Background: Rab31 is a member of the Rab5 subfamily of Rab GTPases.
Results: Manipulation of Rab31 and interacting partners affects trafficking of the EGF receptor (EGFR) to the late endosome.
Conclusion: Rab31, with its regulator and effector, plays an important role in the trafficking of the EGFR from the early to the late endosome.
Significance: Evidence is provided for Rab31 being a key regulator of endocytic traffic of the EGFR.
Keywords: Endocytosis, Endosomes, Epidermal Growth Factor Receptor (EGFR), Rab, Trafficking
Abstract
Rab31 is a member of the Rab5 subfamily of Rab GTPases. Although localized largely to the trans-Golgi network, it shares common guanine nucleotide exchange factors and effectors with other Rab5 subfamily members that have been implicated in endocytic membrane traffic. We investigated whether Rab31 also has a role in the trafficking of the ligand-bound EGF receptor (EGFR) internalized through receptor-mediated endocytosis. We found that loss of Rab31 inhibits, but overexpression enhances, EGFR trafficking to the late endosomes and that the effect of Rab31 silencing could be specifically rescued by overexpression of a silencing-resistant form of Rab31. Rab31 was found to interact with the EGFR by coimmunoprecipitation and affinity pulldown analyses, and the primarily trans-Golgi network-localized Rab31 has increased colocalization with the EGFR in A431 cells 30 min after pulsing with EGF. A glycerol gradient sedimentation assay suggested that Rab31 is sequestered into a high molecular weight complex after stimulation with EGF, as was early endosome antigen 1 (EEA1), a factor responsible for endosomal tethering and fusion events. We found that loss of EEA1 reduced the interaction between Rab31 and the EGFR and abrogated the effect of Rab31 overexpression on the trafficking of the EGFR. Likewise, loss of GAPex5, a Rab31 guanine nucleotide exchange factor that has a role in ubiquitination and degradation of the EGFR, reduced the interaction of Rab31 with the EGFR and its effect on EGFR trafficking. Taken together, our results suggest that Rab31 is an important regulator of endocytic trafficking of the EGFR and functions in an EGFR trafficking complex that includes EEA1 and GAPex5.
Introduction
The superfamily of GTPases consists of GTP-activated regulatory proteins with an intrinsic enzymatic activity that hydrolyzes GTP to GDP. The family of small (20–35 kDa) GTPases include Ras, Rho, and Rab, and these function as molecular switches in the cell, with the latter playing critical roles in membrane transport (1). Rabs regulate vesicular transport in the cell by engaging various effector proteins, such as motor proteins, tethering factors, and fusion components, such as SNARE proteins (2–5). These molecular switches confer specificity to particular vesicular transport steps by virtue of their specific subcellular location along the vesicular transport pathways as well as via a large repertoire of specific interacting proteins (6, 7). These include upstream activators, such as guanine nucleotide exchange factors (GEFs),3 that exchange GDP for GTP and downstream effectors that bind to activated, GTP-bound Rabs (8, 9).
The human genome contains over 60 different Rab and Rab-like genes (10, 11). Rab31 was first cloned from human melanocyte cDNA and has been classified under the Rab5-related subfamily on the basis of sequence homology (12). The Rab5 subfamily of Rab proteins includes Rab5, Rab21, Rab22a, and Rab31 (13). Members of this subfamily have been implicated in a variety of endocytosis-related trafficking steps, including that of cell surface receptors (14–16). Cell surface receptors are endocytosed constantly, both constitutively and when bound by its ligands. The epidermal growth factor receptor (EGFR), for example, exists at a steady state on the cell surface as a monomer. Upon binding of EGF or other ligands, the receptor dimerizes and autophosphorylates, initiating a resultant signaling cascade by serving as a docking site for Src homology 2 or phosphotyrosine B domain-containing proteins (17). At the same time, the ligand-bound EGFR is internalized from the plasma membrane, trafficked to early and late endosomes, and, eventually, degraded in lysosomes, which results in termination of its signaling (18, 19). Alternatively, the receptor may be recycled to the cell surface (20). Many Rabs, including those of the Rab5 subfamily, have been implicated to varying extents in various trafficking steps of the EGFR internalization pathway. For example, Rab5 (21) and Rab 21 (15) have been shown to enhance the movement of the EGFR from the cell surface into early endosomes, whereas Rab22a (the closest paralogue to Rab5 (22)), and also Rab21, have been implicated in the later trafficking steps with a general role in recycling or terminating EGFR signaling in late endosomes/lysosomes (16). In some studies, loss of Rab5 activity (either by siRNA or use of dominant negative Rab5) has also been shown to inhibit the exit of the ligand-bound EGFR from the early endosome (23, 24). Meanwhile, another Rab, Rab7, has been linked to the maturation of EGFR-containing late endosomes to lysosomes (25).
Rab31 has been shown to function in Golgi-endosome trafficking of the mannose 6-phosphate receptor (26, 27) but has not been implicated directly in endocytic trafficking pathways. We have reported previously that Rab31 is localized to the perinuclear region of the cell, colocalizing with trans-Golgi network (TGN) markers such as TGN46 (28). The TGN compartment is a major focal point for many vesicular transport pathways in the cell. It receives retrograde input, on one hand, from the plasma membrane via early/recycling endosomes and, on the other, it channels anterograde traffic from the Golgi network to other compartments in the cell (29). Given its subcellular location at the TGN, Rab31 is, therefore, situated at a crossroad of many trafficking steps. It also shares similar GEFs and effectors with other Rab5 subfamily members, notably GAPex5 and the early endosome antigen 1 (EEA1) (22, 30). GAPex5 contains a Ras-GAP (GTPase-activating protein) domain and also a GEF domain for Rabs that is homologous to yeast Vps9p and has been identified to bind to the EGFR through Cbl, an E3 ubiquitin ligase, upon receptor dimerization (31). EEA1 participates in endosomal tethering and docking (32). Given the possible connections mentioned above, we investigated how Rab31 would affect the EGFR trafficking itinerary in cultured A431 cells, a human epidermoid carcinoma line that has a high level of EGFR expression. We report that Rab31 is important for the trafficking of the EGFR, upon ligand stimulation, to the late endosome compartment via the formation of a trafficking complex with the cargo itself.
EXPERIMENTAL PROCEDURES
Gene Constructs
A human Rab31 (Integrated Molecular Analysis of Genomes (IMAGE) Consortium clone) sequence tag was obtained. The mouse Rab31 expression construct was provided by Prof. Mitsunori Fukuda. pmCherryC1-Rab5a from the laboratory of Dr. Christien Merrifield was obtained from Addgene (catalog no. 27679). pCI-Neo Rab11 was modified from constructs obtained from the laboratory of Prof. Mary McCaffrey. Constructs were inserted into various vector backbones, including pCI-Neo (Promega, Madison, WI), pmCherry-C1 (Clontech), pEGFP-C1 (Clontech), and pGEX-4T-3 (GE Healthcare).
Antibodies
The rabbit antiserum against Rab31 was generated by repeated immunization with GST fused to the C-terminal 37 amino acids of human Rab31. The mouse monoclonal antibody against Rab31 was obtained from Abmart (Shanghai, China). The following commercial primary antibodies were used: EGFR (Merck Millipore, Singapore), EEA1 (BD Biosciences), GAPex5 (Abcam, Cambridge, UK), RIN3 (Abnova, Taipei, Taiwan), the trans-Golgi marker TGN46 (AbD Serotec, Kidlington, UK), Rab11 (Abcam), and γ-tubulin (Sigma). The CD63 hybridoma H5C6 monoclonal antibody developed by J. T. August and J. E. K. Hildreth was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD, National Institutes of Health, and maintained by The University of Iowa Department of Biology (Iowa City, IA).
Cell Culture
A431 cells (ATCC) were grown in high-glucose DMEM (Hyclone, Logan, UT) supplemented with 10% heat-inactivated FBS (Hyclone), 1% antibiotic/antimycotic (Invitrogen), and 1 mm sodium pyruvate (Invitrogen). Transfection was performed with Lipofectamine 2000 (Invitrogen) according to the protocols of the manufacturer. Stably transfected cells were selected with 1.2 mg/ml G418 (Invitrogen) and single cell-cloned.
Expression Silencing
siRNA-mediated silencing was carried out by Lipofectamine RNAiMAX-mediated (Invitrogen) transfection of two 27-mer RNA duplexes (Integrated DNA Technologies, Inc., Coralville, IA), according to the protocols of the manufacturer. siRNAs were on the basis of the following sequences: Rab31, 5′-rGrGrArArTrArCrGrCrTrGrArArTrCrCrArTrArGrGrTrGCC-3′ and 5′-rGrTrGrCrCrTrTrGrTrGrGrArArArTrGrArArCrTrTrCrACA-3′; EEA1, 5′-rGrCrArGrGrArUrUrCrArGrCrArArArGrArArArGrArArCAG-3′ and 5′-rGrUrArUrGrArUrGrArArGrArArArGrGrArGrUrCrUrUrCGA-3′; GAPex5, 5′-rArArGrArArUrCrGrArUrUrArCrCrUrArUrArGrCrArArCUC-3′ and 5′-rGrCrArGrGrArGrGrArGrCrGrUrCrUrGrCrArArGrArArCTG-3′; RIN3, 5′-rCrGrArCrCrArGrCrCrArCrCrUrCrUrUrGrGrArArArUrUGC-3′ and 5′-rCrCrArCrCrArCrUrGrArCrCrUrArGrGrUrGrUrGrArCrCAC-3′; and Rab5a, 5′-rGrUrArCrUrArCrArGrArGrGrArGrCrArCrArArGrCrArGCC-3′ and 5′-rCrCrCrArCrArCrArArCrCrArArCrCrArGrGrArArUrCrAGT-3′. Rab31 silencing was also performed using HuSH 29-mer shRNA (Origene, Rockville, MD transfected into cells.
