Abstract
The iatrogenic risks associated with excessive Mn administration in parenteral nutrition (PN) patients are well documented. Hypermanganesemia and neurotoxicity are associated with the duration of Mn supplementation, Mn dosage, as well as pathological conditions, such as anemia or cholestasis. Recent PN guidelines recommend the biomonitoring of patients if they receive Mn in their PN longer than 30 days. The data in the literature are conflicting about the method for assessing Mn stores in humans as a definitive biomarker of Mn exposure or induced-neurotoxicity has yet to be identified. The biomonitoring of Mn relies on the analysis of whole blood Mn (WB Mn) levels, which are highly variable among human population and are not strictly correlated with Mn-induced neurotoxicity. Alterations in dopaminergic (DAergic) and catecholaminergic metabolism have been studied as predictive biomarkers of Mn-induced neurotoxicity. Given these limitations, this review addresses various approaches for biomonitoring Mn exposure and neurotoxic risk.
Keywords: Manganese, Parenteral Nutrition, Biomonitoring, Neurotoxicity
1. Background
Mn is an essential trace element (Kemmerer et al., 1931), required for normal mammalian physiological processes, such as bone growth, development of cartilage and connective tissues (Hurley, 1981), reproductive function (Keen et al., 1999), neuronal function (Sloot and Gramsbergen, 1994; Takeda et al., 1998), immune function, digestion and defense against free radicals (Aschner et al., 2005; Greger, 1999). Mn supplementation in PN patients is essential (Hardy et al., 2008) to prevent the depletion of endogenous stores and symptoms of deficiency (Hardy, 2009). Despite the classification of Mn as an essential trace element, there is little evidence in humans of Mn deficiency being clinically relevant (Hardy et al., 2008), as no cases of deficiency have been described in humans receiving un-supplemented PN (Frankel, 1993). Most of the evidence for human Mn deficiency is derived from experimental studies where subjects received Mn depleted diets (Hardy et al., 2008). The first case of suspected Mn deficiency was in a male subject who was fed a chemically defined diet as part of an investigation for determining vitamin-K requirements. Mn was inadvertently omitted from the diet for 17 weeks; the subject developed mild dermatitis, reddening of his black hair and beard, slowed growth of hair, nails, and beard, occasional nausea and vomiting, and moderate weight loss. His total diet (food and water) provided only 0.35 mg Mn/d, resulting in 55 and 85% reduction in serum and stool Mn levels, respectively (Doisy, 1974). Friedman et al. (1987) investigated experimental Mn depletion in seven healthy male subjects, from 19 to 22 years of age. The subjects were fed a Mn-adequate diet (2.59 mg Mn/d) for 3 weeks to establish baseline data followed by a purified diet containing 0.11 mg Mn/d for 39 days (depletion), followed by two 5 day periods of 1.53 and 2.55 mg Mn/d (repletion). The appearance of dermatitis, termed Miliaria Crystallina (prickly heat), developed in five of the seven subjects at the end of the depletion period, but disappeared as Mn repletion began (Friedman et al., 1987).
Although, PN was introduced into medical practice in the 1960s (Buchman et al., 2009; Dudrick and Wilmore, 1968), the iatrogenic risk of Mn-induced neurotoxicity associated to PN was only recognized in 1990, when Mehta and Reilly (1990) reported a case of a 32-year old woman medicated with haloperidol, receiving Mn (0.3 mg) daily. After 4 months of Mn supplementation, the patient developed extrapyramidal signs, which were irreversible after haloperidol discontinuation. WB Mn was significantly increased, and 3 days after receiving Mn-free PN all symptoms resolved. WB Mn levels fell to normal limits within 1 month after Mn discontinuation in the PN solution (Mehta and Reilly, 1990).
Mn toxicity upon ingestion is rare as homeostatic mechanisms tightly regulate its absorption and excretion (Santamaria and Sulsky, 2010; Underwood, 1981), ensuring adequate supplies. In contrast, Mn delivered intravenously (IV) bypasses homeostatic mechanisms regulating Mn absorption (Alves et al., 1997; Bertinet et al., 2000; Fitzgerald et al., 1999; Hambidge et al., 1989; Malecki et al., 1996; Mehta and Reilly, 1990; Mirowitz et al., 1991; Reimund et al., 2000). When dietary Mn levels are high, adaptive changes include reduced gastrointestinal (GI) absorption of Mn, enhanced Mn liver metabolism, and increased biliary and pancreatic excretion of Mn (Aschner and Aschner, 2005). For example, when rats were given an oral tracer dose of MnCl2, the amounts found in the stomach, duodenum, and jejunum on a % dose/g tissue basis decreased as dietary Mn increased from 4 to 2000 ppm (Abrams et al., 1976). Some studies suggest that Mn is absorbed through an active transport mechanism (Garcia-Aranda, Wapnir and Lifshitz, 1983), which most likely involves the metal divalent transporter 1, referred as DMT1 (also known as DCT-1 or nramp-2) (Bai et al., 2008). The mechanism underlying the regulation of Mn absorption has not been fully clarified, but an important role has been ascribed to DMT-1 (Garcia et al., 2006; Wang, Li and Zheng, 2006). Mn homeostasis is also believed to be maintained by excretion of excess absorbed Mn through the gut (Davis, Zech and Greger, 1993) as biliary secretion is the main pathway for Mn excretion. Mn biliary elimination is dose-dependent (Malecki et al., 1996). The rate of Mn radioactivity elimination following an IV injection of a tracer dose in bile duct ligated rats was enhanced by IV injection of large amounts of unlabelled Mn, indicating inducible intestinal excretion of Mn (Bertinchamps, Miller and Cotzias, 1966). Dose-dependent elimination of tracer doses of Mn has also been reported in Mn-exposed miners from Chile as compared to control populations. The terminal blood half-time for the active (i.e., Mn-exposed) miners was 15 ± 2 days, compared to 37.5 ± 7.5 days for control individuals and 28.3 ± 8 days for ex-miners with chronic Mn toxicity who had stopped working in mining 2 to 25 years previously (Cotzias et al., 1968). There is a limited knowledge on the molecular mechanisms that mediate Mn elimination. Mn concentration in bile can exceed plasma by 100-fold, suggesting active transport (Crossgrove and Yokel, 2004). Recently, solute carrier 30A10 (SLC30A10) has been identified as a human Mn transporter, highly expressed in the liver. The autosomal recessively inherited disorder associated SLC30A10 deficiency leads to Mn accumulation in liver (Tuschl et al., 2013). No studies have been published on the effect of Mn on SLC30A10 expression.
Since the first report of Mn-induced neurotoxicity, numerous other cases of parkinsonian-like symptoms associated with Mn exposure from parenteral admixtures have been reported (Alves et al., 1997; Bertinet et al., 2000; Ejima et al., 1992; Fitzgerald et al., 1999; Mirowitz and Westrich, 1992; Mirowitz et al., 1991; Nagatomo et al., 1999; Hambidge et al., 1989; Hsieh et al., 2007; Iinuma et al., 2003; Komaki et al., 1999; Ono et al., 1995; Reynolds et al., 1994). Tables 1 and 2 show several cases of hypermanganesemia and induced-neurotoxicity in patients fed by the parenteral route.
Table 1.
SELECTED REPORTS OF HYPERMANGANESEMIA IN PARENTERALLY FED ADULT PATIENTS
| Reference | n | Age Range | Disease | Mn daily dose and duration of intake | Laboratory Findings | Radiological Findings | Clinical Symptoms |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Ejima et al. (1992) | 1 | 62 y | SBS | 2.2b mg (2.3 mo) | Incr WB Mn 3.0–5.6 μg/dL (normal range 0.4–2.0), decr after 15 wk of Mn-free PN | Incr MRI signal (basal ganglia, especially globus pallidus, tectum, and tegmentum of midbrain and pons); MRI signal decr after 22 wk of Mn-free PN. | Parkinsonism w/dysarthria, mild rigidity, hypokinesia, masked face, halting gate. |
| Mirowitz et al. (1991) | 9 | 51–74 y | numerous | 0.3–0.4 mg (mean PN duration 5.3 y, range 5 mo–11 y) | NR | Incr MRI signal (basal ganglia) | 5 pts w/neurologic symptoms: memory loss, confusion weakness, fatigue and imbalance. |
| Mirowitz and Westrich (1992 | 1 | 61 y | GI dyskinesia | 0.4 mg (PN duration 3 y) | NR | Incr MRI signal (globus pallidus); 12 mo after d/c Mn, complete regression MRI signals | No symptoms |
| Alves et al. (1997) | 1 | 63 y | SBS | 1–2 mg (PN duration 19 mo) | Incr serum Mn 114 nmol/L (normal range 10–40) and incr urine Mn 381 nmol/L (normal range 15–60 nmol/24 h) | Incr MRI signal (basal ganglia and white matter); 6 mo after d/c Mn, decr MRI signals | Gait disturbance, dystonic movements |
| Nagatomo et al. (1999) | 1 | 68 y | ulcerative colitis | 20 μmol (PN duration 3 mo) | Incr serum Mn 4.2 mg/dL (normal range 0.4–2.0 mg/dL) and incr urine Mn 9.0 mg/dL (normal range <2.0 mg/dL) | Incr MRI signal (basal ganglia) | Psychiatric symptoms and gait disturbance |
| 1 | 70 y | aspiration pneumonia | 20 μmol (PN duration 4 mo) | Incr serum Mn 5.1 mg/dL; urine Mn 1.0 mg/dL | Incr MRI signal (basal ganglia) | Progressive gait disturbance and confusion | |
| Fitzgerald et al. (1999) | 36 | NR | numerous | 500 μg (< 48h) | RBC Mn: low to normal | 3 selected cases of abnormal MRI | 1 pt was noted to exhibit petit mal seizures, vertigo, gait disturbances and peripheral neuropathy |
| 30 | NR | 500 μg (range 3–30 d) | |||||
| 21 | 14–87 y | 500 μg (range 36–5075 d) | Incr RBC Mn in 15/21 pts in pts w/PN > 37 d (normal range 11 to 23 μg/L) | ||||
| Bertinet et al. (2000) | 15 | 32–74 y | numerous | Median Mn suppl. 0.1 mg (median PN duration 3.8 y) | Decr WB Mn after 1 y of IV Mn withdrawal | 10/15 incr MRI signal (basal ganglia) at the beginning of the PN | No symptoms |
Conc-concentration, d/c - discontinuation, Decr - decreased, GI - gastrointestinal, h-hours, Incr - increased, mo - month, MRI - magnetic resonance imaging, NR - not reported, PN - parenteral nutrition, pts-patients, RBC - red blood cells, SBS - short-bowel disease, suppl. - supplementation, wk - weeks, w/o - without, w/- with, wt - weight, y-years.
