Abstract
Objective
The purpose of this study was to compare the results of the three food-cue paradigms most commonly used for functional neuroimaging studies to determine: i) commonalities and differences in the neural response patterns by paradigm; and, ii) the relative robustness and reliability of responses to each paradigm.
Design and Methods
functional magnetic resonance imaging (fMRI) studies using standardized stereotactic coordinates to report brain responses to food cues were identified using on-line databases. Studies were grouped by food-cue modality as: i) tastes (8 studies); ii) odors (8 studies); and, iii) images (11 studies). Activation likelihood estimation (ALE) was used to identify statistically reliable regional responses within each stimulation paradigm.
Results
Brain response distributions were distinctly different for the three stimulation modalities, corresponding to known differences in location of the respective primary and associative cortices. Visual stimulation induced the most robust and extensive responses. The left anterior insula was the only brain region reliably responding to all three stimulus categories.
Conclusions
These findings suggest visual food-cue paradigm as promising candidate for imaging studies addressing the neural substrate of therapeutic interventions.
Introduction
Obesity affects one-third of American adults and 17% of American children according to data from the National Health and Examination Survey (NHANES, 2009–2010). Obesity increases the risk of developing cardiovascular diseases, diabetes, hypertension, hypercholesterolemia, asthma, arthritis, chronic renal failure and certain types of cancer (1). The underlying cause of obesity, in the vast majority of afflicted persons, is inadequate regulation of food intake. Functional neuroimaging can identify brain structures involved in food craving, food perception and food intake.
Experimental strategies most commonly used for functional neuroimaging studies of feeding-related neural systems contrast food cues with non-food cues or high-calorie food cues with low-calorie food cues (2,3,4,5). In-scanner stimulation methods vary, including: oral delivery of foods or flavored liquids; oro-nasal delivery of food odors; and, visual presentation of food images. These and other methodological variations have contributed to significant between-study variability in the neural systems identified by these studies. Structures implicated in food-intake regulation by functional neuroimaging are numerous, including: anterior insula, inferior frontal and orbitofrontal cortex, medial temporal cortex (amygdala and parahippocampus), nucleus accumbens, and higher-order visual cortex (6). While an extensive constellation of regions likely is required for the complex experiences of food craving, food seeking and food consumption/enjoyment, the collective neuroimaging literature fails to identify strong candidates as regional biomarkers for therapeutic trials.
Coordinate-based meta-analysis is a widely used tool for computing between-study concordance among functional neuroimaging studies, with activation likelihood estimate (ALE) being the most widely applied technique (7,8,9). (For a partial listing of peer-reviewed ALE meta-analyses, see brainmap.org/pubs). Meta-analysis has been applied successfully to the food-cue neuroimaging literature by several investigative teams. A non-quantitative metaanalytic approach (employing visual inspection of plotted coordinates) was applied by Small and Prescott (10) to identify orbitofrontal cortex, insula, and cingulate as common responses to olfactory and gustatory food cues. Quantitative meta-analysis (ALE) was used by van der Laan et al. (11) to assess convergence of neural responses to visual food cues (contrasted with nonfood visual cues) and reported consistent activations in the posterior fusiform gyrus, left lateral orbitofrontal cortex, and insula. Similarly, Tang et al. (12) performed an ALE meta-analysis of studies presenting visual food cues (contrasted with non-food items) in healthy normal weight participants. Convergent activation was identified in the lateral orbitofrontal cortex, anterior and middle insula, amygdala, parahippocampus, precuneus, postcentral gyrus, and visual cortex (fusiform gyrus, and lingual gyri). Veldhuizen et al. (13) performed an ALE meta-analysis of gustatory food cues and found significantly convergent activation in anterior and mid-dorsal insula, parietal operculum, postcentral gyrus, right medial orbitofrontal cortex and mediodorsal thalamus, left lateral orbitofrontal cortex and pre-genual anterior cinculate cortex. None of these meta-analyses, however, compared across the three dominant food-cue-delivery methods nor attempted to prioritize regions as targets (candidate biomarkers) for subsequent therapeutic trials.
In the present study, we performed three ALE meta-analysis, one for each of the most widely used food-cue-delivery paradigms. The primary purpose of this undertaking was to determine which food-cue paradigms produced the most robust and reliable results and, within that paradigm, to determine which region or regions held the most promise as regions of interest for neuroimaging studies of therapeutic interventions.
Methods
fMRI studies of visual, olfactory, and taste food stimuli were identified using on-line electronic databases, including PubMed (http://www.ncbi.nlm.nih.gov/pubmed/) and the BrainMap® database (www.brainmap.org) (14). For PubMed, keywords searches included: “fMRI”; “food” AND “pictures”; “odor” AND “food”; “milkshake”; “meal” and similar terms in various combinations. Additional studies were found by examining the bibliographies of retrieved articles. Inclusion criteria were: papers published in peer-reviewed, English-language journals; whole-brain, voxel-wise analysis of the primary data (i.e., no region-of-interest studies); results reported in standardized stereotactic coordinates; no results filtering other than by statistical significance. Retrieved studies fell into three categories, based upon food-cue delivery method: visual presentation of food images; oral delivery of foods or flavored liquids; and, oro-nasal delivery of food odors. In all included studies, healthy, non-obese subjects were fasted overnight at the time of scanning.
Of fMRI studies using visual food cues, we restricted our sample to studies in which brain activations were induced by viewing pictures of high-caloric-content food (e.g., pizza, hamburgers, ice cream) as contrasted to viewing nonfood pictures (e.g., tools, scenery, flowers, animals) as the control state. Eleven publications reporting 12 experiments were identified which collectively reported 109 brain-activation locations across a total of 201 participants (Table 1a).
