Abstract
Objective
To determine how eye closure, test positions, and stimulus frequencies influence ocular vestibular evoked myogenic potentials.
Study Design
This study used a within-subjects repeated measures design.
Methods
Twenty asymptomatic subjects were each tested on ocular vestibular evoked myogenic potentials in three head/eye conditions at 500 Hz and 1000 Hz using air-conducted sound: 1) Sitting upright, head erect, eyes open, looking up. 2) Lying supine, neck flexed 30 degrees, eyes open and looking up. 3) Lying supine, neck flexed 30 degrees, eyes closed and relaxed. Four dependent variables measured were n10, p16, amplitude, and threshold.
Results
The supine position/ eyes open was comparable to sitting/ eyes open and better than supine/ eyes closed. Eyes closed resulted in lower amplitude, higher threshold, and prolonged latency. Significantly fewer subjects provided responses with eyes closed than with eyes open. No significant differences were found between both eyes open conditions. Both n10 and p16 were lower at 1000 Hz than at 500 Hz. Amplitude and threshold were higher at 1000 Hz than at 500 Hz.
Conclusion
Supine eyes open is a reliable alternative to sitting eyes open in patients who cannot maintain a seated position. Testing at 1000 Hz provides a larger response with a faster onset that fatigues faster than at 500 Hz. The increased variability and decreased response in the eyes closed position suggest that the eyes closed position is not reliable.
Keywords: diagnostic testing, stimulus frequency, labyrinth, VEMP
Introduction
Irregular otolith neurons, present in saccular and utricular maculae, respond to changes in linear acceleration.1 At low intensities, both air conducted sound (ACS) and bone conducted vibration (BCV) selectively activate irregular otolith neurons.2 This finding is the basis for the clinical use of ACS and BCV in assessing otolith function in the utricle and saccule. Either ACS or BCV can be used to assess utricular function by measuring the contralateral vestibulo-ocular reflex (VOR).3 The ocular vestibular evoked myogenic potential (oVEMP) test measures the VOR and can be used clinically as a test of utricular function.4
The oVEMP response is generated by synchronous activity in extraocular muscles. The direction of gaze during recording affects the response.3 Amplitude of the response increases with upward gaze due to the tonic contraction of the inferior oblique muscle.5 Therefore, the optimal method for oVEMP testing is in the seated position with an upward gaze.6 This position, however, is fatiguing and difficult for some patients to maintain. Therefore, the purpose of this study was to determine if other test positions can be used to obtain reliable responses.
Chihara et al.6 reported a decreased response rate associated with oVEMP responses recorded with eyes closed. Recently, Huang et al.7 recorded oVEMP responses to BCV in young subjects, age 21–30, tested in a seated position with eyes closed. The responses obtained were characterized by blunted waveforms and prolonged latencies.7 Therefore, a second goal of this study was to determine if oVEMP tests performed with eyes closed generate reliable responses.
The oVEMP response is best evoked at frequencies between 400 Hz – 1000 Hz.8 The optimal frequency within the 400–1000 Hz range is controversial, however. 9,10, Therefore, a third goal of this study was to examine the influence of test frequency on the response.
Methods
Subjects
Twenty asymptomatic adult subjects with no complaints of vertigo or neurologic disorders participated in this study (11 females, 9 males; mean age = 36.9, standard deviation of age = 13.2, age range = 22–59). Subjects were screened with otoscopy prior to participation. The study was approved by the Institutional Review Board for Human Subjects Research for Baylor College of Medicine and Affiliated Hospitals. All subjects gave written informed consent before participating.
Experimental Design
Subjects were tested on oVEMP using ACS. Both the right and left ears were tested in three head/eye conditions at two different stimulus frequencies, 500 Hz and 1000 Hz. Every subject was tested in each head/eye condition and each frequency for a total of 6 trials per subject. The conditions tested were: 1) Sitting upright, head erect, eyes open, looking up (sit EO). 2) Lying supine, neck flexed 30 degrees, eyes open and looking up (supine EO). 3) Lying supine, neck flexed 30 degrees, eyes closed and relaxed (EC). Subjects were tested with the neck flexed 30 degrees because this is the position used by clinicians. For all trials, subjects wore headphones through which they received monaural tone bursts delivered at 7 Hz with 100 sweeps per trial. Responses to tone bursts were measured and recorded in the contralateral extraocular muscles. At 500 Hz, subjects received tone bursts starting at 105 dB nHL. The intensity was decreased in 5 dB increments over the subsequent trials until no repeatable ocular response was seen. At 1000 Hz, subjects received tone bursts starting at 115 dB nHL. The intensity was decreased in 5 or 10 dB increments until no repeatable ocular response was seen.
