Abstract
Alzheimer’s disease (AD) exhibits extensive oxidative stress throughout the body, being detected peripherally as well as associated with the vulnerable regions of the brain affected in disease. Abundant evidence not only demonstrates the full spectrum of oxidative damage to neuronal macromolecules, but also reveals the occurrence of oxidative events early in the course of the disease and prior to the formation of the pathology, which support an important role of oxidative stress in AD. As a disease of abnormal aging, AD demonstrats oxidative damage at levels that significantly surpass that of elderly controls, which suggests the involvement of additional factor(s). Structurally and functionally damaged mitochondria, which are more proficient at producing reactive oxygen species but less so in ATP, are also an early and prominent feature of the disease. Since mitochondria are also vulnerable to oxidative stress, it is likely that a vicious downward spiral involving the interactions between mitochondrial dysfunction and oxidative stress contributes to the initiation and/or amplification of reactive oxygen species that is critical to the pathogenesis of AD.
Keywords: Alzheimer disease, oxidative stress, mitochondrial dysfunction, mitochondrial fission, mitochondrial fusion, DLP1
1. Introduction
Alzheimer’s disease (AD) is the most prevalent form of dementia that predominantly affects the elderly populations and is characterized by selective neuronal death and two pathologic hallmarks, i.e., senile plaques formed by extracellular deposits of amyloid-β (Aβ) peptides and neurofibrillary tangles (NFTs) composed of intracellular aggregations of hyperphosphorylated tau protein [1]. The majority of AD cases are sporadic. Less than 10% of AD cases are caused by genetic mutations in three genes including amyloid-β protein precursor (APP), presenilin 1, and presenilin 2, which are involved in the production of Aβ peptide. Down’s syndrome (DS) patients, who carry an extra copy of chromosome 21, develop AD-type dementia with pathological hallmarks in the brain, likely due to the extra copy of the APP gene located on this chromosome. A vast research effort has been made to study Aβ overproduction and/or tau hyperphosphorylation. However, their contribution to the onset and pathogenesis of this devastating disease are still controversial.
There were 26.6 million cases of AD in the world in 2006 and it was projected that the worldwide prevalence of AD will grow four fold to 106.8 million with 1 in 86 people living with AD by the year 2050 [2]. The increase is a result of the aging population worldwide which signifies the fact that AD is a disease of aging and aging represents the single biggest risk factor for AD. Increased oxidative stress has been implicated in the aging process. Oxidative stress is the redox state resulting from an imbalance between the generation and detoxification of reactive oxygen species (ROS). ROS are unavoidable physiological byproducts which act as a double-edged sword in the biological system [3]: they can serve crucial functions such as signaling molecules under carefully controlled situations, but can do damage to biological system when present in excess amount since they are capable of oxidizing all major biomolecules including nucleic acid (DNA, RNA), protein, and lipids. The brain is highly susceptible to oxidative imbalance due to its high energy demand, high oxygen consumption, rich abundance of easily peroxidizable polyunsaturated fatty acids, high level of potent ROS catalyst iron, and relative paucity of antioxidants and related enzymes. It is no wonder that oxidative imbalance and subsequent oxidative stress mediated damage to biomolecules is extensively reported in AD and increasing evidence suggest that oxidative imbalance plays a critical role in the disease [4]. As the main source of ROS generation and also a major target of oxidative damage, progressive impairment of mitochondrial function has also been implicated in aging and AD [5]. The interaction between oxidative stress and mitochondrial dysfunction likely forms a vicious downward spiral that amplifies the deficits and likely plays an important role in the pathogenesis of AD.
2. Evidence of Oxidative Stress in Alzheimer’s Disease
ROS are generated under normal conditions and their levels are kept relatively low by the delicate balance between the rate of their production and the rate of their clearance by antioxidant and related enzymes. Thus, either enhanced ROS production or impaired antioxidant system will tip the cellular redox balance to oxidative imbalance and cause ROS overproduction. ROS are usually highly reactive, unstable and have a very short half-life, thus making them difficult to measure directly. Oxidized biomolecule products generated by ROS are much more stable and commonly used as ROS markers. In addition, ROS could also be assessed indirectly by measuring antioxidant levels or antioxidant enzyme activity. In fact, oxidative imbalance and significant increase of its by-products have been consistently reported in AD.
2.1 Lipid peroxidation
A large body of research has demonstrated that lipid peroxidation is greatly enhanced in AD. Lipid peroxidation refers to the process in which lipids are attacked by ROS through a free radical chain reaction mechanism to generate lipid peroxidation products. By far, the most extensive lipid peroxidation products studied in AD are reactive aldehydes including 4-hydroxynonal, malondialdehyde (MDA), and 2-propenal (acrolein), and chemically and metabolically stable isoprostanoids including F2-isoprostanes and F4-neuroprostanes. It was reported that the 4-hydroxynonal levels are significantly elevated in hippocampus [6–8], entorhinal cortex [8], temporal cortex [8], amygdala [6, 7], parahippocampal gyrus [9], ventricular fluid [10], and plasma [11] in AD patients compared with age-matched control subjects. Significant increase of MDA was also reported in hippocampus [6], pyriform cortex [6], temporal cortex [12, 13], occipital cortices [14], and erythrocytes [15] from AD patients. Several studies found no change of basal MDA [16–20] but significant higher stimulated MDA production in various AD brain regions [16, 19]. In addition, acrolein, the most reactive of the α,β-unsaturated aldehydes, was found to be elevated in AD hippocampus/parahippocampal gyrus [21–23], amygdala [22], superior and middle temporal gyrus [23], and cerebellum [23]. F2-isoprostanes and F4-neuroprostanes are stable products generated from peroxidation of arachidonic acids and docosahexaenoic acid, respectively. Their levels were found to be significantly increased in frontal/temporal poles [24], cerebrospinal fluid [25–27], urine [27], and plasma [27] of AD patients.
2.2 Protein oxidation
Oxidative modifications of proteins, resulting either from direct ROS attack, or from the reaction with glycation, glycoxidation, and lipid peroxidation products binding, have also been extensively reported in AD. The most widely studied markers of protein oxidation are protein carbonyls and 3-nitrotyrosine. A significant increase of protein carbonyl content was reported in hippocampus [28, 29], parietal lobe [30], and superior middle temporal gyrus in AD patients [29]. However, it still remains controversial whether there is elevation of overall brain carbonyl levels in AD [17, 30, 31]. Significant increase of the carbonyl level of specific proteins such as creatine kinase, glutamine synthase, and ubiquitin carboxy-terminal hydrolase L-1 was detected in different AD brain regions including hippocampus, frontal and temporal lobes, inferior parietal lobe, and cerebellum [29, 32–34]. The other major protein oxidative modification, 3-nitrotyrosine, is the end product of the interaction of peroxynitrite with tyrosine residues and was found to be significantly increased in AD in various brain regions [34–40] and cerebrospinal fluid [37].
2.3 DNA/RNA oxidation
Numerous studies have provided evidence that oxidative damage of DNA/RNA was increased in AD. Oxidative damage of DNA can cause DNA double strand breaks, DNA/DNA or DNA/protein crosslinking, and base modification. High levels of DNA breaks were found in both AD hippocampus [41] and AD cerebral cortex [42]. The most widely used DNA oxidative markers in AD are 8-hydroxydeoxyguanosine (8-OHdG) and 8-hydroxyguanosine (8-OHG), which is the product of guanine oxidation. Guanine is the base most readily attacked by oxidative stress due to its low oxidation potential compared with the other three DNA bases. AD brains demonstrated significant increase of 8OHdG and 8-OHG in both mitochondrial DNA and nuclear DNA compared with age-matched controls [30, 35, 43]. RNA is largely single stranded and is usually subjected to similar oxidative damage/modifications as DNA. Predominant oxidation of cytoplasmic RNA rather than nuclear DNA was reported in AD [44]. Significant higher levels of oxidized rRNA or mRNAs were also documented in AD [45–47].
2.4 Antioxidants
In addition to the widespread increase of oxidative biomolecule products, significant decrease of antioxidant levels or antioxidant enzyme activity has been repetitively reported. The plasma levels of antioxidants such as albumin, bilirubin, uric acid, lycopene, vitamin A, vitamin C, and vitamin E were found to be decreased in AD patients [48, 49]. Significant decrease in the activity of antioxidant enzymes such as superoxide dismutase, catalase, glutathione peroxidase, and heme oxygenase were also reported in different AD brain areas including frontal and temporal cortex, although the expression levels of some of them were increased [13, 50–52].
