Abstract
The dentate gyrus of the hippocampus plays a pivotal role in pattern separation, a process required for the behavioral task of contextual discrimination. One unique feature of the dentate gyrus that contributes to pattern separation is adult neurogenesis, where newly born neurons play a distinct role in neuronal circuitry. Moreover, the function of neurogenesis in this brain region differs in adolescent and adult mice. The signaling mechanisms that differentially regulate the distinct steps of adult neurogenesis in adolescence and adulthood remain poorly understood. We used mice lacking RAS-GRF1 (GRF1), a calcium-dependent exchange factor that regulates synaptic plasticity and participates in contextual discrimination performed by mice, to test whether GRF1 plays a role in adult neurogenesis. We show Grf1 knockout mice begin to display a defect in neurogenesis at the onset of adulthood (~2 months of age), when wild-type mice first acquire the ability to distinguish between closely related contexts. At this age, young hippocampal neurons in Grf1 knockout mice display severely reduced dendritic arborization. By 3 months of age, new neuron survival is also impaired. BrdU labeling of new neurons in 2 month-old Grf1 knockout mice shows they begin to display reduced survival between 2 and 3 weeks after birth, just as new neurons begin to develop complex dendritic morphology and transition into using glutamatergic excitatory input. Interestingly, GRF1 expression appears in new neurons at the developmental stage when GRF1 loss begins to effect neuronal function. In addition, we induced a similar loss of new hippocampal neurons by knocking down expression of GRF1 solely in new neurons by injecting retrovirus that express shRNA against GRF1 into the dentate gyrus. Together, these findings show that GRF1 expressed in new neurons promotes late stages of adult neurogenesis. Overall our findings show GRF1 to be an age-dependent regulator of adult hippocampal neurogenesis, which contributes to ability of mice to distinguish closely related contexts.
Keywords: dentate gyrus, knockout mice, Ras, contextual discrimination, pattern separation
Background
The subgranular zone of the dentate gyrus is distinct because it is one of two sites in the brain where new neurons are continually generated throughout adulthood (Altman and Das, 1967; Alvarez-Buylla and Lim, 2004). Newborn cells traverse multiple phases including proliferation, differentiation, and survival en route to integration into existing hippocampal circuitry (Ming and Song, 2005; Pathania et al., 2010; Ming and Song, 2011). The unique feature of newborn neurons is their enhanced excitability, which arises before they fully develop into mature granule cell neurons. This property is theorized to provide a selective advantage for the recruitment of new neurons into circuits encoding new learning events (Aimone et al., 2010). One of the established contributions of adult neurogenesis to brain function is its role in an animal’s ability to perform pattern separation, which is involved in contextual discrimination learning (Clelland et al., 2009; Aimone and Gage, 2011; Sahay et al., 2011; Kheirbek et al., 2012a).
The rate of neurogenesis in the dentate gyrus can be both positively and negatively regulated by external factors to influence hippocampal function. This can occur by altering either the proliferation rate of neural precursor stem cells, or the survival of partially differentiated cells. For example, antidepressants such as fluoxetine increase the rate of neurogenesis (Santarelli et al., 2003), which is required for the drug to be effective.
The biochemical regulators for each of the phases of adult neurogenesis are not completely defined. Moreover, the process is age-dependent, such that the rate of neurogenesis is most robust during adolescence, but then decreases steadily as an animal ages (Seki and Arai, 1995; Ben Abdallah et al., 2010). Interestingly, the functions of new neurons produced in the young and the adult differ (Wei et al., 2011; Martinez-Canabal et al., 2013), implying mechanisms that regulate their production are also age-dependent.
Ras-GRF1(GRF1) and Ras-GRF2(GRF2) constitute a family of calcium-activated guanine nucleotide exchange factors with the capacity to activate both RAS and RAC GTPases (Feig, 2011) . They regulate synaptic plasticity in the CA1 hippocampus in an age-dependent manner (Tian et al., 2004; Tian and Feig, 2006). In particular, beginning at ~1-month of age, when the hippocampus first begins to contribute to learning and memory, GRF1 mediates NMDA receptor-stimulated LTD, while GRF2 mediates NMDA receptor-stimulated LTP in the CA1 hippocampus (Li et al., 2006; Jin and Feig, 2010). However, at 2-months of age GRF1 begins to contribute to LTP mediated by calcium-permeable AMPA receptors in this hippocampal region (Jin et al., 2013). Their influence on behavior matches these age dependent contributions to synaptic plasticity, where GRF2 contributes to contextual fear conditioning when mice acquire this form of learning at 1 month of age (Jin et al., 2013) and GRF1 contributes to contextual discrimination when mice acquire this ability at 2-months of age (Giese et al., 2001).
Based on the association between a role for GRF1 in contextual discrimination, and the importance of adult born neurons for this task, we hypothesized that GRF1 could also be associated with the process of adult hippocampal neurogenesis. In this study, we show GRF1 contributes to the late stage survival of new hippocampal neurons during adult neurogenesis in the dentate gyrus beginning at 2 months of age, concomitant with the ability of mice to distinguish closely related contexts.
Materials and Methods
Animals
Male Ras-Grf1 homozygous knockout (Grf1 KO) mice and WT littermate mice, generated as described previously (Giese et al., 2001) and backcrossed onto a C57BL/6J background for more than 10 generations, were used in this study. All mice were housed in a temperature and light-controlled colony room (12-h light/dark cycle) with food and water ad libitum.
Materials
The following antibodies were used: Doublecortin (Santa Cruz; sc-8066; 1:500), GRF1 C-18 (Santa Cruz; sc-244; 1:1000), BrdU (Accurate Chemical and Scientific; OBT0030; 1:400), NeuN (Millipore; MAB377; 1:2000), S100 (Sigma; HPA015768; 1:2000), Ki-67 (BioCare Medical; CRM325A; 1:500), and GFP (Abcam; ab13970; 1:1000). DAPI (Sigma; D9542; 1:10,000) or Nuclear Fast Red (Vector Labs; H3403) were used to counterstain for nuclei. 5-bromo-2-deoxyuridine (BrdU, Sigma; B9285) was used for labeling newborn neurons. 3-3'-diaminobenzadine (DAB)-based staining kits were purchased from Vector Labs (Elite ABC Kit, PK-7100; Peroxidase Substrate Kit; SK-4100). The plasmid encoding for GRF1miRNA (pSM2c) and the MSCV-based retrovirus vector was purchased from Open Biosystems (cat # EAV 4679; GRF1miRNA sequence V2MM-77224). Retro-X Concentrator reagent was purchased from Clontech (cat # 631455).
BrdU injections
For cell survival experiments, 1mo (young adult;WT n = 13 and GRF1-KO n=11) or 2mo (adult; WT n = 10 and GRF1-KO n=13) old mice received two intraperitoneal (i.p.) injections (50 mg/kg in 0.9% NaCl,8 hours apart) over 3 days. Animals were perfused at either 1 week (early survival; 1mo+1week: WT n = 6 and GRF1-KO n = 5; 2mo+1week: WT n = 5 and GRF1-KO n = 6) or 4 weeks (long term survival; 1mo+4weeks: WT n = 7 and GRF1-KO n = 6; 2mo+4weeks: WT n = 5 and GRF1-KO n = 7) after the last BrdU injection.
Fluoxetine injections
For cell survival experiments following treatment with either saline or chronic fluoxetine (FLX), 2-month old mice (WT n = 5 and Grf1-KO n = 5) received BrdU injections as described above. 24 hours after the last BrdU injection, mice were injected once daily with either sterile saline (0.9%) or FLX (Sigma, 18 mg/kg in 0.9% saline) for 4 weeks. Animals were perfused at the end of the treatment paradigm and tissue was processed for BrdU staining as described below.
