Background: Metabolic stresses, including hyperinsulinemia, promote insulin resistance.
Results: Monoclonal antibodies raised against Ser(P)/Thr(P) residues in IRS1 were used to quantify phosphorylation in response to insulin or agents that model metabolic stress.
Conclusion: Similar IRS1 Ser(P)/Thr(P) residues are increased by insulin or metabolic stress, and some correlate significantly with reduced IRS1 tyrosine phosphorylation.
Significance: Metabolic stress co-opts insulin-dependent IRS1 phosphorylation to aggravate insulin resistance.
Keywords: Cell Signaling, Insulin Resistance, Phosphotyrosine Signaling, Protein Phosphorylation, Signal Transduction, CHO Cells, IRS1, Insulin Signaling, Metabolic Stress, Monoclonal Antibodies
Abstract
IRS1 and IRS2 are key substrates of the insulin receptor tyrosine kinase. Mass spectrometry reveals more than 50 phosphorylated IRS1 serine and threonine residues (Ser(P)/Thr(P) residues) in IRS1 from insulin-stimulated cells or human tissues. We investigated a subset of IRS1 Ser(P)/Thr(P) residues using a newly developed panel of 25 phospho-specific monoclonal antibodies (αpS/TmAbIrs1). CHO cells overexpressing the human insulin receptor and rat IRS1 were stimulated with insulin in the absence or presence of inhibitors of the PI3K → Akt → mechanistic target of rapamycin (mTOR) → S6 kinase or MEK pathways. Nearly all IRS1 Ser(P)/Thr(P) residues were stimulated by insulin and significantly suppressed by PI3K inhibition; fewer were suppressed by Akt or mTOR inhibition, and none were suppressed by MEK inhibition. Insulin-stimulated Irs1 tyrosine phosphorylation (Tyr(P)Irs1) was enhanced by inhibition of the PI3K → Akt → mTOR pathway and correlated with decreased Ser(P)-302Irs1, Ser(P)-307Irs1, Ser(P)-318Irs1, Ser(P)-325Irs1, and Ser(P)-346Irs1. Metabolic stress modeled by anisomycin, thapsigargin, or tunicamycin increased many of the same Ser(P)/Thr(P) residues as insulin, some of which (Ser(P)-302Irs1, Ser(P)-307Irs1, and four others) correlated significantly with impaired insulin-stimulated Tyr(P)Irs1. Thus, IRS1 Ser(P)/Thr(P) is an integrated response to insulin stimulation and metabolic stress, which associates with reduced Tyr(P)Irs1 in CHOIR/IRS1 cells.
Introduction
Insulin resistance promotes hyperglycemia, hyperinsulinemia, dyslipidemia, and hypertension that can progress to nonalcoholic fatty liver disease, type 2 diabetes, and cardiovascular disease (1, 2). Although many genetic and physiological factors contribute to insulin resistance, animal and human studies suggest that dysregulated signaling through the insulin receptor substrates IRS12 and IRS2 is mechanistically important (3–6). The activated insulin receptor kinase phosphorylates tyrosine residues in IRS1 and IRS2, stimulating the recruitment of SH2 domain proteins, including the class I PI3K and GRB2·SOS (son of sevenless guanine nucleotide exchange factor) (6). Numerous mechanisms regulate the strength or duration of this signaling, including variable expression or stability of the insulin receptor isoforms A and B, or of their proximal downstream components, including IRS1, IRS2, IRS3, IRS4, PI3K, and Akt (7). Diverse regulatory proteins and pathways, including GRB10 (8), phosphatidylcholine transfer protein (9), MG53/TRIM72 (muscle-specific mitsugumin 53/tripartite motif 72) (10), the cullin RING ligase CRL7 (cullin RING E3 ubiquitin ligase 7) (11), and the Lin28/Let-7 axis (12), can each affect these proximal components to promote insulin resistance. Protein and lipid phosphatases, including PP2A, PTPN1 (tyrosine-protein phosphatase nonreceptor type 1, also known as PTP1B), and PTEN (phosphatase and tensin homolog), can also negatively regulate signaling through the insulin receptor → IRS → PI3K pathway (13–15).
Although tyrosine phosphorylation of IRS1 generates the insulin signal, IRS1 is heavily phosphorylated upon serine and threonine residues (Ser(P)/Thr(P) residues) before, during, and after insulin stimulation (6, 16, 17). Numerous studies over the past 15 years have focused upon specific Ser(P)/Thr(P) residues as a general mechanism to modulate insulin signaling (6). These investigations provide evidence for a conserved and highly regulated signaling process that can modulate physiological insulin sensitivity or contribute to pathological insulin resistance (5). Nonetheless, a complete understanding of this regulatory mechanism remains incomplete and challenging because of the large number of Ser(P)/Thr(P) residues found within IRS1 in human/animal tissues.
An extensive literature has characterized the relationship in cultured cells between IRS1 Ser/Thr phosphorylation and signaling by downstream insulin-stimulated Ser/Thr kinase pathways, including Akt → GSK3β, Akt → SIK2, Akt → mTOR → S6K1, and MEK → ERK1/2 pathways, each of which can affect IRS1 Ser/Thr phosphorylation (6). Moreover, in disease states multisite IRS1 Ser/Thr phosphorylation may be mediated by heterologous kinases, including energy-sensing AMP-activated protein kinase; sympathetic-activated G protein-coupled receptor kinase 2; lipid/inflammatory stimulated JNK, IKKβ (inhibitor of nuclear factor κB kinase β), or mPLK (pelle-like kinase/interleukin-1 receptor-associated kinase); or novel and conventional PKCs (6). Chronic hyperinsulinemia during insulin resistance might compound this dysregulation by adding the effects of insulin upon IRS1 Ser(P)/Thr(P) residues (18).
More than 50 IRS1 Ser(P)/Thr(P) residues have been identified in various MS/MS studies (19–25), and most have been studied individually or in small groups, including Ser-24Irs1 (26–28), Ser-267Irs1 (29), Ser-302Irs1 (30), Ser-307Irs1 (31–36), Ser-318Irs1 (37, 38), Ser-332Irs1 (39), Ser-357Irs1 (40), Ser-408Irs1 (41), Ser-522Irs1 (19), Ser-612Irs1 (34, 42–44), Ser-632Irs1 (34, 42, 45), Ser-662Irs1 (42, 44), Ser-731Irs1 (42), Ser-789Irs1 (46, 47), and Ser-1097Irs1/Ser-1101IRS1 (48, 49). Because a limited number of phospho-specific antibodies were generated in early studies, the phosphorylation of a few Ser/Thr residues (Ser-302Irs1, Ser-307Irs1, Ser-318Irs1, Ser-612Irs1, and Ser-632Irs1) has been interrogated in many different contexts, whereas many others have never been examined (6). Early work showed that anisomycin-stimulated JNK is associated with inhibition of insulin-stimulated Tyr(P)Irs1 (31). Mutational analysis suggests that rodent Ser(P)-307Irs1 (Ser(P)-312IRS1 in humans) is a principal target of JNK, which appears to disrupt the interaction between the activated insulin receptor and the PTB domain of IRS1 (31, 50). Consequently, Ser(P)-307Irs1 is widely investigated and has frequently been implicated in insulin resistance under conditions of physiologic and metabolic stress, including obesity, hyperinsulinemia/insulin resistance, neuronal dysregulation, and ER stress, in mouse models and humans (6, 45, 51–56). However, mice carrying a mutant form of Irs1 in which Ser-307Irs1 is replaced by Ala-307Irs1 display insulin resistance rather than hypersensitivity, suggesting that Ser-307Irs1 or its phosphorylation is important to normal insulin signaling (57). Regardless, mice bearing a muscle specific Irs1 transgene containing three mutations, S307AIrs1 along with S302AIrs1 and S612AIrs1, are protected from fat-induced insulin resistance and display increased muscle insulin-sensitivity in hyperinsulinemic-euglycemic clamp studies (58). Cell-based experiments with compound at S302AIrs1, S307AIrs1, and S318AIrs1 mutations reveal a complex role of Ser(P)/Thr(P) in the regulation of Tyr(P)Irs1 and downstream glucose uptake (17).
To investigate more comprehensively the multisite Ser/Thr phosphorylation of Irs1, we generated sequence- and phospho-specific monoclonal antibodies (αpS/TmAbIrs1) against 26 Ser(P)/Thr(P) residues identified in Irs1 by MS/MS (19). We used the αpS/TmAbIrs1 to interrogate Irs1 phosphorylation in CHO cells overexpressing the human insulin receptor and rat Irs1 (CHOIR/Irs1) (19, 57). We assayed the contribution of the MEK → ERK and PI3K cascades to Ser(P)/Thr(P) and Tyr(P)Irs1 during insulin stimulation using small molecule inhibitors of MEK (59), PI3K (60), Akt (61), mTORC1 (62), mTORC1/2 (63), and S6K (64). We further investigated Ser/Thr phosphorylation of Irs1 by the application of metabolic stress-mimicking agents to activate insulin-stimulated and heterologous kinase pathways. The results of these studies highlight the major insulin-signaling pathways that mediate Irs1 Ser/Thr phosphorylation, identify several Ser(P)/Thr(P) residues that correlate negatively with insulin-stimulated Tyr(P)Irs1, and provide evidence that metabolic stress pathways perturb the global pattern of Irs1 Ser/Thr phosphorylation in a fashion akin to insulin itself.
EXPERIMENTAL PROCEDURES
Mass Spectrometric Analysis
MS/MS analysis was used previously to identify Ser(P)/Thr(P) residues in rat Irs1 (19). Originally this analysis was conducted on Irs1 isolated from unstimulated and insulin-stimulated CHOIR/Irs1 cells but was subsequently extended to Irs1 isolated from mouse liver (data not shown). A set of 50 MS/MS-identified sites was used for the generation of phosphopeptide antigens.
Generation of IRS1 Ser(P)/Thr(P) Specific Antibodies
Phosphopeptides (15-mers) purchased from various sources (Sigma-Genosys, Harvard University core facility, or Synpep) that centered around the Ser(P)/Thr(P) residue of interest were coupled to maleimide-activated keyhole limpet hemocyanin (Thermo Scientific) according to the manufacturer's protocol. The conjugates were injected into BALB/c mice, which were tested for antibody production by screening of serum on Western blots of CHO cell extracts. Spleen cells from positive mice were used to generate hybridomas. At least 200 clones were screened against membrane-immobilized phosphopeptides in a dot-blot format for each site. Positive clones were verified by immunoblotting and ELISAs using hyperphosphorylated Irs1 from okadaic acid-treated CHOIR/Irs1 cells grown in 10% FBS. The reactive hybridomas were cloned at limiting dilution twice to produce monoclonal cell lines. All mouse work and subcloning was subcontracted to the Hybridoma Development Services Core facility at St. Louis University Health Sciences Center. All screening (except the initial dot blot) was completed in our laboratory.
Cell Culture
CHOIR/Irs1 cells were grown in Ham's F-12 medium (Invitrogen) supplemented with 10% FBS. Hybridoma cell lines were maintained in RPMI (Invitrogen) supplemented with 10% FBS (heat-inactivated), 10% hybridoma growth factor supplement (PAA Laboratories, Inc.), 1× hypoxanthine and thymidine (HT) media supplement (Sigma) and 1% penicillin/streptomycin (Invitrogen).
Antibodies
Supernatants from hybridoma cell lines were incubated with protein G beads (Amersham Biosciences). The antibody-bound beads were washed once with 250 mm Tris (pH 7.5, 250 mm NaCl, 10% glycerol) and then with 150 mm Tris (pH 7.5, 250 mm NaCl, 10% glycerol). The monoclonal antibodies were eluted with 0.1 m glycine (pH 2.9) into 1 m Tris (pH 9.0, 250 mm NaCl, 10% glycerol). The eluent was concentrated, frozen in aliquots, and stored at −20 °C. Five commercially available αpS/TIrs1 polyclonal antibodies (Cell Signaling) were used to investigate phosphorylation sites not represented by our library (rat numbering): αpS336Irs1, αpS612Irs1, αpS632/S635Irs1, αpS789Irs1, and αpS1100Irs1. Some antibodies generated in this work are available from Millipore: total Irs1 (catalog no. 05-1085), αpS302Irs1 (catalog no. 05-1086), αpS307Irs1 (catalog no. 05-1087), αpS318Irs1 (mabs138), αpS522Irs1 (catalog no. 05-1921), and αpS632Irs1 (catalog no. 05-1568).
Polyclonal antibodies were obtained from Cell Signaling against ERK and Thr(P)-202/Tyr(P)-204ERK; Akt, Thr(P)-308Akt and Ser(P)-473Akt; GSK3β and Ser(P)-9GSK3β; FOXO1 and Ser(P)-256FOXO1; S6K and Thr(P)-389S6K; S6 and Ser(P)-235/236S6; mTOR and Ser(P)-2448mTOR. Polyclonal antibodies were obtained from Cell Signaling against PERK and Thr(P)-980PERK; p38 MAP kinase and Thr(P)-180/Y182p38; and from Millipore against JNK and Thr(P)-183/Y185JNK.