Table 2.
SELECTED REPORTS OF HYPERMANGANESEMIA IN PARENTERALLY FED CHILDREN AND ADOLESCENT PATIENTS
| Reference | n | Age Range | Disease | Mn daily dose and duration of intake | Laboratory Findings | Radiological Findings | Clinical Symptoms |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Fell et al. (1992) | 57 | 1–162 mo | Numerous 46/57 w/o cholestasis | wt<10 Kg-1 μmol/Kg wt>10 Kg-0.8 μmol/Kg (median PN duration 1.25 mo) |
45/57 Incr WB Mn 615–1840 nmol/L (normal range 72–210 nmol/L); Higher Mn associated w/cholestasis. | 2 selected cases of abnormal MRI Incr MRI signal (globus pallidus, nuclei subthalamic) |
2 pts w/dystonic limb movements and abnormal posturing; 4 pts died |
| Reynolds et al. (1994) | 1 | 7 mo | SBS, PN associated liver disease | 44–55 μg/Kg (PN duration 17 mo) | Incr WB Mn 1740 nmol/L (normal range 73–210 nmol/L). | MRI abnormalities compatible w/deposition of a paramagnetic metal | Developmental delay, abnormal dystonic movements of both arms, microcephaly |
| 53 | NR | numerous | 44–55 μg/Kg (PN duration > 6 wk) | Incr WB Mn in 35/53 w/evidence of cholestatic liver disease > 360 nmo/L | NR | NR | |
| Ono et al. (1995) | 1 | 5 y | Intractable diarrhea and recurrent pancreatitis | 10 μmol (PN duration > 2y) | Incr WB Mn 135 μg/dL (normal range 14.6 + 4.7), 5 mo after d/c Mn, decr WB Mn to 20 μg/dL | Incr MRI signal (basal ganglia) | Nonspecific headache and amnesia |
| Komaki et al. (1998) | 1 | 2 y | Intractable vomiting and diarrhea | 82 μg/Kg (PN duration 14 mo) | Incr WB Mn 9.7 μg/dL (normal range < 2.5 μg/dL), 3 mo after d/c Mn, decr WB Mn to normal levels | Incr MRI signal (globus pallidus, thalamus), 3 mo after d/c Mn MRI abnormalities regressed | Tremor and generalized tonic seizures, psychomotor retardation, hyperactivity; 3 mo after d/c Mn, seizures and tremor completely disappeared |
| Hsieh et al. (2007) | 1 | 10 y | SBS | 8 μg/kg (PN duration 3 mo) | Incr WB Mn 3.7 μg/dL (normal range 0.4 – 1.4 μg/L) | Incr MRI signal (basal ganglia) | Tonic-clonic seizures |
| Iinuma et al. (2003) | 7 | 41–249 mo (mean 93 mo) | numerous | 15.7–91.5 μg/kg (PN duration 18–37 mo) | 4/7 Incr WB Mn 3.1–5.1 μg/dL (normal range 1.8–2.4) | 6/7 pts incr MRI signal, 12 mo after d/c Mn, 5/7 pts decr MRI signals | No symptoms |
Conc-concentration, d/c - discontinuation, Decr - decreased, GI - gastrointestinal, h-hours, Incr - increased, mo - month, MRI - magnetic resonance imaging, NR - not reported, PN - parenteral nutrition, pts-patients, RBC - red blood cells, SBS - short-bowel disease, suppl. - supplementation, wk - weeks, w/o - without, w/- with, wt - weight, y-years.
2. Modulating Factors of Mn Induced Neurotoxicity
Parenteral Mn Dosage
The dosage of parenteral Mn is recognized as an important risk factor for the development of hypermanganesemia and subsequent neurotoxicity. A broad range of daily adult Mn dosages, extending from a low dose of 0.18–0.91 μmol/d (0.01–0.05 mg/d) to a high of 40 μmol/d (2.2 mg/d) (Wretlind, 1972) has been previously recommended. Most of the case reports of Mn intoxication were in adults receiving > 500 μg/d of parenteral Mn (Alves et al., 1997; Dickerson, 2001; Ejima et al., 1992; Ono et al., 1995; Reimund et al., 2000; Taylor and Manara, 1994) or pediatric patients receiving > 40 μg/kg/d (Fell et al., 1996; Reynolds et al., 1994). The above adult dosage is significantly greater than the total estimated Mn absorbed dose of ~115 μg/d from food and drinking water, and 0.5 μg/d from inhaled Mn (ATSDR, 2000; Santamaria and Sulsky, 2010).
Duration of Mn Supplementation
Several reports describe an association between long-term PN and increased WB Mn levels (Alves et al., 1997; Iinuma et al., 2003; Komaki et al., 1999; Siepler et al., 2003) and brain Mn accumulation, particularly in the basal ganglia. Recent in vivo studies suggest that dopamine active transporter (DAT) plays an important role in Mn accumulation in the striatum (Erikson et al., 2005). Inhibition of DAT function in weanling male Sprague–Dawley rats attenuates Mn accumulation in the globus pallidus during chronic exposure (Anderson et al., 2007).
Increased brain Mn levels can be detected by T1-weighted magnetic resonance imaging (MRI) (Iinuma et al., 2003). A recent analysis of post-mortem data describes the cumulative effect of Mn supplementation in patients who received long-term PN for short bowel syndrome (SBS) (Howard et al., 2007). Hypermanganesemia may also be observed after a short course of PN, in patients receiving > 500 μg daily; for example, elevated Mn levels in red blood cells (RBC) of 2 patients were detected after 14 and 18 days of PN (Fitzgerald et al., 1999). These findings suggest a potential toxicity from the administration of high Mn doses in PN and argue that the routine addition of doses higher than 500 μg daily may pose a risk of Mn-induced neurotoxicity even after short-term administration.
Co-morbidities associated with Mn exposure
Hypermanganesemia can occur as a result of liver disease and decreased biliary excretion (Ikeda et al., 2000), as bile is the major excretory route for Mn. Patients on long-term PN may develop biliary stasis or obstructive jaundice (Angsten et al., 2012; Fallon et al., 2010; Graham et al., 1984; Jones et al., 1993; Pierro et al., 1989; Sax et al., 1986; Shattuck et al., 1993), resulting in excess tissue Mn accumulation (Alves et al., 1997; Fell et al., 1996; Ikeda et al., 2000; Witzleben et al., 1968). Elevated Mn levels have also been seen in patients suffering from chronic liver failure (with inability to excrete Mn via the biliary system) and undergoing PN supplementation (McKinney et al., 2004; Mehta and Reilly, 1990).
Iron (Fe) deficiency (ID) can increase brain Mn levels (Heilig et al., 2005), as it is associated with high concentrations of serum transferrin receptors (TfR) (Kivivuori et al., 2000; Punnonen et al., 1994), which are transporters present at the blood-brain barrier (BBB) that mediate brain influx of both Fe and Mn (Aschner and Aschner, 1991; Erikson et al., 2002).
Animal studies have demonstrated that ID enhances Mn absorption across the GI tract, independent of body Mn stores (Chandra and Shukla, 1976; Shukla et al., 1976). An inverse association has been also demonstrated between DMT1 levels and Mn absorption in humans (Finley and Davis, 1999). Competition between Mn and Fe for intestinal absorption likely occurs by way of DMT1 (Aschner et al., 2005; Bai et al., 2008; Rouault and Cooperman, 2006). DMT1 expression is regulated by Fe status (Thompson et al., 2007) and its levels greatly increase in the duodenum in response to ID (Gunshin et al., 2001). DMT1 is also present in the plasma membranes of astrocytes (Au et al., 2008). Erikson and Aschner (2006) showed that increased Mn uptake in primary astrocyte cultures, with altered Fe status is mediated primarily DMT-1 (Erikson and Aschner, 2006).
3. Toxicological Evaluation Mn Levels in PN
Levels of safe and adequate quantities of Mn in PN are not known (Alves et al., 1997). PN guidelines are based on a Mn oral reference dose (RfD) considering also Mn bioavailability by the oral route. The United States Environmental Protection Agency (US EPA, 1996) used estimates of Mn levels in typical western and vegetarian diets to calculate Mn RfD in food. The RfD represents an estimate of the daily exposure to which the human population (including sensitive subpopulations) may be continually exposed over a lifetime without an appreciable risk of deleterious effects (Goldhaber, 2003).
The RfD for Mn was calculated by dividing the no-observed-adverse-effect level (NOAEL) by the product of the total amount of uncertainty and modifying factors that reflect the limitations of the data used (Nance, 2005). The NOAEL is the highest experimental dose at which there is no statistically or biologically significant increase in frequency or severity of adverse health effects, as seen in the exposed population compared with an appropriate unexposed population (ECETOC, 2002). In contrast to numerous reports describing Mn toxicity following inhalation exposure at high doses in humans, there are relatively few reports on manganism arising from water or dietary sources. Four studies have reported toxicity from ingestion of drinking water containing high levels of Mn. Kawamura and coworkers (1941) documented outbreaks of manganism in Japan and Greece, respectively, due to consumption of well-water contaminated with extremely high levels of Mn (1.8 to 14 mg/L) (Kawamura et al., 1941). Wasserman and coworkers (2006, 2011) also reported adverse impact of Mn exposure associated with water consumption on child developmental outcomes in Bangladesh (Wasserman et al., 2006; Wasserman et al., 2011). In another study, neurologic symptoms were reported in individuals who consumed drinking water containing Mn levels of 1.8–2.3 mg/mL in Greece (Kondakis et al., 1989). Mn toxicity has been reported in an individual who consumed high amounts of Mn supplements for several years (Banta and Markesbery, 1977). In animals, the toxicity of ingested Mn is low and signs of a toxic response generally appear only after concentrations higher than 1,000 μg/g diet are consumed (Hurley, 1981).
