Background: The physiological role of Reelin proteolysis is largely uncharacterized.
Results: An “uncleavable” Reelin mutant remains active for a long time, and the cleaved Reelin fragment localizes differently than full-length Reelin.
Conclusion: Extracellular and intracellular Reelin cleavage is required for halting downstream signaling and transport of the cleaved product.
Significance: Inhibition of Reelin cleavage will be beneficial for treating neuropsychiatric diseases.
Keywords: ADAM ADAMTS, Brain, Endosomes, Neurons, Protein Degradation, Signal Transduction, Dab1, Reelin
Abstract
Reelin is a secreted glycoprotein that plays essential roles in the brain. Reelin is specifically cleaved at two distinct sites, called N-t and C-t, with the former being the major one. N-t cleavage can occur both in the extracellular space and in the endosomes, although the physiological importance of endosomal N-t cleavage has not been investigated. In this study, we first determined the exact N-t cleavage site catalyzed by a protease secreted by cerebral cortical neurons. Cleavage occurred between Pro-1244 and Ala-1245 within Reelin repeat 3. A Reelin mutant in which Pro-1244 was replaced with aspartate (Reelin-PD) was resistant to a protease secreted by cultured cerebral cortical neurons, and its biological activity stayed active longer than that of wild-type Reelin. Interestingly, Reelin-PD remained in the intracellular compartments longer than wild-type Reelin and persistently activated downstream signaling. Therefore, N-t cleavage of Reelin is required for halting the signaling machinery in the extracellular space as well as within endosomes of target neurons. We established a monoclonal antibody specific to uncleaved Reelin protein and found that it is localized in the vicinity of Reelin-producing cells, whereas the N-terminal fragment diffuses, or is transported, to distant regions. These data demonstrate that N-t cleavage of Reelin plays critical roles in regulating the duration and range of Reelin functions both in the extracellular milieu and in the intracellular compartments.
Introduction
Secreted proteins play important roles in controlling various events in brain development and function, including neuronal differentiation, migration, morphology, and plasticity (1–3). The activity of extracellular proteins must be attenuated appropriately to avoid hyperactivation of downstream pathways or diffusion to unwanted targets (4, 5). Inactivation of signaling proteins in the extracellular milieu can be achieved through several mechanisms. For example, the activity of bone morphogenetic protein and Wnt are antagonized by the specific binding proteins Chordin (6) and Tsukushi (7), respectively. Additionally, Hedgehog signaling is limited by binding to the signaling-incompetent binding protein glypican 3 and subsequent endocytosis (8). However, proteolysis is arguably the most frequently used system to limit the activity of extracellular signaling proteins (9–11). Therefore, clarifying the mechanism of specific proteolysis is important to fully understand the function of secreted signaling proteins.
Reelin is a large secreted glycoprotein with 3461 amino acid residues in mice (12) that has been implicated in neuronal cell migration, dendrite development, and synaptic plasticity (1, 13–15). A number of studies have suggested that insufficient Reelin activity causes and/or deteriorates neuropsychiatric diseases such as Alzheimer diseases and schizophrenia (16–21). Therefore, augmentation of Reelin activity may represent a therapeutic option for these diseases. However, the mechanisms involved in regulating Reelin activity are largely unknown. Furthermore, there have been no methods developed to augment Reelin activity except for the direct injection of partially purified Reelin protein into the brain (19).
Reelin protein contains an N-terminal region (NTR),4 eight Reelin repeats (RRs), and a C-terminal region (Fig. 1A) (12, 22). The NTR is required for multimerization, which is a prerequisite for its biological activity (23). The central region (RR3-RR6) is necessary for binding to Reelin receptors (24, 25), apolipoprotein E receptor 2 (ApoER2), and very low-density lipoprotein receptors (VLDLR) (26–28). The C-terminal region binds to unidentified membrane molecule(s) and participates in the activation of downstream signaling (29, 30). Reelin is specifically cleaved at two sites, the N-t and C-t sites (Fig. 1A) (31, 32). In the brain, cleavage occurs predominantly at the N-t site, which has often been referred to as occurring “between RR2 and RR3” (24, 32). Reelin, through ApoER2 and VLDLR, induces tyrosine phosphorylation of the intracellular adaptor protein Dab1 (33, 34). This event is an essential component of Reelin signaling (33, 34). Reelin is internalized in an ApoER2/VLDLR-dependent manner (24, 28), but this is not necessary for Dab1 phosphorylation (35, 36). We showed previously that N-t cleavage virtually abolishes the ability of Reelin to induce Dab1 phosphorylation (10). Phosphorylated Dab1 is quickly degraded in the cytoplasm in a proteasome-dependent manner (37–40), and the amount of Dab1 has been utilized as one of the indications of Reelin signaling (26, 33, 37, 40). N-t cleavage is catalyzed by an unidentified metalloprotease(s) that bind(s) heparin (10). Abnormal N-t cleavage has been implicated in Alzheimer disease (41) and epilepsy (42). Recent studies from our laboratory and other laboratories indicate that a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) 4 is able to cleave Reelin at the N-t site (43, 44), although its contribution to Reelin metabolism in vivo remains uncertain.
FIGURE 1.
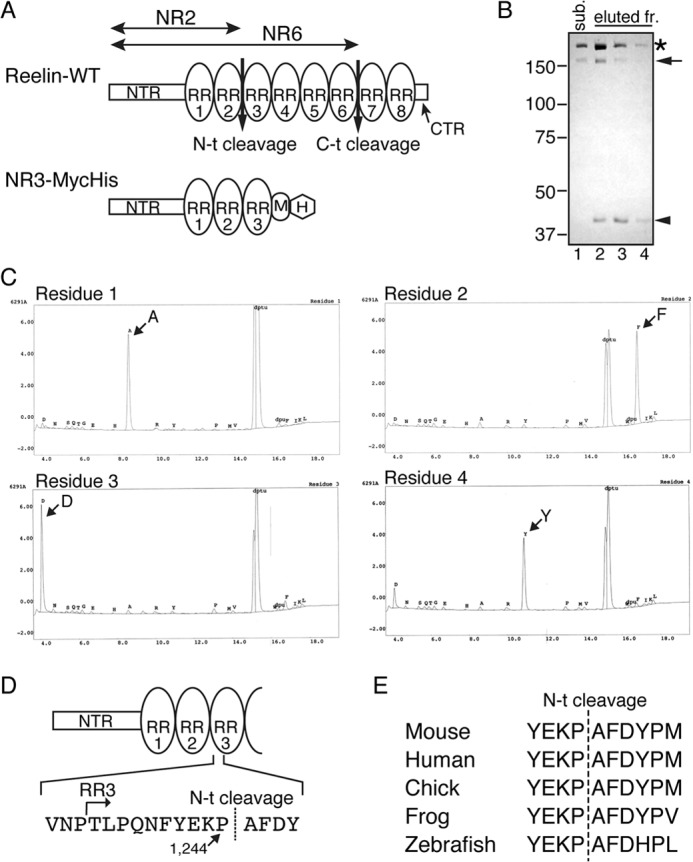
Determination of the Reelin N-t cleavage site. A, schematic of Reelin and the artificial substrate NR3-MycHis. Reelin-WT consists of the NTR, eight tandem RRs (oval), and the C-terminal region (CTR). NR3 consists of the NTR, RR1, RR2, and RR3, followed by a Myc epitope (M) and His6 tag (H). B, purification of the N-t cleavage product. NR3-MycHis protein was incubated with the N-t site protease fraction obtained from cultured cortical neurons, and the reaction mixture was subjected to nickel-agarose column chromatography. The substrate NR3-MycHis (sub., lane 1) and the eluted fractions (eluted fr.) from the column chromatography (lanes 2–4) were analyzed by SDS-PAGE followed by Coomassie Brilliant Blue staining. The asterisk, arrow, and arrowhead denote full-length NR3-MycHis, and the N-terminal and C-terminal products of N-t cleavage, respectively. The C-terminal product of lanes 2-4 were excised from the gel and subjected to Edman sequencing. Positions of molecular mass markers (kDa) are shown on the left. C, automatic Edman sequencing profiles of the C-terminal product of N-t cleavage. D, schematic of the N-t cleavage site and its surrounding sequence. E, comparison of the primary sequence surrounding the N-t cleavage site among various animal species.
