CD synthase | |
|---|---|---|---|---|---|---|---|
| Liver | 42 | 42 | 7 | 7 | 7 | 180 | 21 |
| Kidney | 180 | 180 | 42 | 42 | 42 | 42 | 21 |
| Brain | 180 | 42 | 42 | 21 | 180 | 21 | 21 |
acidic and neutral sphingomyelinases: A-SMase & N-SMase; acidic, neutral, and alkaline ceramidases: Ac-CDase, N-CDase and Al-CDase.
Table 2. Significant increase observed for each enzyme activity compared to respective 180-day-old tissue during aging.
| A-SMase | N-SMase | Ac-CDase | N-CDase | Al-CDase | SM synthase | CD synthase | |
|---|---|---|---|---|---|---|---|
| Liver | 510 d- | 510 d- | 510 db | 510 d- | 510 d- | 510 d- | 510 dc |
| 720 d- | 720 dc | 720 dc | 720 dc | 720 db | 720 dc | 720 dc | |
| Kidney | 510 d- | 510 d- | 510 dc | 510 db | 510 d- | 510 d- | 510 d- |
| 720 dc | 720 db | 720 dc | 720 dc | 720 db | 720 d- | 720 db | |
| Brain | 510 db | 510 d- | 510 d- | 510 d- | 510 dc | 510 d- | 510 dc |
| 720 dc | 720 d- | 720 dc | 720 dc | 720 dc | 720 dc | 720 dc |
bP<0.05, cP<0.01 compared to 180-day-tissue activity. “-”, not signifiant. acidic and neutral sphingomyelinases: A-SMase & N-SMase; acidic, neutral, and alkaline ceramidases: Ac-CDase, N-CDase and Al-CDase.
Increase in CDase activity during development and aging
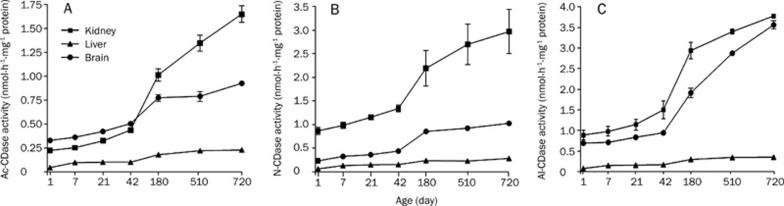
CDases can regulate the cellular ceramide level by hydrolyzing ceramide to sphingosine and free fatty acids. This enzyme not only regulates the levels of ceramide, but also that of sphingosine. We measured the activity of CDases (Ac-CDase, N-CDase and Al-CDase) in rat kidney, liver and brain tissue homogenates to understand the relative activity changes of CDases during development and aging. It was found that the activities of all isoforms of CDases were increased in three rat tissues compared to 1-day-old tissues (Figure 2). The earliest changes were observed in the liver. The activity of Ac-CDase, N-CDase, and Al-CDase increased significantly from day 7 in the liver, and day 42 in the kidney (Table 1). In the brain, the activity of Al-CDase increased from day 180, Ac-CDase from day 42 and N-CDase from day 21 (Table 1).
Figure 2.
CDase activity in rat kidney, liver and brain tissues. Ac-CDase (A), N-CDase (B) and Al-CDase (C) activities were measured from 7-day, 21-day, 42-day, 180-day, 510-day, and 720-day-old rat kidney, liver and brain tissues. Rat tissue homogenates were incubated with [N-palmitoyl-1-14C]-sphingosine. Lipids were extracted and radioactivity of reaction product 14C-palmitate was counted in a liquid scintillation counter.
On day 710, the CDase activities of kidney and brain were higher by almost 11-fold as compared to 710-day-old liver tissue (Figure 2). Collectively, the kidney and brain showed comparatively higher CDases activities, while the liver showed relatively lower CDase activities. Compared to the 180-day-old tissue, three CDase activities in the 710-day-old brain, kidney and liver tissues were significantly increased (Figure 2 and Table 2). In particular, the activity of Ac-CDase in the kidney and brain was further increased in 710-day-old tissues comparison to 180-day tissues and that of Al-CDase in the brain (Figure 2).