Reverse Transcription PCR
Total RNA was harvested from cells using an RNA isolation kit (Qiagen, Singapore). One-step RT-PCR was performed using an RT-PCR kit (Qiagen), and products were run on a 1% agarose gel and imaged. The following primers for amplification were used: Rab5a, 5′-CGC GAA TTC ATG GCT AGT CGA GGC GC-3′ (forward) and 5′-CCT GTC GAC TTA GTT ACT ACA ACA CTG ATT CC-3′ (reverse); Rab31, 5′-CTC GAA TTC AAT GAT GGC GAT ACG GGA GCT C-3′ (forward) and 5′-TCG GTC GAC TCA ACA GCA CCG GCG GCT-3′ (reverse); GAPex5, 5′-GTC GAA TTC ATG GTG AAA CTA GAT ATT CAT ACT CTG-3′ (forward) and 5′-CCT CCC ATC AAC AAA TTG TGT ATC TTC-3′ (reverse); and RIN3, 5′-GCG GAA TTC A ATG ATC CGA CAC GCC GGG GCG-3′ (forward) and 5′-CTA GCC ACC ACC CGG TGC AGG ATC-3′ (reverse).
EGF Pulse-Chase
Cells were serum-starved overnight in basal DMEM. 0.25–0.5 μg/ml Texas Red (TxR) or FITC-tagged EGF (Molecular Probes, Invitrogen) or non-conjugated EGF (Peprotech, Rocky Hill, NJ) was incubated with cells on ice for 20 min, followed by a 5-min incubation at 37 °C for internalization. Cells were then acid-washed (150 mm NaCl and 50 mm glycine (pH 3)) before being returned into complete DMEM at 37 °C for the chase and fixed at various time points or harvested for cell lysate.
Western Blot Analysis and Collection of Cell Lysate
For Western blot analysis, cells were lysed with lysis buffer (50 mm Tris (pH 8), 1 mm EDTA (pH 8), 150 mm NaCl, and 1% Triton X-100 with protease inhibitor mixture (Roche Diagnostics)) for 60 min. Lysates were then subjected to reducing SDS-PAGE, electroblotted onto Hybond C-extra (Amersham Biosciences, UK) nitrocellulose membranes, and probed with the respective antibodies.
Immunocytochemistry and Immunofluorescence Microscopy
For immunofluorescence microscopy, cells plated on coverslips and subjected to various treatments were fixed with 4% paraformaldehyde, followed by sequential incubation with the primary and secondary antibodies. Fluorescence labeling was visualized using the Carl Zeiss 710 (Oberkochen, Germany) confocal imaging system. Images were collected in separate z sections, and the final images presented were typically from collapsed z stacks. Image processing was done using Zen 2010 software (Carl Zeiss). Calculation of the overlap coefficient was performed using Zen 2010 software (Carl Zeiss). Particle size was measured using ImageJ (National Institutes of Health, Bethesda, MD).
Glycerol Gradient Sedimentation
Samples were lysed with lysis buffer containing 10 mm HEPES, 2 mm MgCl2, 10 mm KCl, 0.5 mm EDTA, 150 mm NaCl, and 1% TritonX-100. Samples were treated with or without GTPγS (Merck Millipore) and diluted to a final solution containing 0.5% Triton X-100. 2 mg of lysate was loaded onto 11 ml of 5–45% glycerol gradients. Centrifugation was for 18 h at 38,000 rpm in a Beckman SW41Ti rotor maintained at 4 °C. 1-ml fractions were collected, and proteins were precipitated in 20% tricarboxylic acid, followed by a cold acetone wash. Standards were applied to a glycerol gradient and run in parallel.
Coimmunoprecipitation
To purify interacting partners, 2 μg of Rab31 antibody was incubated at 4 °C overnight with 1 mg of cell lysate loaded with 1 mm GTPγS at room temperature for 20 min and then bound to 50 μl of protein A beads (GE Healthcare). After incubation, beads were washed with 10 column volumes of lysis buffer. Elution of bound proteins was performed with loading buffer at 72 °C. Eluted proteins were analyzed by SDS-PAGE and Western blot analysis.
GST Affinity Pulldown
GST fusion proteins of the Rabs were expressed in Escherichia coli DH5α cells and purified by standard protocols. In brief, cells were harvested by centrifugation, resuspended, and sonicated. The supernatant was incubated with glutathione beads (GE Healthcare) and eluted in elution buffer with a final concentration of 50 mm Tris (pH 8), 0.1% Triton X-100, and 20 mm glutathione.
To purify interacting partners, GST fusion proteins were added to 1 mg of cell lysate, loaded with 1 mm GTPγS at room temperature for 20 min, and then bound to 50 μl of glutathione beads overnight at 4 °C. After incubation, beads were washed with 10 column volumes of lysis buffer. Elution of bound proteins was performed with loading buffer at 72 °C. Eluted proteins were analyzed by SDS-PAGE and Western blot analysis.
Statistical Analysis
Statistical analysis was performed using unpaired Student's t test. Data shown in bar graphs represent the mean of three independent experiments assayed in triplicate. Error bars indicate mean ± S.E. of three independent experiments.
RESULTS
Rab31 Affects the Endocytic Trafficking of the Ligand-bound EGFR
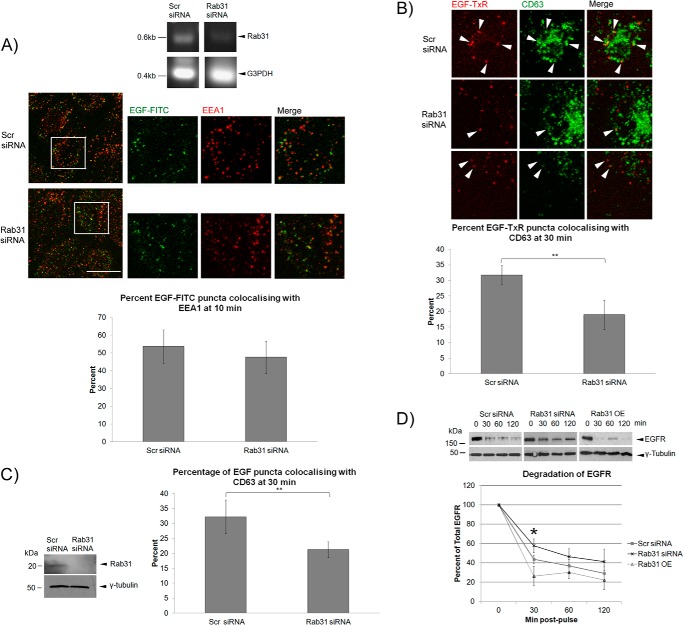
By silencing Rab31 in A431 cells, we have shown previously that loss of Rab31 inhibits the endocytic traffic of the ligand-stimulated EGFR. Rab31 silencing delayed the entry of the ligand-bound EGFR into late endosomes, as seen by the reduced fraction of large EGFR-positive puncta at 60 min post-pulse and as quantified by colocalization with the late endosome marker CD63/Lamp3 (33). When further investigating this phenomenon, we found that loss of Rab31 did not affect the colocalization between the ligand-bound EGFR (as indicated by the labeled EGF signal) and EEA1 at 10 min post-pulse, suggesting that Rab31 is not required for the initial endocytosis and trafficking of the ligand-bound EGFR into the early endosome (Fig. 1A), a role that is commonly attributed to Rab5 (21, 34). Instead, loss of Rab31 resulted in a significantly diminished entry of ligand-bound EGFR into the late endosome compartment because there is less colocalization between the ligand-bound EGFR and the late endosome marker CD63 at 30 min (Fig. 1B). This time of 30 min corresponds to the transition of the internalized EGFR between early and late endosomes (36) and suggests that the absence of Rab31 impairs early to late endosome transport of the ligand-bound EGFR. To ascertain that the effect of Rab31 manipulations on endocytic traffic of ligand-bound EGFR was not specific only to A431 cells, which have exceptionally high levels of EGFR, we also performed similar experiments in HeLa cells. As with A431 cells, Rab31 silencing in HeLa cells inhibited late endosome traffic of the ligand-bound EGFR (Fig. 1C). To confirm that Rab31 impacts the endocytic trafficking of the ligand-bound EGFR to late endosomes, we looked at the eventual degradation of the EGFR after an EGF pulse. We found that at 30 min, loss of Rab31 reduced the rate at which the EGFR is degraded by 14%, whereas overexpression of Rab31 increased the rate by 17% (Fig. 1D).
FIGURE 1.
Loss of Rab31 hinders endocytic trafficking of the ligand-bound EGFR. A and B, A431 cells were transfected with scrambled (Scr) or Rab31 siRNA and analyzed for EGF trafficking after 48 h. A, top panel, the extent of Rab31 knockdown was assessed by RT-PCR. The endogenous levels of Rab31 protein were low, and Western blot analysis was not useful. Center panel, cells were pulsed with 0.5 μg/ml EGF-FITC (green), followed by a 10-min chase before fixation and immunofluorescence analysis with the colabeling of early the endosome marker EEA1 (red). The individual and merged fluorescence signals of the areas enclosed in white squares are shown enlarged ×2 on the right. Scale bar = 20 μm. Bottom panel, the number of EGF-FITC puncta positive for EEA1 was quantified and presented graphically as a percentage of total EGF-FITC puncta counted. 27 cells in three independent experiments were analyzed, and data are shown as mean ± S.E. B, cells were pulsed with 0.5 μg/ml EGF-TxR (red) followed by a 30-min chase before fixation and immunofluorescence analysis with the colabeling of the late endosome marker CD63 (green). Top panel, arrowheads show EGF-TxR puncta colocalizing with CD63. Scale bar = 20 μm. Bottom panel, the number of EGF-TxR puncta also positive for CD63 was quantified and presented graphically as a percentage of total EGF-TxR puncta counted. 27 cells in three independent experiments were analyzed, and data are shown as mean ± S.E. **, p < 0.01; Student's t test. C, HeLa cells were transfected with scrambled or Rab31 siRNA, and analysis was performed after 48 h. Left panel, knockdown of Rab31 in HeLa cells was quantified by Western blot analysis. Right panel, cells were pulsed with 0.5 μg/ml EGF-FITC followed by a 30-min chase before fixation and immunofluorescence analysis with the colabeling of the late endosome marker CD63. The number of EGF-FITC puncta also positive for CD63 was quantified and presented graphically as a percentage of total EGF-FITC puncta counted. 35 cells in three independent experiments were analyzed, and data are shown as mean ± S.E. **, p < 0.01; Student's t test. D, HeLa cells overexpressing Rab31 (Rab31 OE) and those that were transfected with scrambled or Rab31 siRNA were pulsed with 0.5 μg/ml EGF. At the various time points indicated, lysates were harvested and probed by Western blot analysis for total EGFR and γ-tubulin. Shown is a representative set of data from six independent experiments. Levels of EGFR were normalized against γ-tubulin and plotted graphically as a percentage of EGFR at 0 min after pulse. *, p < 0.05 between scrambled and Rab31 siRNA or scrambled and Rab31 OE at 30 min.
