Background: Transcription factor NF-κB is involved in various biological processes and regulated at multiple levels.
Results: KLF6 modulates NF-κB-mediated transcription by promoting binding of p65 to promoters of downstream genes.
Conclusion: KLF6 is a co-activator of NF-κB-mediated transcription of selected downstream genes.
Significance: Our study reveals a new regulatory mechanism for NF-κB-mediated transcriptional activation in the nucleus.
Keywords: DNA-binding Protein, Interleukin, Kruppel-like factor (KLF), NF-κB (NF-κB), Signal Transduction, Transcription, Tumor Necrosis Factor (TNF)
Abstract
The transcription factor NF-κB plays a pivotal role in a broad range of physiological and pathological processes, including development, inflammation, and immunity. How NF-κB integrates activating signals to expression of specific sets of target genes is of great interest. Here, we identified Krüppel-like factor 6 (KLF6) as a co-activator of NF-κB after TNFα and IL-1β stimulation. Overexpression of KLF6 enhanced TNFα- and IL-1β-induced activation of NF-κB and transcription of a subset of downstream genes, whereas knockdown of KLF6 had opposite effects. KLF6 interacted with p65 in the nucleus and bound to the promoters of target genes. Upon IL-1β stimulation, KLF6 was recruited to promoters of a subset of NF-κB target genes in a p65-dependent manner, which was in turn required for the optimal binding of p65 to the target gene promoters. Our findings thus identified KLF6 as a previously unknown but essential co-activator of NF-κB and provided new insight into the molecular regulation of p65-dependent gene expression.
Introduction
Nuclear factor κB (NF-κB)2 is a family of structurally homologous transcription factors critically involved in various biological processes, including development, inflammation, and immunity(1). The mammalian NF-κB family includes five proteins: p65 (RelA), RelB, c-Rel, p50, and p52, which bind to κB sites of downstream gene promoters as homo- or hetero-dimmers. All NF-κB family members contain a Rel-homology domain (RHD) at the N terminus that mediates DNA binding and dimerization, whereas only p65, RelB, and c-Rel contain a transcriptional activation domains (TAD) at the C terminus that mediates transcription of downstream genes. NF-κB is sequestered in the cytoplasm by inhibitor of κBα (IκBα) and p100, two proteins which play primary roles in the canonical and noncanonical NF-κB pathways, respectively (1, 2). p100 binds to and sequesters RelB in the cytoplasm in unstimulated cells and undergoes phosphorylation-dependent partial degradation upon certain stimulation, leading to formation of the noncanonical NF-κB, RelB/p52 heterodimer that subsequently enters the nucleus to activate transcription of downstream genes. IκBα is associated with the canonical NF-κB, p65/p50 heterodimer in unstimulated cells, and treatment with various stimuli such as TNFα and IL-1β results in phosphorylation and subsequent proteosome-mediated degradation of IκBα, releasing the p65/p50 complex into nucleus (3, 4).
Although the key steps by which NF-κB is activated in the cytoplasm have been proposed, and the importance of NF-κB nuclear translocation is greatly appreciated, it remains less clear how NF-κB functions in the nucleus to differentially regulate the transcription of thousands of genes in distinct types of cells upon various stimulations. In addition to the diversity of NF-κB dimers that exert different DNA binding affinity and specificity, it has been suggested that various co-repressors and co-activators play critical roles in the process. Ribosomal protein S3 (RPS3) has been identified as a non-Rel subunit of p65 homodimer and p65/p50 heterodimer that regulates NF-κB-mediated transcription of selected target genes (5). The evolutionarily conserved Akirin proteins are localized in the nucleus and required for NF-κB-mediated transcription of IL-6, IκBα, IP-10, but not KC or IκBδ in MEFs upon stimulation with IL-1β or LPS, though the exact mechanisms of which are unknown (6). The deacetylase SIRT6 modulates the transcription of NF-κB-dependent genes that are involved in organismal life span, while Nurr1 recruits the CoREST corepressor to clear the promoter-binding p65, resulting in transcriptional repression in microglia and astrocytes (7, 8). A few transcription factors including Sp1, AP-1, IRFs, STAT3, and CEBP/β are also reported to synergize with NF-κB to regulate transcription of downstream genes (9). Whether and how other proteins are involved in nuclear NF-κB-mediated transcription is of great interest.
The Krüppel-like factors (KLFs) are transcriptional regulators that regulate diverse processes including development, homeostasis, tumorigenesis, and cell malignancy. Seventeen KLF family members have been characterized in the human genome so far (10). The C termini of KLFs are highly conserved and consist of 81 amino acid residues characterized with the C2H2 zinc finger, which binds to “GC-box” or “CACCC-box” motifs in the promoters and/or mediates protein-protein interactions. The N-terminal regions of KLFs are much more variable and mediate transcriptional activation or repression (11). For example, KLF1 interacts with and is acetylated by the coactivators p300 and CBP, which alters the ability of KLF1 to interact with SWI/SNF to regulate tissue-specific expression of β-globin (12), while KLF3 and KLF8 interact with the co-repressor protein C-terminal-binding protein (CtBP) to repress transcription(13). However, little is known about the roles of KLFs in regulation of NF-κB-mediated transcription.
In this study, we identified KLF6 as a co-activator of NF-κB essential for NF-κB-mediated transcription of selected downstream genes after TNFα- and IL-1β stimulation. KLF6 interacts with p65 in the nucleus and binds to the promoters of p65 target genes, which is required for the optimal binding of p65 to the promoters and subsequent transcription of a subset of downstream genes, including MCP1, CXCL2, and IL-8. Our findings thus uncovered a new regulatory mechanism for NF-κB-mediated transcriptional activation in the nucleus.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies
Recombinant human TNFα and IL-1β (Peprotech); mouse monoclonal antibodies against Flag (Sigma), HA (Covance), and β-actin (Sigma); rabbit anti-p65 (Santa Cruz Biotechnology) and rabbit anti-LMNB1 (protein-tech) were purchased from the indicated manufacturers. Rabbit polyclonal serum against KLF6 was made against KLF6 (aa1–200) of human origin and the anti-KLF6 IgG was purified by affinity chromatography. Mouse anti-IκBα was previously described (14).
Constructs
NF-κB, ISRE, and IRF1 promoter luciferase reporter plasmids, mammalian expression plasmids for Flag-tagged IκBα, TRADD, MyD88, TRAF2, TRAF6, TAK1, TAB1, and IKKβ were previously described (14–16). Mammalian cDNA expression plasmids were purchased from Origene Inc. Flag-p65, Flag-p50, Flag- or HA-tagged KLF6, and its mutants and isoforms were constructed by standard molecular biology techniques.
Transfection and Reporter Gene Assays
HEK293 cells (1 × 105) were seeded on 24-well plates and transfected on the following days by standard calcium phosphate precipitation method. In the same experiment, empty control plasmid was added to ensure that each transfection receives the same amount of total DNA. To normalize for transfection efficiency, 0.01 μg of pRL-TK Renilla luciferase reporter plasmid was added to each transfection. Luciferase assays were performed using a dual-specific luciferase assay kit (Promega), and the firefly luciferase activities were normalized based on Renilla luciferase activities.
RNAi Experiments
Double-strand oligonucleotides corresponding to the target sequences were cloned into the pSuper.RetroRNAi plasmid (Oligoengine Inc.). The following sequences were targeted for human KLF6: KLF6-RNAi #1: CAGGAAAGTTTACACCAAA; KLF6-RNAi #2: CTTTAACGGCTGCAGGAAA.