Insulin Stimulated Irs1 Ser(P)/Thr(P) residues and Tyr(P)Irs1
CHOIR/Irs1 cells were grown to 85% confluence followed by a 24-hour in serum-free medium. Cells were incubated for 30-min with 30 nm insulin. The cells were then placed on ice, washed thoroughly with PBS, and incubated with lysis buffer (50 mm Tris, pH 7.5, 1% Triton, 0.1% SDS, 0.1 mm sodium pyrophosphate, 0.5 mm NaF, 150 mm NaCl, and 1 mm EDTA) containing 1 × phosphatase and protease inhibitors pellets (Roche). Cleared lysates were mixed with SDS loading buffer and resolved using 8% SDS-PAGE, transferred onto nitrocellulose, and probed with the indicated antibodies.
Kinase Inhibition
Inhibitors were incubated with serum-starved CHOIR/Irs1 cells (above) for 30 min prior to insulin stimulation: PD98059, wortmannin, rapamycin, and PP242 from Sigma; LY294002 from Santa Cruz Biotechnology; PP-103, GDC-0491, and PIK-90 from Selleck; and AKT-VIII from EMD Biosciences. DG2, a gift of Dr. Kevan Shokat, is available from EMD.
Heterologous Regulation of IRS1 Ser(P)/Thr(P) residues and Tyr(P)Irs1 by Metabolic Stress Agents
The indicated agents (Sigma) were added to serum-starved CHOIR/Irs1 cultures (above) with or without subsequent insulin stimulation (30 nm for 30 min). Anisomycin (5 μg/ml) was added 60 min prior to insulin stimulation, whereas thapsigargin (300 nm) or tunicamycin (10 μg/ml) were added 3 h prior.
Imaging and Quantitation of Western Blots
Western blots were imaged using ECL and Kodak Image Station 4000MM Pro; signals were quantitated using Carestream Molecular Imaging software V5.0. The median background surrounding each band was subtracted from the total signal (sum of pixel values) to give a “net signal” for analysis. In inhibition studies, the inhibited net signal was normalized by the insulin-stimulated net signal observed in the absence of inhibitors. Phosphorylation curves, obtained at a range of inhibitor concentrations, were fitted to the following hyperbolic function,
using SigmaPlot V12.5, where Kid is the ED50 of inhibition (ED50), and Kin is the maximal fold change at infinite inhibitor concentration. Fitted curves with r2 > 0.7 were taken to indicate a reportable effect. The fitted parameter Kin, determined for each kinase inhibitor, was used to estimate the maximal inhibition of Ser(P)/Thr(P) (or stimulation of Tyr(P)Irs1) at saturating kinase inhibitor concentration. The fitted parameter Kid was used to estimate the concentration of inhibitor that half-maximally inhibited Ser(P)/Thr(P) (i.e. Kid = ED50pS/T), or that half-maximally stimulated Tyr(P)Irs1 (Kid = ED50pTyr). Pearson correlation between the parameters describing inhibition or stimulation was calculated using the Analyze-it plug-in for Microsoft Excel (version 3.20.2).
RESULTS
Generation and Validation of Phospho-specific Antibodies against Irs1
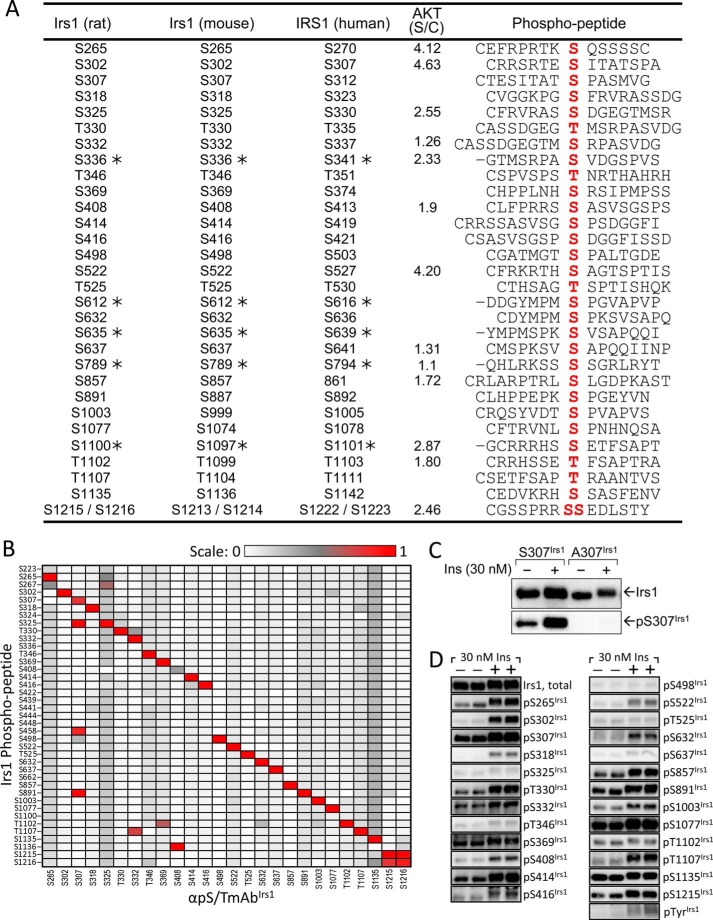
We developed a library of monoclonal antibodies (αpS/TmAbIrs1) against 26 Ser(P)/Thr(P) residues in rat Irs1. Hybridoma lines producing each antibody were generated from spleen cells of mice immunized with keyhole limpet hemocyanin-conjugated phosphopeptide antigens (Fig. 1A), with peptide sequences based upon our previous MS/MS identification of Ser(P)/Thr(P) residues in rat Irs1 from CHOIR/Irs1 cells (19) (Fig. 1A). We validated the sequence specificity and phospho-specificity of each αpS/TmAbIrs1 using both peptide-based ELISA assays (Fig. 1B) and immunoblotting of insulin-stimulated CHOIR/Irs1 cells (Fig. 1, C and D). None of the αpS/TmAbIrs1 reacted with nonphosphorylated peptides, demonstrating that each was phospho-specific (data not shown). All of the αpS/TmAbIrs1, except αpS408Irs1, reacted most strongly by ELISA with the immunizing phosphopeptide, confirming specificity; αpS408Irs1 reacted most strongly with Ser(P)-1136, which has a similar surrounding sequence (Fig. 1B). The αpS307Irs1, αpS332Irs1, αpS369Irs1, and αpS1215Irs1 each reacted less strongly with a second or third phosphopeptide (Fig. 1B); however, immunoblotting of wild type and mutant Irs1 proteins in insulin-stimulated CHO cells confirmed that αpS307Irs1 required Ser(P)-307 to recognize Irs1 (Fig. 1C). The αpS1216Irs1 behaved identically to αpS1215Irs1 (Fig. 1B) and was excluded from further analysis. The set of 25 αpS/TmAbIrs1 thus validated was not exhaustive but covered greater than half of all MS/MS-identified sites distributed across the Irs1 protein. To provide additional coverage for some experiments, we used five commercially available polyclonal αpS/TIrs1 antibodies (against rat Ser(P)-336Irs1, Ser(P)-612Irs1, Ser(P)-632/S635Irs1, Ser(P)-789Irs1, and Ser(P)-1100Irs1) without further in-house validation (identified with asterisks in Fig. 1A).
FIGURE 1.
Validation of monoclonal antibodies (αpS/TmAbIrs1) generated against MS/MS-identified Irs1 phosphopeptides (19). A, Irs1 Ser(P)/Thr(P) residues (rat, mouse, and human numbering) and their surrounding amino acid sequences (phosphopeptide, phosphorylated residue in red) used for mAb production. The Akt motifs were identified at a medium threshold by GPS 3.0 using rat Irs1 (CAA41264) as the target (91). The ratio of score to cutoff (S/C) is reported. B, the sequence specificity of each αpS/TmAbIrs1 was verified in an ELISA assay against 15-residue phosphopeptides based upon the seven amino- and carboxyl-terminal residues surrounding the Ser(P)/Thr(P) of interest in rat Irs1. C, CHO cell lines expressing human insulin receptor and either wild type or mutant (A307) rat Irs1 were serum-starved overnight and then stimulated with 30 nm insulin for 30 min. Extracts were blotted with total Irs1 and αpS307Irs1 antibodies. D, CHOIR/Irs1 cells were treated with 30 nm insulin for 30 min. Irs1 was immunoblotted with the indicated αpS/TmAbIrs1; each column represents a separate cell extract, with the same extract being used for each antibody in that column.
To validate use of our αpS/TmAbIrs1 library on biological samples, lysates of CHOIR/Irs1 cells treated without or with insulin were immunoblotted using antibodies against total Irs1, phosphotyrosine (αpY), or each αpS/TmAbIrs1. Insulin stimulated the phosphorylation of Irs1 on tyrosine (Tyr(P)Irs1) and on all Ser(P)/Thr(P) residues, save Ser(P)-498Irs1, Thr(P)-525Irs1, Thr(P)-1102Irs1, and Ser(P)-1135Irs1 (Fig. 1D).
Insulin-dependent Ser(P)/Thr(P) Residues
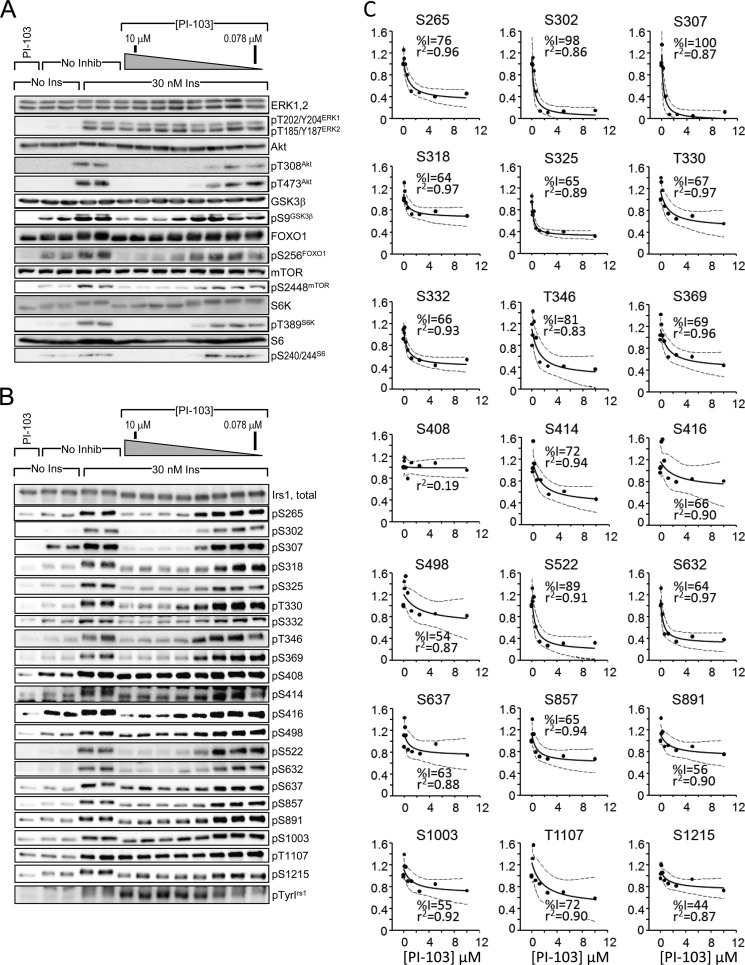
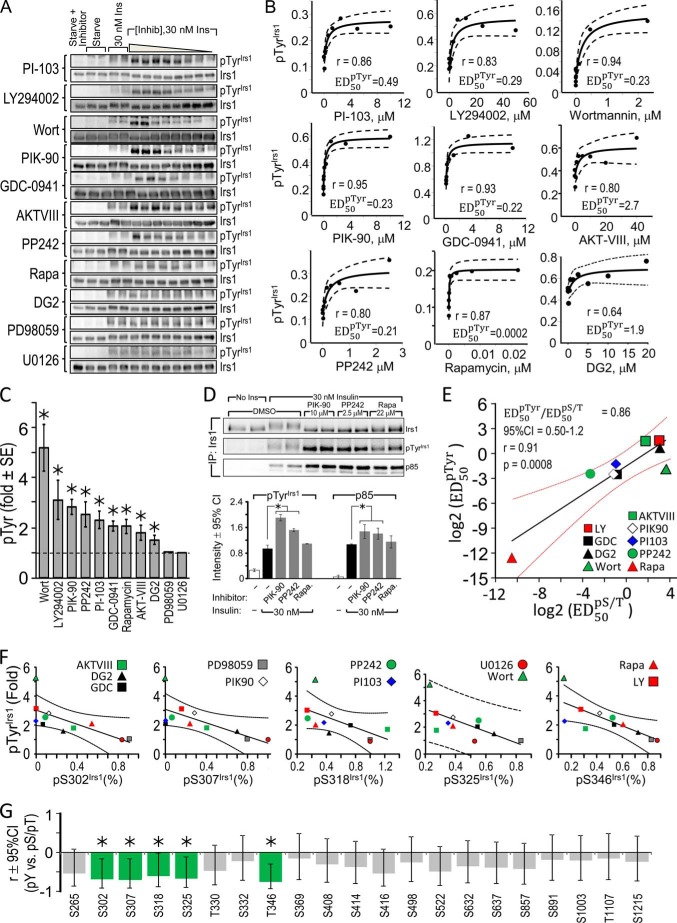
To investigate the basis of insulin-stimulated Irs1 Ser(P)/Thr(P) residues, we used inhibitors to block various insulin-stimulated kinases in CHOIR/Irs1 cells, including class I PI3 kinase (PI-103, LY294002, wortmannin, PIK-90, or GDC-0941), Akt (AKT-VIII), mTORC1 and mTORC2 (PP242), mTORC1 alone (rapamycin), S6K (DG2), or MEK (PD98059 or U0126) (Figs. 2A and 3, A–J). First, we verified the specificity of each inhibitor and determined the “global ED50TP,” or 50% effective inhibitory dose (see “Experimental Procedures”), by quantitating the insulin-stimulated phosphorylation of known inhibitor target protein(s), including ERK, Akt, GSK3β, FOXO1, mTOR, S6K, or S6. The strongest and most complete inhibition was obtained with PI-103, which targets the catalytic p110α subunit of PI3K, mTORC1/2, and nuclear DNA-dependent serine/threonine protein kinase (Fig. 2A). Treatment with PI-103 reduced by more than 50% the phosphorylation of all “target proteins” save ERK (global ED50TP = 0.59 μm) (Fig. 2A; see Fig. 5A). By contrast, the MEK-specific inhibitors PD98059 and U0126 strongly reduced ERK phosphorylation without significant effects upon components of the PI3K cascade (Fig. 3, I and J; see Fig. 5A). Each of the remaining four PI3K inhibitors inhibited phosphorylation of Thr(P)-308Akt and Ser(P)-473Akt but displayed variable effects upon phosphorylation of Akt targets GSK3β, FOXO1, and mTOR; all PI3K inhibitors assayed also inhibited S6K and S6 phosphorylation (Figs. 2A and 3, A–D; see Fig. 5A). The Akt inhibitor (AKT-VIII) reduced Akt, mTOR, S6K, and S6 phosphorylation but had small effects upon the other tested proteins (Fig. 3E; see Fig. 5A). PP242, a catalytic inhibitor of mTOR in both mTORC1 and mTORC2 complexes, also reduced strongly Akt, S6K, and S6 phosphorylation (Fig. 3F; see Fig. 5A); however, rapamycin, the allosteric mTORC1 inhibitor, reduced S6K and S6 phosphorylation without effect upon Akt, at least in part because mTORC2 remains active to phosphorylate Ser-473Akt (65, 66) (Fig. 3G; see Fig. 5A). The mTORC1 substrate S6K is thought to be a major autologous feedback regulator of IRS1 signaling (67, 68). However, the S6K-specific inhibitor DG2 blocked S6 phosphorylation without enhancing the insulin-stimulated phosphorylation of Akt or its substrates GSK3 and FOXO1 in CHOIR/Irs1 cells (Fig. 3H; see Fig. 5A).