Table 1.
| a. Studies included in visual food cues meta-analysis | |||||
|---|---|---|---|---|---|
| Food vs. Nonfood | Visual Food Stimuli |
Hours fasted |
n | Foci | Nonfood stimuli |
| Uher et al., 2006 | Roasted chicken, hamburger, chocolate cake, strawberries, etc. | 24 | 18 | 3 | Armchair, brushes, car flower, etc. |
| Beaver et al., 2006 | Chocolate cake, ice cream sundae, rotten meat, moldy bread, etc. | 2 | 14 | 14 | Videocassette Iron, etc. |
| Cornier et al., 2007 | Waffles, Chocolate cake, Cookies, etc. | Overnight | 22 | 7 | Animals, Trees, Books, etc. |
| Fuhrer et al., 2008 | Food | 1 and 14 hrs | 12 | 12 | Nonfood items |
| Killgore et al., 2003 | French fries, ice cream, cheeseburgers, cake, etc. | 6 | 13 | 18 | Rocks, bricks, trees flowers, etc. |
| Killgore et al., 2006 | French fries, ice cream, cheeseburgers, cake, etc. | 6 | 8 | 23 | Rocks, bricks, trees flowers, etc. |
| Malik et al., 2008 | Food | 3 | 20 | 20 | Scenery |
| Rothemund et al., 2007 | Hamburgers, pancakes, etc. | 1.5 | 13 | 1 | Rocks and flowers |
| Santel et al., 2006 | High-caloric food | 12 | 10 | 3 | Objects |
| Schienle et al., 2009 | French fries, ice cream, cake, chips, etc. | >12 | 17 | 3 | Household items |
| Simmons et al., 2005 | Cheeseburger, spaghetti Cookies, etc. | NR | 9 | 6 | House, mall, school, etc. |
| b. Studies included in the taste food cues meta-analysis | |||||
|---|---|---|---|---|---|
| Food vs. Nonfood | Taste Food Stimuli |
Hours fasted |
n | Foci | Nonfood stimuli |
| Smeets et al., 2010 | Orangeade | 2 | 10 | 2 | Not reported |
| Kringelbach et al., 2003 | Chocolate milk tomato juice | 6 | 10 | 5 | Tasteless solution |
| Smeets et al., 2006 | Chocolate milk | Overnight | 24 | 18 | Water |
| Araujo et al., 2004 | Vegetable oil | 3 | 12 | 3 | Tasteless solution |
| Araujo et al., 2003 | MSGIMP + MSG +IMP | NR | 10 | 5 | Tasteless solution |
| Rolls et al., 2007 | Chocolate | 2,3 | 16 | 6 | Tasteless solution |
| Araujo et al., 2003 | Sucrose and strawberry | NR | 11 | 19 | Tasteless solution |
| Haase et al., 2007 | Sucrose | NR | 18 | 31 | Water |
| c. Studies included in the olfactory food cues meta-analysis | |||||
|---|---|---|---|---|---|
| Food/pleasant odor vs. Nonfood/unpleasant odor |
Odor Food Stimuli |
Hours fasted |
n | Foci | Nonfood stimuli |
| McCabe et al., 2007 | Vegetable | NR | 12 | 1 | Odorless solution |
| Small et al., 2005 | Chocolate, lavender, butanol, farnesol | NR | 10 | 11 | Odorless solution |
| de Araujo et al., 2003 | Strawberry | NR | 24 | 11 | Odorless solution |
| Bragula et al., 2010 | Fat, sweet, | 24 | 10 | 28 | Non appetizing odors |
| Gotfried et al., 2003 | Pleasant | NR | 17 | Non pleasant odors | |
| Royet et al., 2003 | Pleasant | NR | 14 | 12 | Unpleasant |
| Reske et al., 2010 | Vanilla, rotten yeast | NR | 15 | 13 | Air |
| Plailly et al., 2007 | Familiar odors | NR | 16 | 10 | Unfamiliar odors |
For the contrast between food and nonfood stimuli, 14 experiments from 11 studies, with a total of 249 participants and 124 reported coordinates were included. The abbreviation NR refers to the fact that the information was not reported in the study.
For the contrast between food and nonfood pictures, 11 experiments from 8 studies, with a total of 146 participants and 89 reported coordinates were included. The abbreviation NR refers to the fact that the information was not reported in the study.
For the contrast between food/pleasant odor and nonfood/unpleasant odor, 8 experiments from 8 studies, with a total of 131 participants and 79 reported coordinates were included. The abbreviation NR refers to the fact that the information was not reported in the study.
Of fMRI studies of oral/gustatory food stimuli, we restricted our sample to studies in which brain activations were induced by oral delivery of flavored foods (milkshake, chocolate, fat) as contrasted with tasting water or other tasteless solutions. Eight publications reporting 11 experiments were identified which collectively reported 89 brain-activation locations from a total of 146 participants (Table 1b).
Of fMRI studies of olfactory food cues, we restricted our sample to studies in which brain activations were induced by smelling food or appetizing odors (e.g., chocolate, vanilla, vegetables) as contrasted with odorless vapors or unpleasant odors. Eight publications reporting a total of eight experiments were identified which collectively reported 79 brain-activation locations from a total of 118 participants (Table 1c).
