Abstract
Background
Although excess body weight has been associated with cancers of the gastric cardia, relationships with gastric cancer at other anatomic subsites are not well defined. Furthermore, subsite-specific associations with attained height have not been fully assessed.
Methods
In 1995–1996, 483,700 Whites enrolling in the multi-state NIH-AARP Diet and Health Study self-reported height and weight. Gastric cancers occurring through December 31, 2006 were ascertained from regional population-based registries. We used Cox regression models to estimate cancer hazard ratios (HRs) for sex-specific tertiles of height and weight and for body mass index (BMI) categories of the World Health Organization.
Results
One thousand incident cancers (48% localized to the cardia, 4% fundus, 6% corpus, 3% greater curvature, 6% lesser curvature, 10% antrum, 2% pylorus, 5% overlapping lesion, and 16% unspecified) occurred an average of 5.4 years after enrollment. After controlling for effects of age, sex, education and smoking, we found an inverse association between height and total noncardia cancers (i.e., fundus, corpus, greater and lesser curvatures, antrum, and pylorus), with HRs vs. tertile 1 of 0.65 and 0.71 for tertiles 2 and 3, respectively (p-trend=0.016). Trends were consistent for individual noncardia subsites. In contrast, although weight and BMI were each associated with risk of cardia cancer, neither was associated with total noncardia cancer nor individual subsites.
Conclusion
Noncardia gastric cancer is associated with short stature but not with high body weight nor obesity. The excess risk for shorter adults would be consistent with the known association of chronic H. pylori infection with growth retardation during childhood.
Keywords: BMI, cardia, gastric cancer, height, noncardia, weight
INTRODUCTION
Although incidence rates have decreased, gastric cancer remains the second leading cause of cancer related mortality worldwide [1]. Gastric cardia and noncardia cancer have distinct epidemiological and clinical characteristics [2]. In particular, seropositivity for Helicobacter pylori is positively associated with noncardia cancer but inversely associated with cardia cancer [3].
Noncardia gastric cancer incidence rates among Whites in the United States (U.S.) have been declining in older adults but rising in younger persons [4]. Moreover, subsite-specific analyses indicate a shifting distribution by anatomic subsite, with a significant increase of corpus cancer among younger and middle-aged Whites [5].
The association between attained height and overall gastric cancer risk has been previously examined, but findings have been inconsistent [6, 7]. Furthermore, subsite-specific associations have been insufficiently investigated. Based on the established association of excess body weight with risk of cardia cancer [8], we hypothesized that anthropometric factors may be related to the incidence patterns in noncardia cancer as well. To examine this hypothesis, we evaluated subsite-specific associations of height, weight, and body mass index (BMI) with gastric cancer among Whites, including nearly twice as many cases as our previous reports from the same U.S. cohort [9, 10].
MATERIALS AND METHODS
Study population
The U.S. National Institutes of Health (NIH)-AARP Diet and Health Study design has been described in detail elsewhere [11]. In brief, the cohort was established in 1995–1996 by inviting 3.5 million AARP members aged 50–69 years residing in six states (California, Florida, Louisiana, New Jersey, North Carolina and Pennsylvania) and two metropolitan areas (Atlanta, Georgia and Detroit, Michigan) to complete a baseline questionnaire on demographic, anthropometric, dietary, and lifestyle characteristics. The study was approved by the Special Studies Institutional Review Board of the U.S. National Cancer Institute, and consent was assumed for participants who completed and returned the questionnaire.
A total of 566,401 self-administered questionnaires were returned with satisfactory data. Our analysis is restricted to White responders (n=516,914). We excluded 33,214 subjects with cancer at baseline, proxy respondents, and those missing data for BMI. The resulting cohort consisted of 483,700 participants (290,291 men and 193,409 women).
Case ascertainment and cohort follow-up
Incident cancers, including gastric cancer cases, were identified by probabilistic linkage with population-based cancer registries in the original recruitment areas, and three common states of relocation (Arizona, Texas, and Nevada). Cancer sites were identified by anatomical site and histological code of the International Classification of Disease for Oncology (ICD-O, 3rd edition). Tumors with ICD-O codes C16.0-C16.9 were classified as gastric cancers and, for this analysis, those with site codes C16.1–C16.6 were grouped as total noncardia.
