Background: ROP18 is a Toxoplasma secreted Ser/Thr protein kinase important for acute virulence.
Results: ROP18 phosphorylates host p65 at Ser-468 and target this protein to the ubiquitin-dependent degradation.
Conclusion: ROP18 inhibits the host NF-κB pathway by promoting p65 degradation.
Significance: These findings reveal a novel molecular mechanism by which type I strain manipulates the host immune system to facilitate infection.
Keywords: Cell Biology, NF-κB (NF-KB), NF-kB Transcription Factor, Parasite, Parasitology, Pathogenesis, Signal Transduction
Abstract
The obligate intracellular parasite Toxoplasma gondii secretes effector molecules into the host cell to modulate host immunity. Previous studies have shown that T. gondii could interfere with host NF-κB signaling to promote their survival, but the effectors of type I strains remain unclear. The polymorphic rhoptry protein ROP18 is a key serine/threonine kinase that phosphorylates host proteins to modulate acute virulence. Our data demonstrated that the N-terminal portion of ROP18 is associated with the dimerization domain of p65. ROP18 phosphorylates p65 at Ser-468 and targets this protein to the ubiquitin-dependent degradation pathway. The kinase activity of ROP18 is required for p65 degradation and suppresses NF-κB activation. Consistently, compared with wild-type ROP18 strain, ROP18 kinase-deficient type I parasites displayed a severe inability to inhibit NF-κB, culminating in the enhanced production of IL-6, IL-12, and TNF-α in infected macrophages. In addition, studies have shown that transgenic parasites carrying kinase-deficient ROP18 induce M1-biased activation. These results demonstrate for the first time that the virulence factor ROP18 in T. gondii type I strains is responsible for inhibiting the host NF-κB pathway and for suppressing proinflammatory cytokine expression, thus providing a survival advantage to the infectious agent.
Introduction
Toxoplasma gondii is an obligate intracellular parasite and an important opportunistic human pathogen, particularly in patients with primary or acquired defects in T cell-mediated immunity (1, 2). The clinical symptoms of T. gondii infections in humans range from mild flu-like symptoms in most people to severe complications in immunocompromised individuals or, after transplacental transmission, to a fetus (3, 4). Because tachyzoites infect any cell type and tissue and replication leads to cell lysis, this parasite has a tremendous potential to cause disease. The innate immune response limits parasite growth and promotes the development of adaptive immunity, which is required for long term resistance to infection (2, 5). In turn, T. gondii interferes with the host signaling pathways in the infected cells, enabling the parasite to evade the innate immune response.
NF-κB is a family of dimeric transcription factors and central components of innate and adaptive immunity responsible for the activation of many genes required in infection, stress, and injury. The NF-κB family of transcription factors is comprised of five members: p50 (NF-κB1), p52 (NF-κB2), p65 (RelA), RelB, and c-Rel (6). In the absence of inflammatory stimuli, NF-κB is maintained in an inactive form through binding to an inhibitor of NF-κB (IκB).4 Once the cells are stimulated, IκB is rapidly phosphorylated, ubiquitinated, and degraded, facilitating the subsequent translocation of NF-κB to the nucleus to induce a wide array of genes critical in the immune response and inflammation (7, 8).
There have been several controversial studies claiming both the inhibition and activation of host NF-κB pathway by T. gondii (9). Some studies have shown that type I strains inhibit NF-κB pathway and the recruitment and activation of immune cells, resulting in the enhanced survival of the parasites (10–14). These studies showed that infection of mammalian cells with the type I strain results in the activation of IκB kinase and degradation of IκB, an event involved in the nuclear translocation of p65/RelA to activate NF-κB. However, despite the initiation of NF-κB signaling, infection with T. gondii did not lead to the activation of NF-κB but to its termination. The reason for disabling NF-κB is associated with blocking of p65 translocation to the nucleus (10, 12, 13). Other studies have demonstrated that T. gondii activates NF-κB, which up-regulates the expression of anti-apoptotic genes to facilitate the replication of the pathogen (15–17). These results suggested that type I strains promoted the phosphorylation of IκB and induced nuclear translocation of p65 (14, 16, 18). Therefore, studies of the nuclear translocation of p65 in type I strain infection have yielded conflicting results. Importantly, none of these studies demonstrated which effectors of the type I strain manipulate the host NF-κB signaling to elicit a survival response during infection. The ROP18 kinase has been identified as a key virulence determinant conferring a high mortality phenotype of type I strains. Accordingly, we screened the ROP18 interacting host proteins using the yeast two-hybrid method. To our surprise, p65 was found as a target protein of ROP18. Then we sought to elucidate the relationship between the kinase activity of ROP18 and p65 degradation. Furthermore, we investigated ROP18-mediated host NF-κB suppression and the phenotype of infected macrophages.
Our results presented here showed that ROP18 phosphorylates p65 at Ser-468 to promote its ubiquitin-dependent degradation; thus, the nuclear localization of p65 cannot be obviously observed in cells infected with type I strains, consistent with previous studies (9, 12, 13, 19, 20). Our data demonstrated that infection with T. gondii type I results in p65 ubiquitin-dependent degradation, which blocks the nuclear translocation of p65 and induces the consequent termination of the NF-κB pathway. Therefore, the study presented here gave a reasonable explanation for the initiation and termination of NF-κB pathway by T. gondii type I infection (10). Consistently, compared with wild-type ROP18 strain, kinase-deficient ROP18 type I parasites displayed a severe inability to inhibit the NF-κB pathway, culminating in the enhanced production of IL-6, IL-12, and TNF-α. In addition, transgenic parasites carrying kinase-deficient ROP18 parasites induced M1-biased activation. The data indicated for the first time that the T. gondii type I virulence factor ROP18 is responsible for inhibiting the host NF-κB pathway and for suppressing proinflammatory cytokine expression, thereby providing a survival advantage to this infectious agent.
EXPERIMENTAL PROCEDURES
Ethics Statement
Ethical permission was obtained from the Institutional Review Board of the Institute of Biomedicine at Anhui Medical University (permit number AMU 26–093628), which records and regulates all research activities in the school. The Institutional Review Board of the Anhui Medical University approved both animals and humans protocols. The approval from the Institutional Review Board includes the permission of using mouse under euthanasia, and all the experimental procedures were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Cells, Mice, and Parasites
The 3–4-week-old female KunMing mice or BALB/c mice used in this study were obtained from the Experimental Animal Center of Anhui Province. All animal experiments were conducted with the approval of the Animal Care and Use Committee of Anhui Medical University. The wild-type ROP18-Ty1 RH strain (overexpressing wild-type ROP18 RH strain) and the kinase-deficient ROP18-Ty1 RH strain (overexpressing kinase-deficient ROP18 RH strain) were kindly provided by Professor J. F. Dubremetz (Universite de Montpellier, Montpellier, France). The Δku80Δhxgprt RH strain and ROP18 knock-out Δku80 RH strain were kindly provided by Professor John C. Boothroyd (Stanford University School of Medicine). The HFF (human foreskin fibroblast cell line), RAW264.7(mouse macrophage cell line), U937(human macrophage cell line), and HEK293T cell lines were purchased from the American Type Culture Collection (Manassas, VA) and cultured in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin in a humidified 5% CO2 atmosphere. All parasite strains and cell lines were routinely assessed for mycoplasma contamination, and no contamination was detected.
Reagents and Plasmids
Amplification of the open reading frame encoding T. gondii ROP18 (GenBankTM ID AM075204.1) was achieved through RT-PCR of the whole T. gondii tachyzoite RNA (21, 22). Point mutations were introduced using the QuikChange method (Stratagene). The plasmid vectors pGBKT7 (Clontech), pGADT7 (Clontech), 3× FLAG (Sigma), and pEGFP-C2 (BD Biosciences) were used to generate mammalian and yeast expression constructs carrying full-length ROP18, the N-terminal portion of ROP18 (amino acids 1–252), ROP18Δ27-WT (lacking signal peptide), ROP18Δ27-MUT (D394A, lacking signal peptide), ROP18Δ240 (lacking amino acids 1–240), p65-N1 (amino acids 1–285), p65-N2 (amino acids 1–190), p65-M (amino acids 190–285), and p65-C (amino acids 285–550). Site-directed mutagenesis was performed using a standard molecular biology protocol. All constructs were confirmed through DNA sequencing. The full-length and deletion mutants for ROP18 or p65 were cloned into the bacterial expression plasmids pGEX-6P-1 (GE Healthcare) and pET-28a (Novagen). Lipopolysaccharides (LPS) were from Sigma, rhTNF-α and rmTNF-α were from R&D Systems (Minneapolis, MN), TRIzol reagent and PrimeScriptTM RT reagent kit were from Invitrogen, MG132 was from Millipore, three kinds of p65 antibodies were purchased from Abcam (ab7970, ab177895) and Upstate (06-418), anti-FLAG M2 gel, monoclonal anti-Ty1, monoclonal anti-GFP, and monoclonal anti-FLAG antibody M2 were from Sigma. Anti-SAG1 was from ViroStat, anti-CD86, anti-PD-L1, anti-PD-L2, and anti-MHC2 were from BD Biosciences, and anti-ubiquitin (#3936) and phospho-p65 Ser-468 (#3039) were from Cell Signaling. The IL-6, IL-12p40, and TNF-α levels were measured using an ELISA kit (R&D Systems). The reporter genes (3×κB-Luc and pFR-Luc) were kindly provided by Pro. Wancheng Li (University of Nebraska Medical Center).
