CASE SUMMARY
History
A 6-year-old African American boy with X-linked chronic granulomatous disease (CGD) presented with a two week history of progressive, erythematous facial plaques.
The patient had taken oral voriconazole for 5 months for a fungal pneumonia. Two months prior to evaluation, the voriconazole dose was increased from 7 mg/kg to 9 mg/kg twice daily. The patient’s mother deferred a skin biopsy, and fluocinolone 0.025% ointment twice a day was prescribed along with strict sun protection measures. Eventually, the lesions resolved after voriconazole was discontinued. The patient returned 7 months later with a similar facial eruption that recurred after voriconzole was reinitiated at 9.3 mg/kg twice daily. The skin lesions progressed despite treatment with fluocinolone 0.025% ointment. His other medications were micafungin 45 mg intravenous daily and trimethoprim-sulfamethoxazole (TMP-SMX) 300 mg/60 mg by mouth twice a day.
Physical Examination
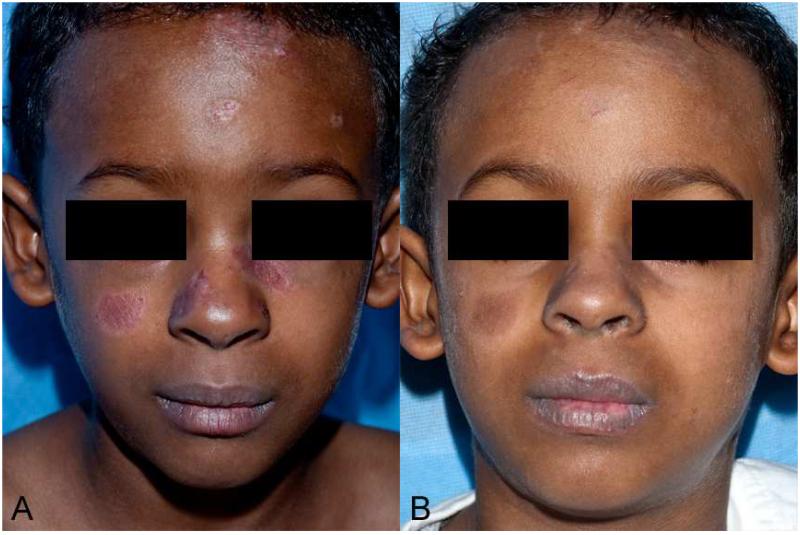
The patient was a well-developed boy with Fitzpatrick skin phototype V. Multiple erythematous oval plaques with irregular, elevated scaly pink borders were present on the forehead, central cheeks, and nose, most with violaceous centers (Fig 1A).
Fig 1. Discoid lupus erythematosus-like lesions in a patient with CGD following treatment with voriconazole.
A, Multiple erythematous to violaceous plaques with irregular, elevated scaly pink borders on the forehead, central cheeks, and nose. B, Resolution of lesions with post-inflammatory pigment alteration 4 weeks after voriconazole was discontinued.
Histopathology
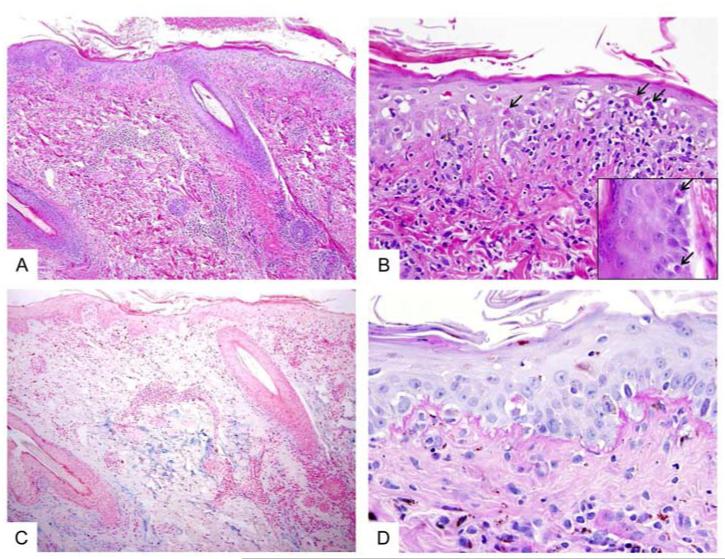
A punch biopsy of lesional skin revealed a superficial and deep perivascular lymphocytic infiltrate (Fig 2A). There was hyperparakeratosis, basal vacuolar changes, and scattered necrotic keratinocytes in the epidermis and along the basal layers of follicular infundibulum (Fig 2B). Alcian blue stain demonstrated abundant mucin in the reticular dermis (Fig 2C). PAS-D stain highlighted a thickened epidermal basement membrane (Fig 2D).