Coimmunoprecipitation and Western Blot Analysis, Retrovirus-mediated Construction of KLF6-RNAi Stable Cell Line
Quantitative Real-time PCR (qPCR)
Total RNA was isolated from cells using Trizol reagent (TAKARA) and subjected to qPCR analysis to measure expression of mRNA. The mRNA levels of specific genes were normalized to GAPDH mRNA. Gene-specific primer sequences were as following: MCP1: 5′-AGAATCACCAGCAGCAAGTGTCC-3′, 5′-TCCTGAACCCACTTCTGCTTGG-3′; CXCL2: 5′-CAAGAACATCCAAAGTGTGA-3′, 5′-CCATTCTTGAGTGTGGCTAT-3′; IL8: 5′-GAGAGTGATTGAGAGTGGACCAC-3′, 5′-CACAACCCTCTGCACCCAGTTT-3′; IκBα: 5′-GTCCTTGGGTGCTGATGT-3′, 5′-GAGAATAGCCCTGGTAGGTAA-3′; GAPDH: 5′-GAGTCAACGGATTTGGTCGT-3′, 5′-GACAAGCTTCCCGTTCTCAG-3′.
Chromatin Immunoprecipitation (ChIP) Assays
ChIP assays were performed as previously described (20, 21). Immunoprecipitation was performed with 1 μg of p65 antibody (C-20) or 5 μg of KLF6 antibody, and the immune complexes were absorbed with protein A beads blocked with bovine serum albumin and salmon sperm DNA (Millipore). Gene-specific primer sequences were as following: MCP1: 5′-GACCCCGGGAGGAATGAAGAAA-3′, 5′-CAGAGGGGCTATGGGGAAAATGA-3′; IL8: 5′-TGATAAGGAACAAATAGGAAGTG-3′, 5′-GTGTGCTCTGCTGTCTCTGA-3′; CXCL2: 5′-CTCGCAGGCGGTTATCTCGGTATC-3′, 5′-GGGGGTCGGGGCACTCACG-3′; IκBα: 5′-TAGCAGAGGACGAAGCCAGT-3′, 5′-TGGCTGGGGATTTCTCTG-3′.
Apoptosis Assays
HCT116 cells were treated with TNFα (100 ng/ml) together with Smac mimetic (100 nm) (Xiaodong Wang, NIBS, Beijing) (22) for 6 or 12 h followed by staining with annexin V-FITC in PBS for 15 min and propidium iodide (PI) for 3 min. The cells were washed and subjected to flow cytometry using a FACS caliber flow cytometer (XDP, Beckman Counter).
RESULTS
Overexpression of KLF6 Potentiates TNFα- and IL-1β-induced NF-κB Activation
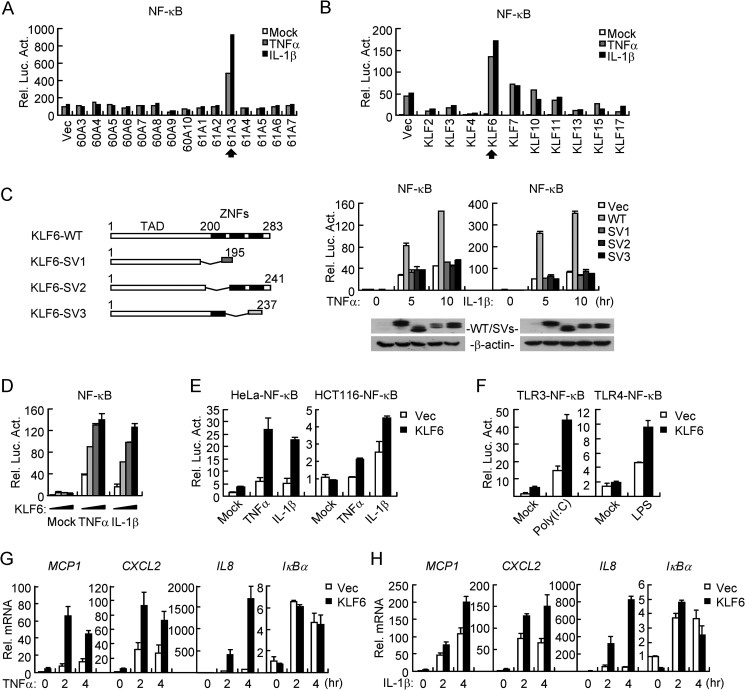
To identify additional regulators involved in TNFα- or IL-1β-induced activation of NF-κB, we screened ∼15,000 independent human cDNA clones by NF-κB reporter assays in HEK293 cells and found that KLF6 (clone 61A3), a tumor suppressor in multiple types of cancers (4, 23), markedly potentiated TNFα- and IL-1β-induced NF-κB activation (Fig. 1A). KLF6 belongs to the KLF family consisting of 17 members in humans. Interestingly, of the ten KLFs that were examined in our screening, only KLF6 substantially increased TNFα- and IL-1β-triggered activation of NF-κB (Fig. 1B). KLF6 is ubiquitously expressed in mammalian tissues and its splicing variants KLF6-SV1, KLF6-SV2, and KLF6-SV3 have been shown to function in different pathways (10). We thus made Flag-tagged expression plasmids encoding human KLF6 or its splicing variants independently of the clone 61A3 and examined their ability to activate NF-κB by reporter assays. However, only the full-length KLF6 potentiated TNFα- and IL-1β-induced NF-κB activation in a dose-dependent manner in HEK293, HeLa, and HCT116 cells (Fig. 1, C–E). In addition, overexpression of KLF6 also potentiated poly(I:C)-triggered TLR3-mediated and LPS-induced TLR4-mediated NF-κB activation (Fig. 1F). Results from quantitative real-time PCR (qPCR) experiments indicated that KLF6 promoted TNFα- and IL-1β-induced transcription of NF-κB downstream genes MCP1, CXCL2, and IL8 but not IκBα (Fig. 1, G and H). These data suggest that KLF6 regulates NF-κB-mediated transcription of a subset of downstream genes after TNFα and IL-1β stimulation.
FIGURE 1.
Overexpression of KLF6 potentiates TNFα- and IL-1β-induced NF-κB activation. A, identification of KLF6 (61A3) as a positive regulator of TNFα- and IL-1β-induced NF-κB activation. Human and mouse expression cDNA clones (Origene Inc.) (0.05 μg) were individually transfected into HEK293 cells (5 × 104) together with an NF-κB luciferase reporter (0.02 μg). Twenty hours after transfection, cells were treated with TNFα (20 ng/ml) or IL-1β (10 ng/ml) or left untreated for 10 h before reporter assays were performed. B, KLF6 but not other members of the KLF family potentiated TNFα- and IL-1β-induced NF-κB activation. Experiments were performed as in A except that indicated plasmids were used. C, KLF6 but not its splicing variants potentiated TNFα- and IL-1β- induced NF-κB activation. HEK293 cells are transfected with KLF6-WT and KLF6 SV1/2/3 expression plasmid (0.05 μg) together with an NF-κB luciferase reporter (0.02 μg). Twenty hours after transfection, cells were treated with TNFα (20 ng/ml) or IL-1β (10 ng/ml) or left untreated for 5 or 10 h before reporter assays were performed. (WT: wild-type; SV1: splicing variant 1; SV2: splicing variant 2; SV3: splicing variant 3). D, overexpression of KLF6 potentiates TNFα- and IL-1β induced NF-κB activation in a dose-dependent manner. HEK293 cells (1 × 105) were transfected with Flag-KLF6 expression plasmid (0, 0.01, 0.02, or 0.05 μg) together with an NF-κB luciferase reporter (0.02 μg). Twenty hours after transfection, cells were treated with TNFα (20 ng/ml) or IL-1β (10 ng/ml) or left untreated for 10 h before reporter assays were performed. E, overexpression of KLF6 potentiates TNFα- and IL-1β-induced activation of NF-κB in HeLa and HCT116 cells. HeLa or HCT116 cells (5 × 105) were transfected with NF-κB luciferase reporter (0.04 μg) and Flag-KLF6 plasmid (0.05 μg) by lipofatamine 2000. Twenty hours after transfection, cells were treated with TNFα (20 ng/ml) or IL-1β (10 ng/ml) or left untreated for 10 h before reporter assays were performed. F, overexpression of KLF6 potentiates poly(I:C)- or LPS-induced activation of NF-κB in TLR3–293 or TLR4–293 cells. TLR3/4–293 cells were treated as in E except cells are stimulated with poly(I:C) (10 μg/ml) or LPS (1 μg/ml) for 6 h. G and H, overexpression of KLF6 potentiates TNFα- and IL-1β-induced expression of MCP1, CXCL2, and IL8. HEK293 cells (4 × 105) were transfected with an empty control vector or Flag-KLF6 plasmid (0.5 μg). Twenty hours after transfection, cells were treated with TNFα (20 ng/ml) (G) or IL-1β (10 ng/ml) (H) or left untreated for the indicated time points before qPCR was performed.
