Abstract
Background
Mycosis fungoides is a rare, but potentially devastating malignancy. It classically presents with cutaneous patches and plaques and can progress to tumors on the skin with lymph node, blood, and visceral involvement. While most patients with MF have a relatively benign disease course, a subset of patients will develop progressive disease that is often fatal.
Objective
The aim of this study is to identify genetic markers in early MF limited to the skin (stages IA-IIA) that distinguish those patients who will have progressive disease from those who will not, so that early appropriate treatment may be instituted.
Methods
The study includes eighteen patients who were diagnosed with early stage MF at the time of biopsy and had follow up to determine which patients developed progressive disease. RNA was extracted from skin biopsy specimens and analyzed for expression of CD3, FOXP3, IFNγ, IL-4, IL-13, KIR3DL2, MICB, PLS3, and STAT4 by quantitative real-time polymerase chain reaction.
Results/Conclusions
Reduced expression of FOXP3 and STAT4 and increased expression of IL-4 relative to CD3 expression levels were significantly associated with MF progression. Further studies will be needed to fully assess the usefulness of these genetic markers to predict disease progression and guide treatment options in patients diagnosed with early MF.
Introduction
Mycosis fungoides (MF) is the most common form of cutaneous T-cell lymphoma (CTCL). It classically progresses from patches to infiltrated plaques and ultimately tumors on the skin, often with extracutaneous involvement.1 Although 80–90% of those affected with early MF have a favorable prognosis (73–97% survival rate at 5 years), 10–20% of patients can progress onto more advanced disease with a marked increase in mortality (26% survival at 5 years).2–4
One of the difficulties with this malignancy is the inability to reliably predict which patients with clinically early MF will develop progressive disease versus those who will not. It is thought that as MF progresses, it becomes more Th2 polarized by accumulating malignant T-cells that secrete Th2 cytokines such as IL-45, IL-10, and IL-13.6 Additionally, decreased numbers of FOXP3+ regulatory T-cells in skin lesions of MF have been reported to be associated with a worse prognosis.7–9 As MF progresses FOXP3 may also increase as it has also been shown that FOXP3 can be expressed on transformed malignant T-cells.10 More recent studies have identified the expression of the microRNA processing protein, Dicer11, and the microRNA let-7a12 as being independent prognostic indicators of survival. Additionally, increased expression of Th17 genes13 and also elevated serum IgE, large Pautrier’s micoabscesses, and a dermal infiltrate composed of numerous hyperchromatic or large atypical cells and reduced proportion of CD8+ cells have been associated with an increased risk of disease progression.14
Several genes are known to be aberrantly expressed by malignant T-cells in CTCL and may serve as genetic markers of tumor burden including the overexpressed MIC-B, KIR3DL2, and PLS3/T-plastin.15 A reduction of STAT4 has also been associated with malignant T-cells.16 Accordingly, we hypothesize that patients with early stage MF who are prone to have more aggressive disease will have increased mRNA expression of tumor-associated markers (KIR3DL2, MIC-B, PLS3), decreased FOXP3 and STAT4 expression, and increased Th2 cytokines compared to patients with less aggressive disease.
Materials and Methods
This retrospective study utilized the CTCL tissue repository at Johns Hopkins School of Medicine and was approved by the Johns Hopkins Institutional Review Board. Eighteen subjects were selected based on the diagnosis of MF limited to the skin (Stage IA-IIA) and established, long-term follow up (median 44.5 months). Patients were grouped into disease “progressors” (n=8) and “non-progressors” (n=10) according to an increase in TNMB status during follow-up with a single Dermatologist (E.V.). RNA was extracted from each of the lesional skin samples and quantitative real-time polymerase chain reaction (RT-PCR) was performed to identify expression levels of FOXP3, IFNγ, IL-4, IL-13, KIR3DL2, MIC-B, PLS3, and STAT4. CD3 expression was also measured as a control for Tcell infiltrate, and the expression of the aforementioned genes were evaluated relative to CD3 expression.