3. Oxidative Stress as an Early Event in Alzheimer’s Disease
It is known that AD has a long latent period before symptoms appear and a diagnosis can be made. Recent studies demonstrated that the onset of AD is commonly preceded by an interim phase known as mild cognitive impairment (MCI), when there is no significant increase of senile plaques and NFTs [53–55]. Indeed, MCI subjects exhibited significant oxidative imbalance compared with age-matched controls. For example, the levels of the isoprostane 8,12-iso-iPF2α-VI were significantly increased in cerebrospinal fluid, plasma, and urine of MCI subjects compared with age-matched control subjects, whereas the levels of AD cerebrospinal fluid markers such as Aβ or tau remained unchanged [56]. Enhanced overall protein peroxidation as well as oxidative modification of specific proteins was also found in hippocampus and superior and middle temporal gyri from MCI subjects [57, 58]. Previous and more recent studies showed significant decreased levels of non-enzymatic antioxidants such as uric acid, vitamin C, vitamin E, vitamin A, lutein, zeaxanthin, β-cryptoxanthin, and α-carotene, and reduced activity of antioxidant enzymes such as superoxide dismutase, glutathione peroxidase, and glutathione reductase in MCI patients [59, 60]. Since MCI subjects are at high risk to progress to early AD and the widespread oxidative damage in MCI could precede the pronounced AD neuropathological alterations, these facts strongly suggest that oxidative imbalance appears at the very early stage of AD and is probably a central feature of the pathogenesis of AD. In fact, it was also reported that lipid peroxidation product, isoprostane 8,12-iso-iPF2alpha-VI, is increased in the urine, blood, and cerebrospinal fluid of AD patients and correlated with the severity of the disease [27], further suggesting that oxidative stress also plays an important role in the progression of the disease.
Patients with DS, caused by trisomy of chromosome 21 and characterized by enhanced Aβ production in the brain and increased incidence of developing AD in middle ages [61], demonstrated predictable progression of AD-type pathology with age. Significant oxidative stress occurs in young DS patients in the absence of significant AD pathologic hallmarks such as senile plaques and NFTs [62–65]. In vitro cultured DS neurons derived from fetal DS also demonstrated higher levels of ROS and significant oxidative damage [66]. The results that ROS overproduction preceded neuronal death, and that antioxidants could greatly enhance neuronal viability, strongly suggested that DS neurons had a preset oxidative imbalance, which was probably responsible for neuronal deficits and subsequent pathological changes during the progression of DS.
Depending on the stability of oxidation modification products, two distinct distribution patterns of oxidative modification were revealed by detailed in situ studies: Products of lipid peroxidation or protein glycation often cause crosslinked molecules which are resistant to degradation at the site of generation. These modifications likely represent cumulative oxidative damage during the course of the disease. In situ detection of these modifications indicates that they were widely present in neurons with and without associated pathology [36, 67, 68]. On the contrary, oxidized DNA/RNAs are rapidly turned over, the levels of which likely reflect steady state oxidative stress. However, RNA oxidation is prominent in cells without pathology but less abundant in neurons with pathology in AD brain [69–72]. These data suggest that oxidative stress occurs earlier than, and likely contributes to the formation of AD-associated pathology and furthermore, AD-associated pathology may play a protective role in quenching ROS production. Such a notion is also supported by studies in DS patients where oxidative stress gradually decreased when amyloid pathology increased with age [73]. Nevertheless, one most recent live imaging study demonstrated active ROS production followed by neuronal death in the proximity to amyloid plaques in the brain of APP/PS1 transgenic mice [74]. Whether this observation can be directly translated into the human situation is unclear since many therapeutics effective in AD mouse models failed to have any clinical benefit in human patients. Overall, these studies suggest that oxidative stress is not only an early event during the course of the disease, but also precedes the AD pathology which suggests a central role of oxidative stress in the pathogenesis of AD.
Evidence further supporting the causative role of oxidative imbalance in the pathogenesis of AD comes from studies showing that antioxidant vitamin deficiency alone is sufficient to induce neurological deficits similar to those in AD. For example, it has been extensively reported that the deficiency of vitamin E, one of the most important fat-soluble antioxidants, caused dementia and other neurological symptoms with an increased risk of developing AD [75, 76], and the addition of vitamin E could reverse the neurologic dysfunction [76]. In addition to vitamin E, the deficiencies of other vitamins that have antioxidant activity were also reported to impair brain function. The lack of folate, a water-soluble vitamin B9 that is important for the development and normal function of the central nervous system [77], resulted in cognitive decline, dementia, depression and other neurological symptoms [78, 79]. And, the treatment with folic acid could significantly alleviate neurological deficits in those folic acid deficiency patients [80]. Dementia, cognitive impairment and other AD like neurological symptoms have also been found in subjects with vitamin B12 [81] and vitamin D deficiency [82]. Taken together, these findings suggest that oxidative imbalance is an early event and likely plays an important role in the pathogenesis of AD.
4. Mitochondrial Dysfunction and Oxidative Stress in Alzheimer’s Disease
Mitochondria are the major source of oxidative stress because the unavoidable electron leakage during electron transfer leads to the constant production of superoxide anion which, despite the presence of an efficient mitochondrial/cellular antioxidant system, is responsible for 90% of the endogenous ROS. It is suggested that dysfunctional mitochondria are less efficient producers of ATP but more efficient producers of ROS, which could represent a major source of oxidative imbalance observed in AD [83, 84]. Indeed, mitochondrial dysfunction is a prominent and early feature of AD [85], and almost all aspects of mitochondrial function have been reported to be impaired in AD.
4.1 Reduced energy metabolism
Reduced energy metabolism in the diseased brain is one of the best documented abnormalities in AD. In fact, low glucose metabolism at baseline and longitudinal glucose metabolism decline is viewed as a sensitive measure useful for monitoring change in cognition and functionality in AD and MCI, and is being increasingly adopted to assist diagnosis and used to predict future cognitive decline [86–88].
4.2 Alterations in the key enzymes in oxidative phosphorylation
A genome-wide transcriptomic study suggests that cerebral glucose metabolic decline in AD is associated with reduced neuronal expression of nuclear genes encoding subunits of the mitochondrial electron transport chain. Indeed, several key enzymes of oxidative metabolism including α-ketoglutarate dehydrogenase complex, pyruvate dehydrogenase complex, and cytochrome oxidase all demonstrated reduced expression and/or activity in AD [84, 89–94], alteration of which significantly correlated with clinical state and plaque counts [95]. Increased oxidative stress likely caused further reduction in activity of some of these enzymes such as that of α-ketoglutarate dehydrogenase complex.
4.3 Calcium dyshomeostasis
Dysfunctional mitochondria contribute to calcium dyshomeostasis through impaired buffering capacity or direct impact on the endoplasmic reticulum calcium channels [96]. Indeed, calcium mishandling was reported in peripheral cells from AD patients such that endoplasmic reticulum (ER) develops calcium overload and calcium uptake is diminished [97, 98]. Indirect evidence suggests elevated intracellular calcium in AD brain since calmodulin-dependent kinase and calpain are elevated in vulnerable neurons early in the disease process [99–102]. Most recently, it was demonstrated that the function of mitochondria-associated ER membranes and ER-mitochondrial communication are increased significantly in fibroblasts from patients with both the familial and sporadic forms of AD [103]. Consistently, increased expression of several ER-mitochondria interface proteins were also reported in early stage of AD [104]. These suggest that altered MAM will likely contribute to calcium dyshomeostasis and mitochondrial dysfunction.
4.4 Mitochondrial DNA (mtDNA)
Elevated levels of sporadic mutations in the mtDNA including the most common 5-kb deletion were demonstrated in the brain of AD patients [105, 106]. In fact, several of the mutations in the mtDNA control regions were unique to AD [107–110]. Due to the absence of protective proteins such as histone, the relative paucity of efficient DNA repair system and the close proximity to site of ROS generation, mtDNA is vulnerable to ROS attack. Analysis of oxidized nucleoside revealed three-fold increase in oxidative damage in the mtDNA in AD brain [111], which probably is the cause of the increased mutations. Consistent with the systemic increase of oxidative stress, mutations in mtDNA were also found in blood samples from AD and lymphoblastoid lines derived from AD blood samples. Many of these mutations occur at sites of known mtDNA transcription and replication regulatory element and thus cause reduced transcript levels of crucial mitochondrial proteins that is deleterious to mitochondrial function [107, 112]. A causal role of mtDNA alterations in some of AD-related deficits such as oxidative stress and biochemical deficits in cytochrome oxidase activity and calcium handling were demonstrated in cybrids where mtDNA from AD patients were transferred into cell lines devoid of mtDNA [113].
4.5 Apoptotic pathway
Mitochondria lie in the center of the intrinsic apoptotic pathway. Although it remains controversial whether apoptosis plays a major role in neurodegeneration in AD [114], it is clear that many components of the intrinsic apoptotic pathway are activated or altered in AD brain [115]. It is of interest to note that the activation of these molecules may not necessarily lead to apoptosis. For example, it appears that caspase 3 is cleaved and activated in AD which is linked to tau cleavage and NFT formation [116].
5. Abnormal Mitochondrial Dynamics and Oxidative Stress in Alzheimer’s Disease
The normal function of mitochondria is dependent upon their intact structure to keep the proper electrochemical gradient. Structurally damaged mitochondria, as evidenced by broken cristae and partial or near complete loss of the internal structure, were abundant and represent a prominent feature in vulnerable neurons in biopsied AD brain as revealed by electron microscopy [105], which is likely the structural basis of the significant mitochondrial dysfunction in AD. A slight but significant increase in mitochondrial size along with a significant decrease in mitochondrial number in these neurons was noted [105]. The size and number of mitochondria are regulated by the dynamic process of mitochondrial fission and fusion [117], therefore, this study was the first that implicated the involvement of abnormal mitochondrial dynamics in AD.