FIGURE 2.
Nearly global inhibition of insulin-stimulated Irs1 S/T phosphorylation by PI3K inhibitor PI-103. CHOIR/Irs1 cells were treated without or with PI-103 (0.078 μm or 0.156, 0.312, 0.625, 1.25, 2.50, 5.00, or 10.0 mm) for 30 min before insulin (30 nm) treatment. A and B, cell extracts were immunoblotted with total or phospho-specific target protein antibodies (A) or total Irs1 and each αpS/TmAbIrs1 (B). C, insulin-stimulated Ser/Thr phosphorylation in the presence of PI-103 was normalized by the intensity measured in the absence of PI-103. The normalized response was fitted to an hyperbolic curve, f(x) = y0 · (kid + x · kin)/(kid + x). For each Ser(P)/Thr(P), the maximal inhibition (%I) by PI-103 and the square of the correlation coefficient (r2) are indicated; blue dotted lines indicate the 95% confidence interval of the fitted curve. Curves with r2 > 0.7 and %I > 50 are taken to indicate a reportable effect. No Inhib, no inhibition; No Ins, no insulin; Ins, insulin.
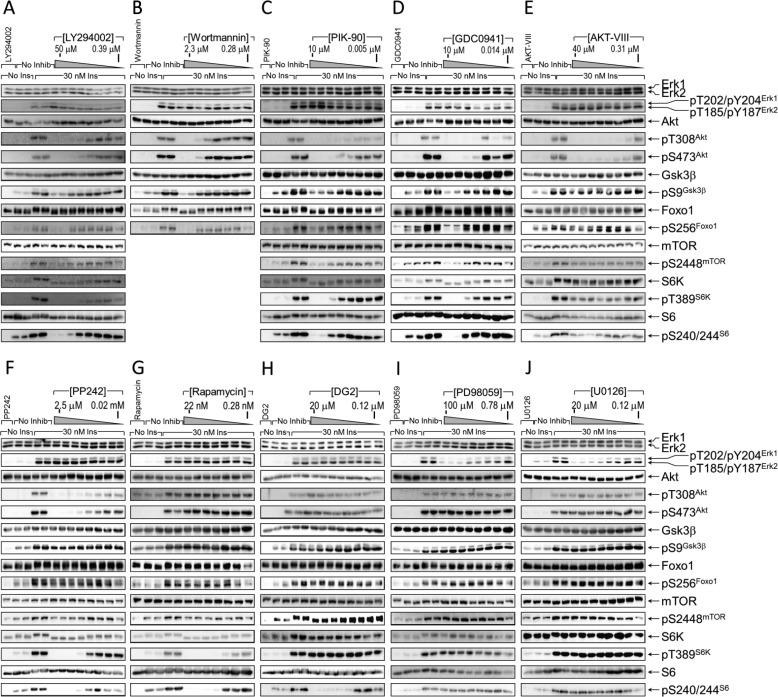
FIGURE 3.
Inhibition of insulin-stimulated Ser/Thr kinase pathways. A–J, CHOIR/Irs1 cells were treated for 30 min without or with the indicated kinase inhibitor at concentrations increasing sequentially 2-fold between the boundaries shown on each figure. Then the cells were treated with insulin (30 min, 30 nm), and the cleared lysates were immunoblotted with the indicated antibodies. The bands were quantified on a Kodak Image Station 4000MM Pro, and the data were analyzed using Carestream Molecular Imaging software version 5.0. No Inhib, no inhibition; No Ins, no insulin; Ins, insulin.
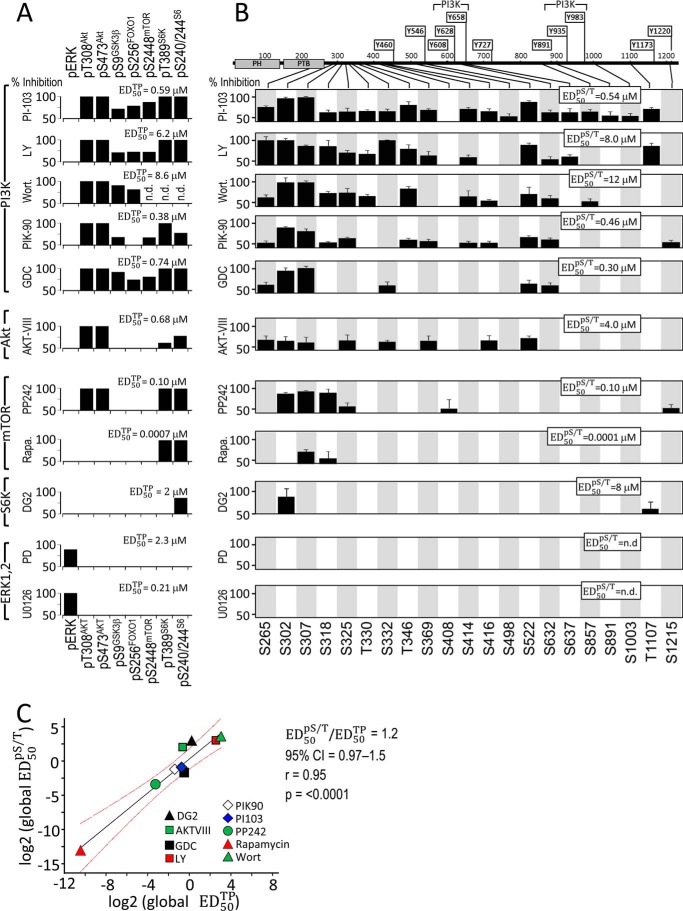
FIGURE 5.
Inhibitors of insulin-stimulated Ser/Thr kinases have similar effects upon known inhibitor target proteins and Irs1 Ser(P)/Thr(P) residues. CHOIR/Irs1 cells were treated with the indicated kinase inhibitors (concentration ranges in Fig. 3) for 30 min before insulin stimulation (30 nm, 30 min), and the cleared cell extracts were analyzed on immunoblots (shown in Fig. 3 and Fig. 4). Insulin-stimulated phosphorylation in the presence of each inhibitor was normalized to that measured in its absence, and the normalized data were fitted to f(x) = y0 · (kid + x · kin)/(kid + x) to assay significant inhibition (%I). Individual target protein or Ser(P)/Thr(P) residues that reached the cutoffs (%I > 50; r2 > 0.7) are shown as black bars. A, the global ED50TP (ED50 for inhibition of target proteins) was determined for each inhibitor by fitting the data for the target proteins simultaneously. B, the sets of Irs1 Ser(P)/Thr(P) residues significantly inhibited by each compound (black bars) were analyzed in a similar way to determine the global ED50pS/T. C, linear regression of the global ED50pS/T (log2) on the global ED50TP (log2) values determined for each inhibitor. The slope of the line is the ratio of ED50 values. Red lines show the 95% confidence interval of the fitted line. Wort., wortmannin; LY, LY294002; Rapa., rapamycin.
PI3K-dependent Irs1 Ser(P)/Thr(P)
Next we investigated the effect of PI3K inhibitors upon insulin-stimulated Irs1 Ser(P)/Thr(P). To establish the scope of Ser(P)/Thr(P) under PI3K control, we determined the maximal effect of each inhibitor upon insulin-stimulated Irs1 Ser/Thr phosphorylation as detected by our αpS/TmAbIrs1. The results were fitted to hyperbolic curves to identify significant inhibitory effects (r2 > 0.7) and to calculate the global ED50pS/T, the inhibitor concentration that reduced all of the significantly inhibited Ser(P)/Thr(P) residues by 50% (Figs. 2B and 4, A–J; see Fig. 5B).
FIGURE 4.
Effect upon insulin-stimulated Irs1 Ser(P)/Thr(P) following inhibition of insulin-stimulated Ser/Thr kinase pathways. A–J, CHOIR/Irs1 cells were pretreated with the indicated kinase inhibitors for 30 min before insulin stimulation (30 nm) for an additional 30 min. Cleared lysates were subject to immunoblotting with the indicated αpS/TmAbIrs1 to determine the effect upon Irs1 Ser/Thr phosphorylation. The bands were quantified on a Kodak Image Station 4000MM Pro, and the data were analyzed using Carestream Molecular Imaging software version 5.0. No Inhib, no inhibition; No Ins, no insulin; Ins, insulin.
PI-103 produced the most complete inhibition of Irs1 Ser(P)/Thr(P) residues of any tested compound, because all insulin-stimulated sites except Ser(P)-408Irs1 and Ser(P)-1215Irs1 were >50% inhibited (Fig. 2, B and C; summarized in Fig. 5B). In addition to indirectly inhibiting Akt downstream of PI3K, PI-103 directly inhibits mTORC1 and mTORC2 complexes that regulate Akt and other kinases (69). More specific PI3K inhibitors yielded less complete Ser(P)/Thr(P) inhibition: LY294002 and wortmannin inhibited fewer Ser(P)/Thr(P) residues than PI-103 (Figs. 4, A and B, and 5B); PIK-90 inhibited 12 and GDC-0941 inhibited six of the tested Ser(P)/Thr(P) residues (Figs. 4, C and D, and 5B). All five PI3K inhibitors consistently inhibited Ser(P)-265Irs1, Ser(P)-302Irs1, Ser(P)-307Irs1, Ser(P)-522Irs1, and Ser(P)-632Irs1 (Fig. 5B); and each of the PI3K inhibitors, except GDC-0941, blocked Ser(P)-318Irs1, Thr(P)-346Irs1, and Ser(P)-414Irs1. All the PI3K inhibitors, save wortmannin and GDC-0941, also blocked Ser(P)-332Irs1, Ser(P)-369Irs1, and Thr(P)-1107Irs1 (Fig. 5B).