Knockdown of KLF6 Impairs TNFα- and IL-1β-triggered NF-κB Activation
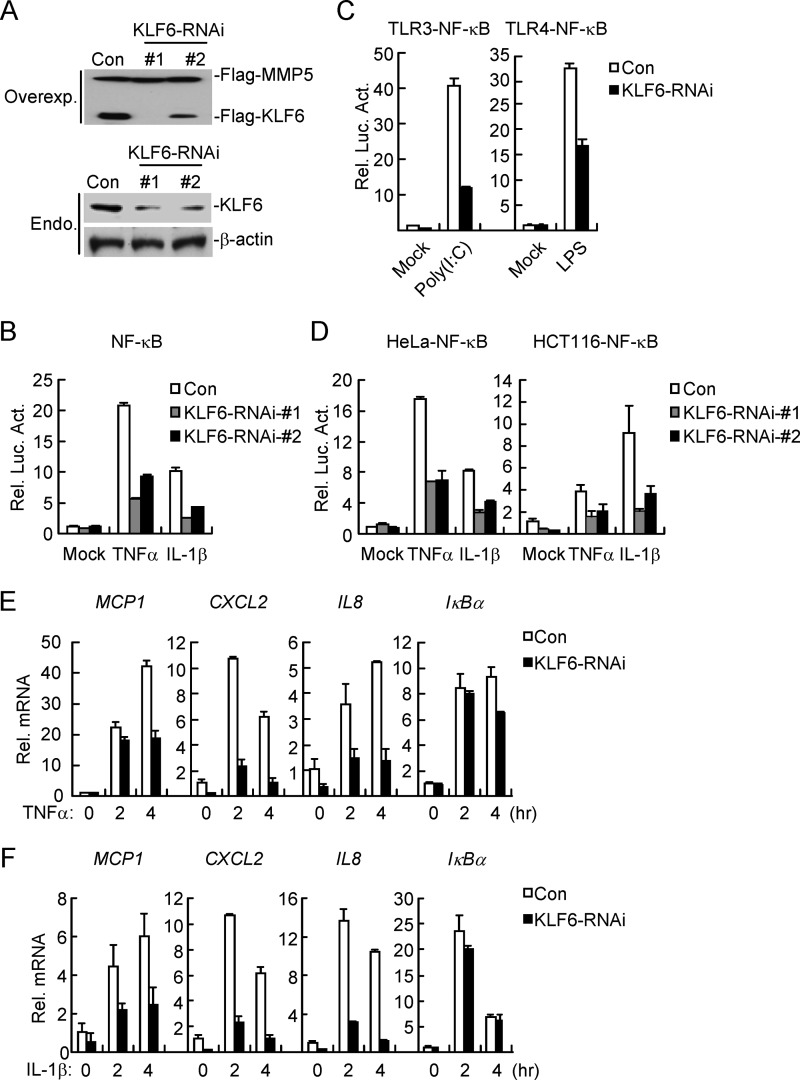
We next determined the effect of KLF6 knockdown on TNFα- and IL-1β-induced NF-κB activation by reporter assays. We constructed two human KLF6-RNAi plasmids, both of which could markedly inhibit expression of transfected KLF6 in HEK293 cells or endogenous KLF6 in HCT116 cells (Fig. 2A). In reporter assays, knockdown of KLF6 inhibited TNFα- and IL-1β-induced NF-κB activation in HEK293, HeLa, and HCT116 cells, and the degrees of inhibition were correlated with the efficiencies of knockdown of KLF6 by each RNAi plasmids (Fig. 2, B and D). We selected KLF6-RNAi-#1 plasmid for additional experiments described below, and similar results were obtained with KLF6-RNAi-#2. We found that knockdown of KLF6 also inhibited poly(I:C)- or LPS-induced NF-κB activation in TLR3 or TLR4-293 cell lines (Fig. 2C). Results from qPCR experiments suggested that knockdown of KLF6 inhibited TNFα- and IL-1β-induced expression of NF-κB downstream genes MCP1, CXCL2, and IL8 but not IκBα (Fig. 2, E and F), indicating that KLF6 mediates the transcription of a subset of downstream genes of NF-κB. However, neither overexpression nor knockdown of KLF6 had noticeable effects on IFNγ-induced IRF1 promoter activation (data not shown). Taken together, these data suggest that KLF6 plays a crucial role in the regulation of TNFα- and IL-1β-induced NF-κB-mediated transcription of a subset of downstream genes.
FIGURE 2.
Knockdown of KLF6 impairs TNFα- and IL-1β-triggered NF-κB activation. A, efficiencies of KLF6-RNAi plasmids on KLF6 expression. In the upper panels, HEK293 cells (4 × 105) were transfected with expression plasmids for Flag-KLF6 (0.2 μg), Flag-MMP5 (0.05 μg) and the indicated RNAi plasmids (2 μg each). Twenty-four hours after transfection, cell lysates were analyzed by immunoblots with anti-Flag. In the lower panels, HCT116 cells (1 × 106) were transfected with a control or the indicated KLF6-RNAi plasmids (10 μg each). Thirty-six hours later, cells were lysed, and the cell lysates were analyzed by immunoblots with anti-KLF6 or anti-β-actin. B, knockdown of KLF6 inhibits TNFα- and IL-1β-induced NF-κB activation. HEK293 cells (1 × 105) were transfected with the indicated KLF6-RNAi (0.5 μg each) and NF-κB luciferase reporter (0.02 μg) plasmids. Thirty-six hours after transfection, cells were treated with TNFα (20 ng/ml) or IL-1β (10 ng/ml) or left untreated for 10 h before luciferase assays were performed. C, knockdown of KLF6 potentiates poly(I:C)- or LPS-induced activation of NF-κB in TLR3–293 or TLR4–293 cells. TLR3/4–293 cells (5 × 105) were transfected with the indicated KLF6-RNAi (1 μg each) and NF-κB luciferase reporter (0.02 μg) plasmids by lipofectamine 2000. Thirty-six hours after transfection, cells were treated with poly(I:C) (10 μg/ml) or LPS (1 μg/ml) for 6 h before luciferase assays were performed. D, knockdown of KLF6 inhibits TNFα- and IL-1β-induced activation of NF-κB in HeLa and HCT116 cells. HeLa and HCT116 cells were treated as in C except cells are stimulated for TNFα (20 ng/ml) or IL-1β (10 ng/ml) for 10 h. E and F, knockdown of KLF6 inhibits TNFα- and IL-1β-induced expression of MCP1, CXCL2, and IL8. HEK293 cells (4 × 105) were transfected with a control or KLF6-RNAi plasmid (3 μg). Thirty-six hours after transfection, cells were left untreated or treated with TNFα (20 ng/ml) (E) or IL-1β (10 ng/ml) (F) for the indicated time points before qPCR was performed.