For all experiments, results (peak coordinates) and associated meta-data (including experimental design) were entered into the BrainMap database (14) using the Scribe software application (www.brainmap.org) to allow filtering of experimental parameters and metaanalytic pre-processing. Coordinates reported in MNI spaces were converted to Talairach coordinates using the Lancaster’s transform (15). Meta-analyses were performed using GingerALE 2.1, which included modifications to the ALE algorithm (16,8) described by Eickhoff et al. (7) and Turkeltaub et al. (9). Three meta-analysis were performed, one for each of the above-described groups of data. Each collection of studies/experiments was used to compute an ALE map that was statistically contrasted to an ALE null-distribution map. The null distribution map was derived from a permutation procedure and computed using the same number of experiments and reported coordinates as the experimental map. This map represented the null-hypothesis that there was a random spatial association between the results of the experiments. The ALE analysis implemented a random effects inference (i.e., the inference detects the above-chance concurrence between experiments, and not on the clustering of coordinates within experiments). Statistical significance was corrected for multiple comparisons. ALE maps were thresholded at P<0.05 using the option false discovery rate (FDR) pN with an extent threshold greater than 200 mm3. All ALE results were reported in Talairach space, and the candidate anatomical labels for these regions were determined using a validated, fully automated algorithm (17).
Results
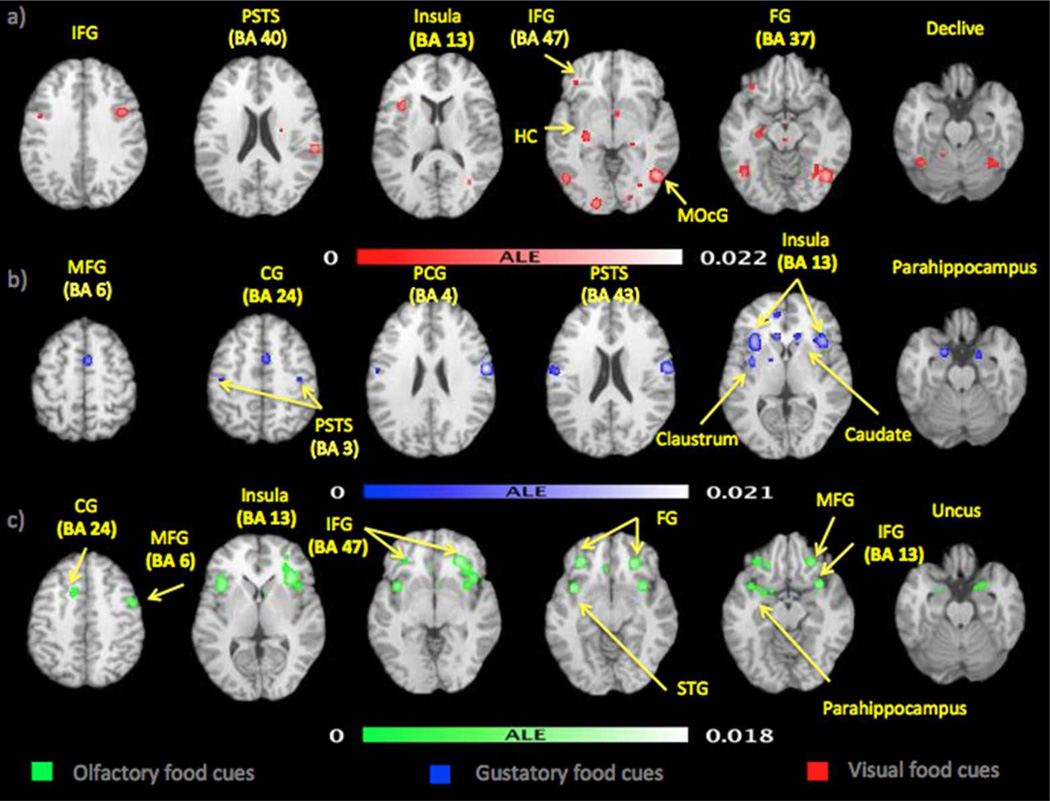
Contrasting food pictures with nonfood pictures yielded 11 clusters of significant convergence (Table 2a, Figure 1) with a total volume of 17,332 mm3 and maximum ALE value of 0.0219. The most robust activation convergence was in higher-order visual cortex (right fusiform gyrus), in keeping with the visual nature of the stimuli. Additional lateralized convergent activations were observed in the left insula, right postcentral gyrus, right precuneus, left inferior frontal gyrus, left middle occipital gyrus and left hippocampus. Bilateral convergent activations were observed in the fusiform gyrus, declive, parahippocampus and superior temporal gyrus.
Table 2.