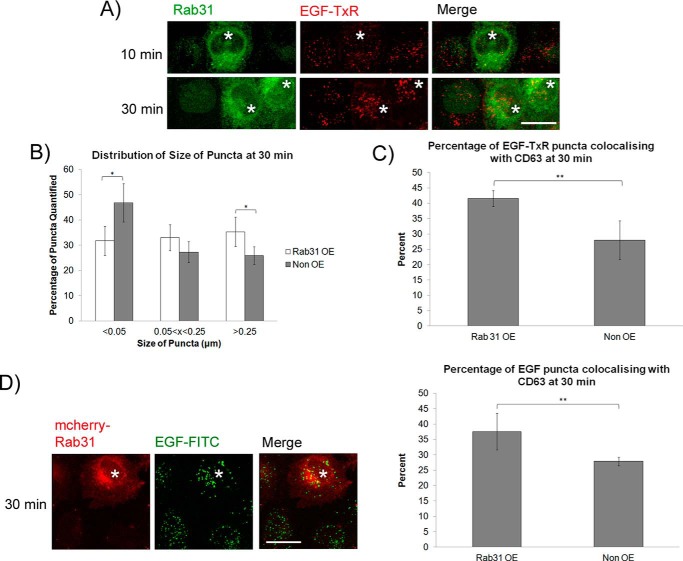
We asked whether an increase in Rab31 levels could have a converse effect on the endocytic transport of the EGFR. Stable overexpression of Rab31 (Rab31 OE) did not affect the steady-state levels or plasma membrane localization of the EGFR, as gauged by Western blotting and immunofluorescence when compared with cells transfected with the vector control (data not shown). When the cells were pulsed and chased with EGF-TxR, cells overexpressing Rab31 exhibited larger EGF-TxR punctate structures representative of the internalized EGFR, as observed by immunofluorescence. The difference was only evident at 30 min, not at 10 min, post-pulse, suggesting that Rab31 overexpression does not impact the initial internalization of the EGFR from the plasma membrane (Fig. 2A). There were significantly larger puncta in the Rab31 OE cells compared with non-overexpressing cells in the same population (Fig. 2B). Moreover, overexpression of Rab31 increased the percentage of EGF-TxR puncta that were positive for CD63 at 30 min (Fig. 2C). These experiments were repeated in HeLa cells, and similar results were obtained (Fig. 2D). These results, beyond complementing the Rab31 silencing experiments, suggest that Rab31 positively influences the endocytic trafficking of the ligand-bound EGFR and that this influence may be proportionately enhanced by increasing Rab31 levels.
FIGURE 2.
Overexpression of Rab31 enhances endocytic trafficking of the ligand-bound EGFR to the CD63-containing late endosome. A–C, A431 cells were stably transfected with Rab31. Cells were pulsed with 0.5 μg/ml EGF-TxR followed by a chase at various time points before fixation and immunofluorescence analysis. A, the effect of overexpressing Rab31 (asterisks) on the endocytosis of EGF-TxR (red) was observed by labeling for Rab31 (green) after 10- and 30-min chases. Scale bar = 20 μm. B, sizes of EGF-TxR puncta in Rab31-OE and non-OE cells from the same population were quantified using ImageJ, and the size distribution is represented graphically as a bar chart. 15 cells in three independent experiments were analyzed, and data are shown as mean ± S.E. *, p < 0.05; Student's t test. C, the number of EGF-TxR puncta also positive for CD63 in Rab31 OE and non-OE cells from the same population was quantified and presented graphically as a percentage of total EGF-TxR puncta counted. 32 cells in three independent experiments were analyzed, and data are shown as mean ± S.E. **, p < 0.01; Student's t test. D, HeLa cells were stably transfected with mcherry-Rab31. Cells were pulsed with 0.5 μg/ml EGF-FITC followed by a chase at various time points before fixation and immunofluorescence analysis. Left panel, the effect of overexpressing Rab31 (red, asterisks) on the endocytosis of EGF-FITC (green) was observed after a 30-min chase. Scale bar = 20 μm. Right panel, the number of EGF-FITC puncta also positive for CD63 was quantified and presented graphically as a percentage of total EGF-TxR puncta counted. 35 cells in three independent experiments were analyzed, and data are shown as mean ± S.E. **, p < 0.01; Student's t test.
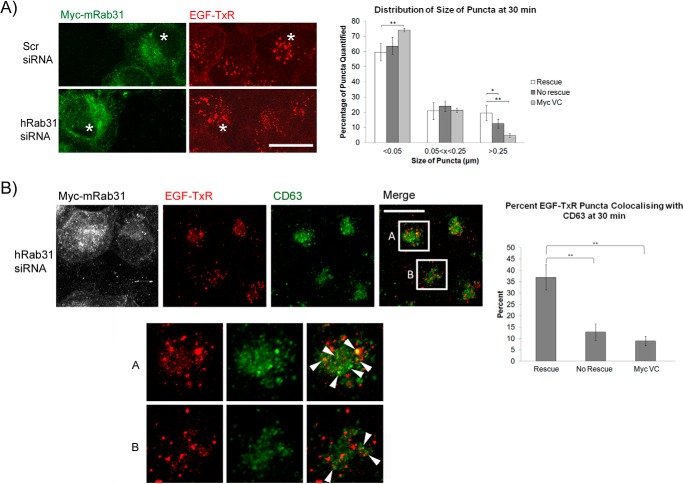
To verify that Rab31 is indeed the critical factor depleted in the silencing experiments, we attempted to rescue Rab31-silenced cells by transfecting the cells with Myc-tagged mouse Rab31 (Myc-mRab31), which differs in sequence from human Rab31 targeted by our siRNA. Overexpression of silencing-resistant Myc-mRab31 could indeed rescue the ligand-bound EGFR trafficking defect caused by loss of Rab31. After a 30-min chase with EGF-TxR, cells in which the rescue of the Rab31 knockdown phenotype had occurred (as evidenced by the expression of Myc-tagged mRab31) displayed larger EGF-TxR puncta compared with cells in the same population that did not express Myc-mRab31 (Fig. 3A). We also quantified the percentage of EGF-TxR puncta that were positive for CD63 after a 30-min chase (Fig. 3B, arrowheads). In cells with Myc-mRab31 overexpression, the percentage of EGF-TxR and CD63 colocalization was increased significantly compared with cells in the same population with no Myc-mRab31 expression. The percentage was also significantly higher compared with cells transfected with the empty vector. Taken together, our results suggest that Rab31 plays a specific role in the endocytic trafficking of the ligand-bound EGFR.
FIGURE 3.
Rescue of Rab31 silencing restores the endocytic trafficking defect of the ligand-bound EGFR. A431 cells were transfected with scrambled (Scr) or Rab31 siRNA for 48 h before subsequent transfection with siRNA silencing-resistant Myc-tagged mouse Rab31 (Myc-mRab31) or empty Myc vector (Myc VC). Cells were pulsed 24 h later with 0.5 μg/ml EGF-TxR followed by chase and fixation at various time points. A, left panel, immunofluorescence analysis for EGF-TxR (red) and Myc-mRab31 (green). The asterisks indicate Myc-mRab31-overexpressing cells with larger EGF-TxR puncta compared with non-overexpressing cells. Scale bar = 20 μm. Right panel, sizes of EGF-TxR puncta in cells with (Rescue), and without Myc-mRab31 expression (No rescue), and cells transfected with empty Myc vector after Rab31 siRNA treatment were quantified using ImageJ, and the size distribution is represented graphically as a bar chart. 15 cells in three independent experiments were analyzed, and data are shown as mean ± S.E. *, p < 0.05; **, p < 0.01; Student's t test. B, left panel, top, the effect of silencing Rab31 and subsequent rescue of the ligand-bound EGFR trafficking phenotype was also determined by assessing the amount of EGF-TxR (red) that has entered the CD63-containing (green) late endosome. Box A encloses the central region of a representative cell with Myc-mRab31 expression (pseudocolored white), whereas box B encloses a representative cell without Myc-mRab31 expression. Left panel, bottom, individual and merged fluorescence signals of the boxed areas, magnified ×2. Arrowheads indicate some EGF-TxR puncta (red) that are also positive for CD63 (green). Scale bar = 20 μm. Right panel, the percentage of EGF-TxR puncta that are positive for CD63 in cells with or without Myc-mRab31 expression and cells transfected with empty Myc vector after Rab31 siRNA treatment were quantified and presented graphically as a percentage of total EGF-TxR puncta counted. 29 cells in three independent experiments were analyzed, and data are shown as mean ± S.E. **, p < 0.01; Student's t test.
Rab31 Associates with an EGFR Trafficking Complex
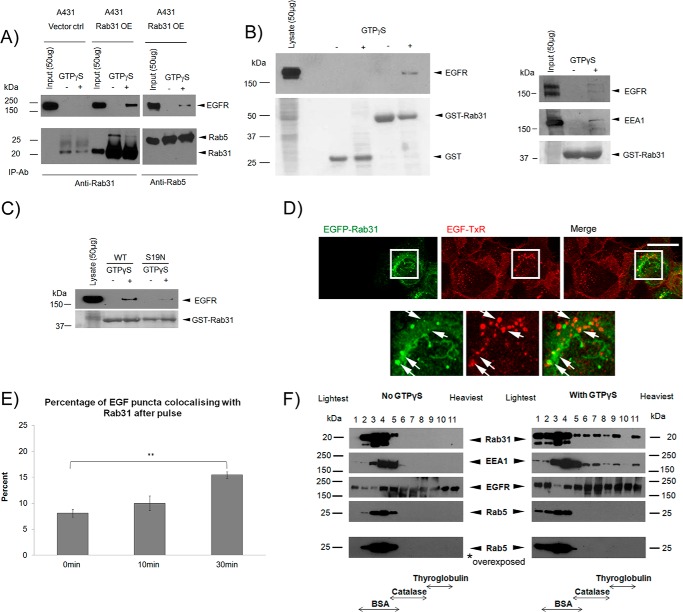
We sought to determine how Rab31 could influence the trafficking of the ligand-bound EGFR. We found that Rab31 associated with EGFR in a GTP-dependent manner by coimmunoprecipitation with anti-Rab31 antibodies (Fig. 4A). Rab5, in comparison, coimmunoprecipitated less EGFR. Similarly, we were able to perform an affinity pulldown of EGFR using GST-Rab31, but not GST, in the presence of a non-hydrolysable GTP analog, GTPγS, in both A431 and HeLa cells (Fig. 4B). In contrast, little or no EGFR was pulled down using GST-tagged Rab31S19N, a dominant negative, GDP-locked mutant of Rab31 (Fig. 4C), suggesting that Rab31 must be in its active, GTP-bound form to associate with the EGFR.