Mitochondria are highly dynamic organelles that undergo continual fission and fusion events which regulate the morphology and distribution of mitochondria [118]. Mitochondrial fission and fusion are regulated by mitochondrial fission proteins such as dynamin-like protein 1 protein (DLP1, also referred to as DRP1), Fis1 [119, 120], and Mff [121], and mitochondrial fusion proteins such as Mitofusin 1 (Mfn1), Mitofusin 2 (Mfn2), and optic atrophy protein 1 (OPA1) [119, 120]. The majority of DLP1 resides in the cytoplasm. However, during fission, DLP1 is recruited to the mitochondrial surface and appears as punctate spots. Fis1, Mff, Mfn1, and Mfn2 are mitochondrial transmembrane proteins localized to the outer mitochondrial membrane [122, 123], while OPA1 is localized to the inner mitochondrial membrane. In support of the involvement of altered mitochondrial dynamics in AD, recent studies demonstrated significant changes in the expression of almost all mitochondrial fission and fusion proteins including DLP1, OPA1, Mfn1/2, and Fis1 in the brain from AD patients [124–128]. Mitochondrial DLP1, the fraction critical for mitochondrial fission, is increased in AD brain [124]. Consistently, DLP1 phosphorylation at Ser616 and S-nitrosylation of DLP1, which either facilitate DLP1 translocation to mitochondria or activate the GTPase activity of DLP1 and thus facilitate mitochondrial fission, are also increased in AD [124, 129]. Interestingly, DLP1 interacts with Aβ monomer and oligomers and phosphorylated tau in AD brain tissues [126, 130], although it remains to be determined how such interactions may impact mitochondrial dynamics. More detailed analysis revealed significantly reduced mitochondrial length but increased mitochondrial width with a significant increase in overall size that resulted in a rounder and fatter (i.e., swollen) morphology of mitochondria in vulnerable neurons from biopsied AD brains [131]. Although larger mitochondria are normally the result of inhibited fission or enhanced fusion, similarly swollen mitochondria were abundant in the brain of fusion-deficient Mfn2 knockout mice [132].
While admittedly non-conclusive due to the “snap-shot” nature of the morphometric study based on electron microscopy micrographs, considering the biochemical data, these studies collectively suggested that there is likely enhanced fission which leads to structurally damaged and swollen mitochondria in vulnerable neurons in the AD brain. Such a notion is supported by in vitro and animal models of AD [133]. For example, neurons treated with oligomeric Aβ or overexpressing familial AD-causing mutant APP demonstrate mitochondrial fragmentation and structural damages [124, 131, 134]. Feany’s group demonstrated that tau overexpression causes mitochondrial elongation in Drosophila through actin-stabilization mediated DLP1 mislocalization [135], however, overexpression of caspase cleaved tau or tau hyperphosphorylation (i.e., cardinal features of human tauopathies including AD) causes mitochondrial fragmentation in mammalian cells [136–140]. Damaged and swollen mitochondria are also documented in the brain of Tg2576 and APP/PS1 mice [141]. Interestingly, the 3-dimension reconstruction of consecutive electron micrographs revealed the presence of swollen mitochondria connected by very narrow membrane segments resembling “beads-on-the-string” pattern in these AD mouse models [141]. This peculiar “beads-on-the-string” pattern may reflect enhanced fission caught in action before the final scission or enhanced fission with arrested final scission, the distinction of which requires techniques that can overcome the snap-shot nature of an electron microscopy study such as in vivo imaging on live animals.
While it remains to be determined whether and how excessive fission causes structural damage to mitochondria in AD, it is clear that excessive fission is sufficient to cause many deleterious consequences seen in AD including increased oxidative stress [142]. It was reported that ROS was overproduced along with mitochondrial fragmentation when neuronal cells were exposed to high glucose concentrations [142]. The inhibition of mitochondrial pyruvate uptake, an effective means to stop ROS increase, did not prevent mitochondrial fragmentation while genetic inhibition of mitochondrial fission by dominant negative mutant form of DLP1 prevented ROS production in high glucose conditions, suggesting that mitochondrial fragmentation plays a critical role in ROS overproduction and oxidative imbalance [142]. More recently, we and others demonstrated that mitochondrial electron-transport-chain complex inhibitors such as rotenone, 1-methyl-4-phenylpyridinium, and 3-nitropropionic acid cause fragmentation of the mitochondrial network along with increased ROS production [143–145]. Antioxidants could only partially alleviate electron-transport-chain complex inhibitors induced mitochondrial fragmentation [144, 145], while inhibition of mitochondrial fission could significantly reduce ROS overproduction and block mitochondrial/cellular dysfunction [145], further suggesting that mitochondrial function and dynamics are intimately related, and mitochondrial morphological dynamics are essential for the maintenance of ROS balance. Indeed, ROS overproduction in AD models could be efficiently prevented or rescued by the inhibition of mitochondrial fission or the promotion of mitochondrial fusion, demonstrating the contribution of abnormal mitochondrial dynamics to oxidative imbalance in AD [124, 127, 131, 146]. Defects in mitochondrial fission and fusion can also increase ROS indirectly through negative impact on bioenergetics, calcium handling, and mtDNA integrity. For example, mitochondrial fission plays crucial role in the assembly of electron transport complexes and controls the dynamic features of mitochondrial nucleoids [147, 148]. Unopposed fission leads to rapid accumulation of mtDNA mutations and decreased calcium buffering capacity [149, 150]. The balance of mitochondria fission and fusion is also sensitive to oxidative imbalance. Most recent studies demonstrated that, through regulation of mitochondrial fission and fusion proteins such as DLP1 and Mfn2, both endogenous [151] and exogenous [152] application of ROS might directly impair mitochondrial fission and fusion balance, induce mitochondrial fragmentation and further cause subsequent mitochondrial dysfunction including ROS overproduction and thus form a vicious cycle that amplifies oxidative stress which perhaps plays an important role in the oxidative imbalance in AD.
6. Conclusion
The importance of oxidative stress in the pathogenesis of AD is supported by the early occurrence and the widespread nature of oxidative damages in AD. Increased oxidative stress also characterizes the aging process. However, that oxidative stress is significantly increased in the AD brain compared to age-matched elderly controls indicates that AD is not a normal part of aging which thus suggests that additional factors must be involved in initiating and/or amplifying oxidative stress during the onset and progression of the disease. Among many potential initiators/sources, mitochondria likely play a critical, if not central, role because of their primacy in energy metabolism and redox homeostasis. Defects in mitochondrial dynamics, either due to the response to genetic deficits or metabolic or environmental alterations, will make mitochondria less versatile in responding to the changing needs of cells which likely have particularly debilitating effects on neurons. The resultant mitochondrial dysfunction and ensuing oxidative stress and their interactions have the potential to form a vicious downward spiral that becomes a ubiquitous causative feature of cell malfunction and degeneration (Figure 1). Clearly, more studies are needed to understand the initiation of oxidative stress and the critical contribution of mitochondrial dysfunction in AD.
Figure 1.
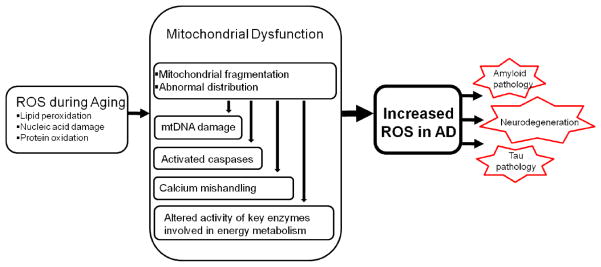
Mitochondrial dysfunction is likely involved in the initiation and/or amplification of oxidative stress during the onset and progression of Alzheimer disease. Oxidative stress can negatively impact mitochondrial integrity and function. Defects in mitochondrial dynamics, either due to the response to genetic deficits or metabolic or environmental alterations, will make mitochondria less versatile in responding to the changing needs of neurons which will exacerbate mitochondrial dysfunction in chronic condition. The resultant mitochondrial dysfunction and ensuing greater oxidative stress and their interactions have the potential to form a vicious downward spiral that becomes a ubiquitous causative feature of cell malfunction, insufficient compensation and degeneration in AD.
Highlights.
Oxidative stress is an early and prominent feature of AD.
Structurally and functionally damaged mitochondria are characteristics of AD.
Mitochondrial fragmentation contributes to mitochondrial damage and dysfunction in AD.
A vicious downward spiral involving ROS and mitochondrial deficits is critical to AD pathogenesis.
Acknowledgments
Work in the authors’ laboratories is supported by the National Institutes of Health (NS083385 to XZ) and the Alzheimer’s Association (IIRG-10-173358 to XZ and IIRG-10-173471 to GP).