| a. Summary of ALE results for visual food cues | |||||||
|---|---|---|---|---|---|---|---|
| Cluster # | Volume (mm3) |
Extrema Value (*10−3) |
X | Y | Z | Label | BA |
| 1 | 1632 | 21.9 | 44 | −64 | −8 | Right fusiform gyrus | 37 |
| 2 | 728 | 11.6 | −40 | −58 | −16 | Left cerebellum | - |
| 9.4 | −34 | −58 | −26 | Left cerebellum | - | ||
| 3 | 672 | 11.6 | 32 | −58 | −18 | Right cerebellum | - |
| 4 | 472 | 13.5 | −26 | −24 | −8 | Left hippocampus | - |
| 5 | 424 | 13.8 | −16 | −94 | −6 | Left lingual gyrus | 17 |
| 6 | 416 | 13.9 | 60 | −28 | 18 | Right postcentral gyrus | 40 |
| 7 | 344 | 11.9 | 40 | 6 | 32 | Right precentral gyrus | 9 |
| 8 | 320 | 10.6 | −46 | −68 | −6 | Left middle occipital gyrus | 37 |
| 9 | 288 | 10.7 | −34 | 14 | 12 | Left insula | 13 |
| 10 | 256 | 9.9 | −36 | 30 | −8 | Left inferior frontal gyrus | 47 |
| 11 | 240 | 10.7 | −52 | −30 | 4 | Left superior temporal gyrus | 22 |
| b. Summary of ALE results for taste food cues | |||||||
|---|---|---|---|---|---|---|---|
| Cluster # | Volume (mm3) |
Extrema Value (*10−3) |
X | Y | Z | Label | BA |
| 1 | 1768 | 19.3 | −32 | 16 | 6 | Left insula | 13 |
| 9.1 | −40 | 12 | 12 | Left insula | 13 | ||
| 2 | 1176 | 14.8 | 38 | 16 | 2 | Right insula | 13 |
| 3 | 1088 | 20.6 | 58 | −6 | 22 | Right precentral gyrus | 4 |
| 4 | 680 | 12.9 | −58 | −10 | 16 | Left postcentral gyrus | 43 |
| 5 | 384 | 12.9 | 6 | 6 | −4 | Right caudate head | - |
| 6 | 376 | 13.5 | 18 | −4 | −18 | Right amygdala | - |
| 7 | 360 | 12.8 | −18 | 0 | −18 | Left parahippocampal gyrus | 34 |
| 8 | 360 | 11.2 | 6 | 2 | 50 | Right medial frontal gyrus | 6 |
| 9 | 328 | 11.3 | −8 | 44 | 6 | Left cingulate | 32 |
| 10 | 280 | 10.9 | −6 | 20 | 40 | Left cingulate gyrus | 32 |
| 11 | 272 | 10.7 | −34 | −4 | 4 | Left claustrum | - |
| 12 | 248 | 10.9 | −8 | 22 | 2 | Left caudate head | - |
| 13 | 200 | 9.2 | 12 | 22 | 0 | Right caudate head | - |
| 14 | 200 | 10.4 | −54 | 12 | 10 | Left frontal gyrus | 44 |
| c. Summary of ALE results for odor food cues | |||||||
|---|---|---|---|---|---|---|---|
| Cluste # | Volume (mm3) |
Extrema Value (*10−3) |
X | Y | Z | Label | BA |
| 1 | 4088 | 17.6 | 34 | 24 | 0 | Right inferior frontal gyrus | 47 |
| 14.2 | 26 | 32 | −6 | Right inferior frontal gyrus | 47 | ||
| 14.1 | 24 | 30 | −10 | Right inferior frontal gyrus | 47 | ||
| 13.2 | 42 | 14 | −2 | Right insula | 13 | ||
| 11.8 | 32 | 8 | −12 | Right inferior frontal gyrus | 13 | ||
| 2 | 976 | 12.9 | −38 | 14 | 4 | Left insula | 13 |
| 3 | 536 | 13.5 | −36 | 6 | −8 | Left extra-nuclear | 13 |
| 4 | 496 | 12.6 | −30 | 32 | −10 | Left inferior frontal gyrus | 47 |
| 5 | 360 | 10.4 | −12 | 10 | 46 | Left medial frontal gyrus | 32 |
| 6 | 296 | 8.7 | −22 | −2 | −16 | Left amygdala | - |
| 7.0 | −30 | 4 | −14 | Left parahippocampal gyrus | 34 | ||
| 7 | 280 | 9.0 | 48 | 0 | 42 | Right precentral gyrus | 6 |
Note that the responses labeled “cerebellar” likely represent mislabeling of responses from the inferior visual cortex, which lies immediately adjacent to the superior cerebellum.
Figure 1.
Significant ALE clusters are shown for the visual (red), taste (blue) and odor (green) food cues meta-analysis.
Contrasting food tastes with tasteless solutions produced 14 significant ALE clusters (Table 2b, Figure 1) summing a volume of 13,332 mm3 and a maximum ALE value of 0.0193. The most robust activation convergence was in the insula, bilaterally, in the presumed location of primary gustatory cortex. Strong activations were also observed in the region of the sensorimotor mouth representation, likely reflecting stimulation of the oral mucosa and manipulation of the food in the mouth. Additionally, the cingulate gyrus, parahippocampal gyrus, postcentral gyrus, caudate, claustrum, insula, medial frontal gyrus, precentral gyrus, thalamus and lentiform nucleus were activated. The majority of ALE clusters showed bilateral activations.
Contrasting food odors or non-food appetizing odors with odorless stimuli or unpleasant odors produced 7 significant ALE clusters with a total volume of 11,981 mm3 and maximum ALE value of 0.0176 (Figure 1 and Table 2c). The inferior frontal gyrus and anterior insula were the regions of most extensive and robust activation convergence, in the region of primary olfactory cortex. Other strong activations included amygdala and parahippocampus, regions with known olfactory projections.