Based on a composite of data from several epidemiological studies, the NOAEL was derived at 10 mg/d (0.14 mg/kg/d for 70 kg adult) for chronic human consumption of Mn in the diet (US EPA, 1996). However, the US EPA stated that there are significant concerns about possible adverse neurological effects at doses not far from the range of essentiality. Because of this concern, the US EPA recommended that a modifying factor of 3 be applied when assessing risk from Mn in drinking water or soil. A modified RfD of 0.05 mg/kg/d is recommended for Mn from drinking water or soil (Goldhaber, 2003).
The Mn acceptable daily intake (ADI) was established based on median intake because data are insufficient to calculate the recommended dietary allowance (RDA) (Gropper and Smith, 2013). Progress in the field of Mn nutrition has been hampered because of the lack of a practical method for assessing Mn status as inter-individual variations in Mn retention can be large (Davidsson et al., 1989). Blood Mn levels appear to reflect body Mn status of rats fed deficient or adequate amounts of Mn (Keen et al., 1983), but consistent changes in blood or plasma Mn levels have not been observed in depleted or repleted human subjects (Freeland-Graves et al., 1988; Friedman et al., 1987).
The US National Research Council (NRC) has established an estimated safe and acceptable dietary intake (ESADDI) of 2–5 mg/d for adults (Greger, 1998). The first guidelines for Mn supplementation in PN were developed by the American Medical Association Nutrition Advisory Group (AMA NAG) (AMA, 1979). In 1988, the pediatric parenteral trace element requirements were reevaluated by the Committee on Clinical Practice Issues of the American Society for Clinical Nutrition (ASCN) and the pediatric dosage of Mn was reduced to 1 μg/Kg/d (from 2 to 10 μg/Kg/d) about 10 years after the original AMA NAG recommendations were published (Greene et al., 1988). The European Society of Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the European Society for Clinical Nutrition and Metabolism (ESPEN) recommended 1μg/kg/d with a maximum of 50 μg/d for children receiving long-term PN (Koletzko et al., 2005).
In 2002, the published literature indicated a broad range in adults Mn supplementation in PN. Takagi et al. (2002) suggested a safe optimal Mn dose for adult patients undergoing PN at 1 μmol/d based on MRI observations (Takagi et al., 2002). Later, in 2004 the AMA guidelines were revisited by the American Society of Parenteral and Enteral Nutrition (ASPEN) and the daily intake of Mn for adults was reduced to 60–100 μg/d (Mirtallo et al., 2004). Although based on toxicological data, the Mn levels suggested in the recent ASPEN guidelines do not pose a significant risk for Mn overexposure in patients without risk factors, other sources of exposure should be considered in PN patients, such as the contamination of PN with Mn from raw components of PN (Buchman et al., 2009; Dickerson, 2001; Jetton et al., 1976; Malecki et al., 1996). Table 3 summarizes the guidelines issued for the parenteral administration of Mn.
Table 3.
GUIDELINES ISSUED FOR ADMNISTRATION OF Mn BY PARENTERAL ROUTE
| AMA (1979) | Children | 2–10 μg/kg/d |
| Adults | 0.15and 0.8 mg/d | |
| ASCN (1988) | Infants (pre-termand Term) and Children | 1 μg/Kg/d |
| ASPEN(2004) | Infants (pre-termand Term) and Children | 1 μg/Kg/d |
| Adults | 60–100 μg/d |
AMA: American Medical Association ASCN: American Society for Clinical Nutrition ASPEN: American Society of Parenteral and Enteral Nutrition
4. Biomonitoring of Mn in Patients Undergoing PN
Biomonitoring is based on a systematic collection of biological samples for analysis of concentrations of compounds, metabolites or specific non-adverse biological effect parameters, with the objective of assessing exposure and health risk in exposed subjects, comparing the data observed with the reference level and — if necessary — leading to corrective actions (Zielhuis, 1984). The World Health Organization (WHO) defined a biomarker as a chemical, its metabolite, or the product of an interaction between a chemical and some target molecule or cell that is measurable in the human body (WHO, 2006). Biomarker research assumes that toxicant-induced diseases are progressive and that injury proceeds from entry of the toxicant into target cells, which induces subcellular biochemical events, to cell- and organ-level events that eventually induce irreversible or persistent organism dysfunction (Silbergeld and Davis, 1994); such effect markers are generally preclinical indicators of abnormalities (Grandjean and Landrigan, 2006) (Figure 1).
Figure 1.
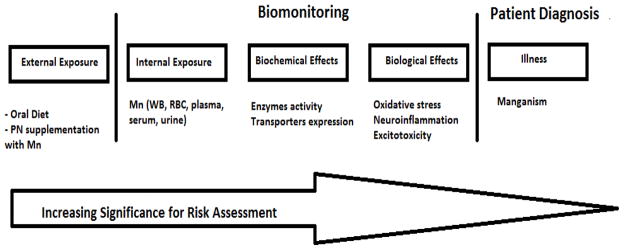
Biomonitoring paradigm of Mn in patients undergoing PN
Biomarkers may be classified into three types: biomarkers of exposure (internal dose), of effect or of susceptibility (Amorim, 2003; Costa, 1996). Exposure biomarkers reflect the internal dose of a xenobiotic and may be an exogenous compound (or a metabolite) within the body, an interactive product between the compound (or metabolite) and an endogenous component, or another event related to the exposure (Grandjean and Landrigan, 2006). A biomarker of effect is a characteristic that can be objectively measured and evaluated as an indicator of normal or pathogenic biologic processes (Atkinson, 2001).
Biomarkers of susceptibility serve as indicators of a particular sensitivity of individuals to the effect of a xenobiotic or to the effects of a group of such compounds (Gil and Pla, 2001). Biochemical events can be modified by genetically determined individual differences which may play a role as modifiers not only of long-term outcomes, but also of early biochemical changes. Examples of biomarkers of individual susceptibility to Mn include Fe status and markers of redox status (Smargiassi and Mutti, 1999), the latter playing a key role in Mn neurotoxicity.
4.1. Classical Approach of Mn Biomonitoring in PN Patients: Biomarkers of Exposure
Mn concentrations in Body Fluids
WB Mn levels are linked to the external administration of Mn by the PN route; for example a study in adult patients receiving PN showed that WB Mn changed in a dose-dependent manner. Takagi et al. (2002) reported that WB Mn was significantly higher when PN patients were administered 2 or 20 μmol Mn/d compared to 0 or 1 μmol/d during PN exposure (Takagi et al., 2002).
A reasonable biomarker of Mn exposure should display an acceptable threshold or cut-off value above which a Mn exposed individual can be differentiated from unexposed individuals (Zheng et al., 2011). Although WB Mn analysis is the preferred screening method, the high variability in normal human Mn levels makes it unsuitable for individual biological monitoring (Apostoli et al., 2000). A review by Iyengar and Woittiez (1988) reported median Mn values of 13.6 (8.0–18.7) μg/L in WB, 0.63 (0.54–1.76) μg/L in serum and 0.6 (0.5–9.8) μg/L in urine from a population covering 100,000 individuals from 55 countries (Iyengar and Woittiez, 1988). A comprehensive review on Mn from the USA quotes 4 – 15 μg/L for WB, whereas a more recent North American publication quotes a slightly higher range of 7– 16 μg/L as the normal levels in WB (Hardy et al., 2008). Albeit the high variability in WB Mn levels, a community based study in Québec showed an association between higher WB Mn (above 7.5 μg/L) levels and motor deficits (Mergler et al., 1999).
The utility of serum Mn has been questioned as a marker for whole body Mn as it was reported that intra-cerebral Mn levels were elevated in the presence of normal serum values (Alves et al., 1997). Several authors do not consider serum and WB Mn levels to be suitable indicators and suggest that the Mn content of RBCs may better reflect tissue accumulation as it is not directly influenced by current IV supplementation. WB matrix and WB Mn level is affected by IV delivery of Mn (Keen et al., 1983). Accordingly, in patients receiving PN, WB Mn is influenced by the pattern of PN (i.e. intermittent PN vs. continuous whole-day PN), as it may lead to changes in the distribution of Mn between tissues and blood and result in individual variation in response to parenterally administered Mn (Iinuma et al., 2003). RBC accounts for about 60–80% of Mn in the WB (Buchet et al., 1976; Hagenfeldt et al., 1973; Mirowitz et al., 1991), their turnover is slower than that of other cellular components (Milne et al., 1990) and RBC Mn levels are not directly dependent on current IV supplementation which makes them better indicators of Mn exposure (Bertinet et al., 2000).
Urinary Mn levels may also serve as a biomarker of exposure. However, biliary excretion is the main pathway by which Mn is excreted with most of the element ultimately being excreted in the feces (Aschner et al., 2005; Davis et al., 1993). Urinary Mn excretion is low, representing only about 0.01 ± 1% of the absorbed dose (Apostoli et al., 2000), and about 6% of the total excreted amount (Smargiassi and Mutti, 1999). Greger et al. (1990) and Davis and Greger (1992) failed to demonstrate a correlation between urinary Mn concentration and dietary intake in adult male and female volunteers (Davis and Greger, 1992; Greger et al., 1990). Mn in urine shows a poor correlation with either recent or cumulative dose (Lucchini et al., 1995; Smargiassi and Mutti, 1999).
Magnetic Resonance Imaging (MRI)
MRI intensity in the globus pallidus is a useful means for the detection of brain Mn accumulation (Finkelstein et al., 2008; Takagi et al., 2002). The Mn (II) ion has five unpaired electrons in the 3d orbit, causing the shortening of T1-relaxation time and an increase in signal intensity on T1-weighted MRI (Kim, 2004). In clinical settings, most patients undergoing PN are asymptomatic and diagnosed only on the basis of abnormal T1 signal intensity in the globus pallidus and occasionally in the tegmentum of the brainstem (Mirowitz et al., 1991; Saitoh et al., 1996).