RNA isolation
Four to 6 mm skin biopsy specimens obtained from patch or plaque lesions were snap frozen in liquid nitrogen, and stored at −80°C. The biopsies were thawed, ground, and immediately placed into Trizol (Invitrogen, Grand Island, NY) for RNA extraction. RNA was isolated as per manufacturer’s protocol (Invitrogen, Grand Island, NY).
cDNA preparation and quantitative real-time polymerase chain reaction
Determination of relative gene expression was performed by quantitative RT-PCR using an ABI 7500 real time PCR system (Applied Biosystems, Foster City, CA). cDNA was prepared from the isolated RNA using random hexamers to prime reverse transcription (Ready- to-Go You-Prime First Strand Beads; GE Healthcare, United Kingdom) following the manufacturer’s protocol. Multiplexed quantitative determination was completed in triplicate wells using approximately 1:30 of the cDNA per well and primer/probe sets for the FAM labeled target genes and VIC labeled endogenous reference gene (GAPDH) with the standard ABI chemistry and reagents. Relative transcripts were determined by the formula: 1/2(CTtarget - CTcontrol). Primers for CD3, FOXP3, IFNγ, IL-4, IL-13, KIR3DL2, MIC-B, PLS3, and STAT4 were obtained from ABI Corporation (Foster City, CA). For gene expression that was too low to register a recorded value by quantitative RT-PCR, a value of 0.05 lower than the lowest recorded value was assigned. As we utilized non-parametric statistical analyses, these data were grouped in the lowest category and the lack of a true value did not affect outcomes. IL-13 transcripts were rarely detected in any tissues samples and were therefore were not included in our analysis.
Statistical analysis
Statistical analysis was performed with SPSS 20.0 for Windows (SPSS, Inc., Chicago, IL). The Mann Whitney U test was performed to evaluate for significant associations between single continuous variables and clinical outcome. Fishers’ exact test and logistic regression were utilized for evaluation of dichotomous variables and association with clinical outcome.
Results
Demographic and Clinical Data
Clinical characteristics, disease stage, and disease progression are summarized in Table 1. Of the 18 patients studied, the male to female ratio was 1.6:1 (male= 11, female= 7). Two patients (11.1%) were diagnosed at stage IA, 11 (61.1%) were diagnosed at stage IB, and 5 (27.8) at stage IIA. After diagnosis, 8 of the 18 (44%) progressed to more advanced disease. 6 patients developed tumors, 1 became erythrodermic, and 1 progressed directly to lymph node involvement. The increased age of patients in the non-progressor group (median, 66.5 years, range 51 to 81) was not statistically different from patients in the progressor group (median, 61.5 years, range, 32 to 69; p= 0.102). There was also a non-significant increase in females in the progressor group (50%) as compared to the non-progressor group (30%, p=0.35). There was no statistically significant difference in initial stage between the progressors or non-progressors (Stage IA 12.5% vs 10%, p=0.71; Stage IB 50% vs 70% p=0.35; Stage IIA, 37.5% vs 20%, p=0.38).
Table 1.
Study Population
| ID# | Progression* | Sex | Age | Stage | TNMB |
|---|---|---|---|---|---|
|
| |||||
| 1 | No | F | 67 | IB | T2bN0B0xM0 |
| 2 | No | M | 62 | IB | T2bN0B0xM0 |
| 3 | No | M | 51 | IA | T1bN0B0xM0 |
| 4 | No | M | 68 | IB | T2bN0B0xM0 |
| 5 | No | M | 65 | IIA | T2bN1xB0aM0 |
| 6 | No | F | 76 | IB | T2bN0B0xM0 |
| 7 | No | M | 81 | IIA | T2bNXB0xM0 |
| 8 | No | F | 68 | IB | T2bN0B0bM0 |
| 9 | No | M | 53 | IB | T2bN0B0aM0 |
| 10 | No | M | 66 | IB | T2bN0B0xM0 |
| 11 | Yes | M | 65 | IB | T2bN0B0xM0 |
| 12 | Yes | F | 40 | IB | T2bN0B0aM0 |
| 13 | Yes | M | 58 | IIA | T2bN1aB0aM0 |
| 14 | Yes | F | 69 | IIA | T2bN0B0xM0 |
| 15 | Yes | F | 67 | IIA | T2bN1aB0xM0 |
| 16 | Yes | M | 65 | IB | T2bN0B0xM0 |
| 17 | Yes | M | 49 | IA | T1bN0B0xM0 |
| 18 | Yes | F | 32 | IB | T2bN0B1aM0 |
Defined as increasing in disease stage over the course of follow-up (median 44.5 months); M= male; F= female.
