Abstract
Aim:
To evaluate the role of glutamate receptors in the dorsal hippocampus (DH) in the motivational component of morphine withdrawal.
Methods:
NMDA receptor antagonist D-AP5 (5 μg/0.5 μL per side) or AMPA receptor antagonist NBQX (2 μg/0.5 μL per side) was microinjected into DH of rats. Conditioned place aversion (CPA) induced by naloxone-precipitated morphine withdrawal were assessed.
Results:
Preconditioning microinjection of D-AP5 or NBQX into the DH impaired the acquisition of CPA in acute morphine-dependent rats. However, intra-DH microinjection of D-AP5 or NBQX after conditioning but before the testing session had no effect on the expression of CPA.
Conclusion:
Our results suggest that NMDA and AMPA receptors in the dorsal hippocampus are involved in the acquisition, but not in the expression, of the negative motivational components of acute morphine withdrawal in rats.
Keywords: NMDA receptors; AMPA receptors; hippocampus; morphine withdrawal; conditioned place aversion; Rats, Sprague-Dawley; microinjections
Introduction
The development of opiate abuse, defined as a compulsive drug-taking associated with a loss of control, is driven by two distinct motivational factors: the rewarding effects of the drug (positive reinforcement) and the avoidance of withdrawal symptoms (negative reinforcement). Recently, the mechanisms of negative reinforcement have received considerable attention1, 2. Withdrawal from acute or chronic opiate exposure elicits a broad range of somatic symptoms and arouses assorted negative emotional states (eg, anxiety, irritability, and dysphoria). Clinical evidence suggests that the affective aspect of opiate withdrawal may play a more important role than its physical counterpart in driving drug craving and relapse to compulsive drug use by functioning as a negative motivation3, 4. An increasing number of studies have focused on elucidating the motivational effects of drug withdrawal in animals. One popular method for such research involves studying a conditioned place aversion (CPA) paradigm, in which the negative affective component of opiate withdrawal is paired with a particular environment by conditioning training. The animals then avoid the previously paired environment when re-exposed to this environment in a drug-free state, reflecting the expression of the negative motivational effect of opiate withdrawal.
Glutamate, the predominant excitatory neurotransmitter in the central nervous system, is crucial for the development of major aspects of addiction, including craving, relapse and withdrawal5, 6. Recent research supports the involvement of the glutamatergic system in the negative motivational component of opiate withdrawal. For instance, systemic administration of the NMDA-receptor antagonist MK-801, AMPA-receptor antagonist GYKI 52466 and glutamate release inhibitor riluzole impaired motivational signs of morphine withdrawal7, 8. Furthermore, the inhibition of glutamate receptors in the central nucleus of the amygdala diminished morphine withdrawal-induced CPA9.
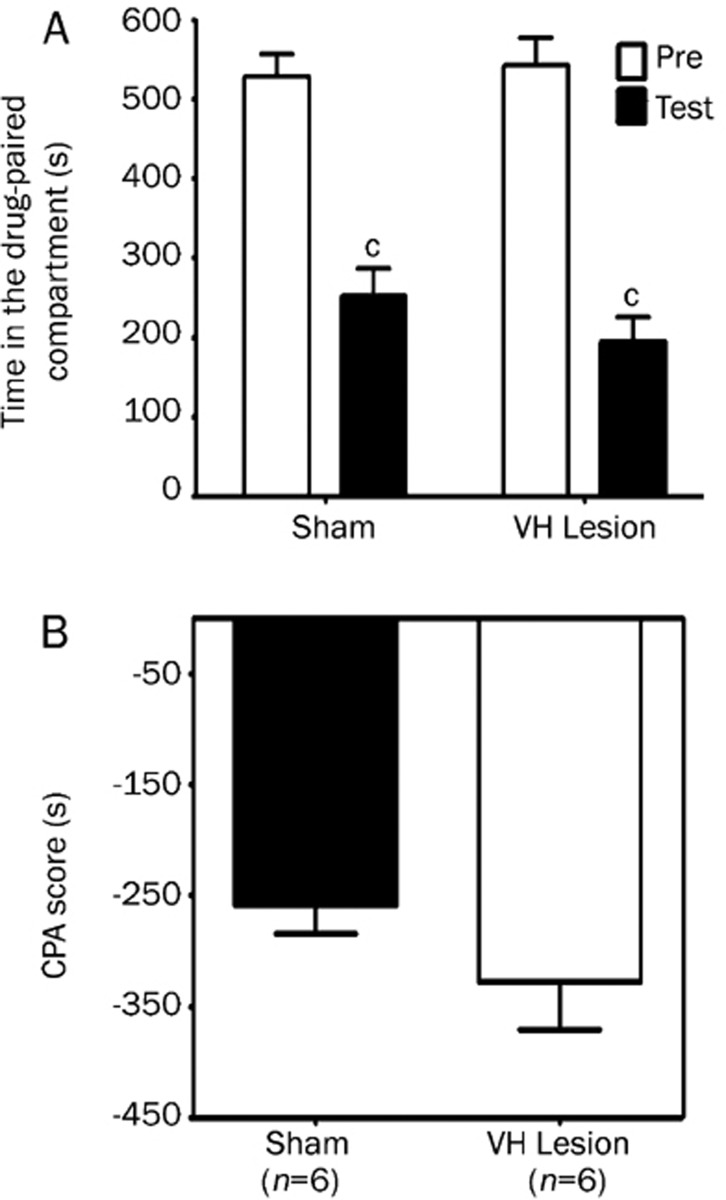
The hippocampus has the highest density of glutamatergic input in the brain and is anatomically connected with other brain regions that have been implicated in the motivational components of opiate withdrawal, such as the amygdala9, 10, 11. Immunohistochemical studies have shown that opiate withdrawal increases the expression of c-fos mRNA, an immediate-early gene used as a marker of neuronal activity, in the hippocampal CA1 region12. Furthermore, we recently found that excitotoxic lesions of the dorsal hippocampus, but not the ventral hippocampus (Figure S1 and Figure S2), impaired morphine withdrawal-induced CPA, indicating the involvement of the dorsal hippocampus in the motivational effects of opiate withdrawal. In addition, Guo et al. reported that acute or chronic morphine treatment decreased the extracellular glutamate concentration in the hippocampus, whereas naloxone-precipitated withdrawal enhanced the release of glutamate in the hippocampus13. These findings suggest the possibility that the glutamatergic system in the dorsal hippocampus may be involved in the motivational components of morphine withdrawal.