4.2. Alternative Approaches: Subclinical Biomarkers predictive of Mn-induced Neurotoxicity
Neurobehavioral Tests
During the early stages of pathogenesis, Mn-induced neuropsychological impairment is subtle (Bowler et al., 2007; Mergler and Baldwin, 1997; Roels et al., 1987), characterized by alterations in motor function and response speed, as well as memory and more complex cognitive function impairments (Bowler et al., 2003; Laohaudomchok et al., 2011; Sinczuk-Walczak et al., 2001). The first manifestations of poisoning by Mn are usually subjective (Emara et al., 1971) (Figure 2). Biomarkers should provide information about neurotoxic effects-induced by Mn, as the preliminary early neurotoxic effects are subtle (Mergler and Baldwin, 1997), as they usually precede the emergence of more serious adverse effects. Neurophysiological assessments rely on sensitive tools to assess neurophysiological functions and neuropsychological performance; Mn exposure may lead to poorer hand-eye coordination, motor slowing, increased tremor, reduced response speed, olfactory enhancement, mood changes and possible memory and intellectual deficits (Iregren, 1994; Mergler et al., 1999).
Figure 2.

Progression of neurofunctional changes in response to Mn exposure (adapted from Mergler et al., 1997).
Neuroimaging Biomarker: 1H proton magnetic resonance spectroscopy (1H MRS)
Recent studies with 1H proton magnetic resonance spectroscopy (1H MRS), a noninvasive technique used for studying brain metabolites (Zheng et al., 2011), show an increase in γ-aminobutyric acid (GABA) content in the thalamus region of smelters occupationally exposed to Mn (Dydak et al., 2011). Classically, Mn neurotoxicity is characterized by deregulation of glutamatergic (Bagga and Patel, 2012; Garcia et al., 2006), GABAergic (Bagga and Patel, 2012; Erikson et al., 2002; Fitsanakis et al., 2006; Stanwood et al., 2009) and dopaminergic (DAergic) systems (Fitsanakis et al., 2006; Stanwood et al., 2009). Striatal GABA concentrations in rats are altered after long-low exposure to Mn (Gwiazda et al., 2002). Conflicting data exists as to whether Mn accumulation leads to decreases or increases in regional GABA levels (Erikson et al., 2002; Fitsanakis et al., 2006). For example, exposure to 6 mg Mn/kg/d led to a significant increase in brain Mn concentrations and significant decrease in GABA concentrations (Chandra et al., 1982). Another report showed that rats exposed to 20 mg Mn/kg/d had significantly increased brain Mn and GABA concentrations (Lipe et al., 1999). Accordingly, it appears that a relationship exists between the severity of Mn exposure and GABA concentrations, with lower Mn exposure leading to decreased GABA, and higher Mn exposure leading to increased GABA concentrations (Erikson et al., 2002; Fitsanakis et al., 2006). Further research is needed to study the effect of Mn exposure on GABA content in populations with higher risk of developing Mn-induced neurotoxicity before applying this methodology to biomonitor PN patients.
Hematologic and Urinary Biomarkers related to catecholaminergic system
Research strategies in development for a biomarker of Mn-induced neurotoxicity have focused mainly on the effects of Mn on the DAergic and GABAergic systems (Calabresi et al., 2001). Several studies reported alterations in homovanillic acid (HVA) (Ai et al., 1998; Siqueira and Moraes, 1989) and vanillylmandelic acid (VMA) (Ai et al., 1998) levels in populations exposed to higher Mn levels (Table 4). In humans, VMA is the major end product of norepinephrine and epinephrine metabolism, whereas HVA is the main end product of dopamine (DA) metabolism (Eisenhofer et al., 2004; Kvetnansky et al., 2009). The high prevalence of increased HVA levels suggest an increased activity of monoamine oxidase B (MAO B) (Smargiassi and Mutti, 1999). However, this finding was not replicated in a separate study of 11 men randomly selected in a ferromanganese-alloy plant, where a tendency towards lower platelet MAO B activity was found in the exposed workers (Smargiassi et al., 1995). MAO B cannot be recommended for monitoring early biochemical events of Mn exposure as platelet MAO B activity can be modified by genetically determined individual differences (Smargiassi and Mutti, 1999), such as differences in the genotype of transcription factor AP-2 beta (Pivac et al., 2006). Mn also affects dihydropteridine reductase (DHPR) activity, an enzyme required for regeneration of the cofactor tetrahydrobiopterin (BH4), an essential cofactor in enzymes involved in aromatic amino acid hydroxylation, such as phenylalanine and tyrosine hydroxylases (Butler et al., 1978). Altindag et al. (2003) found that Mn in the sulfate form, led to statistically significant decreases in DHPR activity (Altindag et al., 2003). These results are also consistent with a previous study from our group where we noted increased tyrosine and phenylalanine levels in the brains of rats exposed to Mn (Santos et al., 2012).
Table 4.
BIOCHEMICAL BIOMARKERS OF Mn-INDUCED NEUROTOXICITY
| Study | Study Population | Alterations in the exposed population |
|---|---|---|
|
| ||
| Ai et al. (1998) | Male welders (n=39), control group: non-occupationally exposed to Mn (n=19) | Incr HVA and Incr VMA (sig) |
| Siqueira and Moraes (1989) | Male workers occupationally exposed to Mn in a ferromanganese alloy plant (n=40), control group: non-occupationally exposed to Mn (n=25) | HVA levels not sig. different among the groups |
| Montes et al. (2008) | Population living close to a mine and mineral processing plant in Mexico (n=300) | Positive correl. (sig) prolactin and WB Mn |
| Alessio et al. (1989) | Male workers employed in a ferrous-Mn foundry (n=14), control group: non-occupationally exposed to Mn (n=14) | Incr Prolactin (sig) |
| Buchet (1993) | Male workers exposed to Mn-containing dust in a dry alkaline battery plant or an Mn oxide and salt producing plant (n=68), control group: non-occupationally exposed to Mn (n=35) | Positive correl. (sig) HVA and urine Mn |
| Roels et al. (1992) | Male workers (n=92) in a dry alkaline factory, exposed to MnO2, control group: non-occupationally exposed to Mn (n=101) | Incr Prolactin (not sig) |
| Takser et al. (2004) | Pregnant women at delivery (n=87) | Positive correl. (sig) prolactin and Mn cord blood levels |
Sig - significant, correl-correlation; incr - increased; Decr - decreased
Mn exposure in monkeys (Dydak et al., 2011) and rats (Fitsanakis et al., 2008) reduces striatal DA content. The DAergic function can also be assessed indirectly by measuring prolactin levels (Takser et al., 2004), as DA is produced by tubero-infundibular neurons and is the major factor controlling prolactin synthesis and release. Once taken up by the capillaries of the hypophysial portal system, DA acts directly on pituitary and neuronal DA receptors to inhibit prolactin secretion (Chang and Shin, 1997). Table 4 shows data from several studies in which prolactin levels were used to assess Mn-induced neurotoxicity (Alessio et al., 1989; Montes et al., 2008; Roels et al., 1992; Smargiassi and Mutti, 1999; Takser et al., 2004).
5. Summary
PN guidelines recommend the biomonitoring of patients if they receive Mn with their PN for >30 days (Mirtallo et al., 2004). The data in the literature are conflicting about the best method for assessing Mn stores in humans and a definitive biomarker of Mn exposure or induced-neurotoxicity has not been identified or validated. Biomarkers of Mn exposure, such as WB Mn levels, are of limited use in evaluating Mn-induced neurotoxicity. Accordingly, periodic brain MRI examination may be required to monitor excess Mn accumulation in the brain of patients receiving PN (Iinuma et al., 2003). However, MRI is not used as a routine method for diagnosis due to high costs and accessibility. Furthermore the relationship between hyperintensities in the basal ganglia and the onset of subclinical Mn neurotoxicity has yet to be determined (Finkelstein et al., 2008). Several authors also suggest that Mn accumulation detected by MRI does not necessarily correlate with the degree of clinically apparent neurological abnormalities (Masumoto et al., 2001). RBC Mn concentrations appear to be a promising biomarker of Mn exposure. Mn and Fe share similar transport mechanisms in cells of erythroid tissue, duodenal mucosa, kidney and blood-brain barrier (Chua and Morgan, 1997). The mechanisms of Mn transport into erythroid cells were investigated using rabbit reticulocytes and mature erythrocytes. High affinity Mn2+ transport occurred in reticulocytes, but not erythrocytes with Km of 0.4 μM. Low affinity Mn2+ transport occurred in erythrocytes as well as in reticulocytes and had Km of ~20 and 50 μM for the two cell types, respectively. The direction of Mn2+ transport was reversible, resulting in Mn2+ efflux. The uptake of Mn-Tf occurred only with reticulocytes and was dependent on receptor-mediated endocytosis (Chua et al., 1996). The permeability constant for inward and outward movement of Mn(II) in mature normal human RBC are 2.87 ± 0.13 × 10−9 and 1.38 ± 0.21 × 10−9 cm./sec, respectively. This slower rate of outward movement is consistent with the finding that 40 to 60 per cent of the Mn(II) taken up by the RBC is non-ultrafilterable. Less than 5 to 10 per cent of the Mn(II) appears to be bound to the stroma. This findings suggest that the entry and exit of Mn(II) is a process of passive diffusion involving no carriers, transport, or metabolic linkage (Weed and Rothstein, 1960). Research is needed to ascertain the relationship between Mn exposure and the impact in different exposure indices such as RBC, WB and urine Mn. Additional research is also needed to establish the putative relationships between Mn exposure, internal dose and alterations in neurotransmitter metabolites (HVA, VMA) and prolactin.
Supplementary Material
Highlights.
Hypermanganesemia and neurotoxicity are associated with the duration of Mn supplementation.
Whole blood Mn levels are not well correlated with Mn-induced neurotoxicity.