Fig 2. Histopathology of lupus-like lesions.
A, Skin biopsy shows a mild perivascular chronic inflammatory infiltrate in the superficial and deep dermis. The overlying epidermis shows hyperparakeratosis and interface vacuolar changes. (H&E stain, original magnification = 100x). B, Higher magnification highlights frequent necrotic/dyskeratotic keratinocytes (arrows) in the epidermis and along the infundibular portions of hair follicles (inset). (H&E stain, original magnification = 400x; inset = 600x). C, An Alcian Blue stained section shows abundant mucin deposition in the reticular dermis. (Alcian blue stain, original magnification = 100x). D, PAS-D stained section shows irregularly thickened basement membrane along the dermal-epidermal junction. (PAS-D stain, original magnification = 600x)
Significant Diagnostic Studies
A potassium hydroxide preparation of skin scrapings was negative for fungal elements. Laboratory studies revealed a positive antinuclear antibody (ANA) of 2.3 EU (reference range 0-0.9 EU). Anti-dsDNA was negative.
Diagnosis
Voriconazole-induced discoid lupus erythematosus (DLE)-like lesions in the setting of chronic granulomatous disease.
FOLLOW-UP
Voriconazole was discontinued a second time, and posaconazole was initiated for antifungal coverage. After one month, the lesions had significantly improved (Fig 1B). Interestingly, the patient’s mother, a carrier of X-linked CGD, had a chronic palmar rash that was subsequently biopsied demonstrating lupus-like histology. Her ANA, anti-ENA, and anti-histone antibodies were negative.
DISCUSSION
CGD is an immunodeficiency resulting from a defect in the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex responsible for generating reactive oxygen species after pathogen phagocytosis. Impaired phagocytosis results in increased susceptibility to catalase-positive bacteria and fungi.1
Voriconazole is a second-generation triazole commonly used for antifungal prophylaxis in CGD patients and is indicated for invasive aspergillosis and other serious fungal or Candida infections.2 It is fungicidal by inhibiting 13-α-sterol demethylase.2,3
Adverse effects of voriconazole include visual disturbances (21%), transaminase elevation (12.4%), and skin rash (7%), including photosensitivity.3 In initial clinical trials, photosensitivity was reported in 1-2% of patients on long-term voriconazole. Subsequent reports have described numerous photosensitive reactions including pseudoporphyria,4 photoaging,5 facial erythema, cheilitis,6-9 DLE-like lesions,7,10-11 and multiple lentigines.5,12-14 In addition, actinic keratoses, squamous cell carcinomas, 12,15 and melanoma in situ13 have been reported in patients on long-term voriconazole therapy. Product labeling now recommends discontinuing voriconazole if skin cancer develops.3
The mechanism of voriconazole-induced photosensitivity is unclear. Although the drug itself does not absorb UVA or UVB radiation, it has been postulated that the drug’s principal metabolite, N-oxide, could be responsible for its phototoxic effects. However, in vitro absorption spectra of N-oxide voriconazole suggest absorption in the UVB range,22 whereas broadband, predominantly UVA-mediated, photosensitivity is associated with voriconazole in vivo.6
Voriconazole-induced photosensitivity is well documented in the CGD population.9-11,13,15-17,24 However, TMP-SMX, another photosensitizing drug, is also frequently used in CGD patients. It has been hypothesized that combining voriconazole and TMP-SMX may exacerbate photosensitivity.17 Voriconazole is both a substrate and inhibitor of the P450 enzymes CYP2C19, CYP2C9, and CYP3A4. 2,3,8 As sulfamethoxazole inhibits CYP2C9 in vitro,25 combining voriconazole with TMP-SMX could lead to elevated voriconazole levels and thus exacerbate photosensitity.17 However, conflicting data exist regarding plasma voriconazole levels and photosensitivity reactions in children,18-20 and specific toxic levels of voriconazole remain undefined. Further investigation is needed to determine the relationship between voriconazole metabolism and photosensitivity risk.