KLF6 Is Dispensable for TNFα- and IL-1β-triggered Nuclear Translocation of NF-κB
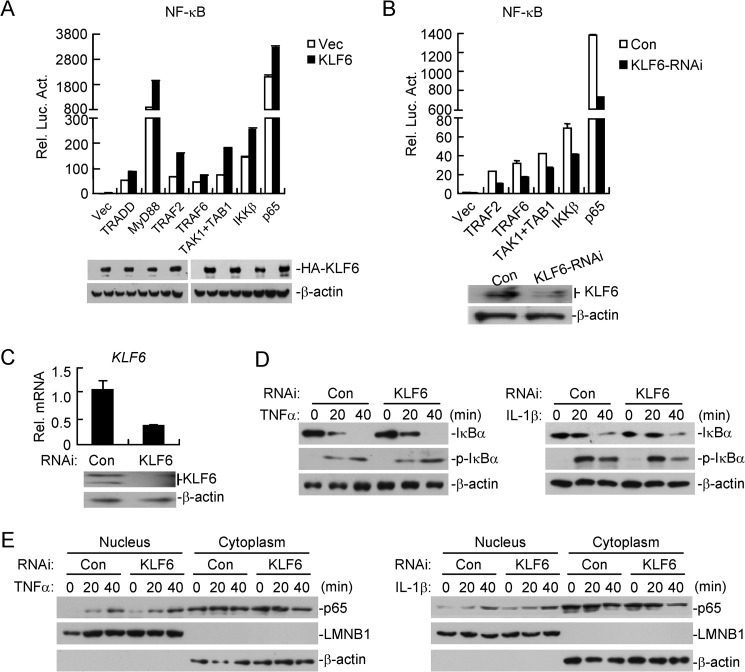
Various molecules have been reported to regulate TNFα- and IL-1β-triggered NF-κB activation, including TRADD, MyD88, TRAF2, TRAF6, TAK1/TABs, IKKβ, and p65. To determine the molecular order of KLF6 in regulating NF-κB activation, we transfected plasmids encoding these molecules in the presence or absence of KLF6 and performed reporter assays. Results indicated that KLF6 enhanced NF-κB activation mediated by p65- and its upstream molecules (Fig. 3A). Conversely, knockdown of KLF6 had the opposite effects (Fig. 3B), suggesting that KLF6 functions at the level of or downstream of p65. To further confirm this conclusion, we established HCT116 cell lines stably transfected with a control or KLF6 RNAi plasmid and examined TNFα- or IL-1β-induced phosphorylation and degradation of IκBα, two hallmarks of canonical NF-κB activation. As shown in Fig. 3D, knockdown of KLF6 had minimal effects on TNFα- or IL-1β-triggered phosphorylation and degradation of IκBα. In addition, TNFα- or IL-1β-induced nuclear translocation of p65 was comparable between the cells stably transfected with a control or KLF6-RNAi plasmid (Fig. 3E). These data collectively suggest that KLF6 is dispensable for NF-κB nuclear translocation upon TNFα or IL-1β stimulation.
FIGURE 3.
KLF6 is dispensable for TNFα- and IL-1β-triggered nuclear translocation of NF-κB. A, overexpression of KLF6 potentiates TRADD-, MyD88-, TRAF2-, TRAF6-, TAK1/TAB1-, IKKβ-, or p65-mediated NF-κB activation. HEK293 cells (1 × 105) were transfected with an empty control vector or HA-tagged KLF6 expression plasmid (0.05 μg) and the indicated plasmids (0.1 μg each). Reporter assays were performed 20 h after transfection. The lysates were analyzed by immunoblots with anti-HA or anti-β-actin. B, knockdown of KLF6 inhibits TAK1/TAB1-, TRAF2-, TRAF6-, IKKβ-, or p65-mediated NF-κB activation. KLF6-RNAi or control cell line, which was constructed in 293 cells (1 × 105) was transfected with the indicated plasmids (0.1 μg each) and NF-κB reporter plasmid (0.02 μg). Reporter assays were performed 20 h after transfection. The efficiency of the knockdown of KLF6 was analyzed by immunoblots. C, construction of a KLF6-RNAi stable cell line. HCT116 cells were stably transfected with control RNAi or KLF6-RNAi by retroviral-mediated gene transfer. The cells (4 × 105) were analyzed by qPCR or immunoblots. D, knockdown of KLF6 has no marked effects on TNFα- or IL-1β-triggered phosphorylation and degradation of IκBα. The cells in C (2 × 105) were treated with TNFα (20 ng/ml) or IL-1β (10 ng/ml) for the indicated time points before immunoblot analysis was performed. E, knockdown of KLF6 has no marked effects on p65 translocation. Cells in C (2 × 105) were treated with TNFα (20 ng/ml) or IL-1β (10 ng/ml) for the indicated time points. The cytoplasm extracts were prepared in 1 ml of homogenization buffer, whereas the nuclear extracts were prepared in 0.2 ml of nuclear lysis buffer. Equal volumes of the cytoplasm and nuclear extracts were loaded for immunoblot analysis with the indicated antibodies.