FIGURE 4.
Rab31 associates with EGFR in a high molecular weight complex that includes its effector EEA1 but not Rab5. A, EGFR was coimmunoprecipitated (IP) with Rab31 or Rab5 antibody (ab), respectively, using 1 mg of lysates from cells transfected with vector alone (Vector ctrl) and Rab31 (Rab31 OE), respectively, with or without GTPγS loading. B, 1 mg of A431 cell lysate (left panel) or HeLa cell lysate (right panel) with and without GTPγS was incubated with 20 μg of GST or GST-Rab31 and glutathione beads, and the ability of the GST fusion proteins to pull down EGFR was analyzed by Western blot analysis. The GST fusion proteins were visualized with Ponceau S stain. C, 1 mg of A431 cell lysate with and without GTPγS was incubated with 20 μg of GST-Rab31 or GST-Rab31S19N (S19N) and glutathione beads, and the ability of the GST fusion proteins to pull down EGFR was analyzed by Western blot analysis. The GST proteins were visualized with Ponceau S stain. D, A431 cells stably transfected with EGFP-tagged Rab31 (EGFP-Rab31) were pulsed with 0.5 μg/ml EGF-TxR, fixed after 30 min, and analyzed for colocalization between EGFP-Rab31 (green) and EGF-TxR (red). Bottom panel, the boxed areas in the upper panel, enlarged ×2. Arrows indicate structures positive for both EGFP-Rab31 and EGF-TxR. Scale bar = 20 μm. E, the percentage of EGF-TxR-positive puncta that are also positive for EGFP-Rab31 was quantified from cells fixed after a 0-, 10-, and 30-min chase and graphically represented as a percentage of total EGF-TxR puncta counted. 34 cells in three independent experiments were analyzed, and data are shown as mean ± S.E. **, p < 0.01; Student's t test. F, 2 mg of A431 Rab31 OE lysates with and without GTPγS loading was resolved by glycerol gradient sedimentation. Fractions were collected, and TCA was precipitated and analyzed by Western blot analysis for Rab31, EGFR, Rab5, and EEA1 (an effector for both Rab5 and Rab31). The membrane blot for Rab5 was also overexposed. The position in the gradient that contains the molecular size markers BSA (67 kDa), catalase (240 kDa), and thyroglobulin (660 kDa) is indicated.
We looked more closely at how Rab31 might be associated with the EGFR, which is not conventionally known to traverse the TGN (where most of Rab31 is located) during its endocytic traffic. Using an immunofluorescence analysis of A431 cells stably transfected with EGFP-Rab31 and pulse-chased with EGF-TxR, we observed that a portion of the EGFP-Rab31 signal associates with the EGF-positive punctate structures, indicative of endosomes bearing the ligand-bound EGFR (Fig. 4D). This association was particularly evident after a 30-min chase, a period when the ligand-bound EGFR is likely to be transiting between early and late endosomes (36). We quantified the percentage of EGF-TxR puncta that were also immunopositive for Rab31 and found that the percentage increases upon EGF pulse and is statistically significant at 30 min (Fig. 4E). This suggests that a portion of Rab31 may be recruited to the endocytic membrane, rather than the TGN, and may associate with a trafficking complex containing the ligand-bound EGFR. Likewise, we observed a percentage of colocalization between Rab31 and EGF in an orthogonal projection of HeLa cells transfected with mCherry-Rab31 and pulse-chased with EGF-FITC (data not shown), suggesting that this phenomenon is not specific only to A431 cells.
That Rab31 may indeed be part of an EGFR-containing trafficking complex was also suggested by a glycerol gradient sedimentation analysis of proteins from Rab31-overexpressing A431 lysate. Like the early endosome-residing Rab5, Rab31 was found mainly in the lighter fractions of the gradient. However, in the presence of GTPγS and after a pulse-chase with EGF, Rab31 was seen in higher molecular weight fractions, including fractions in which thyroglobulin (a 660-kDa dimeric protein) was found (Fig. 4F). There was, however, no discernible extension of Rab5 to the high molecular weight fractions, even when the blot was overexposed, suggesting that this effect of entering higher molecular weight fractions with GTPγS is specific to Rab31. Taken together, the results suggest that, after EGF stimulation, Rab31 becomes associated with a high molecular weight complex that likely contains EGFR during the time when ligand-bound EGFR is likely to be transferred between early and late endosomes.
Rab31-mediated EGFR Trafficking Occurs Downstream of Rab5
Rab5 has been suggested to play a role in both the early and later stages of EGFR trafficking, including internalization and entry into the early endosome as well as the transition between the early to late endosome (24, 34). Our results so far are consistent with Rab31 playing a role in the later stages of EGFR trafficking, probably during transit into late endosomes, by associating with a high molecular weight complex that that likely contains EGFR. Thus, we sought to determine whether there was any interplay between Rab5 and Rab31 in EGFR endocytic transport. We transfected cells with mCherry-Rab5 or Rab31 and quantified the percentage of EGF-FITC puncta positive for Rab5 and Rab31 at various time points post-pulse. We found that Rab5 remains associated with EGF puncta up to 60 min post-pulse, with a slight decrease between 10 and 30 min (Fig. 5A). In contrast, we found that the association between Rab31 and EGF only increased significantly at 30 min. Together, the results suggest that, although Rab5 appears to associate with both EGFR-carrying early and late endosomes and may play a role in both early and later stages of EGFR trafficking, Rab31 is more likely to be involved only at the early to late endosome transit stage.
FIGURE 5.
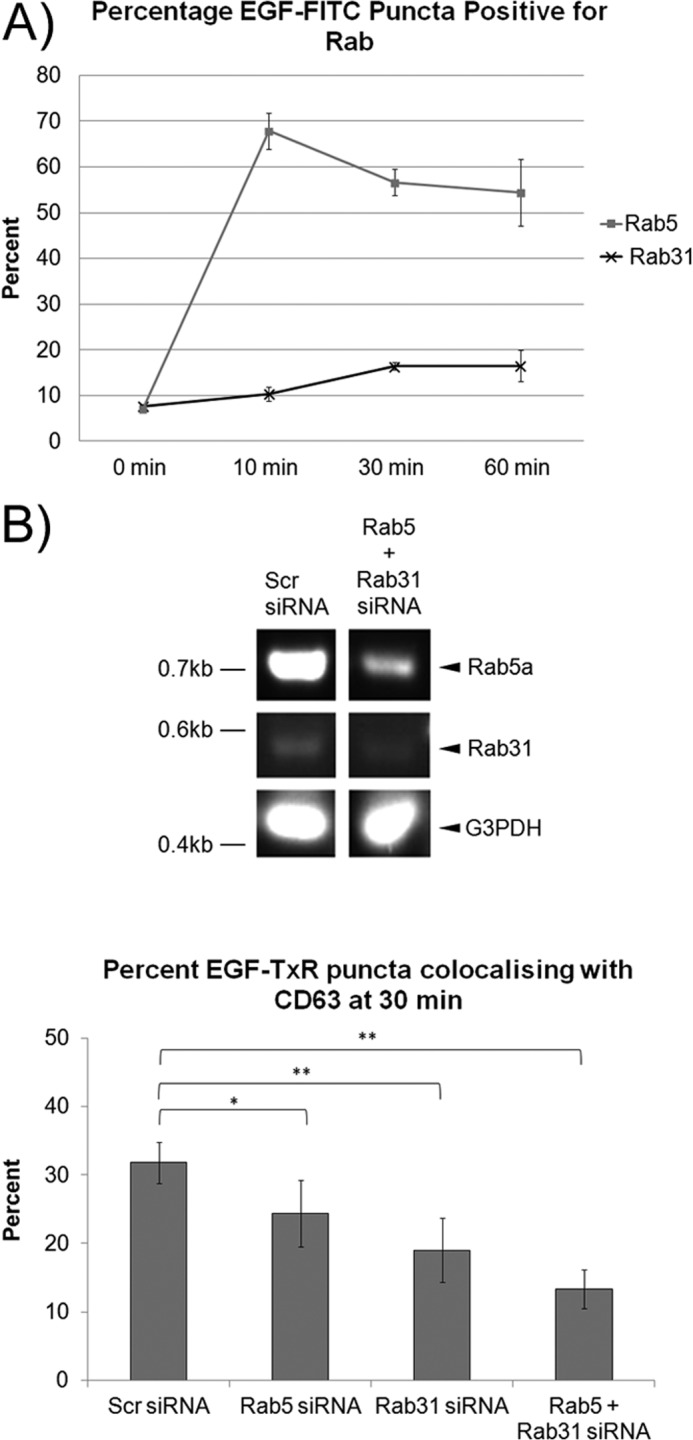
Rab5 appears to act upstream of Rab31 in EGFR trafficking. A, A431 cells were transfected with either mCherry-Rab5 or mCherry-Rab31. Cells were pulsed with 0.5 μg/ml EGF-FITC and fixed at the various time points indicated for immunofluorescence analysis. The percentage of EGF-FITC positive puncta that were positive for either Rab5 or Rab31 was quantified from cells fixed after a 0-, 10-, 30-, and 60-min chase and presented graphically as a percentage of total EGF-FITC puncta counted. 29 cells in three independent experiments were analyzed, and data are shown as mean ± S.E. B, A431 cells were transfected with scrambled (Scr), Rab5, or Rab31 siRNA as indicated, and subsequent analyses were performed after 48 h. Top panel, the extent of knockdown was assessed by RT-PCR. Bottom panel, cells were pulsed with 0.5 μg/ml EGF-TxR and fixed at 30 min. Cells were immunostained for CD63, and the percentages of EGF-TxR puncta that were also positive for CD63 were quantified and presented graphically as a percentage of total EGF-TxR puncta counted. 27 cells in three independent experiments were analyzed, and data are shown as mean ± S.E.*, p < 0.05; **, p < 0.01; Student's t test.