Stimulus and Recording Parameters
Gold cup electrodes were used for recording. Active electrodes centered horizontally at the orbital midline were placed 1 cm inferior to each lower eyelid at the superior edge of the infraorbital margin. Two reference electrodes were placed approximately 1.5 cm inferior to each active electrode. One ground electrode was placed at the hairline at the midline forehead. Facial skin was cleaned prior to electrode placement to minimize impedance. Surface potentials were recorded using the Natus Viking EDX desktop-based system.
Responses were present when a repeatable negative-positive biphasic waveform resembling the standard oVEMP response was seen.11 For each of the trials, the dependent measures were the n10 latency to onset (ms) – the first negative component of the waveform occurring at approximately 10 ms after stimulus onset, p16 (ms) – the positive component which follows at approximately 16 ms after stimulus onset, maximum amplitude (µV) – the voltage difference between the voltages of n10 and p16 at the maximum intensity tested, and the threshold of the ocular response (dB) – the lowest intensity at which a repeatable response was observed. A repeatable response was present when the n10 and p16 changed ≤ 2 ms between trials.
Statistical Analysis
Changes in dependent measures of interest over various conditions of eye (open or closed), head (lying down or sitting up) and frequency (500 or 1000 Hz) was assessed using mixed, multilevel statistical techniques (Proc Glimmix in SAS). A separate model was fitted to each dependent variable. Comparisons were made between males and females. Within each model, within and between (by gender) subjects effects were tested. Interaction effects were included in each model and tested. At each condition, and for each outcome variable, paired t-test was used to compare results between left and right ear. We compared the frequency of patients who could provide a response or not provide a response, in EC vs EO conditions, using McNemar’s test. P<0.05 was considered significant. All analyses were performed in SAS statistical software.
Results
Within each subject responses from the left and right ears did not differ significantly. Few significant differences between males and females were found: on the right side – the n10 latencies in EC at 500 Hz (p = 0.045) and 1000 Hz (p = 0.02) were longer in females, and amplitude in sit EO at 1000 Hz was higher in males (p = 0.003).