Figure S1.
Effects of excitotoxic lesions of the ventral hippocampus on the acquisition of conditioned place aversion induced by naloxone-precipitated morphine withdrawal in morphine-treated rats. Ibotenic acid (10 μg/μL) was microinjected into the ventral hippocampus (0.15 μL per site) using the following coordinates: (a) AP, -4.9 mm, ML, ±4.8 mm, DV, -5.5 and -4.6 mm; (b) AP, -5.2 mm, ML, ±4.0 mm, DV, -7.5 and -4.4; (c) AP, +5.5 mm, ML, ±5.0 mm, DV, -6.1 and -5.3 mm. The columns show the time spent in the drug-paired compartment in the preconditioning (Pre) or testing (test) sessions (A) and the CPA score (B). Data are expressed as means±SEM. cP<0.01 compared with the corresponding preconditioning session (Student's t test). VH: ventral hippocampus.
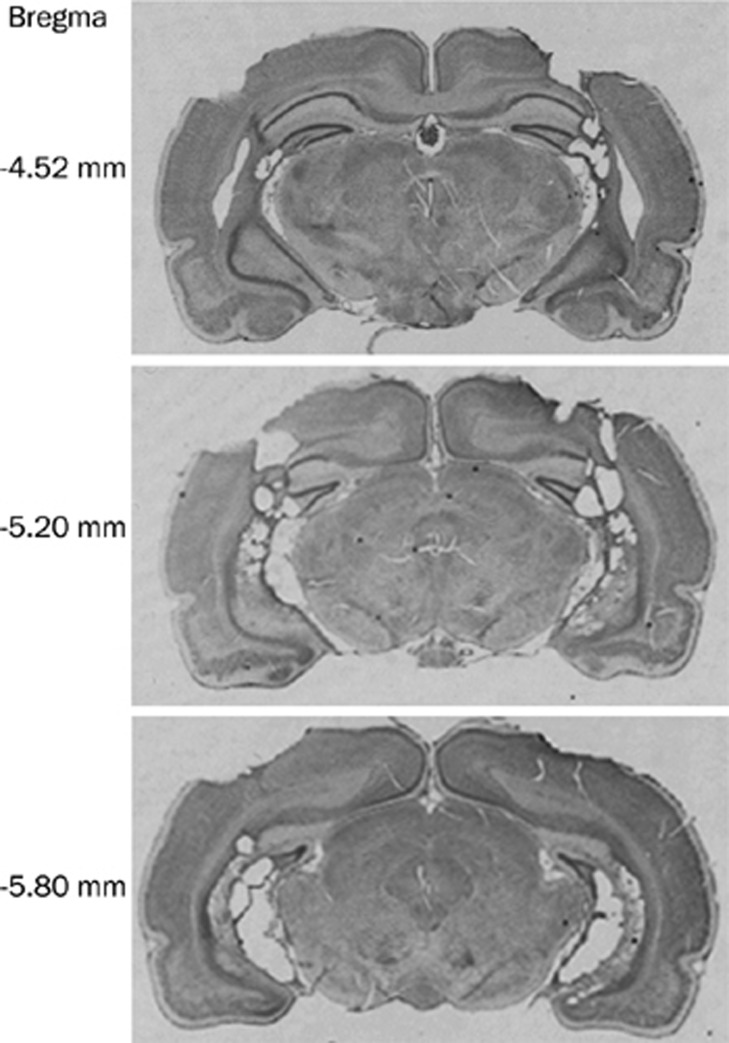
Figure S2.
Representative photographs of cresyl violet-stained coronal sections showing the ventral hippocampus lesions.
We here investigate the role of glutamate receptors within the dorsal hippocampus during the acquisition and expression of conditioned place aversion induced by acute morphine withdrawal.
Materials and methods
Subjects
Sprague-Dawley male rats (clean grade) weighing 220−250 g were obtained from the Laboratory Animal Center, Chinese Academy of Sciences (Shanghai, China). Rats were housed 2–3 per cage and maintained on a 12-h light/dark cycle with access to food and water ad libitum. All experimental procedures were in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Surgery
Rats were anesthetized using sodium pentobarbital (50 mg/kg, ip), treated with atropine sulfate (0.2 mg/kg, ip) and then placed in a stereotaxic apparatus (Narishige, Tokyo, Japan) with the incisor bar set at -3.3 mm relative to the interaural line. The scalp was incised and retracted to expose the skull. Small burr holes were drilled through the skull to allow the guide cannulae to pass into the brain. The guide cannulae (23 gauge) were implanted bilaterally into the dorsal hippocampus using the following coordinates: anteroposterior (AP), -3.80 mm from bregma; mediolateral (ML), ±2.5 mm from the midline, dorsoventral (DV), -1.5 mm from the skull14. Three surgical screws were implanted into the skull as anchors. The cannulae and the screws were affixed to the skull with dental cement. Insect pins (0.5 mm beyond the tip of guide cannula) were inserted into the cannulae to maintain patency and were removed only for the infusions. All rats were given 7–10 d for postsurgical recovery before commencing behavioral training.