Cohort members were followed annually through the U.S. Postal Service national database for address changes and the U.S. Social Security Administration Death Master File and the National Death Index Plus for updated vital status. Follow-up for each subject began on the date of questionnaire return and continued until the date of cancer diagnosis, date of censoring due to loss to follow-up, date of death, or December 31, 2006, whichever came first.
Exposure assessment
Self-reported height and weight were obtained from the baseline questionnaire, and BMI was derived as weight in kilograms/height in square meters. Height and weight were analyzed as tertiles according to sex-specific distributions. Average heights for men and women, respectively, were 66.8 and 61.1 inches for tertile 1, 69.6 and 63.6 inches for tertile 2, and 72.7 and 66.6 inches for tertile 3. Average weights for men and women, respectively, were 158.2 and 124.4 pounds for tertile 1, 185.4 and 150.4 pounds for tertile 2, and 225.9 and 195.1 pounds for tertile 3. For BMI, we used predefined World Health Organization standard categories: underweight, less than 18.5 kg/m2; normal, 18.5 to less than 25; overweight, 25 to less than 30; obese, 30 to less than 35; and morbidly obese 35 or greater.
Statistical analysis
We used multivariable Cox hazards regression to estimate hazard ratios (HR) and 95% confidence intervals (CI) of height, weight, and BMI for combined and individual noncardia cancer subsites. Referent categories were tertile 1 for height and weight, and normal for BMI. All models were adjusted for age (continuous), sex, education (less than high school, completion of high school, some post-high school training, completion of college, or completion of graduate school), cigarette use (never smokers, former smokers who smoked up to 20 cigarettes/day, former smokers who smoked more than 20 cigarettes/day, current smokers who smoke up to 20 cigarettes/day, or current smokers who smoke more than 20 cigarettes/day). Missing values were included as a separate category for education (2.4%) and smoking (3.4%). Tests for linear trend with height or weight were performed by assigning values of 1, 2, and 3 to their tertiles and modeling as ordinal variables.