Abbreviations
- AD
Alzheimer’s disease
- Aβ
amyloid-β
- APP
amyloid-β protein precursor
- DS
Down’s syndrome
- DLP1
dynamin-like protein 1 protein
- ER
endoplasmic reticulum
- 8-OHdG
8-hydroxydeoxyguanosine
- 8-OHG
8-hydroxyguanosine
- MDA
malondialdehyde
- MCI
mild cognitive impairment
- mtDNA
mitochondrial DNA
- Mfn1
Mitofusin 1
- Mfn2
Mitofusin 2
- NFTs
neurofibrillary tangles
- OPA1
optic atrophy protein 1
- ROS
reactive oxygen species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Smith MA. Alzheimer disease. Int Rev Neurobiol. 1998;42:1–54. doi: 10.1016/s0074-7742(08)60607-8. [DOI] [PubMed] [Google Scholar]
- 2.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 3.Ferrer MD, Sureda A, Mestre A, Tur JA, Pons A. The double edge of reactive oxygen species as damaging and signaling molecules in HL60 cell culture. Cell Physiol Biochem. 2010;25:241–252. doi: 10.1159/000276558. [DOI] [PubMed] [Google Scholar]
- 4.Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S, Atwood CS, Petersen RB, Smith MA. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 2001;60:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- 5.Swerdlow RH. Brain aging, Alzheimer’s disease, and mitochondria. Biochim Biophys Acta. 2011;1812:1630–1639. doi: 10.1016/j.bbadis.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lovell MA, Ehmann WD, Butler SM, Markesbery WR. Elevated thiobarbituric acid-reactive substances and antioxidant enzyme-activity in the brain in Alzheimer’s disease. Neurology. 1995;45:1594–1601. doi: 10.1212/wnl.45.8.1594. [DOI] [PubMed] [Google Scholar]
- 7.Markesbery WR, Lovell MA. Four-hydroxynonenal, a product of lipid peroxidation, is increased in the brain in Alzheimer’s disease. Neurobiol Aging. 1998;19:33–36. doi: 10.1016/s0197-4580(98)00009-8. [DOI] [PubMed] [Google Scholar]
- 8.Montine KS, Reich E, Neely MD, Sidell KR, Olson SJ, Markesbery WR, Montine TJ. Distribution of reducible 4-hydroxynonenal adduct immunoreactivity in Alzheimer disease is associated with APOE genotype. J Neuropathol Exp Neurol. 1998;57:415–425. doi: 10.1097/00005072-199805000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Markesbery WR, Lovell MA. Four-hydroxynonenal, a product of lipid peroxidation, is increased in the brain in Alzheimer’s disease. Neurobiol Aging. 1998;19:33–36. doi: 10.1016/s0197-4580(98)00009-8. [DOI] [PubMed] [Google Scholar]
- 10.Lovell MA, Ehmann WD, Mattson MP, Markesbery WR. Elevated 4-hydroxynonenal in ventricular fluid in Alzheimer’s disease. Neurobiol Aging. 1997;18:457–461. doi: 10.1016/s0197-4580(97)00108-5. [DOI] [PubMed] [Google Scholar]
- 11.McGrath LT, McGleenon BM, Brennan S, McColl D, McIlroy S, Passmore AP. Increased oxidative stress in Alzheimer’s disease as assessed with 4-hydroxynonenal but not malondialdehyde. QJM-Mon J Assoc Phys. 2001;94:485–490. doi: 10.1093/qjmed/94.9.485. [DOI] [PubMed] [Google Scholar]
- 12.Palmer AM, Burns MA. Selective increase in lipid peroxidation in the inferior temporal cortex in Alzheimer’s disease. Brain Res. 1994;645:338–342. doi: 10.1016/0006-8993(94)91670-5. [DOI] [PubMed] [Google Scholar]
- 13.Marcus DL, Thomas C, Rodriguez C, Simberkoff K, Tsai JS, Strafaci JA, Freedman ML. Increased peroxidation and reduced antioxidant enzyme activity in Alzheimer’s disease. Exp Neurol. 1998;150:40–44. doi: 10.1006/exnr.1997.6750. [DOI] [PubMed] [Google Scholar]
- 14.Miranda MD, de Bruin VMS, Vale MR, Viana GSB. Lipid peroxidation and nitrite plus nitrate levels in brain tissue from patients with Alzheimer’s disease. Gerontology. 2000;46:179–184. doi: 10.1159/000022156. [DOI] [PubMed] [Google Scholar]
- 15.Bermejo P, Gomez-Serranillos P, Santos J, Pastor E, Gil P, Martin-Aragon S. Determination of malonaldehyde in Alzheimer’s disease: a comparative study of high-performance liquid chromatography and thiobarbituric acid test. Gerontology. 1997;43:218–222. doi: 10.1159/000213853. [DOI] [PubMed] [Google Scholar]
- 16.Hajimohammadreza I, Brammer M. Brain membrane fluidity and lipid peroxidation in Alzheimer’s disease. Neurosci Lett. 1990;112:333–337. doi: 10.1016/0304-3940(90)90226-y. [DOI] [PubMed] [Google Scholar]
- 17.Hayn M, Kremser K, Singewald N, Cairns N, Nemethova M, Lubec B, Lubec G. Evidence against the involvement of reactive oxygen species in the pathogenesis of neuronal death in Down’s syndrome and Alzheimer’s disease. Life Sci. 1996;59:537–544. doi: 10.1016/0024-3205(96)00334-7. [DOI] [PubMed] [Google Scholar]
- 18.Lyras L, Cairns NJ, Jenner A, Jenner P, Halliwell B. An assessment of oxidative damage to proteins, lipids, and DNA in brain from patients with Alzheimer’s disease. J Neurochem. 1997;68:2061–2069. doi: 10.1046/j.1471-4159.1997.68052061.x. [DOI] [PubMed] [Google Scholar]
- 19.McIntosh LJ, Trush MA, Troncoso JC. Increased susceptibility of Alzheimer’s disease temporal cortex to oxygen free radical-mediated processes. Free Radic Biol Med. 1997;23:183–190. doi: 10.1016/s0891-5849(96)00573-4. [DOI] [PubMed] [Google Scholar]
- 20.Fernandes MAS, Proenca MT, Nogueira AJA, Grazina MMM, Oliveira LMV, Fernandes AIP, Santiago B, Santana I, Oliveira CR. Influence of apolipoprotein E genotype on blood redox status of Alzheimer’s disease patients. Int J Mol Med. 1999;4:179–186. doi: 10.3892/ijmm.4.2.179. [DOI] [PubMed] [Google Scholar]
- 21.Calingasan NY, Uchida K, Gibson GE. Protein-bound acrolein: a novel marker of oxidative stress in Alzheimer’s disease. J Neurochem. 1999;72:751–756. doi: 10.1046/j.1471-4159.1999.0720751.x. [DOI] [PubMed] [Google Scholar]
- 22.Lovell MA, Xie CS, Markesbery WR. Acrolein is increased in Alzheimer’s disease brain and is toxic to primary hippocampal cultures. Neurobiol Aging. 2001;22:187–194. doi: 10.1016/s0197-4580(00)00235-9. [DOI] [PubMed] [Google Scholar]
- 23.Williams TI, Lynn BC, Markesbery WR, Lovell MA. Increased levels of 4-hydroxynonenal and acrolein, neurotoxic markers of lipid peroxidation, in the brain in Mild Cognitive Impairment and early Alzheimer’s disease. Neurobiol Aging. 2006;27:1094–1099. doi: 10.1016/j.neurobiolaging.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Pratico D, MYLV, Trojanowski JQ, Rokach J, Fitzgerald GA. Increased F2-isoprostanes in Alzheimer’s disease: evidence for enhanced lipid peroxidation in vivo. FASEB J. 1998;12:1777–1783. doi: 10.1096/fasebj.12.15.1777. [DOI] [PubMed] [Google Scholar]
- 25.Montine TJ, Markesbery WR, Morrow JD, Roberts LJ., 2nd Cerebrospinal fluid F2-isoprostane levels are increased in Alzheimer’s disease. Ann Neurol. 1998;44:410–413. doi: 10.1002/ana.410440322. [DOI] [PubMed] [Google Scholar]
- 26.Roberts LJ, 2nd, Montine TJ, Markesbery WR, Tapper AR, Hardy P, Chemtob S, Dettbarn WD, Morrow JD. Formation of isoprostane-like compounds (neuroprostanes) in vivo from docosahexaenoic acid. J Biol Chem. 1998;273:13605–13612. doi: 10.1074/jbc.273.22.13605. [DOI] [PubMed] [Google Scholar]
- 27.Pratico D, Clark CM, Lee VM, Trojanowski JQ, Rokach J, FitzGerald GA. Increased 8,12-iso-iPF2alpha-VI in Alzheimer’s disease: correlation of a noninvasive index of lipid peroxidation with disease severity. Ann Neurol. 2000;48:809–812. [PubMed] [Google Scholar]
- 28.Hensley K, Hall N, Subramaniam R, Cole P, Harris M, Aksenov M, Aksenova M, Gabbita SP, Wu JF, Carney JM, Lovell M, Markesbery WR, Butterfield DA. Brain regional correspondence between Alzheimer’s disease histopathology and biomarkers of protein oxidation. J Neurochem. 1995;65:2146–2156. doi: 10.1046/j.1471-4159.1995.65052146.x. [DOI] [PubMed] [Google Scholar]
- 29.Aksenov MY, Aksenova MV, Butterfield DA, Geddes JW, Markesbery WR. Protein oxidation in the brain in Alzheimer’s disease. Neuroscience. 2001;103:373–383. doi: 10.1016/s0306-4522(00)00580-7. [DOI] [PubMed] [Google Scholar]
- 30.Lyras L, Cairns NJ, Jenner A, Jenner P, Halliwell B. An assessment of oxidative damage to proteins, lipids, and DNA in brain from patients with Alzheimer’s disease. J Neurochem. 1997;68:2061–2069. doi: 10.1046/j.1471-4159.1997.68052061.x. [DOI] [PubMed] [Google Scholar]