Conditioned place aversion
Apparatus
A CPA apparatus [60 cm (length)×30 cm (width)×30 cm (height)] made of Plexiglas was divided into two equal-size compartments by a partition with an opening (10×10 cm) at one end, which allowed rats free access to each compartment. Two compartments were distinguished by visual and tactile cues: one compartment had white walls with a textured floor, whereas the other compartment had black walls with a smooth floor. Another partition without an opening was used to confine the rat to a given compartment in the conditioning session of the CPA procedure.
Procedures
The method to establish conditioned place aversion was similar to that employed by White et al15. The CPA procedure consisted of three sessions: pre-conditioning, conditioning and testing. In the pre-conditioning session, rats were allowed to freely explore the entire apparatus for 15 min. Time spent in each compartment was determined automatically using a computer system (AniLab v2.0, AniLab Software & Instrument Co Ltd, Ningbo, China). There was no significant difference between the time spent in the smooth floor compartment (474.9±18.0 s, n=62) and that in the textured floor compartment (425.1±18.0 s, n=62) during the pre-conditioning session. Conditioning took place over the next 2 d. On the first day, the rats were injected with saline (1 mL/kg, sc) and then returned to home cages. Four hours later, they were again given saline and then confined to one or the other compartment in a counterbalanced manner for 30 min. On the second day, the rats were injected with either morphine (10 mg/kg, sc) or saline (1 mL/kg, sc) and then returned to their home cages. Four hours later, they were injected with naloxone (0.3 mg/kg, sc) and then confined to the compartment they did not occupy on the first day for 30 min. This compartment will be referred to as the “drug-paired compartment”. In the testing session (24 h after the conditioning trial), all rats were allowed to freely explore the entire apparatus for 15 min and the amount of time spent in each compartment was recorded. CPA score is defined as time in the drug–paired compartment during the testing session minus time during the preconditioning session.
Drugs and intracerebral microinjection
Morphine hydrochloride was purchased from Qinghai Pharmaceutical General Factory (Qinghai, China). Naloxone hydrochloride, D-2-Amino-5-phosphonovaleric acid (D-AP5) and NBQX disodium salt were supplied by Sigma-Aldrich (St Louis, MO, USA). D-AP5 and NBQX were dissolved in PBS and other drugs were dissolved in saline before use. Bilateral microinfusions were made through 31-gauge injection cannulae (2.0 mm beyond the tip of guide cannulae) at a rate of 0.5 μL/min using a microsyringe pump (Harvard Apparatus, South Natick, MA, USA) over 1 min. The injection cannulae were kept in place for an additional 2 min. The dose of D-AP5 and NBQX was chosen based on pilot experiments and previous studies16, 17. To determine the effects of D-AP5 and NBQX on the acquisition of CPA, D-AP5 and NBQX were microinjected into the dorsal hippocampus 10 min before paring on the second day of the conditioning session. To determine the effects of D-AP5 and NBQX on the expression of CPA, D-AP5 and NBQX were microinjected into the dorsal hippocampus 20 min before the testing session.
Histology
After behavioral testing, all rats were deeply anesthetized with sodium pentobarbital and perfused transcardially with PBS, followed by 10% formalin. The brains were removed and stored in a 30% sucrose/10% formalin solution for 2–3 d. Coronal sections (50 μm thick) were cut on a cryostat (Leica, Nussloch, Germany), stained with cresyl violet and then examined by light microscopy to determine the injection site.
Data analysis
Data are represented as mean±SEM. All data were analyzed with one-way ANOVA or Student's t tests, where appropriate. Post hoc testing was performed with Newman-Keuls Test. Differences with P<0.05 were considered statistically significant.
Results
Histology
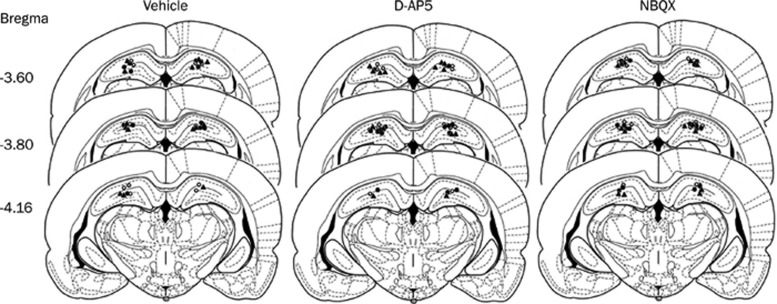
The injection sites for all rats included in the analysis of this experiment (no rats from the initial 62 were discarded) are illustrated in Figure 1. All injection sites were located in the dorsal hippocampus. Injection sites for vehicle, D-AP5 and NBQX were basically the same; thus no difference among three conditions resulted from differential injection sites.
Figure 1.
Schematic representation of injection sites in the dorsal hippocampus for all rats included in the experiments (○, preconditioning microinjection in saline-treated rats; ○, preconditioning microinjection in morphine-treated rats; , pretesting microinjection in morphine-treated rats). Numbers to the left of the coronal sections represent the distance (mm) from bregma in the anterior-posterior axis.