Latency (n10 and p16)
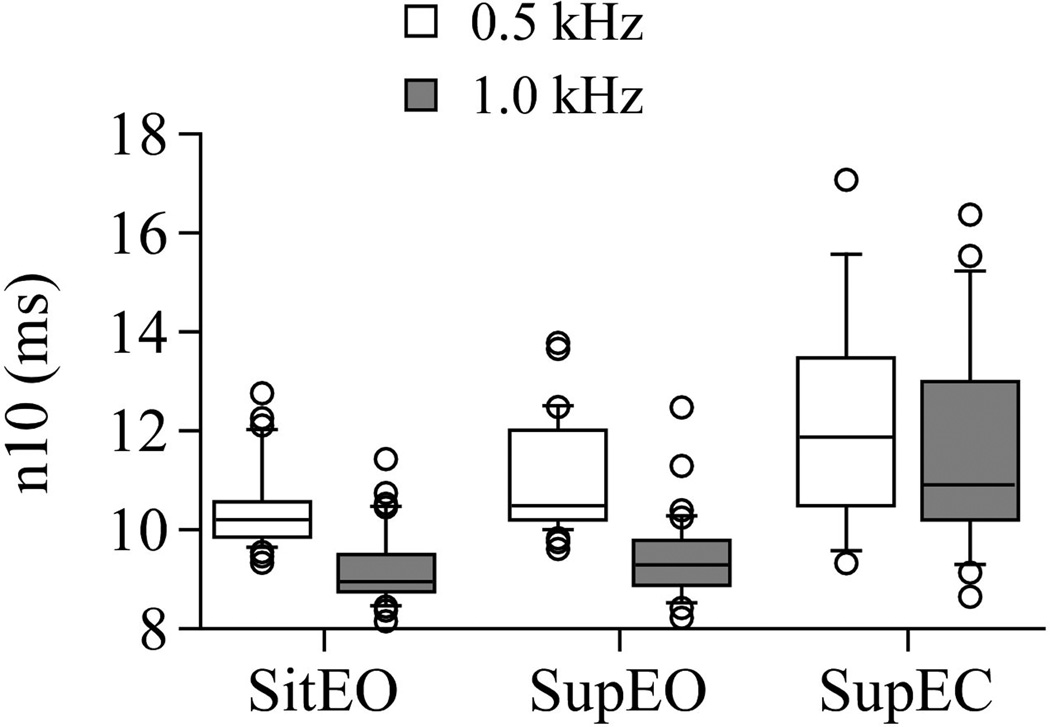
The n10 latencies were not significantly different between both EO conditions (Sit EO 500 vs. Supine EO 500 right p =0.26. Sit EO 1000 Hz vs. Supine EO1000 Hz left p = 0.62, right p = 0.5). In both EO conditions, n10 was significantly shorter at 1000 Hz than at 500 Hz (Sit EO: left p=0.01; right p = 0.0008; Supine EO: left p = 0.0001; right p < 0.0001). The n10 latencies were significantly shorter in sit EO than in supine EC (500 Hz left p = 0.0001, right p = 0.02; 1000 Hz left p < 0.0001, right p < 0.001). On the left, n10 latencies were significantly shorter in supine EO than in supine EC (500 Hz p = 0.0097; 1000 Hz p<0.0001). On the right, the difference between n10 of supine EO and EC was significant at 1000 Hz (p = 0.001). In EC, the finding of shorter n10 latencies at 1000 Hz compared to 500 Hz was not significant (left p = 0.08, right p = 0.09). See Figure 1.
Figure 1.
Plots showing n10 in each test position and frequency: Sitting eyes open (sit EO), Supine eyes open (Sup EO), Supine eyes closed (Sup EC). Center horizontal bars are medians, rectangle ends are interquartile ranges, error bars are 10th and 90th deciles, and circles are outliers. The latency of oVEMP onset is increased in supine EC compared to both EO conditions and the n10 values are more variable in supine EC than in both EO conditions. Latency is decreased at 1000 Hz when compared to 500 Hz.
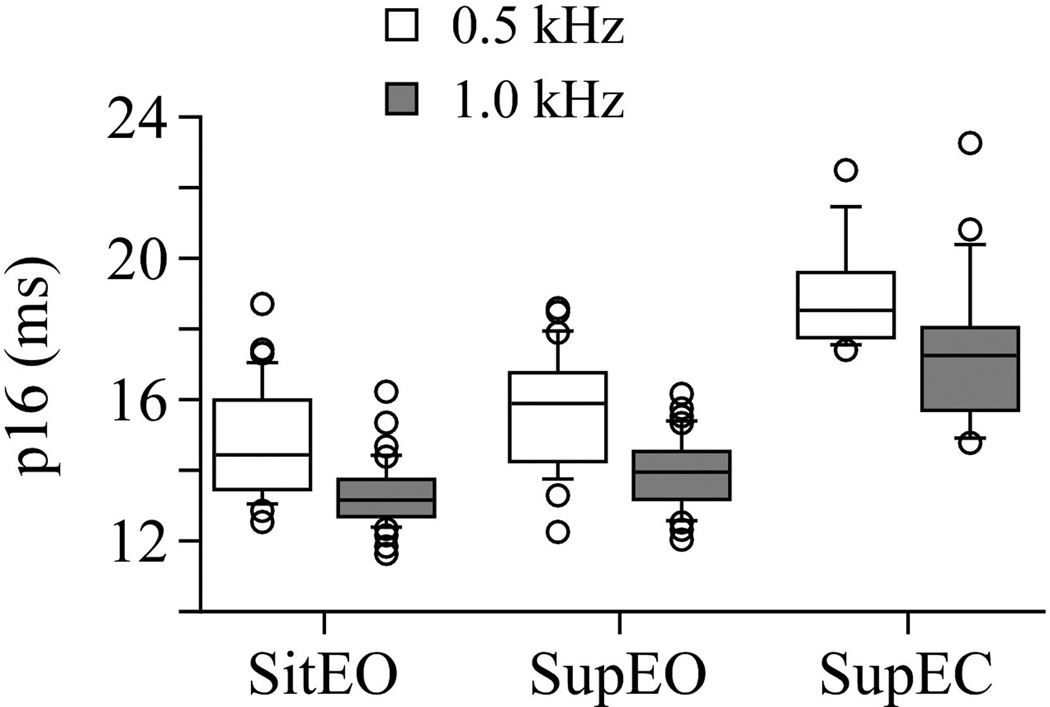
Significant differences were not present in p16 between either EO condition (sit EO 500 vs. supine EO 500 at left and at right, p=0.24; sit EO 1000 vs. supine EO 1000 left, p = 0.138, right p = 0.18). In both EO conditions, p16 was significantly shorter at 1000 Hz compared to 500 Hz (Sit EO: left p = 0.0018; right p = 0.001; Supine EO: left p=0.0016; right p =0.0008). The p16 latencies were significantly shorter in sit EO than in supine EC (500 Hz left p = 0.002, right p = 0.0003; 1000 Hz left p < 0.0001, right p = 0.0002). The p16 latencies were significantly shorter in supine EO than in supine EC (500 Hz left p = 0.0001, right p = 0.01; 1000 Hz left p < 0.0001; right p < 0.0001). In EC, findings of shorter p16 at 1000 Hz were not significant (left p = 0.16, right p = 0.16). See Figure 2.