The exact N-t cleavage site is unknown. We reasoned that precise determination of the cleavage site has at least four implications. First, analysis of the primary sequence surrounding the cleavage site may be helpful in identifying the protease(s) mediating N-t cleavage. Second, introducing point mutations at the cleavage site may produce an “uncleavable” Reelin mutant, which would be an indispensable tool for investigating the physiological significance of N-t cleavage. Third, it may be possible to produce antibodies capable of recognizing either the uncleaved or cleaved forms of Reelin. Such antibodies would be necessary to clarify the localization of full-length Reelin or its cleaved fragments in situ. Fourth, comparison of the primary sequence surrounding the cleavage site with other species may reveal whether or not N-t cleavage is evolutionarily conserved, which may shed light on the evolutionary changes in the function of Reelin in the brain.
Therefore, we aimed to determine the exact site of N-t cleavage and found that it occurs within RR3 between Pro-1244 and Ala-1245. We then produced an uncleavable Reelin mutant and a monoclonal antibody that specifically recognizes uncleaved Reelin protein. Analyses using these tools clearly showed that N-t cleavage plays important roles in regulating the duration and range of Reelin signaling. Most importantly, N-t cleavage is a prerequisite for the clearance of internalized Reelin from the endosome, which is required for halting the Dab1-phosphorylating machinery.
EXPERIMENTAL PROCEDURES
Reagents, Antibodies, and Animals
The following antibodies were purchased: anti-Reelin G10 and anti-phosphotyrosine 4G10 from Millipore, anti-Reelin AF3820 from R&D Systems, anti-Myc (clone 9E10) and anti-β-actin (clone AC-15) from Sigma, and anti-Rab11 antiserum from Abcam. Anti-Dab1 was produced and affinity-purified in our laboratory as described previously (45). The biotinylated cholera toxin B subunit was purchased from Sigma. The active fraction containing the N-t site protease was obtained from the culture supernatant of primary cultured mouse cortical neurons as described previously (10). The prestained molecular weight marker was purchased from Bio-Rad. Jcl:ICR mice were obtained from Charles River Laboratories Japan. Reeler mice (B6C3Fe-a/a-rl) were purchased from The Jackson Laboratory. For primary neuron culture, reeler mice were backcrossed into the Jcl:ICR strain. All experimental protocols using animals were approved by the Animal Care and Use Committee of Nagoya City University and were performed according to the guidelines of the National Institutes of Health of Japan.
DNA Constructs
The expression vector for the artificial substrate used in the determination of the N-t cleavage site (NR3-MycHis, Fig. 1A) was constructed using pcDNA3.1-MycHis (Invitrogen) by utilizing PCR. The Reelin mutants were constructed using pcDNA3.1Zeo(+) (Invitrogen) by PCR. Detailed procedures will be provided upon request.
Cell Culture and Transfection
Culturing of HEK293T cells, transfection of plasmid DNA using Lipofectamine 2000 (Invitrogen), and recovery and preservation of culture supernatant were performed as described previously (29). Primary cortical neurons were prepared and cultured as described previously (29). Briefly, 24- or 12-well polystyrene dishes or glass coverslips were coated with 0.005% poly-l-lysine overnight and subsequently washed with water. Cerebral cortices were removed from embryonic day 15 mice, incubated with 0.25% trypsin (Invitrogen) and 0.1% DNase I (Roche), and triturated with a fire-polished Pasteur pipette. The neurons were cultured in Neurobasal medium supplemented with 2% B27, 50 units/ml penicillin/streptomycin, and 2 mm l-glutamine (all from Invitrogen).
For preparation of reeler cortical neurons, reeler heterozygous male and female mice were mated, and, 14–16 days after the first mating day, the female mouse was sacrificed for removal of the embryos. Genomic DNA was extracted as described previously (46) with some modifications. A small section of the tail of each embryo was removed and boiled in 40 mm NaOH/1 mm EDTA for 30 min and subsequently neutralized with an equal amount of 50 mm Tris (pH 4.5). An aliquot (1 μl) of this solution was used as a template for a PCR (33 cycles of 96 °C for 15 s, 57 °C for 15 s, and 72 °C for 20 s) using the primers AGAATAAATCATACGTTCATTGGTG and CGTGAAGACATTTACTTATGTCAG. The embryos were maintained at 4 °C until their genotypes were determined.
Purification and Edman Sequencing of the Cleaved Product of NR3-MycHis
NR3-MycHis protein (18 μg) was incubated with the N-t protease that had been partially purified from the culture supernatant of primary cultured cortical neurons for 24 h. The reaction mixture was applied to a nickel-agarose column chromatography setup in an ÄKTA purification system (GE Healthcare), and the cleaved product was purified according to the protocol of the manufacturer. The purified fraction was separated by SDS-PAGE, and the proteins were transferred to a polyvinylidene difluoride membrane. The membrane was stained with Coomassie Brilliant Blue, and the bands of cleaved product were excised. The primary sequence of N terminus was determined using automatic Edman sequencing with a Procise 494 HT protein sequencing system (Applied Biosystems).
Assay for Reelin Biological Activity
The amount of Dab1 and its phosphorylation state were measured as described previously (29). After culturing for 4 days, cortical neurons were incubated with Reelin-containing medium for various durations and subsequently lysed with SDS-PAGE sample buffer. Samples were analyzed by Western blotting with anti-Dab1 and anti-phosphotyrosine antibodies. Western blotting with anti-β-actin antibody was performed as a loading control.
Monoclonal Antibody Establishment
A synthesized peptide (YEKPAFDYC, the cysteine residue was for conjugation) was conjugated with keyhole limpet hemocyanin, dissolved in water (0.8 mg/ml), mixed with an equal amount of Freund adjuvant (Sigma), and emulsified using a sonicator (Branson). A female reeler mouse was immunized intraperitoneally with the antigen twice with a 2-week interval. The spleen was removed after 2 weeks of final immunization, and lymphocytes were prepared and fused with PAI myeloma cells. The resultant cells were cultured in 96-well plates with GIT medium (Wako, Japan) in the presence of hypoxanthine-aminopterin-thymidine. ELISA and Western blotting were employed to screen for monoclonal antibodies against full-length Reelin protein.
Immunocytochemistry
Cortical neurons cultured on the coverslips were fixed with 4% paraformaldehyde in PBS at room temperature for 10 min. For staining without membrane permeabilization, cells were incubated with anti-Reelin AF3820 (×1000) and biotinylated cholera toxin B subunit (2 μg/ml) in PBS for 2 h, washed with PBS four times, incubated with Alexa Fluor 488-conjugated anti-goat IgG and Alexa Fluor 594-conjugated streptavidin (Invitrogen) for 1 h, washed with PBS four times, and mounted. For staining under permeabilized conditions, cells were treated with PBS containing 0.05% Tween 20 (PBS-T) for 20 min, incubated with anti-Reelin AF3820 (×1000) and anti-Rab11 (×200) in PBS-T containing 2% bovine serum albumin for 2 h, washed with PBS-T four times, incubated with Alexa Fluor 488- or Alexa Fluor 594-conjugated secondary antibodies for 1 h, washed with PBS-T four times, and mounted. They were then observed with a fluorescent microscope system (BZ9000, Keyence, Japan).
Immunohistochemistry
Preparation of frozen sections was performed as described previously (45). Antigen retrieval was performed in 10 mm citrate (pH 6.0) at 105 °C for 5 min. The sections were washed with PBS and permeabilized with PBS-T for 20 min. The sections were then incubated with the indicated primary antibodies dissolved in PBS-T containing 2% bovine serum albumin for 2 h at room temperature or overnight at 4 °C, washed with PBS-T four times, incubated with Alexa Fluor 488- or Alexa Fluor 594-conjugated secondary antibodies for 1 h, washed with PBS-T four times, and mounted. They were observed with a fluorescent microscope system (BZ9000).