Effects of intra-DH injection of D-AP5 or NBQX on the acquisition of conditioned place aversion induced by naloxone-precipitated morphine withdrawal
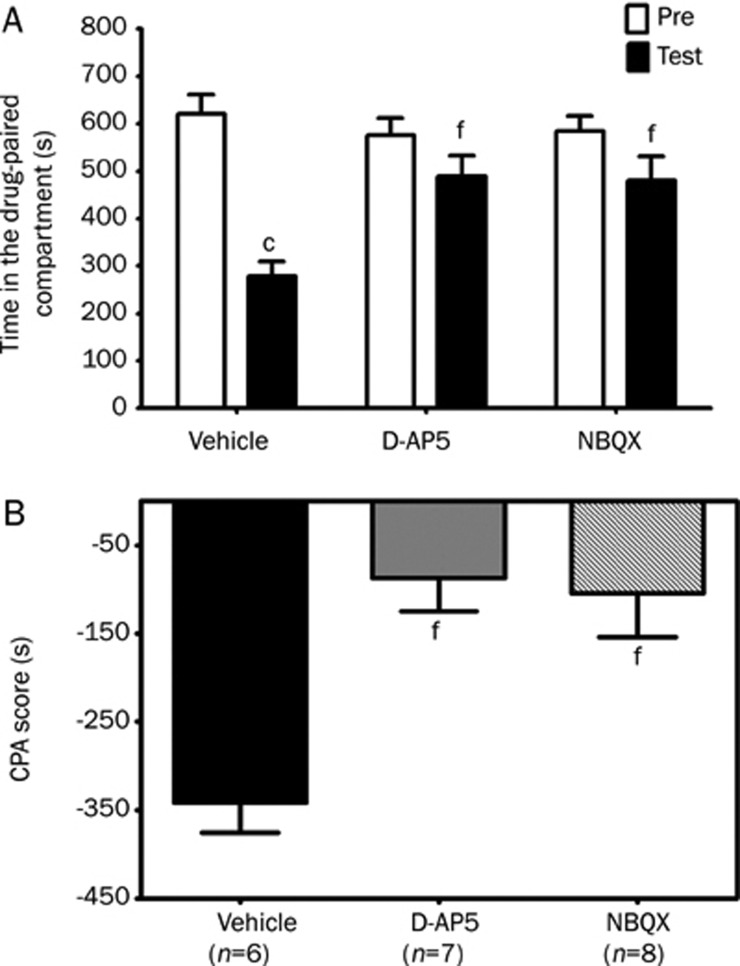
Conditioned place aversion (CPA) is a form of associative learning in which an animal avoids an environment that had been paired previously with drug withdrawal. The hippocampus is vital for mediating the encoding and consolidation of associative memory processes which are mediated particularly by glutamate receptors18, 19, 20. To determine whether glutamate receptors in the dorsal hippocampus are involved in the acquisition of morphine withdrawal-induced CPA, the competitive NMDA receptor antagonist D-AP5 (5 μg/0.5 μL per side) or the competitive AMPA receptor antagonist NBQX (2 μg/0.5 μL per side) was bilaterally microinfused into the dorsal hippocampus 10 min before naloxone injection during the conditioning session. In the rats microinjected with vehicle into the bilateral DH, CPA was dramatically induced by naloxone injection following a single exposure to morphine, consistent with previous studies15, 21. In intra-DH vehicle-injected rats, the time spent in the drug-paired compartment during the testing session was significantly shorter at 278.8±30.8 s (P<0.01, Student's t test) than that spent during the preconditioning session (620.7±40.3 s) (Figure 2A). Microinjection of D-AP5 and NBQX into the DH of the morphine-treated rats attenuated the acquisition of CPA induced by naloxone-precipitated morphine withdrawal. In the rats microinjected with D-AP5 or NBQX into the DH, there were no significant differences (P>0.05, Student's t test) between the time spent in the drug-paired compartment during the testing session (489.0±43.7 s and 479.8±51.2 s, respectively) and that spent during the preconditioning session (575.7±36.1 s and 583.9±32.8 s, respectively) (Figure 2A). CPA scores revealed an inhibition of morphine withdrawal-induced CPA behavior by the intra-DH injection of D-AP5 or NBQX (Figure 2B). One-way ANOVA indicated a significant difference among groups (F(2, 18)=10.09; P<0.01). Post hoc comparison by the Newman-Keuls test showed that D-AP5 (-86.7±38.1 s, P<0.01) and NBQX (-104.1±49.5 s, P<0.01) produced a significant attenuation of CPA score compared with the vehicle-injected morphine-treated group (-341.9±33.6 s).
Figure 2.
Effects of intra-DH infusion of D-AP5 or NBQX on the acquisition of conditioned place aversion induced by naloxone-precipitated morphine withdrawal in morphine-treated rats. D-AP5 (5 μg/0.5 μL per side), NBQX (2 μg/0.5 μL per side) or vehicle (0.5 μL/side) was bilaterally microinfused into the dorsal hippocampus 10 min before pairing on the second day of the conditioning session. The columns show the time spent in the drug-paired compartment during the preconditioning (Pre) or testing (test) sessions (A) and the CPA score (B). The CPA score is defined as time in the drug–paired compartment during the testing session minus the time spent during the preconditioning session. Data are expressed as means±SEM. cP<0.01 compared with the corresponding preconditioning session (Student's t test); fP<0.01 compared with vehicle injected rats (one-way ANOVA with Newman-Keuls post hoc test).