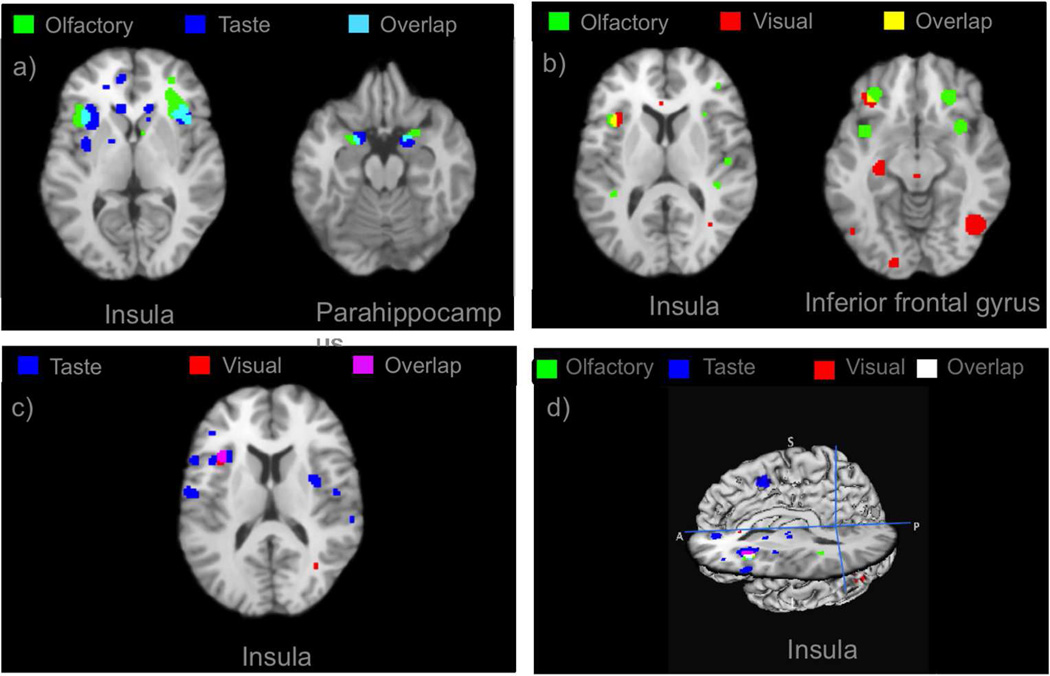
The insula and parahippocampus were activated by both olfactory and taste food cues (Figure 2a). The insula and inferior frontal gyrus were commonly activated by olfactory and visual stimulation (Figure 2b). The insula was activated by both taste and visual presentation of food cues (Figure 2c). The left anterior insula was the only structure that was commonly activated by all three modalities (Figure 2d).
Figure 2.
(a) Overlay of ALE results for olfactory and taste food cues and their overlaps. Insula and parahippocampus are commonly activated. (b) Overlay of ALE results for visual and odor food cues and their overlaps. Insula and inferior frontal gyrus are commonly activated. (c) Overlay of ALE results for visual and taste food cues and their overlaps. Insula is the only common area.
Discussion
Quantitative, coordinate-based meta-analysis was used to compare the collective findings of the three food-cue paradigms most extensively applied in functional neuroimaging studies of the neural systems engaged in food-intake regulation. Brain-response distributions were distinctly different for each of the three modalities, corresponding to known differences in the locations of the respective primary and associative cortices. Responses in (and around) primary olfactory and gustatory cortex were observed for odor and taste stimuli, respectively. The most extensive and statistically reliable food-cue-specific responses, however, were observed in higher-order visual cortex (fusiform gyrus), rather than in brain regions more commonly associated with the hedonic properties of food or appetite regulation. The anterior insula – in the region of primary gustatory cortex – was the only brain region to demonstrate significant responses to all three stimulus-delivery modalities.
Responses to Visual Food Cues
Meta-analysis of neural responses to visual food cues gave the most robustly convergent responses as determined both by peak statistical significant (ALE value) and by the volume of brain exhibiting a significant response (Table 2a). When using visual food cues as stimuli, the regions showing the most reliable and significant activation lay within the visual system proper (occipital lobe), rather than in regions more traditionally regarded as involved in food-appetite regulation. Specifically, the region most strongly activated was the fusiform gyrus, which is a component of a nonretinotopic, high-level object-representation network. What is particularly striking about this activation is that all included studies used familiar, visually complex, nonfood objects as control stimuli, so the intense activation cannot be attributed to differential stimulus complexity or other irrelevant attributes. Rather, this finding argues strong that food images are “pop-out” stimuli, that reliably evoke significantly more intense and spatially extensive, obligatory processing than non-food images (11). A similar effect is observed, for example, with familiar faces (18).
From an evolutionary perspective, a species’ survival depends on its ability to find food and to rapidly and reliably identify potentially edible items. Evolutionarily early species (e.g., aquatic species) rely almost entirely on chemosensation (taste) to detect and localize food. On land, olfaction rapidly evolved into the dominant modality for food detection and remains so for most species. Many primate species, including humans, have highly evolved visual systems. For most primates, visual search is a far more effective means of discovering food than olfaction or taste (19,20). Thus, it is not surprising that the visual system proved so effective at producing food-specific responses, i.e., at discriminating food from non-food items, in human subjects. What is, perhaps, more surprising is that the strongest visually induced food-specific responses were within higher-order visual cortex (object-recognition regions), rather than in regions more specific to eating. The robustness of the responses of visual regions to food cues, however, should not be construed as indicting that the visual system is solely involved in food detection.
The sight of food evokes physiological, emotional and cognitive responses (11). Visual perception of food cues prepares the body for food ingestion, an anticipatory physiological response called the “cephalic phase” of eating (21). The consequent emotional responses enhance the desire to ingest food (22), and triggers pleasure, which has been proposed as a biological mechanism evolved to encourage survival behaviors (23). In addition, food cues evoke cognitive processes, such as memory retrieval and hedonic evaluation, based on previous experiences with food (23). Exposure to food cues can trigger cognitive processes such as selfregulation (24) or overeating due to the override of satiety signals (25) by the food cues.
Responses to Taste and Odor Food Cues
Oral stimulation with foods or flavored liquids also gave very robust and extensive convergent activations across experiments (Table 2b). The most robust responses were in the anterior insula, bilaterally, that is, in the primary gustatory cortex. Strong responses were also observed in sensorimotor cortices (in regions representing the mouth), as well as in subcortical motor-control regions (caudate head). These likely reflect both the stimulation of oral mucosa by food/liquid contact and manipulation of the food substances by the tongue, and oropharynx. Emotional, mnemonic and attention-related regions (amygdala, parahippocampus, anterior cingulate) were also activated, likely reflecting hedonic aspects of food ingestion.