- 31.Smith CD, Carney JM, Starke-Reed PE, Oliver CN, Stadtman ER, Floyd RA, Markesbery WR. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc Natl Acad Sci U S A. 1991;88:10540–10543. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aksenova MV, Aksenov MY, Payne RM, Trojanowski JQ, Schmidt ML, Carney JM, Butterfield DA, Markesbery WR. Oxidation of cytosolic proteins and expression of creatine kinase BB in frontal lobe in different neurodegenerative disorders. Dement Geriatr Cogn Disord. 1999;10:158–165. doi: 10.1159/000017098. [DOI] [PubMed] [Google Scholar]
- 33.Aksenov M, Aksenova M, Butterfield DA, Markesbery WR. Oxidative modification of creatine kinase BB in Alzheimer’s disease brain. J Neurochem. 2000;74:2520–2527. doi: 10.1046/j.1471-4159.2000.0742520.x. [DOI] [PubMed] [Google Scholar]
- 34.Castegna A, Aksenov M, Thongboonkerd V, Klein JB, Pierce WM, Booze R, Markesbery WR, Butterfield DA. Proteomic identification of oxidatively modified proteins in Alzheimer’s disease brain. Part II: dihydropyrimidinase-related protein 2, alpha-enolase and heat shock cognate 71. J Neurochem. 2002;82:1524–1532. doi: 10.1046/j.1471-4159.2002.01103.x. [DOI] [PubMed] [Google Scholar]
- 35.Good PF, Werner P, Hsu A, Olanow CW, Perl DP. Evidence of neuronal oxidative damage in Alzheimer’s disease. Am J Pathol. 1996;149:21–28. [PMC free article] [PubMed] [Google Scholar]
- 36.Smith MA, Richey Harris PL, Sayre LM, Beckman JS, Perry G. Widespread peroxynitrite-mediated damage in Alzheimer’s disease. J Neurosci. 1997;17:2653–2657. doi: 10.1523/JNEUROSCI.17-08-02653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tohgi H, Abe T, Yamazaki K, Murata T, Ishizaki E, Isobe C. Alterations of 3-nitrotyrosine concentration in the cerebrospinal fluid during aging and in patients with Alzheimer’s disease. Neurosci Lett. 1999;269:52–54. doi: 10.1016/s0304-3940(99)00406-1. [DOI] [PubMed] [Google Scholar]
- 38.Butterfield DA, Kanski J. Brain protein oxidation in age-related neurodegenerative disorders that are associated with aggregated proteins. Mech Ageing Dev. 2001;122:945–962. doi: 10.1016/s0047-6374(01)00249-4. [DOI] [PubMed] [Google Scholar]
- 39.Castegna A, Thongboonkerd V, Klein JB, Lynn B, Markesbery WR, Butterfield DA. Proteomic identification of nitrated proteins in Alzheimer’s disease brain. J Neurochem. 2003;85:1394–1401. doi: 10.1046/j.1471-4159.2003.01786.x. [DOI] [PubMed] [Google Scholar]
- 40.Reed TT, Pierce WM, Jr, Turner DM, Markesbery WR, Butterfield DA. Proteomic identification of nitrated brain proteins in early Alzheimer’s disease inferior parietal lobule. J Cell Mol Med. 2009;13:2019–2029. doi: 10.1111/j.1582-4934.2008.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson AJ, Su JH, Cotman CW. DNA damage and apoptosis in Alzheimer’s disease: colocalization with c-Jun immunoreactivity, relationship to brain area, and effect of postmortem delay. J Neurosci. 1996;16:1710–1719. doi: 10.1523/JNEUROSCI.16-05-01710.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mullaart E, Boerrigter ME, Ravid R, Swaab DF, Vijg J. Increased levels of DNA breaks in cerebral cortex of Alzheimer’s disease patients. Neurobiol Aging. 1990;11:169–173. doi: 10.1016/0197-4580(90)90542-8. [DOI] [PubMed] [Google Scholar]
- 43.Mecocci P, Macgarvey U, Beal MF. Oxidative damage to mitochondrial-DNA is increased in Alzheimer’s disease. Ann Neurol. 1994;36:747–751. doi: 10.1002/ana.410360510. [DOI] [PubMed] [Google Scholar]
- 44.Nunomura A, Perry G, Pappolla MA, Wade R, Hirai K, Chiba S, Smith MA. RNA oxidation is a prominent feature of vulnerable neurons in Alzheimer’s disease. J Neurosci. 1999;19:1959–1964. doi: 10.1523/JNEUROSCI.19-06-01959.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shan X, Tashiro H, Lin CLG. The identification and characterization of oxidized RNAs in Alzheimer’s disease. J Neurosci. 2003;23:4913–4921. doi: 10.1523/JNEUROSCI.23-12-04913.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ding QX, Markesbery WR, Chen QH, Li F, Keller JN. Ribosome dysfunction is an early event in Alzheimer’s disease. J Neurosci. 2005;25:9171–9175. doi: 10.1523/JNEUROSCI.3040-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Honda K, Smith MA, Zhu XW, Baus D, Merrick WC, Tartakoff AM, Hattier T, Harris PL, Siedlak SL, Fujioka H, Liu Q, Moreira PI, Miller FP, Nunomura A, Shimohama S, Perry G. Ribosomal RNA in Alzheimer disease is oxidized by bound redox-active iron. J Biol Chem. 2005;280:20978–20986. doi: 10.1074/jbc.M500526200. [DOI] [PubMed] [Google Scholar]
- 48.Foy CJ, Passmore AP, Vahidassr MD, Young IS, Lawson JT. Plasma chain-breaking antioxidants in Alzheimer’s disease, vascular dementia and Parkinson’s disease. QJM. 1999;92:39–45. doi: 10.1093/qjmed/92.1.39. [DOI] [PubMed] [Google Scholar]
- 49.Kim TS, Pae CU, Yoon SJ, Jang WY, Lee NJ, Kim JJ, Lee SJ, Lee C, Paik IH, Lee CU. Decreased plasma antioxidants in patients with Alzheimer’s disease. Int J Geriatr Psychiatry. 2006;21:344–348. doi: 10.1002/gps.1469. [DOI] [PubMed] [Google Scholar]
- 50.Omar RA, Chyan YJ, Andorn AC, Poeggeler B, Robakis NK, Pappolla MA. Increased expression but reduced activity of antioxidant enzymes in Alzheimer’s disease. J Alzheimers Dis. 1999;1:139–145. doi: 10.3233/jad-1999-1301. [DOI] [PubMed] [Google Scholar]
- 51.Dore S. Decreased activity of the antioxidant heme oxygenase enzyme: implications in ischemia and in Alzheimer’s disease. Free Radic Biol Med. 2002;32:1276–1282. doi: 10.1016/s0891-5849(02)00805-5. [DOI] [PubMed] [Google Scholar]
- 52.Venkateshappa C, Harish G, Mahadevan A, Srinivas Bharath MM, Shankar SK. Elevated oxidative stress and decreased antioxidant function in the human hippocampus and frontal cortex with increasing age: implications for neurodegeneration in Alzheimer’s disease. Neurochem Res. 2012;37:1601–1614. doi: 10.1007/s11064-012-0755-8. [DOI] [PubMed] [Google Scholar]
- 53.Mufson EJ, Chen EY, Cochran EJ, Beckett LA, Bennett DA, Kordower JH. Entorhinal cortex beta-amyloid load in individuals with mild cognitive impairment. Exp Neurol. 1999;158:469–490. doi: 10.1006/exnr.1999.7086. [DOI] [PubMed] [Google Scholar]
- 54.Markesbery WR, Schmitt FA, Kryscio RJ, Davis DG, Smith CD, Wekstein DR. Neuropathologic substrate of mild cognitive impairment. Arch Neurol. 2006;63:38–46. doi: 10.1001/archneur.63.1.38. [DOI] [PubMed] [Google Scholar]
- 55.Price JL, McKeel DW, Jr, Buckles VD, Roe CM, Xiong C, Grundman M, Hansen LA, Petersen RC, Parisi JE, Dickson DW, Smith CD, Davis DG, Schmitt FA, Markesbery WR, Kaye J, Kurlan R, Hulette C, Kurland BF, Higdon R, Kukull W, Morris JC. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging. 2009;30:1026–1036. doi: 10.1016/j.neurobiolaging.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pratico D, Clark CM, Liun F, Rokach J, Lee VY, Trojanowski JQ. Increase of brain oxidative stress in mild cognitive impairment: a possible predictor of Alzheimer disease. Arch Neurol. 2002;59:972–976. doi: 10.1001/archneur.59.6.972. [DOI] [PubMed] [Google Scholar]
- 57.Butterfield DA, Poon HF, St Clair D, Keller JN, Pierce WM, Klein JB, Markesbery WR. Redox proteomics identification of oxidatively modified hippocampal proteins in mild cognitive impairment: insights into the development of Alzheimer’s disease. Neurobiol Dis. 2006;22:223–232. doi: 10.1016/j.nbd.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 58.Keller JN, Schmitt FA, Scheff SW, Ding Q, Chen Q, Butterfield DA, Markesbery WR. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology. 2005;64:1152–1156. doi: 10.1212/01.WNL.0000156156.13641.BA. [DOI] [PubMed] [Google Scholar]
- 59.Rinaldi P, Polidori MC, Metastasio A, Mariani E, Mattioli R, Cherubini A, Catani M, Cecchetti R, Senin U, Mecocci P. Plasma antioxidants are similarly depleted in mild cognitive impairment and in Alzheimer’s disease. Neurobiol Aging. 2003;24:915–919. doi: 10.1016/s0197-4580(03)00031-9. [DOI] [PubMed] [Google Scholar]