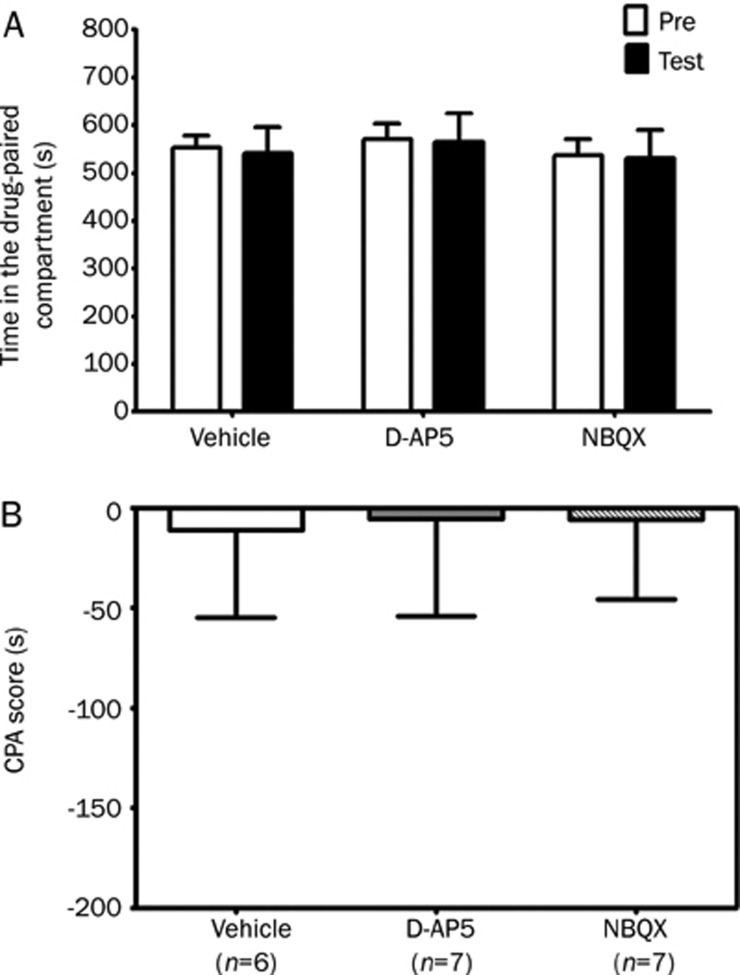
To examine whether intra-DH injection of D-AP5 or NBQX produced conditioned place preference (CPP) or CPA in the absence of morphine treatment, D-AP5 or NBQX was injected into the bilateral dorsal hippocampus of saline-treated rats. No significant differences (P<0.05, Student's t test) were observed in the vehicle, D-AP5 or NBQX-injected groups between the time spent in the drug-paired compartment during the testing session (542.5±53.0 s, 565.6±59.8 s and 532.0±58.2 s, respectively) and that spent during the preconditioning session (553.4±25.4 s, 571.0±32.3 s and 537.9±33.4 s, respectively) (Figure 3A). One-way ANOVA indicated no significant differences in CPA score among groups (F(2, 17)=0.005; P>0.05) (Figure 3B). These data showed that neither CPP nor CPA was induced by intra-DH injection of vehicle, D-AP5 or NBQX, suggesting that D-AP5 and NBQX alone have no motivational effects when injected at this dose into the dorsal hippocampus.
Figure 3.
Effects of intra-DH infusion of D-AP5 or NBQX on conditioned place aversion in saline-treated rats. The columns show the time spent in the drug-paired compartment in the preconditioning (Pre) or testing (test) sessions (A) and the CPA score (B). D-AP5 (5 μg/0.5 μL per side), NBQX (2 μg/0.5 μL per side) or vehicle (0.5 μL/side) was bilaterally microinfused into the dorsal hippocampus 10 min before pairing on the second day of the conditioning session. Data are expressed as means±SEM.
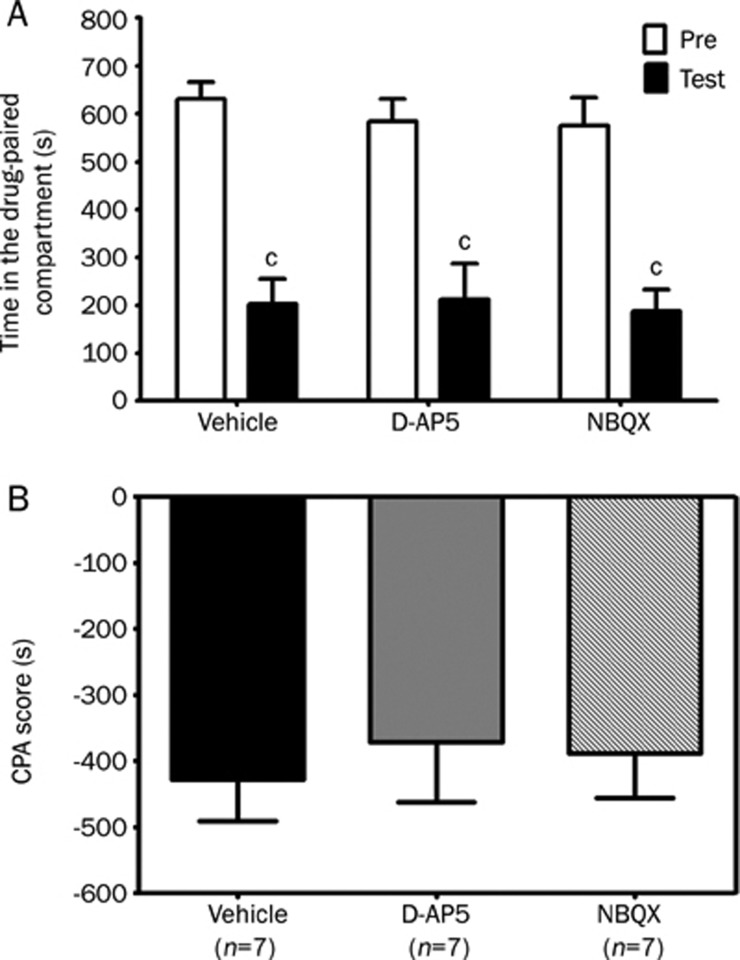
Effects of intra-DH injection of D-AP5 or NBQX on the expression of conditioned place aversion induced by naloxone-precipitated morphine withdrawal
Considerable evidence has shown that the dorsal hippocampus is involved in contextual memory retrieval in several Pavlovian conditioning tasks, such as fear conditioning and morphine-induced CPP22, 23, 24, 25. In the CPA testing session, re-exposure to the training context elicited retrieval of the morphine withdrawal memory and the negative motivation induced by the morphine withdrawal-paired environment was then expressed as aversion. To examine the effects of D-AP5 or NBQX on the expression of the negative motivational effect of morphine withdrawal, each of the drugs was bilaterally microinjected into the dorsal hippocampus 20 min before the testing session. Figure 4 indicates that the intra-DH injection of D-AP5 or NBQX did not induce a change in the expression of CPA in the morphine-treated rats. In the vehicle, D-AP5 or NBQX-injected groups, significant differences (P<0.01, Student's t test) were observed between the time spent in the drug-paired compartment during the testing session (202.8± 52.8 s, 212.5±74.0 s and 187.4±46.2 s, respectively) and the time spent during the preconditioning session (630.6±35.5 s, 583.9±46.8 s and 575.8±58.8 s, respectively), indicative of the formation of morphine-withdrawal induced CPA(Figure 4A). One-way ANOVA indicated no significant difference in CPA score among the groups (F(2, 18)=0.15; P>0.05) (Figure 4B).