Akt Inhibition
Akt is a key node in the PI3K pathway that is activated by PDK1 → Thr(P)-308Akt and mTORC2 → Ser(P)-473Akt (8, 66). Akt-mediated IRS1 Ser(P)/Thr(P) residues are implicated in the regulation of IRS1 tyrosine phosphorylation (21). To establish which PI3K-dependent Irs1 Ser(P)/Thr(P) residues also required Akt activity, we treated the CHOIR/Irs1 cells with AKT-VIII, an inhibitor that interacts with the pleckstrin homology domain of Akt to prevent its phosphorylation and activation by PDK1 (70). Eight insulin-stimulated Ser(P)/Thr(P) residues detected by our αpS/TmAbIrs1 were significantly inhibited by AKT-VIII, including five predicted Akt sites: Ser(P)-265Irs1, Ser(P)-302Irs1, Ser(P)-325Irs1, Ser(P)-332Irs1, and Ser(P)-522Irs1 (Fig. 1A), and three others: Ser(P)-307Irs1, Ser(P)-369Irs1, and Ser(P)-416Irs1 (Figs. 4E and 5B). Each of these Ser(P)/Thr(P) residues was also inhibited by PI-103, but only three of the predicted Akt sites (Ser(P)-265Irs1, Ser(P)-302Irs1, and Ser(P)-522Irs1) and Ser(P)-307Irs1 were inhibited by all five of the PI3K inhibitors, with the other Ser(P)/Thr(P) residues displaying mixed responses. In total, seven Ser(P)/Thr(P) residues sensitive to at least one of the more specific PI3K inhibitors were less than 50% inhibited by AKT-VIII, suggesting that both AKT and additional kinases downstream of PI3K are involved (Fig. 5B).
mTOR Inhibition
mTOR assembles into two molecular complexes, mTORC1 and mTORC2, with distinct functions in insulin signaling (66, 69). mTORC1 mediates insulin regulation of protein, lipid, and nucleotide synthesis required for cell growth and proliferation, whereas mTORC2 promotes full activation of Akt by phosphorylating Ser-473Akt (8, 66, 71). Previous studies in mouse fibroblasts demonstrate that rapamycin-sensitive mTORC1 promotes phosphorylation of Ser-302Irs1, Ser-307Irs1, Ser-522Irs1, Ser-612Irs1, and Ser-632Irs1, associated with Irs1 degradation (28, 67, 68). In 3T3-L1 cells, mTORC1 associates with Irs1 through its RAPTOR (regulatory-associated protein of mTOR) subunit and phosphorylates Ser-636/Ser-639IRS1 (mouse Ser-632/Ser-635Irs1) (72).
The catalytic mTOR inhibitor PP242 and the allosteric mTORC1-specific inhibitor rapamycin were used to investigate insulin-stimulated Irs1 Ser(P)/Thr(P) residues (8, 63). Inhibition of both mTORC1 and mTORC2 by PP242 inhibited Ser(P)-302Irs1, Ser(P)-307Irs1, Ser(P)-318Irs1, Ser(P)-325Irs1, Ser(P)-408Irs1, and Ser(P)-1215Irs1 (Fig. 4, F and G, and 5B). Notably, Ser(P)-522Irs1 and Ser(P)-632Irs1 were not inhibited, and Ser-612Irs1 was not tested. By comparison, rapamycin inhibited only Ser(P)-307Irs1 and Ser(P)-318Irs1, with both Ser(P)-302Irs1 and Ser(P)-632Irs1 missing the cutoffs for significance (Fig. 4, F and G, and 5B). Thus, based upon the specificities of PP242 and rapamycin, Ser(P)-302Irs1, Ser(P)-325Irs1, Ser(P)-408Irs1, and Ser(P)-1215Irs1 appeared to be phosphorylated predominantly via mTORC2, rather than mTORC1, in CHOIR/Irs1 cells; however, direct phosphorylation remains provisional because these sites poorly match the predicted mTOR substrate motifs, and both mTORC1 and mTORC2 regulate Akt activity during insulin stimulation (8, 69).
S6 Kinase Inhibition
Under conditions of unrestrained mTORC1 activation, S6K1 mediates extensive Ser/Thr phosphorylation of Irs1 and can directly phosphorylate Ser-302Irs1 and Ser-522Irs1 in vitro (68). Additionally, S6K1 promotes the in vitro and in vivo phosphorylation of both Ser-265Irs1 and Ser-1097Irs1 (49, 73). We used the ATP-competitive inhibitor DG2 to specifically inhibit S6K during insulin stimulation; however, this resulted in the inhibition of only Ser(P)-302Irs1 (Figs. 4H and 5B). Because Ser(P)-302Irs1 was not significantly blocked by the mTORC1 inhibitor rapamycin, this result could reflect either the stringency of the numerical cutoffs required to achieve significance or a partially rapamycin-resistant biological process.
MAP Kinase Inhibition
ERK is activated by the RAS → RAF → MEK kinase cascade, activated by GRB2·SOS association with either Irs1 or Shc (74–77). Previous studies suggest that chemical inhibitors of MEK1/2 reduce insulin-stimulated Ser(P)-632Irs1 (34, 78). We used the MEK inhibitors PD98059 or U0126 to inhibit insulin-stimulated ERK activity in CHOIR/Irs1 cells (Fig. 4, I and J, and 5B); although Ser(P)-632Irs1 was weakly inhibited by PD98059, none of the Irs1 Ser(P)/Thr(P) residues were inhibited significantly (r2 > 0.7 and >50%) (Fig. 4, I and J, and 5B). Thus, compared with other insulin-stimulated kinases, ERK only weakly phosphorylated Irs1 during insulin stimulation.
Relation between Inhibitor Target Protein Phosphorylation/Activity and Irs1 Ser(P)/Thr(P)
Next, we correlated for each of the inhibitors the concentration required for half-maximal inhibition of the set of significantly inhibited Irs1 Ser(P)/Thr(P) (called the global ED50pS/T) with the dose required for half-maximal inhibition of each proximal target protein (called the global ED50TP) (Fig. 5, A and B). This analysis revealed a highly significant correlation between the inhibition of insulin-stimulated Irs1 Ser(P)/Thr(P) residues and that of the known inhibitor target proteins: the log2 transformed ED50 values were related linearly by a slope of unity and Pearson correlation coefficient (r) of 0.95 (p < 0.0001) (Fig. 5C). We conclude that Irs1 was phosphorylated via kinases in the targeted cascades.
Relation between Irs1 Ser/Thr Phosphorylation and Insulin-stimulated Tyr(P)Irs1
The hypothesis that individual Ser(P)/Thr(P) residues generally inhibit insulin-stimulated Tyr(P)Irs1 is widely accepted (6, 54). Consistent with this hypothesis, increasing concentrations of all the tested inhibitors, excepting the MEK inhibitors, enhanced insulin-stimulated Tyr(P)Irs1 (Fig. 6A). Quantitation of these results reveals the hyperbolic relation between Tyr(P)Irs1 and inhibitor concentration (Fig. 6B). The maximal fold increase of Tyr(P)Irs1 ranged between 1.5- and 5-fold (Fig. 6C). Three inhibitors, PIK-90, PP242, and rapamycin, were tested to confirm that increased Tyr(P)Irs1 indeed gave rise to enhanced p85 association with Irs1 in immunoprecipitates (Fig. 6D).
FIGURE 6.
Inhibition of insulin-stimulated Ser/Thr kinases enhances insulin-stimulated Tyr(P)Irs1. A, Tyr(P)Irs1 determined in CHOIR/Irs1 cells treated with kinase inhibitors. Cells were treated for 30 min without or with the indicated kinase inhibitor at concentrations increasing sequentially 2-fold between the boundaries shown in Fig. 4 before incubation with insulin (30 nm, 30 min). Cleared lysates were subject to immunoblotting with antibodies against Irs1 or Tyr(P). The bands were quantified on a Kodak Image Station 4000MM Pro, and the data were analyzed using Carestream Molecular Imaging software version 5.0. B, the insulin-stimulated Tyr(P)Irs1 at each inhibitor concentration was normalized to that observed in the absence of inhibitor, and the normalized data were fitted to f(x) = y0 · (kid + x · kin)/(kid + x) to determine the maximal fold enhancement of Tyr(P)Irs1 and ED50pTyr. Dotted lines show the 95% confidence interval of the fitted curves. C, calculated maximal fold enhancement of insulin-stimulated Tyr(P)Irs1 by each kinase inhibitor. Error bars show 95% confidence interval. *, p < 0.05. D, CHOIR/Irs1 cells were treated without or with inhibitors PIK-90, PP242, or rapamycin for 30 min before insulin stimulation (30 nm, 30 min). Lysates were immunoprecipitated with total Irs1 monoclonal antibody and immunoblotted with antibodies against Irs1, Tyr(P), or the p85 subunit of PI3K. The bands were quantified on a Kodak Image Station 4000MM Pro, and the data were analyzed using Carestream Molecular Imaging software version 5.0. The average signals (± S.D.) in the absence or presence of kinase inhibitors for three separate experiments are shown; *, p < 0.05. E, linear regression of the ED50pTyr (log2) on the global ED50pS/T (log2) values determined for each inhibitor; the slope of the line is the ratio of ED50 values (± 95% confidence interval in red). F, Pearson correlation (r ± 95% confidence interval) establishing inverse relation between the maximal inhibition (%I) of particular Irs1 Ser(P)/Thr(P) and stimulation (fold) of Tyr(P)Irs1 determined from kinase inhibitor data. G, summary of Pearson correlation for all assayed Ser(P)/Thr(P) residues. Green bars highlight significant (p < 0.05) negative r, indicating Irs1 Ser(P)/Thr(P) residues associated with reduced Tyr(P)Irs1; gray bars are not significant. Inhib, inhibition; Ins, insulin; Wort, wortmannin; CI, confidence interval; IP, immunoprecipitation; Rapa, rapamycin.
We exploited the effects of graded Ser/Thr kinase inhibition to determine the relation between Irs1 Ser(P)/Thr(P) residues and Tyr(P)Irs1 during insulin stimulation. To do so, we calculated the ED50pTyr (see “Experimental Procedures”) as a measure of the inhibitor dosage required for half-maximal enhancement of Irs1 tyrosine phosphorylation. The ED50pTyr of the various inhibitors correlated significantly with the global ED50pS/T (ED50pTyr/ED50pS/T = 0.86; Pearson r = 0.91; p = 0.0008) (Fig. 4E). This close relation between the effective doses for Ser(P)/Thr(P) inhibition and Tyr(P)Irs1 enhancement supports the idea that Ser(P)/Thr(P) residues on IRS1 regulate (mostly negatively) Tyr(P)Irs1 that coordinates the downstream insulin signal.
For individual Ser(P)/Thr(P) residues, we calculated the Pearson correlation between the maximal Ser(P)/Thr(P) inhibition and the maximal Tyr(P)Irs1 enhancement achieved using each kinase inhibitor. All the Ser(P)/Thr(P) residues correlated negatively with Tyr(P)Irs1; however, significant correlation (p < 0.05) was found for only a subset of sites, including Ser(P)-302Irs1, Ser(P)-307Irs1, Ser(P)-318Irs1, Ser(P)-325Irs1, and Ser(P)-346Irs1 (Fig. 6F; summary in Fig. 6G). Because the negative correlation of these Ser(P)/Thr(P) residues with Tyr(P)Irs1 integrates the responses to chemically distinct inhibitors, this subset of Ser(P)/Thr(P) residues might be particularly relevant for down-regulating Irs1 signaling. However, mechanism(s) linking these Ser(P)/Thr(P) residues to the regulation of Tyr(P)Irs1 cannot be resolved from this analysis; indeed, it remains possible that Tyr(P)Irs1 is inhibited by the aggregate effect of many Ser(P)/Thr(P) residues. Regardless, we note that degradation of Irs1 was never observed in the time interval of our experiments.
Metabolic Stress Stimulates Irs1 Ser(P)/Thr(P) and Inhibits Insulin-stimulated Tyr(P)Irs1
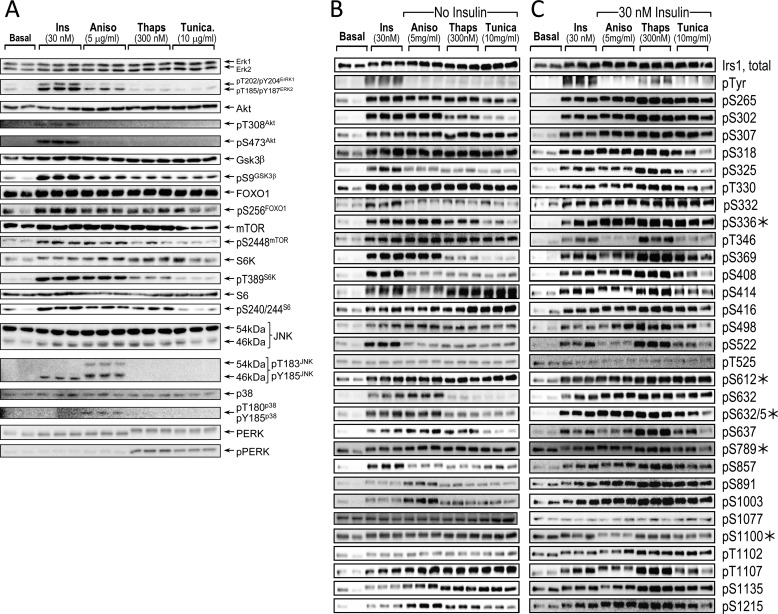
Next, we stimulated Irs1 Ser/Thr phosphorylation by inducing metabolic stress with anisomycin, thapsigargin, or tunicamycin in CHOIR/Irs1 cells. Anisomycin inhibits protein synthesis and activates JNK and p38 MAP kinases (79, 80); thapsigargin noncompetitively inhibits sarco-/endoplasmic reticulum Ca2+ ATPases, increasing cytosolic Ca2+ and ER stress (63); and tunicamycin inhibits N-linked protein glycosylation, which also promotes ER stress (81). Induction of ER stress stimulates the phosphorylation and activation of PERK (PKR-like endoplasmic reticulum kinase). We measured phosphorylation of JNK, p38, and PERK to verify the effect of metabolic stress agents in CHOIR/Irs1 cells. As expected, anisomycin stimulated JNK and p38 phosphorylation, whereas thapsigargin or tunicamycin stimulated PERK phosphorylation (Fig. 7A). All three agents also moderately stimulated the inhibitory phosphorylation of GSK3β, albeit without upstream phosphorylation of Akt on Thr-308 or Ser-473. Consistent with previous reports, both anisomycin and thapsigargin stimulated the phosphorylation/activation of mTOR and S6K (Fig. 7A) (28).