Tissue preparation and sectioning
All mice were deeply anesthetized with ketamine/xylazine and transcardially perfused with 0.1 M phosphate buffer (PB) followed by 4% paraformaldehyde (PFA 4%) dissolved in PB 0.1M. Brains were extracted and post-fixed in PFA 4% for 24 h. Brains were transferred to 30% sucrose for 48–72 h before slicing 30 μm coronal sections through the extent of the hippocampus using a cryostat. Sections were stored in cryoprotectant at −20°C until use. Each immunohistochemical analysis was conducted from a 1-in-12 series of 30 µm sections spanning the rostrocaudal extent of the DG(1- in 6 series if not enough cells found for phenotypic quantification).
Retrovirus preparation, stereotactic injection, and analysis
Preparation
A MSCV-based, VSV-G pseudotyped retrovirus encoding for GFP (LMP-GFP) was prepared as previously described (Tashiro et al., 2006b) with minor modifications. For the creation of the retrovirus encoding for GRF1miRNA (LMP-GRFmi-GFP), the GRF1miRNA hairpin sequence was cut from the pSM2c vector and inserted into the cloning site of LMP-GFP using EcoRI and XhoI restriction sites according to manufacturer's instructions. Following retrovirus production; collection, amplification, and purification of the virus was performed using Retro-X Concentrator reagent according to manufacturer's instructions. In brief, viral supernatant was collected at 48 and 72 hours after transfection. Supernatant was centrifuged at 500 × g for 10 min and filtered through a 0.45µm low protein binding PVDF membrane. 1 volume Retro-X Concentrator reagent was added to 3 volumes viral supernatant and incubated overnight at 4 °C. The next day, the sample was centrifuged for 45 minutes at 1,500 × g at 4°C. The resulting pellet was resuspended in 1:100 of the original supernatant volume using sterile PBS. Titering was performed as described (Tashiro et al., 2006b). Retroviral stocks used for in vivo experiments were similarly concentrated at 1e7 units/mL.
Stereotactic Injection
2-month old male C57Bl/6J mice housed under standard conditions were used for stereotactic injections of retrovirus into the dentate gyrus (DG) region of the hippocampus as described (Gu et al., 2011). DG was targeted (anterioposterior = −2.0 mm from bregma; lateral = ± 1.6mm; ventral = −2.5mm; anterioposterior = −3.0mm from bregma; lateral = ±2.5mm; ventral = −3.2mm) using a stereotaxic frame (BENCHmark). LMP-GFP and LMP-GRF1mi-GFP viruses (1.5ul per site, 0.25ul/min of rate) were injected in the left and right DG respectively.
Immunohistochemistry
Free-floating sections were rinsed extensively in Phosphate Buffer Saline with 0.25% Triton X- 100 (PBS-T). Sections were blocked for 1h at room temperature in PBS-T with 5% normal goat serum (or 3% donkey serum for DCX). Primary antibodies were diluted in the blocking solution, incubated overnight at 4°C, and rinsed 3 times for 15 minutes in PBS-T. For each marker every 12th section (360um spacing) was processed through the rostrocaudal extent of the dentate gyrus.
Ki-67
Sections were incubated overnight in primary antibody followed by incubation in appropriate biotinylated secondary antibodies (Vector Labs, 1:400–1:1000) for 90 minutes, rinsed in PBST before incubating in avidin-biotin-peroxidase complex solution for an additional 90 minutes. Sections were rinsed in PBST and developed for 10 min in DAB solution, rinsed in PBST-Azide to stop the reaction, and mounted on slides. Slides were taken through a series of dehydration washes, counterstained with Nuclear Fast Red and coverslipped.
BrdU-NeuN-S100
Sections were denatured in 2N HCl for 50 minutes at room temperature and then neutralized twice in 0.1M borate buffer, pH 8.5, rinsed again in PBST, blocked in 5% normal goat serum (NGS) in PBST for 1 hour, and incubated overnight at 4°c in a mixture of rat anti-BrdU antibody in PBST + 5% normal serum. The following day, sections were incubated for 1.5h at room temperature in a mixture of secondary reagents (Jackson ImmunoResearch): goat anti-rat Cy3 (1:2000), goat anti-mouse 647 (1:500), and goat anti-rabbit DyLight 488 (1:1000) in PBST + 5% normal serum. Sections were mounted on slides and coverslipped using DAPI mounting media to label cell nuclei and stored at 4°C.
GFP
Sections were incubated overnight at 4°c in primary antibody followed by incubation in goat anti-chicken DyLight 488 (Jackson ImmunoResearch, 1:1000) at room temperature in PBST + 5% normal serum for 90 minutes. Slices were rinsed in PBST, mounted with DAPI, and coverslipped.
Quantification and Image analysis
All cell quantifications were conducted from a 1-in-12 series (1- in 6 series if not enough cells found for phenotypic quantification) of labeled sections spanning the rostrocaudal extent of the dentate gyrus. Cell quantifications of labeled-cells were conducted by an experimenter blind to the experimental conditions.
Ki67+
Ki-67+ cells were counted using a Nikon 80i microscope with ×40 objective. The surface area of the granule cell layer (GCL) was outlined and measured using ImageJ software. The reference volume of the GCL was calculated as the sum of the traced areas multiplied by the distance between sampled sections (360μm). The total number of cells was calculated as previously described (Trouche et al., 2009).
DCX+ quantification
DCX+ cell quantification was performed in essentially the same manner. Cell bodies of DCX+ cells were quantified within the entire DG of each slice. The reference volume of the GCL was calculated as the sum of the traced areas multiplied by the distance between sampled sections (360μm).
Sholl analysis
Images were acquired using a Nikon A1R confocal microscope at 40× objective. A z-stack (1μm step size) was acquired from slices (30μm) from 2-month old WT and Grf1 KO mice immunostained with DCX. Slides were coded and images were acquired and quantified in a blinded manner. Maximal projected images were created from the resulting z-stacks and the dendritic arbor including the cell body of origin was traced. Dendritic complexity and total dendritic length were analyzed using the NeuronJ plug-in for ImageJ (NIH, Bethesda MD). Sholl analysis was performed using the Sholl analysis plug-in for ImageJ. A series of concentric circles of increasing radii (10μm intervals) was drawn around the neuron with the cell body center as the focal point. 8–10 neurons from each animal (N = 5 animals/genotype) were analyzed.
BrdU+ nuclei quantification
A Nikon A1R confocal laser-scanning microscope was used for BrdU+ nuclei quantification and BrdU co-expression with other markers (NeuN and S100). The settings for PMT, laser power, gain and offset were identical between experimental groups. Each BrdU+ cell within the granular cell layer (GCL) and, adjacent subgranular zone (SGZ) defined as a 2-cell body wide zone was analyzed for colocalization with each marker by collection of an image stack at 2µm step intervals over the entire z-axis using a ×40 objective. The reference volume of the GCL was calculated and the total number of BrdU+ cells was determined.
Determination of cell phenotype
To determine the mean percentage of BrdU+ cells co-expressing either NeuN (postmitotic neurons) or S100 (as mainly an astrocytic marker), four randomly selected animals were sampled for each genotype. An average of 58 BrdU+ cells per mouse were randomly chosen for analysis. Colocalization was confirmed by performing z-stack acquisitions using ImageJ software. The mean number of cells for each phenotype was obtained by multiplying the average fraction for each phenotype by the individual BrdU+ cell count for each animal.
Quantification of GFP immunoreactive cell (GFP+)
GFP+ cells were counted using a Nikon 80i fluorescent microscope with ×40 objective. To measure the density of new cells, GFP+ cells were totaled and divided by the number of sections containing any GFP+ cells. Although reliable infection rates were seen between mice using the same retroviral stock, absolute numbers of GFP+ cells could vary. To reduce this variability, densities recorded at 4 weeks were normalized to the densities recorded at 7 days as previously described (Tashiro et al., 2006a).
Statistical analysis
Results are expressed as mean ± SEM. Prism5 software was used for data analysis. Differences between groups were assessed by ANOVAs or unpaired t-tests. Posthoc multiple comparisons using Bonferroni's correction were performed unless otherwise indicated. For all comparisons, values of p<0.05 were considered significant.