Figure 4.
Effects of intra-DH infusion of D-AP5 or NBQX on the expression of conditioned place aversion induced by naloxone-precipitated morphine withdrawal in morphine-treated rats. D-AP5 (5 μg/0.5 μL per side), NBQX (2 μg/0.5 μL per side) or vehicle (0.5 μL per side) was bilaterally microinfused into the dorsal hippocampus 20 min before the testing session. The columns show the time spent in the drug-paired compartment in the preconditioning (Pre) or testing (test) sessions (A) and the CPA score (B). Data are expressed as means±SEM. cP<0.01 compared with the corresponding preconditioning session (Student's t test).
Discussion
The hippocampus is an important part of the limbic system that has been widely reported to be involved in many important aspects of addiction, including craving and relapse. However, very few studies have addressed the role of the hippocampus in opiate withdrawal. We recently found that excitotoxic lesions of the dorsal hippocampus, but not the ventral hippocampus, impaired CPA induced by morphine withdrawal (supplemental data in Figure S1 and Figure S2), suggesting that there is a functional dissociation between the dorsal and ventral hippocampus with respect to the motivational effect of morphine withdrawal. This finding was consistent with previous studies showing that the dorsal hippocampus and ventral hippocampus play different roles in a variety of learning and memory tasks, such as fear conditioning, cocaine place conditioning and spatial learning26, 27, 28. The possible mechanisms underlying this functional dissociation remain unclear.
In the present study, we found that a preconditioning intra-DH injection of the competitive NMDA receptor antagonist D-AP5 or the AMPA receptor antagonist NBQX attenuated acute morphine withdrawal-induced CPA, whereas pretesting injections of the same did not. These results suggest that the glutamatergic system of the dorsal hippocampus is required for the formation, but not the expression, of the negative motivational aspects of opiate withdrawal. Our present results are consistent with previous findings that the central glutamate system is involved in the motivational aspects of opiate withdrawal7, 8, 9 and extend these findings by revealing that the dorsal hippocampus is a key area of the brain for this involvement. In addition, the present study provides further insight into which transmitter system in the dorsal hippocampus is responsible for the motivational effect of morphine withdrawal.
CPA is a Pavlovian conditioning paradigm wherein drug withdrawal is paired with a particular environment, triggering an association between the negative affective consequences of withdrawal and this context. Once the animals are re-exposed to the paired environment in a drug-free state, this association leads to avoidance of the paired environment. Thus, CPA reflects a process of learning and memory. The persistent changes in behavior and psychological function that occur as a consequence of experience are thought to be mediated by the reorganization or strengthening of synaptic connections in specific neural circuits, and synaptic plasticity is thus thought to play a critical role in learning and memory. Long-term potentiation (LTP), a form of activity-dependent synaptic plasticity that strengthens active synapses, has long been thought to be a possible neural mechanism underling learning and memory29, 30.
The hippocampus is a key region of the brain for both the encoding and the consolidation of associative memory18, 19, 20. Extensive evidence supports the idea that the glutamatergic system, acting via NMDA and AMPA receptors, plays a crucial role in the formation and maintenance of hippocampal LTP31, 32, 33, 34. For instance, substantial evidence indicates that NMDA-dependent actin polymerization is important for consolidation of the early phase of LTP into the late phase in adult rats in vivo35, 36 and that increases in glutamate transmission by AMPA receptors can lead to a stabilization of dendritic spine morphology, LTP and memory37. Therefore, one possible explanation for the effects of glutamate antagonists D-AP5 or NBQX on morphine withdrawal-induced CPA observed in this study involves the disruption of associative learning during CPA training, which may lead to an impairment of the aversive morphine withdrawal memory formed by the association between naloxone-precipitated morphine withdrawal and environmental cues. Supporting this model, several studies have demonstrated that the inhibition of glutamate receptors in the hippocampus impaired the acquisition and consolidation of fear conditioning38, 39, 40, possibly due to the blockade of glutamate receptor-dependent synaptic plasticity41, 42.
In the present study, we demonstrate that the intra-DH injection of D-AD5 and NBQX blocks the acquisition of morphine withdrawal-induced place aversion without affecting its expression. Our results thus reveal that the acquisition and the expression of CPA rely on different molecular mechanisms. Several previous studies have also shown that the glutamatergic system is distinctly involved in the acquisition and the expression of certain behaviors. For example, Quinn et al reported that blockade of the dorsal hippocampus NMDA receptors disrupted the acquisition of two forms of fear conditioning while differentially affecting their expression39. They also found that intra-DH injection of D-AP5 did not diminish the expression of contextual fear conditioning. Additionally, previous studies have shown that in the dorsal hippocampus, NMDA receptors were involved only in the acquisition, but not the expression, of morphine induced-CPP43, whereas dopamine and GABA receptors were required for the expression of morphine induced-CPP23, 24, 25. Although our study clearly showed that NMDA and AMPA receptors in the dorsal hippocampus are involved in the acquisition, but not the expression, of morphine withdrawal-induced CPA, further work is needed to address the mechanisms by which NMDA and AMPA receptors modulate synaptic plasticity (eg, actin rearrangement and LTP) in response to place aversion conditioning and to determine the neurotransmitters that are involved in the expression of CPA.