Generation of Over-expressing ROP18-WT and MUT Type I T. gondii in ROP18 Knock-out Background
The 5′-UTR region of ROP18 from type I Toxoplasma genomic DNA was amplified by PCR (forward, 5′-GAACATCTGCTTCAGCAGTTGCACAGGGACGACGATCT-3′; the BglII site was underlined; reverse, 5′-CATGCCATGGCACAACTTTCACACAAACTGGACTGGGGTG-3′; the NcoI site was underlined). The 3′-UTR region of ROP18 was amplified with primers (forward, 5′-TCCCCCGGGAAGACTCAAAATGAAAAGGGGAACGTGGCG-3′; the SamI site is underlined; reverse, 5′-GCTCTAGACTGCTCACGCGCCCTTGTAGTTGT-3′; the XbaI site is underlined). Genomic DNA containing the ROP18 wild-type or mutant genes were amplified from the wild-type ROP18-Ty1 or the kinase-deficient ROP18-Ty1 RH strains (kindly provided by Professor J. F. Dubremetz, Universite de Montpellier, France) by PCR (forward, 5′-CATGCCATGGCTTCAGCAGTTGCACAGGGACGACGATCT-3′; the NcoI site is underlined; reverse, 5′-TCCCCCGGGGATAACAATTTCACACAGGAAACAGCT-3′ the SamI site is underlined) to generate a 6.95-kb-long fragment and digested as NcoI/SamI fragment. Then the PCR product of 5′-UTR digested with NcoI was connected with the NcoI/SamI fragment and was connected with the 3′-UTR digested with SamI. The 5′-UTR-TUB promoter-ROP18 -Ty1-HXGPRT-3′-UTR was amplified by PCR with the primers (forward, 5′-GAACATCTGCTTCAGCAGTTGCACAGGGACGACGATCT-3′; the BglII site was underlined; 5′-GCTCTAGACTGCTCACGCGCCCTTGTAGTTGT-3′; the XbaI site was underlined) and subcloned as the BglII/XbaI fragment. The 100-μg fragment was transfected into the Δku80Δhxgprt RH strain parasites (kindly provided by Professor John C. Boothroyd, Stanford University) by electroporation. Electroporation was done in a 2-mm cuvette (Bio-Rad) with 2 mm ATP (MP Biomedicals) and 5 mm GSH (EMD) in a Gene Pulser Xcell (Bio-Rad) with the following settings: 25 μm FD, 1.25 kV, ∞Ω. Stable integrants were selected in media with 50 μg/ml mycophenolic acid (Axxora) and 50 μg/ml xanthine (Alfa Aesar) and cloned by limiting dilution. Expression of ROP18 wild-type and mutant (D394A) were confirmed by PCR, immunofluorescence, and Western blotting.
In Vitro Phosphorylation Assay
The His-tagged full-length p65 and its truncates (p65-N1 and p65-C) were expressed in Escherichia coli strain BL21 (DE3) and purified by using Ni2+-Sepharose beads (Qiagen) as previously described (23, 24). For in vitro phosphorylation assay, anti-GFP immunoprecipitates from 293T cells transiently expressing ROP18-GFP were incubated with purified recombinant full-length p65 and its truncates (200 μg each) in kinase buffer (25 mm HEPES, pH 7.2, 1 mm DTT, 50 mm NaCl, 2 mm EGTA, 5 mm MgSO4) with 50 μm ATP and 0.5 μCi of [32P]ATP. The reaction mixtures (50 μl) were incubated at 30 °C for 30 min and terminated by adding SDS-PAGE sample buffer. Proteins were then fractionated on SDS-PAGE. The gel was stained with Coomassie Brilliant Blue and subsequently incubated with x-ray film (25).
Yeast Two-hybrid Analysis
All p65 variants constructed in pGADT7 (BD Bioscience) were mated with the AH109 strain transformed with pGBKT7-ROP1825–251. Briefly, the yeast cells were transformed with the bait construct using the lithium acetate method followed by the selection of bait-containing, auxotrophic yeast cells via the appropriate nutritional marker in the selection medium. The bait-containing cells were subsequently transformed with the library constructs, and the resulting transformants were grown on medium for the selection of either the expression of both AD and BD vectors or an interaction between the expressed fusion proteins via nutritional reporter gene expression. Clones expressing all three reporter genes, HIS3, ADE2, and x-gal, were further analyzed. Interactions between bait and prey were selected via colony growth on plates lacking tryptophan, leucine, and histidine.
GST Pulldown Assay
GST-ROP18 or GST was purified and conjugated to glutathione-Sepharose 4B beads. Purified His-p65-M was incubated with GST-ROP18-conjugated or GST-conjugated beads at 4 °C for 2 h. Subsequently, the beads were washed 3 times with pre-cooled PBS containing 1% Triton X-100 followed by 3 washes with PBS. The bound proteins were fixed in Laemmli loading buffer, incubated at 100 °C for 10 min, and subjected to 10% SDS-PAGE followed by immunoblot analysis.
Luciferase Assay
The cells were seeded onto 24-well plates and transfected with reporter plasmids and other plasmids as indicated. After transfection, the cells were treated with the indicated reagents or left untreated. Luciferase activity was evaluated using a Luciferase Assay System (Promega, Madison, WI) according to the manufacturer's protocol. The assays were performed in triplicate.
Immunofluorescence
The cells were harvested and plated onto glass coverslips. Subsequently, the cells were fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100 in PBS, and blocked using a solution containing 10% bovine serum albumin. The coverslips were incubated with anti-Ty1, anti-p65, and anti-GFP at 4 °C overnight. FITC-conjugated goat anti-mouse IgG, rhodamine-conjugated goat anti-rabbit IgG, and DAPI dye were used for antigen and DNA visualization. The images were captured using an Olympus BX60 Upright Fluorescence microscope with the appropriate filters and objectives and with identical acquisition parameters for each experiment.
Immunoprecipitation and Immunoblot Analysis
The 293T or HFF cells and parasites were lysed in lysis buffer (50 mm HEPES, pH 7.4, 150 mm NaCl, 2 mm EGTA, 1% Triton X-100, 1 mm phenylmethylsulfonyl fluoride, 10 g/ml leupeptin, and 10 g/ml pepstatin A) containing a protease inhibitor mixture (Sigma). The cell lysates were separated through SDS-PAGE, transferred to nitrocellulose membranes, probed with the corresponding antibodies, and developed using an ECL kit. For immunoprecipitation, the cell lysates were precleared using anti-FLAG M2 affinity gel for 4 h with rotation at 4 °C. The immunoprecipitants were washed three times with lysis buffer and three times with PBS and then eluted through boiling with Laemmli loading sample buffer. The eluates were separated using 10% SDS-PAGE and transferred to nitrocellulose membranes for immunoblotting.
Flow Cytometric Analysis
A total of 2 × 106 U937 cells were stained with phosphatidylethanolamine-conjugated anti-CD86, anti-CD273 (PD-L2), or anti-CD274 (PD-L1) antibodies or phosphatidylethanolamine-Cy5-conjugated anti-MHC Class II antibodies. The stained cells were analyzed on a FACS Canto II flow cytometer (BD Biosciences) using FCS Express 4 Plus Research Edition software.
ELISA Analysis
RAW264.7 cells were seeded (106 per well) onto 12-well plates and incubated overnight at 37 °C in 5% CO2. The cells were infected with freshly lysed T. gondii tachyzoites at an m.o.i. of 1 and treated or untreated with10 ng/ml TNF-α or 100 ng/ml LPS. After 6 h, the supernatants were collected and analyzed using an ELISA for mouse IL-12p40, TNF-α, or IL-6 (R&D Systems). The experiments were repeated three times, and the data represent the mean of five wells ± S.E.
RT-PCR
Total RNA was prepared from RH, ROP18-WT, ROP18-MUT, and ROP18 knock-out RH strains using TRIzol (Invitrogen) purification according to the manufacturer's instructions. Reverse transcription of purified RNA was performed using SuperScript III (Invitrogen). The RT-PCR was performed to assess ROP18 gene expression, normalized to the expression of the SAG1 using primers: ROP18, forward (5′-ATG TTT TCG GTA CAG CGG CCA CCT CTT A-3′) and reverse (5′-TTA TTC TGT GTG GAG ATG TT-3′); SAG1, forward (5′-ACA GAG TTG TAT GGT CAC GG-3′) and reverse (5′-TCG TCC CGG AAC AGT ACT GAT TCG-3′).