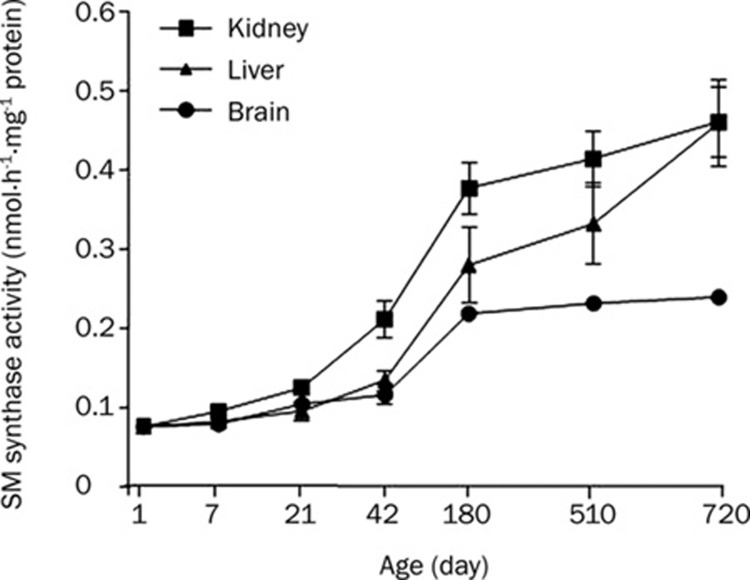
Change in SM synthase activity during development and aging
SM synthase could contribute to the accumulation of ceramide depending on its relative activity to SMase. SM synthase (phosphatidylcholine:ceramide phosphorylcholine transferase) catalyzes the transfer of the phosphocholine head group of phosphatidylcholine to ceramide, thus forming SM7. To determine whether development and aging affected the activity of this enzyme, we measured the SM synthase activity in the same set of tissues using [N-palmitoyl-1-14C]-sphingosine as the substrate. We observed that the SM synthase activity increased with development in the brain from day 21, kidney from day 42 and liver from day 180 (Figure 3). During aging, the activity further increased in the 710-day-old liver and brain tissues compared to the 180-day-old tissues. However, no significant increase was observed in the kidney from day 180 (Figure 3 and Table 2). Though SM synthase activity was increased in developing and aging tissues, the rate of conversion of ceramide to SM was relatively lower than that of SMase, for example 0.610±0.022 versus 9.783±0.638 in the liver (Figures 1 and 3). This suggested that the level of accumulated cellular ceramide had increased during development and aging of the tissues due to the higher activity of SMase than SM synthase. Furthermore, the ceramide content in the kidney increaseed further during aging because SM synthase activity was not increased but A-SMase activity was continuously increased even after day 180 in the kidney (Figure 1B).
Figure 3.
SM synthase activity in rat kidney, liver and brain tissues. SM synthase activities were measured from 7-day, 21-day, 42-day, 180-day, 510-day, and 720-day-old rat kidney, liver and brain tissues. Rat tissue homogenates were incubated with [N-palmitoyl-1-14C]-sphingosine. Lipids were extracted and [N-palmitoyl-1-14C]-SM isolated on TLC by autoradiography and quantitated using a liquid scintillation counter.