Decreased expression of either STAT4 or FOXP3 are associated with progressive MF
mRNA expression of CD3, FOXP3, IFNγ, IL-4, IL-13, KIR3DL2, MIC-B, PLS3, and STAT4 in the skin specimens was evaluated by quantitative RT-PCR to determine the association between gene expression and disease progression in MF. We utilized CD3 expression as a surrogate marker to control for T-lymphocyte infiltrate size and compared gene expression relative to CD3 expression. There was no significant difference in CD3 expression between the progressors and non-progressors. We identified a reduction in average expression of both STAT4 and FOXP3 in the skin from patients with eventual disease progression compared to those with stable disease [STAT4: 0.03 +/− SEM 0.007 vs. 0.065 +/− SEM 0.013 (p=0.05); FOXP3: 0.093 +/− SEM 0.039 vs. 0.267 +/− SEM 0.076 (p=0.05)] (Table 2 and Fig. 1). There was no association between the expression levels of the other genes (IFNγ, IL-4, IL-13, KIR3DL2, MIC-B, PLS3) relative to CD3 expression between progressors and non-progressors (Table 2).
Table 2.
Gene Expression in Progressive and Non-Progressive Mycosis Fungoides
| Gene expression relative to T-cell infiltrate (CD3) | Mean Relative mRNA Expression | p-Value | |
|---|---|---|---|
|
| |||
| Progressors (n=8) | Non-progressors (n=10) | ||
| FOXP3/CD3 | 0.093 (SEM 0.039) | 0.267 (SEM 0.076) | p=0.05 |
| IFNγ/CD3 | 0.089 (SEM 0.057) | 0.112 (SEM 0.057) | p=0.86 |
| IL4/CD3 | 0.00013 (SEM 0.00009) | 0.00017 (SEM 0.00016) | p=0.08 |
| KIR3DL2/CD3 | 0.052 (SEM 0.026) | 0.051 (SEM 0.025) | p=1.00 |
| MICB/CD3 | 0.032 (SEM 0.013) | 0.041 (SEM 0.008) | p=0.11 |
| PLS3/CD3 | 82.57 (SEM 36.81) | 180.62 (SEM 76.23) | p=0.68 |
| STAT4/CD3 | 0.03 (SEM 0.007) | 0.065 (SEM 0.013) | p=0.05 |
Figure 1. STAT4 and FOXP3 are decreased in progressive MF.
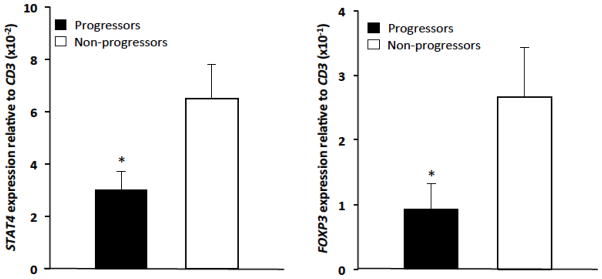
qRT-PCR expression of STAT4 and FOXP3 relative to CD3 in MF progressors and non progressors (*p=0.05).
The signature of elevated IL-4 and decreased STAT4 and FOXP3 is associated with progressive MF
For further evaluation of these potential markers, each gene was assigned a cut-off value, converting them into dichotomous variables. There was a significant association of disease progression with FOXP3/CD3 expression of < 0.1 (p=0.02, Table 3). Similarly, a relative expression of IL-4/CD3 > 1 ×10−5 was also associated with disease progression (p=0.03, Table 3). Although not statistically significant, STAT4/CD3 < 0.05 was associated with disease progression. When utilizing a combination of the genes most associated with MF progression in our data, we found the expression signature of FOXP3/CD3 < 0.1, STAT4/CD3 < 0.05, and IL-4/CD3 > 1 ×10−5 together was significantly associated with disease progression in MF (p=0.04; Table 3).
Table 3.
Progressive Disease Associated with the Expression of Specific Genes
| Tumor Marker | Cut-off Value | p-value |
|---|---|---|
|
| ||
| FOXP3/CD3 | <0.1 | p=0.02 |
| STAT4/CD3 | <0.05 | p=0.06 |
| IL4/CD3 | >1 ×10−5 | p=0.03 |
| FOXP3/CD3, STAT4/CD3, IL-4/CD3 | as above | p=0.04 |
Through the assignment of a cut-off value, dichotomous variables were generated from the gene expression data.