This review addresses various approaches for biomonitoring Mn exposure and neurotoxic risk.
Acknowledgments
The authors acknowledge FCT (Foundation for Science and Technology of Portugal; SFRH/BD/64128/2009) and the National Institute of Environmental Health Sciences (ES R01 10563 (MA)).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Abrams E, Lassiter JW, Miller WJ, Neathery MW, Gentry RP, Scarth RD. Absorption as a factor in manganese homeostasis. J Anim Sci. 1976;42:630–6. doi: 10.2527/jas1976.423630x. [DOI] [PubMed] [Google Scholar]
- Ai LB, Chua LH, New AL, Lee BL, Liu YM, Chia SE, et al. Urinary homovanillic acid (HVA) and vanillymandelic acid (VMA) in workers exposed to manganese dust. Biol Trace Elem Res. 1998;64:89–99. doi: 10.1007/BF02783327. [DOI] [PubMed] [Google Scholar]
- Alessio L, Apostoli P, Ferioli A, Lombardi S. Interference of manganese on neuroendocrinal system in exposed workers. Preliminary report. Biol Trace Elem Res. 1989;21:249–53. doi: 10.1007/BF02917260. [DOI] [PubMed] [Google Scholar]
- Altindag ZZ, Baydar T, Engin AB, Sahin G. Effects of the metals on dihydropteridine reductase activity. Toxicol In Vitro. 2003;17:533–37. doi: 10.1016/s0887-2333(03)00136-x. [DOI] [PubMed] [Google Scholar]
- Alves G, Thiebot J, Tracqui A, Delangre T, Guedon C, Lerebours E. Neurologic disorders due to brain manganese deposition in a jaundiced patient receiving long-term parenteral nutrition. J Parenter Enteral Nutr. 1997;21:41–5. doi: 10.1177/014860719702100141. [DOI] [PubMed] [Google Scholar]
- AMA. Guidelines for essential trace element preparations for parenteral use. JAMA. 1979;241:2051–4. [PubMed] [Google Scholar]
- Amorim L. Os biomarcadores e a sua aplicação na avaliação da exposição aos agentes químicos ambientais. Rev Bras Epidemiol. 2003;6:158–70. [Google Scholar]
- Anderson JG, Cooney PT, Erikson KM. Inhibition of DAT function attenuates manganese accumulation in the globus pallidus. Environ Toxicol Pharmacol. 2007;23:179–184. doi: 10.1016/j.etap.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angsten G, Finkel Y, Lucas S, Kassa AM, Paulsson M, Lilja HE. Improved outcome in neonatal short bowel syndrome using parenteral fish oil in combination with omega-6/9 lipid emulsions. J Parenter Enteral Nutr. 2012;36:587–95. doi: 10.1177/0148607111430507. [DOI] [PubMed] [Google Scholar]
- Apostoli P, Lucchini R, Alessio L. Are current biomarkers suitable for the assessment of manganese exposure in individual workers? Am J Ind Med. 2000;37:283–90. doi: 10.1002/(sici)1097-0274(200003)37:3<283::aid-ajim6>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Aschner M, Aschner JL. Manganese neurotoxicity: cellular effects and blood-brain barrier transport. Neurosci Biobehav Rev. 1991;15:333–40. doi: 10.1016/s0149-7634(05)80026-0. [DOI] [PubMed] [Google Scholar]
- Aschner M, Erikson KM, Dorman DC. Manganese dosimetry: species differences and implications for neurotoxicity. Crit Rev Toxicol. 2005;35:1–32. doi: 10.1080/10408440590905920. [DOI] [PubMed] [Google Scholar]
- Aschner J, Aschner M. Nutritional aspects of Mn homeostasis. Mol Aspects Med. 2005:353–62. doi: 10.1016/j.mam.2005.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson AJ, DeGruttola VG, DeMets DL, Downing GJ, Hoth DF, Oates JA, et al. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- ATSDR. Toxicological Profile for Manganese. Atlanta Georgia: US Department of Health and Human Services, Agency for Toxic Substances and Disease Registry; 2000. pp. 1–466. [Google Scholar]
- Au C, Benedetto A, Aschner M. Manganese transport in eukaryotes: the role of DMT1. Neurotoxicology. 2008;29:569–76. doi: 10.1016/j.neuro.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagga P, Patel AB. Regional cerebral metabolism in mouse under chronic manganese exposure: implications for Manganism. Neurochem Int. 2012;60:177–85. doi: 10.1016/j.neuint.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Bai SP, Lu L, Luo XG, Liu B. Kinetics of manganese absorption in ligated small intestinal segments of broilers. Poult Sci. 2008;87:2596–604. doi: 10.3382/ps.2008-00117. [DOI] [PubMed] [Google Scholar]
- Banta RG, Markesbery WR. Elevated manganese levels associated with dementia and extrapyramidal signs. Neurology. 1977;27:213–6. doi: 10.1212/wnl.27.3.213. [DOI] [PubMed] [Google Scholar]
- Bertinchamps AJ, Miller ST, Cotzias GC. Interdependence of routes excreting manganese. Am J Physiol. 1966;211:217–24. doi: 10.1152/ajplegacy.1966.211.1.217. [DOI] [PubMed] [Google Scholar]
- Bertinet DB, Tinivella M, Balzola FA, de Francesco A, Davini O, Rizzo L, et al. Brain manganese deposition and blood levels in patients undergoing home parenteral nutrition. J Parenter Enteral Nutr. 2000;24:223–7. doi: 10.1177/0148607100024004223. [DOI] [PubMed] [Google Scholar]
- Bowler RM, Gysens S, Diamond E, Booty A, Hartney C, Roels HA. Neuropsychological sequelae of exposure to welding fumes in a group of occupationally exposed men. Int J Hyg Environ Health. 2003;206:517–29. doi: 10.1078/1438-4639-00249. [DOI] [PubMed] [Google Scholar]
- Bowler RM, Nakagawa S, Drezgic M, Roels HA, Park RM, Diamond E, et al. Sequelae of fume exposure in confined space welding: a neurological and neuropsychological case series. Neurotoxicology. 2007;28:298–311. doi: 10.1016/j.neuro.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Buchet JP, Lauwerys R, Roels H, De Vos C. Determination of manganese in blood and urine by flameless atomic absorption spectrophotometry. Clin Chim Acta. 1976;73:481–6. doi: 10.1016/0009-8981(76)90151-0. [DOI] [PubMed] [Google Scholar]
- Buchman AL, Howard LJ, Guenter P, Nishikawa RA, Compher CW, Tappenden KA. Micronutrients in parenteral nutrition: too little or too much? The past, present, and recommendations for the future. Gastroenterology. 2009;137:S1–6. doi: 10.1053/j.gastro.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Butler IJ, Koslow SH, Krumholz A, Holtzman NA, Kaufman S. A disorder of biogenic amines in dihydropteridine reductase deficiency. Ann Neurol. 1978;3:224–30. doi: 10.1002/ana.410030307. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Ammassari-Teule M, Gubellini P, Sancesario G, Morello M, Centonze D, et al. A synaptic mechanism underlying the behavioral abnormalities induced by manganese intoxication. Neurobiol Dis. 2001;8:419–32. doi: 10.1006/nbdi.2000.0379. [DOI] [PubMed] [Google Scholar]
- Chandra SV, Malhotra KM, Shukla GS. GABAergic neurochemistry in manganese exposed rats. Acta Pharmacol Toxicol (Copenh) 1982;51:456–8. doi: 10.1111/j.1600-0773.1982.tb01053.x. [DOI] [PubMed] [Google Scholar]
- Chandra SV, Shukla GS. Role of iron deficiency in inducing susceptibility to manganese toxicity. Arch Toxicol. 1976;35:319–23. doi: 10.1007/BF00570272. [DOI] [PubMed] [Google Scholar]
- Chang A, Shin SH. Relationships between dopamine-induced changes in cytosolic free calcium concentration ([Ca2+]i) and rate of prolactin secretion. Elevated [Ca2+]i does not indicate prolactin release. Endocrine. 1997;7:343–9. doi: 10.1007/BF02801329. [DOI] [PubMed] [Google Scholar]
- Chua AC, Morgan EH. Manganese metabolism is impaired in the Belgrade laboratory rat. J Comp Physiol B. 1997;167:361–9. doi: 10.1007/s003600050085. [DOI] [PubMed] [Google Scholar]
- Chua AC, Stonell LM, Savigni DL, Morgan EH. Mechanisms of manganese transport in rabbit erythroid cells. J Physiol. 1996;493:99–112. doi: 10.1113/jphysiol.1996.sp021367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG. Biomarker research in neurotoxicology: the role of mechanistic studies to bridge the gap between the laboratory and epidemiological investigations. Environ Health Perspect. 1996;104 (Suppl 1):55S–67. doi: 10.1289/ehp.96104s155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotzias GC, Horiuchi K, Fuenzalida S, Mena I. Chronic manganese poisoning. Clearance of tissue manganese concentrations with persistance of the neurological picture. Neurology. 1968;18:376–82. doi: 10.1212/wnl.18.4.376. [DOI] [PubMed] [Google Scholar]
- Crossgrove J, Yokel RA. Manganese distribution across the blood-brain barrier III. The divalent metal transporter-1 is not the major mechanism mediating brain manganese uptake. Neurotoxicology. 2004;25:451–60. doi: 10.1016/j.neuro.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Davidsson L, Cederblad A, Lonnerdal B, Sandstrom B. Manganese absorption from human milk, cow’s milk, and infant formulas in humans. Am J Dis Child. 1989;143:823–7. doi: 10.1001/archpedi.1989.02150190073024. [DOI] [PubMed] [Google Scholar]
- Davis CD, Greger JL. Longitudinal changes of manganese-dependent superoxide dismutase and other indexes of manganese and iron status in women. Am J Clin Nutr. 1992;55:747–52. doi: 10.1093/ajcn/55.3.747. [DOI] [PubMed] [Google Scholar]