FIGURE 7.
Stimulation of Ser/Thr kinases and Irs1 Ser(P)/Thr(P) residues by insulin or agonists of metabolic stress. A, serum-starved CHOIR/Irs1 cells were left untreated (Basal), or treated with insulin (30 nm, 30 min), anisomycin (5 μg/ml, 60 min), thapsigargin (300 nm, 180 min), or tunicamycin (10 μg/ml, 180 min). The cleared lysates were immunoblotted with antibodies against total protein or the indicated phosphospecific antibodies. B, the lysates in A were immunoblotted with antibodies to total Irs1, Tyr(P), and 25 αpS/TmAbIrs1 plus five commercially available Irs1 phosphospecific polyclonal antibodies (*). C, CHOIR/Irs1 cells were treated as in A, but with the subsequent addition of insulin (30 nm, 30 min) to those cells treated with anisomycin, thapsigargin, or tunicamycin. Bands were quantified on a Kodak Image Station 4000MM Pro, and the data were analyzed using Carestream Molecular Imaging software version 5.0. Ins, insulin; Aniso, anisomycin; Thaps, thapsigargin; Tunica, tunicamycin.
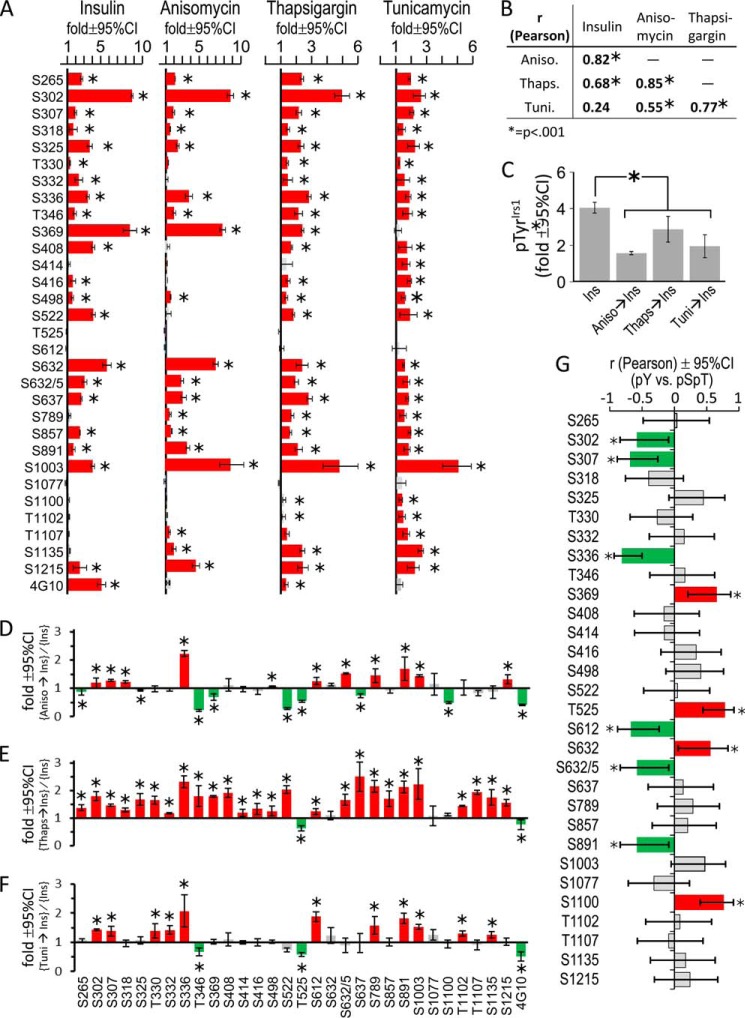
As previously shown, anisomycin, thapsigargin, or tunicamycin stimulated Ser-307Irs1 phosphorylation (50, 51, 57); however, analysis with our αpS/TmAbIrs1 plus five commercially available polyclonal antibodies (Fig. 1A) revealed that most Irs1 Ser(P)/Thr(P) residues were increased by metabolic stress (Figs. 7B and 8A). The Ser(P)/Thr(P) patterns induced by anisomycin, thapsigargin, and tunicamycin differed somewhat but were each significantly correlated (p < 0.001) (Fig. 8B). In general, these patterns also mimicked the appearance of insulin stimulation (Figs. 7B and 8A). The Ser(P)/Thr(P) pattern induced by anisomycin was most similar to that of insulin (Pearson r = 0.82, p < 0.001), whereas those produced by thapsigargin (r = 0.68, p < 0.001) or tunicamycin (r = 0.24, p > 0.05) were less correlated (Fig. 8B).
FIGURE 8.
The effect of metabolic stress upon basal and insulin-stimulated Irs1 phosphorylation. A, CHOIR/Irs1 cells were treated with insulin (30 nm, 30 min), anisomycin (5 μg/ml, 60 min), thapsigargin (300 nm, 3 h), or tunicamycin (10 μg/ml, 60 min). Irs1 Ser(P)/Thr(P) residues stimulated by insulin or agonists of metabolic stress (blots in Fig. 7B) were quantified and normalized to the phosphorylation in untreated CHOIR/Irs1 cells to determine the fold stimulation (± 95% confidence interval) above basal. Red bars indicate significant stimulation (*, p < 0.05); gray bars are not significant. B, Pearson correlation (r) of insulin- and metabolic stress-induced Irs1 Ser(P)/Thr(P) patterns shown in A. C, fold (versus basal) Tyr(P)Irs1 stimulated by insulin alone (30 nm, 30 min) or by insulin preceded by anisomycin (5 μg/ml, 60 min), thapsigargin (300 nm, 3 h), or tunicamycin (10 μg/ml, 60 min) treatment. D–F, the Irs1 Ser(P)/Thr(P) residues stimulated by insulin alone or insulin preceded by metabolic stress (blots in Fig. 7C) were quantified, normalized to the basal phosphorylation in untreated cells, and expressed as the ratio (metabolic stress plus insulin)/(insulin alone) (fold ± 95% confidence interval). Red bars indicate significantly greater Ser/Thr phosphorylation in cells pretreated with the indicated agent, and green bars significantly less (*, p < 0.05). G, Pearson correlation (r ± 95% confidence interval) of Irs1 Ser(P)/Thr(P) residues in cells treated with insulin, or with anisomycin, thapsigargin, or tunicamycin followed by insulin, with the fold insulin-stimulated Tyr(P)Irs1 observed under these conditions; green bars indicate Ser(P)/Thr(P) residues that correlate significantly with less Tyr(P)Irs1; red bars indicate Ser(P)/Thr(P) residues that correlate significantly with more Tyr(P)Irs1 (*, p < 0.05); gray bars are not significant. Ins, insulin; Aniso, anisomycin; Thaps, thapsigargin; Tuni, tunicamycin; CI, confidence interval.
We examined the effect of these stress-induced basal Ser(P)/Thr(P) patterns on insulin-stimulated phosphorylation of Irs1 (Fig. 7C). As expected for an insulin-desensitizing effect of metabolic stress, insulin-stimulated Tyr(P)Irs1 was diminished significantly—by approximately half or greater—by anisomycin or tunicamycin pretreatment, although less by pretreatment with thapsigargin (Fig. 8C). Consistent with the relative Tyr(P)Irs1 achieved in each case, insulin further stimulated only 11 of 30 Ser(P)/Thr(P) residues assayed in anisomycin- or tunicamycin-treated cells, whereas it retained ability to augment nearly all Ser(P)/Thr(P) residues (26 of 30) in thapsigargin-treated cells (Fig. 8, D–F). Six Ser(P)/Thr(P) residues that were significantly stimulated by insulin alone (Ser(P)-265Irs1, Ser(P)-325Irs1, Thr(P)-346Irs1, Ser(P)-369Irs1, Ser(P)-522Irs1, and Ser(P)-637Irs1; Fig. 8A) were significantly less stimulated by insulin in cells pretreated with anisomycin, which diminished Tyr(P)Irs1 the most (by 58%) (Fig. 8D). Moreover, even in thapsigargin-treated cells, in which Tyr(P)Irs1 was diminished the least (24%), the average insulin-stimulated augmentation of Ser(P)/Thr(P) (versus insulin alone, n = 26 sites) was less than 1-fold (∼70%) (Fig. 8E). Thus, Ser/Thr phosphorylation that was promoted by metabolic stress in the basal period supplanted the normal program of Irs1 Ser/Thr phosphorylation stimulable by insulin.
To identify Ser(P)/Thr(P) residues that associate most strongly with reduced Tyr(P)Irs1 during drug-induced metabolic stress, we calculated the correlation between insulin-stimulated Tyr(P)Irs1 and each Ser(P)/Thr(P) observed in the absence or presence of anisomycin, thapsigargin, or tunicamycin (from data in Fig. 7C). Significant negative correlations were observed between Tyr(P)Irs1 and Ser(P)-302Irs1, Ser(P)-307Irs1, Ser(P)-336Irs1, Ser(P)-612Irs1, Ser(P)-632/5Irs1, and Ser(P)-891Irs1 (Fig. 8G). Of these, all but Ser(P)-612Irs1 were significantly stimulated by insulin alone (Fig. 8A); Ser(P)-302Irs1 and Ser(P)-307Irs1 were also negatively correlated to Tyr(P)Irs1 in the analysis of inhibitor data (Fig. 6F). By contrast, four Ser(P)/Thr(P) residues, Ser(P)-369Irs1, Thr(P)-525Irs1, Ser-632Irs1, and Ser(P)-1100Irs1, correlated positively with Tyr(P)Irs1 during metabolic stress (Fig. 8G), and of these only Ser(P)-369Irs1 and Ser(P)-632Irs1 were significantly stimulated by insulin alone (Fig. 8A). Negative correlation of Ser(P)/Thr(P) residues with Tyr(P)Irs1 during metabolic stress owed mostly to retained stimulation (augmentation) by insulin in both anisomycin and tunicamycin-treated cells and does not necessarily indicate involvement in the reduction of Tyr(P)Irs1. Regardless, our data suggest that metabolic stress desensitizes Tyr(P)Irs1 in a fashion similar to insulin—potentially through such “common” insulin and stress-induced Ser(P)/Thr(P) residues as Ser(P)-302Irs1 and Ser(P)-307Irs1.
DISCUSSION
We used our αpS/TmAbIrs1 library to quantify about half of all Irs1 Ser(P)/Thr(P) so far identified via mass spectrometry, both during insulin stimulation and under conditions of drug-induced metabolic stress. Numerous cell-based studies reveal Irs1 Ser(P)/Thr(P) to be a physiologically integrative mechanism that modulates in vivo insulin sensitivity (6). In our CHOIR/Irs1 cell-based system, insulin was clearly an important input to Irs1 Ser/Thr phosphorylation, because the vast majority of Ser(P)/Thr(P) residues assayed were stimulated by insulin and were diminished by inhibition of the insulin-stimulated PI3K → Akt → mTOR cascade. Moreover, the Irs1 Ser(P)/Thr(P) patterns produced during drug-induced metabolic stress correlated significantly with that stimulated by insulin. These data suggest that Irs1 Ser(P)/Thr(P) is foremost a feedback mechanism that develops during insulin stimulation but that this mechanism can be co-opted by metabolic stress, such as ER stress or inflammation, to inhibit insulin signaling and promote metabolic disease (6, 17, 19, 20, 22, 23, 34). An implicit corollary is that hyperinsulinemia may be an important physiologic mediator of insulin resistance in animals, and there is some experimental evidence that this is so (18).
Activation of PI3K signaling by insulin is the unique province of the IRS proteins, and PI3K activity was required for nearly all insulin-stimulated Ser(P)/Thr(P) detectable by our αpS/TmAbIrs1 library. By contrast, a significant effect of MAP kinase inhibition was not observed. Because earlier reports identified a contribution of MEK → ERK signaling to Irs1 Ser(P)/Thr(P), the pattern of phosphorylation might depend upon the cell or tissue background or upon the method of detection (72). Nonetheless, it is noteworthy that insulin-stimulated MAP kinase activity can be mediated via pathways other than IRS and remains unimpaired in insulin-resistant human tissue (82).