RESULTS
Identification of p65 as a ROP18-interacting Host Protein
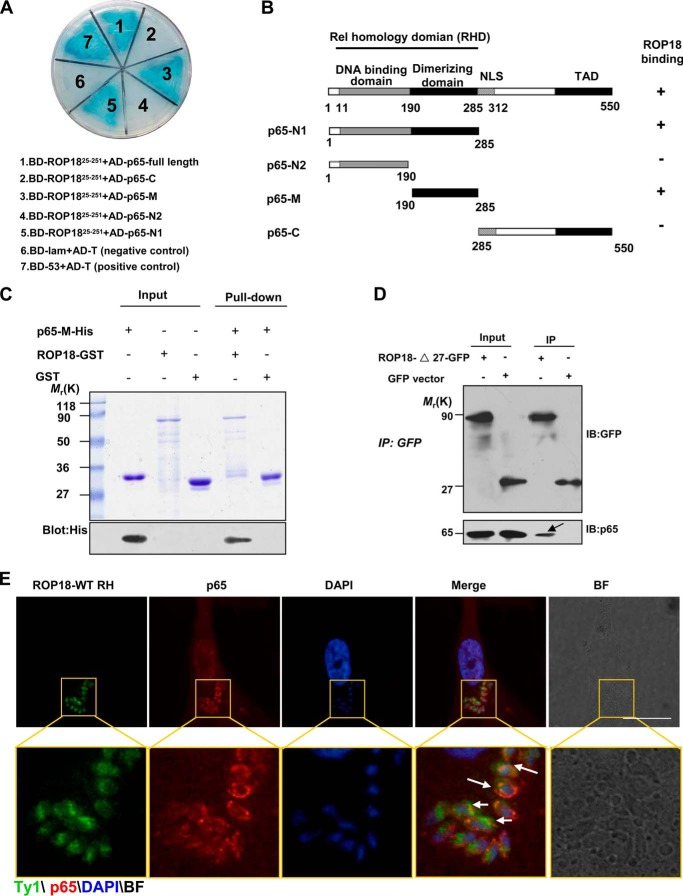
To identify host proteins targeted by the virulence factor ROP18, we performed a systematic yeast two-hybrid screening in which the cDNA fragment harboring the N-terminal portion of ROP18 (amino acids 1–252) was used as bait. Several positive clones encoding p65®elA were identified. In addition, the previously confirmed ROP18-interacting protein (e.g. ATF β) was screened out (21, 22). We further performed co-transformation experiments using p65 truncates to identify the region involved in the association with ROP18. According to the structure analysis of p65, four p65 deletions were generated as illustrated in Fig. 1B. The p65 full-length and deletion mutants were co-transformed with ROP1825–251 into yeast cells using β-galactosidase activity (lacZ reporter) as a measure of protein-protein interaction (Fig. 1A). As summarized in Fig. 1B, human p65 binds to ROP18 via its dimerization domain (amino acids 190–285). Thus, the yeast genetic assay suggested that human p65 is a novel potential binding partner for Toxoplasma virulence factor ROP18. To explore whether ROP18 directly interacts with p65, we performed an in vitro GST pulldown assay using recombinant ROP18-GST and His6-p65-M (amino acids 190–285) proteins expressed in E. coli. ROP18-GST, but not GST, was able to pull down His6-p65-M, demonstrating that ROP18 physically bound to the dimerization domain of p65 (Fig. 1C). To further confirm this result, we transfected ROP18-Δ27-GFP (GFP-tagged ROP18 lacking the signal peptide) into 293T cells for immunoprecipitation assay using an anti-GFP antibody. The results suggested that ROP18 indeed interacts with p65 in vivo (Fig. 1D). To address the physiological relevance of this interaction in mammalian cells, we used overexpressing wild-type ROP18 Ty1-tagged RH strain (26) and infected the primary HFFs with the parasites. Interestingly, we observed that p65 was recruited around the parasites and co-distributed with ROP18 (Fig. 1E), confirming co-localization of ROP18 with p65 in the cytosol of the infectious HFF cells. Therefore, these results indicate that ROP18 interacts directly with p65 in vivo.
FIGURE 1.
Identification of p65 as a ROP18-interacting host protein. A, identification of the interaction of ROP18 with p65 using a yeast two-hybrid system. Screening plasmids expressing the N-terminal portion of ROP18 (amino acids 25–251, lacking signal peptide and kinase domain) fused to the GAL4 DNA binding domain were cotransfected with a plasmid expressing full-length or truncated versions of p65 fused to the GAL4 transactivation domain. Interactions were detected through growth on medium containing x-α-gal and lacking Ade, Trp, Leu, and His. Yeast cells co-transformed with BD-53 and AD-T were used as positive controls, and yeast cells co-transformed with BD-lam and AD-T were used as the negative controls. The results revealed that the dimerizing domain of p65 interacts with ROP18. B, schematic illustration of the full-length and truncated versions of p65. RHD, Rel homology domain (containing DNA binding domain and dimerizing domain); NLS, nuclear localization signal; TAD, transactivation domain. C, confirmation of the site of p65 binding to ROP18 using in vitro pulldown assays. GST-ROP18 purified on glutathione beads was used as an affinity matrix for absorbing His6-tagged p65-M fragments (dimerization domain of p65, amino acids 190–285). The SDS-PAGE gel was stained with Coomassie Brilliant Blue (upper panel) and subsequently blotted with anti-His antibody (lower panel). D, verifying the interaction of ROP18 with p65 through immunoprecipitation. 293T cells were transfected with ROP18-Δ27-GFP or control GFP vector. At 24 h after transfection, the cells were immunoprecipitated with anti-GFP. Starting fractions (Input) and immunoprecipitates (IP) were analyzed by SDS-PAGE and Western blotting using GFP and p65 antibodies. The arrow indicates that ROP18 (upper panel) and p65 (lower panel) were co-immunoprecipitated. E, HFF cells grown on glass coverslips were infected with or without ROP18-WT-Ty1 RH strain at an m.o.i. of 1 for 24 h. Double immunofluorescence was performed using mouse monoclonal anti-Ty1 (green) and rabbit polyclonal anti-p65 (red). A distinctive pattern of p65 and ROP18 localization was observed around the parasite. BF, brightfield. Bars, 5 μm.
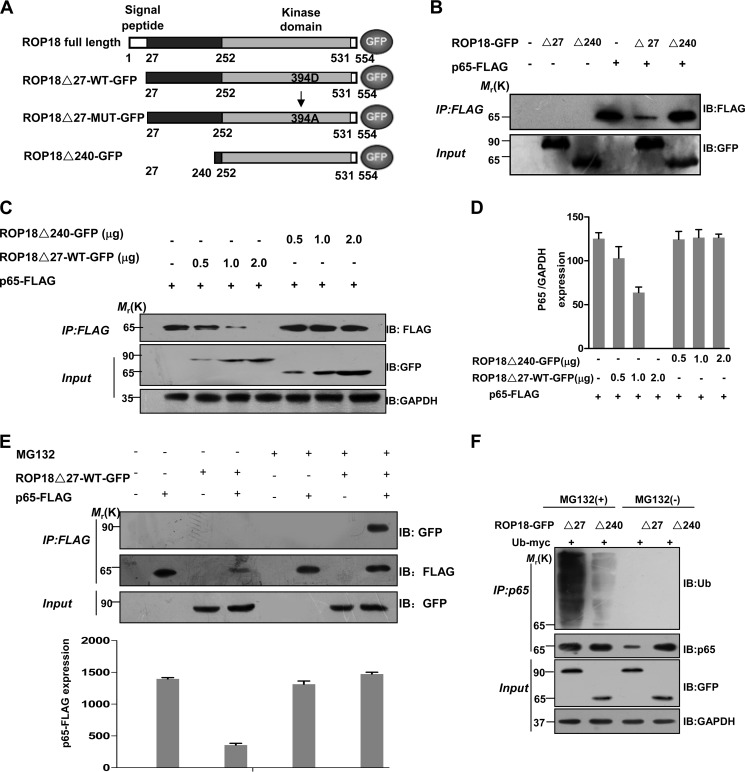
ROP18 Interacts with p65 and Mediates Its Proteasome-ubiquitin-dependent Degradation
To further assess the specificity of this interaction, we expressed GFP-tagged ROP18 lacking the signal peptide (ROP18Δ27-WT-GFP) and the N-terminal deletion of ROP18 (ROP18Δ240-GFP) together with FLAG-tagged p65 in 293T cells (Fig. 2A). Interestingly, we observed that the overexpression of ROP18Δ27-WT-GFP rather than ROP18Δ240-GFP dramatically reduced the level of FLAG-p65 (Fig. 2B). In addition, ROP18Δ27-WT-GFP, but not ROP18Δ240-GFP, reduced the levels of p65 in a dose-dependent fashion in dually transfected 293T cells (Fig. 2, C and D). To determine whether the ROP18-dependent decrease in p65 protein level is mediated through proteasomal degradation, FLAG-p65 was coexpressed with ROP18Δ27-WT-GFP in the presence of the proteasome inhibitor MG132. Under this condition, the level of p65 was stabilized, and the interaction between ROP18Δ27-WT-GFP and p65 was confirmed through coimmunoprecipitation (Fig. 2E). Additionally, the cells were co-transfected with the various ROP18 plasmids and myc-tagged ubiquitin for charactering the effect of ROP18-mediated p65 proteasomal degradation. As the result, ubiquitination of p65 was significantly increased by ROP18Δ27-WT-GFP, but not ROP18Δ240-GFP, in the presence of MG132 (Fig. 2F). Taken together, these results support that the N-terminal portion of ROP18 associates with p65 and targets it to a proteasome-ubiquitin-dependent degradation pathway.
FIGURE 2.
ROP18 interacts with p65 and mediates its proteasome-ubiquitin-dependent degradation. A, schematic illustration of ROP18 and its mutant GFP-tagged expression vectors. ROP18Δ27-WT-GFP means lacking signal peptide (amino acids 1–27) ROP18 wild-type GFP-tagged plasmid; ROP18Δ27-MUT-GFP means lacking signal peptide kinase-deficient ROP18 GFP-tagged plasmid; ROP18Δ240-GFP means lacking N-terminal portion of ROP18 (amino acids 1–240). B, lysates of 293T cells transiently cotransfected with 2 μg of the indicated GFP-tagged ROP18 and/or 2 μg of FLAG-tagged p65 expression vectors were immunoprecipitated (IP) with the indicated antibodies and detected through Western blotting (IB) with the indicated antibodies. C, lysates of 293T cells transiently cotransfected with the indicated volumes of the GFP-tagged ROP18Δ27-WT or ROP18Δ240 and/or 2 μg of FLAG-tagged p65 expression vectors were immunoprecipitated with the indicated antibodies and detected through Western blotting with the indicated antibodies. D, the levels of p65 in the above experiment were quantified using the mean ratio of p65 to GAPDH for triplicate results. Error bars represent the means ± S.D. of triplicates. E, lysates of 293T cells transiently cotransfected with 2 μg of GFP-tagged ROP18Δ27-WT and/or 2 μg of FLAG-tagged p65 expression vectors in the absence or presence of MG132 (10 μm) for 12 h were immunoprecipitated with the indicated antibodies and detected through Western blotting. The levels of p65 in the above experiment were quantified for triplicate results. Error bars represent the means ± S.D. of triplicates. F, ROP18-p65 interaction increases p65 ubiquitination. 293T cells were transfected as indicated and cultured for 24 h, then the cells were treated with or without MG132 MG132 (10 μm) for 4 h before the cells were harvested. After immunoprecipitation of p65, its ubiquitination was shown by immunoblotting.