- Davis CD, Zech L, Greger JL. Manganese metabolism in rats: an improved methodology for assessing gut endogenous losses. Proc Soc Exp Biol Med. 1993;202:103–8. doi: 10.3181/00379727-202-43518. [DOI] [PubMed] [Google Scholar]
- Dickerson RN. Manganese intoxication and parenteral nutrition. Nutrition. 2001;17:689–93. doi: 10.1016/s0899-9007(01)00546-9. [DOI] [PubMed] [Google Scholar]
- Doisy EA. Effects of deficiency in manganese upon plasma levels of clotting proteins and cholesterol in man. In: Hoekstra WG, Suttie JW, Ganther HE, Mertz W, editors. Trace Element Metabolism in Animals. Baltimore: University Park Press; 1974. pp. 668–70. [Google Scholar]
- Dudrick SJ, Wilmore DWVH, Rhoads JE. Long-term total parenteral nutrition with growth, development and positive nitrogen balance. Surgery. 1968;64:134–42. [PubMed] [Google Scholar]
- Dydak U, Jiang YM, Long LL, Zhu H, Chen J, Li WM, et al. In vivo measurement of brain GABA concentrations by magnetic resonance spectroscopy in smelters occupationally exposed to manganese. Environ Health Perspect. 2011;119:219–24. doi: 10.1289/ehp.1002192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECETOC. Technical Report 85. European Centre for Ecotoxicology and Toxicology of Chemicals; 2002. Recognition of, and differentiation between, adverse and non-adverse effects in toxicology studies; pp. 1–56. [Google Scholar]
- Eisenhofer G, Kopin IJ, Goldstein DS. Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev. 2004;56:331–49. doi: 10.1124/pr.56.3.1. [DOI] [PubMed] [Google Scholar]
- Ejima A, Imamura T, Nakamura S, Saito H, Matsumoto K, Momono S. Manganese intoxication during total parenteral nutrition. Lancet. 1992;339:426. doi: 10.1016/0140-6736(92)90109-g. [DOI] [PubMed] [Google Scholar]
- Emara AM, el-Ghawabi SH, Madkour OI, el-Samra GH. Chronic manganese poisoning in the dry battery industry. Br J Ind Med. 1971;28:78–82. doi: 10.1136/oem.28.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson KM, Aschner M. Increased manganese uptake by primary astrocyte cultures with altered iron status is mediated primarily by divalent metal transporter. Neurotoxicology. 2006;27:125–30. doi: 10.1016/j.neuro.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Shihabi ZK, Aschner JL, Aschner M. Manganese accumulates in iron-deficient rat brain regions in a heterogeneous fashion and is associated with neurochemical alterations. Biol Trace Elem Res. 2002;87:143–56. doi: 10.1385/BTER:87:1-3:143. [DOI] [PubMed] [Google Scholar]
- Erikson KM, John CE, Jones SR, Aschner M. Manganese accumulation in striatum of mice exposed to toxic doses is dependent upon a functional dopamine transporter. Environ Toxicol Pharmacol. 2005;20:390–394. doi: 10.1016/j.etap.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Fallon EM, Le HD, Puder M. Prevention of parenteral nutrition-associated liver disease: role of omega-3 fish oil. Curr Opin Organ Transplant. 2010;15:334–40. doi: 10.1097/mot.0b013e3283394879. [DOI] [PubMed] [Google Scholar]
- Fell JM, Reynolds AP, Meadows N, Khan K, Long SG, Quaghebeur G, et al. Manganese toxicity in children receiving long-term parenteral nutrition. Lancet. 1996;347:1218–21. doi: 10.1016/s0140-6736(96)90735-7. [DOI] [PubMed] [Google Scholar]
- Finkelstein Y, Zhang N, Fitsanakis VA, Avison MJ, Gore JC, Aschner M. Differential deposition of manganese in the rat brain following subchronic exposure to manganese: a T1-weighted magnetic resonance imaging study. Isr Med Assoc J. 2008;10:793–8. [PMC free article] [PubMed] [Google Scholar]
- Finley JW, Davis CD. Manganese deficiency and toxicity: are high or low dietary amounts of manganese cause for concern? Biofactors. 1999;10:15–24. doi: 10.1002/biof.5520100102. [DOI] [PubMed] [Google Scholar]
- Fitsanakis VA, Au C, Erikson KM, Aschner M. The effects of manganese on glutamate, dopamine and gamma-aminobutyric acid regulation. Neurochem Int. 2006;48:426–33. doi: 10.1016/j.neuint.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Fitsanakis VA, Zhang N, Anderson JG, Erikson KM, Avison MJ, Gore JC, et al. Measuring brain manganese and iron accumulation in rats following 14 weeks of low-dose manganese treatment using atomic absorption spectroscopy and magnetic resonance imaging. Toxicol Sci. 2008;103:116–24. doi: 10.1093/toxsci/kfn019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald K, Mikalunas V, Rubin H, McCarthey R, Vanagunas A, Craig RM. Hypermanganesemia in patients receiving total parenteral nutrition. J Parenter Enteral Nutr. 1999;23:333–6. doi: 10.1177/0148607199023006333. [DOI] [PubMed] [Google Scholar]
- Frankel D. Supplementation of trace elements in parenteral nutrition: rationale and recommendations. Nutrition Research. 1993;13:583–96. [Google Scholar]
- Freeland-Graves JH, Behmardi F, Bales CW, Dougherty V, Lin PH, Crosby JB, et al. Metabolic balance of manganese in young men consuming diets containing five levels of dietary manganese. J Nutr. 1988;118:764–73. doi: 10.1093/jn/118.6.764. [DOI] [PubMed] [Google Scholar]
- Friedman BJ, Freeland-Graves JH, Bales CW, Behmardi F, Shorey-Kutschke RL, Willis RA, et al. Manganese balance and clinical observations in young men fed a manganese-deficient diet. J Nutr. 1987;117:133–43. doi: 10.1093/jn/117.1.133. [DOI] [PubMed] [Google Scholar]
- Garcia-Aranda JA, Wapnir RA, Lifshitz F. In vivo intestinal absorption of manganese in the rat. J Nutr. 1983;113:2601–7. doi: 10.1093/jn/113.12.2601. [DOI] [PubMed] [Google Scholar]
- Garcia SJ, Gellein K, Syversen T, Aschner M. A manganese-enhanced diet alters brain metals and transporters in the developing rat. Toxicol Sci. 2006;92:516–25. doi: 10.1093/toxsci/kfl017. [DOI] [PubMed] [Google Scholar]
- Gil F, Pla A. Biomarkers as biological indicators of xenobiotic exposure. J Appl Toxicol. 2001;21:245–55. doi: 10.1002/jat.769. [DOI] [PubMed] [Google Scholar]
- Goldhaber SB. Trace element risk assessment: essentiality vs. toxicity Regul Toxicol Pharmacol. 2003;38:232–42. doi: 10.1016/s0273-2300(02)00020-x. [DOI] [PubMed] [Google Scholar]
- Graham MF, Tavill AS, Halpin TC, Louis LN. Inhibition of bile flow in the isolated perfused rat liver by a synthetic parenteral amino acid mixture: associated net amino acid fluxes. Hepatology. 1984;4:69–73. doi: 10.1002/hep.1840040112. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368:2167–78. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- Greene HL, Hambidge KM, Schanler R, Tsang RC. Guidelines for the use of vitamins, trace elements, calcium, magnesium, and phosphorus in infants and children receiving total parenteral nutrition: report of the Subcommittee on Pediatric Parenteral Nutrient Requirements from the Committee on Clinical Practice Issues of the American Society for Clinical Nutrition. Am J Clin Nutr. 1988;48:1324–42. doi: 10.1093/ajcn/48.5.1324. [DOI] [PubMed] [Google Scholar]
- Greger JL. Dietary standards for manganese: overlap between nutritional and toxicological studies. J Nutr. 1998;128:368S–71. doi: 10.1093/jn/128.2.368S. [DOI] [PubMed] [Google Scholar]
- Greger JL. Nutrition versus toxicology of manganese in humans: evaluation of potential biomarkers. Neurotoxicology. 1999;20:205–12. [PubMed] [Google Scholar]
- Greger JL, Davis CD, Suttie JW, Lyle BJ. Intake, serum concentrations, and urinary excretion of manganese by adult males. Am J Clin Nutr. 1990;51:457–61. doi: 10.1093/ajcn/51.3.457. [DOI] [PubMed] [Google Scholar]
- Gropper S, Smith J. Advanced Nutrition and Human Metabolism. 6. Belmont: Yolanda Cossio; 2013. [Google Scholar]
- Gunshin H, Allerson CR, Polycarpou-Schwarz M, Rofts A, Rogers JT, Kishi F, et al. Iron-dependent regulation of the divalent metal ion transporter. FEBS Lett. 2001;509:309–16. doi: 10.1016/s0014-5793(01)03189-1. [DOI] [PubMed] [Google Scholar]
- Gwiazda RH, Lee D, Sheridan J, Smith DR. Low cumulative manganese exposure affects striatal GABA but not dopamine. Neurotoxicology. 2002;23:69–76. doi: 10.1016/s0161-813x(02)00002-5. [DOI] [PubMed] [Google Scholar]
- Hagenfeldt K, Plantin LO, Diczfalusy E. Trace elements in the human endometrium. 2. Zinc, copper and manganese levels in the endometrium, cervical mucus and plasma. Acta Endocrinol (Copenh) 1973;72:115–26. [PubMed] [Google Scholar]
- Hambidge KM, Sokol RJ, Fidanza SJ, Goodall MA. Plasma manganese concentrations in infants and children receiving parenteral nutrition. J Parenter Enteral Nutr. 1989;13:168–71. doi: 10.1177/0148607189013002168. [DOI] [PubMed] [Google Scholar]