Lupus-like skin lesions have been reported in carriers of CGD and, less commonly, in patients with CGD.10,11,26-36 ANA serologies in these patients are usually negative.26-34 The pathogenesis of the lupus-like lesions is not fully understood. Decreased superoxide production in monocytes and neutrophils in CGD carriers correlates with the development of lupus-like skin lesions.34 The abnormal respiratory burst results in delayed clearance of certain bacteria, fungi, and damaged cells. It has been hypothesized that impaired clearance of apoptotic cells could lead to chronic inflammation,35,36 and these findings may be more pronounced in sun-exposed skin.36 Alternatively, repeated antigen stimulation by nonphagocytosed organisms may theoretically lead to overproduction of autoantibodies. However, the frequently negative autoimmune serologies oppose this theory.27,28
Two prior cases of lupus-like eruptions associated with voriconazole have been reported in CGD patients.10,11 A 10-month-old Caucasian child with CGD developed lupus-like lesions two months after starting voriconazole. The lesions resolved after discontinuing voriconazole.10 A 28 month-old boy with CGD also developed lupus-like lesions one year after initiating TMP-SMX and voriconazole.11 Our case confirms the association of lupus-like eruption and voriconazole treatment in patients with CGD by demonstrating lesion recurrence after drug rechallenge. In addition, together with prior reports,14 this case illustrates that dark-skinned individuals are also susceptible to photosensitive reactions during voriconazole treatment.
It is unclear if voriconazole directly induces a lupus-like reaction in CGD patients or if the phototoxic effect of the drug unmasks an underlying predisposition to lupus-like lesions. Of note, one case of voriconazole-associated DLE-like lesions has been reported in an immunocompetent patient.7 Although CGD patients are at higher risk of developing DLE-like lesions, we believe the use of voriconazole in this setting may further increase the risk. Additional studies are needed to better define the association of lupus-like reactions in patients on voriconazole, including patients with CGD.
KEY TEACHING POINTS.
-
-
We report a case of a 6-year-old African American boy with X-linked chronic granulomatous disease (CGD) who developed discoid lupus erythematosus-like skin lesions after starting voriconazole.
-
-
Voriconazole is commonly used to treat fungal infections in patients with CGD.
-
-
Lupus erythematosus-like skin lesions have been reported in carriers of X-linked CGD and, less commonly, in patients with CGD.
-
-
Voriconazole is a significant cause of drug-induced photosensitivity, and may play a role in unmasking an underlying predisposition to lupus-like skin lesions.
-
-
Photoprotective measures and routine exams to monitor for skin toxicity, including skin cancer, are prudent during voriconazole treatment.