We examined possible effect modification by sex for the associations between height and noncardia cancer as well as individual gastric cancer subsites. Statistical tests for interaction evaluated the significance of categorical cross-product terms in the multivariable models. The proportional hazard assumption was tested by including time-dependent variables in the models. All analyses were performed with SAS 9.1.3 (SAS Institute, Cary, NC). All tests were two-sided and a p-value of less than 0.05 was considered statistically significant.
RESULTS
The 483,700 White participants contributed 4,669,852 person-years of observation. The average time from enrollment to gastric cancer diagnosis was 5.4 years (standard deviation, 3 years). There were 786 gastric cancer cases ascertained in men and 214 in women. Of these 1000 total cancers, 478 (48%) were localized to the cardia, 42 (4%) fundus, 58 (6%) corpus, 30 (3%) greater curvature, 64 (6%) lesser curvature, 98 (10%) antrum, 16 (2%) pylorus, 55 (5%) overlapping, and 159 (16%) unspecified.
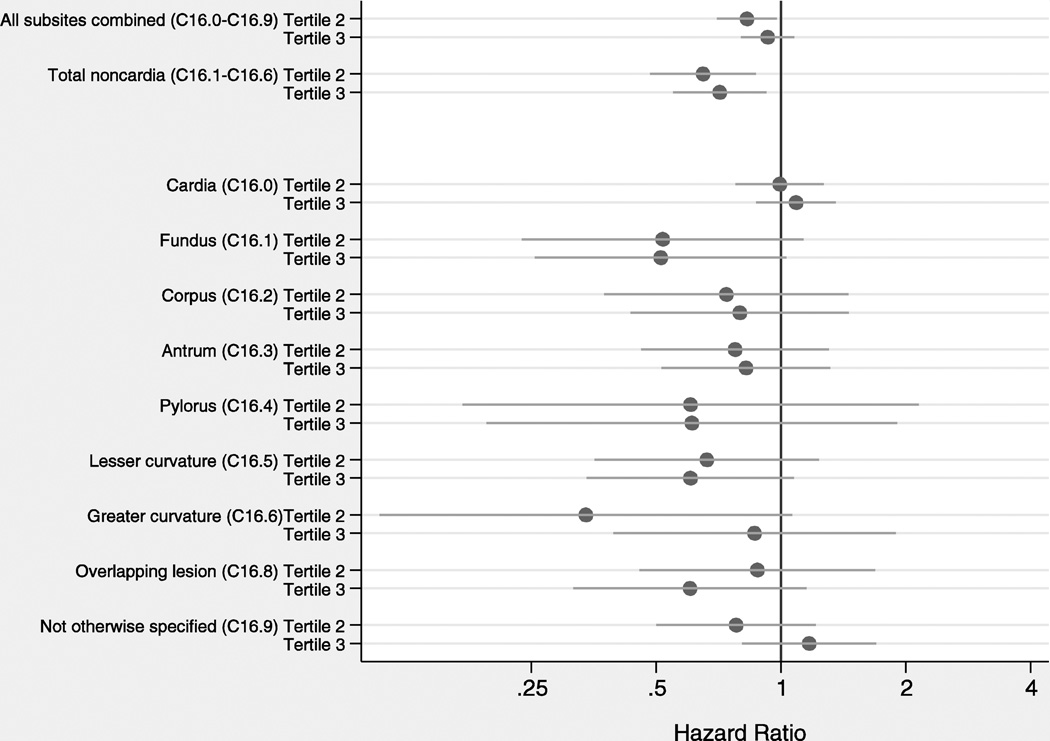
Figure 1 presents adjusted HR for associations between attained height and gastric cancer risk for combined and individual anatomic subsites. For all gastric cancer combined, there was no significant trend across tertiles (p-trend=0.47). The risk of cardia cancer was not associated with height. Risks of individual subsites of noncardia gastric cancer were inversely associated, but not statistically significant. For total noncardia cancer, individuals in the second and third tertiles of height had lower gastric cancer risk vs. the first tertile (p-trend=0.016). As compared to crude risk estimates, HRs adjusted for confounders were somewhat attenuated. Furthermore, there were no significant multiplicative interactions between height and sex (data not shown).
Figure 1.
Adjusted hazard ratios and 95% confidence intervals for associations between attained height and gastric cancer risk by anatomic subsite