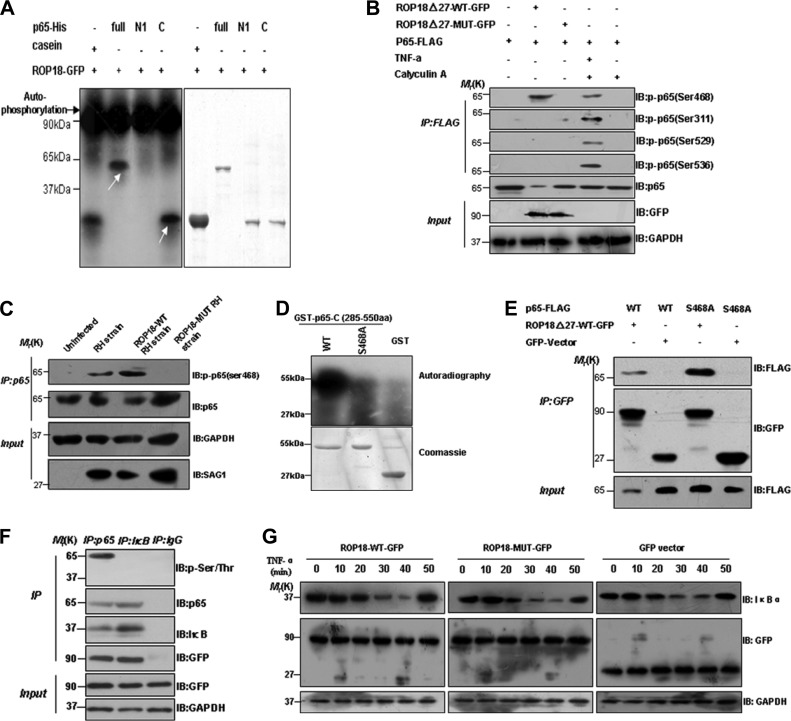
ROP18 Phosphorylates p65 at Ser-468 to Promotes Its Degradation
Although the kinase activity of ROP18 is essential for the virulence of type I parasites (22, 26–28), the biological significance of the kinase activity of ROP18 and NF-κB remains unknown. To determine if ROP18 phosphorylates p65, eukaryotic expression ROP18 was incubated with recombinant p65 and its truncates in a kinase assay in vitro (Fig. 3A). The observed autophosphorylation band confirmed that the eukaryotic expression of ROP18 has kinase activity (Fig. 3A, black arrow). Incubation of the recombinant p65 and its truncate proteins with [32P]ATP and GFP-tagged ROP18 resulted in the incorporation of 32P into the full-length and C-terminal portion of recombinant of p65 (Fig. 3A, white arrow). Thus, the results showed that ROP18 primarily phosphorylates the C- terminal portion of p65. To define the residues of p65 were phosphorylated by ROP18, we co-transfected the cells with GFP-tagged-ROP18-WT or MUT (kinase- deficient) and p65-FLAG plasmids and treated the cells with or without TNF-α. Calyculin A was used as the phosphatase inhibitor to block dephosphorylation. The results demonstrated that wild-type but not kinase-deficient ROP18 phosphorylated p65 at Ser-468, whereas other phosphor-p65 antibodies did not give rise to any signal (Fig. 3B), indicating that ROP18 may phosphorylate p65 at Ser-468. To determine whether ROP18 phosphorylates p65 at Ser-468 in vivo, HFF cells were infected at an m.o.i. of ∼1 with ROP18-WT RH, ROP18-MUT RH, or RH strain. After immunoprecipitation of p65, Western blotting revealed the Ser-468 phosphorylation of p65 from the ROP18-WT RH strain and RH strain compared with the ROP18-MUT RH strain (Fig. 3C). To confirm if Ser-468 is ROP18 substrate, we generated non-phosphorylate-able p65 by mutating serine 468 to alanine and repeated the in vitro phosphorylation experiment. We conclude that Ser-468 of p65 is the main substrate of ROP18 due to the fact that the phosphorylation of p65 S468A mutant proteins was significantly decreased by ROP18 in vitro judged by the 32P incorporation (Fig. 3D). To further evaluate whether ROP18 phosphorylation regulates p65 degradation in vivo, we constructed FLAG-tagged non-phosphorylate-able p65 S468A mutant and transfected them into 293T cells. Western blotting revealed that p65 S468A mutant, compared with p65-WT, was obviously detected in the ROP18 immunoprecipitation (Fig. 3E, upper panel). Our results strongly suggested that ROP18 phosphorylates p65 at Ser-468, facilitating p65 degradation.
FIGURE 3.
ROP18 phosphorylates p65 at serine 468 to promote its degradation. A, bacterially expressed His-full-length p65 and its truncates (p65-N1 and p65-C) as substrates. Both His-p65 full-length and truncate proteins were purified on Ni2+-Sepharose beads and phosphorylated in vitro using [32P]ATP and eukaryotic express ROP18 as described. Samples were separated by SDS-PAGE gel and stained with Coomassie Brilliant Blue-stained (right) and subsequently incubated with x-ray film. The autoradiogram was also shown (left). Casein was used as a positive control. Note that in the presence of ROP18, the dramatic autophosphorylation of ROP18 (black arrow) and the incorporation of 32P into full-length and the C-terminal portion of p65 protein were observed (white arrow). B, ROP18 phosphorylates p65 at serine 468. 293T cells were co-transfected with ROP18-Δ27-WT-GFP or ROP18-Δ27-MUT-GFP and p65-FLAG. At 24 h after transfection, the cells were treated with or without TNF-α (10 ng/ml) for 6 h and immunoprecipitated (IP) with FLAG antibodies. Calyculin A was used as the phosphatase inhibitor to block dephosphorylation. The p65 phosphorylation was detected by the indicated phospho-p65 antibodies. IB, immunoblot. C, using wild-type and kinase-deficient ROP18 expressing transgenic strains to determine that ROP18 phosphorylates p65 at Ser-468. The HFF cells were infected with the indicated parasites at an m.o.i. of 1. At 12 h after infection, immunoprecipitation (IP) of p65 from infected cell lysates was detected with phospho-p65 Ser-468 antibody. D, ROP18 phosphorylates p65 serine 468 in vitro. Bacterially expressed GST-p65-C fragments (amino acids 285–550) as substrates, both wild-type and S468A mutant GST-p65-C proteins, were purified on glutathione-agarose beads and phosphorylated in vitro using [32P]ATP and eukaryotic express ROP18 as described. Samples were separated by SDS-PAGE gel and stained with Coomassie Brilliant Blue (lower) and subsequently incubated with x-ray film. The autoradiogram was also shown (upper). Note that in the presence of ROP18 there was dramatic incorporation of 32P into wild-type, but not S468A mutant, GST-p65-C protein. E, verifying that ROP18 phosphorylation Ser-468 of p65 regulates it degradation. 293T cells were co-transfected with FLAG-tagged p65-wild-type or S468A mutant and ROP18-Δ27-WT-GFP. At 24 h after transfection, the cells were immunoprecipitated with anti-GFP and immunoblotted with the indicated antibodies, respectively. F, verifying that ROP18 kinase selectively phosphorylates p65 but not IκBα. 293T cells were transfected with ROP18Δ27-WT-GFP plasmid and immunoprecipitated with p65 or IκBα antibody. IgG antibody was used as the negative control. After immunoprecipitation, p65 and IκBα phosphorylations were detected by phospho-Ser/Thr antibody immunoblotting. G, ROP18 did not influence THF-α-induced IκBα degradation. Cells were transfected with ROP18 wild-type, kinase-deficient expression plasmid, and control vector, then treated with THF-α for the indicated times. Western blotting was performed on the cell extracts to check the degradation of IκBα.
As stated in the Introduction, p65 is maintained in an inactive form through binding to an inhibitor of NF-κB (IκBα) in the absence of inflammatory stimuli. Having demonstrated that p65 is a bona fide substrate of ROP18, we wanted to address whether ROP18 kinase also phosphorylates IκBα in vivo. To this end, we overexpressed ROP18Δ27-WT-GFP in 293T cells and immunoprecipitated with p65 or IκBα antibody followed by phosphoserine/threonine immunoblot analysis. This result revealed the presence of phosphorylated p65 only, supporting a selective phosphorylation of p65 rather than IκBα by ROP18 kinase (Fig. 3F). Further studies were subjected to confirmation of the effects of ROP18 kinase on IκBα degradation. Our results did not reveal any differences between ROP18 wild-type, ROP18 kinase-deficient, and control cells during TNF-α stimulation (Fig. 3G). These results together demonstrated that ROP18 performs its function via direct phosphorylation of p65 at Ser-468 to promote the substrate degradation.