The loss of Rab31 caused a more significant inhibition of colocalization between ligand-bound EGFR and CD63 than a loss of Rab5, suggesting that Rab5 only plays a partial role in this trafficking step (Fig. 5B). Double Rab5 and Rab31 silencing further reduced the percentage of colocalization. This suggests that, although Rab5 may play a role in both the early and later stages of EGFR trafficking, Rab31 is more essential in the later trafficking of ligand-bound EGFR between the early and late endosome. This is despite the fairly low percentage of EGF puncta that are positive for Rab31, which may simply reflect the transient nature of the association of Rab31 with EGFR-containing membrane structures.
EEA1 Is Part of the Rab31-EGFR Trafficking Complex and Plays a Role in Rab31-EGFR Association
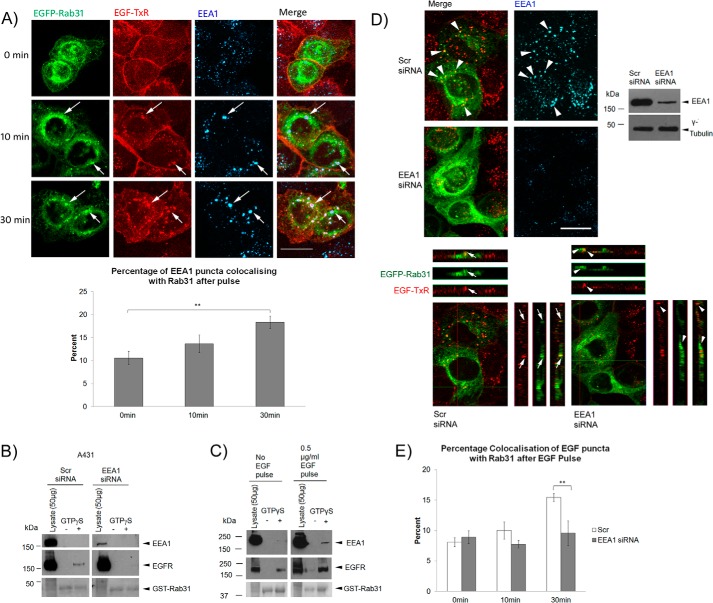
Having shown that Rab31 participates in the transition of EGFR from the early to late endosome in an EGFR trafficking complex, we sought to determine which other components could be important in mediating the role of Rab31. Our glycerol gradient sedimentation results suggest that the trafficking complex is of high molecular weight and likely includes more than just the EGFR. EEA1 is a known effector of Rab31 and interacts with the GTP-bound form of Rab31. In the glycerol gradient sedimentation of the A431 lysate, we observed that, along with Rab31, the presence of EEA1 also appeared to be extended to the higher molecular weight fractions upon EGF pulse (Fig. 4F). Using an immunofluorescence analysis of A431 cells stably expressing EGFP-Rab31, we observed that, after a pulse-chase with EGF-TxR, a portion of the EEA1 puncta colocalized with Rab31 together with EGF-TxR (Fig. 6A). This percentage increased gradually and was significant after a 30-min chase with EGF (Fig. 6A, bottom panel). This mirrors the colocalization data between Rab31 and EGFR and suggests that EEA1 may be associated together with Rab31 and EGFR, likely in high molecular weight trafficking complexes.
FIGURE 6.
EEA1 mediates the Rab31-EGFR interaction in the trafficking complex. A, top panel, A431 EGFP-Rab31 OE cells (green) were pulsed with 0.5 μg/ml EGF-TxR (red), fixed at various time points, and colabeled using EEA1 antibodies (blue). Arrows indicate some structures positive for EGFP-Rab31, EGF-TxR, and EEA1. Scale bar = 20 μm. Bottom panel, the percentage of EEA1 puncta that are positive for Rab31 was quantified from cells fixed after a 0-, 10-, and 30-min chase and presented graphically as a percentage of total EGF-TxR puncta counted. 33 cells in three independent experiments were analyzed, and data are shown as mean ± S.E. **, p < 0.01; Student's t test. B, A431 cells were transfected with scrambled (Scr) or EEA1 siRNA. 1 mg of lysates with and without GTPγS was incubated with 20 μg of GST-Rab31 and glutathione beads. The ability of GST-Rab31 to pull down EGFR was analyzed by Western blot analysis. Ponceau S staining of the GST proteins used is shown. C, cells were pulsed with 0.5 μg/ml EGF before harvesting after a 30-min chase. 1 mg of lysates with and without GTPγS was incubated with 20 μg of GST-Rab31 and glutathione beads. The ability of GST-Rab31 to pull down EGFR and EEA1 was analyzed by Western blot analysis. Ponceau S staining of the GST proteins used is shown. D, A431 EGFP-Rab31 OE cells (green) were transfected with scrambled or EEA1 siRNA. Cells were pulsed 48 h later with 0.5 μg/ml EGF-TxR (red), fixed after 30 min, and colabeled for EEA1 (blue). Top panels, Knockdown of EEA1 was assessed by immunofluorescence (left) and Western blot analysis (right). Arrowheads also indicate points of colocalization between EGFP-Rab31, EGF-TxR, and EEA1 puncta. Scale bar = 20 μm. Bottom panels, orthogonal projection of the three-dimensional stacked confocal images shown in the top left panel. In cells transfected with scrambled siRNA, there is colocalization between EGFP-Rab31 (green) and EGF-TxR (red) (arrows). In cells with EEA1 knockdown, EGFP-Rab31 and EGF-TxR appeared delocalized (arrowheads). E, the percentage of EGF-TxR-positive puncta that are also positive for EGFP-Rab31 was quantified from scrambled and EEA1 siRNA-transfected cells fixed after a 0-, 10-, and 30-min chase and graphically represented as a percentage of total EGF-TxR puncta counted. 29 cells in three independent experiments were analyzed, and data are shown as mean ± S.E. **, p < 0.01; Student's t test.
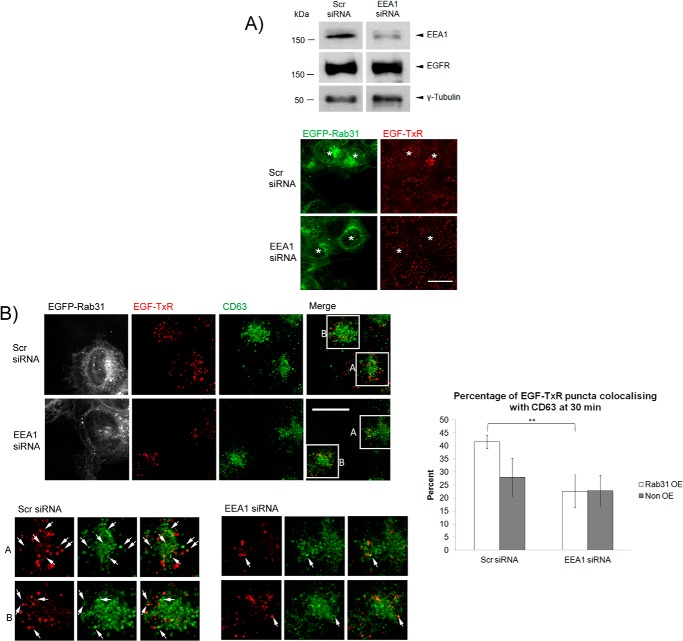
We further probed the role of EEA1 in Rab31-EGFR association. With a loss of EEA1, GST-Rab31 was unable to pull down the EGFR, even in the presence of GTPγS (Fig. 6B). This suggests that EEA1 could facilitate the interaction between Rab31 and the EGFR. (We should note that, although EEA1 interaction itself was not detected here, we were able to detect GST-Rab31 pulldown of EEA1 when cells were first pulsed with EGF before harvesting of the cell lysate for the assay (Fig. 6C)). Furthermore, after a 30-min pulse-chase with EGF-TxR, there was a delocalization between EGFP-Rab31 and EGF-TxR when EEA1 was silenced, compared with cells in the control population (Fig. 6, D and E). This supports our postulation that EEA1 is important for the interaction between Rab31 and the EGFR in the trafficking complexes.
We next looked at how the loss of EEA1 might affect the Rab31-mediated enhancement of ligand-bound EGFR endocytic trafficking. When EEA1 was silenced, there appeared to be little difference in the size of EGF puncta between cells overexpressing Rab31 (asterisks) and non-overexpressing cells (Fig. 7A), unlike cells in the control population. We also quantified the percentage of EGF-TxR puncta that were positive for CD63 (Fig. 7B) and found that there was a significantly higher percentage of colocalization in cells with Rab31 overexpression compared with cells without overexpression in the control population but not in the EEA1-silenced population. This suggests that the Rab31-mediated effect on EGFR trafficking to the CD63 late endosome compartment is abrogated with a loss of EEA1. Overall, our results suggest that Rab31 associates with the ligand-bound EGFR and enhances its trafficking and that this association requires the presence of EEA1.
FIGURE 7.
EEA1 is important for the Rab31-mediated enhancement of ligand-bound EGFR endocytic trafficking. A, A431 EGFP-Rab31 OE cells were transfected with scrambled (Scr) or EEA1 siRNA and analyzed after 48 h. Top panel, the levels of EEA1 and EGFR were analyzed by Western blot analysis. Bottom panel, cells were pulsed with 0.5 μg/ml EGF-TxR (red) and fixed at 30 min for comparison of the puncta sizes between cells overexpressing EGFP-Rab31 (green, asterisks) and non-overexpressing cells in the same populations. Scale bar = 20 μm. B, left panels, scrambled and EEA1 siRNA-treated cells were immunostained for CD63 (pseudocolored green) along with EGF-TxR (red) and EGFP-Rab31 (pseudocolored white). Left lower panels, individual and merged fluorescence signals of the boxed areas, magnified ×2. Box A represents cells with Rab31 overexpression, whereas box B represents cells with no overexpression. Arrows indicate some structures positive for both EGF-TxR and CD63. Scale bar = 20 μm. Right panel, the percentage of EGF-TxR puncta that are positive for CD63 were quantified and graphically represented as a percentage of total EGF-TxR puncta counted. 36 cells in three independent experiments were analyzed, and data are shown as mean ± S.E. **, p < 0.01; Student's t test.