- 60.Torres LL, Quaglio NB, de Souza GT, Garcia RT, Dati LM, Moreira WL, Loureiro AP, de Souza-Talarico JN, Smid J, Porto CS, Bottino CM, Nitrini R, Barros SB, Camarini R, Marcourakis T. Peripheral oxidative stress biomarkers in mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis. 2011;26:59–68. doi: 10.3233/JAD-2011-110284. [DOI] [PubMed] [Google Scholar]
- 61.Coyle JT, Oster-Granite ML, Gearhart JD. The neurobiologic consequences of Down syndrome. Brain Res Bull. 1986;16:773–787. doi: 10.1016/0361-9230(86)90074-2. [DOI] [PubMed] [Google Scholar]
- 62.Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S, Atwood CS, Petersen RB, Smith MA. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 2001;60:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- 63.Jovanovic SV, Clements D, MacLeod K. Biomarkers of oxidative stress are significantly elevated in Down syndrome. Free Radic Biol Med. 1998;25:1044–1048. doi: 10.1016/s0891-5849(98)00137-3. [DOI] [PubMed] [Google Scholar]
- 64.Pallardo FV, Degan P, d’Ischia M, Kelly FJ, Zatterale A, Calzone R, Castello G, Fernandez-Delgado R, Dunster C, Lloret A, Manini P, Pisanti MA, Vuttariello E, Pagano G. Multiple evidence for an early age pro-oxidant state in Down Syndrome patients. Biogerontology. 2006;7:211–220. doi: 10.1007/s10522-006-9002-5. [DOI] [PubMed] [Google Scholar]
- 65.Cenini G, Dowling ALS, Beckett TL, Barone E, Mancuso C, Murphy MP, LeVine H, Lott IT, Schmitt FA, Butterfield DA, Head E. Association between frontal cortex oxidative damage and beta-amyloid as a function of age in Down syndrome. Biochim Biophys Acta. 2012;1822:130–138. doi: 10.1016/j.bbadis.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Busciglio J, Yankner BA. Apoptosis and increased generation of reactive oxygen species in Down’s syndrome neurons in vitro. Nature. 1995;378:776–779. doi: 10.1038/378776a0. [DOI] [PubMed] [Google Scholar]
- 67.Takeda A, Smith MA, Avila J, Nunomura A, Siedlak SL, Zhu X, Perry G, Sayre LM. In Alzheimer’s disease, heme oxygenase is coincident with Alz50, an epitope of tau induced by 4-hydroxy-2-nonenal modification. J Neurochem. 2000;75:1234–1241. doi: 10.1046/j.1471-4159.2000.0751234.x. [DOI] [PubMed] [Google Scholar]
- 68.Sayre LM, Zelasko DA, Harris PL, Perry G, Salomon RG, Smith MA. 4-Hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer’s disease. J Neurochem. 1997;68:2092–2097. doi: 10.1046/j.1471-4159.1997.68052092.x. [DOI] [PubMed] [Google Scholar]
- 69.Nunomura A, Perry G, Pappolla MA, Wade R, Hirai K, Chiba S, Smith MA. RNA oxidation is a prominent feature of vulnerable neurons in Alzheimer’s disease. J Neurosci. 1999;19:1959–1964. doi: 10.1523/JNEUROSCI.19-06-01959.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nunomura A, Chiba S, Kosaka K, Takeda A, Castellani RJ, Smith MA, Perry G. Neuronal RNA oxidation is a prominent feature of dementia with Lewy bodies. Neuroreport. 2002;13:2035–2039. doi: 10.1097/00001756-200211150-00009. [DOI] [PubMed] [Google Scholar]
- 71.Nunomura A, Chiba S, Lippa CF, Cras P, Kalaria RN, Takeda A, Honda K, Smith MA, Perry G. Neuronal RNA oxidation is a prominent feature of familial Alzheimer’s disease. Neurobiol Dis. 2004;17:108–113. doi: 10.1016/j.nbd.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 72.Nunomura A, Tamaoki T, Motohashi N, Nakamura M, McKeel DW, Jr, Tabaton M, Lee HG, Smith MA, Perry G, Zhu X. The earliest stage of cognitive impairment in transition from normal aging to Alzheimer disease is marked by prominent RNA oxidation in vulnerable neurons. J Neuorpathol Exp Neurol. 2012;71:233–241. doi: 10.1097/NEN.0b013e318248e614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nunomura A, Perry G, Hirai K, Aliev G, Takeda A, Chiba S, Smith MA. Neuronal RNA oxidation in Alzheimer’s disease and Down’s syndrome. Ann N Y Acad Sci. 1999;893:362–364. doi: 10.1111/j.1749-6632.1999.tb07855.x. [DOI] [PubMed] [Google Scholar]
- 74.Xie H, Hou S, Jiang J, Sekutowicz M, Kelly J, Bacskai BJ. Rapid cell death is preceded by amyloid plaque-mediated oxidative stress. Proc Natl Acad Sci U S A. 2013;110:7904–7909. doi: 10.1073/pnas.1217938110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aslam A, Misbah SA, Talbot K, Chapel H. Vitamin E deficiency induced neurological disease in common variable immunodeficiency: two cases and a review of the literature of vitamin E deficiency. Clin Immunol. 2004;112:24–29. doi: 10.1016/j.clim.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 76.Howard L, Ovesen L, Satya-Murti S, Chu R. Reversible neurological symptoms caused by vitamin E deficiency in a patient with short bowel syndrome. Am J Clin Nutr. 1982;36:1243–1249. doi: 10.1093/ajcn/36.6.1243. [DOI] [PubMed] [Google Scholar]
- 77.Reynolds EH. Folic acid, ageing, depression, and dementia. BMJ. 2002;324:1512–1515. doi: 10.1136/bmj.324.7352.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Botez MI, Fontaine F, Botez T, Bachevalier J. Folate-responsive neurological and mental disorders: report of 16 cases. Neuropsychological correlates of computerized transaxial tomography and radionuclide cisternography in folic acid deficiencies. Eur Neurol. 1977;16:230–246. doi: 10.1159/000114904. [DOI] [PubMed] [Google Scholar]
- 79.Reynolds EH, Rothfeld P, Pincus JH. Neurological disease associated with folate deficiency. Br Med J. 1973;2:398–400. doi: 10.1136/bmj.2.5863.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Godfrey PSA, Toone BK, Carney MWP, Flynn TG, Bottiglieri T, Laundy M, Chanarin I, Reynolds EH. Enhancement of recovery from psychiatric illness by methylfolate. Lancet. 1990;336:392–395. doi: 10.1016/0140-6736(90)91942-4. [DOI] [PubMed] [Google Scholar]
- 81.Blundo C, Marin D, Ricci M. Vitamin B12 deficiency associated with symptoms of frontotemporal dementia. Neurol Sci. 2011;32:101–105. doi: 10.1007/s10072-010-0419-x. [DOI] [PubMed] [Google Scholar]
- 82.Llewellyn DJ, Lang IA, Langa KM, Melzer D. Vitamin D and cognitive impairment in the elderly U.S. population. J Gerontol A Biol Sci Med Sci. 2011;66:59–65. doi: 10.1093/gerona/glq185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Castellani R, Hirai K, Aliev G, Drew KL, Nunomura A, Takeda A, Cash AD, Obrenovich ME, Perry G, Smith MA. Role of mitochondrial dysfunction in Alzheimer’s disease. J Neurosci Res. 2002;70:357–360. doi: 10.1002/jnr.10389. [DOI] [PubMed] [Google Scholar]
- 84.Gibson GE, Sheu KF, Blass JP. Abnormalities of mitochondrial enzymes in Alzheimer disease. J Neural Transm. 1998;105:855–870. doi: 10.1007/s007020050099. [DOI] [PubMed] [Google Scholar]
- 85.Wang X, Su B, Zheng L, Perry G, Smith MA, Zhu X. The role of abnormal mitochondrial dynamics in the pathogenesis of Alzheimer’s disease. J Neurochem. 2009;109(Suppl 1):153–159. doi: 10.1111/j.1471-4159.2009.05867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yamane T, Ikari Y, Nishio T, Ishii K, Ishii K, Kato T, Ito K, Silverman DH, Senda M, Asada T, Arai H, Sugishita M, Iwatsubo T the J-ADNI Study Group. Visual-statistical interpretation of 18F-FDG-PET images for characteristic Alzheimer patterns in a multicenter study: inter-rater concordance and relationship to automated quantitative evaluation. AJNR Am J Neuroradiol. 2013 doi: 10.3174/ajnr.A3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shokouhi S, Claassen D, Kang H, Ding Z, Rogers B, Mishra A, Riddle WR I for The Alzheimer’s Disease Neuroimaging. Longitudinal progression of cognitive decline correlates with changes in the spatial pattern of brain 18F-FDG PET. J Nucl Med. 2013;54:1564–1569. doi: 10.2967/jnumed.112.116137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Landau SM, Harvey D, Madison CM, Koeppe RA, Reiman EM, Foster NL, Weiner MW, Jagust WJ I Alzheimer’s Disease Neuroimaging. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging. 2011;32:1207–1218. doi: 10.1016/j.neurobiolaging.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chandrasekaran K, Giordano T, Brady DR, Stoll J, Martin LJ, Rapoport SI. Impairment in mitochondrial cytochrome oxidase gene expression in Alzheimer disease. Brain Res Mol Brain Res. 1994;24:336–340. doi: 10.1016/0169-328x(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 90.Cottrell DA, Blakely EL, Johnson MA, Ince PG, Turnbull DM. Mitochondrial enzyme-deficient hippocampal neurons and choroidal cells in AD. Neurology. 2001;57:260–264. doi: 10.1212/wnl.57.2.260. [DOI] [PubMed] [Google Scholar]