RESULTS
Determination of the N-t Cleavage Site of Reelin
The culture supernatant of primary cultured mouse cerebral cortical neurons contains an enzyme (likely only one enzyme) of unknown identity that catalyzes N-t cleavage (10). Previously, we established a method to partially purify this protease (10), and preliminary experiments revealed that this protease cleaves Reelin mutants lacking the C-terminal half of Reelin5. Thus, we made an artificial substrate consisting of the NTR through to the RR3 with Myc- and His6 tags at the C terminus (NR3-MycHis, Fig. 1A). Approximately 18 μg of NR3-MycHis protein was prepared from the culture supernatant of transfected HEK293T cells and incubated with the partially purified protease. The post-digestion C-terminal fragment of NR3-MycHis was purified using nickel-chelating column chromatography (Fig. 1B, arrowhead) and subjected to Edman sequencing. The N-terminal sequence of the cleaved product was then determined to be AFDY (Fig. 1C), indicating that the N-t cleavage occurred between Pro-1244 and Ala-1245, 10 residues downstream of the beginning of RR3 (Fig. 1D). The surrounding sequence of this cleavage site is well conserved through vertebrates (Fig. 1E).
Proline 1244 Is Required for N-t Cleavage
From the alignment of the RR sequences (Fig. 2A), we noted that RR3 is distinctive because of the presence of a Pro residue (Pro-1244, circled in Fig. 2A) where acidic residues are located in all other RRs. Thus, we expressed a mutant Reelin protein in which Pro-1244 was replaced with Asp (Reelin-PD). Wild-type Reelin (Reelin-WT) and Reelin-PD were secreted from cells to the same extent, as revealed by Western blotting using anti-NTR antibody G10 (Fig. 2B, lanes 1 and 2). The N-terminal fragment generated by N-t cleavage (NR2) was detected only in medium containing Reelin-WT (Fig. 2B, lane 1), indicating that a protease present in the supernatant of HEK293T cells can weakly cleave Reelin-WT but not Reelin-PD. When these proteins were incubated with the culture supernatant of cortical neurons, Reelin-WT was cleaved at the N-t site, thereby generating the NR2 fragment (Fig. 2B, lane 3). Conversely, Reelin-PD remained intact (Fig. 2B, lane 4), indicating that Pro-1244 is critically involved in the recognition motif of the N-t site protease secreted from cortical neurons.
FIGURE 2.
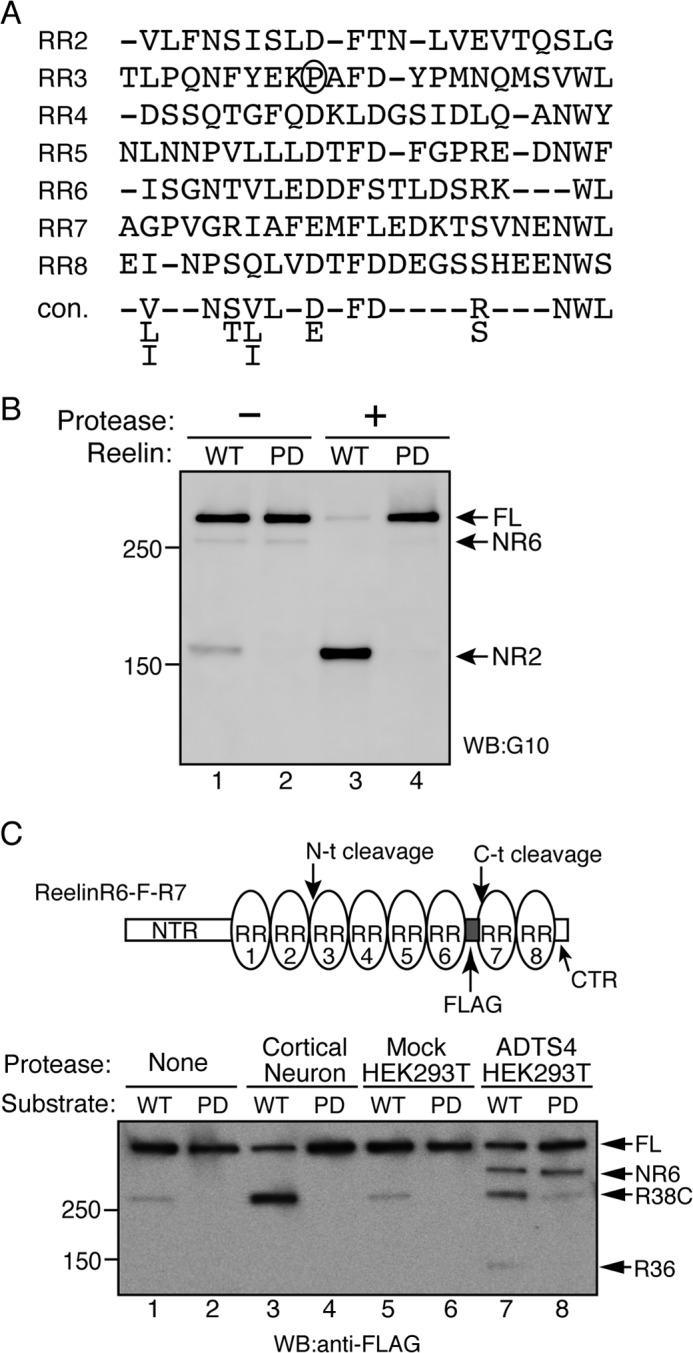
Pro-1244 is necessary for N-t cleavage. A, alignment of the partial primary sequences of the RRs according to Ichihara et al. (22). The consensus sequence (con.) is provided at the bottom of the alignment. Pro-1244 of RR2 is circled. B, Pro-1244 is required for N-t cleavage. Reelin-WT (lanes 1 and 3) or its mutant, Reelin-PD (Pro-1244 substituted with aspartic acid, lanes 2 and 4), were incubated for 24 h with the control buffer (lanes 1 and 2) or partially purified N-t site protease (lanes 3 and 4). The reaction mixtures were analyzed by Western blotting (WB) using anti-Reelin NTR antibody G10. Positions of molecular mass markers (kDa) are shown on the left. FL, full-length. C, N-t cleavage by ADAMTS-4 is inhibited significantly by PD mutation. The schematic of the substrate used in this assay (ReelinR6-F-R7) is shown on the top. ReelinR6-F-R7 (WT, lanes 1, 3, 5, and 7) or its PD mutant (PD, lanes 2, 4, 6, and 8) were incubated with control buffer (None, lanes 1 and 2), the N-t protease partially purified from the culture supernatant of cortical neurons (Cortical Neuron, lanes 3 and 4), the culture supernatant of mock-transfected HEK293T cells (Mock HEK293T, lanes 5 and 6), or that of ADAMTS-4-transfected HEK293T cells (ADTS4 HEK293T, lanes 7 and 8) at 37 °C for 24 h. The reaction mixtures were separated by SDS-PAGE and analyzed by Western blotting with anti-FLAG antibody. Positions of full-length substrate (FL), NR6, R38C, and R36 fragments are indicated by arrows. Positions of molecular mass markers (kDa) are shown at the left.
We and another group reported recently that ADAMTS-4 can catalyze the N-t cleavage of Reelin (43, 44). To determine whether Reelin-PD could be cleaved by ADAMTS-4, Reelin with a FLAG epitope between RR6 and RR7 (ReelinR6-F-R7, 43) and its PD version were used as substrates (Fig. 2C). When ReelinR6-F-R7 was incubated with the culture supernatant of primary cortical neurons, N-t cleavage occurred, and the R38C fragment was generated (Fig. 2C, lane 3). ADAMTS-4 expressed by HEK293T cells cleaved both the N-t and C-t sites (43), and, as a result, the NR6, R38C, and R36 fragments were generated (Fig. 2C, lane 7). When the PD version of ReelinR6-F-R7 was incubated with ADAMTS-4, the amount of NR6 fragment was not affected, whereas that of R38C and R36 decreased markedly (Fig. 2C, lane 8). Therefore, Pro-1244 is important for recognition by ADAMTS-4 in N-t cleavage. Importantly, ADAMTS-4 weakly cleaved Reelin-PD (Fig. 2C, lane 8), as evidenced by the slight increase in the amounts of R38C and R36 compared with the control (Fig. 2C, lanes 6 and 8). Because the protease secreted from cortical neurons did not catalyze the N-t cleavage of the PD mutant (Fig. 2C, lane 4), it is strongly suggested that ADAMTS-4 is not the N-t protease secreted from cortical neurons, as suggested previously (43).