The Rab31 GEF GAPex5, but Not RIN3, Plays a Role in the Rab31-EGFR Trafficking Complex
GAPex5 is a GEF for Rab31 and, thus, acts to activate Rab31 by enhancing the exchange of GDP for GTP. It has also been shown to be involved in the early steps of ligand-bound EGFR internalization by interacting with Cbl, which itself binds to the EGFR upon stimulation with the ligand (31). As such, GAPex5 stands as a good candidate for a possible mediator of the formation of the Rab31-EGFR trafficking complex.
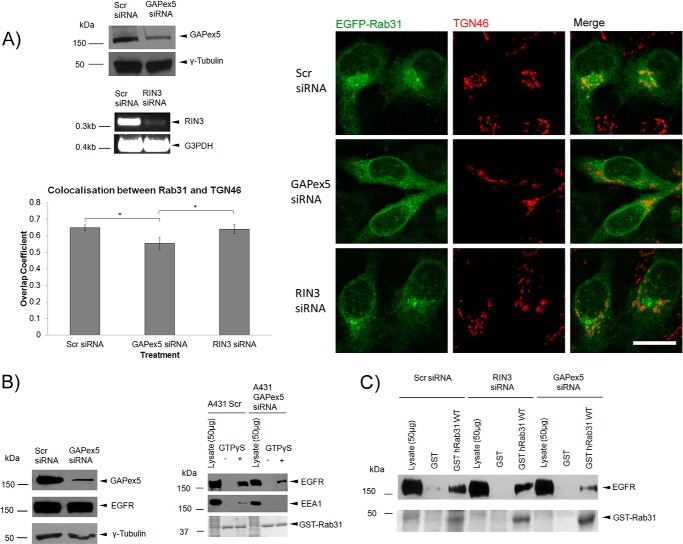
In our initial studies with silencing of GAPex5 in Rab31-overexpressing A431 cells, we observed that loss of GAPex5 resulted in a distinct dispersal of Rab31 from the TGN, as seen by a delocalization with the trans-Golgi marker TGN46 (Fig. 8A). This effect was specific to GAPex5 because silencing of another GEF of Rab31, RIN3 (37), did not bring about a similar effect in A431 cells. The dispersal of Rab31 from the TGN did not result in its corresponding accumulation in other vesicular compartments, which indicated that the membrane association of Rab31 was lost.
FIGURE 8.
The guanine nucleotide exchange factor GAPex5 is important for Rab31-EGFR interaction. A, A431 Rab31 OE cells were transfected with scrambled (Scr), GAPex5, or RIN3 siRNA and assayed after 48 h. Cell lysates were analyzed by Western blot analysis for GAPex5 knockdown and RT-PCR for RIN3 knockdown. Cells were fixed and immunostained for localization of EGFP-Rab31 (green) and TGN46 (red), a marker for the trans-Golgi network. Scale bar = 20 μm. Colocalization between EGFP-Rab31 and TGN46 was quantified using Zen 2010 software for calculation of the overlap coefficient. 27 cells in three independent experiments were analyzed, and data are shown as mean ± S.E. *, p < 0.05; Student's t test. B, A431 cells were transfected with GAPex5 siRNA and harvested 48 h later. Left panel, the extent of GAPex5 knockdown and the levels of the EGFR were assessed by Western blot analysis. Right panel, a GST-Rab31 affinity pulldown assay was performed with the harvested lysates. Cells were pulsed with 0.5 μg/ml EGF before harvesting after a 30-min chase. 1 mg of lysates with and without GTPγS was incubated with 20 μg of GST-Rab31 and glutathione beads. The ability of GST-Rab31 to pull down EGFR and EEA1 was analyzed by Western blot analysis. Ponceau S staining of the GST proteins used is shown. C, 1 mg of A431 cell lysate harvested 48 h after transfection with relevant siRNA was incubated with 20 μg of GST or GST-Rab31 and glutathione beads in the presence of GTPγS, and the ability of the fusion proteins to pull down the EGFR was analyzed by Western blot analysis. GST proteins were visualized with Ponceau S stain.
Thus, we looked at how silencing of GAPex5 would affect the Rab31-EGFR interaction. Loss of GAPex5 resulted in a reduced pulldown of both EEA1 and EGFR by GST-Rab31, even in the presence of GTPγS (Fig. 8B). Interestingly, as with the dispersal of Rab31 from the TGN, this effect was specific to GAPex5 because RIN3 silencing did not produce the same result (Fig. 8C). This suggests that the reduced Rab31-EGFR interaction seen with GAPex5 knockdown was not due to a reduction in Rab31 activation resulting from a general loss of GEF activity but was, rather, a GAPex5-specific effect.
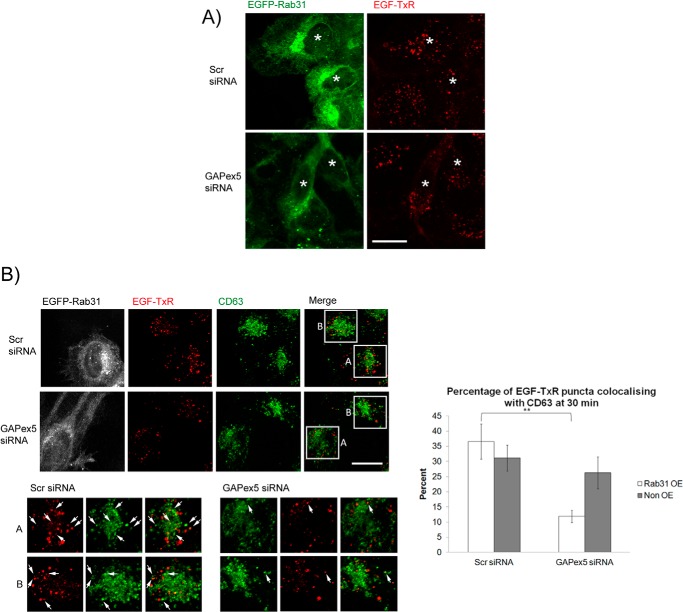
As with EEA1 silencing, the loss of GAPex5 abrogated Rab31-mediated enhancement of ligand-bound EGFR trafficking (Fig. 9A). In the control population, there was a significantly higher percentage of colocalization between EGF-TxR and CD63 in cells with Rab31 overexpression compared with cells without overexpression. In contrast, in the GAPex5-silenced population, there was no significant difference between Rab31-overexpressing and non-overexpressing cells (Fig. 9B). Together, the results suggest that the Rab31 GEF GAPex5, but not RIN3, may have a specific role in Rab31-EGFR trafficking in bringing together the interaction between Rab31, the EGFR, and EEA1 in the trafficking complex.
FIGURE 9.
The guanine nucleotide exchange factor GAPex5 is important for the Rab31-mediated enhancement of ligand-bound EGFR endocytic trafficking. A, A431 EGFP-Rab31 OE cells were transfected with scrambled (Scr) or GAPex5 siRNA. Cells were pulsed with 0.5 μg/ml EGF-TxR and fixed at 30 min for analysis of the size of EGF-TxR puncta (red) in cells that do (green, asterisks) or do not overexpress EGFP-Rab31. Scale bar = 20 μm. B, left panels, scrambled and GAPex5 siRNA-treated cells were immunostained for CD63 (pseudocolored green) along with EGF-TxR (red) and EGFP-Rab31 (pseudocolored white). The left bottom panels show individual and merged fluorescence signals of the boxed areas, magnified ×2. Box A encloses the central area of cells with Rab31 overexpression, whereas box B encloses central areas of cells with no Rab31 overexpression. Arrows indicate some structures positive for both EGF-TxR and CD63. Scale bar = 20 μm. Right panel, the percentage of EGF-TxR puncta that are also positive for CD63 were quantified and are graphically presented as a percentage of total EGF-TxR puncta counted. 41 cells in three independent experiments were analyzed, and data are shown as mean ± S.E. **, p < 0.01; Student's t test.
The Role of Rab31 in EGFR Trafficking Is Focused on the Degradative, Not the Recycling, Pathway
Up to this point, we focused on the role of Rab31 in the degradative trafficking pathway of ligand-bound EGFR. So far, our data suggest that Rab31 is involved in a later step in EGFR trafficking, after Rab5, and involves the movement of the ligand-bound EGFR from early to late endosomes. Notably, though, upon stimulation by EGF, there is also a fraction of the ligand-bound EGFR that is recycled back to the cell surface (20, 38). We investigated how Rab31 might play a role in this process or whether its effect is limited specifically to the degradative pathway.
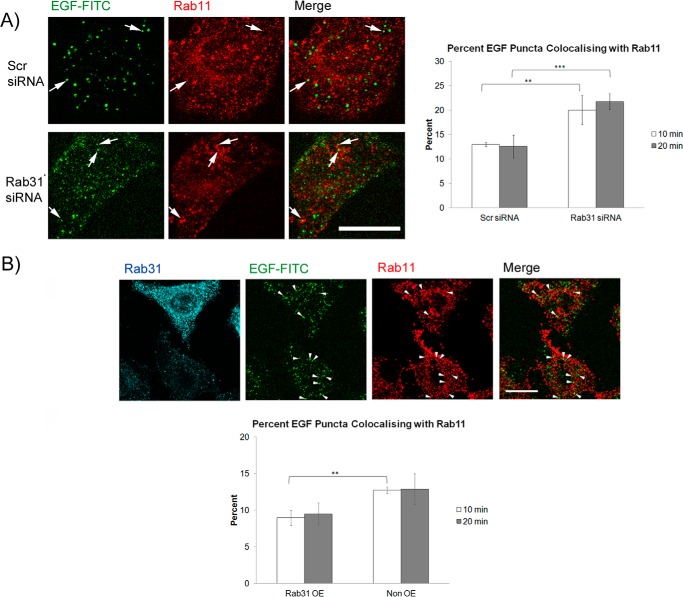
After a pulse with EGF-FITC, we determined the rate of recycling of the EGFR in cells as reflected by the percentage of EGF/EGFR puncta colocalizing with Rab11, a marker for recycling endosomes. Because Rab11 was present at higher levels in HeLa cells compared with A431 (hardly detectable by immunofluorescence), we used HeLa cells for this set of experiments. Silencing of Rab31 significantly increased the percentage of EGF-FITC puncta colocalizing with Rab11 at 10 and 20 min compared with controls (Fig. 10A), whereas overexpression of Rab31 decreased the percentage (Fig. 10B). Our results suggest that, by enhancing the movement of the ligand-bound EGFR from early to late endosomes, Rab31 may channel more ligand-bound EGFR to the degradative pathway, thus indirectly reducing the percentage of EGFR recycled to the cell surface.