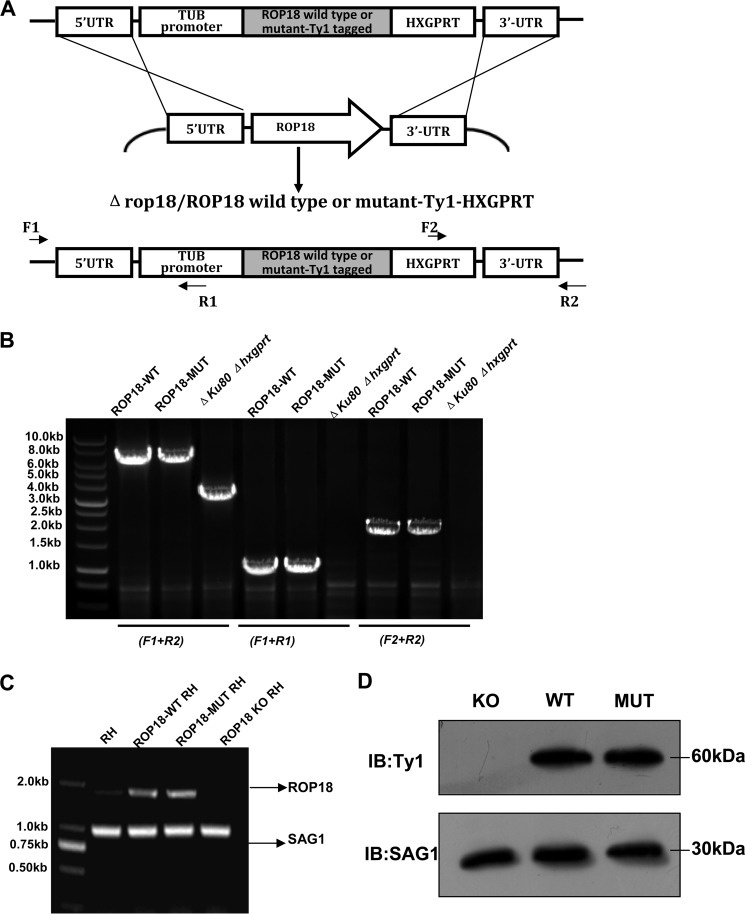
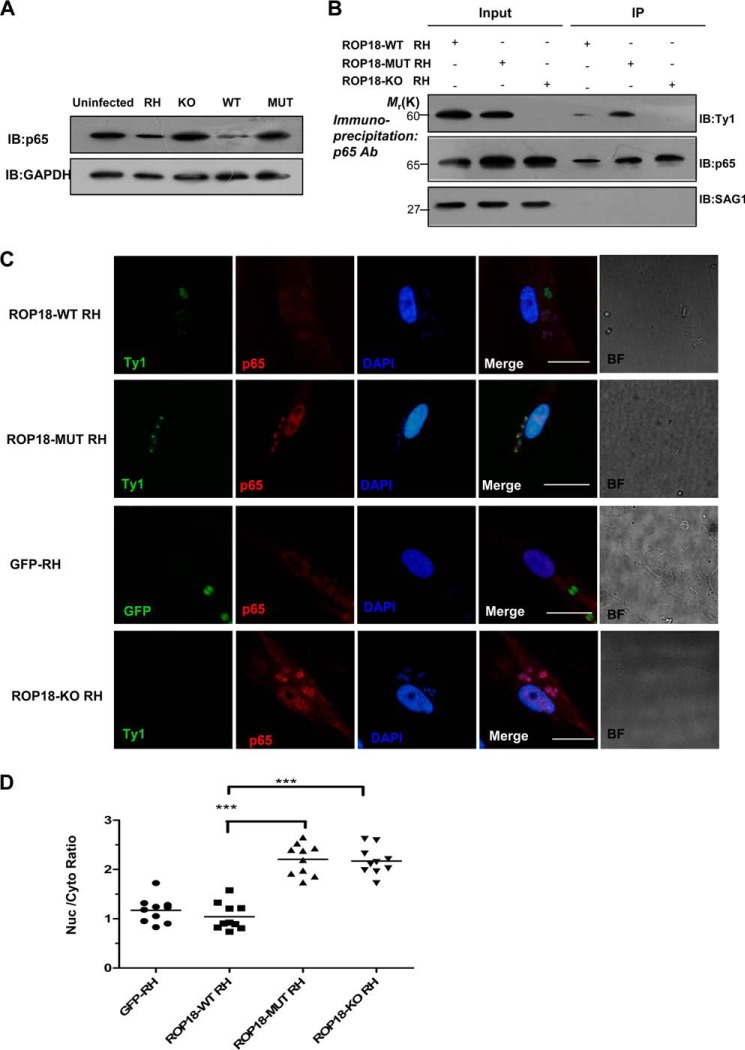
The Kinase Activity of ROP18 in the RH Strain Is Responsible for p65 Degradation
To assess whether ROP18 kinase activity plays a role in type I parasite-mediated pathogenicity, we constructed the transgenic strain parasites expressing wild-type ROP18-Ty1-tagged (ROP18-WT RH strain) or kinase-deficient ROP18-Ty1-tagged (ROP18-MUT RH strain) in ROP18 knock-out background. Clones were also tested by PCR for the presence of 5′ integration and 3′ integration and the insertion of TUB promoter-ROP18 -Ty1-HXGPRT cassette into ROP18 locus. Parental Δku80Δhxgprt RH strain was included as the control (Fig. 4, A and B). The expression of ROP18 or its mutant in RH, ROP18-WT RH, and ROP18-MUT RH strains was tested by RT-PCR and Western blotting (Fig. 4, C and D). To determine whether parasite infection also reduced p65 expression levels, HFF cells were infected with ROP18-WT, ROP18-MUT, or ROP18-KO RH parasites, and the result showed that p65 expression was consistently reduced in cells infected with ROP18-WT RH strain but not those infected with ROP18-MUT or ROP18-KO RH strain (Fig. 5A). Additionally, we analyzed ROP18 co-immunoprecipitates with endogenous p65 in infected cells to determine the interaction of ROP18 with p65 in vivo. HFF cells were infected at an m.o.i. of ∼1 with ROP18-WT, ROP18-MUT, or ROP18-KO RH strain. Western blotting showed that ROP18 from the ROP18-MUT, compared with ROP18-WT, was obviously detectable in the p65 immunoprecipitation (Fig. 5B, upper panel). In addition, ROP18-WT RH strain induced significant degradation of p65, whereas infection with ROP18-MUT strain did not reduce the levels of nuclear p65 in HFF cells (Fig. 5, C and D). Collectively, these results provide the first evidence that the host cellular factor p65 is a target of ROP18 in the context of T. gondii type I pathogenesis.
FIGURE 4.
Generation of ROP18 WT and MUT expressing type I T. gondii in rop18 knock-out background. A, the ROP18 coding sequence of a virulent type I (RH Δku80Δhxgprt) strain was replaced with TUB-promoter-ROP18-Ty1-tagged-HXGPRT by homologous recombination. B, primers were used to evaluate the generation of ROP18-WT or MUT Ty1-tagged RH strain in rop18 knock-out background. The 5′-integration was verified with primer F1 and R1; the 3′-integration was verified with primer F2 and R2; the insertion of TUB promoter-ROP18 -Ty1-HXGPRT cassette into ROP18 locus was verified with primer F1 and R2. C, expression of wild-type ROP18 and its mutant in ROP18-WT or MUT RH strains were determined by RT-PCR. Expression levels of SAG1 were analyzed and shown as loading controls. Data was representative of two independent experiments. D, expression of wild-type ROP18 and its mutant ROP18-WT or MUT RH strains were determined by Western blotting. Expression levels of SAG1 antigen were analyzed and shown as loading controls. Data are representative of two independent experiments. IB, immunoblot.
FIGURE 5.
Expressing wild-type ROP18 transgenic strains led to p65 degradation. A, the HFF cells were infected with the indicated parasites at an m.o.i. of 1. At 12 h after infection, the cells were lysed and subjected to Western blotting (IB) with anti-p65 (upper panel). The p65 expression levels were normalized against GAPDH (lower panel). B, kinase-deficient ROP18 forms a more stable interaction with p65 than wild-type ROP18 in infected cells. HFF cells were infected with the indicated strains for 12 h. Immunoprecipitation of p65 from infected cell lysates was detected with a polyclonal rabbit anti-p65. Input and immunoprecipitation (IP) fractions were resolved through SDS-PAGE and blotted for ROP18 using mouse monoclonal anti-Ty1. C, HFF cells grown on glass coverslips were infected with ROP18-WT RH strain, ROP18 MUT RH strain, ROP18-KO RH strain, or GFP-RH strain (stable expressing GFP RH strain) at an m.o.i. of 1 for >12 h. Double immunofluorescence was performed with mouse monoclonal anti-Ty1 (green) and rabbit polyclonal anti-p65 (red). A dramatic reduction of nuclear p65 was observed in ROP18-WT RH strain-infected cells compared with ROP18-MUT or ROP18-KO RH strain. BF, brightfield. Bars, 5 μm. D, the level of nuclear p65 in infected cells was quantified using the mean ratio of nuclear to cytoplasmic p65 (after subtraction of background signal) in at least 10 infected cells per stain. ***, p < 0.001.
The Kinase Activity of ROP18 Suppresses NF-κB Activation
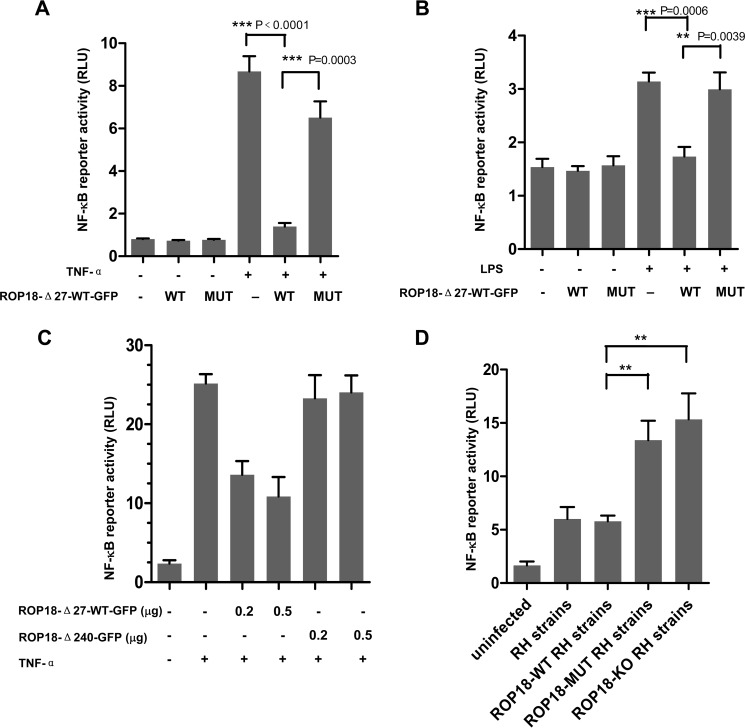
To investigate the effect of ROP18 on NF-κB-mediated target gene activation, luciferase reporter assays were performed. The cells were transiently transfected with a luciferase reporter construct containing three copies of the NF-κB binding site (3×κB-Luc) together with ROP18-WT or ROP18-MUT vectors and treated with TNF-α for 6 h. The reporter assays showed that the overexpression of ROP18-WT significantly inhibited TNF-α-induced NF-κB activation (Fig. 6A) and considerably reduced NF-κB transcriptional activity induced by LPS (Fig. 6B). Additionally, transfection with ROP18-Δ27-WT-GFP or ROP18Δ240-GFP plasmids at different doses revealed that ROP18-Δ27-WT-GFP inhibited the activity of κB-luciferase in a dose-dependent manner. However, in cells expressing ROP18Δ240-GFP (without p65 interacting domain), the activity of κB-luciferase was largely unaffected (Fig. 6C). Additional experiments employing ROP18-WT or ROP18-MUT or ROP18-KO RH strain consistently showed that ROP18 WT-RH strain induced a dramatic inhibition of NF-κB activity compared with ROP18-MUT and ROP18-KO parasites (Fig. 6D). Taken together, the results suggested that the kinase activity of ROP18 is required for the inhibition of NF-κB activation.