Results
Newborn neurons in 2-month old but not 1-month old Grf1 knockout mice display reduced levels of dendritic complexity
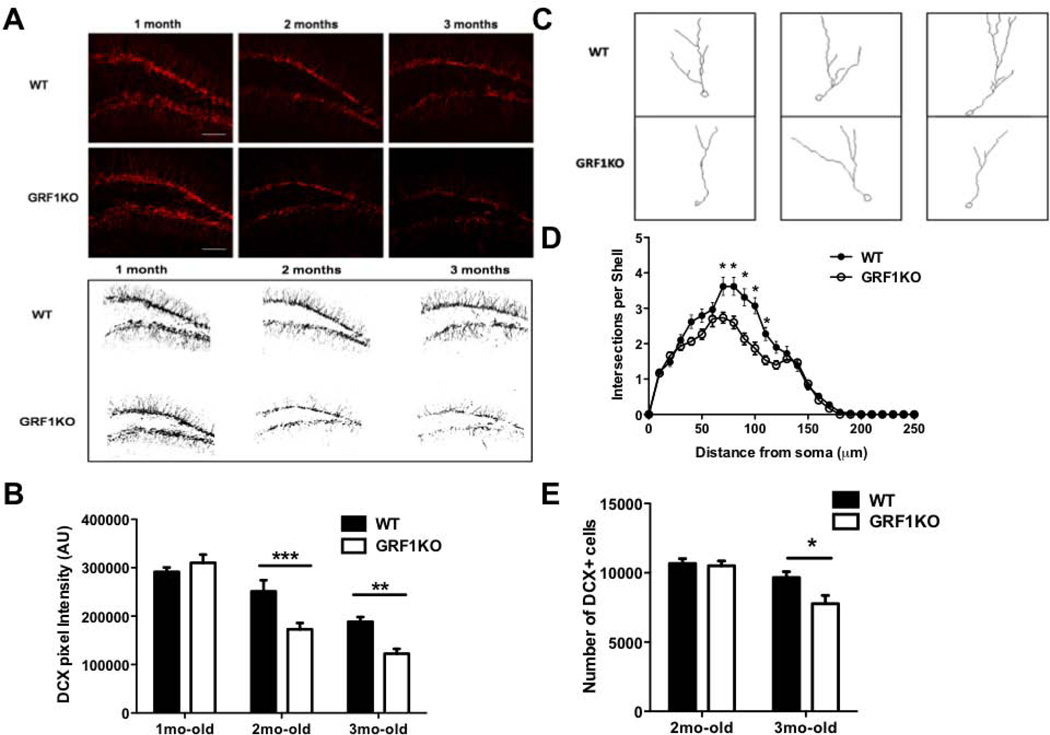
To begin to characterize a potential role for GRF1 in adult hippocampal neurogenesis, the dentate gyrus of WT and Grf1 KO mice were stained with doublecortin (DCX), a marker for new neurons at an intermediate stage of development. Because GRF1 function is age-dependent, we selected mice at 1 month of age when GRF1 first participates in NMDA receptor-mediated LTD in the CA1 (Li et al., 2006), 2 months of age when it begins to participate in calcium-permeable AMPA receptor-mediated LTP (Tian and Feig, 2006; Jin et al., 2013), and 3 months of age. Interestingly, we found that Grf1 knockout mice display a reduced level of total DCX+ staining at 2 and 3 months, but not at 1 month of age (Figs. 1A and B). In particular, staining of DCX+ neurons was similar in 1-month old WT and GRF1 knockout mice. However, while WT mice showed a characteristic decrease in DCX staining with age associated with a decreased rate of neurogenesis (Ben Abdallah et al., 2010), this loss of staining with age was enhanced in Grf1 knockout mice.
Figure 1. Absence of GRF1 alters hippocampal neurogenesis after 2 months of age.
A. Top: Doublecortin (DCX) immunostaining in the dentate gyrus from 1, 2 and 3 month-old wild-type and GRF1-KO mice. Bottom: representative images of anatomically matched slices highlighting overall DCX signal intensity in WT and Grf1-KO mice at 1, 2, and 3 months of age. B. The DCX signal intensity in A is quantified (n =7–14 slices) (Two-way ANOVA, F2,50 = 7.21, P < 0.005; 2-month post-test; t =3.774, P < 0.01; 3 month post-test, t = 3.435, P < 0.01). C. Representative images of DCX-positive cells containing tertiary dendrites in WT and Grf1-KO mice at 2, months of age. D. Sholl analysis of data from C. n = 29–30 cells/4–5 mice/genotype, repeated measures ANOVA, genotype × distance interaction F(25,1482) = 5.33, p<0.001). E. The number of DCX-immunoreactive cells in A is quantified (Genotype effect: F(1,16) = 5.32 , p<0.05; Age effect: F(1,16) = 18.07, p < 0.001; 2-month old: t8 = 0.25, p>0.05; 3-month old: t8 = 3.01, p<0.05). Graphs show means ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001.
To determine whether suppressed DCX staining was due to a decreased complexity of neuron architecture and/or a decreased number of new neurons in Grf1 knockout mice, dendritic morphology was assessed by Sholl analysis and cell number was quantified by counting DCX positive nuclei along the entire dentate gyrus. At 2 months of age, Grf1 knockout mice exhibit decreased dendrite branching (Figs. 1C and D), but no significant change in the number of new neurons (Fig. 1E). However, at 3 months of age the number of DCX positive neurons were reduced in Grf1 knockout mice compared to control mice (Fig 1E). Thus, GRF1 has no significant effect on either the morphology or number of new neurons in juvenile animals, but begins to have an effect on new neuron morphology once animals approach adulthood at 2-months of age and then new neuron cell number by 3-months of age.
GRF1 is important for long-term survival of new hippocampal neurons in 2-month old but not 1-month old mice
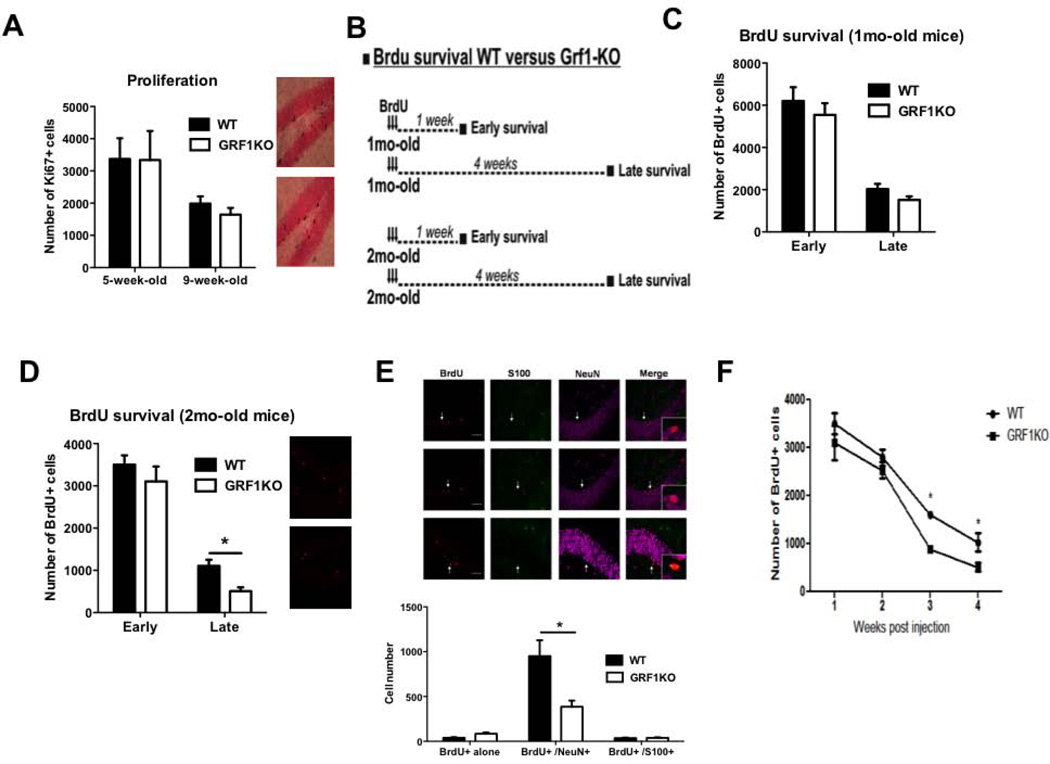
We first determined whether GRF1 influences new neuron cell number by regulating the proliferation rate during early neurogenesis and/or by contributing to cell survival during an early or late initial critical period (Tashiro et al., 2006a). To this end, the proliferative capacity of newborn progenitor cells in WT and Grf1 KO mice was evaluated using the proliferation marker Ki-67 staining. Quantitative analysis of Ki-67 labeled cells showed no difference in proliferative levels between progenitor cells from WT and Grf1 KO mice in either 1 or 2 month old animals (Fig. 2A). This finding is consistent with the previous result showing a normal number of DCX stained neurons in 2 month old Grf1 KO mice and implies that the defect causing reduced DCX cell numbers in 3-month-old Grf1 knockout mice is reduced cell survival.