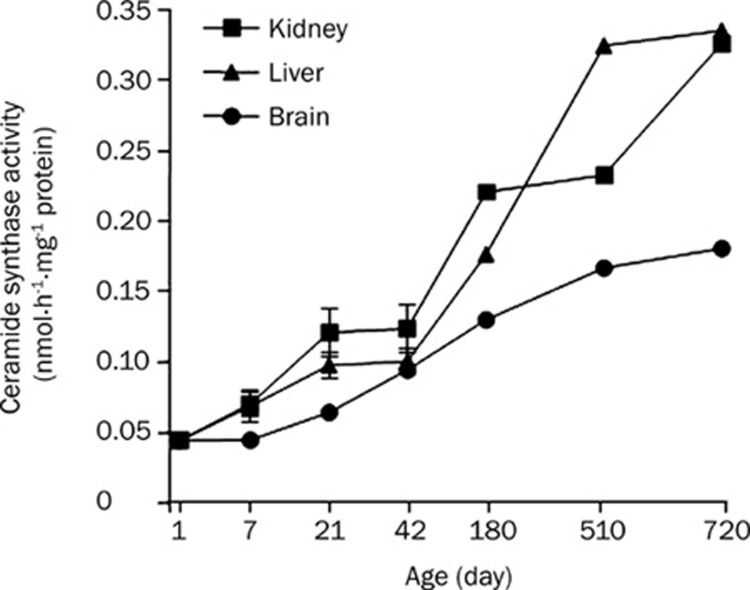
Change in ceramide synthase activity during development and aging
Next, we measured the ceramide synthase activity in the same set of tissues. We found that the enzyme activity was increased with development from day 21 in the rat kidney, liver and brain (Figure 4). We also observed that ceramide synthase activity in those tissues was drastically lower compared to other sphingolipid catabolic enzyme activities like SMase and CDase; however, a further increase in ceramide synthase was observed in three tissues from day 180 (Figures 4 and 5). Thus, the rate of ceramide generation from sphingosine might be low and comparable to the rate of sphingomyelin generation from ceramide.
Figure 4.
Ceramide synthase activity in rat kidney, liver and brain tissues. Ceramide synthase activities were measured from 1-day, 7-day, 21-day, 42-day, 180-day, 510-day, and 720-day-old rat kidney, liver and brain tissues. Rat tissue homogenates were incubated with [3H]-sphingosine. Lipids were extracted and [3H]-ceramide isolated on TLC by autoradiography and quantitated using a liquid scintillation counter.
Figure 5.
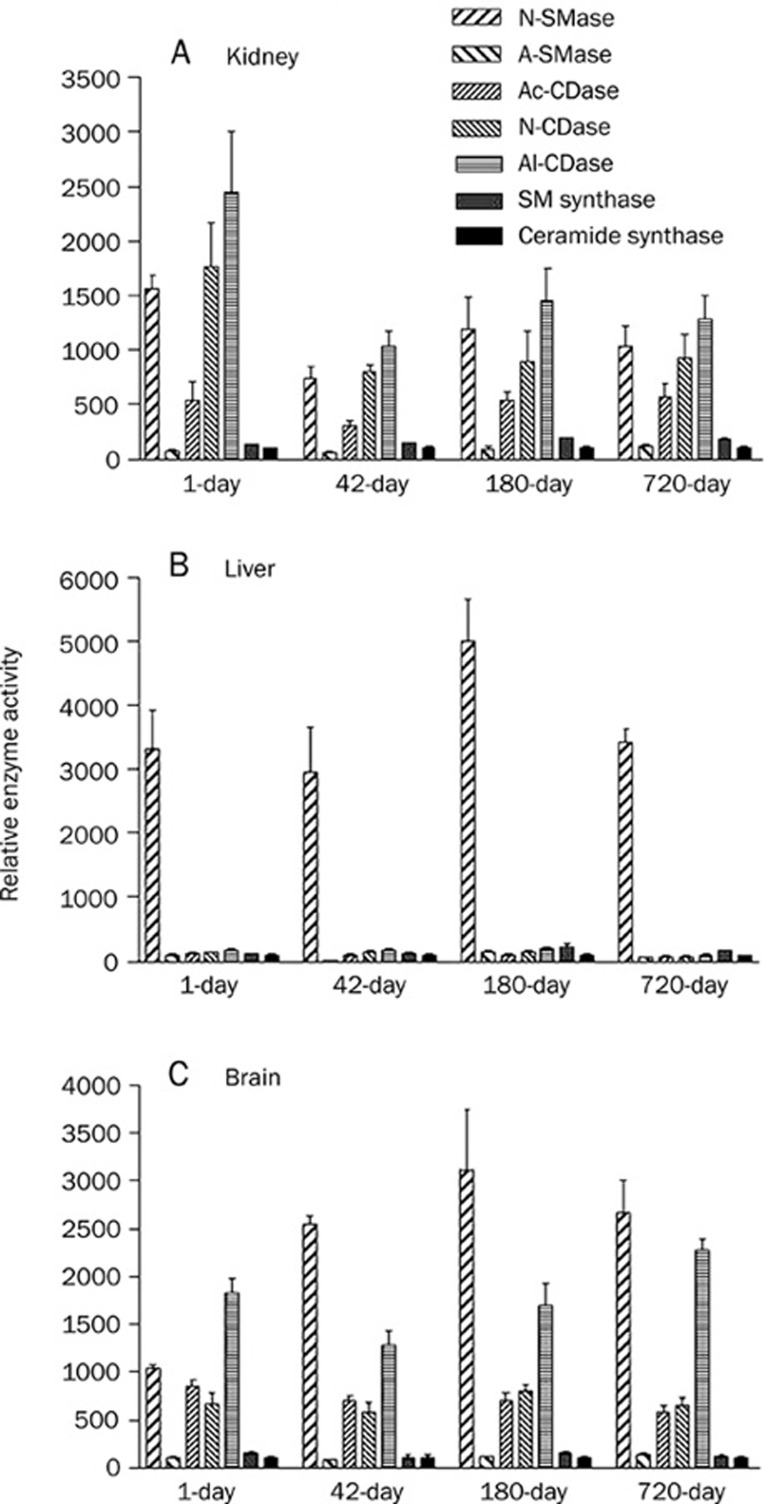
Relative sphingolipid metabolic enzyme activities in 1-day-old, 42-day-old, 180-day-old, and 720-day-old rat tissues of kidney (A), liver (B) and brain (C). Sphingolipid metabolic enzyme activities were compared by assuming activity of ceramide synthase as 100%.
Comparison of sphingolipid metabolic enzyme activities during development and aging
We studied the relative enzyme activities of the 1-day-old, 42-day-old 180-day-old and 710-day-old tissues of rat kidney, liver and brain, because all the tested enzyme activities increased during development and/or aging. For this analysis we assumed ceramide synthase activity as 100% because this activity amounted to very low as compared to other measured activities; we compared the enzyme activity to that of