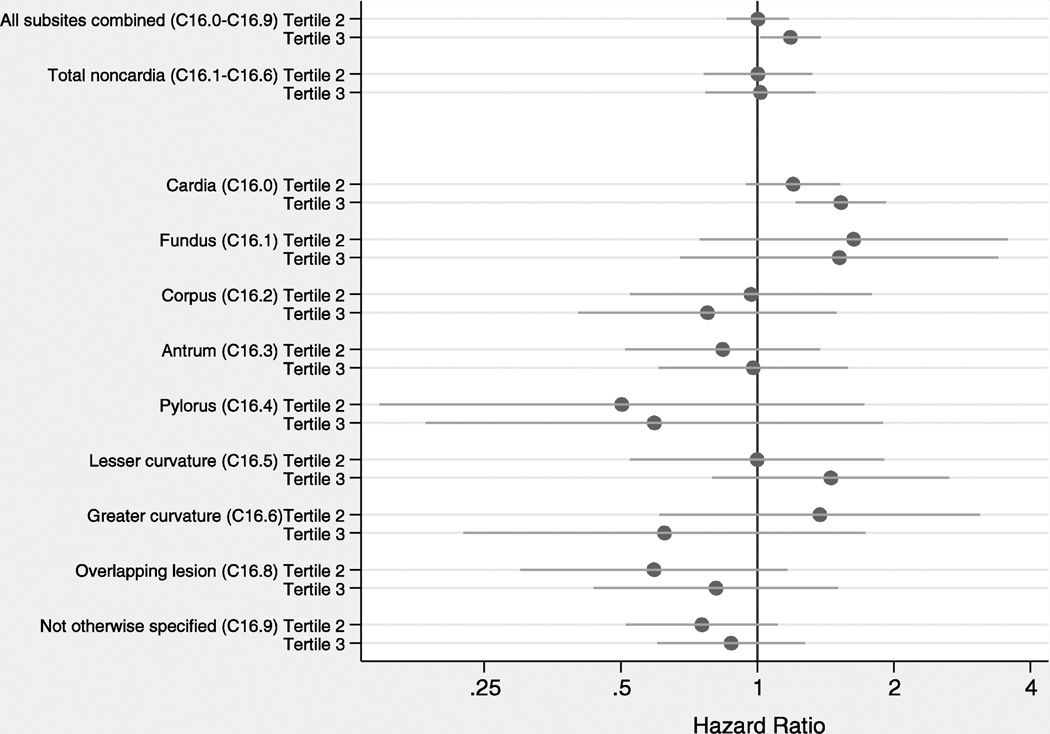
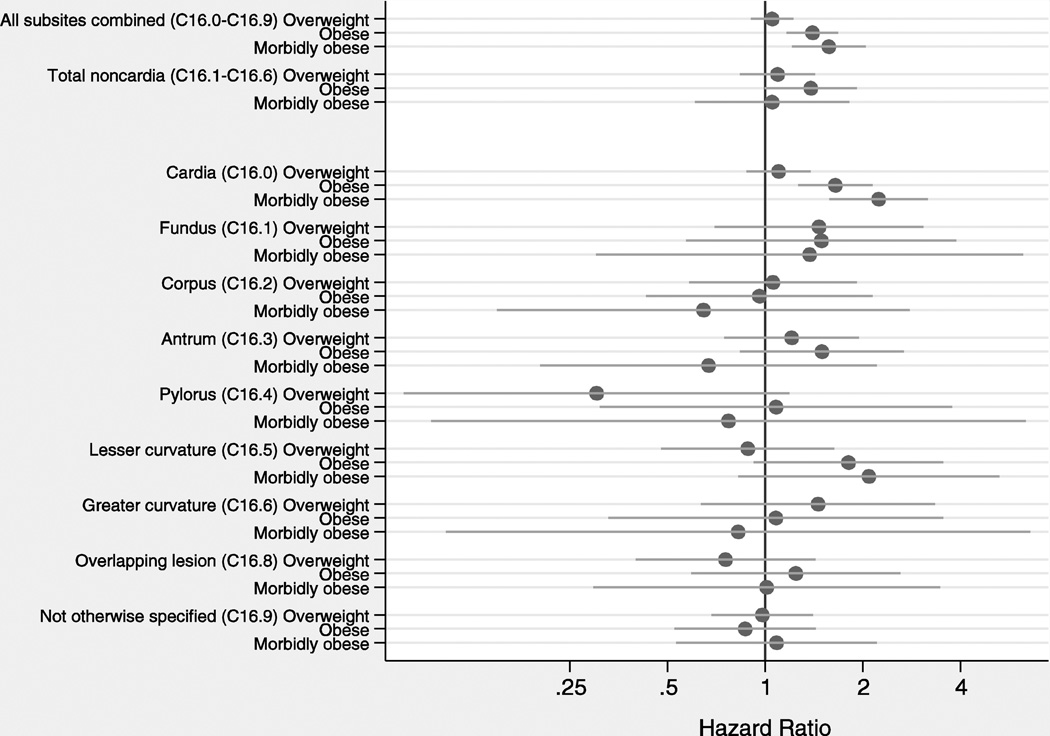
Excess weight was associated with increased gastric cancer risk for all subsites combined (p-trend=0.028) and for cardia cancer specifically (p-trend=0.0002), while no consistent associations were found for noncardia subsites (Figure 2). Patterns were similar for associations with BMI (Figure 3). The proportional hazard assumption was satisfied for all models (data not shown).
Figure 2.
Adjusted hazard ratios and 95% confidence intervals for associations between weight and gastric cancer risk by anatomic subsite
Figure 3.
Adjusted hazard ratios and 95% confidence intervals for associations between body mass index and gastric cancer risk by anatomic subsite
DISCUSSION
To our knowledge, this study represents the first attempt to investigate whether associations of height, weight, and BMI with gastric cancer vary by detailed anatomical subsite. In this large cohort, we found an inverse association between attained height and total noncardia gastric cancer, with a consistent decreased risk across individual subsites. Excess body weight was not related to noncardia cancers, but was associated with a significantly increased risk of cardia cancer.
Height is a complex trait, involving a variety of genetic, hormonal and environmental factors [12]. Interestingly, chronic H. pylori infection, the primary risk factor for noncardia gastric cancer [13], is a suspected cause of growth retardation during childhood [14, 15]. The possible mechanisms through which this bacterial infection may affect growth include lower food intake, impaired nutrient absorption and direct nutrient loss [16, 17]. Inadequate childhood nutrition may also affect susceptibility to H. pylori infection, perhaps by reducing immune competence [18] or by high metabolic demands of the inflammatory response [19]. Nevertheless, it cannot be completely dismissed that H. pylori infection may be a surrogate marker for other factors associated with socio-economic deprivation in early life.