Figure 2. Loss of newborn neurons in Grf1-KO occurs during late stage of neuronal survival.
A. Left part: The number of Ki67+ cells is similar between wild-type and Grf1-KO mice at 5 weeks of age (1month + 1week group) or 9 weeks of age (1mo+4weeks and 2mo+1week were combined since their number of Ki67+ cell was similar). Genotype effect: F(1,30) = 1.54, p = 0.22; see Table S1. Right part: Ki67 immunostaining within the subgranular zone of the DG from 9week-old wild-type (top) and Grf1-KO (bottom) mice. B. Design of BrdU survival experiments. Briefly, 1-month and 2-month old wild-type and Grf1-KO mice received BrdU injections over 3 days. Animals were perfused at either 1 week (early survival) or 4 weeks (long term survival) after the last BrdU injection. C. Quantification of BrdU+ cells 1mo-old WT and Grf1-KO mice (Genotype × Time interaction F(1,20) = 0.03, p=0.86). D Quantification of BrdU+ cells in 2-month old WT and Grf1 KO mice. Genotype effect: F(1,19) = 4.78, p<0.05; Time effect: F(1,19) = 120.9, p<0.001, "Early": t9 = 0.39, p>0.05, "Late": t10 = 3.98, p<0.001). E. Top: representative images of the phenotypic distribution of BrdU-positive cells(red) colocalizing with either with neuronal (NeuN, purple) or astrocytic (S100, green) markers in 2mo-old wild-type and Grf1-KO mice 4 weeks after BrdU injections. Scale bar = 50μm. Bottom: the number of newborn Brdu+/NeuN-/S100- and Brdu+/NeuN-/S100+ is unchanged in Gfr1-KO mice. However, we find a significantly smaller number of BrdU+/ NeuN+/S100− cells in the DG of GRF1-KO mice compared to WT animals (Genotype × Phenotype interaction: F(2,30) = 12.01, p < 0.001; BrdU+/NeuN+, t10 = 5.75, p<0.05; BrdU+/ S100+ (t10 = 0.03, p>0.05; BrdU only (t10 = 0.45, p>0.05). F. BrdU was injected into 2 month old wild-type and Grf1-KOmice. Mice were perfused either 1 to 4 weeks later and surviving BrdU-stained neurons were quantified (Genotype, F(1,35) = 11.91, p<0.05; age, F(3,35) = 76.16, p<0.001; 2mo+3wks, t8 = 7.67, p < 0.001; 2mo+4wks, t10 = 3.98, p < 0.001). Graphs show means ± s.e.m.
To test whether newborn cell survival was influenced by GRF1, BrdU injections were performed to label and follow the life span of newborn neurons. The experimental set-up is depicted in Figure 2B, where either 1-month (juvenile) or 2-months-old (young adult) mice were injected with BrdU and then sacrificed 1-week or 4-weeks later to examine the potential effect of the loss of GRF1 on the early and/or late survival phase of newborn cells. Figure 2C and 2D show that the absolute number of 1-week-old BrdU+ cells was unaltered in Grf1 KO juvenile and young adult mice, which is consistent with results from Ki67 staining in Figure 2A. Moreover, as previously documented by others, the number of surviving cells in WT animals decreased significantly 4-weeks after birth. In Grf1 KO mice injected with BrdU at 1-month of age (Fig. 2C), no significant difference in newborn cell survival was detected 4 weeks later, consistent with DCX staining of 2-month old mice showing normal numbers of new neurons at an intermediate stage of development. However, Grf1 KO mice injected with BrdU at 2 months of age (Fig. 2D) show a sharp decrease in survival 28 days later, again consistent with DCX staining of 3-month-old mice showing decreased numbers of new neurons. These effects were not attributable to a difference in dentate gyrus volume between wild-type and GRF1 KO mice (unpaired t-tests; 1mo-old, p=0.66; 2mo-old, p=0.67; 3mo-old, p=0.18, see Table S1). To confirm that the newborn cell loss is neuron specific, we used triple-label immunohistochemistry to detect colocalization of BrdU with either NeuN, a neuronal marker, or S100, an astrocytic cell marker. As expected, we found that the dentate gyrus of 2 month-old BrdU-injected Grf1-KO mice contained significantly fewer BrdU-positive cells co-stained with NeuN, but not S100, 4 weeks after BrdU injection compared to WT controls (Fig. 2E).
To more precisely reveal the time in new neuron development when GRF1 affects survival, the previous experiments were repeated except that 2 month-old BrdU-injected mice were sacrificed 2 and 3 weeks after BrdU injection, in addition to 4 weeks later. Figure 2F shows accelerated neuron loss can first be observed at 3-weeks after cell birth consistent with GRF1 promoting survival late in the differentiation process of new neurons. Overall, these findings show that GRF1 first influences cell morphology of developing new neurons at 2 months of age and then as the animal ages, it has a more profound effect by promoting neuron survival.
GRF1-dependent survival is cell autonomous
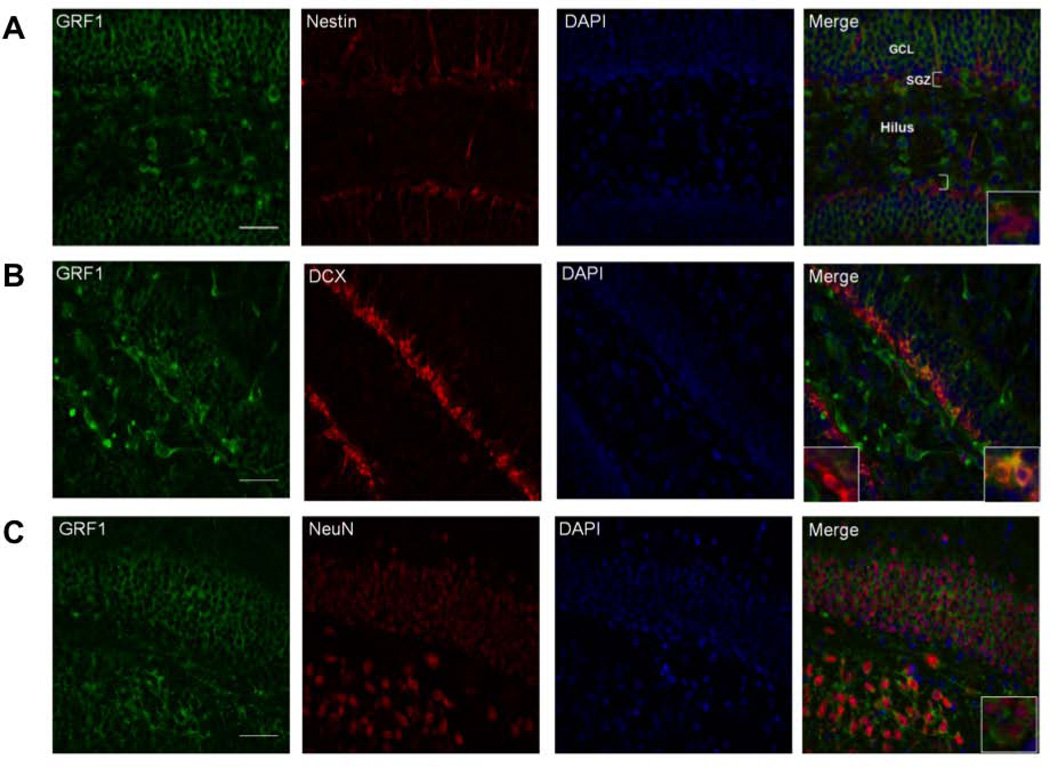
GRF1 is expressed throughout the granule cell population of the dentate gyrus. To begin to address whether GRF1 plays a role within the developing new neurons themselves, we performed double-label immunohistochemistry to test for the presence of GRF1 in newborn neurons at various stages of their development (Fig. 3). Hippocampal brain slices were co-stained with antibodies to GRF1 and Nestin to detect GRF1 expression in early progenitor stem cells (Fukuda et al., 2003), to GRF1 and DCX to detect GRF1 expression in early differentiating cells between 1 and 3 weeks of age (Brown et al., 2003), and to GRF1 and NeuN to detect GRF1 in mature neurons (Kuhn et al., 1996). GRF1 staining did not overlap with Nestin (Fig. 3A). However GRF1 expression did overlap with a subset of DCX+ neurons (Fig.3B) and with the majority of NeuN+ neurons (Fig.3C). These findings indicate that GRF1 is mainly expressed within a late stage population of differentiated neurons.