Acknowledgements
This publication was supported by the National Institutes of Health (NIH) Intramural Research Program, Center of Cancer Research, National Cancer Institute. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Funding Sources: This research was supported by the Intramural Program of NIH, Center for Cancer Research, National Cancer Institute
ABBREVIATIONS
- CGD
Chronic granulomatous disease
- TMP-SMX
Trimethoprim-sulfamethoxazole
- ANA
Antinuclear antibody
- DLE
Discoid lupus erythematosus
Footnotes
Conflicts of Interest Disclosure: None declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Holland SM. Chronic granulomatous disease. Clin Rev Allergy Immunol. 2010;38(1):3–10. doi: 10.1007/s12016-009-8136-z. [DOI] [PubMed] [Google Scholar]
- 2.Theuretzbacher U, Ihle F, Derendorf H. Pharmacokinetic/pharmacodynamic profile of voriconazole. Clin Pharmacokinet. 2006;45(7):649–63. doi: 10.2165/00003088-200645070-00002. [DOI] [PubMed] [Google Scholar]
- 3.Voriconazole (Vfend) product labeling information. Pfizer; New York, NY: 2010. [Google Scholar]
- 4.Tolland JP, McKeown PP, Corbett JR. Voriconazole-induced pseudoporphyria. Photodermatol Photoimmunol Photomed. 2007 Feb;23(1):29–31. doi: 10.1111/j.1600-0781.2007.00263.x. [DOI] [PubMed] [Google Scholar]
- 5.Racette AJ, Roenigk HH, Jr, Hansen R, et al. Photoaging and phototoxicity from long-term voriconazole treatment in a 15-year-old girl. J Am Acad Dermatol. 2005;52(5 Suppl. 1):S81–S85. doi: 10.1016/j.jaad.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 6.Haylett AK, Felton S, Denning DW, Rhodes LE. Voriconazole-induced photosensitivity: photobiological assessment of a case series of 12 patients. Br J Dermatol. 2012 Jan;168(1):179–85. doi: 10.1111/j.1365-2133.2012.11196.x. [DOI] [PubMed] [Google Scholar]
- 7.Denning DW, Griffiths CE. Mucocutaneous retinoid-effects and facial erythema related to the novel triazole antifungal agent voriconazole. Clin Exp Dermatol. 2001;26(8):648–653. doi: 10.1046/j.1365-2230.2001.00909.x. [DOI] [PubMed] [Google Scholar]
- 8.Epaulard O, Leccia MT, Blanche S, Chosidow O, Mamzer-Bruneel MF, Ravaud P, Thiebaut A, Villier C, Lortholary O. Phototoxicity and photocarcinogenesis associated with voriconazole. Med Mal Infect. 2011 Dec;41(12):639–45. doi: 10.1016/j.medmal.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 9.Rubenstein M, Levy ML, Metry D. Voriconazole-induced retinoid-like photosensitivity in children. Pediatr Dermatol. 2004;21(6):675–678. doi: 10.1111/j.0736-8046.2004.21614.x. [DOI] [PubMed] [Google Scholar]
- 10.Gomez-Moyano E, Vera-Casaño A, Moreno-Perez D, Sanz-Trelles A, Crespo-Erchiga V. Lupus erythematosus-like lesions by voriconazole in an infant with chronic granulomatous disease. Pediatr Dermatol. 2010;27(1):105. doi: 10.1111/j.1525-1470.2009.01058.x. [DOI] [PubMed] [Google Scholar]
- 11.Geller L, Raciti PM, Mercer SE, Phelps RG. Lupus-like lesions in a 28-month-old boy with chronic granulomatous disease on long-term voriconazole prophylaxis. J Cutan Pathol. 2011 Aug;38(8):677–8. doi: 10.1111/j.1600-0560.2011.01714.x. [DOI] [PubMed] [Google Scholar]