the ceramide synthase activity of the respective aged tissue (Figure 5). We found that the activity of N-SMase in all liver tissues was higher than that of the other enzymes (Figure 5B). N-SMase activity in the liver was higher than that of the kidney and brain tissues (Figure 1). On the other hand, N-SMase, N-CDase and Al-CDase activities in kidney tissues were higher than that of other enzymes (Figure 5A). In the brain, the activities of N-SMase and Al-CDase were relatively higher than that of other enzymes (Figure 5C). SM synthase activity was relatively lower and similar to ceramide synthase activity in any of the tissues tested.
Discussion
Cutler and Mattson suggested a major role for sphingolipid metabolism in regulating the rate of development and lifespan, based on several lines of evidence6. For example, stress conditions are related to both aging and sphingolipid metabolism. Calorie restriction, the representative model of lifespan expanding animal model, decreases sphingolipid synthesis18. Alterations in sphingolipid metabolism increase the risk and progression of age-related disease6. Finally, sphingolipids, including ceramide and sphingosine, accumulate in several tissues (eg liver and brain) during aging7, 19. Several lines of evidence support the postulation that age-related neurodegenerative diseases like Alzheimer's disease are related to sphingolipid metabolism: (1) β-amyloid protein (Aβ1-42) and human immunodeficient virus type 1 gp120 activates N-SMase in human primary neurons, resulting in apoptosis20, 21, 22, 23. (2) Ceramides significantly accumulate in the brain of Alzheimer's disease patients24. (3) Sphingomyelin inhibits γ-secretase activity23. However, changes in sphingolipid metabolic enzyme activities during development and aging have been poorly studied. In the present study, we conducted a systemic analysis of sphingolipid metabolic enzyme activities from 1-day-old to 710-day-old rat tissues.