Discussion
While most patients diagnosed with early stage MF will have an indolent disease course, there is a subset of patients who will experience progressive disease with an associated increased mortality. Currently, it is not possible to accurately predict what course an individual patient will follow, and given this widely variable spectrum of disease outcome, improved predictors of MF progression would be helpful to identify those requiring more intensive therapy. We hypothesized that the development of progressive MF would be associated with an altered expression of several proposed tumor-associated markers previously identified in MF (KIR3DL2, MIC-B, and PLS3),15 polar Th cytokines (IFN γ, IL-4, IL-13)5, 6 and decreased STAT416 and FOXP3 (previously shown to be associated with poorer outcome in MF).7–9
In this study, we identified decreased expression of STAT4 and FOXP3, relative to CD3 expression, in patients with early stage MF who had eventual progression of disease. Through the assignment of cut-off values for FOXP3, STAT4, and IL-4 all relative to CD3, we were able to identify an expression signature of three genes (FOXP3/CD3 < 0.1, STAT4/CD3 < 0.05, and IL-4/CD3 > 1 ×10−5) that shows significant association with progressive disease.
This mRNA expression signature of a decrease in FOXP3 and STAT4 and an increase in IL- 4 is consistent with known mechanisms of tumorigenesis. For example, FOXP3 is the most specific available marker for regulatory T-cells (T reg) and has been shown to be present in various neoplasms, including MF.9 However, the role of FOXP3 in MF and CTCL is quite complex.17 T reg cells in early MF infiltrates appear to have a beneficial effect on the disease, perhaps by directly inhibiting neoplastic T-cells.7,9 However, under some circumstances such as large cell transformation, neoplastic T-cells may acquire T reg-like properties including expression of FOXP3, and this can be deleterious through suppression of the anti-tumor immune response.10 In this study we observed that diminished FOXP3 mRNA expression in lesional skin of clinically early MF was associated with subsequent progression of disease. This suggests that the MF infiltrate of progressors contains fewer non-neoplastic FOXP3+ cells than non-progressors. Thus the inferred decrease of T reg cells, as measured by decreased FOXP3, may account for the more aggressive disease course found in patients with progressive MF 8. Although more studies examining this theory and the prognostic value of FOXP3 expression in early MF are needed, these data offer additional support for assessing mRNA expression of FOXP3 in the evaluation of early MF patients.
Similarly, a reduction in both expression and phosphorylation of STAT4 has been identified in CTCL. 16, 18–20 STAT proteins are cytoplasmic transcription factors involved in cytokine signaling.21 Because expression of STAT4 is activated by IL-12 and required for Th1 differentiation, 22–24 it is suggested that the dysregulation of the STAT4 signal transduction pathway in MF results in a shift to Th2 polarization and more aggressive disease.19, 20 When looking at patients with a leukemic variant of CTCL, Sézary Syndrome, STAT4 protein is markedly decreased,18, 20 suggesting that decreased STAT4 denotes more progressive disease.
Interleukin 4 (IL-4) is a well-known cytokine produced by Th2 cells, which induces Th2 cell proliferation.25 In MF, the transition from a Th1 to a Th2 cytokine profile is thought to mark progression to more advanced disease.5, 26,27 Thus, IL-4 has been implicated as an early indicator of disease progression and a predictor of advanced MF.5 This concept of increasing Th2 polarization as MF progresses is disputed, however. Recently, IL-4 was shown to be decreased in late-stage MF when compared to normal skin, although an increase in IL-4 was still observed in early MF.6 The use of IL-4 as a biomarker in early MF is promising, yet further studies are needed to clarify the utility of IL-4 in predicting disease progression.
Lastly, it is interesting to note that there was a non-significant trend with female gender and decrease in age in those whose MF progressed from early stage disease. These data are in keeping with the findings of Sun, et al, who reported increased aggressivity of MF disease in younger females.28 Additional studies to support younger age and female gender bias towards early MF progression to more aggressive disease are needed.
Conclusion
Early MF can have a widely divergent clinical course, and those with progressive disease have a markedly worse prognosis with a 5-year survival of 26% for advanced disease2. Therefore, it is essential that we identify markers for those with early stage MF that will help predict who will go on to develop advanced disease. In this study we show that decreased mRNA expression of FOXP3/CD3 and STAT4/CD3 and increased IL-4/CD3 can be useful as potential early markers of eventual clinical progression. Given our small cohort, additional studies and evaluation will be needed to further clarify the role of these markers. It is also imperative that we continue the identification of other possible markers early in disease, in order to accurately diagnose patients with greater risk of morbidity and steer appropriate treatment.
Acknowledgments
Funding: L.Y.M. is supported by the Dermatology Foundation, the NIH/NCI (K12CA090625), and the Vanderbilt Department of Medicine/Dermatology. C.M.E. is supported by R01CA148950 and the Vanderbilt Department of Pathology, Microbiology and Immunology. This project was also supported by the CTSA award No. UL1TR000445 from the National Center for Advancing Translational Sciences, and the American Cancer Society (IRG-58-009-51).