Odors also induced highly significant and extensive convergent activations (Table 2c), including the inferior frontal gyrus (prepiriform cortex), amydala and parahippocampus (piriform cortex) and anterior insula bilaterally. The role of these regions in olfaction is well established. By comparison to visual food cues, it should be noted that the most commonly used control stimuli for taste and odor stimuli are the absence of an effective stimulus (i.e. tasteless or odorless substances), while visual control stimuli were highly effective stimuli (i.e., non-food pictures.)
Convergent Responses Across Food-Cue Modalities
Anatomical convergence of gustatory and olfactory food-cue responses (Figure 2a) in insula, parahippocampus and orbitofrontal cortex is not surprising. Primary gustatory cortex (anterior insula and frontal operculum) and primary olfactory cortex (amygdala, uncus, parahippocampus) are adjacent to one another and are mutually connected functionally and anatomically. The co-activation of the anterior insula by olfactory and gustatory food cues stimuli is consistent with the presence of projections from the olfactory neurons from oral cavity and afferents from the gut to the anterior insula (26). However, gustatory and olfactory stimulus paradigms likely do not recruit fully independent brain networks because of the retronasal olfaction pathway by which there is a perception of odors emanating from the oral cavity during food ingestion. A previous meta-analysis of uni-modal gustatory stimulation studies was performed by Veldhuizen et al. (13) demonstrating the engagement of insula on pure gustatory stimulation, indicating that true insular activation was induced by all three types of food cues.
Anatomical convergence of visual responses with olfactory response in orbitofrontal cortex (Figure 2b) and with both olfactory and gustatory (Figure 2c,d) in anterior insula is more striking and pragmatically promising. Although anatomical projections from the fusiform gyrus to the anterior insula has not yet been reported, reliable co-activation (as observed here) implies at least indirect connectivity (27,28). This suggests that anterior insula likely is a hub region via which visual stimuli (and other modalities) induce physiological and psychological responses to food.
The anterior insula is known to engage in a wide range of tasks, including attention, memory, interoception, emotion, olfaction and gustation (29). Functional connectivity studies (30) showed that the anterior insula is part of a neural network of reward circuitry that included the thalamus, caudate, among others (31). Moreover, the left anterior insula is involved in different types of craving types, such as smoking, cocaine, sexual arousal, drug use, and gambling (32,33,34,35,36,37). In addition, it has been published that lesion of the insular cortex leads to recovery of nicotine addiction (38), suggesting that the insula is a key brain structure implicated in (food) craving-reward, processing and modulation of hedonic response, beyond food sensory stimuli integration. Furthermore, our findings identified the left anterior insula as opposed to the whole insula. Taken together, we speculate that overeating and obesity may be a result of dysfunction of craving-reward circuitry, sharing a common pathway with addiction.
The Visual Food-Cue Paradigm: Strengths and Opportunities
Of the three most widely used food-cue paradigms, visual presentation is by far the simplest to implement. MRI-compatible visual-display hardware and software for stimulus delivery and experiment management are commercially available from numerous vendors. Picture stimuli are readily created and can be digitally stored and shared. Digital pictures can be presented for durations as brief and at repetition rates as rapid as desired, without being impeded by stimulus-delivery mechanics. These characteristics allow picture-based food-cue experiments to be readily implemented for clinical trials and to exploit ongoing advances in fMRI experimental design and analysis strategies, including event-related designs. Further, the occipital lobe (lingual gyrus) is far less prone to susceptibility artifacts than the medial temporal lobe and inferior frontal brain regions recruited by taste and odor stimuli, simplifying fMRI data acquisition and analysis.
Delivery of taste stimuli is far more complex, requiring calibrated solutions to be compounded and delivered into the mouth of the subject, typically through flexible tubes held between the lips. Tasting the solution, swallowing, rinsing the mouth and repeating with an alternate taste or control substance is an inherently slow process, which limits the rate of data acquisition and the range of experimental-design possibilities. Oral delivery of foods and flavored liquids also introduces the possibility of in-scanner aspiration, a risk not entailed with other food-cue stimulus modalities. While imaging studies using oral delivery of food cues were certainly required to map the neural populations recruited by food intake, it is less clear that this delivery modality is well suited for the transition to therapeutic trials using functional imaging to study mechanisms of action and as potential neural predictors of therapeutic outcome.
Odor stimulus delivery is yet more complicated, requiring a ventilation system capable of delivering odor-bearing gases rapidly and at calibrated concentrations and of equally rapidly removing the odorants without detectible residue. Such systems are not commercially available and must be constructed in-house. This makes them poorly suited for therapeutic trials.
Collectively, present results and the practicalities of stimulus delivery and experimental design argue strongly for the use of visual food-cue paradigms when studying neural responses to potential therapies. This would apply both to studies exploring the neural mechanisms of action of pharmacologic, behavioral or surgical therapies and to studies using imaging as a rapid neural biomarker to predict longer-term therapeutic outcomes. Visual-food-cueresponsive regions in lingual/fusiform gyrus, anterior insula and orbitofrontal cortex all offer promising candidate regions of interest.