Figure 3. GRF1 expression first appears in a subset of DCX expressing new hippocampal neurons.
Confocal images of the dentate gyrus stained for GRF1 and A. Nestin (Inset; GRF1-/Nestin+). B. DCX(GRF1-/DCX+, left inset GRF1+/DCX+, right inset;) and C. NeuN (Inset; GRF1+/NeuN+). Scale bars = 50μm.
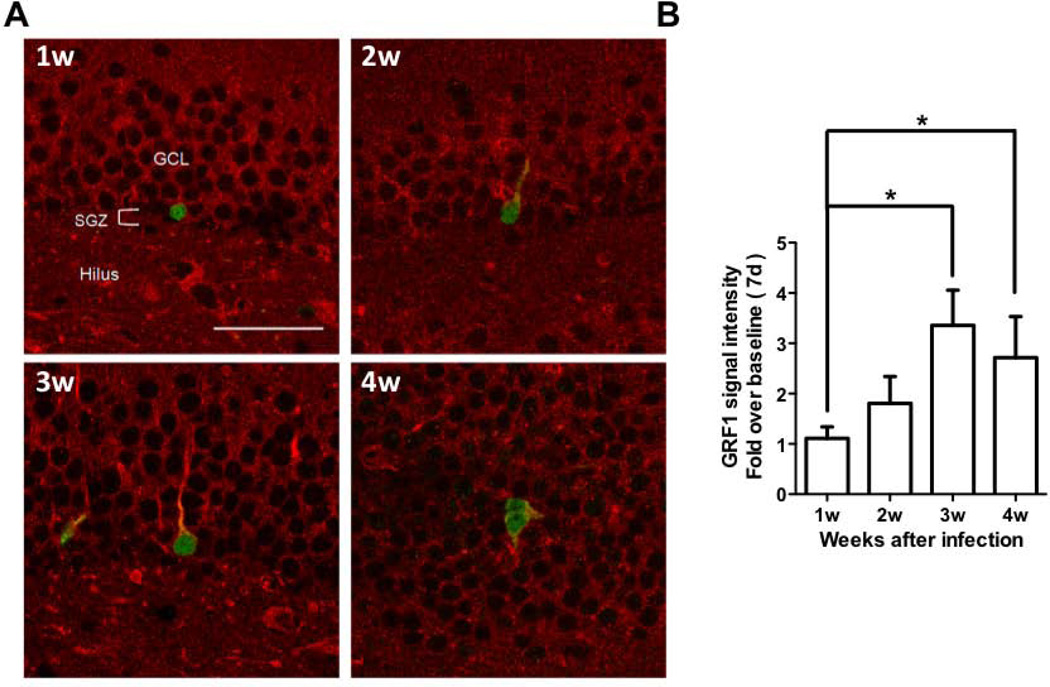
To further assess whether GRF1 expression is developmentally regulated within newborn neurons, the appearance of GRF1 in developing newborn neurons was assessed by infecting proliferating stem cells in vivo with a GFP-expressing retrovirus. Following infection, mice were sacrificed in successive weeks and hippocampal brain slices were co-stained with GRF1 and GFP antibodies. Figure 4A shows representative images where GRF1 expression begins to be detected at ~ 2-weeks after birth when early differentiation begins. Quantification of GRF1 staining intensity within GFP-positive cells is depicted in Figure 4B. Together, the two previous experiments suggest that GRF1 expression is elevated during the later stages of differentiation between weeks 2 and 3, approximately the same time the effect of GRF1 on cell survival is first observed (see Fig. 2F).
Figure 4. GRF1 expression increases as an adult born hippocampal neuron develops.
A. Confocal images of newborn neurons infected with a virus expressing GFP for 1 to 4 weeks and immunostained for GRF1 and GFP. Scale bar = 50μm B. Quantification of GRF1 expression in developing new neurons infected with a virus expressing GFP (Kruskal-Wallis test, p=0.0053; Dunn's multiple comparison test, 7 vs 21, p<0.05, 7 vs. 28, p<0.05). Graphs show means ± s.e.m.
Since the mouse model used was a global Grf1 knockout mouse, we sought to determine whether the phenotypes observed were due, at least in part, to the loss of GRF1 present within the new neurons. To this end, we generated retroviruses expressing GFP plus either a miRNA against Grf1, shown previously to specifically knockdown expression levels of Grf1 (Jin et al., 2013), or a scrambled miRNA as a negative control. The experimental and control viruses were injected unilaterally into the dentate gyrus for each animal for direct comparison of survival in the same brain. The fate of these neurons over time was quantified by sacrificing mice at 7 or 28 days after injection and quantifying GFP labeled cells. Figures 5A and B show that 1 week after injection the same number of control virus- and miRNA Grf1 virus-infected cells were present, however, 28 days later, while the number of control virus-expressing cells decreased as expected, the number of Grf1 virus infected cells decreased significantly more. These results mimic those found in Grf1 knockout mice implying that the loss of GRF1 expression in the developing neurons was responsible, at least in part, for the decrease in cell survival observed in the Grf1 knockout mice.
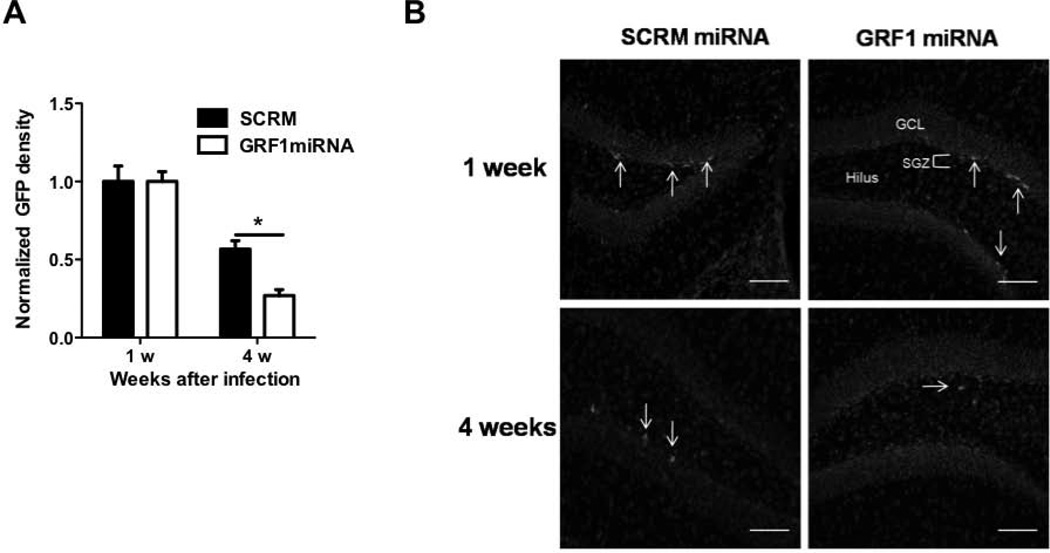
Figure 5. GRF1 regulation of adult neurogenesis occurs in a cell-autonomous manner.