FIGURE 6.
Kinase activity of ROP18 suppresses NF-κB activation. A, 293T cells were transfected with a luciferase reporter construct containing three copies of the NF-κB binding site (3×κB-Luc) together with ROP18Δ27-WT-GFP, ROP18Δ27-MUT-GFP, or empty expression vectors for 24 h and treated with or without TNF-α (10 ng/ml) for 6 h before the luciferase assays were performed. RLU, relative light units. B, RAW264.7 cells were transfected with a luciferase reporter construct containing three copies of the NF-κB binding site (3×κB-Luc) together with ROP18Δ27-WT-GFP, ROP18Δ27-MUT-GFP, or empty expression vectors for 24 h and stimulated with or without LPS (100 ng/ml) for 6 h before the luciferase assays were performed. C, dose-dependent effect of ROP18 on TNF-α-induced NF-κB reporter gene activity. The cells were transiently co-transfected with NF-κB-Luc reporter plasmid and different amounts of the indicated plasmids or empty expression vectors. At 24 h after transfection, the cells were treated with TNF-α (10 ng/ml) for 8 h. D, 293T cells were infected with or without the indicated different RH strains at an m.o.i. of 1 for >12 h before the luciferase assays were performed. Data are the means ± S.D. of at least three independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001, compared with the control vectors.
The Kinase Activity of ROP18 Suppresses the Expressions of NF-κB Target Genes in Vitro
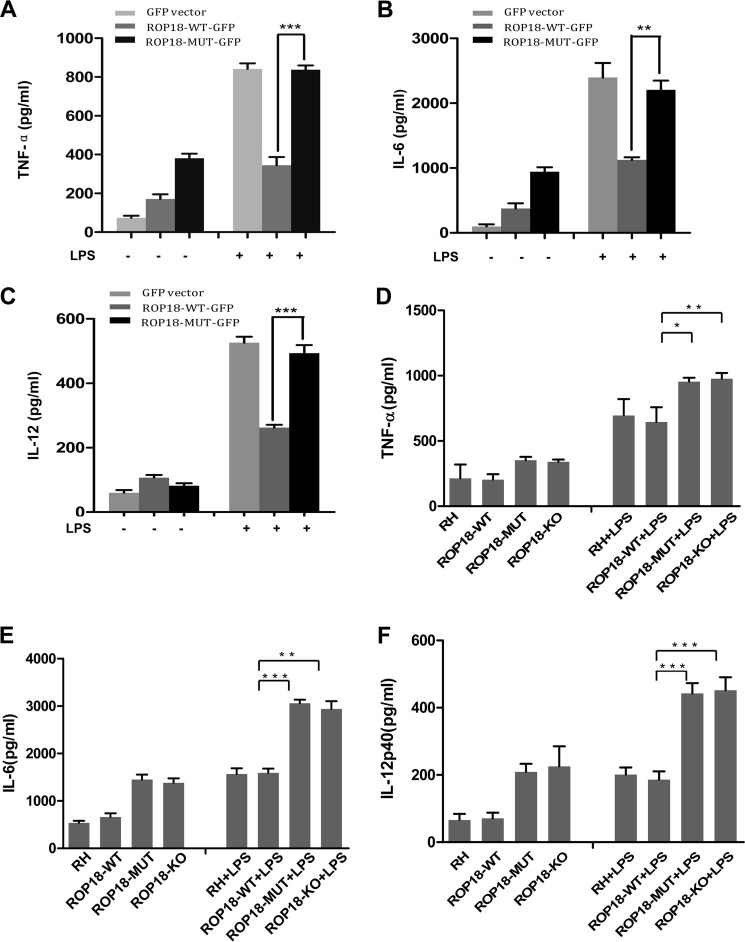
Previous results have shown that infection with type I RH tachyzoites inhibits macrophage responses to LPS and other TLR ligands, and this inhibition phenotype is associated with the NF-κB (19, 29–31). Therefore, we examined whether ROP18 kinase is the effector to manipulate host NF-κB. RAW264.7 cells were transfected with ROP18-WT, ROP18-MUT, or empty vectors and stimulated with LPS for 6 h. The macrophages stimulated by LPS produced robust amounts of IL-6, IL-12p40, and TNF-α, whereas cells expressing ROP18-WT induced low levels of IL-6, IL-12p40, and TNF-α compared with ROP18-MUT (Fig. 7, A–C). Furthermore, U937 cells infected with different strains were stimulated with LPS for 6 h. Although cells infected with ROP18-WT RH displayed an effective ability to suppress LPS-induced cytokine production, cells infected with ROP18-MUT or ROP18-KO RH strains strongly enhanced LPS-induced IL-6, IL-12p40, and TNF-α production (Fig. 7, D–F). Thus, we conclude that the interaction of ROP18 with p65 mediates p65 degradation, thereby down-regulating the expression of NF-κB target genes.
FIGURE 7.
Kinase activity of ROP18 affects the expressions of NF-κB target genes in vitro. A and C, RAW264.7 cells were transfected with the indicated ROP18Δ27-WT-GFP, ROP18Δ27-MUT-GFP, or empty expression vectors for 24 h. Subsequently, the cells were treated with or without LPS (100 ng/ml) for 6 h. Concentrations of TNF-α (A), IL-6 (B), and IL-12 p40 (C) in the culture supernatants were measured through ELISA. Indicated values are the means ± S.D. of triplicates. *, p < 0.05; **, p < 0.01; ***, p < 0.001, compared with the vector control. D–F, U937 cells were infected with ROP18 WT, MUT, or KO RH strain at an m.o.i. of 1 for over 12 h. Subsequently, the cells were treated with or without LPS (100 ng/ml) for 6 h. Concentrations of TNF-α (D), IL-6 (E), and IL-12p40 (F) in the culture supernatants were measured through ELISA. The indicated values are the means ± S.D. of triplicates. *, p < 0.05; **, p < 0.01; ***, p < 0.001, compared with the vector control.
M1 Polarization Infected with ROP18 Kinase-deficient Strain
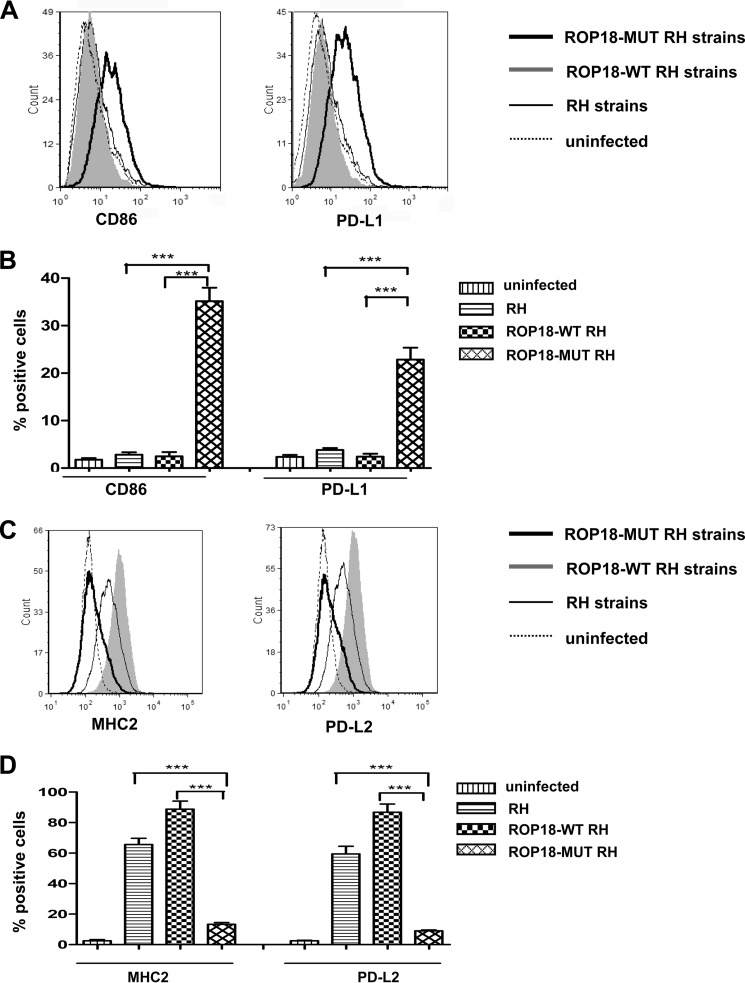
T. gondii primarily infects nucleated cells, including macrophages, and associated mononuclear phagocytes in vivo. Recent literature has stated that type I and type III strains elicit a gene expression profile similar to that of alternatively activated macrophages (termed M2), whereas type II strains induce a classically activated phenotype (termed M1) (29). M1 macrophages typically express chemokines and cytokines that activate antimicrobial activity in cells, whereas M2 macrophages secrete anti-inflammatory molecules that down-regulate TH1 type responses (2, 32). To determine the association of ROP18 with a M1/M2-polarized phenotype, macrophages were infected with ROP18-WT, ROP18-MUT, or RH strains. FACS assays showed that ROP18-MUT tachyzoites, unlike ROP18-WT RH and RH strains, elicited the expression of the M1-associated markers (CD86 and PD-L1), which is consistent with the inability of this strain to inhibit the NF-κB pathway (Fig. 8, A and B). The M2 signature (MHC2 and PD-L2) was clearly noted in macrophages infected with ROP18-WT RH and RH strains (Fig. 8, C and D). Thus, the kinase activity of ROP18 contributes to the M1/M2-biased phenotype of the host macrophages.