Limitations of this study
There are a number of limitations associated with the data and methodology of this study, which should be considered when interpreting our results. First, the available number of publications included in these meta-analyses is relatively small, which necessitated inclusion of heterogeneous stimulation paradigms (such as type of food and duration of exposure), imageacquisition protocols and image-analysis methods, among others. Second, the ALE analysis algorithm did not account for the statistical significance or spatial extent of the included responses. Finally, to our surprise, none of the three meta-analyses revealed activation in the hypothalamus, which has been strongly implicated in satiety. Further, only one of the studies included in these three meta-analyses reported hypothalamic activation. Duration of preimaging fasting proved not to be an explanatory factor. The most likely explanation is that the hypothalamus is a small structure located in a region of high magnetic susceptibility, limiting the detectability of neural responses when using fMRI. Studies that have reported robust hypothalamic responses (39,40) have generally used acquisition protocols customized for this purpose.
Conclusions
The results of the visual food cues meta-analysis suggested that this paradigm is a simple and robust tool to probe the neural mechanisms involved in eating behavior. Our results support the notion that the anterior insula plays an important role in craving-reward processing and modulation of hedonic response, likely beyond food stimuli integration. Future fMRI studies could probe the left anterior insula and fusiform gyrus to determine whether it plays a role in eating behavior, eating disorders, obesity and diabetes.
What is already known about this subject?
Obesity affects one-third of American adults and 17% of American children. Prevalence is rapidly rising. The neural networks underlying food perception and food craving have been detailed by numerous functional neuroimaging studies using food cues as in-scanner stimuli.
Published neuroimaging studies have varied widely in their food cue presentation methods, including: visual presentation of food images; oral delivery of food or flavored liquids; and, oro-nasal delivery of food odors. These and other methodological variations have contributed to significant between-study variability in reported results.
On the other hand, virtually all of the functional neuroimaging studies in this domain analyzed data and reported results using well-validated methods, including voxel-wise analyses within standardized anatomical reference spaces, making them amenable to quantitative meta-analysis.
What does this study add?
Coordinate-based meta-analyses were used to identify regional brain responses most reliably recruited for each of the three most commonly used food-cue delivery strategies: oral foods, food odors, and food images.
Brain response distributions were distinctly different for each of the three modalities, corresponding to known differences in location of the respective primary and associative cortices. The only brain region showing significant responses to all three stimulus-delivery modalities was the left anterior insula. The most widespread, robust and reliable food-cuespecific responses were elicited by visual food cues in higher-order visual cortex. This suggests visual food-cue paradigms as strong candidates for studies of therapeutic interventions.
Acknowledgements
Claudia Huerta, Angela Laird and Peter Fox conceptualized the study and designed the meta-analyses. Claudia Huerta and Pooja Sarkar performed the literature searches, selected the data subsets and carried out the meta-analyses. Claudia Huerta generated the figures and tables and wrote the manuscript. All authors edited the manuscript. This study was funded by R01 MH074457 (PTF and ARL, PIs).
Footnotes
Conflict of interest
None declared
References
- 1.Cecchini M, Sassi F, Lauer JA, Lee YY, Guajardo-Barron V, Chisholm D. Tackling of unhealthy diets, physical inactivity, and obesity: health effects and cost-effectiveness. Lancet. 2010;376:1775–1784. doi: 10.1016/S0140-6736(10)61514-0. [DOI] [PubMed] [Google Scholar]
- 2.Killgore WDS, Young AD, Femia LA, Bogorodzki P, Rogowska J, Yurgelun-Todd DA. Cortical and limbic activation during viewing of high- versus low-calorie foods. NeuroImage. 2003;19:1381–1394. doi: 10.1016/s1053-8119(03)00191-5. [DOI] [PubMed] [Google Scholar]
- 3.Killgore WDS, Yurgelun-Todd DA. Sex Differences in Cerebral Responses to Images of High vs Low Calorie Food. Neuroreport. 2010;21:354–358. doi: 10.1097/WNR.0b013e32833774f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.LaBar KS, Gitelman DR, Parrish TB, Kim YH, Nobre AC, Mesulam MM. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behav Neurosci. 2001;115:493–500. doi: 10.1037/0735-7044.115.2.493. [DOI] [PubMed] [Google Scholar]
- 5.de Araujo IE, Rolls ET. Representation in the Human Brain of Food Texture and Oral Fat. The Journal of Neuroscience. 2004;24:3086–3093. doi: 10.1523/JNEUROSCI.0130-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benarroch EE. Neural control of feeding behavior: Overview and clinical correlations. Neurology. 2010;74:1643–1650. doi: 10.1212/WNL.0b013e3181df0a3f. [DOI] [PubMed] [Google Scholar]
- 7.Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Human Brain Mapping. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. NeuroImage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- 9.Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P. Minimizing withinexperiment and within-group effects in activation likelihood estimation meta-analyses. Human Brain Mapping. 2011 doi: 10.1002/hbm.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Small DM, Prescott J. Odor/taste integration and the perception of flavor. Experimental Brain Research Experimentelle Hirnforschung Experimentation Cerebrale. 2005;166:345–357. doi: 10.1007/s00221-005-2376-9. [DOI] [PubMed] [Google Scholar]
- 11.van der Laan LN, de Ridder DTD, Viergever MA, Smeets PAM. The first taste is always with the eyes: a meta-analysis on the neural correlates of processing visual food cues. NeuroImage. 2011;55:296–303. doi: 10.1016/j.neuroimage.2010.11.055. [DOI] [PubMed] [Google Scholar]