- 91.Maurer I, Zierz S, Moller HJ. A selective defect of cytochrome c oxidase is present in brain of Alzheimer disease patients. Neurobiol Aging. 2000;21:455–462. doi: 10.1016/s0197-4580(00)00112-3. [DOI] [PubMed] [Google Scholar]
- 92.Nagy Z, Esiri MM, LeGris M, Matthews PM. Mitochondrial enzyme expression in the hippocampus in relation to Alzheimer-type pathology. Acta Neuropathol (Berl) 1999;97:346–354. doi: 10.1007/s004010050997. [DOI] [PubMed] [Google Scholar]
- 93.Parker WD, Jr, Mahr NJ, Filley CM, Parks JK, Hughes D, Young DA, Cullum CM. Reduced platelet cytochrome c oxidase activity in Alzheimer’s disease. Neurology. 1994;44:1086–1090. doi: 10.1212/wnl.44.6.1086. [DOI] [PubMed] [Google Scholar]
- 94.Parker WD, Jr, Parks J, Filley CM, Kleinschmidt-DeMasters BK. Electron transport chain defects in Alzheimer’s disease brain. Neurology. 1994;44:1090–1096. doi: 10.1212/wnl.44.6.1090. [DOI] [PubMed] [Google Scholar]
- 95.Bubber P, Haroutunian V, Fisch G, Blass JP, Gibson GE. Mitochondrial abnormalities in Alzheimer brain: mechanistic implications. Ann Neurol. 2005;57:695–703. doi: 10.1002/ana.20474. [DOI] [PubMed] [Google Scholar]
- 96.Gibson GE, Chen HL, Xu H, Qiu L, Xu Z, Denton TT, Shi Q. Deficits in the mitochondrial enzyme alpha-ketoglutarate dehydrogenase lead to Alzheimer’s disease-like calcium dysregulation. Neurobiol Aging. 2012;33:1121, e1113–1124. doi: 10.1016/j.neurobiolaging.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Peterson C, Gibson GE, Blass JP. Altered calcium uptake in cultured skin fibroblasts from patients with Alzheimer’s disease. N Engl J Med. 1985;312:1063–1065. doi: 10.1056/NEJM198504183121618. [DOI] [PubMed] [Google Scholar]
- 98.Ito E, Oka K, Etcheberrigaray R, Nelson TJ, McPhie DL, Tofel-Grehl B, Gibson GE, Alkon DL. Internal Ca2+ mobilization is altered in fibroblasts from patients with Alzheimer disease. Proc Natl Acad Sci U S A. 1994;91:534–538. doi: 10.1073/pnas.91.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nixon RA. The calpains in aging and aging-related diseases. Ageing Res Rev. 2003;2:407–418. doi: 10.1016/s1568-1637(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 100.Keller JN, Guo Q, Holtsberg FW, Bruce-Keller AJ, Mattson MP. Increased sensitivity to mitochondrial toxin-induced apoptosis in neural cells expressing mutant presenilin-1 is linked to perturbed calcium homeostasis and enhanced oxyradical production. J Neurosci. 1998;18:4439–4450. doi: 10.1523/JNEUROSCI.18-12-04439.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kruman I, Guo Q, Mattson MP. Calcium and reactive oxygen species mediate staurosporine-induced mitochondrial dysfunction and apoptosis in PC12 cells. J Neurosci Res. 1998;51:293–308. doi: 10.1002/(SICI)1097-4547(19980201)51:3<293::AID-JNR3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 102.McKee AC, Kosik KS, Kennedy MB, Kowall NW. Hippocampal neurons predisposed to neurofibrillary tangle formation are enriched in type II calcium/calmodulin-dependent protein kinase. J Neuropathol Exp Neurol. 1990;49:49–63. doi: 10.1097/00005072-199001000-00006. [DOI] [PubMed] [Google Scholar]
- 103.Area-Gomez E, Del Carmen Lara Castillo M, Tambini MD, Guardia-Laguarta C, de Groof AJ, Madra M, Ikenouchi J, Umeda M, Bird TD, Sturley SL, Schon EA. Upregulated function of mitochondria-associated ER membranes in Alzheimer disease. EMBO J. 2012;31:4106–4123. doi: 10.1038/emboj.2012.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hedskog L, Pinho CM, Filadi R, Ronnback A, Hertwig L, Wiehager B, Larssen P, Gellhaar S, Sandebring A, Westerlund M, Graff C, Winblad B, Galter D, Behbahani H, Pizzo P, Glaser E, Ankarcrona M. Modulation of the endoplasmic reticulum-mitochondria interface in Alzheimer’s disease and related models. Proc Natl Acad Sci U S A. 2013;110:7916–7921. doi: 10.1073/pnas.1300677110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen RB, Perry G, Smith MA. Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Corral-Debrinski M, Horton T, Lott MT, Shoffner JM, McKee AC, Beal MF, Graham BH, Wallace DC. Marked changes in mitochondrial DNA deletion levels in Alzheimer brains. Genomics. 1994;23:471–476. doi: 10.1006/geno.1994.1525. [DOI] [PubMed] [Google Scholar]
- 107.Coskun PE, Beal MF, Wallace DC. Alzheimer’s brains harbor somatic mtDNA control-region mutations that suppress mitochondrial transcription and replication. Proc Natl Acad Sci U S A. 2004;101:10726–10731. doi: 10.1073/pnas.0403649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lin MT, Simon DK, Ahn CH, Kim LM, Beal MF. High aggregate burden of somatic mtDNA point mutations in aging and Alzheimer’s disease brain. Hum Mol Genet. 2002;11:133–145. doi: 10.1093/hmg/11.2.133. [DOI] [PubMed] [Google Scholar]
- 109.Coskun P, Wyrembak J, Schriner SE, Chen HW, Marciniack C, Laferla F, Wallace DC. A mitochondrial etiology of Alzheimer and Parkinson disease. Biochim Biophys Acta. 2012;1820:553–564. doi: 10.1016/j.bbagen.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Coskun PE, Wyrembak J, Derbereva O, Melkonian G, Doran E, Lott IT, Head E, Cotman CW, Wallace DC. Systemic mitochondrial dysfunction and the etiology of Alzheimer’s disease and down syndrome dementia. J Alzheimers Dis. 2010;20(Suppl 2):S293–310. doi: 10.3233/JAD-2010-100351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mecocci P, MacGarvey U, Beal MF. Oxidative damage to mitochondrial DNA is increased in Alzheimer’s disease. Ann Neurol. 1994;36:747–751. doi: 10.1002/ana.410360510. [DOI] [PubMed] [Google Scholar]
- 112.Wallace DC, Stugard C, Murdock D, Schurr T, Brown MD. Ancient mtDNA sequences in the human nuclear genome: a potential source of errors in identifying pathogenic mutations. Proc Natl Acad Sci U S A. 1997;94:14900–14905. doi: 10.1073/pnas.94.26.14900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Khan SM, Cassarino DS, Abramova NN, Keeney PM, Borland MK, Trimmer PA, Krebs CT, Bennett JC, Parks JK, Swerdlow RH, Parker WD, Jr, Bennett JP., Jr Alzheimer’s disease cybrids replicate beta-amyloid abnormalities through cell death pathways. Ann Neurol. 2000;48:148–155. [PubMed] [Google Scholar]
- 114.Zhu X, Raina AK, Perry G, Smith MA. Apoptosis in Alzheimer disease: a mathematical improbability. Curr Alzheimer Res. 2006;3:393–396. doi: 10.2174/156720506778249470. [DOI] [PubMed] [Google Scholar]
- 115.Raina AK, Hochman A, Zhu X, Rottkamp CA, Nunomura A, Siedlak SL, Boux H, Castellani RJ, Perry G, Smith MA. Abortive apoptosis in Alzheimer’s disease. Acta Neuropathol. 2001;101:305–310. doi: 10.1007/s004010100378. [DOI] [PubMed] [Google Scholar]
- 116.Avila J. Alzheimer disease: caspases first. Nat Rev Neurol. 2010;6:587–588. doi: 10.1038/nrneurol.2010.157. [DOI] [PubMed] [Google Scholar]
- 117.Chan DC. Fusion and fission: interlinked processes critical for mitochondrial health. Ann Rev Genet. 2012;46:265–287. doi: 10.1146/annurev-genet-110410-132529. [DOI] [PubMed] [Google Scholar]
- 118.Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol. 2007;8:870–879. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- 119.Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125:1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 120.Knott AB, Perkins G, Schwarzenbacher R, Bossy-Wetzel E. Mitochondrial fragmentation in neurodegeneration. Nat Rev Neurosci. 2008;9:505–518. doi: 10.1038/nrn2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Otera H, Wang CX, Cleland MM, Setoguchi K, Yokota S, Youle RJ, Mihara K. Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. J Cell Biol. 2010;191:1141–1158. doi: 10.1083/jcb.201007152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen H, Chomyn A, Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem. 2005;280:26185–26192. doi: 10.1074/jbc.M503062200. [DOI] [PubMed] [Google Scholar]