In this study, 180-day-old rats were supposed to be young adult rats. Therefore, the changes up to day 180 were interpreted as developmental, while the changes in 520-day- and 710-day-old rats were considered to be aging-dependent changes. We report that N-SMase activity was significantly increased in rat kidney, liver and brain developing tissues. Among the tissues, interestingly, the liver showed higher N-SMase activity than the others. Petkova et al reported an age-induced increase in the activity of rat liver plasma membrane-bound N-SMase25. N-SMase was also shown to be activated eight fold in senescent fibroblasts9 as well as being linked to cell cycle arrest26. In the liver, N-SMase was further increased during aging (Figure 1). Among the 7 enzyme activities tested, only N-SMase showed a 30-fold higher activity than others (Figure 5). This implied that N-SMase played a significant role in liver development and aging. In the kidney, N-SMase and A-SMase increased significantly during aging; in the brain, A-SMase increased during aging. Thus, N-SMase was suggested to be a good candidate for maintenance of aging in the liver. A-SMase in the brain and both N-SMase and A-SMase in the kidney might contribute to aging in these organs, although it must be noted that the relative activity of A-SMase was relatively lower than that of N-SMase.
Ceramidases are key enzymes regulating cellular levels of apoptosis-related sphingolipids, ceramide and sphingosine. Activation of Ac-CDase has been linked to resistance of ceramide-mediated death pathways. For instance, overexpression of Ac-CDase was shown to protect cells from TNF-α-induced apoptosis27. We observed that ceramidase (Ac-CDase, N-CDase and Al-CDase) activities increased specifically in 710-day and/or 520-day-old rat kidney, liver and brain tissues as compared to 180-day-old tissues (Figure 2 and Table 2). Among the isoforms of ceramidases, Al-CDase showed the highest activity and was higher in the kidney and brain than the liver. Al-CDase activity in the 710-day-old kidney was 2.2-fold and 1.4-fold higher than Ac-CDase and N-CDase activities in kidney. Thus, the activities of ceramidases decreased in the following order: Al-CDase > N-CDase > Ac-CDase. Spence et al proposed that N-CDase activity was higher than Ac-CDase activity and that both activities decreased in the following order: kidney > brain > cardiac muscle > cerebellum > liver > spleen > cardiac muscle > lung > psoas muscle28. Activities of the isoforms of ceramidases were found to increase in the following order: kidney > brain > liver, supporting the previous study (Figures 2 and 5). Increase in the ceramidase activity in kidney and brain suggests increased sphingosine generation with aging in the tissues. The lower level of CDase activity in the liver suggests the decreased conversion of ceramide to sphingosine, leading to ceramide accumulation in aging liver tissues.
Sphingolipid anabolic enzyme (SM synthase and ceramide synthase) activities were increased in kidney, liver and brain tissues during development and aging. The activity of SM synthase in those tissues, however, was relatively lower than the catabolic enzyme activities (Figure 5). Lightle et al, reported that aging led to a slight decrease in SM synthase in the microsomes, however, the difference did not reach statistical significance in young rats versus old animals7. Our results showed that SM synthase activity increased during development and aging, but the activity itself was lower than that of catabolic enzyme activities. This suggested that a decreased conversion of ceramide to SM might also contribute to age-related increases in ceramide levels. It was also an indication that aging resulted in a slower synthesis of SM and/or a slower synthesis of SM resulted in aging7.