Previous findings for the association of overall gastric cancer with attained height have been inconsistent. Case-control comparisons have found an inverse association [6, 20], while cohort studies have suggested a weak-to-null association [7, 21–25]. We found an average reduction in noncardia gastric cancer risk of 33% for height tertiles 2 and 3 as compared to tertile 1 (35% for tertile 2 and 29% for tertile 3). This observation is consistent with several independent reports associating H. pylori infection with a roughly 0.5–1 inch decrement in attained height [26–28], presumably due to the pediatric growth velocity relationship described above.
Unfortunately, we could not directly assess potential confounding by H. pylori infection status because we did not have this information. However, under the assumption that all of the cancer risk difference is explained by variation in H. pylori infection and that prevalence is 40% overall [29], our data would imply H. pylori prevalences of about 50% for height tertile 1 and 33% for tertiles 2 and 3. Accordingly, 5/12 (i.e., 1/3×50%/40%) of H. pylori-infected subjects and 5/18 (i.e., 1/3×50%/60%) of H. pylori-uninfected subjects would be in height tertile 1. Based on the height distribution for the NIH-AARP population overall, these considerations lead to estimated averages of 69.4 and 63.4 inches for H. pylori-positive men and women, respectively, and of 70.0 and 64.0 inches for H. pylori-negative men and women, respectively. Thus, indirect estimates from our data lead to the same 0.6 inch difference that has been empirically observed. Importantly, this analysis suggests that the height difference in gastric cancer may merely reflect association with H. pylori infection, rather than any effect on progression from infection to malignancy. Furthermore, the null association with cardia cancer substantiates previous evidence that gastric cancer at this anatomic subsite is largely unrelated to H. pylori infection.
As we previously reported [5], there are subsite-specific differences in gastric cancer incidence trends in the U.S., suggesting that progression to cancer may require different co-factors by anatomic subsite. Our results are consistent with the known association of excess body weight with cardia cancer [8], which may be mediated at least in part by gastroesophageal reflux disease [30]. However, our findings suggest that excess body weight is not associated with risk of noncardia gastric cancer, either as individual or combined subsites.
Our study had several strengths, including its prospective nature and relatively low proportion of subsite-unspecified cancer (16%). However, the analysis was based on self-reported anthropometric variables, which tend to understate weight and overstate height [31, 32]. Nevertheless, there is no reason to expect differential misclassification associated with future development of gastric cancer. Lastly, although our study was large, we still had limited statistical power to examine associations with cancers localized to some anatomic subsites.
In conclusion, patients with noncardia gastric cancer tend to be short in stature. The known association between attained height and H. pylori status seems to be sufficient in magnitude to fully explain the difference in cancer risk. Our study provides additional data regarding the role of early life exposures in gastric carcinogenesis.
ACKNOWLEDGEMENTS
This work was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health. Specific to NIH-AARP: Cancer incidence data from the Atlanta metropolitan area were collected by the Georgia Center for Cancer Statistics, Department of Epidemiology, Rollins School of Public Health, Emory University. Cancer incidence data from California were collected by the California Department of Health Services, Cancer Surveillance Section. Cancer incidence data from the Detroit metropolitan area were collected by the Michigan Cancer Surveillance Program, Community Health Administration, State of Michigan. The Florida cancer incidence data used in this report were collected by the Florida Cancer Data System (FCDC) under contract with the Florida Department of Health (FDOH), the views expressed herein are solely those of the authors and do not necessarily reflect those of the FCDC or FDOH. Cancer incidence data from Louisiana were collected by the Louisiana Tumor Registry, Louisiana State University Medical Center in New Orleans. Cancer incidence data from New Jersey were collected by the New Jersey State Cancer Registry, Cancer Epidemiology Services, New Jersey State Department of Health and Senior Services. Cancer incidence data from North Carolina were collected by the North Carolina Central Cancer Registry. Cancer incidence data from Pennsylvania were supplied by the Division of Health Statistics and Research, Pennsylvania Department of Health, Harrisburg, Pennsylvania, the Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations or conclusions. Cancer incidence data from Arizona were collected by the Arizona Cancer Registry, Division of Public Health Services, Arizona Department of Health Services. Cancer incidence data from Texas were collected by the Texas Cancer Registry, Cancer Epidemiology and Surveillance Branch, Texas Department of State Health Services. We are indebted to the participants in the NIH-AARP Diet and Health Study for their outstanding cooperation. We also thank Sigurd Hermansen and Kerry Grace Morrissey from Westat for outcomes ascertainment and study management and Nathan Arpel at Information Management Services for data support and analysis. The NIH-AARP Diet and Health Study is registered at clinicaltrials.gov as NCT00340015.