Figure 2.
Box and whisker plot showing p16 in each test position and frequency: Sitting eyes open (sit EO), Supine eyes open (Sup EO), Supine eyes closed (Sup EC). Center horizontal bars are medians, rectangle ends are interquartile ranges, error bars are 10th and 90th deciles, and circles are outliers. Latency is increased in supine EC compared to both EO conditions. The p16 values are more variable in supine EC than in the EO conditions. Latency is decreased at 1000 Hz compared to 500 Hz.
Amplitude
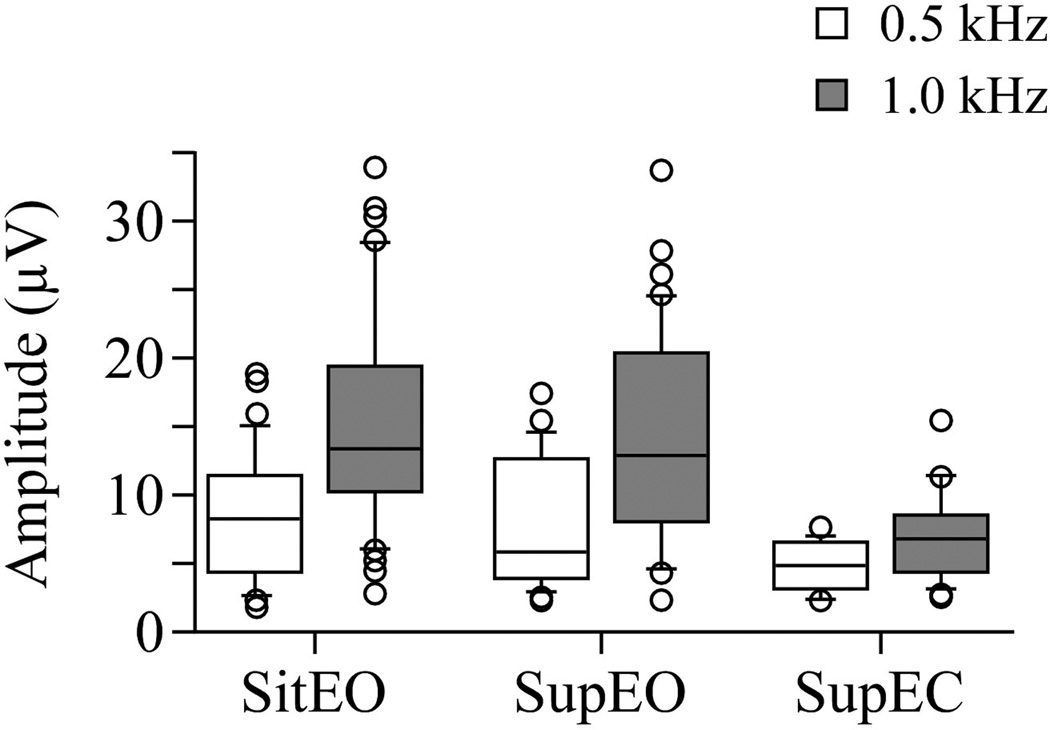
In both EO conditions, amplitude was significantly higher at 1000 Hz compared to 500 Hz (Sit EO: left p < 0.0001; right p < 0.0001; Supine EO: left p < 0.0001; right p < 0.0001). At 1000 Hz, amplitude was significantly higher in sit EO than in supine EC (left p < 0.0001; right p = 0.02). Amplitude was also significantly higher in the supine EO than in EC at 1000 Hz (left p < 0.0001; right p = 0.01). At 500 Hz, no significant differences between amplitude values of all three conditions were found (Sit EO vs. supine EO left p = 0.52, right p = 0.72. Supine EO vs. EC left p = 0.35, right p = 0.23. Sit EO vs. supine EC left p = 0.18, right p = 0.31). In supine EC, responses had significantly higher amplitude at 1000 Hz compared to 500 Hz on the right (p = 0.04), but not on the left (p = 0.67). See Figure 3.