Similar to SM synthase, ceramide synthase activity was also very low. Thus, ceramide generation by ceramide synthase in aging tissues was supposed to be very low, suggesting a minor contribution of ceramide synthesis to ceramide accumulation.
In the present study, we observed that the activities of sphingolipid metabolic enzymes increased differentially in three tested tissues during development and aging. We measured 7 enzyme activities from 3 tissues. Thus 21 enzyme activities were monitored from day 1 to 710. All 21 enzyme activities were increased during development, although significant increases appeared from different time points depending on tissue and enzyme type. On day 710, 18 out of 21 enzyme activities were further increased compared to day 180, suggesting important roles of sphingolipid metabolic enzyme activities in aging.
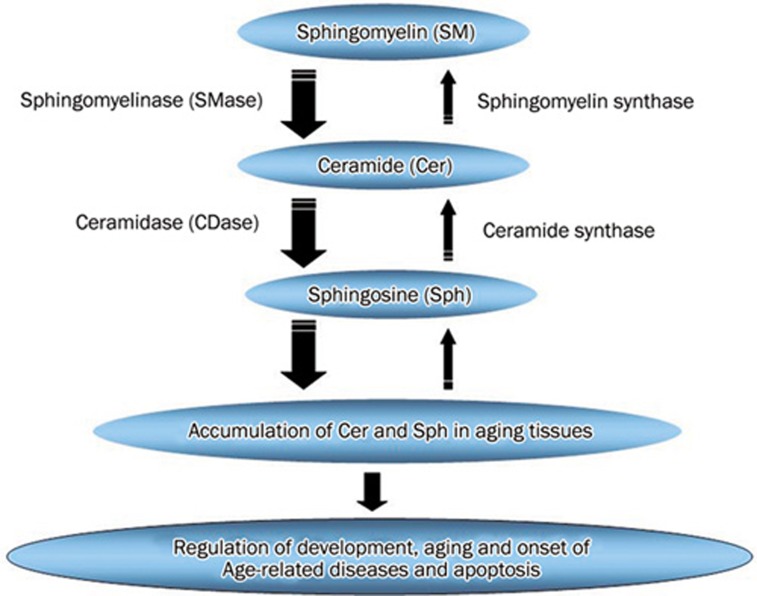
In the present study, N-SMase activity was higher in the liver than in the kidney and brain, while A-SMase and ceramidases (Ac-CDase, N-CDase and Al-CDase) activities were higher in the kidney. We compared these activities in kidney, liver and brain by assuming ceramide synthase activity 100% (Figure 5). We report that (1) N-SMase showed highest activity in the liver, while N-SMase and CDases (especially alkaline and neutral) showed higher activities in kidney and brain tissues and (2) the sphingomyelin synthase and ceramide synthase activities were absolutely and relatively lower than SMase and ceramidases activities in developing and aging tissues. Thus, in the liver N-SMase is postulated to actively produce ceramide, while N-SMase and CDases might produce ceramide and sphingosine in the kidney and brain during aging, thereby leading to an accumulation of ceramide and/or sphingosine. Stress conditions in aged tissues may contribute to the higher activities and the accumulated ceramide/sphingosine induces apoptosis of the cells in aged tissues (Figure 6).
Figure 6.
Biosynthesis and catabolism of sphingolipids.
In summary, on the basis of our results we propose that (1) an accumulation of ceramide/sphingosine occurs with development and aging and plays important roles in regulating an array of physiological processes, including cell proliferation, differentiation, and apoptosis. This increase in the level of ceramide/sphingosine could result from (i) an increased generation from sphingomyelin via mainly N-SMase and minorly A-SMase and ceramidases and (ii) relatively lower activities of SM synthase and ceramide synthase. (2) A differential increase in the sphingolipid metabolic rate in the liver, kidney and brain tissues via differential increased sphingolipid metabolic enzyme activities (SMase, CDase, SM synthase and ceramide synthase) during development and aging may contribute to the varying accumulation of ceramide/sphingosine in the liver, kidney and brain (Figure 6).