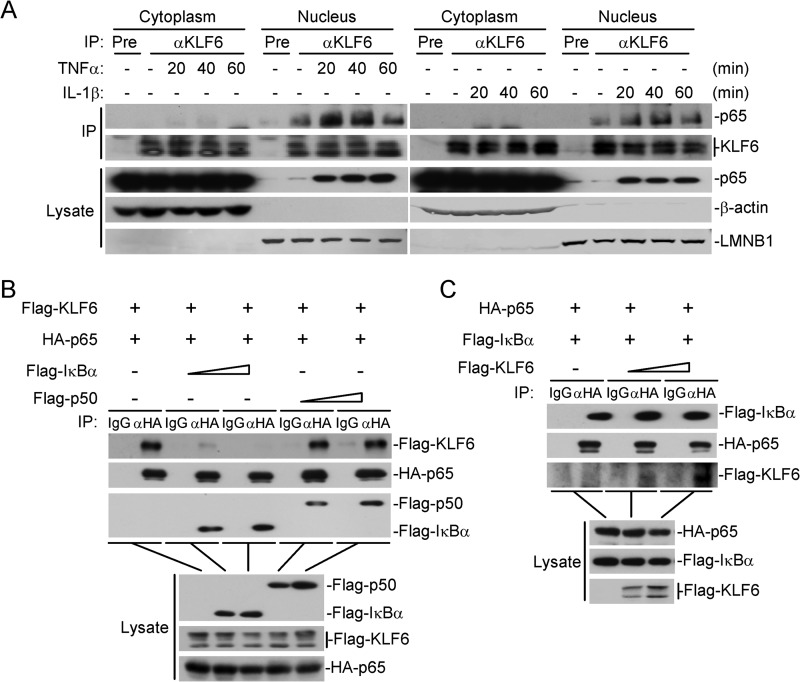
KLF6 Interacts with p65 in the Nucleus
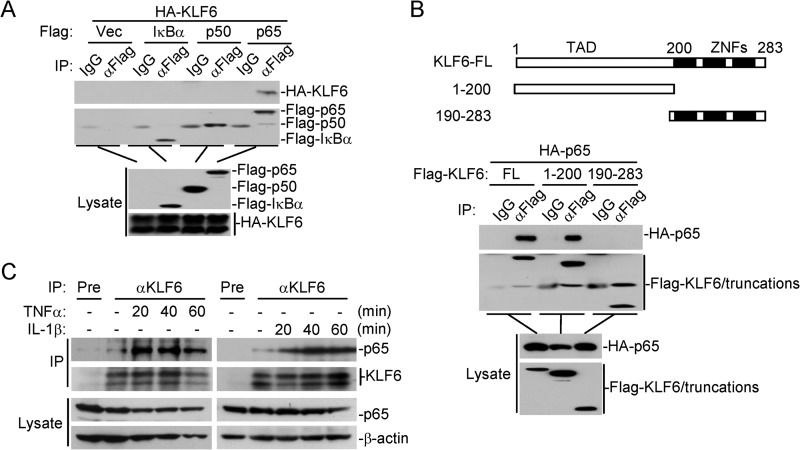
We next examined whether KLF6 interacts with canonical NF-κB protein p65 or p50. In transient transfection and coimmunoprecipitation experiments, overexpression of KLF6 interacted with p65 constitutively but not with p50 and IκBα (Fig. 4A). Results from domain mapping experiments indicated that the N-terminal transcription activation domain of KLF6(aa1–200) was required for their interaction (Fig. 4B). The endogenous KLF6 interacted with p65 weakly without stimulation, and this association was enhanced upon TNFα or IL-1β stimulation (Fig. 4C). It has been reported that KLF6 shuttles between the cytoplasm and nucleus (24). We thus performed cellular fractionation assays to isolate the cytosol and nuclear fractions and examined whether KLF6 interacts with p65 in the nucleus or cytoplasm by coimmunoprecipitation. Consistent with previous studies, KLF6 was found both in the nucleus and the cytoplasm. However, KLF6 only interacted with p65 in the nucleus, and this interaction could be increased following TNFα or IL-1β treatment (Fig. 5A). As a negative feedback regulator, IκBα is synthesized at later times after TNFα or IL-1β stimulation and drives p65/p50 from the nucleus back to the cytoplasm. Interestingly, co-expression of IκBα but not p50 dose-dependently disrupted KLF6-p65 interaction, indicating that IκBα sequesters p65 in the cytoplasm to prevent interaction of p65 with KLF6 (Fig. 5B). Because TNFα- or IL-1β-induced expression and degradation of IκBα was not altered by overexpression or knockdown of KLF6 (Figs. 1, G and H and 3D), we suspected that KLF6 inhibited IκBα-mediated retention of p65 in the cytoplasm by associating with p65 in the nucleus, resulting in retention of p65 in the nucleus for continued transcriptional activation. To test this possibility, we examined IκBα-p65 interaction in the presence or absence of KLF6. As shown in Fig. 5C, however, IκBα-p65 interaction remained unchanged when KLF6 was co-expressed, indicating that KLF6 does not interfere IκBα-p65 interaction and possibly regulates p65 transcriptional activity in the nucleus.
FIGURE 4.
KLF6 interacts with p65. A, KLF6 interacts with p65 in mammalian overexpression system. HEK293 cells (3 × 106) were transfected with the indicated plasmids (5 μg each) for 24 h. Coimmunoprecipitation and immunoblots were performed with the indicated antibodies. B, transcription activation domain of KLF6 is required for its interaction with p65. HEK293 cells (2 × 106) were transfected with HA-p65 (1 μg) and Flag-KLF6 or its truncation mutants (5 μg each) for 20 h before coimmunoprecipitation and immunoblot analysis were performed with the indicated antibodies. KLF6-FL: KLF6-full length. C, endogenous KLF6 interacts with p65. HEK293 cells (3 × 107) were left untreated or treated with TNFα (20 ng/ml) or IL-1β (10 ng/ml) for the indicated time points. Endogenous coimmunoprecipitation and immunoblots were performed with the indicated antibodies.
FIGURE 5.
KLF6 interacts with p65 in the nucleus. A, KLF6 interacts with p65 in the nucleus. HEK293 cells (5 × 107) were left untreated or treated with TNFα (20 ng/ml) or IL-1β (10 ng/ml) for the indicated time points. Cells fractionation was performed, and the cytoplasmic and nuclear proteins were prepared in 1 ml of homogenization buffer or nuclear extraction buffer, respectively, following by coimmunoprecipitation and immunoblot analysis. B, IκBα but not p50 disrupts the p65-KLF6 interaction in a dose-dependent manner. HEK293 cells (3 × 106) were transfected with the indicated plasmids together with IκBα (1 or 2 μg) or p50 (1 or 2 μg) expression plasmids. Coimmunoprecipitation and immunoblots were performed with the indicated antibodies. C, KLF6 does not affect p65-IκBα interaction. HEK293 cells (3 × 106) were transfected with the indicated plasmids together with KLF6-expression plasmids (4 or 8 μg). Coimmunoprecipitation and immunoblots were performed with the indicated antibodies.
KLF6 Binds to the Promoters of a Subset of p65 Target Genes
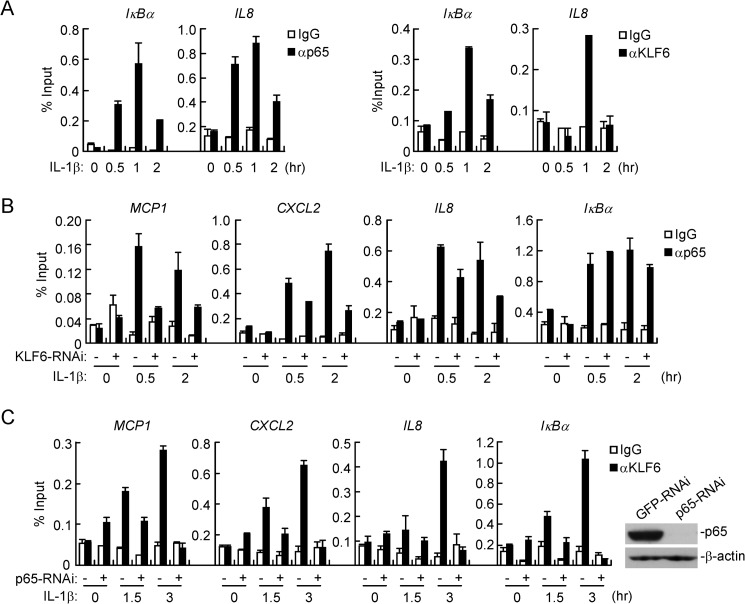
Because KLF6 interacted with p65 in the nucleus and was required for TNFα- and IL-1β-induced expression of a subset of downstream genes, we hypothesized that KLF6 might be recruited to the promoters of these genes to promote p65-dependent transcription. We focused on KLF6-mediated regulation of NF-κB target genes after IL-1β stimulation, as TNFα stimulation similarly induced NF-κB activation and KLF6-p65 interaction as IL-1β. Using ChIP assays, we found that IL-1β stimulation induce binding of p65 and KLF6 to the promoters of IκBα and IL8, all of which are NF-κB target genes downstream of TNFα and IL-1β (Fig. 6A). Interestingly, p65 bound to the promoters of MCP1, CXCL2, and IL8 genes following IL-1β stimulation, which was diminished in cells stably transfected with KLF6-RNAi (Fig. 6B). In addition, IL-1β-induced binding of KLF6 to the promoters of MCP1, CXCL2 and IL8 genes was impaired by knockdown of p65 (Fig. 6C). It should be noted that knockdown of KLF6 did not affect binding of p65 to the promoter of IκBα gene whereas knockdown of p65 impaired binding of KLF6 to the promoter of IκBα gene. These data suggest that KLF6 and p65 are mutually required for their binding to a subset of NF-κB target genes downstream of IL-1β.