Figure 3.
Box and whisker plot showing amplitude in each test position and frequency: Sitting eyes open (sit EO), Supine eyes open (Sup EO), Supine eyes closed (Sup EC). Center horizontal bars are medians, rectangle ends are interquartile ranges, error bars are 10th and 90th deciles, and circles are outliers. Amplitude is decreased in supine EC compared to both EO conditions. Amplitude is significantly increased at 1000 Hz compared to 500 Hz.
Threshold
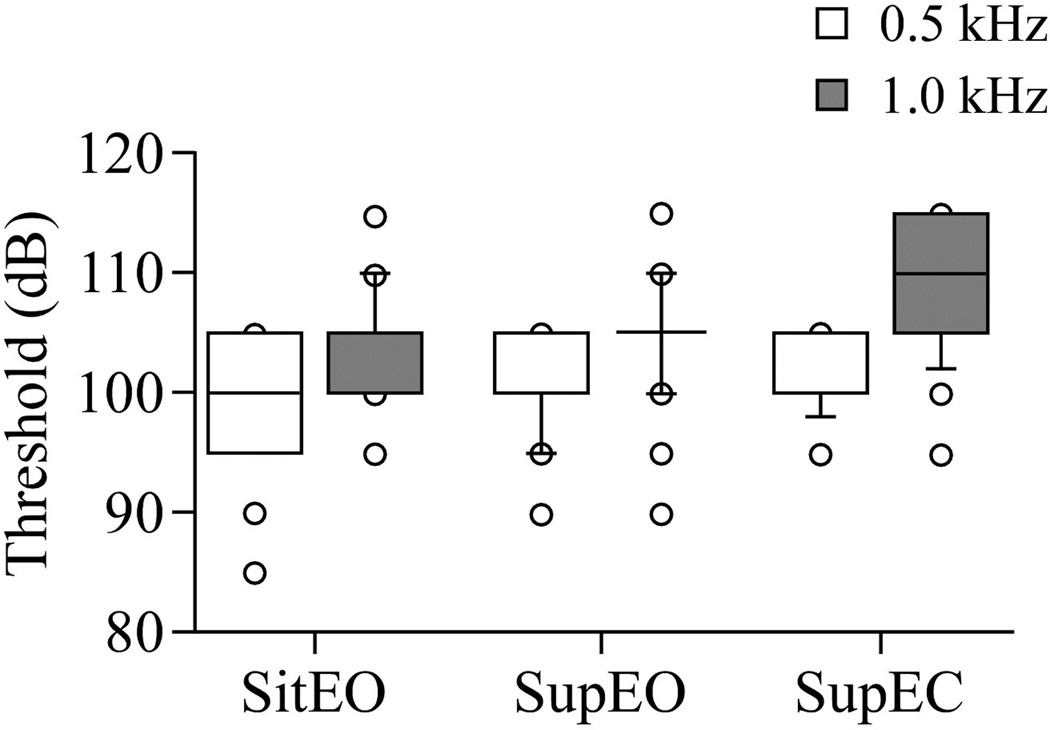
Threshold was significantly higher at 1000 Hz compared to 500 Hz in all three conditions (sit EO: left p < 0.0001; right p < 0.0001; supine EO: left p < 0.0001; right p = 0.01; EC: left p = 0.0001; right p = 0.02). At 1000 Hz, threshold was significantly lower in sit EO than in supine EC (left p < 0.0001; right p = 0.01). Threshold was also significantly lower in supine EO than in EC at 1000 Hz (left p < 0.0001; right p = 0.002). The left oVEMP showed a significant difference in threshold between sit EO and EC at 500 Hz (p = 0.01). The left oVEMP also contained a significant difference between supine EO and EC at 500 Hz (p = 0.03). The right oVEMP, however, showed no significant differences between threshold values in any of the three conditions at 500 Hz (Sit EO vs. supine EO p = 0.37. Supine EO vs. EC p = 0.39. Sit EO vs. supine EC p = 0.18). See Figure 4.