Reelin-PD Remains Biologically Active Longer Than Reelin-WT
Reelin quickly induces Dab1 phosphorylation in cultured cortical neurons, which can last at least for 4 h (33). Phosphorylated Dab1 is quickly degraded in the cytoplasm (37–40), and, thus, the amount of Dab1 reflects the extent of Reelin signaling (26, 33, 37, 40). If N-t cleavage is the major inactivation mechanism for Reelin, the duration of Reelin-PD activity should exceed that of Reelin-WT. To clarify the differences between Reelin-WT and Reelin-PD by excluding the effects of endogenous Reelin, we utilized primary cortical neurons prepared from Reelin-deficient reeler embryos. Because reeler mice rarely mate successfully and it is, therefore, difficult to obtain timed-pregnant female reeler mice, we established a quick genotyping method to select reeler embryos from heterozygote pairs (see “Experimental Procedures”), and primary cultures of cerebral cortical neurons were prepared from them. To these neurons, culture supernatants from control HEK293T cells, Reelin-WT-expressing, or Reelin-PD-expressing cells were added and incubated for 20 min, 4 h, 12 h, and 24 h (Fig. 3A). During incubation with cortical neurons, Reelin-WT in the culture supernatant gradually underwent N-t cleavage, whereas Reelin-PD was quite stable (Fig. 3A, first panel). The amount of phosphorylated Dab1 at the 20-min time point was not different in neurons incubated with Reelin-WT or Reelin-PD (Fig. 3A, third panel). After this time point, the amount of phosphorylated Dab1 in the Reelin-treated neurons decreased and became difficult to quantitate. Therefore, we quantitated the amount of Dab1 protein and used this as the index of Reelin signaling. The amount of Dab1 in neurons incubated with Reelin-PD was comparable with those incubated with Reelin-WT until the 4-h time point (Fig. 3A, second panel, lanes 8–11, and Fig. 3B). At the 12-h time point, the amount of Dab1 in neurons incubated with Reelin-PD (Fig. 3A, second panel, lanes 15 and 16) was lower than that of those incubated with Reelin-WT (Fig. 3A, second panel, lanes 13 and 14). The amount of Dab1 in neurons incubated with Reelin-WT was increased at the 24-h time point compared with the 12-h time point (Fig. 3A, second panel, compare lanes 18 and 19 to lanes 13 and 14). This observation suggests that the Reelin-WT remaining in the culture supernatant was no longer able to induce Dab1 phosphorylation and that, thus, the amount of Dab1 began to recover. On the other hand, the amount of Dab1 in neurons incubated with Reelin-PD continued to decrease during the incubation period (Fig. 3A, second panel, and Fig. 3B). Therefore, it is strongly suggested that the duration of Reelin signaling is regulated by N-t cleavage.
FIGURE 3.
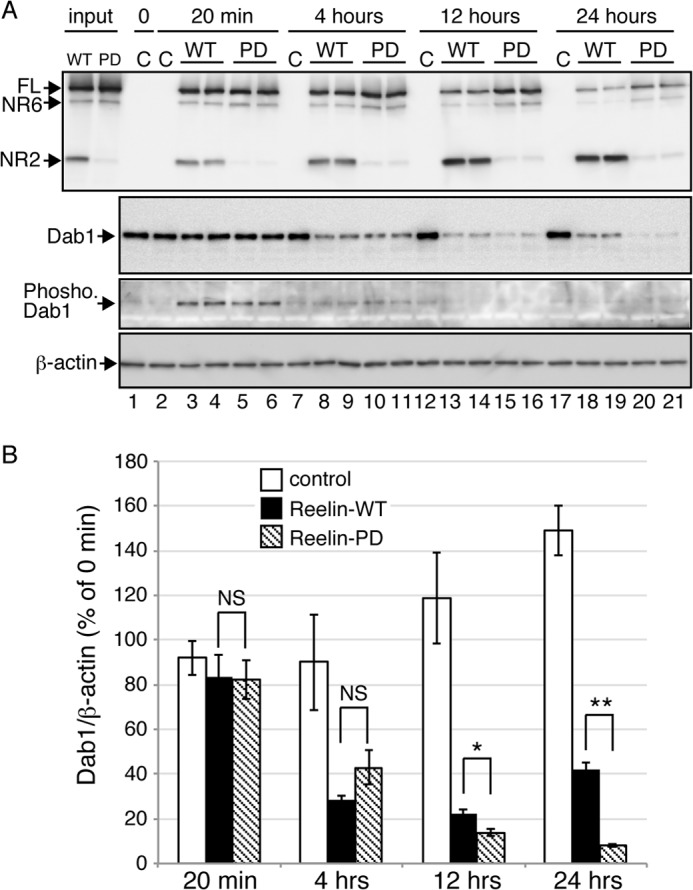
The biological activity of Reelin-PD is sustained longer than that of Reelin-WT. A, primary cortical neurons from reeler mice were incubated with culture medium from control mock-transfected cells (C), Reelin-WT-expressing cells (WT), or Reelin-PD-expressing cells (PD) for the times indicated at the top. Cells were then lysed in SDS-PAGE sample buffer and analyzed by Western blotting. The two left lanes (input) represent the culture medium used to stimulate the neurons. At each time point, two wells of neurons were used for Reelin treatment. B, quantification of the amount of Dab1 protein. Open, black, and hatched bars indicate neurons treated with control, Reelin-WT, and Reelin-PD, respectively. Band intensities were quantified using ImageJ and normalized to those of β-actin. Data are mean ± S.E. and were analyzed using Mann-Whitney U test (n = 8). *, p < 0.05; **, p < 0.01; NS, not significant.
Endosomal Reelin N-t cleavage Is Required for Halting the Dab1-phosphorylating Machinery
N-t cleavage of Reelin has been observed to occur within cells that have internalized Reelin (47). The biological significance of this event is unknown. Moreover, it is unknown whether or not endocytosed Reelin retains its activity. To investigate these questions, we incubated cortical neurons from reeler embryos with conditioned medium containing Reelin-WT or Reelin-PD for 3 h to allow Reelin internalization and then changed to Reelin-free culture medium. At the end of the 3-h incubation with Reelin-WT, the cellular fraction contained both full-length Reelin-WT and the NR2 fragment (Fig. 4A, top panel, lane 2). On the other hand, the lysates of neurons incubated with Reelin-PD contained full-length Reelin-PD and the NR6 fragment but not the NR2 fragment (Fig. 4A, top panel, lane 3). Therefore, Reelin-PD is also resistant to endosomal N-t cleavage. At this time point, Dab1 levels in cells treated with Reelin-PD were indistinguishable from cells treated with Reelin-WT (Fig. 4A, center panel, lanes 2 and 3, and Fig. 4B), further indicating that the activity of Reelin-WT and Reelin-PD are approximately equal in the short term. After 15 h in Reelin-free medium, internalized Reelin-WT was largely degraded (Fig. 4A, top panel, lane 5) and was barely detectable after 24 h (Fig. 4A, top panel, lane 8). On the other hand, a significant amount of Reelin-PD remained in the cellular fraction during this period (Fig. 4A, top panel, lanes 6 and 9). More importantly, in neurons that had been incubated with Reelin-WT, the amount of Dab1 protein began to recover after the 15-h time point (Fig. 4A, center panel, lanes 5 and 8, and Fig. 4B), whereas it continued to decrease in neurons that had been incubated with Reelin-PD (Fig. 4A, center panel, lanes 6 and 9, and Fig. 4B). These results strongly suggest that internalized Reelin-PD remains biologically active for up to 24 h and that the N-t cleavage and/or its subsequent degradation of Reelin in neurons plays a critical role in halting downstream signaling in neurons. Although we observed that a small fraction of internalized Reelin-WT or Reelin-PD was resecreted into the culture medium (Fig. 4C), Reelin of this concentration was too low to induce Dab1 phosphorylation, and the resecreted Reelin did not seem to bind to the neuronal cell surface under the present conditions (Fig. 5, H and I, see below).