A. New neurons were infected with retroviruses expressing GFP along with either a miRNA that knocks down the expression of GRF1 (Jin et al., 2013) or scrambled miRNA. 1 or 4 weeks after virus injection the number of surviving neurons expressing GFP was quantified (Virus × Time interaction, F(1,12) = 4.89, p=0.047; Scrm vs. miRNA 28d, t6 = 3.13, p<0.05). B. Representative images of cells transduced with scrambled (left) or Grf1 miRNA-expressing retrovirus (right) at 1 week (top) or 4 weeks (bottom) after infection, scale bars = 50μm . Graphs show means ± s.e.m.
Fluoxetine-enhancement of neuronal survival is GRF1 independent
Chronic treatment with antidepressants (ADs), such as fluoxetine, have been shown to increase survival of newborn neurons (Malberg et al., 2000; Santarelli et al., 2003). Thus, we tested whether AD-induced increases in neuronal survival requires GRF1, and if AD treatment could reverse the deficit in new neuron survival present in Grf1 KO mice. WT and Grf1 KO mice were injected with fluoxetine daily for 28 days after injection of BrdU to follow new neuron fate (Fig. 6A). Because it takes at least a 2 weeks for fluoxetine to increase proliferation of progenitor cells, which would be well after injected BrdU remains in the brain to mark new neurons in this paradigm, any changes in the number of cells detected in these experiments represents fluoxetine effects only on neuron survival. Figures 6B and C show that, as previously reported, chronic fluoxetine treatment increases survival ~ 50% in WT mice. It also led to ~ 50% increase in the survival of neurons in Grf1 KO mice. Thus, fluoxetine enhanced new cell survival in a GRF1-independent manner. However, because the basal rate of neuron survival was less in Grf1 KO mice, the production of neurons in Grf1 KO mice after chronic fluoxetine treatment was less than that found in WT mice.
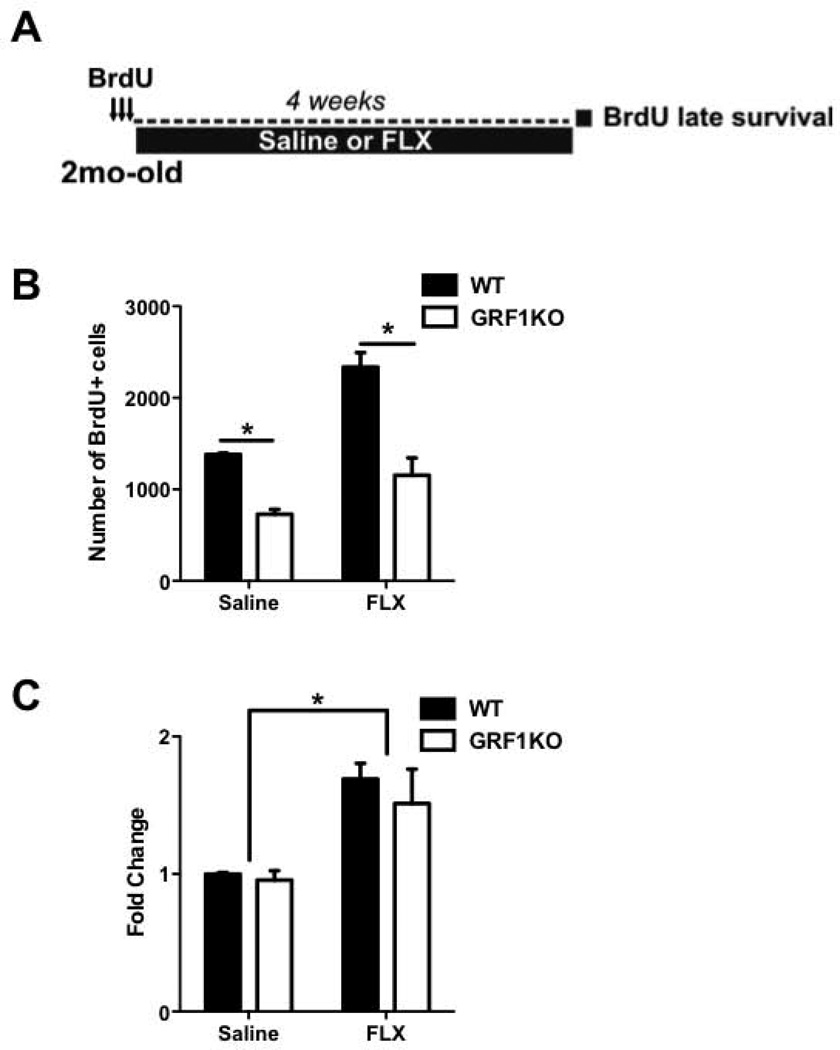
Figure 6. Fluoxetine promotes late survival of newborn cells independent of RasGRF1.
A. Experimental design to test whether GRF1 is involved in the enhanced late survival of new hippocampal cells following 4 weeks chronic antidepressant treatment with fluoxetine. B. Chronic treatment with fluoxetine increases late survival (2-way ANOVA, Treatment effect; F (1,17) = 27.58 p<0.001) in both wild-type and Gfr1KO mice, though it does not reverse the difference between wild-type and Gfr1-KO mice injected with saline (Bonferroni WT vs Grf-KO; t = 3.90,P<0.01) compared to animals injected with fluoxetine (WT vs Grf1-KO; t = 6.75,P<0.001). C. Chronic treatment with fluoxetine results in an enhancement of newborn cell late survival (F(1,17) = 21.98, P<0.05), with a proportional increase in both wild-type and Grf1-KO mice compared to saline-injected animals. Graphs show means ± s.e.m.
Discussion
In this study, we demonstrated that GRF1 plays an important age-dependent role in adult neurogenesis. In particular, Grf1 knockout mice first display a defect at ~ 2 months of age when the dendritic architecture of new neurons becomes less complex than in control mice. Then, at 3 months of age, these mice contain fewer than normal new neurons due to a decrease in their survival. GRF1 influences survival between 14 and 21 days after new neurons are born, at the time when they are beginning to receive inputs from the cortex. Moreover, the effects of GRF1 on cell survival are cell autonomous, since knockdown of GRF1 expression specifically in new neurons mimics the effects observed in Grf1 knockout mice.
Although the term “adult neurogenesis” is commonly used to describe new neuron production in the dentate gyrus of the hippocampus after birth, new postnatal neurons are produced before adulthood. In fact, the rate of new neuron production is greatest during adolescence at ~ 1-month of age and decreases continuously thereafter. Moreover, recent studies suggest that functions of postnatal neurogenesis during adolescence and adulthood differ. For example, inhibition of neurogenesis during adolescence, but not adulthood, negatively affects sociability and fear learning (Wei et al., 2011) as well as learning and memory as assessed by the Morris water maze test (Martinez-Canabal et al., 2013). The particular sensitivity during adolescence may be because the dentate gyrus is still going through structural and functional maturation at this time and may suffer from not only a loss of new neurons but also distortions in hippocampus development. These findings raise the question of whether age-dependent regulators of neurogenesis exist.
Interestingly, GRF1 does not appear to be involved in neurogenesis at 1 month of age when the dentate is still developing, since new neuron numbers and morphology are normal in GRF1 knockout mice at this age. However, GRF1 does begin to play a significant role after dentate gyrus development is mostly complete at 2 months of age. First, we observed a defect in dendritic patterning of new neurons stained with doublecortin, which marks new neurons between 1–3 weeks old. Then, by 3 months of age a decreased number of new neurons appeared. A similar age-dependence of function was previously found for GRF1 in the CA1 region of the hippocampus. Before 1 month of age, when the CA1 is still developing and does not contribute to learning and memory, GRF1 plays no detectable role in synaptic plasticity (Li et al., 2006). However, after that time, it begins to contribute to NMDA receptor mediated LTD and by 2 months of age, GRF1 begins to contribute to LTP mediated by calcium permeable AMPA receptors (Jin et al., 2013).