- 123.Ishihara N, Eura Y, Mihara K. Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity. J Cell Sci. 2004;117:6535–6546. doi: 10.1242/jcs.01565. [DOI] [PubMed] [Google Scholar]
- 124.Wang X, Su B, Lee HG, Li X, Perry G, Smith MA, Zhu X. Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J Neurosci. 2009;29:9090–9103. doi: 10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bossy B, Petrilli A, Klinglmayr E, Chen J, Lutz-Meindl U, Knott AB, Masliah E, Schwarzenbacher R, Bossy-Wetzel E. S-Nitrosylation of DRP1 does not affect enzymatic activity and is not specific to Alzheimer’s disease. J Alzheimers Dis. 20(Suppl 2):S513–526. doi: 10.3233/JAD-2010-100552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Manczak M, Calkins MJ, Reddy PH. Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer’s disease: implications for neuronal damage. Hum Mol Genet. 20:2495–2509. doi: 10.1093/hmg/ddr139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang X, Su B, Fujioka H, Zhu X. Dynamin-like protein 1 reduction underlies mitochondrial morphology and distribution abnormalities in fibroblasts from sporadic Alzheimer’s disease patients. Am J Pathol. 2008;173:470–482. doi: 10.2353/ajpath.2008.071208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang S, Song J, Tan M, Albers KM, Jia J. Mitochondrial fission proteins in peripheral blood lymphocytes are potential biomarkers for Alzheimer’s disease. Eur J Neurol. 2012;19:1015–1022. doi: 10.1111/j.1468-1331.2012.03670.x. [DOI] [PubMed] [Google Scholar]
- 129.Cho DH, Nakamura T, Fang J, Cieplak P, Godzik A, Gu Z, Lipton SA. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science. 2009;324:102–105. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Manczak M, Reddy PH. Abnormal interaction between the mitochondrial fission protein Drp1 and hyperphosphorylated tau in Alzheimer’s disease neurons: implications for mitochondrial dysfunction and neuronal damage. Hum Mol Genet. 2012;21:2538–2547. doi: 10.1093/hmg/dds072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, Casadesus G, Zhu X. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci U S A. 2008;105:19318–19323. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chen H, McCaffery JM, Chan DC. Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell. 2007;130:548–562. doi: 10.1016/j.cell.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 133.Zhu X, Perry G, Smith MA, Wang X. Abnormal mitochondrial dynamics in the pathogenesis of Alzheimer’s disease. J Alzheimers Dis. 2013;33(Suppl 1):S253–262. doi: 10.3233/JAD-2012-129005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Calkins MJ, Manczak M, Mao P, Shirendeb U, Reddy PH. Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer’s disease. Hum Mol Genet. 20:4515–4529. doi: 10.1093/hmg/ddr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.DuBoff B, Gotz J, Feany MB. Tau promotes neurodegeneration via DRP1 mislocalization in vivo. Neuron. 2012;75:618–632. doi: 10.1016/j.neuron.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Quintanilla RA, Matthews-Roberson TA, Dolan PJ, Johnson GV. Caspase-cleaved tau expression induces mitochondrial dysfunction in immortalized cortical neurons: implications for the pathogenesis of Alzheimer disease. J Biol Chem. 2009;284:18754–18766. doi: 10.1074/jbc.M808908200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Quintanilla RA, Dolan PJ, Jin YN, Johnson GV. Truncated tau and Abeta cooperatively impair mitochondria in primary neurons. Neurobiol Aging. 2012;33:619, e625–635. doi: 10.1016/j.neurobiolaging.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cho MH, Kim DH, Choi JE, Chang EJ, Seung Y. Increased phosphorylation of dynamin-related protein 1 and mitochondrial fission in okadaic acid-treated neurons. Brain Res. 2012;1454:100–110. doi: 10.1016/j.brainres.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 139.Schulz KL, Eckert A, Rhein V, Mai S, Haase W, Reichert AS, Jendrach M, Muller WE, Leuner K. A new link to mitochondrial impairment in tauopathies. Mol Neurobiol. 2012;46:205–216. doi: 10.1007/s12035-012-8308-3. [DOI] [PubMed] [Google Scholar]
- 140.Cagalinec M, Safiulina D, Liiv M, Liiv J, Choubey V, Wareski P, Veksler V, Kaasik A. Principles of the mitochondrial fusion and fission cycle in neurons. J Cell Sci. 2013;126:2187–2197. doi: 10.1242/jcs.118844. [DOI] [PubMed] [Google Scholar]
- 141.Trushina E, Nemutlu E, Zhang S, Christensen T, Camp J, Mesa J, Siddiqui A, Tamura Y, Sesaki H, Wengenack TM, Dzeja PP, Poduslo JF. Defects in mitochondrial dynamics and metabolomic signatures of evolving energetic stress in mouse models of familial Alzheimer’s disease. PloS one. 2012;7:e32737. doi: 10.1371/journal.pone.0032737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci U S A. 2006;103:2653–2658. doi: 10.1073/pnas.0511154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Benard G, Bellance N, James D, Parrone P, Fernandez H, Letellier T, Rossignol R. Mitochondrial bioenergetics and structural network organization. J Cell Sci. 2007;120:838–848. doi: 10.1242/jcs.03381. [DOI] [PubMed] [Google Scholar]
- 144.Liot G, Bossy B, Lubitz S, Kushnareva Y, Sejbuk N, Bossy-Wetzel E. Complex II inhibition by 3-NP causes mitochondrial fragmentation and neuronal cell death via an NMDA- and ROS-dependent pathway. Cell Death Differ. 2009;16:899–909. doi: 10.1038/cdd.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang X, Su B, Liu W, He X, Gao Y, Castellani RJ, Perry G, Smith MA, Zhu X. DLP1-dependent mitochondrial fragmentation mediates 1-methyl-4-phenylpyridinium toxicity in neurons: implications for Parkinson’s disease. Aging Cell. 2011;10:807–823. doi: 10.1111/j.1474-9726.2011.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang X, Perry G, Smith MA, Zhu X. Amyloid-beta-derived diffusible ligands cause impaired axonal transport of mitochondria in neurons. Neurodegener Dis. 2010;7:56–59. doi: 10.1159/000283484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Liu W, Acin-Perez R, Geghman KD, Manfredi G, Lu B, Li C. Pink1 regulates the oxidative phosphorylation machinery via mitochondrial fission. Proc Natl Acad Sci U S A. 108:12920–12924. doi: 10.1073/pnas.1107332108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ban-Ishihara R, Ishihara T, Sasaki N, Mihara K, Ishihara N. Dynamics of nucleoid structure regulated by mitochondrial fission contributes to cristae reformation and release of cytochrome c. Proc Natl Acad Sci U S A. 2013;110:11863–11868. doi: 10.1073/pnas.1301951110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Frieden M, James D, Castelbou C, Danckaert A, Martinou JC, Demaurex N. Ca(2+) homeostasis during mitochondrial fragmentation and perinuclear clustering induced by hFis1. J Biol Chem. 2004;279:22704–22714. doi: 10.1074/jbc.M312366200. [DOI] [PubMed] [Google Scholar]
- 150.Chen H, Vermulst M, Wang YE, Chomyn A, Prolla TA, McCaffery JM, Chan DC. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell. 141:280–289. doi: 10.1016/j.cell.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Distelmaier F, Valsecchi F, Forkink M, van Emst-de Vries S, Swarts HG, Rodenburg RJ, Verwiel ET, Smeitink JA, Willems PH, Koopman WJ. Trolox-sensitive reactive oxygen species regulate mitochondrial morphology, oxidative phosphorylation and cytosolic calcium handling in healthy cells. Antioxid Redox Signal. 2012;17:1657–1669. doi: 10.1089/ars.2011.4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li JC, Donath S, Li YR, Qin D, Prabhakar BS, Li PF. miR-30 regulates mitochondrial fission through targeting p53 and the dynamin-related protein-1 pathway. Plos Genet. 2010;6:e1000795. doi: 10.1371/journal.pgen.1000795. [DOI] [PMC free article] [PubMed] [Google Scholar]