The inhibition of PI3K with diverse inhibitors produced somewhat different patterns of insulin-stimulated Irs1 phosphorylation, reflecting in part the effects on downstream kinases that “feed back” upon Irs1 (6). PI-103, which inhibits directly the activity of both class I PI3K and mTOR, inhibited nearly all the Irs1 Ser(P)/Thr(P) residues detected by our αpS/TmAbIrs1. Other, more specific inhibitors of PI3K produced less complete Ser(P)/Thr(P) inhibition; however, these differences vis-à-vis PI-103 could not clearly be ascribed to greater activity of mTORC1 alone (Fig. 5, A and B). Previous work has shown that the PI3K → PKCλ/ζ cascade can mediate phosphorylation Ser-318Irs1, Ser-498Irs1, and Ser-570Irs1 in cultured cells. The use of additional inhibitors of this or other pathways thus has the potential to further resolve the complexity of insulin-stimulated Irs1 Ser/Thr phosphorylation (6). With our well characterized αpS/TmAbIrs1, experiments can further be conducted in murine and human tissues to investigate the regulation of Irs1 Ser(P)/Thr(P) under ordinary and pathological conditions.
Inhibition of the PI3 kinase cascade during insulin stimulation both diminished Irs1 Ser(P)/Thr(P) residues and enhanced Tyr(P)Irs1 (Fig. 6, A–C); compatible with the latter, the insulin-stimulated association of SH2 domain protein p85 with Irs1 was also enhanced (Fig. 6D). The similar inhibitor concentrations required to achieve these dual effects (i.e. ED50pS/T versus ED50pTyr; Fig. 6E) suggest that one or more Irs1 Ser(P)/Thr(P) residues either negatively regulated Tyr(P)Irs1 accumulation or else promoted the dephosphorylation of Irs1 tyrosine residues. Regardless of the exact mechanism(s), the enhancement of Tyr(P)Irs1 apparently stemmed largely from diminished feedback by insulin-stimulated kinases, because Irs1 Ser(P)/Thr(P) residues were low—and generally not further diminished by inhibitors—in the insulin-free basal state (Fig. 4, A–J). A possible exception to this rule was Ser(P)-307Irs1, which was clearly somewhat reduced in the basal state by inhibitors of the PI3K cascade.
By analyzing the relationship between Tyr(P)Irs1 and Ser(P)/Thr(P) signals in the presence of diverse inhibitors, we detected significant negative correlation of Tyr(P)Irs1 with several Ser(P)/Thr(P) residues proximal to the Irs1 PTB domain, including Ser(P)-302Irs1, Ser(P)-307Irs1, Ser(P)-318Irs1, Ser(P)-325Irs1, and Thr(P)-346Irs1. The other insulin-stimulated Ser(P)/Thr(P) residues might also have effects, although they did not reach statistical significance in this experiment. Insulin-stimulated Ser(P)/Thr(P) near the PTB region might preferentially impair interaction of Irs1 with the insulin receptor in CHOIR/Irs1 cells, as proposed originally for Ser(P)-307Irs1 (17, 50). Potentially, additional or distinct Ser(P)/Thr(P) residues could have significant inhibitory effects upon Tyr(P)Irs1 in other cell types or under different physiologic conditions.
A large number of Ser(P)/Thr(P) residues provide the means for multiple kinases to affect Tyr(P)Irs1 and modulate insulin action, yet the PI3 kinase cascade is central to the insulin response in all cells. Prominent events that follow PI3K activation include PDK1 → Thr(P)-308Akt and mTORC2 → p473Akt that activate Akt. Of eight Ser(P)/Thr(P) residues inhibited significantly by an Akt inhibitor (AKT-VIII), five of them, Ser(P)-265Irs1, Ser(P)-302Irs1, Ser(P)-325Irs1, Ser(P)-332Irs1, and Ser(P)-522Irs1, lie within a strong consensus Akt targeting sequence (RXRXXS) (83, 84), supporting previous reports that these sites are phosphorylated directly by Akt (19, 85).
Inhibition of mTOR using rapamycin or PP242 did not yield a long nor identical list of inhibited Ser(P)/Thr(P) residues in CHOIR/Irs1 cells, although each inhibitor significantly enhanced Tyr(P)Irs1 (Fig. 4B). The maximal Tyr(P)Irs1 enhancement achieved with rapamycin was somewhat less than that with PP242. Consistent with these data, mTORC1 inhibition by rapamycin significantly inhibited only Ser(P)-307Irs1 and Ser(P)-318Irs1, although other Ser(P)/Thr(P) residues, such as Ser(P)-302Irs1, were somewhat reduced. In contrast, the catalytic mTORC1 and mTORC2 inhibitor PP242 significantly inhibited six Ser(P)/Thr(P) residues: three by greater than 90% (Ser(P)-302Irs1, Ser(P)-307Irs1, and Ser(P)-318Irs1) and three others by slightly more than 50% (Ser(P)-325Irs1, Ser(P)-408Irs1, and Ser(P)-1215Irs1).
Remarkably, the set of PP242-inhibited sites included four of the five Ser(P)/Thr(P) residues proximal to the Irs1 PTB domain that showed significant anti-correlation with Tyr(P)Irs1 across all conditions. There is currently some uncertainty about the mechanism of mTORC2 regulation by insulin (86), because both positive and negative feedback loops involving Akt and mTORC1 → S6K signaling have been demonstrated in different cell types (66, 87). However, a growing literature connects mTORC2 to the proteasome-mediated degradation of Irs1 in cultured cells (88, 89) and possibly in animal tissues (11). The mechanism described in cultured cells involves mTORC2 phosphorylation and stabilization of Fbxw8 (F-box and WD repeat domain containing 8), the substrate-targeting subunit of cullin-RING ligase CRL7 (88), but also relies upon phosphorylation of Ser-302Irs1, Ser-307Irs1, and Ser-522Irs1 (90). We observed significant inhibition of Ser(P)-302Irs1 (together with Ser(P)-325Irs1, Ser(P)-408Irs1, and Ser(P)-1215Irs1) by PP242, but not by rapamycin, suggesting that these sites lie downstream of mTORC2. Nonetheless, Irs1 phosphorylated at Ser-302Irs1 was previously observed to accumulate during insulin stimulation in Sin1−/− mouse embryo fibroblasts that lack mTORC2 activity (88). Because we observed at least some inhibition of Ser(P)-302Irs1 in rapamycin-treated cells and significant inhibition of Ser(P)-302Irs1 by the S6K-specific inhibitor DG2, a reasonable conclusion is that Ser(P)-302Irs1 in our experiments is mediated by the mTORC1 → S6K pathway. This conclusion is supported by the previously described in vitro and in vivo phosphorylation of Ser-302Irs1 by S6K1 (68).
The paucity of insulin-stimulated Ser(P)/Thr(P) residues inhibited by rapamycin in CHOIR/Irs1 cells is surprising because activation of mTORC1 → S6K signaling in tuberous sclerosis complex (TSC)-deficient mouse embryo fibroblasts causes robust Irs1 Ser(P)/Thr(P) and Irs1 degradation (67). Our studies with DG2 further identified only Ser(P)-302Irs1 as a significant S6K site, and the effect of DG2 to enhance Tyr(P)Irs1 was nominally less than that of other inhibitors. Compatible with these data, we never observed significant disappearance of Irs1 protein during insulin stimulation. We conclude that, at least in our CHOIR/Irs1 cell system, conditions of insulin stimulation do not equate to the dramatically enhanced activation of mTORC1 following TSC deletion. Potentially, chronic in vivo nutrient excess and/or ER stress might better replicate the TSC-deficient mouse embryo fibroblast cell.
Metabolic stress present in the physiologic setting of obesity, such as that from nutrient excess, ER stress, or inflammation, has the potential to impair insulin signaling by altering the ambient Ser(P)/Thr(P) patterns of the IRS proteins (6). To model the effects of metabolic stress, we treated CHOIR/Irs1 cells with anisomycin, thapsigargin, or tunicamycin. These agents directly activate Ser/Thr kinase pathways that mediate nutrient signaling, the ER stress response, and inflammatory cytokine signals in tissues (Fig. 7A). Applied to CHOIR/Irs1 cells in the basal state, anisomycin and thapsigargin had a significant insulin-like effect to cause broad Irs1 Ser/Thr phosphorylation, which was less pronounced in the case of tunicamycin. During subsequent insulin stimulation, drug-induced metabolic stress significantly reduced Tyr(P)Irs1, along with many Ser(P)/Thr(P) residues promoted by the insulin-stimulated PI3K pathway (Fig. 8, D–F). However, analysis of the metabolic stress data uncovered several Ser(P)/Thr(P) residues: Ser(P)-302Irs1, Ser(P)-307Irs1, Ser(P)-336Irs1, Ser(P)-612Irs1, Ser(P)-632/5Irs1, and Ser(P)-891Irs1, that correlated significantly with reduced Tyr(P)Irs1 (Fig. 8G). The simplest explanation is that these Ser(P)/Thr(P) residues, which are mostly increased basally by stress (Fig. 8A), preferentially mediate the inhibition of insulin-stimulated Tyr(P)Irs1, and therefore also the feedback phosphorylation of Irs1 by insulin-stimulated Ser/Thr kinases. However, Ser(P)-612Irs1 was not strongly enhanced by metabolic stress alone, and it remains unclear, mechanistically, how Ser(P)-302Irs1, Ser(P)-307Irs1, and Ser(P)-336Irs1 remain sensitive to insulin stimulation, whereas Tyr(P)Irs1 is reduced during metabolic stress. Indeed, we cannot rule out that Irs1 bearing one or more “stress-augmented” Ser(P)/Thr(P) residues is merely less subject to dephosphorylation during insulin stimulation.
Previous studies in cultured cells reveal a positive effect for some Irs1 Ser(P)/Thr(P) residues on insulin signaling. Insulin-stimulated phosphorylation of human Ser-1223IRS1 (rat Ser-1216Irs1) appears to promote Tyr(P)Irs1 and PI3K binding to Irs1 by suppressing the formation of an Irs1·PTPN11 complex (23). In CHOIR/Irs1 cells, Ser(P)-1215Irs1 (and/or Ser(P)-1216Irs1; Fig. 1B) does not correlate significantly with Tyr(P)Irs1. Previous reports suggest that insulin-stimulated phosphorylation of Ser-302Irs1 and Ser-318Irs1 initially enhances insulin signaling, but at later times correlates with reduced signaling (17). These data suggest that the temporal pattern of IRS1 Ser(P)/Thr(P) has a role in determining “positive” or “negative” outcomes; however, the mechanisms at play in insulin-resistant tissues remain difficult to resolve. Encouragingly, three of the Ser(P)/Thr(P) residues negatively correlated with Tyr(P)Irs1 in CHOIR/Irs1 cell experiments, Ser(P)-302Irs1, Ser(P)-307Irs1, and Ser(P)-612Irs1, show a similar insulin-desensitizing function in mice, as revealed by transgenic mice expressing Irs1 mutated at these sites (58). However, a contradictory positive role in insulin signaling was inferred for Ser-307Irs1 in a separate knock-in mouse line (57). Definitive evidence regarding the role of these and other Ser(P)/Thr(P) residues will likely require improved understanding of the Ser(P)/Thr(P) patterns present within tissues, revealed by our αpS/TmAbIrs1, as well as additional experiments in transgenic or knock-in mice.
This work was supported, in whole or in part, by National Institutes of Health Grants DK38712, DK55326, DK098655 and GM021700 (to M. F. W.). This work was also supported by an award from the Juvenile Diabetes Foundation International.
- IRS1
- human insulin receptor substrate 1
- Irs1
- rat/mouse insulin receptor substrate 1
- GRB
- growth factor receptor-bound protein
- GSK3
- glycogen-synthase kinase 3
- IR
- insulin receptor
- mTOR
- mechanistic target of rapamycin
- mTORC1 (mTOR·RAPTOR·mLST8·PRAS40)
- mTOR complex 1
- mTORC2 (mTOR·RICTOR·mLST8·SIN1)
- mTOR complex 2
- PDK1
- 3-phosphoinositide-dependent kinase
- PTB
- phosphotyrosine-binding
- Tyr(P)Irs1
- IRS1 tyrosine phosphorylation
- S6
- ribosomal S6 protein
- S6K
- ribosomal S6 protein kinase
- TSC
- tuberous sclerosis complex
- ER
- endoplasmic reticulum.