FIGURE 10.
Rab31 indirectly impacts the recycling itinerary of the ligand-bound EGFR. A, HeLa cells were transfected with either scrambled (Scr) or Rab31 siRNA and subsequent assays were performed after 48 h. Left panel, cells were pulsed with 0.25 μg/ml EGF-FITC (green), fixed at 20 min, and analyzed by colabeling for Rab11 (red). Arrows indicate some structures positive for both EGF-FITC and Rab11. Scale bar = 20 μm. Right panel, the percentage of EGF-FITC puncta that are also positive for Rab11 was quantified from cells fixed after a 10- and 20-min chase and presented graphically as a percentage of total EGF-FITC puncta counted. 27 cells in three independent experiments were analyzed, and data are shown as mean ± S.E. **, p < 0.01; ***, p < 0.001; Student's t test. B, top panel, HeLa cells were transfected with Rab31 (blue), pulsed with 0.25 μg/ml EGF-FITC (green), and fixed at 20 min for analysis by coimmunostaining for Rab11 (red). Arrows indicate some structures positive for both EGF-FITC and Rab11. Scale bar = 20 μm. Bottom panel, the percentage of EGF-FITC puncta that are positive for Rab11 was quantified from cells fixed after a 10- and 20-min chase and presented graphically as a percentage of total EGF-FITC puncta counted. 27 cells in three independent experiments were analyzed, and data are shown as mean ± S.E. **, p < 0.01; Student's t test.
DISCUSSION
Rab31 Plays a Role in EGFR Trafficking via an EEA1-containing Trafficking Complex
We have shown previously that Rab31 could coimmunoprecipitate the EGFR, and that Rab31 silencing resulted in a delocalization between the ligand-bound EGFR and the late endosome marker CD63 (33). Here, we have shown further that manipulation of Rab31 levels did not impact the internalization of ligand-bound EGFR or its entry into early endosomes at 10 min after an EGF pulse. Instead, it largely affected the transition between early and late endosomes at 30 min. The effect of Rab31 silencing could be rescued with overexpression of siRNA-resistant Rab31. Together, the results suggest that Rab31 has a specific role in the endocytic trafficking of the ligand-bound EGFR at a time point that is likely to involve its movement between early and late endosomes.
Coimmunoprecipitation and affinity pulldown assays show that Rab31 and EGFR interact and that this interaction could be corroborated by colocalization of immunofluorescence signals in A431 cells. When cells were pulse-chased with EGF-TxR, some EGF puncta were seen to be positive for Rab31, the percentage of which increased gradually to significance at 30 min. EEA1 also colocalized with Rab31 puncta upon EGF stimulation. When EEA1 expression was silenced, the interaction between Rab31 and the EGFR was lost, and the effect of Rab31 overexpression on the ligand-bound EGFR trafficking was also attenuated. Thus, we postulate that Rab31 might be mediating its effect on enhanced trafficking of the EGFR through an EEA1-containing trafficking complex. To date, evidence for the role of EEA1 in endocytosis of EGFR has been varied, with some evidence pointing toward EEA1 being dispensable, at least in the early step of clathrin-mediated internalization of the EGFR from the cell surface (35, 39). Likewise, in our hands, silencing of EEA1 did not abrogate the initial internalization of the EGFR. Our results do suggest that EEA1 is directly involved in the interaction between Rab31 and the EGFR and is important for Rab31-regulated trafficking of the EGFR between early and late endosomes.
These results are intriguing for several reasons. First, it suggests that, although Rab31 is largely localized to the TGN, a fraction of it is found on what are likely to be endosomes, and that this endosomal pool of Rab31 might possibly play a physiological role in the cell. Some groups have suggested that Rab31 emerges from the TGN onto tubulovesicular structures and is responsible for anterograde transport between the TGN and endosomes (26). Here we provide evidence that Rab31 also plays a role in retrograde transport in the endocytic pathway. Second, Rab31 appears to be part of a larger trafficking complex that includes both the EGFR and EEA1. Rab25 and Rab21 are the only Rabs so far that have been shown to interact directly with cell surface receptors, namely integrin (40) and, more recently, the EGFR (17). Third, our results indicate that Rab31 is a key player in the endocytic trafficking pathway of the EGFR. We postulate that the formation of the Rab31-EGFR trafficking complex, together with EEA1, is subsequently essential for the eventual entry of the ligand-bound receptor into the late endosomes. This is perhaps similar to the role that has been postulated recently for Rab21, although, in the case of Rab21, it appears that it functions in a ligand-independent manner as well, enhancing the degradation of the unliganded EGFR (17). The exact mechanism by which the Rab31-EEA1-EGFR trafficking complex mediates the movement of the ligand-bound EGFR from early to late endosomes remains to be fully elucidated.
The Role of a GEF in the Rab31/EEA1-mediated EGFR Trafficking Complex
We have shown previously that loss of GAPex5 phenocopied Rab31 silencing (33). Here we have shown further that overexpression of Rab31 is unable to rescue a loss of GAPex5. One reason for this is likely to be the reduced activation of Rab31 because of a lack of its GEF. However, we have shown here that silencing of RIN3, another GEF for Rab31, does not have the same effect, suggesting that the role of GAPex5 in Rab31-mediated EGFR trafficking may extend beyond its GEF activity. Because GAPex5 has been shown to bind the EGFR via Cbl, a second plausible explanation is that its presence in the EGFR trafficking complex is essential for Rab31 function. It is likely that the presence of GAPex5 in the EGFR-containing complex is responsible for recruiting Rab31 onto the EGFR-carrying endosomes. When there, activated Rab31 can further engage its effector, EEA1. We have not, however, been able to discern an interaction between Rab31 and other components of EGFR signaling, such as Cbl (data not shown). Interestingly, we observed that silencing of GAPex5 resulted in dispersal of Rab31 from the TGN. This dispersal may impact the role of Rab31 in EGFR trafficking as well, possibly by disrupting the cycling of Rab31 between the TGN and the endocytic pathway (26).
Rab31 Is Likely to Work Alongside an Extensive Network of Rabs Involved in EGFR Trafficking
Currently, how Rab31 factors in the EGFR trafficking process in comparison to other Rabs involved has not yet been fully defined. Our results point to the possibility that Rab31 acts downstream of the initial internalization of the EGFR that is coordinated by Rab5. It is tantalizing to speculate that Rab31 might perhaps be recruited to the EGFR trafficking complex subsequent to the involvement of Rab5, which may be responsible for first engaging EEA1 onto EGFR-carrying endosomes. It is also pertinent to note here that Rab5, in some studies, has also been shown to be involved in the trafficking step between the early and late endosome (23, 24). It is not entirely clear to what extent Rab5 is essential for this step. However, we have shown that Rab31 silencing causes a more severe disruption in the trafficking to late endosomes compared with Rab5 silencing. At the very least, Rab31 can be said to function alongside Rab5 in the trafficking of the ligand-bound EGFR.
There are many examples in the literature of the interplay between various Rabs in the ordering of transport steps. For example, Rab5 and Rab7 have been shown to coordinate the maturation of endosomes via a process termed Rab conversion, when the preceding Rab engages various proteins that recruit the subsequent Rab (42). How and when the various Rabs coordinate EGFR-related traffic remains to be fully elucidated. Renzis et al. (43) described the divalent effector Rabenosyn 5, which binds both Rab5 and Rab4 simultaneously, enabling Rab5 and Rab4 membranes to interact, thus allowing the transition between early endosomes and fast recycling endosomes. EEA1, as a common effector for both Rab5 and Rab31, might also fulfil this role because it has two Rab binding domains. Rab5 has a greater affinity for the N-terminal domain, whereas Rab31 has equal affinity for both (30). Alternatively, Zhu et al. (44) identified Rabex5 as a Rab22a effector that is responsible for the recruitment of Rab5 to Rab22a containing early endosomes by acting as a GEF for Rab5. At present, no such dual function GEF/effector has been identified for Rab5 and Rab31.
Lastly, we raised the possibility that Rab31 may indirectly impinge on the recycling of ligand-bound EGFR as well. Because Rab31 appears to mediate the trafficking of the ligand-bound EGFR between early and late endosomes, Rab31 silencing would result in a decrease in channeling to the late endosome-lysosome pathway and, instead, shunt more ligand-bound EGFR to the recycling endosomes, and the converse could be expected for Rab31 overexpression. The extent to which Rab31 determines the rates of recycling versus degradation remains to be fully explored.
In conclusion, our results indicate that Rab31 is a hitherto underappreciated and important player in the endocytic trafficking of the EGFR that functions in an EGFR trafficking complex together with EEA1 and GAPex5. It could conceivably be anticipated that alterations in Rab31 expression or activity, which have been documented in various cancers (46–48), will affect EGFR trafficking and signaling in ways that may contribute to malignancy and metastasis (35).
This work was supported by the National University of Singapore Graduate School for Integrative Sciences and Engineering (NGS).
- GEF
- guanine nucleotide exchange factor
- EGFR
- EGF receptor
- TGN
- trans-Golgi network
- TxR
- Texas Red
- OE
- overexpression.