FIGURE 4.
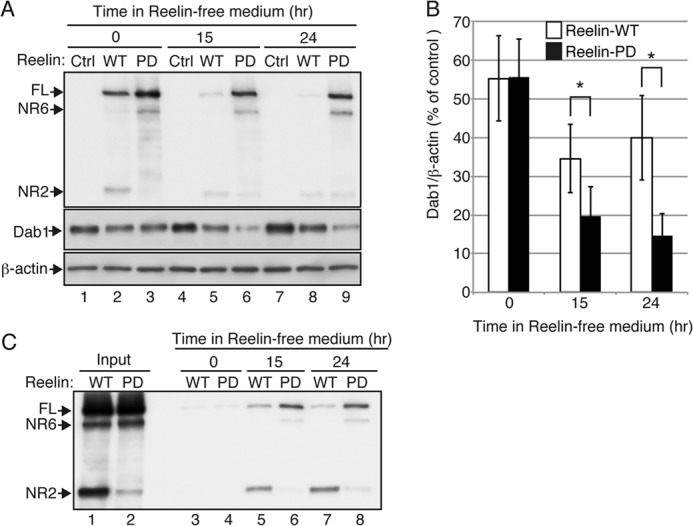
N-t cleavage is required for intracellular degradation of Reelin and halting of the downstream signaling. A, primary cerebral cortical neurons from reeler mice were incubated with control medium (Ctrl, lanes 1, 4, and 7), medium containing Reelin-WT (WT, lanes 2, 5, and 8), or medium containing Reelin-PD (PD, lanes 3, 6, and 9) for 3 h. The neurons were further incubated with Reelin-free medium for 0 (lanes 1, 2, and 3), 15 (lanes 4, 5, and 6), or 24 h (lanes 7, 8, and 9), lysed with SDS-PAGE sample buffer, and analyzed by Western blotting. Anti-Reelin NTR G10 was used for detection of Reelin. FL, full-length. B, quantification of the amount of Dab1 protein. Open and black bars indicate neurons treated with Reelin-WT and Reelin-PD, respectively. Band intensities were quantified using ImageJ and normalized to β-actin. Data are mean ± S.E. and were analyzed using Mann-Whitney U test (n = 4). *, p < 0.05. C, a small amount of Reelin-WT and Reelin-PD is resecreted from neurons. Cortical neurons from reeler mice were incubated with either Reelin-WT (lanes 3, 5, and 7) or Reelin-PD (lanes 4, 6, and 8) for 3 h. The neurons were further incubated for 0 (lanes 3 and 4), 15 (lanes 5 and 6), or 24 h (lanes 7 and 8), and the culture supernatants were collected and analyzed by Western blotting with anti-Reelin NTR G10. The amount of resecreted Reelin is very low compared with that in the original culture medium (Input, lanes 1 and 2).
FIGURE 5.
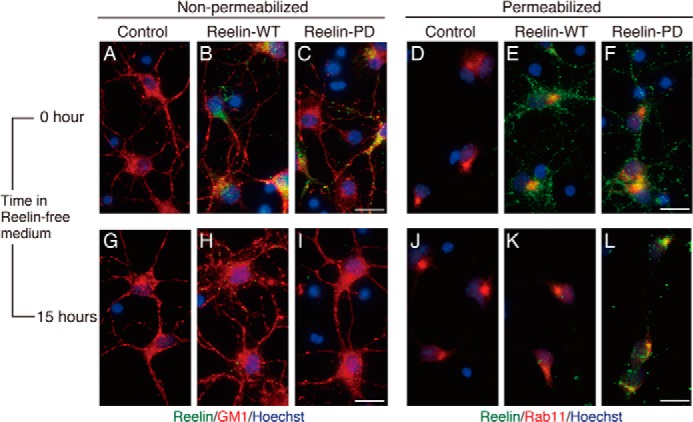
Internalized Reelin-PD is retained in the intracellular compartments longer than Reelin-WT. Primary cerebral cortical neurons from reeler mice were incubated with control medium (A, D, G, and J), medium containing Reelin-WT (B, E, H, and K), or medium containing Reelin-PD (C, F, I, and L) for 3 h. They were further incubated with Reelin-free medium for 0 (A–F) or 15 h (G–L). A–C and G–I, cells were fixed and stained with anti-Reelin NTR AF3820 (green) without membrane permeabilization. Biotinylated cholera toxin B subunit (red) and Hoechst33342 nucleus staining (blue) were used to visualize the neuronal membrane and nucleus, respectively. D–F and J–L, cells were fixed, permeabilized, and stained with AF3820 (green) and anti-Rab11 (red). Nuclei were stained with Hoechst33342 (blue). Scale bars = 20 μm.
To further support the idea that Reelin in the intracellular compartments can signal, we performed immunohistochemical analyses on the localization of Reelin with or without membrane permeabilization. Primary cortical neurons from reeler embryos were incubated with conditioned medium containing Reelin-WT or Reelin-PD for 3 h, fixed, and stained with anti-Reelin antibody. When the neurons were stained without permeabilization, both Reelin-WT and Reelin-PD were detected on the neuronal cell surface (Fig. 5, B and C, respectively). When they were stained after membrane permeabilization, intracellular Reelin protein was detected in both samples, and a few signals overlapped with that of Rab11 (Fig. 5, E and F). Regardless of membrane permeabilization, overall signal intensity was comparable between Reelin-WT and Reelin-PD, indicating that binding to the receptors and subsequent uptake are not affected by the PD mutation. After further incubation with Reelin-free medium for 15 h, very little signal of Reelin-WT was detected on the cell surface (Fig. 5H) or in the intracellular compartments (Fig. 5K), as had been expected from the results of Western blotting (Fig. 4A, top panel, lane 8). On the other hand, Reelin-PD was found in the intracellular compartments of neurons after 15-h incubation in Reelin-free medium (Fig. 5L). Costaining with anti-Rab11 antibody indicated that Reelin-PD protein was mainly localized in the recycling endosome (Fig. 5L). Reelin-PD was hardly detected on the cell surface at this time point (Fig. 5I). Taken together, these results indicate three important points. First, Reelin-PD is also resistant to N-t cleavage in the endosome of neurons. Second, N-t cleavage is a prerequisite for the clearance (i.e. resecretion and/or degradation) of Reelin protein from neurons. Third, N-t cleavage or the clearance of internalized Reelin protein from the endosome is required for halting the Dab1-phosphorylating machinery.
The NR2 Fragment Diffuses or Is Transported to Regions Distant from Reelin-secreting Cells
In the developing mouse brain, the majority of Reelin exists as cleaved fragments (1, 31). It has also been suggested that the NR2 fragment and full-length Reelin may function independently (48). Therefore, it is important to characterize the localization of full-length Reelin and its fragments to understand their roles. However, this has been very difficult because of the lack of antibodies specific for full-length and cleaved Reelin. Thus, we thus attempted to establish an mAb that specifically recognizes Reelin protein that is not cleaved at the N-t site. We immunized reeler mice with the octapeptide consisting of the surrounding sequence of the N-t site. The splenocytes from immunized mice were fused with myeloma cells to generate hybridoma cell lines. We then obtained mAb 2F3, which specifically binds to uncleaved Reelin. When the culture supernatants of HEK293T cells expressing Reelin-WT were separated using SDS-PAGE and probed with mAb 2F3, full-length Reelin and the NR6 fragment were detected, whereas the NR2 fragment was not detected (Fig. 6A, lane 1). The 2F3 antibody did not react with Reelin-PD (Fig. 6A, lane 2), indicating that Pro-1244 is a critical component of its epitope. The culture supernatant of primary cultured mouse cerebral cortex neurons contained only the NR2 fragment (Fig. 6B, lane 3, C.C.), which was not recognized by mAb 2F3 (Fig. 6A, lane 3). The culture supernatant of primary cultured mouse cerebellar granule neurons contained full-length Reelin, NR6, and NR2 fragments (Fig. 6B, lane 4, Cb). Among the samples, only full-length Reelin and the NR6 fragment were detected by mAb 2F3. These results indicate that N-t cleavage occurs at the same site in vivo and that mAb 2F3 only reacts with Reelin protein that is not cleaved at the N-t site.