REFERENCES
- 1. Reaven G. (2004) The metabolic syndrome or the insulin resistance syndrome?: different names, different concepts, and different goals. Endocrinol. Metab. Clin. North Am. 33, 283–303 [DOI] [PubMed] [Google Scholar]
- 2. Biddinger S. B., Kahn C. R. (2006) From mice to men: insights into the insulin resistance syndromes. Annu. Rev. Physiol. 68, 123–158 [DOI] [PubMed] [Google Scholar]
- 3. DeFronzo R. A., Tripathy D. (2009) Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 32, S157–S163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Karlsson H. K., Zierath J. R. (2007) Insulin signaling and glucose transport in insulin resistant human skeletal muscle. Cell Biochem. Biophys. 48, 103–113 [DOI] [PubMed] [Google Scholar]
- 5. Samuel V. T., Shulman G. I. (2012) Mechanisms for insulin resistance: common threads and missing links. Cell 148, 852–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Copps K. D., White M. F. (2012) Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 55, 2565–2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blättler S. M., Cunningham J. T., Verdeguer F., Chim H., Haas W., Liu H., Romanino K., Rüegg M. A., Gygi S. P., Shi Y., Puigserver P. (2012) Yin yang 1 deficiency in skeletal muscle protects against rapamycin-induced diabetic-like symptoms through activation of insulin/IGF signaling. Cell Metab. 15, 505–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hsu P. P., Kang S. A., Rameseder J., Zhang Y., Ottina K. A., Lim D., Peterson T. R., Choi Y., Gray N. S., Yaffe M. B., Marto J. A., Sabatini D. M. (2011) The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science 332, 1317–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ersoy B. A., Tarun A., D'Aquino K., Hancer N. J., Ukomadu C., White M. F., Michel T., Manning B. D., Cohen D. E. (2013) Phosphatidylcholine transfer protein interacts with thioesterase superfamily member 2 to attenuate insulin signaling. Sci. Signal. 6, ra64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Song R., Peng W., Zhang Y., Lv F., Wu H. K., Guo J., Cao Y., Pi Y., Zhang X., Jin L., Zhang M., Jiang P., Liu F., Meng S., Zhang X., Jiang P., Cao C. M., Xiao R. P. (2013) Central role of E3 ubiquitin ligase MG53 in insulin resistance and metabolic disorders. Nature 494, 375–379 [DOI] [PubMed] [Google Scholar]
- 11. Scheufele F., Wolf B., Kruse M., Hartmann T., Lempart J., Muehlich S., Pfeiffer A. F., Field L. J., Charron M. J., Pan Z. Q., Engelhardt S., Sarikas A. (2014) Evidence for a regulatory role of Cullin-RING E3 ubiquitin ligase 7 in insulin signaling. Cell Signal. 26, 233–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhu H., Shyh-Chang N., Segrè A. V., Shinoda G., Shah S. P., Einhorn W. S., Takeuchi A., Engreitz J. M., Hagan J. P., Kharas M. G., Urbach A., Thornton J. E., Triboulet R., Gregory R. I., Altshuler D., Daley G. Q. (2011) The Lin28/let-7 axis regulates glucose metabolism. Cell 147, 81–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mandavia C., Sowers J. R. (2012) Phosphoprotein phosphatase PP2A regulation of insulin receptor substrate 1 and insulin metabolic signaling. Cardiorenal Med. 2, 308–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goldstein B. J., Bittner-Kowalczyk A., White M. F., Harbeck M. (2000) Tyrosine dephosphorylation and deactivation of insulin receptor substrate-1 by protein-tyrosine phosphatase 1B: possible facilitation by the formation of a ternary complex with the Grb2 adaptor protein. J. Biol. Chem. 275, 4283–4289 [DOI] [PubMed] [Google Scholar]
- 15. Gupta A., Dey C. S. (2012) PTEN, a widely known negative regulator of insulin/PI3K signaling, positively regulates neuronal insulin resistance. Mol. Biol. Cell 23, 3882–3898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. White M. F., Maron R., Kahn C. R. (1985) Insulin rapidly stimulates tyrosine phosphorylation of a Mr 185,000 protein in intact cells. Nature 318, 183–186 [DOI] [PubMed] [Google Scholar]
- 17. Weigert C., Kron M., Kalbacher H., Pohl A. K., Runge H., Häring H. U., Schleicher E., Lehmann R. (2008) Interplay and effects of temporal changes in the phosphorylation state of serine-302, -307, and -318 of insulin receptor substrate-1 on insulin action in skeletal muscle cells. Mol. Endocrinol. 22, 2729–2740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mehran A. E., Templeman N. M., Brigidi G. S., Lim G. E., Chu K. Y., Hu X., Botezelli J. D., Asadi A., Hoffman B. G., Kieffer T. J., Bamji S. X., Clee S. M., Johnson J. D. (2012) Hyperinsulinemia drives diet-induced obesity independently of brain insulin production. Cell Metab. 16, 723–737 [DOI] [PubMed] [Google Scholar]
- 19. Giraud J., Haas M., Feener E. P., Copps K. D., Dong X., Dunn S. L., White M. F. (2007) Phosphorylation of Irs1 at SER-522 inhibits insulin signaling. Mol. Endocrinol. 21, 2294–2302 [DOI] [PubMed] [Google Scholar]
- 20. Yi Z., Langlais P., De Filippis E. A., Luo M., Flynn C. R., Schroeder S., Weintraub S. T., Mapes R., Mandarino L. J. (2007) Global assessment of regulation of phosphorylation of insulin receptor substrate-1 by insulin in vivo in human muscle. Diabetes 56, 1508–1516 [DOI] [PubMed] [Google Scholar]
- 21. Giraud J., Leshan R., Lee Y. H., White M. F. (2004) Nutrient-dependent and insulin-stimulated phosphorylation of insulin receptor substrate-1 on serine 302 correlates with increased insulin signaling. J. Biol. Chem. 279, 3447–3454 [DOI] [PubMed] [Google Scholar]
- 22. Langlais P., Yi Z., Finlayson J., Luo M., Mapes R., De Filippis E., Meyer C., Plummer E., Tongchinsub P., Mattern M., Mandarino L. J. (2011) Global IRS-1 phosphorylation analysis in insulin resistance. Diabetologia 54, 2878–2889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Luo M., Reyna S., Wang L., Yi Z., Carroll C., Dong L. Q., Langlais P., Weintraub S. T., Mandarino L. J. (2005) Identification of insulin receptor substrate 1 serine/threonine phosphorylation sites using mass spectrometry analysis: regulatory role of serine 1223. Endocrinology 146, 4410–4416 [DOI] [PubMed] [Google Scholar]
- 24. Yi Z., Luo M., Carroll C. A., Weintraub S. T., Mandarino L. J. (2005) Identification of phosphorylation sites in insulin receptor substrate-1 by hypothesis-driven high-performance liquid chromatography-electrospray ionization tandem mass spectrometry. Anal. Chem. 77, 5693–5699 [DOI] [PubMed] [Google Scholar]
- 25. Yi Z., Luo M., Mandarino L. J., Reyna S. M., Carroll C. A., Weintraub S. T. (2006) Quantification of phosphorylation of insulin receptor substrate-1 by HPLC-ESI-MS/MS. J. Am. Soc. Mass Spectrom. 17, 562–567 [DOI] [PubMed] [Google Scholar]
- 26. Kim J. A., Yeh D. C., Ver M., Li Y., Carranza A., Conrads T. P., Veenstra T. D., Harrington M. A., Quon M. J. (2005) Phosphorylation of Ser24 in the pleckstrin homology domain of insulin receptor substrate-1 by mouse pelle-like kinase/interleukin-1 receptor-associated kinase: cross-talk between inflammatory signaling and insulin signaling that may contribute to insulin resistance. J. Biol. Chem. 280, 23173–23183 [DOI] [PubMed] [Google Scholar]
- 27. Nawaratne R., Gray A., Jørgensen C. H., Downes C. P., Siddle K., Sethi J. K. (2006) Regulation of insulin receptor substrate 1 pleckstrin homology domain by protein kinase C: role of serine 24 phosphorylation. Mol. Endocrinol. 20, 1838–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Carlson C. J., White M. F., Rondinone C. M. (2004) Mammalian target of rapamycin regulates IRS-1 serine 307 phosphorylation. Biochem. Biophys. Res. Commun. 316, 533–539 [DOI] [PubMed] [Google Scholar]
- 29. Gao Z., Zuberi A., Quon M. J., Dong Z., Ye J. (2003) Aspirin inhibits serine phosphorylation of insulin receptor substrate 1 in tumor necrosis factor-treated cells through targeting multiple serine kinases. J. Biol. Chem. 278, 24944–24950 [DOI] [PubMed] [Google Scholar]
- 30. Werner E. D., Lee J., Hansen L., Yuan M., Shoelson S. E. (2004) Insulin resistance due to phosphorylation of IRS-1 at serine 302. J. Biol. Chem. 279, 35298–352305 [DOI] [PubMed] [Google Scholar]
- 31. Aguirre V., Uchida T., Yenush L., Davis R., White M. F. (2000) The c-Jun NH2-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser307. J. Biol. Chem. 275, 9047–9054 [DOI] [PubMed] [Google Scholar]
- 32. Rui L., Aguirre V., Kim J. K., Shulman G. I., Lee A., Corbould A., Dunaif A., White M. F. (2001) Insulin/IGF-1 and TNF-α stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J. Clin. Invest. 107, 181–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gao Z., Hwang D., Bataille F., Lefevre M., York D., Quon M. J., Ye J. (2002) Serine phosphorylation of insulin receptor substrate 1 by inhibitor κB kinase complex. J. Biol. Chem. 277, 48115–48121 [DOI] [PubMed] [Google Scholar]
- 34. Gual P., Grémeaux T., Gonzalez T., Le Marchand-Brustel Y., Tanti J. F. (2003) MAP kinases and mTOR mediate insulin-induced phosphorylation of insulin receptor substrate-1 on serine residues 307, 612 and 632. Diabetologia 46, 1532–1542 [DOI] [PubMed] [Google Scholar]
- 35. Greene M. W., Sakaue H., Wang L., Alessi D. R., Roth R. A. (2003) Modulation of insulin-stimulated degradation of human insulin receptor substrate-1 by serine 312 phosphorylation. J. Biol. Chem. 278, 8199–8211 [DOI] [PubMed] [Google Scholar]
- 36. Bouzakri K., Karlsson H. K., Vestergaard H., Madsbad S., Christiansen E., Zierath J. R. (2006) IRS-1 serine phosphorylation and insulin resistance in skeletal muscle from pancreas transplant recipients. Diabetes 55, 785–791 [DOI] [PubMed] [Google Scholar]
- 37. Moeschel K., Beck A., Weigert C., Lammers R., Kalbacher H., Voelter W., Schleicher E. D., Häring H. U., Lehmann R. (2004) Protein kinase C-ζ-induced phosphorylation of Ser318 in insulin receptor substrate-1 (IRS-1) attenuates the interaction with the insulin receptor and the tyrosine phosphorylation of IRS-1. J. Biol. Chem. 279, 25157–25163 [DOI] [PubMed] [Google Scholar]
- 38. Hennige A. M., Stefan N., Kapp K., Lehmann R., Weigert C., Beck A., Moeschel K., Mushack J., Schleicher E., Häring H. U. (2006) Leptin down-regulates insulin action through phosphorylation of serine-318 in insulin receptor substrate 1. FASEB J. 20, 1206–1208 [DOI] [PubMed] [Google Scholar]
- 39. Liberman Z., Eldar-Finkelman H. (2005) Serine 332 phosphorylation of insulin receptor substrate-1 by glycogen synthase kinase-3 attenuates insulin signaling. J. Biol. Chem. 280, 4422–4428 [DOI] [PubMed] [Google Scholar]
- 40. Waraich R. S., Weigert C., Kalbacher H., Hennige A. M., Lutz S. Z., Häring H. U., Schleicher E. D., Voelter W., Lehmann R. (2008) Phosphorylation of Ser357 of rat insulin receptor substrate-1 mediates adverse effects of protein kinase C-δ on insulin action in skeletal muscle cells. J. Biol. Chem. 283, 11226–11233 [DOI] [PubMed] [Google Scholar]
- 41. Liu Y. F., Herschkovitz A., Boura-Halfon S., Ronen D., Paz K., Leroith D., Zick Y. (2004) Serine phosphorylation proximal to its phosphotyrosine binding domain inhibits insulin receptor substrate 1 function and promotes insulin resistance. Mol. Cell Biol. 24, 9668–9681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mothe I., Van Obberghen E. (1996) Phosphorylation of insulin receptor substrate-1 on multiple serine residues, 612, 632, 662, and 731, modulates insulin action. J. Biol. Chem. 271, 11222–11227 [DOI] [PubMed] [Google Scholar]
- 43. De Fea K., Roth R. A. (1997) Protein kinase C modulation of insulin receptor substrate-1 tyrosine phosphorylation requires serine 612. Biochemistry 36, 12939–12947 [DOI] [PubMed] [Google Scholar]
- 44. Li J., DeFea K., Roth R. A. (1999) Modulation of insulin receptor substrate-1 tyrosine phosphorylation by an Akt/phosphatidylinositol 3-kinase pathway. J. Biol. Chem. 274, 9351–9356 [DOI] [PubMed] [Google Scholar]
- 45. Um S. H., Frigerio F., Watanabe M., Picard F., Joaquin M., Sticker M., Fumagalli S., Allegrini P. R., Kozma S. C., Auwerx J., Thomas G. (2004) Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 431, 200–205 [DOI] [PubMed] [Google Scholar]