FIGURE 6.
KLF6 binds to promoters of a subset of p65 target genes. A, KLF6 and p65 bind to the promoters of IκBα and IL8 genes after IL-1β stimulation. HCT116 cells (5 × 106) were left untreated or treated with IL-1β (10 ng/ml) for the indicated time points, and then ChIP assays were performed with the indicated antibodies. Binding of KLF6 and p65 to the indicated promoters was determined by qPCR with primers specific for the promoters of the indicated genes. B, knockdown of KLF6 diminishes binding of p65 to promoters of MCP1, CXCL2, and IL8 but not IκBα genes. KLF6-RNAi or control RNAi stable cell lines (5 × 106) were left untreated or treated with IL-1β (10 ng/ml) for the indicated time points. ChIP assays were performed with the indicated antibodies. Binding of p65 to the indicated promoters was determined by qPCR. C, knockdown of p65 impairs DNA binding of KLF6 to promoters of MCP1, CXCL2, IL8, and IκBα genes. HCT116 cells (5 × 106) stably transfected with control or KLF6-RNAi plasmids were left untreated or treated with IL-1β (10 ng/ml) for the indicated time points. ChIP assays were performed with the indicated antibodies. Binding of KLF6 to the indicated promoters was determined by qPCR. Knockdown of p65 was measured by immunoblot analysis.
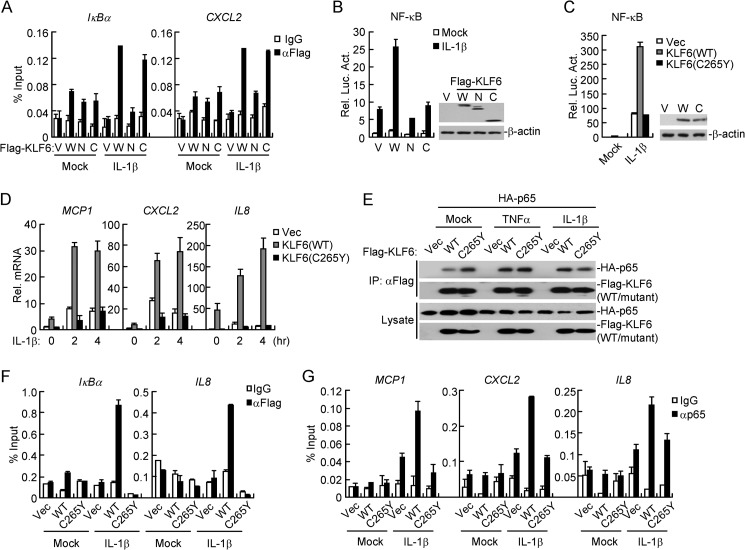
The DNA Binding Activity of KLF6 Is Essential to Promote Binding of p65 to the Promoters of Downstream Genes
KLF6 consists of an N-terminal transcriptional activation domain (TAD) and three C-terminal zinc finger domains (ZNFs), which are responsible for its transcriptional and DNA binding activities, respectively (11). Although the N-terminal TAD interacted with p65 and the C-terminal ZNFs bound to the promoters of IκBα or CXCL2 (Figs. 4B and 7A), neither of these two domains alone potentiated IL-1β-induced activation of NF-κB in reporter assays (Fig. 7B), indicating that the intact KLF6-p65 complex formation is critical for mediating p65-driven expression of target genes. Previously, it has been demonstrated that the Cys265→Tyr265 (C265Y) mutation of KLF6 impairs its association with DNA or proteins (25). In our experiments, we found that KLF6(C265Y) could not enhance NF-κB activation upon IL-1β stimulation or potentiate IL-1β-triggered induction of MCP1, CXCL2, or IL8 (Fig. 7C&D), although KLF6(C265Y) was fully capable of interacting with p65 (Fig. 7E). Consistent with these observations, KLF6(C265Y) lost the binding activity to the promoters of IκBα or IL8 genes upon IL-1β stimulation (Fig. 7F). In addition, KLF6(C265Y) failed to promote binding of p65 to the promoters of MCP1, CXCL2, or IL8 genes (Fig. 7G). These data together suggest that the ZNFs-mediated DNA binding activity of KLF6 is required for optimal binding of p65 to the promoters of target genes.
FIGURE 7.
The DNA binding activity of KLF6 is essential to promote p65 binding to the promoters. A, KLF6-WT and its ZNFs but not its TAD could bind to the promoters of IκBα and CXCL2. HEK293 cells (3 × 106) were transfected with indicated plasmids (5 μg each). Twenty hours after transfection, cells were treated with IL-1β (10 ng/ml) for 30 min. ChIP assays were performed with the indicated antibodies. The binding of Flag-tagged KLF6 or its truncation mutants with indicated promoters were determined by qPCR analysis. V: Vector, W: wild-type of KLF6, N: KLF6(aa1–200), and C: KLF6(aa190–283). B, neither the N terminus nor C terminus of KLF6 potentiates IL-1β-induced NF-κB activation. HEK293 cells (1 × 105) were transfected with the NF-κB luciferase reporter (20 ng) and Flag-KLF6 or its truncation mutants (20 ng each). Twenty hours after transfection, cells were untreated or treated with IL-1β (10 ng/ml) for 10 h before reporter assays were performed. C, wild-type KLF6 but not the KLF6(C265Y) potentiates IL-1β-induced NF-κB activation. HEK293 cells (1 × 105) were transfected with an NF-κB luciferase reporter (0.02 μg) and the indicated plasmids (0.02 μg each). Twenty hours after transfection, cells were untreated or treated with IL-1β (10 ng/ml) for 10 h before reporter assays were performed. The lysates were analyzed by immunoblots with the indicated antibodies. D, wild-type KLF6 but not KLF6(C265Y) mutant potentiates IL-1β-induced transcription of MCP1, CXCL2, and IL8 genes. HEK293 cells (4 × 105) stably transfected with control vector, Flag-KLF6 or Flag-KLF6(C265Y)were untreated or treated with IL-1β (10 ng/ml) for the indicated time points before qPCR analysis was performed. E, KLF6(C265Y) interacts with p65. HEK293 cells (2 × 106) were transfected with HA-tagged p65 (1 μg) and Flag-tagged KLF6 or its C265Y mutant (5 μg each) for 20 h before coimmunoprecipitation and immunoblot analysis was performed with the indicated antibodies. F, wild-type KLF6 but not KLF6(C265Y) mutant could bind to promoters IκBα and IL8 genes after IL-1β stimulation. HEK293 cells (4 × 105) stably transfected with control vector, Flag-KLF6, or Flag-KLF6(C265Y) were stimulated with IL-1β (10 ng/ml) for 30 min. ChIP assays were performed with the indicated antibodies and binding of KLF6 or KLF6(C265Y) to the cognate sites was determined by qPCR. G, overexpression of wild-type KLF6 but not KLF6(C265Y) potentiates IL-1β-induced DNA binding of p65 to promoters of MCP1, CXCL2, and IL8 genes. The experiments were performed as in F except that the p65 antibody was used.