- 12.Tang DW, Fellows LK, Small DM, Dagher A. Food and drug cues activate similar brain regions: a meta-analysis of functional MRI studies. Physiol Behav. 2012;106:317–324. doi: 10.1016/j.physbeh.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Veldhuizen MG, Albrecht J, Zelano C, Boesveldt S, Breslin P, Lundstrom JN. Identification of human gustatory cortex by activation likelihood estimation. Hum Brain Mapp. 2011;32:2256–2266. doi: 10.1002/hbm.21188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox PT, Lancaster JL. Opinion: Mapping context and content: the BrainMap model. Nature Reviews Neuroscience. 2002;3:319–321. doi: 10.1038/nrn789. [DOI] [PubMed] [Google Scholar]
- 15.Lancaster JL, Tordesillas-Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, et al. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Human Brain Mapping. 2007;28:1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, et al. ALE metaanalysis: controlling the false discovery rate and performing statistical contrasts. Human Brain Mapping. 2005;25:155–164. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talairach atlas labels for functional brain mapping. Human Brain Mapping. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fusar-Poli P, Placentino A, Carletti F, Allen P, Landi P, Abbamonte M, et al. Laterality effect on emotional faces processing: ALE meta-analysis of evidence. Neuroscience letters. 2009;452:262–267. doi: 10.1016/j.neulet.2009.01.065. [DOI] [PubMed] [Google Scholar]
- 19.Laska M, Freist P, Krause S. Which senses play a role in nonhuman primate food selection? A comparison between squirrel monkeys and spider monkeys. American journal of primatology. 2007;69:282–294. doi: 10.1002/ajp.20345. [DOI] [PubMed] [Google Scholar]
- 20.Linne Y, Barkeling B, Rossner S, Rooth P. Vision and eating behavior. Obesity research. 2002;10:92–95. doi: 10.1038/oby.2002.15. [DOI] [PubMed] [Google Scholar]
- 21.Drobes DJ, Miller EJ, Hillman CH, Bradley MM, Cuthbert BN, Lang PJ. Food deprivation and emotional reactions to food cues: implications for eating disorders. Biological psychology. 2001;57:153–177. doi: 10.1016/s0301-0511(01)00093-x. [DOI] [PubMed] [Google Scholar]
- 22.Ouwehand C, Papies EK. Eat it or beat it. The differential effects of food temptations on overweight and normal-weight restrained eaters. Appetite. 2010;55:56–60. doi: 10.1016/j.appet.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Berthoud HR, Morrison C. The brain, appetite, and obesity. Annual review of psychology. 2008;59:55–92. doi: 10.1146/annurev.psych.59.103006.093551. [DOI] [PubMed] [Google Scholar]
- 24.Kroese FM, Evers C, De Ridder DT. How chocolate keeps you slim. The effect of food temptations on weight watching goal importance, intentions, and eating behavior. Appetite. 2009;53:430–433. doi: 10.1016/j.appet.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Cornell CE, Rodin J, Weingarten H. Stimulus-induced eating when satiated. Physiology & Behavior. 1989;45:695–704. doi: 10.1016/0031-9384(89)90281-3. [DOI] [PubMed] [Google Scholar]
- 26.Scott JW, Brierley T. A functional map in rat olfactory epithelium. Chem Senses. 1999;24:679–690. doi: 10.1093/chemse/24.6.679. [DOI] [PubMed] [Google Scholar]
- 27.Fox PT, Friston KJ. Distributed processing; distributed functions? NeuroImage. 2012;61:407–426. doi: 10.1016/j.neuroimage.2011.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laird AR, Eickhoff SB, Rottschy C, Bzdok D, Ray KL, Fox PT. Networks of task coactivations. NeuroImage. 2013;80:505–514. doi: 10.1016/j.neuroimage.2013.04.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by metaanalysis. Brain Structure & Function. 2010;214:519–534. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cauda F, Costa T, Torta DM, Sacco K, D’Agata F, Duca S, et al. Meta-analytic clustering of the insular cortex: characterizing the meta-analytic connectivity of the insula when involved in active tasks. NeuroImage. 2012;62:343–355. doi: 10.1016/j.neuroimage.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Camara E, Rodriguez-Fornells A, Ye Z, Munte TF. Reward networks in the brain as captured by connectivity measures. Frontiers in neuroscience. 2009;3:350–362. doi: 10.3389/neuro.01.034.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, et al. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- 33.Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG, et al. Brain metabolic changes during cigarette craving. Archives of general psychiatry. 2002;59:1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- 34.Goudriaan AE, de Ruiter MB, van den Brink W, Oosterlaan J, Veltman DJ. Brain activation patterns associated with cue reactivity and craving in abstinent problem gamblers, heavy smokers and healthy controls: an fMRI study. Addiction biology. 2010;15:491–503. doi: 10.1111/j.1369-1600.2010.00242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kilts CD, Gross RE, Ely TD, Drexler KP. The neural correlates of cue-induced craving in cocaine-dependent women. The American journal of psychiatry. 2004;161:233–241. doi: 10.1176/appi.ajp.161.2.233. [DOI] [PubMed] [Google Scholar]
- 36.Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, et al. Neural activity related to drug craving in cocaine addiction. Archives of general psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- 37.Ko CH, Liu GC, Hsiao S, Yen JY, Yang MJ, Lin WC, et al. Brain activities associated with gaming urge of online gaming addiction. Journal of psychiatric research. 2009;43:739–747. doi: 10.1016/j.jpsychires.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 38.Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends in neurosciences. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y, Gao JH, Liu HL, Fox PT. The temporal resonse of the brain after eating revealed by functional MRI. Nature. 2000;405:1058–1062. doi: 10.1038/35016590. [DOI] [PubMed] [Google Scholar]
- 40.Matsuda M, Liu Y, Mahankali S, Pu Y, Mahankali A, Wang J, DeFronzo RA, Fox PT, Gao JH. Altered hypothalamic function in response to glucose ingestion in obese humans. Diabetes. 1999 Sep;48(9):1801–1806. doi: 10.2337/diabetes.48.9.1801. [DOI] [PubMed] [Google Scholar]