FIGURE 8.
Inducing the M2 phenotype with type I strains expressing wild-type ROP18. A, U937 cells were infected with ROP18-WT RH strain, ROP18-MUT RH strain, or RH strain. At 24 h post-infection, macrophages were stained for CD86 or PD-L1, and fluorescence-activated cell sorting assays were performed (dotted lines, uninfected macrophages; solid lines, macrophages infected with RH strain; heavy lines, macrophages infected with ROP18-MUT RH strain; shaded, macrophages infected ROP18-WT RH strains). B, histograms depict the percentage of positively stained CD86 or PD-L1 cells analyzed in A. Indicated values are the means ± S.D. of triplicates. ***, p < 0.001, compared with the RH strain control. C, U937 cells were infected with ROP18-WT RH strain, ROP18-MUT RH strain, or RH strain. At 24 h post-infection, macrophages were stained for MHC2 or PD-L2, and fluorescence-activated cell sorting assays were performed (dotted lines, uninfected macrophages; solid lines, macrophages infected with RH strain; heavy lines, macrophages infected with ROP18-MUT RH strain; shaded, macrophages infected ROP18-WT RH strain). D, the histograms depict the percentage of positively stained MHC2 or PD-L2 cells analyzed in C. Indicated values are the means ± S.D. of triplicates. ***, p < 0.001, compared with the RH strain control.
DISCUSSION
Transcription factors control many innate immune effectors that enhance or regulate the overall immune response to invading microorganisms. In turn, successful intracellular pathogens have developed strategies to undermine important host cell immune pathways to promote their survival. Recent studies have revealed that, like other pathogens, T. gondii injects virulence factors into host cells using the so-called “kiss and spit” model to provide a survival advantage to parasites (2, 29). ROP16 of type I and type III strains phosphorylates host signal transducers and activators of STAT3 and STAT6, resulting in the prolonged activation of these two transcription factors and the subsequent up-regulation of IL-4 and antagonizing induction of IL-12 (33–35). A dense-granule protein of type II parasite strains, GRA15, integrated to parasitophorous vacuole membrane, activates TRAF6, which activates IκB kinase, leading to the phosphorylation and proteasomal degradation of IκB to the activation of NF-κB pathway. Recent research shows that IRGs (IFN-γ-induced immunity-related GTPases) are crucial for the control of toxoplasmosis in mice. The finding suggests that ROP18 and ROP5 are involved to avoid IRG recruitment, which is unlikely to happen in species that do not have the IRG system such as humans (36–38). Our result here demonstrated that ROP18 in type I strains is involved in the host NF-κB pathway present in murine as well as the human immune system. We confirmed for the first time that the virulence factor ROP18 in type I strains is responsible for inhibiting host NF-κB and for suppressing proinflammatory cytokine, and the disruption of ROP18 kinase activity in type I strain compromised the ability of the parasite to inhibit NF-κB for the degradation of p65 and increased expression of IL-12, IL-6, and TNF-α (Fig. 7). Corresponding to the key role of ROP18 in driving M2 polarization, kinase-deficient ROP18 parasites induced M1-biased activation compared with the wild-type ROP18 strain (Fig. 8).
In addition, we elucidated the molecular mechanisms of the relationship between ROP18 and the host NF-κB pathway. Evidence for the interaction between p65 and ROP18 was obtained through coimmunoprecipitation, GST pulldown, and yeast two-hybrid assays. We further demonstrated that the N-terminal portion of ROP18 is associated with the dimerization domain of p65. ROP18 phosphorylates p65 at Ser-468 and degrades this protein via ubiquitin-dependent degradation pathway and subsequently suppresses NF-κB activation. Kinase-deficient ROP18 forms a more stable interaction with p65 in infected cells (Fig. 5B), suggesting that kinase-deficient ROP18 disabled to induce the phosphorylation and degradation of p65. Previous studies have reported that Ser-468 phosphorylation of p65 allows for binding of the COMMD1 complex and subsequent ubiquitination (39–42). COMMD1 has been identified in a complex with an E3 ubiquitin ligase complex composed of TCEB1/elongin C, CUL2, SOCS1, and RBX1 (40, 41). COMMD1 promotes ubiquitination of p65 and its subsequent proteasomal degradation (39). Therefore, our results implicate that ROP18 phosphorylates p65 at Ser-468 to promote its binding of COMMD1 and subsequent ubiquitination.
The modulation of the NF-κB pathway through T. gondii infection has long been an area of debate, with some studies showing that T. gondii activates NF-κB and other studies showing that T. gondii inhibits NF-κB activation (9). Previous investigations showed that infection with type I strains activates NF-κB in host cells, whereas infection with type I strains results in the activation of IκB kinase and induces the phosphorylation and proteasomal degradation of the inhibitor of NF-κB (IκB) (10, 16, 18). Other studies have shown that infection with type I strains inhibits NF-κB activation, as type I strains do not activate NF-κB compared with LPS and TNF stimulation (10, 12–13). Our immunofluorescence experiments showed that type I strains slightly activate p65 translocation, consistent with the results of previous studies. Interestingly, we also observed that the kinase activity of ROP18 induces p65 ubiquitin-dependent degradation and the subsequent suppression of NF-κB activation. Functionally, p65 ubiquitination is a potential mechanism regulating the termination of NF-κB by the intracellular T. gondii and may also contribute to the oscillation of nuclear p65. The wild-type ROP18 RH strain suppresses NF-κB-regulated proinflammatory cytokines compared with kinase-deficient ROP18 tachyzoites. This result is consistent with previous observations that infections with type I strains inhibit the NF-κB pathway and down-regulate the induction of IL-12, thus limiting protective T helper 1 (Th1)-type cytokine responses (2, 9, 12, 13, 19, 29). Thus, the present results provide a reasonable explanation for this debate. In addition, we further demonstrated that kinase-deficient ROP18 parasites induce M1-biased activation compared with wild-type ROP18 parasites, indicating a ROP18-induced inhibition of host NF-κB pathway and of IL-12 production, thereby causing M2-biased phenotypes. Taken together, our data indicate that ROP18 in type I RH strain is responsible for inhibiting host NF-κB pathways, facilitating the M2-biased response and consequently promoting the survival and proliferation of invading pathogens.
Acknowledgments
We thank Professor John C. Boothroyd (Stanford University School of Medicine) for kindly providing the Δku80Δhxgprt RH strain and ROP18 knock-out Δku80 RH strain and Professor Jean-François Dubremetz (The University of Montpellier, Montpellier, France) for the wild-type ROP18-Ty1 and kinase-deficient ROP18-Ty1 RH strains. We greatly appreciate Professor David Sibley and Dr. Bang Shen (Department of Molecular Microbiology, Washington University School of Medicine) for their generous help and experimental design.
This work was supported by the National Basic Research Program of China (973 program; Grant 2010CB530001; to J. S.), National Natural Science Foundation of China Grants 81271864 and 30801329, Fok Ying Tung College Young Teachers Fund Grant 131033 from the Chinese Ministry of Education, Distinguished Young Scholar of Anhui Province Grants 10040606Y19 and 1308085JGD11 (to J. D.), and National Natural Science Foundation of China Grant 81171605 (to X. X.).
- IκB
- NF-κB
- HFF
- human foreskin fibroblast
- m.o.i.
- multiplicity of infection
- IRG
- IFN-γ-induced immunity-related GTPase.