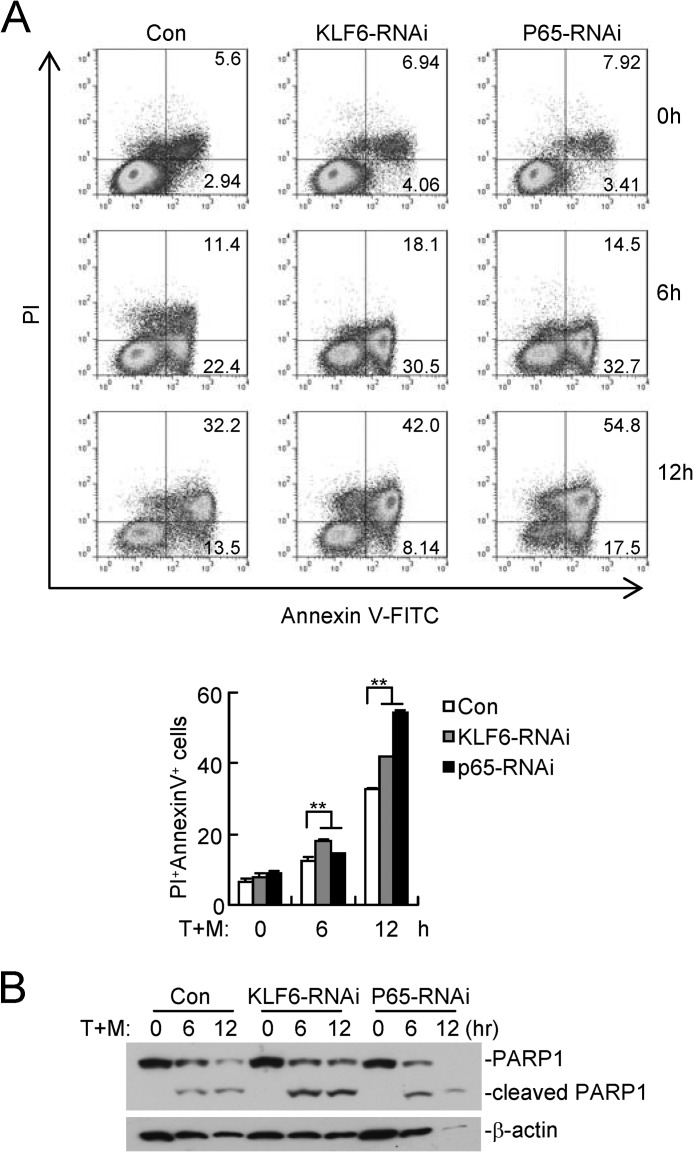
KLF6 Regulates TNFα-triggered Apoptosis
We next examined the physical relevance of KLF6 in regulating TNFα-mediated cellular effects. As shown in Fig. 8A, TNF and Smac mimetic-induced apoptosis of HCT116 cells was substantially enhanced by knockdown of KLF6 or p65. Consistent with these observations, the cleavage of PARP1 was potentiated in KLF6-RNAi compared with control cells (Fig. 8B). These data suggest that KLF6 plays a role in antagonizing apoptosis induced by TNFα and Smac mimetic.
FIGURE 8.
KLF6 antagonizes TNFα-induced apoptosis. A, knockdown of KLF6 or p65 enhanced TNFα and Smac mimetic-induced apoptosis. HCT116 cells stably transfected with the indicated RNAi plasmids were untreated or treated with TNFα (100 μg/ml) and Smac mimetic (100 nm) for 6 or 12 h. The apoptotic cells were stained with annexin V-FITC and PI followed by flow cytometry analysis (upper panel). The flow cytometry data are also presented quantitatively (lower histograph). The graphs show mean ± S.D., n = 3. **, p < 0.01; T+M: TNFα+Smac mimetic. B, knockdown of KLF6 or p65-enhanced TNFα and Smac mimetic-induced cleavages of PARP1. Experiments were performed as in A except that cells were lysed and analyzed by immunoblots with the indicated antibodies.
DISCUSSION
The transcription factor NF-κB is involved in various physiological and pathological processes, including development, inflammation, immunity, and cancers. In this study, we screened a pool of 15,000 independent human and mouse expression clones for potential factors involved in TNFα- or IL-1β-induced activation of NF-κB. This effort identified KLF6, as a positive regulator of TNFα- and IL-1β-induced NF-κB activation. Overexpression of KLF6 potentiated TNFα- and IL-1β-induced expression of a subset of NF-κB target genes, while knockdown of KLF6 had opposite effects. In contrast, neither overexpression nor knockdown of KLF6 had any effects on IFNγ-induced IRF1 activation. These data suggest that KLF6 selectively regulates TNFα- or IL-1β-induced NF-κB activation.
Because knockdown of KLF6 had minimal effects on TNFα- or IL-1β-induced phosphorylation and degradation of IκBα or nuclear translocation of p65, we reasoned that KLF6 might function at the level of or downstream of p65 in the nucleus. This notion was supported by the observations that KLF6 interacted with p65 in the nucleus and was required for optimal binding of p65 to the promoters of a subset of downstream genes. KLF6 contains a TAD domain in its N terminus and three ZNF domains in its C terminus, which are required for its interaction with p65 and the binding to the promoters of p65 target genes, respectively. In addition, the KLF6(C265Y) mutant, which lost DNA binding activity but retained the p65-interacting ability, failed to potentiate IL-1β-induced expression of downstream genes and binding of p65 to the promoters. Our study also demonstrated that p65 was indispensable for optimal binding of KLF6 to the promoters of target genes, as knockdown of p65 diminished IL-1β-induced binding of KLF6 to the promoters of MCP1, CXCL2, IL8, and IκBα genes. A simple interpretation for these observations is that KLF6 binds to the promoters of p65 target genes and interacts with p65 through its respective C-terminal and N-terminal domains, which anchors p65 on the promoters to enhance the transcription of downstream genes, and that the binding of p65 to the promoter facilitates binding of KLF6 to the same promoters (discussed below).
The p65/p50 heterodimer binds to the so-called κB site 5′-GGGRNYYYCC-3′ (where R is a purine, Y is a pyrimidine, and N is an unspecified base), whereas the KLF family proteins recognize the “CACCC-box” that represents the reverse complement sequence for “GGGRN” from the κB site, indicating that KLF6 and p65 may bind to the same DNA sequences on the promoters of target genes. It has been reported that NF-κB binding to the κB sites is transient and quite dynamic, which allows efficient but proper transcription of the target genes. TNFα or IL-1β stimulation induces formation of KLF6 and p65 complex and collaborative binding of these two proteins to the promoters of downstream genes. When one factor is disassociated from the promoters because of the high on/off binding rate, the other would bind the same site, thereby increasing the chance with which KLF6 or p65 binds to the target promoters and induces the transcription of target genes. This explains why KLF6 and p65 are mutually required for promoter binding ability for a subset of downstream genes.
It should be noted that not all p65 target genes, IκBα for instance, were regulated by KLF6; although KLF6 bound to the promoter of the IκBα gene after TNFα or IL-1β stimulation, indicating that other proteins may be involved in the process. Interestingly, knockdown of KLF6 also enhanced apoptosis triggered by TNFα and Smac mimetic, which could be due to impaired KLF6-mediated expression of p65-dependent genes. High throughput ChIP-sequencing may be used for a thorough identification of KLF6/p65-binding promoters in the future. Nonetheless, our study has clearly demonstrated that KLF6 is a co-activator of p65 for transcription of selected downstream genes after TNFα or IL-1β stimulation.
Although much progress has been made to characterize the activation of NF-κB upon various stimulations, how NF-κB in limited forms of dimmers functions to differentially induce transcription of thousands of downstream genes remains largely unclear. Our identification of KLF6 as a co-activator of p65 has thus uncovered a previously unknown mechanism by which NF-κB target genes are regulated in a gene-specific manner. Our findings thus identified KLF6 as a previously unknown but essential co-activators of NF-κB and provided new insight into the molecular regulation of p65-dependent gene expression.