FIGURE 6.
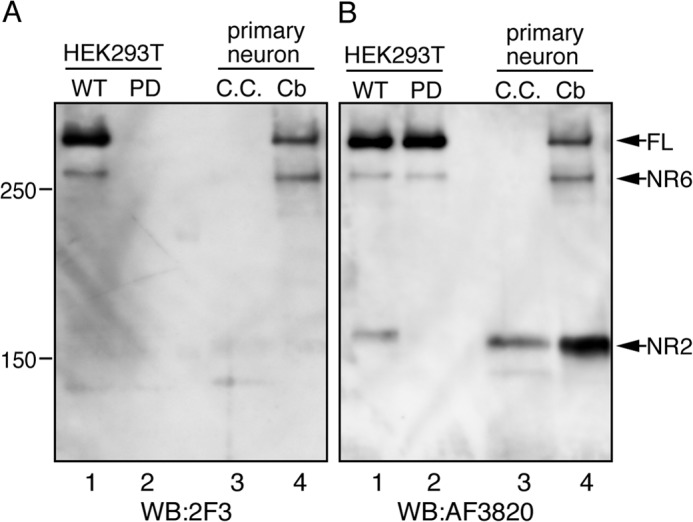
Establishment of a monoclonal antibody that specifically recognizes Reelin protein not cleaved at the N-t site. The culture supernatant of HEK293T cells expressing Reelin-WT (WT, lane 1) or Reelin-PD (PD, lane 2), mouse cerebral cortical primary neurons (C.C., lane 3), and mouse cerebellar primary granule cells (Cb, lane 4) were analyzed by Western blotting (WB) with 2F3 antibody (A) and then reprobed with AF3820 antibody (B). FL, full-length.
To investigate the localization of uncleaved and cleaved Reelin, immunohistochemistry using mAb 2F3 and AF3820 was performed. In the developing cerebral cortex at postnatal day 1, AF3820 staining produced diffuse and discrete signals in addition to strong signals in and near the Reelin-secreting Cajal-Retzius cells (Fig. 7A). On the other hand, staining with mAb 2F3 only produced signals in the vicinity of Cajal-Retzius cells (Fig. 7, B and C). Neither antibody stained reeler brain sections (Fig. 7D), confirming their specificity. This tendency was more evident in the hippocampus. Although mAb 2F3 staining was observed in Reelin-expressing cells and proximal areas (Fig. 7F), many punctate signals were visualized by AF3820 staining in regions distal to Reelin-expressing cells (Fig. 7E, yellow arrows). No staining was observed in reeler brain sections under the present conditions (Fig. 7, H–J). Therefore, although full-length Reelin localizes in or near Reelin-producing cells, the NR2 fragment can diffuse or be transported to distal regions.
FIGURE 7.
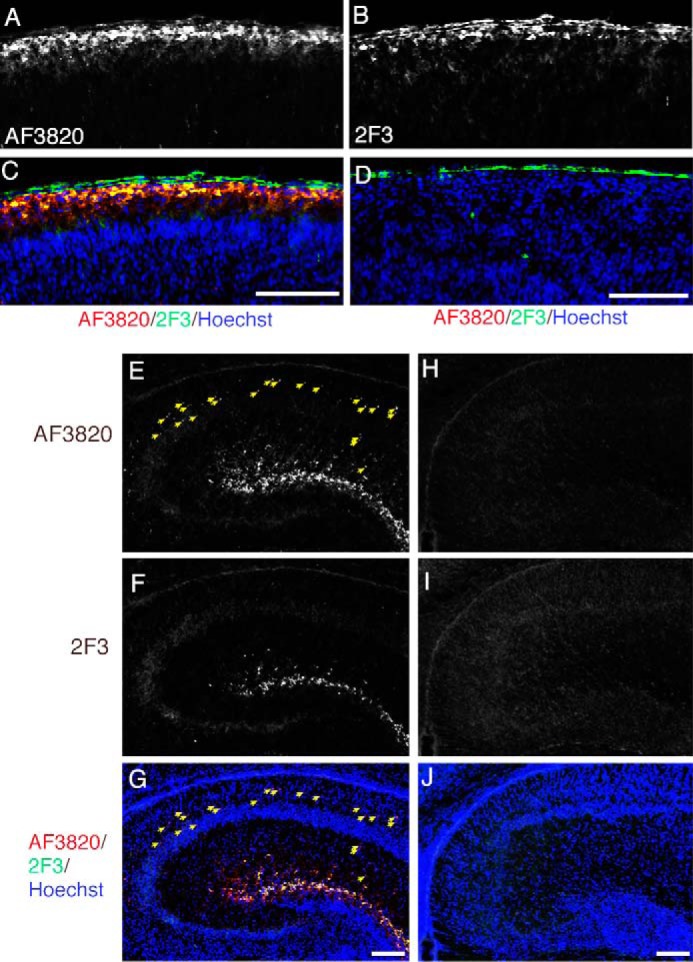
Localization of full-length and cleaved Reelin. Frozen sections from the cerebral cortices of postnatal day 1 (P1) wild-type (A–C) or reeler (D) mice were immunostained with AF3820 (A) and mAb 2F3 (B). C, merged image of A (red), B (green), and Hoechst33342 nucleus staining (blue). D, merged image of signals from AF3820 (red), mAb 2F3 (green), and Hoechst33342 (blue) staining. The pia mater gave a background staining when mAb 2F3 was used (the green signals outside of the brain in C and D). E–J, frozen sections of hippocampi of P1 wild-type (E–G) or reeler (H–J) mice were coimmunostained with AF3820 (E and H) and mAb 2F3 (F and I). The merged images with Hoechst33342 nucleus staining (blue) are shown in G and J. The yellow arrows indicate punctate signals that are positive for AF3820 but not 2F3. Scale bars = 100 μm.
DISCUSSION
We showed previously that N-t cleavage of Reelin greatly diminishes its ability to induce Dab1 phosphorylation (10). Here, we extended this work and made the followings novel observations. First, the N-t cleavage of Reelin occurs between Pro-1244 and Ala-1245. Second, Pro-1244 is critical for N-t cleavage. Third, the N-t cleavage is necessary for clearance of internalized Reelin and, more importantly, for halting the Dab1 phosphorylation machinery. Fourth, localization of the NR2 fragment and full-length Reelin differ considerably in the developing brain. Altogether, this work contributes greatly to our understanding of the mechanism and biological significance of Reelin metabolism.
It has been described frequently that Reelin N-t cleavage occurs between RR2 and RR3. In this study, however, we found that it occurs within RR3 (22). At the cleavage site, there is a unique Pro residue in place of the acidic residues present in all other RRs (Fig. 2A). Furthermore, the surrounding sequence of the N-t cleavage site is completely conserved among vertebrates (Fig. 1E). These facts strongly suggest that N-t cleavage is evolutionally conserved and plays important roles in the brain of many species. In fact, the Reelin fragment generated by N-t cleavage has been observed in the developing avian brain (49).
Substitution of Pro-1244 with Asp results in an uncleavable mutant of Reelin (Reelin-PD, Fig. 2B), indicating that Pro-1244 is critically involved in the recognition motif of the unidentified N-t site protease secreted from cortical neurons. Although ADAMTS-4 is one of the candidate protease that catalyzes N-t cleavage (43, 44), we found that ADAMTS-4 was able to weakly cleave Reelin-PD (Fig. 2C, lane 8). In fact, most of the known substrates of ADAMTS-4 and -5 have acidic residues just upstream of the cleavage site (50–52). These results suggest that ADAMTS-4 and -5 are not likely to be the N-t protease secreted from cortical neurons.