Figure 4.
Box and whisker plot showing threshold in each test position and frequency: Sitting eyes open (sit EO), Supine eyes open (Sup EO), Supine eyes closed (Sup EC). Center horizontal bars are medians, rectangle ends are interquartile ranges, error bars are 10th and 90th deciles, and circles are outliers. Threshold is higher in supine EC than in both EO conditions. Threshold is higher at 1000 Hz compared to 500 kHz indicating that the response fatigues faster at 1000 Hz than 500 Hz.
Sample Size
We analyzed the number of recordable responses obtained in each condition. Significantly fewer subjects provided recordable responses in EC (p < 0.0001): four subjects could not provide any oVEMP responses, eight subjects could provide a response on one side but not the other, and eight subjects provided responses on both the right and left sides. Eight of the subjects who provided responses in EC could provide responses at 1000 Hz but not at 500 Hz. The number of recordable responses in Supine EO and Sit EO did not differ significantly.
Discussion
Both EO conditions involve maintaining an upward gaze by rotating the eye away from its resting position. The lack of significant difference between sit EO and supine EO on all measures indicates that either condition can be used to obtain reliable responses in normal subjects. We reported results only from the contralateral eye. A previous study found a slight difference between the contralateral eyes but not the ipsilateral eyes in the two positions.12 Therefore, that issue remains unclear.
The increased variability and decreased responses obtained in supine EC suggest that supine EC is not a reliable test condition. These findings are consistent with the concept that oVEMP responses are strengthened by activation of the inferior oblique muscle.3 The inferior oblique muscle contributes to elevation and extorsion of the eye. Todd et al. 11 showed that ACS causes extorsion of the contralateral eye. The responses obtained with eyes closed in our study might have been due to extorsion of the eye caused by ACS.
Huang et al.7 recorded oVEMP responses with eyes closed in a seated position using BCV. The difference between their results and ours can be explained as follows. ACS stimulates the saccule while BCV stimulates both the utricle and saccule.11 BCV used in a seated position might be more conducive to obtaining responses with the eyes closed. Because we found no significant differences between sit and supine EO conditions, however, testing subjects in a seated EC position might not have a significant impact on the number of responses obtained with eyes closed.
Huang et al. 7 used ultrasonagraphic measurements to compare the muscles involved during eye closure to those involved during upward gaze. The results showed that oVEMP responses are mostly due to inferior oblique during upward gaze and that the inferior rectus and inferior oblique muscles both contribute to oVEMP responses with eyes closed. This finding may explain why amplitudes are greatly reduced in responses with eyes closed.
We instructed our subjects to relax the eyes during supine EC trials but eye movements during eye closure are variable. Some subjects may have naturally elevated their eyes during the EC trials. Collewijn et al.13 studied the movement of eyes during prolonged eyelid closure and reported observations of Bell’s phenomenon, a natural upward movement of the eye during eyelid closure, in half the subjects tested. Therefore, the variability among our subjects’ responses in supine EC could have been due to individual differences in eye movements during eyelid closure.
The finding that threshold was significantly higher at 1000 Hz than at 500 Hz in both EO conditions indicates that response strength varies based on frequency, and responses fatigue faster at 1000 Hz. The 1000 Hz stimulus, however, provided larger responses with a shorter latency than the 500 Hz stimulus. Therefore, for the general population, the 1000 Hz stimulus might provide a better response. This finding differs somewhat from earlier work suggesting that 500 Hz is the better frequency.14 Our larger sample may account for the difference. More recent work, however, suggests that 500 Hz may be optimal when the subject is tested at threshold.15 Thus the optimal relationship between frequency and intensity remains unknown.
Conclusion
Clinically, patients who are unable to maintain a seated position can be tested on oVEMP in a supine position while maintaining an upward gaze. The variability in EC as well as the finding that significantly fewer subjects could provide responses in EC indicate that it is not a reliable test position. Patients should not be tested using EC. The 1000 Hz stimulus should be favored in situations where a larger response with an earlier onset is preferred. In subjects with higher reflex thresholds, the 500 Hz stimulus is preferred.
Acknowledgements
We would thank the staff at the Center for Balance Disorders for their invaluable technical assistance.
Financial Support: Supported by NIH/NIDCD grant 1R01DC009031 to HSC. No financial interests in outside companies, etc.