Footnotes
Data sharing statement: To gain access to the NIH-AARP data, the correct procedures and proposal application should be followed. Instructions are accessible via the website: http://www.dietandhealth.cancer.gov
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Kim MA, Lee HS, Yang HK, Kim WH. Clinicopathologic and protein expression differences between cardia carcinoma and noncardia carcinoma of the stomach. Cancer. 2005;103:1439–1446. doi: 10.1002/cncr.20966. [DOI] [PubMed] [Google Scholar]
- 3.Webb PM, Law M, Varghese C, Forman D, Yuan JM, Yu M, et al. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut. 2001;49:347–353. doi: 10.1136/gut.49.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson WF, Camargo MC, Fraumeni JF, Correa P, Rosenberg PS, Rabkin CS. Age-Specific Trends in Incidence of Noncardia Gastric Cancer in US Adults. Jama-Journal of the American Medical Association. 2010;303:1723–1728. doi: 10.1001/jama.2010.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camargo MC, Anderson WF, King JB, Correa P, Thomas CC, Rosenberg PS, et al. Divergent trends for gastric cancer incidence by anatomical subsite in US adults. Gut. 2011;60:1644–1649. doi: 10.1136/gut.2010.236737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansson LE, Baron J, Nyren O, Bergstrom R, Wolk A, Lindgren A, et al. Early-life risk indicators of gastric cancer. A population-based case-control study in Sweden. Int J Cancer. 1994;57:32–37. doi: 10.1002/ijc.2910570107. [DOI] [PubMed] [Google Scholar]
- 7.Tretli S, Robsahm TE. Height, weight and cancer of the oesophagus and stomach: a follow-up study in Norway. Eur J Cancer Prev. 1999;8:115–122. doi: 10.1097/00008469-199904000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Yang P, Zhou Y, Chen B, Wan HW, Jia GQ, Bai HL, et al. Overweight, obesity and gastric cancer risk: results from a meta-analysis of cohort studies. Eur J Cancer. 2009;45:2867–2873. doi: 10.1016/j.ejca.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 9.Abnet CC, Freedman ND, Hollenbeck AR, Fraumeni JF, Jr, Leitzmann M, Schatzkin A. A prospective study of BMI and risk of oesophageal and gastric adenocarcinoma. Eur J Cancer. 2008;44:465–471. doi: 10.1016/j.ejca.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Doherty MG, Freedman ND, Hollenbeck AR, Schatzkin A, Abnet CC. A prospective cohort study of obesity and risk of oesophageal and gastric adenocarcinoma in the NIH-AARP Diet and Health Study. Gut. 2012;61:1261–1268. doi: 10.1136/gutjnl-2011-300551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schatzkin A, Subar AF, Thompson FE, Harlan LC, Tangrea J, Hollenbeck AR, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions : the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol. 2001;154:1119–1125. doi: 10.1093/aje/154.12.1119. [DOI] [PubMed] [Google Scholar]
- 12.Silventoinen K. Determinants of variation in adult body height. J Biosoc Sci. 2003;35:263–285. doi: 10.1017/s0021932003002633. [DOI] [PubMed] [Google Scholar]
- 13.International Agency for Research on Cancer. Monographs on the evaluation of carcinogenic risks to humans: Schistosomes, liver flukes and Helicobacter pylori. First ed. Lyon, France: IARC Press; 1994. [PMC free article] [PubMed] [Google Scholar]
- 14.Prentice AM, Darboe MK. Growth and host-pathogen interactions. Nestle Nutr Workshop Ser Pediatr Program. 2008;61:197–210. doi: 10.1159/000113495. [DOI] [PubMed] [Google Scholar]
- 15.Goodman KJ, Correa P, Mera R, Yepez MC, Ceron C, Campo C, et al. Effect of Helicobacter pylori infection on growth velocity of school-age Andean children. Epidemiology. 2011;22:118–126. doi: 10.1097/EDE.0b013e3181fe7e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stephensen CB. Burden of infection on growth failure. J Nutr. 1999;129:534S–538S. doi: 10.1093/jn/129.2.534S. [DOI] [PubMed] [Google Scholar]