- 12.Cowen EW, Nguyen JC, Miller DD, et al. Chronic phototoxicity and aggressive squamous cell carcinoma of the skin in children and adults during treatment with voriconazole. J Am Acad Dermatol. 2010;62(1):31–37. doi: 10.1016/j.jaad.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller DD, Cowen EW, Nguyen JC, McCalmont TH, Fox LP. Melanoma associated with long-term voriconazole therapy: a new manifestation of chronic photosensitivity. Arch Dermatol. 2010;146(3):300–304. doi: 10.1001/archdermatol.2009.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elbaum DJ, Cowen EW. Voriconazole-associated phototoxic effects and lentigo formation in an African American man. Arch Dermatol. 2012;148(8):965–6. doi: 10.1001/archdermatol.2012.705. [DOI] [PubMed] [Google Scholar]
- 15.McCarthy KL, Playford EG, Looke DF, Whitby M. Severe photosensitivity causing multifocal squamous cell carcinomas secondary to prolonged voriconazole therapy. Clin Infect Dis. 2007;44(5):55–6. doi: 10.1086/511685. [DOI] [PubMed] [Google Scholar]
- 16.Frick MA, Soler-Palacín P, Martín Nalda A, Guarner ME, Nadal CF. Photosensitivity in immunocompromised patients receiving long-term therapy with oral voriconazole. Pediatr Infect Dis J. 2010 May;29(5):480–1. doi: 10.1097/INF.0b013e3181d60a82. [DOI] [PubMed] [Google Scholar]
- 17.Frisch S, Askari SK, Beaty SR, Burkemper CN. X-linked chronic granulomatous disease with voriconazole-induced photosensitivity/ photoaging reaction. J Drugs Dermatol. 2010;9(5):562–4. [PubMed] [Google Scholar]
- 18.Soler-Palacín P, Frick MA, Martín-Nalda A, Lanaspa M, Pou L, Roselló E, de Heredia CD, Figueras C. Voriconazole drug monitoring in the management of invasive fungal infection in immunocompromised children: a prospective study. J Antimicrob Chemother. 2012 Mar;67(3):700–6. doi: 10.1093/jac/dkr517. [DOI] [PubMed] [Google Scholar]
- 19.Hansford JR, Cole C, Blyth CC, Gottardo NG. Idiosyncratic nature of voriconazole photosensitivity in children undergoing cancer therapy. J Antimicrob Chemother. 2012 Jul;67(7):1807–9. doi: 10.1093/jac/dks105. [DOI] [PubMed] [Google Scholar]
- 20.Rondeau S, Couderc L, Dominique S, Pramil S, Leguillon C, Masseline B, Favennec L, Marguet C. High frequency of voriconazole-related phototoxicity in cystic fibrosis patients. Eur Respir J. 2012 Mar;39(3):782–4. doi: 10.1183/09031936.00097611. [DOI] [PubMed] [Google Scholar]
- 21.Bernhard S, Kernland Lang K, Ammann RA, Lüer S, Leibundgut K, Diepold M, Aebi C. Voriconazole-induced phototoxicity in children. Pediatr Infect Dis J. 2012 Jul;31(7):769–71. doi: 10.1097/INF.0b013e3182566311. [DOI] [PubMed] [Google Scholar]
- 22.Murayama N, Imai N, Nakane T, Shimizu M, Yamazaki H. Roles of CYP3A4 and CYP2C19 in methyl hydroxylated and N-oxidized metabolite formation from voriconazole, a new anti-fungal agent, in human liver microsomes. Biochem Pharmacol. 2007;73(12):2020–2026. doi: 10.1016/j.bcp.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Stern RS. The risk of squamous cell and basal cell cancer associated with psoralen and ultraviolet A therapy: a 30-year prospective study. J Am Acad DermatolI. 2012;66:553–62. doi: 10.1016/j.jaad.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Vandecasteele SJ, van Wijngaerden E, Peetermans WE. Two cases of severe phototoxic reactions related to longterm outpatient treatment with voriconazole. Eur J Clin Microbiol. 2004;23:656–657. doi: 10.1007/s10096-004-1176-7. [DOI] [PubMed] [Google Scholar]