- 46. Qiao L. Y., Zhande R., Jetton T. L., Zhou G., Sun X. J. (2002) In vivo phosphorylation of insulin receptor substrate 1 at serine 789 by a novel serine kinase in insulin-resistant rodents. J. Biol. Chem. 277, 26530–26539 [DOI] [PubMed] [Google Scholar]
- 47. Horike N., Takemori H., Katoh Y., Doi J., Min L., Asano T., Sun X. J., Yamamoto H., Kasayama S., Muraoka M., Nonaka Y., Okamoto M. (2003) Adipose-specific expression, phosphorylation of Ser794 in insulin receptor substrate-1, and activation in diabetic animals of salt-inducible kinase-2. J. Biol. Chem. 278, 18440–18447 [DOI] [PubMed] [Google Scholar]
- 48. Li Y., Soos T. J., Li X., Wu J., Degennaro M., Sun X., Littman D. R., Birnbaum M. J., Polakiewicz R. D. (2004) Protein kinase Cθ inhibits insulin signaling by phosphorylating IRS1 at Ser1101. J. Biol. Chem. 279, 45304–45307 [DOI] [PubMed] [Google Scholar]
- 49. Tremblay F., Brûlé S., Hee Um S., Li Y., Masuda K., Roden M., Sun X. J., Krebs M., Polakiewicz R. D., Thomas G., Marette A. (2007) Identification of IRS-1 Ser-1101 as a target of S6K1 in nutrient- and obesity-induced insulin resistance. Proc. Natl. Acad. Sci. U.S.A. 104, 14056–14061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Aguirre V., Werner E. D., Giraud J., Lee Y. H., Shoelson S. E., White M. F. (2002) Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J. Biol. Chem. 277, 1531–1537 [DOI] [PubMed] [Google Scholar]
- 51. Ozcan L., Ergin A. S., Lu A., Chung J., Sarkar S., Nie D., Myers M. G., Jr., Ozcan U. (2009) Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 9, 35–51 [DOI] [PubMed] [Google Scholar]
- 52. Jiang G., Dallas-Yang Q., Biswas S., Li Z., Zhang B. B. (2004) Rosiglitazone, an agonist of peroxisome-proliferator-activated receptor γ (PPARγ), decreases inhibitory serine phosphorylation of IRS1 in vitro and in vivo. Biochem. J. 377, 339–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Levkovitz Y., Ben-Shushan G., Hershkovitz A., Isaac R., Gil-Ad I., Shvartsman D., Ronen D., Weizman A., Zick Y. (2007) Antidepressants induce cellular insulin resistance by activation of IRS-1 kinases. Mol. Cell Neurosci. 36, 305–312 [DOI] [PubMed] [Google Scholar]
- 54. Zick Y. (2005) Ser/Thr phosphorylation of IRS proteins: a molecular basis for insulin resistance. Sci. STKE 2005, e4. [DOI] [PubMed] [Google Scholar]
- 55. Morino K., Petersen K. F., Dufour S., Befroy D., Frattini J., Shatzkes N., Neschen S., White M. F., Bilz S., Sono S., Pypaert M., Shulman G. I. (2005) Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J. Clin. Invest. 115, 3587–3593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Talbot K., Wang H. Y., Kazi H., Han L. Y., Bakshi K. P., Stucky A., Fuino R. L., Kawaguchi K. R., Samoyedny A. J., Wilson R. S., Arvanitakis Z., Schneider J. A., Wolf B. A., Bennett D. A., Trojanowski J. Q., Arnold S. E. (2012) Demonstrated brain insulin resistance in Alzheimer's disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J. Clin. Invest. 122, 1316–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Copps K. D., Hancer N. J., Opare-Ado L., Qiu W., Walsh C., White M. F. (2010) Irs1 serine 307 promotes insulin sensitivity in mice. Cell Metab. 11, 84–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Morino K., Neschen S., Bilz S., Sono S., Tsirigotis D., Reznick R. M., Moore I., Nagai Y., Samuel V., Sebastian D., White M., Philbrick W., Shulman G. I. (2008) Muscle-specific IRS-1 Ser → Ala transgenic mice are protected from fat-induced insulin resistance in skeletal muscle. Diabetes 57, 2644–2651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Alessi D. R., Cuenda A., Cohen P., Dudley D. T., Saltiel A. R. (1995) PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J. Biol. Chem. 270, 27489–27494 [DOI] [PubMed] [Google Scholar]
- 60. Knight Z. A., Gonzalez B., Feldman M. E., Zunder E. R., Goldenberg D. D., Williams O., Loewith R., Stokoe D., Balla A., Toth B., Balla T., Weiss W. A., Williams R. L., Shokat K. M. (2006) A pharmacological map of the PI3-K family defines a role for p110α in insulin signaling. Cell 125, 733–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Barnett S. F., Defeo-Jones D., Fu S., Hancock P. J., Haskell K. M., Jones R. E., Kahana J. A., Kral A. M., Leander K., Lee L. L., Malinowski J., McAvoy E. M., Nahas D. D., Robinson R. G., Huber H. E. (2005) Identification and characterization of pleckstrin-homology-domain-dependent and isoenzyme-specific Akt inhibitors. Biochem. J. 385, 399–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sehgal S. N. (2003) Sirolimus: its discovery, biological properties, and mechanism of action. Transplant Proc. 35, 7S–14S [DOI] [PubMed] [Google Scholar]
- 63. Feldman M. E., Apsel B., Uotila A., Loewith R., Knight Z. A., Ruggero D., Shokat K. M. (2009) Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 7, 371–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Okuzumi T., Fiedler D., Zhang C., Gray D. C., Aizenstein B., Hoffman R., Shokat K. M. (2009) Inhibitor hijacking of Akt activation. Nat. Chem. Biol. 5, 484–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zoncu R., Efeyan A., Sabatini D. M. (2011) mTOR: from growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 12, 21–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Humphrey S. J., Yang G., Yang P., Fazakerley D. J., Stöckli J., Yang J. Y., James D. E. (2013) Dynamic adipocyte phosphoproteome reveals that Akt directly regulates mTORC2. Cell Metab. 17, 1009–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Shah O. J., Wang Z., Hunter T. (2004) Inappropriate activation of the TSC/Rheb/mTOR/S6K cassette induces IRS1/2 depletion, insulin resistance, and cell survival deficiencies. Curr. Biol. 14, 1650–1656 [DOI] [PubMed] [Google Scholar]
- 68. Shah O. J., Hunter T. (2006) Turnover of the active fraction of IRS1 involves raptor-mTOR- and S6K1-dependent serine phosphorylation in cell culture models of tuberous sclerosis. Mol. Cell Biol. 26, 6425–6434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sarbassov D. D., Ali S. M., Sabatini D. M. (2005) Growing roles for the mTOR pathway. Curr. Opin. Cell Biol. 17, 596–603 [DOI] [PubMed] [Google Scholar]
- 70. Calleja V., Laguerre M., Parker P. J., Larijani B. (2009) Role of a novel PH-kinase domain interface in PKB/Akt regulation: structural mechanism for allosteric inhibition. PLoS. Biol. 7, e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hay N., Sonenberg N. (2004) Upstream and downstream of mTOR. Genes Dev. 18, 1926–1945 [DOI] [PubMed] [Google Scholar]
- 72. Tzatsos A., Kandror K. V. (2006) Nutrients suppress phosphatidylinositol 3-kinase/Akt signaling via raptor-dependent mTOR-mediated insulin receptor substrate 1 phosphorylation. Mol. Cell Biol. 26, 63–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhang J., Gao Z., Yin J., Quon M. J., Ye J. (2008) S6K directly phosphorylates IRS-1 on Ser-270 to promote insulin resistance in response to TNF-α signaling through IKK2. J. Biol. Chem. 283, 35375–35382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Skolnik E. Y., Batzer A., Li N., Lee C. H., Lowenstein E., Mohammadi M., Margolis B., Schlessinger J. (1993) The function of GRB2 in linking the insulin receptor to Ras signaling pathways. Science 260, 1953–1955 [DOI] [PubMed] [Google Scholar]
- 75. Seger R., Biener Y., Feinstein R., Hanoch T., Gazit A., Zick Y. (1995) Differential activation of mitogen-activated protein kinase and S6 kinase signaling pathways by 12-O-tetracanoylphorbol-13-acetate (TPA) and insulin. J. Biol. Chem. 270, 28325–28330 [DOI] [PubMed] [Google Scholar]
- 76. Boulton T. G., Nye S. H., Robbins D. J., Ip N. Y., Radziejewska E., Morgenbesser S. D., DePinho R. A., Panayotatos N., Cobb M. H., Yancopoulos G. D. (1991) ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell 65, 663–675 [DOI] [PubMed] [Google Scholar]
- 77. White M. F. (1997) The insulin signalling system and the IRS proteins. Diabetologia 40, s2–s17 [DOI] [PubMed] [Google Scholar]
- 78. Bouzakri K., Roques M., Gual P., Espinosa S., Guebre-Egziabher F., Riou J. P., Laville M., Le Marchand-Brustel Y., Tanti J. F., Vidal H. (2003) Reduced activation of phosphatidylinositol-3 kinase and increased serine 636 phosphorylation of insulin receptor substrate-1 in primary culture of skeletal muscle cells from patients with type 2 diabetes. Diabetes 52, 1319–1325 [DOI] [PubMed] [Google Scholar]
- 79. Cano E., Hazzalin C. A., Mahadevan L. C. (1994) Anisomycin-activated protein kinases p45 and p55 but not mitogen-activated protein kinases ERK-1 and -2 are implicated in the induction of c-fos and c-jun. Mol. Cell Biol. 14, 7352–7362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zinck R., Cahill M. A., Kracht M., Sachsenmaier C., Hipskind R. A., Nordheim A. (1995) Protein synthesis inhibitors reveal differential regulation of mitogen-activated protein kinase and stress-activated protein kinase pathways that converge on Elk-1. Mol. Cell Biol. 15, 4930–4938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ding W. X., Ni H. M., Gao W., Hou Y. F., Melan M. A., Chen X., Stolz D. B., Shao Z. M., Yin X. M. (2007) Differential effects of endoplasmic reticulum stress-induced autophagy on cell survival. J. Biol. Chem. 282, 4702–4710 [DOI] [PubMed] [Google Scholar]
- 82. Cusi K., Maezono K., Osman A., Pendergrass M., Patti M. E., Pratipanawatr T., DeFronzo R. A., Kahn C. R., Mandarino L. J. (2000) Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J. Clin. Invest. 105, 311–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Alessi D. R., Andjelkovic M., Caudwell B., Cron P., Morrice N., Cohen P., Hemmings B. A. (1996) Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 15, 6541–6551 [PMC free article] [PubMed] [Google Scholar]
- 84. Xue Y., Ren J., Gao X., Jin C., Wen L., Yao X. (2008) GPS 2.0, a tool to predict kinase-specific phosphorylation sites in hierarchy. Mol. Cell. Proteomics 7, 1598–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Paz K., Liu Y. F., Shorer H., Hemi R., LeRoith D., Quan M., Kanety H., Seger R., Zick Y. (1999) Phosphorylation of insulin receptor substrate-1 (IRS-1) by protein kinase B positively regulates IRS-1 function. J. Biol. Chem. 274, 28816–28822 [DOI] [PubMed] [Google Scholar]
- 86. Xie J., Proud C. G. (2013) Crosstalk between mTOR complexes. Nat. Cell Biol. 15, 1263–1265 [DOI] [PubMed] [Google Scholar]
- 87. Liu P., Gan W., Inuzuka H., Lazorchak A. S., Gao D., Arojo O., Liu D., Wan L., Zhai B., Yu Y., Yuan M., Kim B. M., Shaik S., Menon S., Gygi S. P., Lee T. H., Asara J. M., Manning B. D., Blenis J., Su B., Wei W. (2013) Sin1 phosphorylation impairs mTORC2 complex integrity and inhibits downstream Akt signalling to suppress tumorigenesis. Nat. Cell Biol. 15, 1340–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kim S. J., DeStefano M. A., Oh W. J., Wu C. C., Vega-Cotto N. M., Finlan M., Liu D., Su B., Jacinto E. (2012) mTOR complex 2 regulates proper turnover of insulin receptor substrate-1 via the ubiquitin ligase subunit Fbw8. Mol. Cell 48, 875–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Xu X., Keshwani M., Meyer K., Sarikas A., Taylor S., Pan Z. Q. (2012) Identification of the degradation determinants of insulin receptor substrate 1 for signaling cullin-RING E3 ubiquitin ligase 7-mediated ubiquitination. J. Biol. Chem. 287, 40758–40766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Xu X., Sarikas A., Dias-Santagata D. C., Dolios G., Lafontant P. J., Tsai S. C., Zhu W., Nakajima H., Nakajima H. O., Field L. J., Wang R., Pan Z. Q. (2008) The CUL7 E3 ubiquitin ligase targets insulin receptor substrate 1 for ubiquitin-dependent degradation. Mol. Cell 30, 403–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Cheng Z., Ke Y., Ding X., Wang F., Wang H., Wang W., Ahmed K., Liu Z., Xu Y., Aikhionbare F., Yan H., Liu J., Xue Y., Yu J., Powell M., Liang S., Wu Q., Reddy S. E., Hu R., Huang H., Jin C., Yao X. (2008) Functional characterization of TIP60 sumoylation in UV-irradiated DNA damage response. Oncogene 27, 931–941 [DOI] [PubMed] [Google Scholar]