REFERENCES
- 1. Montoya J. G., Liesenfeld O. (2004) Toxoplasmosis. Lancet 363, 1965–1976 [DOI] [PubMed] [Google Scholar]
- 2. Hunter C. A., Sibley L. D. (2012) Modulation of innate immunity by Toxoplasma gondii virulence effectors. Nat. Rev. Microbiol. 10, 766–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Minbaeva G., Schweiger A., Bodosheva A., Kuttubaev O., Hehl A. B., Tanner I., Ziadinov I., Torgerson P. R., Deplazes P. (2013) Toxoplasma gondii infection in Kyrgyzstan: seroprevalence, risk factor analysis, and estimate of congenital and AIDS-related toxoplasmosis. PLoS Negl. Trop. Dis. 7, e2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ferguson D. J., Bowker C., Jeffery K. J., Chamberlain P., Squier W. (2013) Congenital toxoplasmosis: continued parasite proliferation in the fetal brain despite maternal immunological control in other tissues. Clin. Infect. Dis. 56, 204–208 [DOI] [PubMed] [Google Scholar]
- 5. Tait E. D., Hunter C. A. (2009) Advances in understanding immunity to Toxoplasma gondii. Mem. Inst. Oswaldo Cruz 104, 201–210 [DOI] [PubMed] [Google Scholar]
- 6. Vallabhapurapu S., Karin M. (2009) Regulation and function of NF-κB transcription factors in the immune system. Annu. Rev. Immunol. 27, 693–733 [DOI] [PubMed] [Google Scholar]
- 7. DiDonato J. A., Mercurio F., Karin M. (2012) NF-κB and the link between inflammation and cancer. Immunol. Rev. 246, 379–400 [DOI] [PubMed] [Google Scholar]
- 8. Gilmore T. D., Wolenski F. S. (2012) NF-κB: where did it come from and why? Immunol. Rev. 246, 14–35 [DOI] [PubMed] [Google Scholar]
- 9. Rosowski E. E., Lu D., Julien L., Rodda L., Gaiser R. A., Jensen K. D., Saeij J. P. (2011) Strain-specific activation of the NF-κB pathway by GRA15, a novel Toxoplasma gondii dense granule protein. J. Exp. Med. 208, 195–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shapira S., Harb O. S., Margarit J., Matrajt M., Han J., Hoffmann A., Freedman B., May M. J., Roos D. S., Hunter C. A. (2005) Initiation and termination of NF-κB signaling by the intracellular protozoan parasite Toxoplasma gondii. J. Cell Sci. 118, 3501–3508 [DOI] [PubMed] [Google Scholar]
- 11. Payne T. M., Molestina R. E., Sinai A. P. (2003) Inhibition of caspase activation and a requirement for NF-κB function in the Toxoplasma gondii-mediated blockade of host apoptosis. J. Cell Sci. 116, 4345–4358 [DOI] [PubMed] [Google Scholar]
- 12. Shapira S., Speirs K., Gerstein A., Caamano J., Hunter C. A. (2002) Suppression of NF-κB activation by infection with Toxoplasma gondii. J. Infect. Dis. 185, S66–S72 [DOI] [PubMed] [Google Scholar]
- 13. Butcher B. A., Kim L., Johnson P. F., Denkers E. Y. (2001) Toxoplasma gondii tachyzoites inhibit proinflammatory cytokine induction in infected macrophages by preventing nuclear translocation of the transcription factor NF-κB. J. Immunol. 167, 2193–2201 [DOI] [PubMed] [Google Scholar]
- 14. Leng J., Butcher B. A., Egan C. E., Abi Abdallah D. S., Denkers E. Y. (2009) Toxoplasma gondii prevents chromatin remodeling initiated by TLR-triggered macrophage activation. J. Immunol. 182, 489–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Molestina R. E., Sinai A. P. (2005) Host and parasite-derived IKK activities direct distinct temporal phases of NF-κB activation and target gene expression following Toxoplasma gondii infection. J. Cell Sci. 118, 5785–5796 [DOI] [PubMed] [Google Scholar]
- 16. Molestina R. E., Payne T. M., Coppens I., Sinai A. P. (2003) Activation of NF-κB by Toxoplasma gondii correlates with increased expression of antiapoptotic genes and localization of phosphorylated IκB to the parasitophorous vacuole membrane. J. Cell Sci. 116, 4359–4371 [DOI] [PubMed] [Google Scholar]
- 17. Kim J. Y., Ahn M. H., Jun H. S., Jung J. W., Ryu J. S., Min D. Y. (2006) Toxoplasma gondii inhibits apoptosis in infected cells by caspase inactivation and NF-κB activation. Yonsei Med. J. 47, 862–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Molestina R. E., Sinai A. P. (2005) Detection of a novel parasite kinase activity at the Toxoplasma gondii parasitophorous vacuole membrane capable of phosphorylating host IκBα. Cell Microbiol. 7, 351–362 [DOI] [PubMed] [Google Scholar]
- 19. Robben P. M., Mordue D. G., Truscott S. M., Takeda K., Akira S., Sibley L. D. (2004) Production of IL-12 by macrophages infected with Toxoplasma gondii depends on the parasite genotype. J. Immunol. 172, 3686–3694 [DOI] [PubMed] [Google Scholar]
- 20. Kim L., Butcher B. A., Denkers E. Y. (2004) Toxoplasma gondii interferes with lipopolysaccharide-induced mitogen-activated protein kinase activation by mechanisms distinct from endotoxin tolerance. J. Immunol. 172, 3003–3010 [DOI] [PubMed] [Google Scholar]
- 21. Cheng L., Chen Y., Chen L., Shen Y., Shen J., An R., Luo Q., Du J. (2012) Interactions between the ROP18 kinase and host cell proteins that aid in the parasitism of Toxoplasma gondii. Acta Trop. 122, 255–260 [DOI] [PubMed] [Google Scholar]
- 22. Yamamoto M., Ma J. S., Mueller C., Kamiyama N., Saiga H., Kubo E., Kimura T., Okamoto T., Okuyama M., Kayama H., Nagamune K., Takashima S., Matsuura Y., Soldati-Favre D., Takeda K. (2011) ATF6β is a host cellular target of the Toxoplasma gondii virulence factor ROP18. J. Exp. Med. 208, 1533–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Du J., Cai X., Yao J., Ding X., Wu Q., Pei S., Jiang K., Zhang Y., Wang W., Shi Y., Lai Y., Shen J., Teng M., Huang H., Fei Q., Reddy E. S., Zhu J., Jin C., Yao X. (2008) The mitotic checkpoint kinase NEK2A regulates kinetochore microtubule attachment stability. Oncogene 27, 4107–4114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Du J., Chen L., Luo X., Shen Y., Dou Z., Shen J., Cheng L., Chen Y., Li C., Wang H., Yao X. (2012) 14-3-3ζ cooperates with phosphorylated Plk1 and is required for correct cytokinesis. Front. Biosci. 4, 639–650 [DOI] [PubMed] [Google Scholar]
- 25. Zhou R., Cao X., Watson C., Miao Y., Guo Z., Forte J. G., Yao X. (2003) Characterization of protein kinase A-mediated phosphorylation of ezrin in gastric parietal cell activation. J. Biol. Chem. 278, 35651–35659 [DOI] [PubMed] [Google Scholar]
- 26. El Hajj H., Lebrun M., Arold S. T., Vial H., Labesse G., Dubremetz J. F. (2007) ROP18 is a rhoptry kinase controlling the intracellular proliferation of Toxoplasma gondii. PLoS Pathog. 3, e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Taylor S., Barragan A., Su C., Fux B., Fentress S. J., Tang K., Beatty W. L., Hajj H. E., Jerome M., Behnke M. S., White M., Wootton J. C., Sibley L. D. (2006) A secreted serine-threonine kinase determines virulence in the eukaryotic pathogen Toxoplasma gondii. Science 314, 1776–1780 [DOI] [PubMed] [Google Scholar]
- 28. Saeij J. P., Boyle J. P., Coller S., Taylor S., Sibley L. D., Brooke-Powell E. T., Ajioka J. W., Boothroyd J. C. (2006) Polymorphic secreted kinases are key virulence factors in toxoplasmosis. Science 314, 1780–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Murray P. J. (2011) Macrophages as a battleground for Toxoplasma pathogenesis. Cell Host Microbe 9, 445–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jensen K. D., Wang Y., Wojno E. D., Shastri A. J., Hu K., Cornel L., Boedec E., Ong Y. C., Chien Y. H., Hunter C. A., Boothroyd J. C., Saeij J. P. (2011) Toxoplasma polymorphic effectors determine macrophage polarization and intestinal inflammation. Cell Host Microbe 9, 472–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Harris T. H., Wilson E. H., Tait E. D., Buckley M., Shapira S., Caamano J., Artis D., Hunter C. A. (2010) NF-κB1 contributes to T cell-mediated control of Toxoplasma gondii in the CNS. J. Neuroimmunol. 222, 19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goerdt S., Politz O., Schledzewski K., Birk R., Gratchev A., Guillot P., Hakiy N., Klemke C. D., Dippel E., Kodelja V., Orfanos C. E. (1999) Alternative versus classical activation of macrophages. Pathobiology 67, 222–226 [DOI] [PubMed] [Google Scholar]
- 33. Butcher B. A., Fox B. A., Rommereim L. M., Kim S. G., Maurer K. J., Yarovinsky F., Herbert D. R., Bzik D. J., Denkers E. Y. (2011) Toxoplasma gondii rhoptry kinase ROP16 activates STAT3 and STAT6 resulting in cytokine inhibition and arginase-1-dependent growth control. PLoS Pathog. 7, e1002236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ong Y. C., Reese M. L., Boothroyd J. C. (2010) Toxoplasma rhoptry protein 16 (ROP16) subverts host function by direct tyrosine phosphorylation of STAT6. J. Biol. Chem. 285, 28731–28740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yamamoto M., Standley D. M., Takashima S., Saiga H., Okuyama M., Kayama H., Kubo E., Ito H., Takaura M., Matsuda T., Soldati-Favre D., Takeda K. (2009) A single polymorphic amino acid on Toxoplasma gondii kinase ROP16 determines the direct and strain-specific activation of Stat3. J. Exp. Med. 206, 2747–2760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Niedelman W., Gold D. A., Rosowski E. E., Sprokholt J. K., Lim D., Farid Arenas A., Melo M. B., Spooner E., Yaffe M. B., Saeij J. P. (2012) The rhoptry proteins ROP18 and ROP5 mediate Toxoplasma gondii evasion of the murine, but not the human, interferon-γ response. PLoS Pathog. 8, e1002784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Behnke M. S., Fentress S. J., Mashayekhi M., Li L. X., Taylor G. A., Sibley L. D. (2012) The polymorphic pseudokinase ROP5 controls virulence in Toxoplasma gondii by regulating the active kinase ROP18. PLoS Pathog. 8, e1002992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Steinfeldt T., Könen-Waisman S., Tong L., Pawlowski N., Lamkemeyer T., Sibley L. D., Hunn J. P., Howard J. C. (2010) Phosphorylation of mouse immunity-related GTPase (IRG) resistance proteins is an evasion strategy for virulent Toxoplasma gondii. PLoS Biol. 8, e1000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Geng H., Wittwer T., Dittrich-Breiholz O., Kracht M., Schmitz M. L. (2009) Phosphorylation of NF-κB p65 at Ser-468 controls its COMMD1-dependent ubiquitination and target gene-specific proteasomal elimination. EMBO Rep. 10, 381–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Maine G. N., Mao X., Komarck C. M., Burstein E. (2007) COMMD1 promotes the ubiquitination of NF-κB subunits through a cullin-containing ubiquitin ligase. EMBO J. 26, s447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Maine G. N., Burstein E. (2007) COMMD proteins and the control of the NFκB pathway. Cell Cycle 6, 672–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Huang B., Yang X. D., Lamb A., Chen L. F. (2010) Posttranslational modifications of NF-κB: another layer of regulation for NF-κB signaling pathway. Cell Signal 22, 1282–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]