- Hardy G. Manganese in parenteral nutrition: who, when, and why should we supplement? Gastroenterology. 2009;137:S29–35. doi: 10.1053/j.gastro.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Hardy IJ, Gillanders L, Hardy G. Is manganese an essential supplement for parenteral nutrition? Curr Opin Clin Nutr Metab Care. 2008;11:289–96. doi: 10.1097/MCO.0b013e3282f9e889. [DOI] [PubMed] [Google Scholar]
- Heilig E, Molina R, Donaghey T, Brain JD, Wessling-Resnick M. Pharmacokinetics of pulmonary manganese absorption: evidence for increased susceptibility to manganese loading in iron-deficient rats. Am J Physiol Lung Cell Mol Physiol. 2005;288:L887–93. doi: 10.1152/ajplung.00382.2004. [DOI] [PubMed] [Google Scholar]
- Howard L, Ashley C, Lyon D, Shenkin A. Autopsy tissue trace elements in 8 long-term parenteral nutrition patients who received the current U.S. Food and Drug Administration formulation. J Parenter Enteral Nutr. 2007;31:388–96. doi: 10.1177/0148607107031005388. [DOI] [PubMed] [Google Scholar]
- Hsieh CT, Liang JS, Peng SS, Lee WT. Seizure associated with total parenteral nutrition-related hypermanganesemia. Pediatr Neurol. 2007;36:181–3. doi: 10.1016/j.pediatrneurol.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Hurley LS. Teratogenic aspects of manganese, zinc, and copper nutrition. Physiol Rev. 1981;61:249–95. doi: 10.1152/physrev.1981.61.2.249. [DOI] [PubMed] [Google Scholar]
- Iinuma Y, Kubota M, Uchiyama M, Yagi M, Kanada S, Yamazaki S, et al. Whole-blood manganese levels and brain manganese accumulation in children receiving long-term home parenteral nutrition. Pediatr Surg Int. 2003;19:268–72. doi: 10.1007/s00383-002-0929-6. [DOI] [PubMed] [Google Scholar]
- Ikeda S, Sera Y, Yoshida M, Ohshiro H, Uchino S, Oka Y, et al. Manganese deposits in patients with biliary atresia after hepatic porto-enterostomy. J Pediatr Surg. 2000;35:450–3. doi: 10.1016/s0022-3468(00)90212-4. [DOI] [PubMed] [Google Scholar]
- Iregren A. Using psychological tests for the early detection of neurotoxic effects of low level manganese exposure. Neurotoxicology. 1994;15:671–7. [PubMed] [Google Scholar]
- Iyengar V, Woittiez J. Trace elements in human clinical specimens: evaluation of literature data to identify reference values. Clin Chem. 1988;34:474–81. [PubMed] [Google Scholar]
- Jetton MM, Sullivan JF, Burch RE. Trace element contamination of intravenous solutions. Arch Intern Med. 1976;136:782–4. [PubMed] [Google Scholar]
- Jones MO, Pierro A, Hammond P, Nunn A, Lloyd DA. Glucose utilization in the surgical newborn infant receiving total parenteral nutrition. J Pediatr Surg. 1993;28:1121–5. doi: 10.1016/0022-3468(93)90144-a. [DOI] [PubMed] [Google Scholar]
- Kawamura R, Ikuta H, Fukuzumi S, Yamada R, Tsubaki S, Kodama T, et al. Intoxication by manganese in well water. Kitasato Archives of Experimental Medicine. 1941;18:145–69. [Google Scholar]
- Keen CL, Clegg MS, Lonnerdal B, Hurley LS. Whole-blood manganese as an indicator of body manganese. N Engl J Med. 1983;308:1230. [PubMed] [Google Scholar]
- Keen CL, Ensunsa JL, Watson MH, Baly DL, Donovan SM, Monaco MH, et al. Nutritional aspects of manganese from experimental studies. Neurotoxicology. 1999;20:213–23. [PubMed] [Google Scholar]
- Kemmerer A, Elvehjem C, Hart E. Studies on the relation of manganese to the nutrition of the mouse. J Biol Chem. 1931;92:623–30. [Google Scholar]
- Kim Y. High signal intensities on T1-weighted MRI as a biomarker of exposure to manganese. Ind Health. 2004;42:111–5. doi: 10.2486/indhealth.42.111. [DOI] [PubMed] [Google Scholar]
- Kivivuori SM, Pelkonen P, Ylijoki H, Verronen P, Siimes MA. Elevated serum transferrin receptor concentration in children with juvenile chronic arthritis as evidence of iron deficiency. Rheumatology (Oxford) 2000;39:193–7. doi: 10.1093/rheumatology/39.2.193. [DOI] [PubMed] [Google Scholar]
- Koletzko B, Goulet O, Hunt J, Krohn K, Shamir R. Guidelines on Paediatric Parenteral Nutrition of the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the European Society for Clinical Nutrition and Metabolism (ESPEN) J Pediatr Gastroenterol Nutr. 2005;41 (Suppl 2):S1–87. doi: 10.1097/01.mpg.0000181841.07090.f4. [DOI] [PubMed] [Google Scholar]
- Komaki H, Maisawa S, Sugai K, Kobayashi Y, Hashimoto T. Tremor and seizures associated with chronic manganese intoxication. Brain Dev. 1999;21:122–4. doi: 10.1016/s0387-7604(98)00074-6. [DOI] [PubMed] [Google Scholar]
- Kondakis XG, Makris N, Leotsinidis M, Prinou M, Papapetropoulos T. Possible health effects of high manganese concentration in drinking water. Arch Environ Health. 1989;44:175–8. doi: 10.1080/00039896.1989.9935883. [DOI] [PubMed] [Google Scholar]
- Kvetnansky R, Sabban EL, Palkovits M. Catecholaminergic systems in stress: structural and molecular genetic approaches. Physiol Rev. 2009;89:535–606. doi: 10.1152/physrev.00042.2006. [DOI] [PubMed] [Google Scholar]
- Laohaudomchok W, Lin X, Herrick RF, Fang SC, Cavallari JM, Shrairman R, et al. Neuropsychological effects of low-level manganese exposure in welders. Neurotoxicology. 2011;32:171–9. doi: 10.1016/j.neuro.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipe GW, Duhart H, Newport GD, Slikker W, Ali SF. Effect of manganese on the concentration of amino acids in different regions of the rat brain. J Environ Sci Health B. 1999;34:119–32. doi: 10.1080/03601239909373187. [DOI] [PubMed] [Google Scholar]
- Lucchini R, Selis L, Folli D, Apostoli P, Mutti A, Vanoni O, et al. Neurobehavioral effects of manganese in workers from a ferroalloy plant after temporary cessation of exposure. Scand J Work Environ Health. 1995;21:143–9. doi: 10.5271/sjweh.1369. [DOI] [PubMed] [Google Scholar]
- Malecki EA, Radzanowski GM, Radzanowski TJ, Gallaher DD, Greger JL. Biliary manganese excretion in conscious rats is affected by acute and chronic manganese intake but not by dietary fat. J Nutr. 1996;126:489–98. doi: 10.1093/jn/126.2.489. [DOI] [PubMed] [Google Scholar]
- Masumoto K, Suita S, Taguchi T, Yamanouchi T, Nagano M, Ogita K, et al. Manganese intoxication during intermittent parenteral nutrition: report of two cases. J Parenter Enteral Nutr. 2001;25:95–9. doi: 10.1177/014860710102500295. [DOI] [PubMed] [Google Scholar]
- McKinney AM, Filice RW, Teksam M, Casey S, Truwit C, Clark HB, et al. Diffusion abnormalities of the globi pallidi in manganese neurotoxicity. Neuroradiology. 2004;46:291–5. doi: 10.1007/s00234-004-1179-1. [DOI] [PubMed] [Google Scholar]
- Mehta R, Reilly JJ. Manganese levels in a jaundiced long-term total parenteral nutrition patient: potentiation of haloperidol toxicity? Case report and literature review. J Parenter Enteral Nutr. 1990;14:428–30. doi: 10.1177/0148607190014004428. [DOI] [PubMed] [Google Scholar]
- Mergler D, Baldwin M. Early manifestations of manganese neurotoxicity in humans: an update. Environ Res. 1997;73:92–100. doi: 10.1006/enrs.1997.3710. [DOI] [PubMed] [Google Scholar]
- Mergler D, Baldwin M, Belanger S, Larribe F, Beuter A, Bowler R, et al. Manganese neurotoxicity, a continuum of dysfunction: results from a community based study. Neurotoxicology. 1999;20:327–42. [PubMed] [Google Scholar]
- Milne DB, Sims RL, Ralston NV. Manganese content of the cellular components of blood. Clin Chem. 1990;36:450–2. [PubMed] [Google Scholar]
- Mirowitz SA, Westrich TJ. Basal ganglial signal intensity alterations: reversal after discontinuation of parenteral manganese administration. Radiology. 1992;185:535–6. doi: 10.1148/radiology.185.2.1410368. [DOI] [PubMed] [Google Scholar]
- Mirowitz SA, Westrich TJ, Hirsch JD. Hyperintense basal ganglia on T1-weighted MR images in patients receiving parenteral nutrition. Radiology. 1991;181:117–20. doi: 10.1148/radiology.181.1.1909445. [DOI] [PubMed] [Google Scholar]
- Mirtallo J, Canada T, Johnson D, Kumpf V, Petersen C, Sacks G, et al. Safe practices for parenteral nutrition. J Parenter Enteral Nutr. 2004;28:S39–70. doi: 10.1177/0148607104028006s39. [DOI] [PubMed] [Google Scholar]
- Montes S, Riojas-Rodriguez H, Sabido-Pedraza E, Rios C. Biomarkers of manganese exposure in a population living close to a mine and mineral processing plant in Mexico. Environ Res. 2008;106:89–95. doi: 10.1016/j.envres.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Nagatomo S, Umehara F, Hanada K, Nobuhara Y, Takenaga S, Arimura K, et al. Manganese intoxication during total parenteral nutrition: report of two cases and review of the literature. J Neurol Sci. 1999;162:102–5. doi: 10.1016/s0022-510x(98)00289-5. [DOI] [PubMed] [Google Scholar]
- Nance P. Encyclopedia of Toxycology. Elsevier Inc; 2005. Reference Dose (RfD) pp. 633–4. [Google Scholar]
- Ono J, Harada K, Kodaka R, Sakurai K, Tajiri H, Takagi Y, et al. Manganese deposition in the brain during long-term total parenteral nutrition. J Parenter Enteral Nutr. 1995;19:310–2. doi: 10.1177/0148607195019004310. [DOI] [PubMed] [Google Scholar]