- 25.Wen X, Wang JS, Backman JT, Laitila J, Neuvonen PJ. Trimethoprim and sulfamethoxazole are selective inhibitors of CYP2C8 and CYP2C9, respectively. Drug Metab Dispos. 2002 Jun;30(6):631–5. doi: 10.1124/dmd.30.6.631. [DOI] [PubMed] [Google Scholar]
- 26.Cale CM, Morton L, Goldblatt D. Cutaneous and other lupus-like symptoms in carriers of X-linked chronic granulomatous disease: incidence and autoimmune serology. Clin Exp Immunol. 2007;148(1):79. doi: 10.1111/j.1365-2249.2007.03321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Córdoba-Guijarro S, Feal C, Daudén E, Fraga J, García-Díez A. Lupus erythematosus-like lesions in a carrier of X-linked chronic granulomatous disease. J Eur Acad Dermatol Venereol. 2000;14(5):409–411. doi: 10.1046/j.1468-3083.2000.00113.x. [DOI] [PubMed] [Google Scholar]
- 28.Hafner J, Enderlin A, Seger RA, Wüthrich B, Bruckner-Tudermann L, Panizzoni P, Burg G. Discoid lupus erythematosus-like lesions in carriers of X-linked chronic granulomatous disease. Br J Dermatol. 1992 Oct;127(4):446–7. doi: 10.1111/j.1365-2133.1992.tb00471.x. [DOI] [PubMed] [Google Scholar]
- 29.Ortiz-Romero PL, Corell-Almuzara A, López-Estebaranz J. Lupus-like lesions in a patient with X-linked chronic granulomatous disease and recombinant X chromosome. Dermatology. 1997;195:280–283. doi: 10.1159/000245963. [DOI] [PubMed] [Google Scholar]
- 30.Brandrup F, Koch C, Petri M, Schiødt M, Johansen KS. Discoid lupus erythematosus-like lesions and stomatitis in female carriers of X-linked chronic granulomatous disease. Br J Dermatol. 1981 May;104(5):495–505. doi: 10.1111/j.1365-2133.1981.tb08163.x. [DOI] [PubMed] [Google Scholar]
- 31.Sillevis JH, Weening RS, Krieg SR, Bos JD. Discoid lupus erythematosus-like lesions in carriers of X-linked chronic granulomatous disease. Br J Dermatol. 1990;122:643–650. doi: 10.1111/j.1365-2133.1990.tb07286.x. [DOI] [PubMed] [Google Scholar]
- 32.Manzi S, Urbach A, Mc Cune AB, et al. Systemic lupus erythematosus in a boy with chronic granulomatous disease: case report and review of the literature. Arthritis Rheum. 1991;34:101–105. doi: 10.1002/art.1780340116. [DOI] [PubMed] [Google Scholar]
- 33.Foti C, Cassano N, Martire B, Filotico R, Mastrandrea V, Vena GA. Lupus erythematosus-like lesions in a carrier of X-linked chronic granulomatous disease: a case report and personal considerations. Int J Dermatol. 2004;43(1):840–2. doi: 10.1111/j.1365-4632.2004.01950.x. [DOI] [PubMed] [Google Scholar]
- 34.Kragballe K, Borregaard N, Brandrup F, Koch C, Staehrjohansen K. Relation of monocyte and neutrophil oxidative metabolism to skin and oral lesions in carriers of chronic granulomatous disease. Clin Exp Immunol. 1981 Feb;43(2):390–8. [PMC free article] [PubMed] [Google Scholar]
- 35.De Ravin SS, Naumann N, Cowen EW, Friend J, Hilligoss D, Marquesen M, Balow JE, Barron KS, Turner ML, Gallin JI, Malech HL. Chronic granulomatous disease as a risk factor for autoimmune disease. J Allergy Clin Immunol. 2008;122(6):1097–103. doi: 10.1016/j.jaci.2008.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanford AN, Suriano AR, Herche D, Dietzmann K, Sullivan KE. Abnormal apoptosis in chronic granulomatous disease and autoantibody production characteristic of lupus. Rheumatology. 2006;45:178–81. doi: 10.1093/rheumatology/kei135. [DOI] [PubMed] [Google Scholar]