Footnotes
Conflict of interest: None
Financial Disclosures:: None
References
- 1.Curthoys IS, Manzari L, Smulders YE, Burgess AM. A review of the scientific basis and practical application of a new test of utricular function - ocular vestibular - evoked myogenic potentials to bone-conducted vibration. Acta Otorhinolaryngol Ital. 2009;29:179–186. [PMC free article] [PubMed] [Google Scholar]
- 2.Curthoys IS, Vulovic V, Manzari L. Ocular vestibular-evoked myogenic potential (oVEMP) to test utricular function: neural and oculomotor evidence. Acta Otorhinolaryngol Ital. 2012;32:41–45. [PMC free article] [PubMed] [Google Scholar]
- 3.Rosengren SM, McAngus Todd NP, Colebatch JG. Vestibular-evoked potentials produced by stimulation with bone-conducted sound. Clin Neurophysiol. 2005;116:1938–1948. doi: 10.1016/j.clinph.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Iwasaki S, Chihara Y, Smulders YE, Burgess AM, Halmagyi GM, Curthoys IS, Murofushi T. The role of the superior vestibular nerve in generating ocular vestibular-evoked myogenic potentials to bone conducted vibration at Fz. Clin Neurophysiol. 2009;120:588–593. doi: 10.1016/j.clinph.2008.12.036. [DOI] [PubMed] [Google Scholar]
- 5.Rosengren, Sally M, et al. Why do oVEMPs become larger when you look up? Explaining the effect of gaze elevation on the ocular vestibular evoked myogenic potential. Clin Neurophysiol. 2012;124:785–791. doi: 10.1016/j.clinph.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Chihara Y, Iwasaki S, Munetaka U, Toshihisa M. Vestibular-evoked extraocular potentials by air-conducted sound: Another clinical test for vestibular function. Clin Neurophysiol. 2007;118:2745–2751. doi: 10.1016/j.clinph.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Huang Y, Yang T, Young Y. Feasibility of ocular vestibular-evoked myogenic potentials (oVEMPs) recorded with eyes closed. Clin Neurophysiol. 2012;123:376–381. doi: 10.1016/j.clinph.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 8.Kantner C, Gurkov R. Characteristics and clinical applications of ocular vestibular evoked myogenic potentials. Hearing Research. 2012;294:55–63. doi: 10.1016/j.heares.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Park HJ, Lee I, Shin JE, Lee YJ, Park MS. Frequency-tuning characteristics of cervical and ocular vestibular evoked myogenic potentials induced by air-conducted tone bursts. Clin Neurophysiol. 2009;121:85–89. doi: 10.1016/j.clinph.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Taylor RL, Bradshaw AP, Halmagyi GM, Welgampola MS. Tuning characteristics of ocular and cervical vestibular evoked myogenic potentials in intact and dehiscent ears. Audiol Neurootol. 2012;17:207–218. doi: 10.1159/000336959. [DOI] [PubMed] [Google Scholar]
- 11.Todd NPM, Rosengren SM, Aw ST, Colebatch JG. Ocular vestibular evoked myogenic potentials (OVEMPs) produced by air and bone conducted sound. Clin Neurophysiol. 2007;118:381–390. doi: 10.1016/j.clinph.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 12.Govender S, Rosengren SM, Colebatch JG. The effect of gaze direction on the ocular vestibular evoked myogenic potential produced by air-conducted sound. Clin Neurophysiol. 2009;120:1386–1391. doi: 10.1016/j.clinph.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 13.Collewijn H, Van Der Steen J, Steinman RM. Human eye movements associated with blinks and prolonged eyelid closure. J Neurophysiol. 1985;54:11–27. doi: 10.1152/jn.1985.54.1.11. [DOI] [PubMed] [Google Scholar]
- 14.Chihara Y, Iwasaki S, Fujimoto C, Ushio M, Yamasoba T, Murofushi T. Frequency tuning properties of ocular vestibular evoked myogenic potentials. Neuroreport. 2009;20:1491–1495. doi: 10.1097/WNR.0b013e3283329b4a. [DOI] [PubMed] [Google Scholar]
- 15.Singh NK, Barman A. Characterizing the frequency tuning properties of air-conduction ocular vestibular evoked myogenic potentials in healthy individuals. Int J Audiol. 2013 doi: 10.3109/14992027.2013.822994. epub ahead of print. [DOI] [PubMed] [Google Scholar]