One aspect of learning and memory that has been attributed to the dentate gyrus, and to adult neurogenesis in particular, is pattern separation (McHugh et al., 2007; Tronel et al., 2010; Sahay et al., 2011; Kheirbek et al., 2012b). This skill is an important component of an animal’s ability to distinguish closely related contexts. This can be examined by a test of contextual discrimination, where an animal is exposed to two similar environments and then given a foot shock in one of them. Afterwards, animals know to freeze in the environment in which they had been shocked but not in the closely related one in which they were not shocked. However, animals acquire contextual discrimination rather late in development. In BL6 mice used in this study it occurs at ~2-months of age (Jin et al, 2013). Thus, although new neurons are generated earlier in development they are not sufficient to generate pattern separation necessary to distinguish closely related contexts. Only when neurogenesis in the dentate gyrus begins to be regulated by GRF1 at 2 months of age does it contribute to pattern separation.
We showed previously that GRF1 is required for animals to display contextual discrimination and that GRF1 does so, at least in part, by promoting a calcium-permeable AMPA receptor-mediated form of LTP in the CA1 region of the hippocampus that appears ~ 2 months of age (Jin et al., 2013). Here we show at approximately the same time, that GRF1 also begins to contribute to adult neurogenesis in the dentate gyrus, which is already known to be required for pattern separation. While we cannot confirm that the defect in adult neurogenesis in GRF1 knockout mice contributes to the defect in contextual discrimination observed in these mice because GRF is missing in other brain regions, these findings suggest that acquisition of contextual discrimination is associated with the appearance of two hippocampal functions for GRF1 at 2 months of age, promotion of LTP in the CA1 and contribution to adult neurogenesis in the dentate.
GRF1 is expressed in surrounding mature dentate gyrus neurons, but the experiments described here show that regulation of new neuron survival is mediated, at least in part, by GRF1 expressed within the developing new neurons. Previous studies have shown that a large number of new neurons are produced in the early phases of neurogenesis, but most do not survive. There are two critical windows during adult neurogenesis when survival appears to be regulated. The first is days 1 to 4 of their life, during the transition from amplifying neuroprogenitors to neuroblasts (Sierra et al., 2010). The second is at the immature neuron stage, when cells first begin to integrate into the circuit and compete for activity-dependent survival (Tashiro et al., 2006a). Since, we found that the loss of GRF1 decreased cell survival only ~14–21 days after birth, it clearly is involved in the second critical period. This is when new neuron dendrites begin to become connected to incoming signals from axons of neurons originating in the cortex, which is consistent with the fact that GRF1 functions in the postsynaptic membrane of new neuron dendrites (Sturani et al., 1997).
One measure of integration with the cortex is the ability to measure NMDA receptor-dependent LTP specifically in new neurons of the dentate. This is done by taking advantage of their reduced threshold for potentiation (Snyder et al., 2001; Ge et al., 2007) . As such, LTP can be induced these new neurons even when GABA receptors are not inhibited. This form of LTP is mediated at least in part by NR2B subunit-containing NMDA receptors, which are known to bind to GRF1 (Krapivinsky et al., 2003). Thus, it was possible that GRF1 is involved in this form of synaptic plasticity. However, we found that LTP was intact in 3-month old Grf1 knockout mice (data not shown). This finding is consistent with our previous studies showing that GRF1 plays a role in NR2B NMDA receptor-mediated LTD, but not LTP (Li et al., 2006). Furthermore, we recently showed that GRF1 is involved in LTP induced by calcium-permeable AMPA receptors (CP-AMPARs) in the CA1 (Jin et al., 2013). The failure to detect a role for GRF1 in LTP in new neurons in the dentate gyrus, implies that CP-AMPARs are not involved in this process. Despite the fact that LTP in new neurons can be induced to normal levels in Grf1 knockout mice, these neurons displayed decreased complexity of their dendritic architecture. This phenotype is consistent with previous studies in cultured spinal neurons, which showed that NR2B, but not NR2A receptors (which function through GRF2) promote dendritic branching in a GRF1 dependent manner (Sepulveda et al., 2010).
A significant (but not totally consistent) body of evidence suggests that anti-depressants function, at least in part, by enhancing adult neurogenesis both at the level of elevated proliferation of precursor cells and increased survival of immature neurons (Hanson et al., 2011). BDNF is thought to be a significant mediator of the effects of antidepressants such as fluoxetine (Castren and Rantamaki, 2010), and there is evidence from tissue culture cell lines that BDNF may signal through GRF1 (Robinson et al., 2005; Talebian et al., 2013). However, we found that fluoxetine maintained the ability to enhance survival of new neurons in Grf1 knockout mice to a degree similar to that found in wild-type mice. Thus, fluoxetine most likely functions through GRF1-independent mechanisms to promote new neuron survival. However, fluoxetine was not able to completely rescue defective neurogenesis in Grf1 KO mice, suggesting that antidepressants may be less effective under conditions where GRF1 levels are suppressed. In fact, GRF1 levels are depressed in epileptic patients (Zhu et al., 2013), who interestingly are at a greater than normal risk for depression (Rubino et al., 2006).
In conclusion, GRF1 becomes a significant contributor to late stage survival of adult born neurons when animals first acquire the ability to distinguish closely related contexts. This role for GRF1 in the dentate gyrus, along with its function in promoting LTP by CP-AMPARs in the CA1 hippocampus, highlight that GRF1 is a multifunctional regulator of the hippocampus that contributes to the process of contextual discrimination, which is a key aspect to hippocampal-dependent learning and memory.
Supplementary Material
Detailed results of the immunoreactive cell quantification for Ki67, BrdU, and DCX in the dentate gyrus of WT and Grf1-KO mice. Phenotypic distribution of BrdU+ cells co-labeled with either NeuN or S100 in the "2mo-old +4week" group. Reference volumes of the dentate gyrus for wild-type and Grf1-KO mice at the ages used are shown. Graphs show means ± s.e.m.
Acknowledgments
This work was supported, in whole or in part, by National Institutes of Health Grants RO1 MH083324 (to L. A. F.) and Grant P30 NS047243 through the Tufts Center for Neuroscience Research.
Footnotes
The authors have no conflict of interest to declare.