REFERENCES
- 1. Colicelli J. (2004) Human RAS superfamily proteins and related GTPases. Sci. STKE 2004, RE13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Markgraf D. F., Peplowska K., Ungermann C. (2007) Rab cascades and tethering factors in the endomembrane system. FEBS Lett. 581, 2125–2130 [DOI] [PubMed] [Google Scholar]
- 3. Seabra M. C., Coudrier E. (2004) Rab GTPases and myosin motors in organelle motility. Traffic 5, 393–399 [DOI] [PubMed] [Google Scholar]
- 4. Li L. (2001) Direct interaction of Rab4 with syntaxin 4. J. Biol. Chem. 276, 5265–5273 [DOI] [PubMed] [Google Scholar]
- 5. Simonsen A. (1999) The Rab5 effector EEA1 interacts directly with syntaxin-6. J. Biol. Chem. 274, 28857–28860 [DOI] [PubMed] [Google Scholar]
- 6. Segev N. (2011) Coordination of intracellular transport steps by GTPases. Semin. Cell. Dev. Biol. 22, 33–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grosshans B. L., Ortiz D., Novick P. (2006) Rabs and their effectors: achieving specificity in membrane traffic. Proc. Natl. Acad. Sci. U.S.A. 103, 11821–11827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nottingham R. M., Pfeffer S. R. (2009) Defining the boundaries: Rab GEFs and GAPs. Proc. Natl. Acad. Sci. U.S.A. 106, 14185–14186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schwartz S. L., Cao C., Pylypenko O., Rak A., Wandinger-Ness A. (2007) Rab GTPases at a glance. J. Cell Sci. 120, 3905–3910 [DOI] [PubMed] [Google Scholar]
- 10. Segev N. (2001) Ypt/Rab GTPases: regulators of protein trafficking. (2011) Sci. STKE 2001, re11. [DOI] [PubMed] [Google Scholar]
- 11. Hutagalung A. H., Novick P. J. (2011) Role of Rab GTPases in membrane traffic and cell physiology. Physiol. Rev. 91, 119–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen D., Guo J., Miki T., Tachibana M., Gahl W. A. (1996) Molecular cloning of two novel rab genes from human melanocytes. Gene 174, 129–134 [DOI] [PubMed] [Google Scholar]
- 13. Pereira-Leal J. B., Seabra M. C. (2001) Evolution of the Rab family of small GTP-binding proteins. J. Mol. Biol. 313, 889–901 [DOI] [PubMed] [Google Scholar]
- 14. Bucci C., Parton R. G., Mather I. H., Stunnenberg H., Simons K., Hoflack B., Zerial M. (1992) The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell 70, 715–728 [DOI] [PubMed] [Google Scholar]
- 15. Simpson J. C. (2004) A role for the small GTPase Rab21 in the early endocytic pathway. J. Cell Sci. 117, 6297–6311 [DOI] [PubMed] [Google Scholar]
- 16. Kauppi M., Simonsen A., Bremnes B. (2002) The small GTPase Rab22 interacts with EEA1 and controls endosomal membrane trafficking. J. Cell Sci. 115, 899–911 [DOI] [PubMed] [Google Scholar]
- 17. Yang X., Zhang Y., Li S., Liu C., Jin Z., Wang Y., Ren F., Chang Z. (2012) Rab21 attenuates EGF-mediated MAPK signaling through enhancing EGFR internalization and degradation. Biochem. Biophys. Res. Commun. 421, 651–657 [DOI] [PubMed] [Google Scholar]
- 18. Sorkin A., Goh L. K. (2009) Endocytosis and intracellular trafficking of ErbBs. Exp. Cell Res. 315, 683–696 [DOI] [PubMed] [Google Scholar]
- 19. Ceresa B. P. (2006) Regulation of EGFR endocytic trafficking by Rab proteins. Histol. Histopathol. 21, 987–993 [DOI] [PubMed] [Google Scholar]
- 20. Masui H., Castro L., Mendelsohn J. (1993) Consumption of EGF by A431 cells: evidence for receptor recycling. J. Cell Biol. 120, 85–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang F. (2004) Analysis of clathrin-mediated endocytosis of epidermal growth factor receptor by RNA interference. J. Biol. Chem. 279, 16657–16661 [DOI] [PubMed] [Google Scholar]
- 22. Mishra A., Eathiraj S., Corvera S., Lambright D. G. (2010) Structural basis for Rab GTPase recognition and endosome tethering by the C2H2 zinc finger of early endosomal autoantigen 1 (EEA1). Proc. Natl. Acad. Sci. U.S.A. 107, 10866–10871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen P. I., Kong C., Su X., Stahl P. D. (2009) Rab5 isoforms differentially regulate the trafficking and degradation of epidermal growth factor receptors. J. Biol. Chem. 284, 30328–30338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dinneen J. L., Ceresa B. P. (2004) Expression of dominant negative Rab5 in HeLa cells regulates endocytic trafficking distal from the plasma membrane. Exp. Cell Res. 294, 509–522 [DOI] [PubMed] [Google Scholar]
- 25. Vanlandingham P. A., Ceresa B. P. (2009) Rab7 regulates late endocytic trafficking downstream of multivesicular body biogenesis and cargo sequestration. J. Biol. Chem. 284, 12110–12124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rodriguez-Gabin A. G., Cammer M., Almazan G., Charron M., Larocca J. N. (2001) Role of rRAB22b, an oligodendrocyte protein, in regulation of transport of vesicles from trans Golgi to endocytic compartments. J. Neurosci. Res. 66, 1149–1160 [DOI] [PubMed] [Google Scholar]
- 27. Rodriguez-Gabin A. G., Yin X., Si Q., Larocca J. N. (2009) Transport of mannose-6-phosphate receptors from the trans-Golgi network to endosomes requires Rab31. Exp. Cell Res. 315, 2215–2230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ng E. L., Wang Y., Tang B. L. (2007) Rab22B's role in trans-Golgi network membrane dynamics. Biochem. Biophys. Res. Commun. 361, 751–757 [DOI] [PubMed] [Google Scholar]
- 29. Gu F., Crump C. M., Thomas G. (2001) Trans-Golgi network sorting. Cell Mol. Life Sci. 58, 1067–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lodhi I. J., Chiang S. H., Chang L., Vollenweider D., Watson R. T., Inoue M., Pessin J. E., Saltiel A. R. (2007) Gapex-5, a Rab31 guanine nucleotide exchange factor that regulates Glut4 trafficking in adipocytes. Cell Metab. 5, 59–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Su X., Kong C., Stahl P. D. (2007) GAPex-5 mediates ubiquitination, trafficking, and degradation of epidermal growth factor receptor. J. Biol. Chem. 282, 21278–21284 [DOI] [PubMed] [Google Scholar]
- 32. Mills I. G., Urbé S., Clague M. J. (2001) Relationships between EEA1 binding partners and their role in endosome fusion. J. Cell Sci. 114, 1959–1965 [DOI] [PubMed] [Google Scholar]
- 33. Ng E. L., Ng J. J., Liang F., Tang B. L. (2009) Rab22B is expressed in the CNS astroglia lineage and plays a role in epidermal growth factor receptor trafficking in A431 cells. J. Cell Physiol. 221, 716–728 [DOI] [PubMed] [Google Scholar]
- 34. Barbieri M. A., Roberts R. L., Gumusboga A., Highfield H., Alvarez-Dominguez C., Wells A., Stahl P. D. (2000) Epidermal growth factor and membrane trafficking. EGF receptor activation of endocytosis requires Rab5a. J. Cell Biol. 151, 539–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chia W. J., Tang B. L. (2009) Emerging roles for Rab family GTPases in human cancer. Biochim. Biophys. Acta 1795, 110–116 [DOI] [PubMed] [Google Scholar]
- 36. Liu N. S., Loo L. S., Loh E., Seet L. F., Hong W. (2009) Participation of Tom1L1 in EGF-stimulated endocytosis of EGF receptor. EMBO J. 28, 3485–3499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kajiho H., Sakurai K., Minoda T., Yoshikawa M., Nakagawa S., Fukushima S. (2011) Characterization of RIN3 as a guanine-nucleotide exchange factor for the Rab5-subfamily GTPase Rab31. J. Biol. Chem. 286, 24364–24373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tong J., Sydorskyy Y., St-Germain J. R., Taylor P., Tsao M. S., Moran M. F. (2013) Odin (ANKS1A) modulates EGF receptor recycling and stability. PloS ONE 8, e64817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen X., Wang Z. (2001) Regulation of intracellular trafficking of the EGF receptor by Rab5 in the absence of phosphatidylinositol 3-kinase activity. EMBO Rep. 2, 68–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mai A., Veltel S., Pellinen T., Padzik A., Coffey E., Marjomäki V., Ivaska J. (2011) Competitive binding of Rab21 and p120RasGAP to integrins regulates receptor traffic and migration. J. Cell Biol. 194, 291–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Deleted in proof.
- 42. Rink J., Ghigo E., Kalaidzidis Y., Zerial M. (2005) Rab conversion as a mechanism of progression from early to late endosomes. Cell 122, 735–749 [DOI] [PubMed] [Google Scholar]
- 43. de Renzis S., Sönnichsen B., Zerial M. (2002) Divalent Rab effectors regulate the sub-compartmental organization and sorting of early endosomes. Nat. Cell Biol. 4, 124–133 [DOI] [PubMed] [Google Scholar]
- 44. Zhu H., Liang Z., Li G. (2009) Rabex-5 is a Rab22 effector and mediates a Rab22-Rab5 signaling cascade in endocytosis. Mol. Biol. Cell 20, 4720–4729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Deleted in proof.
- 46. Kotzsch M., Sieuwerts A. M., Grosser M., Meye A., Fuessel S., Meijer-van Gelder M. E., Smid M., Schmitt M., Baretton G., Luther T., Magdolen V., Foekens J. A. (2008) Urokinase receptor splice variant uPAR-del4/5-associated gene expression in breast cancer: identification of rab31 as an independent prognostic factor. Breast Cancer Res. Treat. 111, 229–240 [DOI] [PubMed] [Google Scholar]
- 47. Grismayer B., Sölch S., Seubert B., Kirchner T., Schäfer S., Baretton G. (2012) Rab31 expression levels modulate tumor-relevant characteristics of breast cancer cells. Mol. Cancer 11, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kunkle B. W., Yoo C., Roy D. (2013) Reverse engineering of modified genes by Bayesian network analysis defines molecular determinants critical to the development of glioblastoma. PloS ONE 8, e64140. [DOI] [PMC free article] [PubMed] [Google Scholar]