Author contribution
Yuan-yuan HOU designed and preformed the research and wrote the paper. Yao LIU helped with histology analysis. Shuo KANG and Chuan YU helped with animal training. Zhi-qiang CHI provided consultation. Jing-gen LIU revised the paper.
Acknowledgments
This study was supported by the National Basic Research Program grant from the Ministry of Science and Technology of China (No G2003CB515400) and (No 2009CB522000), National Science Fund for Distinguished Young Scholar from the National Natural Science Foundation of China (No 30425002) and a fund granted by the Chinese Academy of Sciences (No KSCXI/YW/R/68).
References
- Koob GF. Neurobiology of addiction. Toward the development of new therapies. Ann N Y Acad Sci. 2000;909:170–85. doi: 10.1111/j.1749-6632.2000.tb06682.x. [DOI] [PubMed] [Google Scholar]
- Hutcheson DM, Everitt BJ, Robbins TW, Dickinson A. The role of withdrawal in heroin addiction: enhances reward or promotes avoidance. Nat Neurosci. 2001;4:943–7. doi: 10.1038/nn0901-943. [DOI] [PubMed] [Google Scholar]
- Jasinski DR, Johnson RE, Kocher TR. Clonidine in morphine withdrawal. Differential effects on signs and symptoms. Arch Gen Psychiatry. 1985;42:1063–6. doi: 10.1001/archpsyc.1985.01790340041006. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Johnson RE, Jasinski DR.Clinical procedures for the assessment of abuse potentialIn: Bozarth MA, editors. Methods of assessing the reinforcing properties of abused drugs. New York: Springer-Verlag; 1987p573–90.
- Tzschentke TM, Schmidt WJ. Glutamatergic mechanisms in addiction. Mol Psychiatry. 2003;8:373–82. doi: 10.1038/sj.mp.4001269. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Lalumiere RT, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology. 2009;56 Suppl 1:169–73. doi: 10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki Y, Jin C, Suemaru K, Kawasaki H, Shibata K, Choshi T, et al. Effect of glutamate receptor antagonists on place aversion induced by naloxone in single-dose morphine-treated rats. Br J Pharmacol. 2005;145:751–7. doi: 10.1038/sj.bjp.0706228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Araki H, Kawasaki Y, Nagata M, Suemaru K, Shibata K, et al. The glutamate release inhibitor riluzole attenuates the formation of conditioned place aversion induced by naloxone in rats undergoing a single morphine exposure. Brain Res. 2006;1069:120–6. doi: 10.1016/j.brainres.2005.11.058. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Nakagawa T, Yamamoto R, Maeda A, Minami M, Satoh M. Involvement of glutamate receptors within the central nucleus of the amygdala in naloxone-precipitated morphine withdrawal-induced conditioned place aversion in rats. Jpn J Pharmacol. 2002;88:399–406. doi: 10.1254/jjp.88.399. [DOI] [PubMed] [Google Scholar]
- Kelsey JE, Arnold SR. Lesions of the dorsomedial amygdala, but not the nucleus accumbens, reduce the aversiveness of morphine withdrawal in rats. Behav Neurosci. 1994;108:1119–27. doi: 10.1037//0735-7044.108.6.1119. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann N Y Acad Sci. 2000;911:369–91. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Frenois F, Cador M, Caille S, Stinus L, Le Moine C. Neural correlates of the motivational and somatic components of naloxone-precipitated morphine withdrawal. Eur J Neurosci. 2002;16:1377–89. doi: 10.1046/j.1460-9568.2002.02187.x. [DOI] [PubMed] [Google Scholar]
- Guo M, Xu NJ, Li YT, Yang JY, Wu CF, Pei G. Morphine modulates glutamate release in the hippocampal CA1 area in mice. Neurosci Lett. 2005;381:12–5. doi: 10.1016/j.neulet.2005.01.071. [DOI] [PubMed] [Google Scholar]
- Paxinos G., and, Watson C.The Rat Brain in Stereotaxic Coordinates (5th ed), Amsterdam: Elsevier Academic Press; 2005
- White DA, Hwang ML, Holtzman SG. Naltrexone-induced conditioned place aversion following a single dose of morphine in the rat. Pharmacol Biochem Behav. 2005;81:451–8. doi: 10.1016/j.pbb.2005.04.002. [DOI] [PubMed] [Google Scholar]
- McDannald M, Kerfoot E, Gallagher M, Holland PC. Amygdala central nucleus function is necessary for learning but not expression of conditioned visual orienting. Eur J Neurosci. 2004;20:240–8. doi: 10.1111/j.0953-816X.2004.03458.x. [DOI] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Sprunger D, Wright-Hardesty K, Rudy JW. The role of dorsal hippocampus and basolateral amygdala NMDA receptors in the acquisition and retrieval of context and contextual fear memories. Behav Neurosci. 2007;121:721–31. doi: 10.1037/0735-7044.121.4.721. [DOI] [PubMed] [Google Scholar]
- Small SA, Nava AS, Perera GM, DeLaPaz R, Mayeux R, Stern Y. Circuit mechanisms underlying memory encoding and retrieval in the long axis of the hippocampal formation. Nat Neurosci. 2001;4:442–9. doi: 10.1038/86115. [DOI] [PubMed] [Google Scholar]
- McNaughton N, Hebb WJ. Pandemonium and catastrophic hypermnesia: the hippocampus as a suppressor of inappropriate associations. Cortex. 2003;39:1139–63. doi: 10.1016/s0010-9452(08)70882-7. [DOI] [PubMed] [Google Scholar]