Acknowledgments
We thank Xiao-Dong Wang (NIBS, Beijing) for providing Smac Mimetic. We also thank Yan-Yi Wang (Wuhan Institute of Virology), Yu Liu, Shu Li, Rui Chen, Ming-Ming Hu, Xiang Wang, and Shang-Ze Li (Wuhan University) for technical assistance and stimulating discussions.
This study was supported by the Ministry of Science and Technology of China (2012CB910201, 2010CB911800, 2014CB542600), and the National Natural Science Foundation of China (31221061, 31130020, 31371427, and 91029302).
- NF-κB
- nuclear factor κB
- KLF6
- Krüppel-like factor 6
- TNFα
- tumor necrosis factor α
- IL-1β
- interleukin 1β
- TAD
- transcriptional activation domains
- IκBα
- inhibitor of κBα
- SVs
- splicing variants
- ChIP
- chromatin immunoprecipitation
- ZNFs
- zinc finger domains
- RARP1
- polymerase 1.
REFERENCES
- 1. Vallabhapurapu S., Karin M. (2009) Regulation and function of NF-κB transcription factors in the immune system. Annu. Rev. Immunol. 27, 693–733 [DOI] [PubMed] [Google Scholar]
- 2. Ghosh S., May M. J., Kopp E. B. (1998) NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16, 225–260 [DOI] [PubMed] [Google Scholar]
- 3. Baldwin A. S., Jr. (1996) The NF-κB and IκB proteins: new discoveries and insights. Annu. Rev. Immunol. 14, 649–683 [DOI] [PubMed] [Google Scholar]
- 4. May M. J., Ghosh S. (1997) Rel/NF-κB and IκB proteins: an overview. Semin. Cancer Biol. 8, 63–73 [DOI] [PubMed] [Google Scholar]
- 5. Wan F., Anderson D. E., Barnitz R. A., Snow A., Bidere N., Zheng L., Hegde V., Lam L. T., Staudt L. M., Levens D., Deutsch W. A., Lenardo M. J. (2007) Ribosomal protein S3: a KH domain subunit in NF-κB complexes that mediates selective gene regulation. Cell 131, 927–939 [DOI] [PubMed] [Google Scholar]
- 6. Goto A., Matsushita K., Gesellchen V., El Chamy L., Kuttenkeuler D., Takeuchi O., Hoffmann J. A., Akira S., Boutros M., Reichhart J. M. (2008) Akirins are highly conserved nuclear proteins required for NF-κB-dependent gene expression in Drosophila and mice. Nature Immunology 9, 97–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kawahara T. L., Michishita E., Adler A. S., Damian M., Berber E., Lin M., McCord R. A., Ongaigui K. C., Boxer L. D., Chang H. Y., Chua K. F. (2009) SIRT6 links histone H3 lysine 9 deacetylation to NF-κB-dependent gene expression and organismal life span. Cell 136, 62–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saijo K., Winner B., Carson C. T., Collier J. G., Boyer L., Rosenfeld M. G., Gage F. H., Glass C. K. (2009) A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell 137, 47–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oeckinghaus A., Hayden M. S., Ghosh S. (2011) Crosstalk in NF-κB signaling pathways. Nat. Immunol. 12, 695–708 [DOI] [PubMed] [Google Scholar]
- 10. DiFeo A., Martignetti J. A., Narla G. (2009) The role of KLF6 and its splice variants in cancer therapy. Drug Resistance Updates: Reviews and Commentaries in Antimicrobial and Anticancer Chemotherapy 12, 1–7 [DOI] [PubMed] [Google Scholar]
- 11. Bieker J. J. (2001) Kruppel-like factors: three fingers in many pies. J. Biol. Chem. 276, 34355–34358 [DOI] [PubMed] [Google Scholar]
- 12. Zhang W., Bieker J. J. (1998) Acetylation and modulation of erythroid Kruppel-like factor (EKLF) activity by interaction with histone acetyltransferases. Proc. Natl. Acad. Sci. U. S. A. 95, 9855–9860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Vliet J., Turner J., Crossley M. (2000) Human Kruppel-like factor 8: a CACCC-box binding protein that associates with CtBP and represses transcription. Nucleic Acids Res. 28, 1955–1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu L. J., Liu T. T., Ran Y., Li Y., Zhang X. D., Shu H. B., Wang Y. Y. (2012) The E3 ubiquitin ligase MIB1 negatively regulates basal IκBα level and modulates NF-κB activation. Cell Res. 22, 603–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xu L. G., Wang Y. Y., Han K. J., Li L. Y., Zhai Z., Shu H. B. (2005) VISA is an adapter protein required for virus-triggered IFN-β signaling. Mol. Cell 19, 727–740 [DOI] [PubMed] [Google Scholar]
- 16. Li Q., Yan J., Mao A. P., Li C., Ran Y., Shu H. B., Wang Y. Y. (2011) Tripartite motif 8 (TRIM8) modulates TNFα- and IL-1β-triggered NF-κB activation by targeting TAK1 for K63-linked polyubiquitination. Proc. Natl. Acad. Sci. U. S. A. 108, 19341–19346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lei C. Q., Zhang Y., Xia T., Jiang L. Q., Zhong B., Shu H. B. (2013) FoxO1 negatively regulates cellular antiviral response by promoting degradation of IRF3. J. Biol. Chem. 288, 12596–12604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lei C. Q., Zhong B., Zhang Y., Zhang J., Wang S., Shu H. B. (2010) Glycogen synthase kinase 3beta regulates IRF3 transcription factor-mediated antiviral response via activation of the kinase TBK1. Immunity 33, 878–889 [DOI] [PubMed] [Google Scholar]
- 19. Li Y., Chen R., Zhou Q., Xu Z., Li C., Wang S., Mao A., Zhang X., He W., Shu H. B. (2012) LSm14A is a processing body-associated sensor of viral nucleic acids that initiates cellular antiviral response in the early phase of viral infection. Proc. Natl. Acad. Sci. U. S. A. 109, 11770–11775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Berkovich E., Monnat R. J., Jr., Kastan M. B. (2008) Assessment of protein dynamics and DNA repair following generation of DNA double-strand breaks at defined genomic sites. Nature Protocols 3, 915–922 [DOI] [PubMed] [Google Scholar]
- 21. Wang X., Zhu K., Li S., Liao Y., Du R., Zhang X., Shu H. B., Guo A. Y., Li L., Wu M. (2012) MLL1, a H3K4 methyltransferase, regulates the TNFα-stimulated activation of genes downstream of NF-κB. J. Cell Sci. 125, 4058–4066 [DOI] [PubMed] [Google Scholar]
- 22. Wang L., Du F., Wang X. (2008) TNF-α induces two distinct caspase-8 activation pathways. Cell 133, 693–703 [DOI] [PubMed] [Google Scholar]
- 23. Narla G., Heath K. E., Reeves H. L., Li D., Giono L. E., Kimmelman A. C., Glucksman M. J., Narla J., Eng F. J., Chan A. M., Ferrari A. C., Martignetti J. A., Friedman S. L. (2001) KLF6, a candidate tumor suppressor gene mutated in prostate cancer. Science 294, 2563–2566 [DOI] [PubMed] [Google Scholar]
- 24. Rodriguez E., Aburjania N., Priedigkeit N. M., DiFeo A., Martignetti J. A. (2010) Nucleo-cytoplasmic localization domains regulate Kruppel-like factor 6 (KLF6) protein stability and tumor suppressor function. PloS One 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim C. A., Berg J. M. (1996) A 2.2 Å resolution crystal structure of a designed zinc finger protein bound to DNA. Nat. Struct. Biol. 3, 940–945 [DOI] [PubMed] [Google Scholar]