- Pierro A, Carnielli V, Filler RM, Smith J, Heim T. Metabolism of intravenous fat emulsion in the surgical newborn. J Pediatr Surg. 1989;24:95–101. doi: 10.1016/s0022-3468(89)80310-0. [DOI] [PubMed] [Google Scholar]
- Pivac N, Knezevic J, Mustapic M, Dezeljin M, Muck-Seler D, Kozaric-Kovacic D, et al. The lack of association between monoamine oxidase (MAO) intron 13 polymorphism and platelet MAO-B activity among men. Life Sci. 2006;79:45–9. doi: 10.1016/j.lfs.2005.12.030. [DOI] [PubMed] [Google Scholar]
- Punnonen K, Irjala K, Rajamaki A. Iron-deficiency anemia is associated with high concentrations of transferrin receptor in serum. Clin Chem. 1994;40:774–6. [PubMed] [Google Scholar]
- Reimund JM, Dietemann JL, Warter JM, Baumann R, Duclos B. Factors associated to hypermanganesemia in patients receiving home parenteral nutrition. Clin Nutr. 2000;19:343–8. doi: 10.1054/clnu.2000.0120. [DOI] [PubMed] [Google Scholar]
- Reynolds AP, Kiely E, Meadows N. Manganese in long term paediatric parenteral nutrition. Arch Dis Child. 1994;71:527–8. doi: 10.1136/adc.71.6.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roels H, Lauwerys R, Genet P, Sarhan MJ, de Fays M, Hanotiau I, et al. Relationship between external and internal parameters of exposure to manganese in workers from a manganese oxide and salt producing plant. Am J Ind Med. 1987;11:297–305. doi: 10.1002/ajim.4700110307. [DOI] [PubMed] [Google Scholar]
- Roels HA, Ghyselen P, Buchet JP, Ceulemans E, Lauwerys RR. Assessment of the permissible exposure level to manganese in workers exposed to manganese dioxide dust. Br J Ind Med. 1992;49:25–34. doi: 10.1136/oem.49.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouault TA, Cooperman S. Brain iron metabolism. Semin Pediatr Neurol. 2006;13:142–8. doi: 10.1016/j.spen.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Saitoh Y, Kimura S, Nezu A, Ohtsuki N, Kobayashi T, Osaka H, et al. Hyperintense brain lesions on T1-weighted MRI after parenteral nutrition. No To Hattatsu Brain and Development. 1996;28:39–43. [PubMed] [Google Scholar]
- Santamaria AB, Sulsky SI. Risk assessment of an essential element: manganese. J Toxicol Environ Health A. 2010;73:128–55. doi: 10.1080/15287390903337118. [DOI] [PubMed] [Google Scholar]
- Santos D, Batoreu MC, Almeida I, Ramos R, Sidoryk-Wegrzynowicz M, Aschner M, et al. Manganese Alters Rat Brain Amino Acids Levels. Biol Trace Elem Res. 2012;150:337–41. doi: 10.1007/s12011-012-9504-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sax HC, Talamini MA, Brackett K, Fischer JE. Hepatic steatosis in total parenteral nutrition: failure of fatty infiltration to correlate with abnormal serum hepatic enzyme levels. Surgery. 1986;100:697–704. [PubMed] [Google Scholar]
- Shattuck KE, Grinnell CD, Rassin DK. Amino acid infusions induce reversible, dose-related decreases in bile flow in the isolated rat liver. J Parenter Enteral Nutr. 1993;17:171–6. doi: 10.1177/0148607193017002171. [DOI] [PubMed] [Google Scholar]
- Shukla GS, Chandra SV, Seth PK. Effect of manganese on the levels of DNA, RNA, DNase and RNase in cerebrum, cerebellum and rest of brain regions of rat. Acta Pharmacol Toxicol (Copenh) 1976;39:562–9. doi: 10.1111/j.1600-0773.1976.tb03206.x. [DOI] [PubMed] [Google Scholar]
- Siepler JK, Nishikawa RA, Diamantidis T, Okamoto R. Asymptomatic hypermanganesemia in long-term home parenteral nutrition patients. Nutr Clin Pract. 2003;18:370–3. doi: 10.1177/0115426503018005370. [DOI] [PubMed] [Google Scholar]
- Silbergeld EK, Davis DL. Role of biomarkers in identifying and understanding environmentally induced disease. Clin Chem. 1994;40:1363–7. [PubMed] [Google Scholar]
- Sinczuk-Walczak H, Jakubowski M, Matczak W. Neurological and neurophysiological examinations of workers occupationally exposed to manganese. Int J Occup Med Environ Health. 2001;14:329–37. [PubMed] [Google Scholar]
- Siqueira ME, Moraes EC. Homovanillic acid (HVA) and manganese in urine of workers exposed in a ferromanganese alloy plant. Med Lav. 1989;80:224–8. [PubMed] [Google Scholar]
- Sloot WN, Gramsbergen JB. Axonal transport of manganese and its relevance to selective neurotoxicity in the rat basal ganglia. Brain Res. 1994;657:124–32. doi: 10.1016/0006-8993(94)90959-8. [DOI] [PubMed] [Google Scholar]
- Smargiassi A, Mergler D, Bergamaschi E, Vettori MV, Lucchini R, Apostoli P. Peripheral markers of catecholamine metabolism among workers occupationally exposed to manganese (Mn) Toxicol Lett. 1995;77:329–33. doi: 10.1016/0378-4274(95)03314-9. [DOI] [PubMed] [Google Scholar]
- Smargiassi A, Mutti A. Peripheral biomarkers and exposure to manganese. Neurotoxicology. 1999;20:401–6. [PubMed] [Google Scholar]
- Stanwood GD, Leitch DB, Savchenko V, Wu J, Fitsanakis VA, Anderson DJ, et al. Manganese exposure is cytotoxic and alters dopaminergic and GABAergic neurons within the basal ganglia. J Neurochem. 2009;110:378–89. doi: 10.1111/j.1471-4159.2009.06145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi Y, Okada A, Sando K, Wasa M, Yoshida H, Hirabuki N. Evaluation of indexes of in vivo manganese status and the optimal intravenous dose for adult patients undergoing home parenteral nutrition. Am J Clin Nutr. 2002;75:112–8. doi: 10.1093/ajcn/75.1.112. [DOI] [PubMed] [Google Scholar]
- Takeda A, Ishiwatari S, Okada S. In vivo stimulation-induced release of manganese in rat amygdala. Brain Res. 1998;811:147–51. doi: 10.1016/s0006-8993(98)00881-6. [DOI] [PubMed] [Google Scholar]
- Takser L, Mergler D, de Grosbois S, Smargiassi A, Lafond J. Blood manganese content at birth and cord serum prolactin levels. Neurotoxicol Teratol. 2004;26:811–5. doi: 10.1016/j.ntt.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Taylor S, Manara AR. Manganese toxicity in a patient with cholestasis receiving total parenteral nutrition. Anaesthesia. 1994;49:1013. doi: 10.1111/j.1365-2044.1994.tb04339.x. [DOI] [PubMed] [Google Scholar]
- Thompson K, Molina RM, Donaghey T, Schwob JE, Brain JD, Wessling-Resnick M. Olfactory uptake of manganese requires DMT1 and is enhanced by anemia. FASEB J. 2007;21:223–30. doi: 10.1096/fj.06-6710com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuschl K, Clayton PT, Gospe SM, Samshad G, Ibrahim S, Singhi P, et al. The first inborn error of manganese metabolism caused by mutations in SLC30A10, a newly identified manganese transporter. The Lancet. 2013;381:S110. [Google Scholar]
- Underwood EJ. The incidence of trace element deficiency diseases. Philos Trans R Soc Lond B Biol Sci. 1981;294:3–8. doi: 10.1098/rstb.1981.0085. [DOI] [PubMed] [Google Scholar]
- US EPA. Manganese (CASRN 7439-96-5) US Environmental Protection Agency; 1996. [Google Scholar]
- Wang X, Li GJ, Zheng W. Upregulation of DMT1 expression in choroidal epithelia of the blood-CSF barrier following manganese exposure in vitro. Brain Res. 2006;1097:1–10. doi: 10.1016/j.brainres.2006.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Parvez F, Ahsan H, Levy D, Factor-Litvak P, et al. Water manganese exposure and children’s intellectual function in Araihazar, Bangladesh. Environ Health Perspect. 2006;114:124–9. doi: 10.1289/ehp.8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Parvez F, Factor-Litvak P, Ahsan H, Levy D, et al. Arsenic and manganese exposure and children’s intellectual function. Neurotoxicology. 2011;32:450–7. doi: 10.1016/j.neuro.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed RI, Rothstein A. The uptake of divalent manganese ion by mature normal human red blood cells. J Gen Physiol. 1960;44:301–14. doi: 10.1085/jgp.44.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Principles for evaluating health risks in children associated with exposure to chemicals World Health Organization. Environmental Health Criteria. 2006;237:1–329. [Google Scholar]
- Witzleben C, Pitlick P, Bergmeyer J. Acute manganese overload: A new experimental model of intrahepatic cholestasis. Am J Pathol. 1968;53:409–23. [PMC free article] [PubMed] [Google Scholar]
- Wretlind A. Complete intravenous nutrition. Theoretical and experimental background. Nutr Metab. 1972;14:1S–57. [PubMed] [Google Scholar]
- Zheng W, Fu SX, Dydak U, Cowan DM. Biomarkers of manganese intoxication. Neurotoxicology. 2011;32:1–8. doi: 10.1016/j.neuro.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielhuis RL. Recent and potential advances applicable to the protection of workers’ health—biological monitoring. In: Berlin A, Yodaiken RE, Henman BA, editors. Assessment of toxic agents at the workplace—roles of ambient and biological monitoring. Boston: Martinus Nijhoff Publishers; 1984. pp. 84–94. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