- Kennedy PJ, Shapiro ML. Retrieving memories via internal context requires the hippocampus. J Neurosci. 2004;24:6979–85. doi: 10.1523/JNEUROSCI.1388-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azar MR, Jones BC, Schulteis G. Conditioned place aversion is a highly sensitive index of acute opioid dependence and withdrawal. Psychopharmacology (Berl) 2003;170:42–50. doi: 10.1007/s00213-003-1514-y. [DOI] [PubMed] [Google Scholar]
- Maren S, Holt W. The hippocampus and contextual memory retrieval in Pavlovian conditioning. Behav Brain Res. 2000;110:97–108. doi: 10.1016/s0166-4328(99)00188-6. [DOI] [PubMed] [Google Scholar]
- Rezayof A, Zarrindast MR, Sahraei H, Haeri-Rohani A. Involvement of dopamine receptors of the dorsal hippocampus on the acquisition and expression of morphine-induced place preference in rats. J Psychopharmacol. 2003;17:415–23. doi: 10.1177/0269881103174005. [DOI] [PubMed] [Google Scholar]
- Rezayof A, Razavi S, Haeri-Rohani A, Rassouli Y, Zarrindast MR. GABA(A) receptors of hippocampal CA1 regions are involved in the acquisition and expression of morphine-induced place preference. Eur Neuropsychopharmacol. 2007;17:24–31. doi: 10.1016/j.euroneuro.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Massoudi R, Sepehri H, Rezayof A. Involvement of GABA(B) receptors of the dorsal hippocampus on the acquisition and expression of morphine-induced place preference in rats. Physiol Behav. 2006;87:31–8. doi: 10.1016/j.physbeh.2005.08.041. [DOI] [PubMed] [Google Scholar]
- McEown K, Treit D. The role of the dorsal and ventral hippocampus in fear and memory of a shock-probe experience. Brain Res. 2009;1251:185–94. doi: 10.1016/j.brainres.2008.11.041. [DOI] [PubMed] [Google Scholar]
- Meyers RA, Zavala AR, Neisewander JL. Dorsal, but not ventral, hippocampal lesions disrupt cocaine place conditioning. Neuroreport. 2003;14:2127–31. doi: 10.1097/00001756-200311140-00023. [DOI] [PubMed] [Google Scholar]
- Pothuizen HH, Zhang WN, Jongen-Relo AL, Feldon J, Yee BK. Dissociation of function between the dorsal and the ventral hippocampus in spatial learning abilities of the rat: a within-subject, within-task comparison of reference and working spatial memory. Eur J Neurosci. 2004;19:705–12. doi: 10.1111/j.0953-816x.2004.03170.x. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–9. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation — a decade of progress. Science. 1999;285:1870, 4. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Riedel G, Platt B, Micheau J. Glutamate receptor function in learning and memory. Behav Brain Res. 2003;140:1–47. doi: 10.1016/s0166-4328(02)00272-3. [DOI] [PubMed] [Google Scholar]
- Watt AJ, Sjostrom PJ, Hausser M, Nelson SB, Turrigiano GG. A proportional but slower NMDA potentiation follows AMPA potentiation in LTP. Nat Neurosci. 2004;7:518–24. doi: 10.1038/nn1220. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Malenka RC. Expression mechanisms underlying NMDA receptor-dependent long-term potentiation. Ann N Y Acad Sci. 1999;868:515–25. doi: 10.1111/j.1749-6632.1999.tb11320.x. [DOI] [PubMed] [Google Scholar]
- MacDonald JF, Jackson MF, Beazely MA. Hippocampal long-term synaptic plasticity and signal amplification of NMDA receptors. Crit Rev Neurobiol. 2006;18:71–84. doi: 10.1615/critrevneurobiol.v18.i1-2.80. [DOI] [PubMed] [Google Scholar]
- Maletic-Savatic M, Malinow R, Svoboda K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science. 1999;283:1923–7. doi: 10.1126/science.283.5409.1923. [DOI] [PubMed] [Google Scholar]
- Matus A. Actin-based plasticity in dendritic spines. Science. 2000;290:754–8. doi: 10.1126/science.290.5492.754. [DOI] [PubMed] [Google Scholar]
- Lamprecht R, LeDoux J. Structural plasticity and memory. Nat Rev Neurosci. 2004;5:45–54. doi: 10.1038/nrn1301. [DOI] [PubMed] [Google Scholar]
- Bast T, Zhang WN, Feldon J. Dorsal hippocampus and classical fear conditioning to tone and context in rats: effects of local NMDA-receptor blockade and stimulation. Hippocampus. 2003;13:657–75. doi: 10.1002/hipo.10115. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Loya F, Ma QD, Fanselow MS. Dorsal hippocampus NMDA receptors differentially mediate trace and contextual fear conditioning. Hippocampus. 2005;15:665–74. doi: 10.1002/hipo.20088. [DOI] [PubMed] [Google Scholar]
- Burman MA, Gewirtz JC. Hippocampal activity, but not plasticity, is required for early consolidation of fear conditioning with a short trace interval. Eur J Neurosci. 2007;25:2483–90. doi: 10.1111/j.1460-9568.2007.05493.x. [DOI] [PubMed] [Google Scholar]
- Levenson J, Weeber E, Selcher JC, Kategaya LS, Sweatt JD, Eskin A. Long-term potentiation and contextual fear conditioning increase neuronal glutamate uptake. Nat Neurosci. 2002;5:155–61. doi: 10.1038/nn791. [DOI] [PubMed] [Google Scholar]
- Maren S. Synaptic mechanisms of associative memory in the amygdala. Neuron. 2005;47:783–6. doi: 10.1016/j.neuron.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Lashgari R, Rezayof A, Motamedi F, Nazari-Serenjeh F. NMDA receptors of dorsal hippocampus are involved in the acquisition, but not in the expression of morphine-induced place preference. Eur J Pharmacol. 2007;568:192–8. doi: 10.1016/j.ejphar.2007.04.015. [DOI] [PubMed] [Google Scholar]