- 17.Furuta T, Shirai N, Xiao F, Takashima M, Hanai H. Effect of Helicobacter pylori infection and its eradication on nutrition. Aliment Pharmacol Ther. 2002;16:799–806. doi: 10.1046/j.1365-2036.2002.01222.x. [DOI] [PubMed] [Google Scholar]
- 18.Chandra RK. Immunocompetence as a Functional Index of Nutritional-Status. British Medical Bulletin. 1981;37:89–94. doi: 10.1093/oxfordjournals.bmb.a071682. [DOI] [PubMed] [Google Scholar]
- 19.Crimmins EM, Finch CE. Infection, inflammation, height, and longevity. Proc Natl Acad Sci U S A. 2006;103:498–503. doi: 10.1073/pnas.0501470103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munoz N, Plummer M, Vivas J, Moreno V, De Sanjose S, Lopez G, et al. A case-control study of gastric cancer in Venezuela. Int J Cancer. 2001;93:417–423. doi: 10.1002/ijc.1333. [DOI] [PubMed] [Google Scholar]
- 21.Song YM, Smith GD, Sung J. Adult height and cause-specific mortality: a large prospective study of South Korean men. Am J Epidemiol. 2003;158:479–485. doi: 10.1093/aje/kwg173. [DOI] [PubMed] [Google Scholar]
- 22.Tran GD, Sun XD, Abnet CC, Fan JH, Dawsey SM, Dong ZW, et al. Prospective study of risk factors for esophageal and gastric cancers in the Linxian general population trial cohort in China. Int J Cancer. 2005;113:456–463. doi: 10.1002/ijc.20616. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka T, Nagata C, Oba S, Takatsuka N, Shimizu H. Prospective cohort study of body mass index in adolescence and death from stomach cancer in Japan. Cancer Sci. 2007;98:1785–1789. doi: 10.1111/j.1349-7006.2007.00583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green J, Cairns BJ, Casabonne D, Wright FL, Reeves G, Beral V. Height and cancer incidence in the Million Women Study: prospective cohort, and meta-analysis of prospective studies of height and total cancer risk. Lancet Oncol. 2011;12:785–794. doi: 10.1016/S1470-2045(11)70154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The Emerging Risk Factors Collaboration. Adult height and the risk of cause-specific death and vascular morbidity in 1 million people: individual participant meta-analysis. Int J Epidemiol. 2012;41:1419–1433. doi: 10.1093/ije/dys086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murray LJ, McCrum EE, Evans AE, Bamford KB. Epidemiology of Helicobacter pylori infection among 4742 randomly selected subjects from Northern Ireland. Int J Epidemiol. 1997;26:880–887. doi: 10.1093/ije/26.4.880. [DOI] [PubMed] [Google Scholar]
- 27.Moayyedi P, Forman D, Duffett S, Mason S, Brown J, Crocombe W, et al. The association between Helicobacter pylori infection and adult height. European Journal of Epidemiology. 2005;20:455–465. doi: 10.1007/s10654-004-6634-0. [DOI] [PubMed] [Google Scholar]
- 28.Shiotani A, Miyanishi T, Uedo N, Iishi H. Helicobacter pylori infection is associated with reduced circulating ghrelin levels independent of body mass index. Helicobacter. 2005;10:373–378. doi: 10.1111/j.1523-5378.2005.00343.x. [DOI] [PubMed] [Google Scholar]
- 29.Cardenas VM, Graham DY. Smoking and Helicobacter pylori infection in a sample of U.S. adults. Epidemiology. 2005;16:586–590. doi: 10.1097/01.ede.0000165365.52904.4a. [DOI] [PubMed] [Google Scholar]
- 30.Carneiro F, Chaves P. Pathologic risk factors of adenocarcinoma of the gastric cardia and gastroesophageal junction. Surg Oncol Clin N Am. 2006;15:697–714. doi: 10.1016/j.soc.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 31.Rowland ML. Self-reported weight and height. Am J Clin Nutr. 1990;52:1125–1133. doi: 10.1093/ajcn/52.6.1125. [DOI] [PubMed] [Google Scholar]
- 32.McAdams MA, Van Dam RM, Hu FB. Comparison of self-reported and measured BMI as correlates of disease markers in US adults. Obesity. 2007;15:188–196. doi: 10.1038/oby.2007.504. [DOI] [PubMed] [Google Scholar]