References
- Aimone JB, Deng W, Gage FH. Adult neurogenesis: integrating theories and separating functions. Trends Cogn Sci. 2010;14(7):325–337. doi: 10.1016/j.tics.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimone JB, Gage FH. Modeling new neuron function: a history of using computational neuroscience to study adult neurogenesis. Eur J Neurosci. 2011;33(6):1160–1169. doi: 10.1111/j.1460-9568.2011.07615.x. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Postnatal neurogenesis in the guinea-pig. Nature. 1967;214(5093):1098–1101. doi: 10.1038/2141098a0. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41(5):683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- Ben Abdallah NM, Slomianka L, Vyssotski AL, Lipp HP. Early age-related changes in adult hippocampal neurogenesis in C57 mice. Neurobiol Aging. 2010;31(1):151–161. doi: 10.1016/j.neurobiolaging.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467(1):1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Castren E, Rantamaki T. The role of BDNF and its receptors in depression and antidepressant drug action: Reactivation of developmental plasticity. Dev Neurobiol. 2010;70(5):289–297. doi: 10.1002/dneu.20758. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325(5937):210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig LA. Regulation of Neuronal Function by Ras-GRF Exchange Factors. Genes Cancer. 2011;2(3):306–319. doi: 10.1177/1947601911408077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda S, Kato F, Tozuka Y, Yamaguchi M, Miyamoto Y, Hisatsune T. Two distinct subpopulations of nestin-positive cells in adult mouse dentate gyrus. J Neurosci. 2003;23(28):9357–9366. doi: 10.1523/JNEUROSCI.23-28-09357.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54(4):559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese KP, Friedman E, Telliez JB, Fedorov NB, Wines M, Feig LA, Silva AJ. Hippocampus-dependent learning and memory is impaired in mice lacking the Ras-guanine-nucleotide releasing factor 1 (Ras-GRF1) Neuropharmacology. 2001;41(6):791–800. doi: 10.1016/s0028-3908(01)00096-x. [DOI] [PubMed] [Google Scholar]
- Gu Y, Janoschka S, Ge S. Studying the integration of adult-born neurons. J Vis Exp. 2011;(49) doi: 10.3791/2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson ND, Owens MJ, Nemeroff CB. Depression, antidepressants, and neurogenesis: a critical reappraisal. Neuropsychopharmacology. 2011;36(13):2589–2602. doi: 10.1038/npp.2011.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SX, Arai J, Tian X, Kumar-Singh R, Feig LA. Acquisition of Contextual Discrimination Involves the Appearance of a Ras-GRF1/p38 Map Kinase-Mediated Signaling Pathway that Promotes LTP. J Biol Chem. 2013 doi: 10.1074/jbc.M113.471904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SX, Feig LA. Long-term potentiation in the CA1 hippocampus induced by NR2A subunit-containing NMDA glutamate receptors is mediated by Ras-GRF2/Erk map kinase signaling. PLoS One. 2010;5(7):e11732. doi: 10.1371/journal.pone.0011732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheirbek MA, Klemenhagen KC, Sahay A, Hen R. Neurogenesis and generalization: a new approach to stratify and treat anxiety disorders. Nat Neurosci. 2012a;15(12):1613–1620. doi: 10.1038/nn.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheirbek MA, Tannenholz L, Hen R. NR2B-dependent plasticity of adult-born granule cells is necessary for context discrimination. J Neurosci. 2012b;32(25):8696–8702. doi: 10.1523/JNEUROSCI.1692-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapivinsky G, Krapivinsky L, Manasian Y, Ivanov A, Tyzio R, Pellegrino C, Ben-Ari Y, Clapham DE, Medina I. The NMDA receptor is coupled to the ERK pathway by a direct interaction between NR2B and RasGRF1. Neuron. 2003;40(4):775–784. doi: 10.1016/s0896-6273(03)00645-7. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16(6):2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Tian X, Hartley DM, Feig LA. Distinct roles for Ras-guanine nucleotide-releasing factor 1 (Ras-GRF1) and Ras-GRF2 in the induction of long-term potentiation and long-term depression. J Neurosci. 2006;26(6):1721–1729. doi: 10.1523/JNEUROSCI.3990-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20(24):9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Canabal A, Akers KG, Josselyn SA, Frankland PW. Age-dependent effects of hippocampal neurogenesis suppression on spatial learning. Hippocampus. 2013;23(1):66–74. doi: 10.1002/hipo.22054. [DOI] [PubMed] [Google Scholar]
- McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317(5834):94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70(4):687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathania M, Yan LD, Bordey A. A symphony of signals conducts early and late stages of adult neurogenesis. Neuropharmacology. 2010;58(6):865–876. doi: 10.1016/j.neuropharm.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson KN, Manto K, Buchsbaum RJ, MacDonald JIS, Meakin SO. Neurotrophin-dependent tyrosine phosphorylation of Ras guanine-releasing factor 1 and associated neurite outgrowth is dependent on the HIKE domain of TrkA. Journal of Biological Chemistry. 2005;280(1):225–235. doi: 10.1074/jbc.M410454200. [DOI] [PubMed] [Google Scholar]
- Rubino T, Vigano D, Premoli F, Castiglioni C, Bianchessi S, Zippel R, Parolaro D. Changes in the expression of G protein-coupled receptor kinases and beta-arrestins in mouse brain during cannabinoid tolerance: a role for RAS-ERK cascade. Mol Neurobiol. 2006;33(3):199–213. doi: 10.1385/MN:33:3:199. [DOI] [PubMed] [Google Scholar]
- Sahay A, Wilson DA, Hen R. Pattern separation: a common function for new neurons in hippocampus and olfactory bulb. Neuron. 2011;70(4):582–588. doi: 10.1016/j.neuron.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Seki T, Arai Y. Age-related production of new granule cells in the adult dentate gyrus. Neuroreport. 1995;6(18):2479–2482. doi: 10.1097/00001756-199512150-00010. [DOI] [PubMed] [Google Scholar]
- Sepulveda FJ, Bustos FJ, Inostroza E, Zuniga FA, Neve RL, Montecino M, van Zundert B. Differential roles of NMDA Receptor Subtypes NR2A and NR2B in dendritic branch development and requirement of RasGRF1. J Neurophysiol. 2010;103(4):1758–1770. doi: 10.1152/jn.00823.2009. [DOI] [PubMed] [Google Scholar]
- Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7(4):483–495. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Kee N, Wojtowicz JM. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J Neurophysiol. 2001;85(6):2423–2431. doi: 10.1152/jn.2001.85.6.2423. [DOI] [PubMed] [Google Scholar]
- Sturani E, Abbondio A, Branduardi P, Ferrari C, Zippel R, Martegani E, Vanoni M, Denis-Donini S. The Ras Guanine nucleotide Exchange Factor CDC25Mm is present at the synaptic junction. Exp Cell Res. 1997;235(1):117–123. doi: 10.1006/excr.1997.3660. [DOI] [PubMed] [Google Scholar]
- Talebian A, Robinson-Brookes K, MacDonald JIS, Meakin SO. Ras Guanine Nucleotide Releasing Factor 1 (RasGrf1) Enhancement of Trk Receptor-Mediated Neurite Outgrowth Requires Activation of Both H-Ras and Rac. Journal of Molecular Neuroscience. 2013;49(1):38–51. doi: 10.1007/s12031-012-9847-9. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Sandler VM, Toni N, Zhao C, Gage FH. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature. 2006a;442(7105):929–933. doi: 10.1038/nature05028. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Zhao C, Gage FH. Retrovirus-mediated single-cell gene knockout technique in adult newborn neurons in vivo. Nat Protoc. 2006b;1(6):3049–3055. doi: 10.1038/nprot.2006.473. [DOI] [PubMed] [Google Scholar]
- Tian X, Feig LA. Age-dependent participation of Ras-GRF proteins in coupling calcium permeable AMPA-type glutamate receptors to Ras/Erk signaling in cortical neurons. J Biol Chem. 2006 doi: 10.1074/jbc.M512060200. [DOI] [PubMed] [Google Scholar]
- Tian X, Gotoh T, Tsuji K, Lo EH, Huang S, Feig LA. Developmentally regulated role for Ras-GRFs in coupling NMDA glutamate receptors to Ras, Erk and CREB. Embo J. 2004;23(7):1567–1575. doi: 10.1038/sj.emboj.7600151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronel S, Fabre A, Charrier V, Oliet SH, Gage FH, Abrous DN. Spatial learning sculpts the dendritic arbor of adult-born hippocampal neurons. Proc Natl Acad Sci U S A. 2010;107(17):7963–7968. doi: 10.1073/pnas.0914613107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouche S, Bontempi B, Roullet P, Rampon C. Recruitment of adult-generated neurons into functional hippocampal networks contributes to updating and strengthening of spatial memory. Proc Natl Acad Sci U S A. 2009;106(14):5919–5924. doi: 10.1073/pnas.0811054106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Meaney MJ, Duman RS, Kaffman A. Affiliative behavior requires juvenile, but not adult neurogenesis. J Neurosci. 2011;31(40):14335–14345. doi: 10.1523/JNEUROSCI.1333-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Wang L, Xiao Z, Xiao F, Luo J, Zhang X, Peng X, Wang X, Sun H. Decreased expression of Ras-GRF1 in the brain tissue of the intractable epilepsy patients and experimental rats. Brain Res. 2013;1493:99–109. doi: 10.1016/j.brainres.2012.11.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed results of the immunoreactive cell quantification for Ki67, BrdU, and DCX in the dentate gyrus of WT and Grf1-KO mice. Phenotypic distribution of BrdU+ cells co-labeled with either NeuN or S100 in the "2mo-old +4week" group. Reference volumes of the dentate gyrus for wild-type and Grf1-KO mice at the ages used are shown. Graphs show means ± s.e.m.