Footnotes
Conflicts of interest: We have no conflicts of interest
References
- 1.Rappaport H, Thomas LB. Mycosis fungoides: the pathology of extracutaneous involvement. Cancer. 1974;34:1198–1229. doi: 10.1002/1097-0142(197410)34:4<1198::aid-cncr2820340431>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 2.Diamandidou E, Colome M, Fayad L, Duvic M, Kurzrock R. Prognostic factor analysis in mycosis fungoides/Sézary syndrome. J Am Acad Dermatol. 1999 Jun;40(6 Pt 1):914–24. doi: 10.1016/s0190-9622(99)70079-4. [DOI] [PubMed] [Google Scholar]
- 3.Talpur R, Singh L, Daulat S, et al. Long-term Outcomes of 1,263 Patients with Mycosis Fungoides and Sezary Syndrome from 1982 to 2009. Clin Cancer Res. 2012 Sep 15;18(18):5051–60. doi: 10.1158/1078-0432.CCR-12-0604. Epub 2012 Jul 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernengo MG, Quaglino P, Novelli M, et al. Prognostic factors in Sézary syndrome: a multivariate analysis of clinical, haematological and immunological features. Ann Oncol. 1998;9:857–63. doi: 10.1023/a:1008397323199. [DOI] [PubMed] [Google Scholar]
- 5.Papadavid E, Economidou J, Psarra A, et al. The relevance of peripheral blood Thelper 1 and 2 cytokine pattern in the evaluation of patients with mycosis fungoides and Sézary syndrome. Br J Dermatol. 2003 Apr;148(4):709–18. doi: 10.1046/j.1365-2133.2003.05224.x. [DOI] [PubMed] [Google Scholar]
- 6.Chong BF, Wilson AJ, Gibson HM, et al. Immune function abnormalities in peripheral blood mononuclear cell cytokine expression differentiates stages of cutaneous T-cell lymphoma/mycosis fungoides. Clin Cancer Res. 2008 Feb 1;14(3):646–53. doi: 10.1158/1078-0432.CCR-07-0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gjerdrum LM, Woetmann A, Odum N, et al. FOXP3+ regulatory T cells in cutaneous T-cell lymphomas: association with disease stage and survival. Leukemia. 2007 Dec;21(12):2512–8. doi: 10.1038/sj.leu.2404913. Epub 2007 Aug 23. [DOI] [PubMed] [Google Scholar]
- 8.Klemke CD, Fritzsching B, Franz B, et al. Paucity of FOXP3+ cells in skin and peripheral blood distinguishes Sézary syndrome from other cutaneous T-cell lymphomas. Leukemia. 2006 Jun;20(6):1123–9. doi: 10.1038/sj.leu.2404182. [DOI] [PubMed] [Google Scholar]
- 9.Wada DA, Wilcox RA, Weenig RH, Gibson LE. Paucity of intraepidermal FoxP3-positive T cells in cutaneous T-cell lymphoma in contrast with spongiotic and lichenoid dermatitis. J Cutan Pathol. 2010 May;37(5):535–41. doi: 10.1111/j.1600-0560.2009.01381.x. Epub 2009 Aug 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hallermann C, Niermann C, Schulze HJ. Regulatory T-cell phenotype in association with large cell transformation of mycosis fungoides. European journal of haematology. 2007;78:260–3. doi: 10.1111/j.1600-0609.2006.00809.x. [DOI] [PubMed] [Google Scholar]
- 11.Valencak J, Schmid K, Trautinger F, et al. High expression of Dicer reveals a negative prognostic influence in certain subtypes of primary cutaneous T cell lymphomas. Journal of dermatological science. 2011;64:185–90. doi: 10.1016/j.jdermsci.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Maj J, Jankowska-Konsur A, Sadakierska-Chudy A, et al. Altered microRNA expression in mycosis fungoides. The British journal of dermatology. 2012;166:331–6. doi: 10.1111/j.1365-2133.2011.10669.x. [DOI] [PubMed] [Google Scholar]
- 13.Litvinov IV, Jones DA, Sasseville D, et al. Transcriptional profiles predict disease outcome in patients with cutaneous T-cell lymphoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2010;16:2106–14. doi: 10.1158/1078-0432.CCR-09-2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vonderheid EC, Pavlov I, Delgado JC, et al. Prognostic Factors and Risk Stratification Early Mycosis Fungoides. Leukemia & lymphoma. 2013 doi: 10.3109/10428194.2013.790541. [DOI] [PubMed] [Google Scholar]