Reelin induces tyrosine phosphorylation of Dab1 through ApoER2/VLDLR (26, 27, 33, 34), and phosphorylated Dab1 is quickly degraded by the ubiquitin-proteasome system (37–40). Dab1 degradation is critical for the termination of neuronal migration (39). Reelin-WT and Reelin-PD induce Dab1 phosphorylation to the same extent in the short term. However, their long-term effects on Dab1 levels are quite different (Fig. 3). Incubation with Reelin-PD keeps Dab1 levels low for as long as 24 h, indicating that N-t cleavage plays a major role in inactivating the Reelin-Dab1 pathway. In recent years, up-regulation of this pathway has been suggested to be beneficial for the treatment of neuropsychiatric diseases (19, 21, 53, 54). In particular, a single injection of Reelin protein into the ventricle has been shown to have a beneficial effect on synaptic transmission, learning, and memory (19). We expect Reelin-PD to be more potent and durable in such experiments. Expression of Reelin-PD in a mouse disease model is being planned in our laboratory.
One of the major findings of this study is that Reelin-PD is relatively resistant to degradation after endocytosis (Fig. 4). Importantly, Dab1 was maintained at a very low level 24 h after the removal of Reelin-PD, which was not the case with Reelin-WT (Fig. 4, A and B). These results indicate that endocytosed Reelin maintains its ability to induce Dab1 phosphorylation even in intracellular compartments and that N-t cleavage and its subsequent clearance are indispensable aspects of halting downstream Reelin signaling. It has been shown previously that endocytosis of the Reelin-receptor complex is not a prerequisite for Dab1 phosphorylation (35, 36). However, it was unknown whether or not endocytosed Reelin retains its activity. In transfected NIH3T3 cells, Reelin binding to ApoER2 and VLDLR resulted in alterations in their intracellular trafficking, with ApoER2 tending toward degradation, whereas VLDLR was recycled back to the plasma membrane (36). In both cases, Reelin was degraded in the endosome (36). Therefore, it is plausible that Reelin degradation is necessary for the metabolism and trafficking of Reelin receptors.
The determination of the precise site of N-t cleavage enabled the establishment of mAb 2F3, which recognizes only uncleaved Reelin protein (Fig. 6). Immunostaining using mAb 2F3 revealed that uncleaved (and, thus, biologically active) Reelin is exclusively localized in the vicinity of Reelin-secreting cells both in the cerebral cortex and hippocampus (Fig. 7). On the other hand, the antibody recognizing NTR produced punctate staining that was not 2F3-positive (Fig. 7, C and G). Interestingly, the anti-NTR signal did not produce a gradient staining pattern. Rather, punctate staining was observed in regions devoid of Reelin-secreting cells (Fig. 7E, yellow arrows). Therefore, the relocation of the NR2 fragment may not be due to simple diffusion. Rather, it is likely to be transported to specific regions. Alternatively, the NR2 fragment may be anchored to specific regions by an uncharacterized mechanism. It has been reported that the Reelin NR2 fragment negatively regulates dendritic maturation in an ApoER2/VLDLR-independent manner in the cerebral cortex (48). How the localization of the NR2 fragment is regulated and whether it plays a role in the hippocampus are important questions to be answered. Recently, it has been recognized that endocytosis and endosomal trafficking play critical roles in various aspects of neuronal development (55). Further elucidation of Reelin proteolysis, metabolism, and trafficking will lead to a clearer understanding of its versatile and seemingly complicated functions.
Acknowledgments
We thank Prof. Junken Aoki (Tohoku University, Japan) for assistance with monoclonal antibody production. We also thank Profs. Junichi Takagi (Osaka University, Japan), Katsuhiko Mikoshiba (Brain Science Institute, RIKEN, Japan), Ken-ichiro Kubo, and Kazunori Nakajima (Keio University, Japan) for valuable comments.
This work was supported by Ministry of Education, Culture, Sports, Science, and Technology Grants KAKENHI 22390016 and 23123519 (to M. H.) and 22890155 and 24790081 (to T. K.), by grants from the Center for Intellectual Property Strategies of Japan Science and Technology Agency and from the Takeda Science Foundation (to M. H.).
M. Koie, K. Okumura, A. Hisanaga, T. Kamei, K. Sasaki, M. Deng, A. Baba, T. Kohno, and M. Hattori, unpublished observations.
- NTR
- N-terminal region
- RR
- Reelin repeat
- VLDLR
- very low density lipoprotein receptor
- ADAMTS
- a disintegrin and metalloproteinase with thrombospondin motifs.
REFERENCES
- 1. Tissir F., Goffinet A. M. (2003) Reelin and brain development. Nat. Rev. Neurosci. 4, 496–505 [DOI] [PubMed] [Google Scholar]
- 2. Tiberi L., Vanderhaeghen P., van den Ameele J. (2012) Cortical neurogenesis and morphogens: diversity of cues, sources and functions. Curr. Opin. Cell Biol. 24, 269–276 [DOI] [PubMed] [Google Scholar]
- 3. Park H., Poo M. M. (2013) Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 14, 7–23 [DOI] [PubMed] [Google Scholar]
- 4. Baker K. A., Moore S. W., Jarjour A. A., Kennedy T. E. (2006) When a diffusible axon guidance cue stops diffusing: roles for netrins in adhesion and morphogenesis. Curr. Opin. Neurobiol. 16, 529–534 [DOI] [PubMed] [Google Scholar]
- 5. Rogers K. W., Schier A. F. (2011) Morphogen gradients: from generation to interpretation. Annu. Rev. Cell Dev. Biol. 27, 377–407 [DOI] [PubMed] [Google Scholar]
- 6. Sasai Y., Lu B., Steinbeisser H., Geissert D., Gont L. K., De Robertis E. M. (1994) Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell 79, 779–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ohta K., Ito A., Kuriyama S., Lupo G., Kosaka M., Ohnuma S., Nakagawa S., Tanaka H. (2011) Tsukushi functions as a Wnt signaling inhibitor by competing with Wnt2b for binding to transmembrane protein Frizzled4. Proc. Natl. Acad. Sci. U.S.A. 108, 14962–14967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Capurro M. I., Xu P., Shi W., Li F., Jia A., Filmus J. (2008) Glypican-3 inhibits Hedgehog signaling during development by competing with patched for Hedgehog binding. Dev. Cell 14, 700–711 [DOI] [PubMed] [Google Scholar]
- 9. Adams R. H., Lohrum M., Klostermann A., Betz H., Püschel A. W. (1997) The chemorepulsive activity of secreted semaphorins is regulated by furin-dependent proteolytic processing. EMBO J. 16, 6077–6086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kohno S., Kohno T., Nakano Y., Suzuki K., Ishii M., Tagami H., Baba A., Hattori M. (2009) Mechanism and significance of specific proteolytic cleavage of Reelin. Biochem. Biophys. Res. Commun. 380, 93–97 [DOI] [PubMed] [Google Scholar]
- 11. Bingol B., Sheng M. (2011) Deconstruction for reconstruction: the role of proteolysis in neural plasticity and disease. Neuron 69, 22–32 [DOI] [PubMed] [Google Scholar]
- 12. D'Arcangelo G., Miao G. G., Chen S. C., Soares H. D., Morgan J. I., Curran T. (1995) A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature 374, 719–723 [DOI] [PubMed] [Google Scholar]
- 13. Herz J., Chen Y. (2006) Reelin, lipoprotein receptors and synaptic plasticity. Nat. Rev. Neurosci. 7, 850–859 [DOI] [PubMed] [Google Scholar]
- 14. Knuesel I. (2010) Reelin-mediated signaling in neuropsychiatric and neurodegenerative diseases. Prog. Neurobiol. 91, 257–274 [DOI] [PubMed] [Google Scholar]