Author contribution
Dong-soon IM designed research; Santosh J SACKET performed research; Santosh J SACKET, Hae-young CHUNG, Fumikazu OKAJIMA and Dong-soon IM analyzed data; Santosh J SACKET and Dong-soon IM wrote the paper.
Acknowledgments
This work was supported for two years by Pusan National University Research Grant.
References
- Harman D. Aging: overview. Ann N Y Acad Sci. 2001;928:1–21. doi: 10.1111/j.1749-6632.2001.tb05631.x. [DOI] [PubMed] [Google Scholar]
- Johnson FB, Sinclair DA, Guarente L. Molecular-biology of aging. Cell. 1999;96:291–302. doi: 10.1016/s0092-8674(00)80567-x. [DOI] [PubMed] [Google Scholar]
- Pena LA, Fuks Z, Kolesnick R. Stress-induced apoptosis and the sphingomyelin pathway. Biochem Pharmacol. 1997;53:615–21. doi: 10.1016/s0006-2952(96)00834-9. [DOI] [PubMed] [Google Scholar]
- Perry DK, Hannun YA. The role of ceramide in cell signaling. Biochim Biophys Acta. 1998;1436:233–43. doi: 10.1016/s0005-2760(98)00145-3. [DOI] [PubMed] [Google Scholar]
- Pettus BJ, Chalfant CE, Hannun YA. Ceramide in apoptosis: an overview and current perspectives. Biochim Biophys Acta. 2002;1585:114–25. doi: 10.1016/s1388-1981(02)00331-1. [DOI] [PubMed] [Google Scholar]
- Cutler RG, Mattson MP. Sphingomyelin and ceramide as regulators of development and lifespan. Mech Ageing Dev. 2001;122:895–908. doi: 10.1016/s0047-6374(01)00246-9. [DOI] [PubMed] [Google Scholar]
- Lightle SA, Oakley JI. Nikolova-Karakashian MN. Activation of sphingolipid turnover and chronic generation of ceramide and sphingosine in liver during aging. Mech Ageing Dev. 2000;120:111–25. doi: 10.1016/s0047-6374(00)00191-3. [DOI] [PubMed] [Google Scholar]
- Kavok NS, Krasilnikova OA, Babenko NA. Increase in diacylglycerol production by liver and liver cell nuclei at old age. Exp Gerontol. 2003;38:441–7. doi: 10.1016/s0531-5565(02)00246-2. [DOI] [PubMed] [Google Scholar]
- Venable ME, Lee JY, Smyth MJ, Bielawska A, Obeid LM. Role of ceramide in cellular senescence. J Biol Chem. 1995;270:30701–8. doi: 10.1074/jbc.270.51.30701. [DOI] [PubMed] [Google Scholar]
- Feuillan PP, Aguilera G. Regulation of aldosterone in the 7-day-old rat. Endocrinology. 1996;137:3992–8. doi: 10.1210/endo.137.9.8756576. [DOI] [PubMed] [Google Scholar]
- Igarashi Y, Hakomori S. Enzymatic synthesis of N, N-dimethyl-sphingosine: demonstration of the sphingosine: N-methyltransferase in mouse brain. Biochem Biophys Res Commun. 1989;164:1411–6. doi: 10.1016/0006-291x(89)91827-5. [DOI] [PubMed] [Google Scholar]
- Liu B, Hannun YA. Sphingomyelinase assay using radiolabeled substrate. Methods Enzymol. 2000;311:164–7. doi: 10.1016/s0076-6879(00)11077-8. [DOI] [PubMed] [Google Scholar]
- Nikolova-Karakashian M, Merrill AH., Jr Ceramidases. Methods Enzymol. 2000;311:194–201. doi: 10.1016/s0076-6879(00)11081-x. [DOI] [PubMed] [Google Scholar]
- Luberto C, Hannun YA. Sphingomyelin synthase, a potential regulator of intracellular levels of ceramide and diacylglycerol during SV40 transformation. Does sphingomyelin synthase account for the putative phosphatidylcholine-specific phospholipase C. J Biol Chem. 1998;273:14550–9. doi: 10.1074/jbc.273.23.14550. [DOI] [PubMed] [Google Scholar]