- 15.Capriotti E, Vonderheid EC, Thoburn CJ, Wasik MA, Bahler DW, Hess AD. Expression of T-plastin, FoxP3 and other tumor-associated markers by leukemic T-cells of cutaneous T-cell lymphoma. Leuk Lymphoma. 2008 Jun;49(6):1190–201. doi: 10.1080/10428190802064917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tracey L, Villuendas R, Dotor AM, et al. Mycosis fungoides shows concurrent deregulation of multiple genes involved in the TNF signaling pathway: an expression profile study. Blood. 2003 Aug 1;102(3):1042–50. doi: 10.1182/blood-2002-11-3574. Epub 2003 Apr 10. [DOI] [PubMed] [Google Scholar]
- 17.Krejsgaard T, Odum N, Geisler C, et al. Regulatory T cells and immunodeficiency in mycosis fungoides and Sezary syndrome. Leukemia: official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2012;26:424–32. doi: 10.1038/leu.2011.237. [DOI] [PubMed] [Google Scholar]
- 18.Nebozhyn M, Loboda A, Kari L, et al. Quantitative PCR on 5 genes reliably identifies CTCL patients with 5% to 99% circulating tumor cells with 90% accuracy. Blood. 2006 Apr 15;107(8):3189–96. doi: 10.1182/blood-2005-07-2813. Epub 2006 Jan 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nielsen M, Kaltoft K, Nordahl M, et al. Constitutive activation of a slowly migrating isoform of Stat3 in mycosis fungoides: tyrphostin AG490 inhibits Stat3 activation and growth of mycosis fungoides tumor cell lines. Proc Natl Acad Sci U S A. 1997 Jun 24;94(13):6764–9. doi: 10.1073/pnas.94.13.6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Showe LC, Fox FE, Williams D, Au K, Niu Z, Rook AH. Depressed IL-12-mediated signal transduction in T cells from patients with Sezary syndrome is associated with the absence of IL-12 receptor beta 2 mRNA and highly reduced levels of STAT4. J Immunol. 1999;163:4073–4079. [PubMed] [Google Scholar]
- 21.Turkson J. STAT proteins as novel targets for cancer drug discovery. Expert Opin Ther Targets. 2004 Oct;8(5):409–22. doi: 10.1517/14728222.8.5.409. Review. [DOI] [PubMed] [Google Scholar]
- 22.Szabo SJ, Jacobson NG, Dighe AS, Gubler U, Murphy KM. Developmental commitment to the Th2 lineage by extinction of IL-12 signaling. Immunity. 1995;2:665–675. doi: 10.1016/1074-7613(95)90011-x. [DOI] [PubMed] [Google Scholar]
- 23.Nishikomori R, Usui T, Wu CY, Morinobu A, O’Shea JJ, Strober W. Activated STAT4 has an essential role in Th1 differentiation and proliferation that is independent of its role in the maintenance of IL-12R beta 2 chain expression and signaling. J Immunol. 2002;169:4388–4398. doi: 10.4049/jimmunol.169.8.4388. [DOI] [PubMed] [Google Scholar]
- 24.Murphy KM, Ouyang W, Szabo SJ, et al. T helper differentiation proceeds through Stat1-dependent, Stat4-dependent and Stat4-independent phases. Curr Top Microbiol Immunol. 1999;238:13–26. doi: 10.1007/978-3-662-09709-0_2. [DOI] [PubMed] [Google Scholar]
- 25.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 26.Savvateeva MV, Savina MI, Markusheva LI, Samsonov VA. Relative content of cytokines in different tissues in mycosis fungoides. Bull Exp Biol Med. 2002 Aug;134(2):175–6. doi: 10.1023/a:1021148601424. [DOI] [PubMed] [Google Scholar]
- 27.Pawlaczyk M, Sobieska M. A correlation between acute phase proteins and cytokines in patients suffering from mycosis fungoides. Acta Dermatovenerol Alp Panonica Adriat. 2006 Sep;15(3):107–12. [PubMed] [Google Scholar]
- 28.Sun G, Berthelot C, Li Y, Glass DA, et al. Poor prognosis in non-Caucasian patients with early-onset mycosis fungoides. J Am Acad Dermatol. 2009 Feb;60(2):231–5. doi: 10.1016/j.jaad.2008.09.063. [DOI] [PubMed] [Google Scholar]