- Futerman AH, Stieger B, Hubbard AL, Pagano RE. Sphingomyelin synthesis in rat liver occurs predominantly at the cis and medial cisternae of the Golgi apparatus. J Biol Chem. 1990;265:8650–7. [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–7. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Morell P, Radin NS. Specificity in ceramide biosynthesis from long chain bases and various fatty acyl coenzyme A's by brain microsomes. J Biol Chem. 1970;245:342–50. [PubMed] [Google Scholar]
- Tacconi MT, Lligona L, Salmona M, Pitsikas N, Algeri S. Aging and food restriction: effect on lipids of cerebral cortex. Neurobiol Aging. 1991;12:55–9. doi: 10.1016/0197-4580(91)90039-m. [DOI] [PubMed] [Google Scholar]
- Giusto NM, Roque ME, Ilincheta de Boschero MG. Effects of aging on the content, composition and synthesis of sphingomyelin in the central nervous system. Lipids. 1992;27:835–9. doi: 10.1007/BF02535859. [DOI] [PubMed] [Google Scholar]
- Jana A, Pahan K. Human immunodeficiency virus type 1 gp120 induces apoptosis in human primary neurons through redox-regulated activation of neutral sphingomyelinase. J Neurosci. 2004;24:9531–40. doi: 10.1523/JNEUROSCI.3085-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana A, Pahan K. Fibrillar amyloid-beta peptides kill human primary neurons via NADPH oxidase-mediated activation of neutral sphingomyelinase. Implications for Alzheimer's disease. J Biol Chem. 2004;279:51451–9. doi: 10.1074/jbc.M404635200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm MO, Grimm HS, Patzold AJ, Zinser EG, Halonen R, Duering M, et al. Regulation of cholesterol and sphingomyelin metabolism by amyloid-beta and presenilin. Nat Cell Biol. 2005;7:1118–23. doi: 10.1038/ncb1313. [DOI] [PubMed] [Google Scholar]
- Grimm MO, Grimm HS, Hartmann T. Amyloid beta as a regulator of lipid homeostasis. Trends Mol Med. 2007;13:337–44. doi: 10.1016/j.molmed.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Cutler RG, Kelly J, Storie K, Pedersen WA, Tammara A, Hatanpaa K, et al. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer's disease. Proc Natl Acad Sci USA. 2004;101:2070–5. doi: 10.1073/pnas.0305799101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkova DH, Momchilova-Pankova AB, Markovska TT, Koumanov KS. Age-related changes in rat liver plasma membrane sphingomyelinase activity. Exp Gerontol. 1988;23:19–24. doi: 10.1016/0531-5565(88)90016-2. [DOI] [PubMed] [Google Scholar]
- Hofmann K, Tomiuk S, Wolff G, Stoffel W. Cloning and characterization of the mammalian brain-specific, Mg2+-dependent neutral sphingomyelinase. Proc Natl Acad Sci USA. 2000;97:5895–900. doi: 10.1073/pnas.97.11.5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strelow A, Bernardo K, Adam-Klages S, Linke T, Sandhoff K, Kronke M, et al. Overexpression of acid ceramidase protects from tumor necrosis factor-induced cell death. J Exp Med. 2000;192:601–12. doi: 10.1084/jem.192.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence MW, Beed S, Cook HW. Acid and alkaline ceramidases of rat tissues. Biochem Cell Biol. 1986;64:400–4. doi: 10.1139/o86-056. [DOI] [PubMed] [Google Scholar]