- 15. Stranahan A. M., Erion J. R., Wosiski-Kuhn M. (2013) Reelin signaling in development, maintenance, and plasticity of neural networks. Ageing Res. Rev. 12, 815–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chin J., Massaro C. M., Palop J. J., Thwin M. T., Yu G. Q., Bien-Ly N., Bender A., Mucke L. (2007) Reelin depletion in the entorhinal cortex of human amyloid precursor protein transgenic mice and humans with Alzheimer's disease. J. Neurosci. 27, 2727–2733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Durakoglugil M. S., Chen Y., White C. L., Kavalali E. T., Herz J. (2009) Reelin signaling antagonizes beta-amyloid at the synapse. Proc. Natl. Acad. Sci. U.S.A. 106, 15938–15943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kocherhans S., Madhusudan A., Doehner J., Breu K. S., Nitsch R. M., Fritschy J. M., Knuesel I. (2010) Reduced Reelin expression accelerates amyloid-β plaque formation and tau pathology in transgenic Alzheimer's disease mice. J. Neurosci. 30, 9228–9240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rogers J. T., Rusiana I., Trotter J., Zhao L., Donaldson E., Pak D. T., Babus L. W., Peters M., Banko J. L., Chavis P., Rebeck G. W., Hoe H. S., Weeber E. J. (2011) Reelin supplementation enhances cognitive ability, synaptic plasticity, and dendritic spine density. Learn. Mem. 18, 558–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Verbrugghe P., Bouwer S., Wiltshire S., Carter K., Chandler D., Cooper M., Morar B., Razif M. F., Henders A., Badcock J. C., Dragovic M., Carr V., Almeida O. P., Flicker L., Montgomery G., Jablensky A., Kalaydjieva L. (2012) Impact of the Reelin signaling cascade (ligands-receptors-adaptor complex) on cognition in schizophrenia. Am. J. Med. Genet. B Neuropsychiatr. Genet. 159B, 392–404 [DOI] [PubMed] [Google Scholar]
- 21. Teixeira C. M., Martín E. D., Sahún I., Masachs N., Pujadas L., Corvelo A., Bosch C., Rossi D., Martinez A., Maldonado R., Dierssen M., Soriano E. (2011) Overexpression of Reelin prevents the manifestation of behavioral phenotypes related to schizophrenia and bipolar disorder. Neuropsychopharmacology 36, 2395–2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ichihara H., Jingami H., Toh H. (2001) Three novel repetitive units of Reelin. Brain Res. Mol. Brain Res. 97, 190–193 [DOI] [PubMed] [Google Scholar]
- 23. Utsunomiya-Tate N., Kubo K., Tate S., Kainosho M., Katayama E., Nakajima K., Mikoshiba K. (2000) Reelin molecules assemble together to form a large protein complex, which is inhibited by the function-blocking CR-50 antibody. Proc. Natl. Acad. Sci. U.S.A. 97, 9729–9734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jossin Y., Ignatova N., Hiesberger T., Herz J., Lambert de Rouvroit C., Goffinet A. M. (2004) The central fragment of Reelin, generated by proteolytic processing in vivo, is critical to its function during cortical plate development. J. Neurosci. 24, 514–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nogi T., Yasui N., Hattori M., Iwasaki K., Takagi J. (2006) Structure of a signaling-competent Reelin fragment revealed by x-ray crystallography and electron tomography. EMBO J. 25, 3675–3683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Trommsdorff M., Gotthardt M., Hiesberger T., Shelton J., Stockinger W., Nimpf J., Hammer R. E., Richardson J. A., Herz J. (1999) Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell 97, 689–701 [DOI] [PubMed] [Google Scholar]
- 27. Hiesberger T., Trommsdorff M., Howell B. W., Goffinet A., Mumby M. C., Cooper J. A., Herz J. (1999) Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates Tau phosphorylation. Neuron 24, 481–489 [DOI] [PubMed] [Google Scholar]
- 28. D'Arcangelo G., Homayouni R., Keshvara L., Rice D. S., Sheldon M., Curran T. (1999) Reelin is a ligand for lipoprotein receptors. Neuron 24, 471–479 [DOI] [PubMed] [Google Scholar]
- 29. Nakano Y., Kohno T., Hibi T., Kohno S., Baba A., Mikoshiba K., Nakajima K., Hattori M. (2007) The extremely conserved C-terminal region of Reelin is not necessary for secretion but is required for efficient activation of downstream signaling. J. Biol. Chem. 282, 20544–20552 [DOI] [PubMed] [Google Scholar]
- 30. Kohno T., Nakano Y., Kitoh N., Yagi H., Kato K., Baba A., Hattori M. (2009) C-terminal region-dependent change of antibody-binding to the eighth Reelin repeat reflects the signaling activity of Reelin. J. Neurosci. Res. 87, 3043–3053 [DOI] [PubMed] [Google Scholar]
- 31. Lambert de Rouvroit C., de Bergeyck V., Cortvrindt C., Bar I., Eeckhout Y., Goffinet A. M. (1999) Reelin, the extracellular matrix protein deficient in reeler mutant mice, is processed by a metalloproteinase. Exp. Neurol. 156, 214–217 [DOI] [PubMed] [Google Scholar]
- 32. Jossin Y., Gui L., Goffinet A. M. (2007) Processing of Reelin by embryonic neurons is important for function in tissue but not in dissociated cultured neurons. J. Neurosci. 27, 4243–4252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Howell B. W., Herrick T. M., Cooper J. A. (1999) Reelin-induced tyrosine phosphorylation of disabled 1 during neuronal positioning. Genes Dev. 13, 643–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Howell B. W., Herrick T. M., Hildebrand J. D., Zhang Y., Cooper J. A. (2000) Dab1 tyrosine phosphorylation sites relay positional signals during mouse brain development. Curr. Biol. 10, 877–885 [DOI] [PubMed] [Google Scholar]
- 35. Morimura T., Hattori M., Ogawa M., Mikoshiba K. (2005) Disabled1 regulates the intracellular trafficking of Reelin receptors. J. Biol. Chem. 280, 16901–16908 [DOI] [PubMed] [Google Scholar]
- 36. Duit S., Mayer H., Blake S. M., Schneider W. J., Nimpf J. (2010) Differential functions of ApoER2 and very low density lipoprotein receptor in Reelin signaling depend on differential sorting of the receptors. J. Biol. Chem. 285, 4896–4908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Arnaud L., Ballif B. A., Cooper J. A. (2003) Regulation of protein tyrosine kinase signaling by substrate degradation during brain development. Mol. Cell Biol. 23, 9293–9302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bock H. H., Jossin Y., May P., Bergner O., Herz J. (2004) Apolipoprotein E receptors are required for Reelin-induced proteasomal degradation of the neuronal adaptor protein Disabled-1. J. Biol. Chem. 279, 33471–33479 [DOI] [PubMed] [Google Scholar]
- 39. Feng L., Allen N. S., Simo S., Cooper J. A. (2007) Cullin 5 regulates Dab1 protein levels and neuron positioning during cortical development. Genes Dev. 21, 2717–2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Simó S., Cooper J. A. (2013) Rbx2 regulates neuronal migration through different Cullin 5-RING ligase adaptors. Dev. Cell 27, 399–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sáez-Valero J., Costell M., Sjögren M., Andreasen N., Blennow K., Luque J. M. (2003) Altered levels of cerebrospinal fluid Reelin in frontotemporal dementia and Alzheimer's disease. J. Neurosci. Res. 72, 132–136 [DOI] [PubMed] [Google Scholar]
- 42. Tinnes S., Schäfer M. K., Flubacher A., Münzner G., Frotscher M., Haas C. A. (2011) Epileptiform activity interferes with proteolytic processing of Reelin required for dentate granule cell positioning. FASEB J. 25, 1002–1013 [DOI] [PubMed] [Google Scholar]
- 43. Hisanaga A., Morishita S., Suzuki K., Sasaki K., Koie M., Kohno T., Hattori M. (2012) A disintegrin and metalloproteinase with thrombospondin motifs 4 (ADAMTS-4) cleaves Reelin in an isoform-dependent manner. FEBS Lett. 586, 3349–3353 [DOI] [PubMed] [Google Scholar]
- 44. Krstic D., Rodriguez M., Knuesel I. (2012) Regulated proteolytic processing of Reelin through interplay of tissue plasminogen activator (tPA), ADAMTS-4, ADAMTS-5, and their modulators. PLoS ONE 7, e47793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Uchida T., Baba A., Pérez-Martínez F. J., Hibi T., Miyata T., Luque J. M., Nakajima K., Hattori M. (2009) Downregulation of functional Reelin receptors in projection neurons implies that primary Reelin action occurs at early/premigratory stages. J. Neurosci. 29, 10653–10662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Truett G. E., Heeger P., Mynatt R. L., Truett A. A., Walker J. A., Warman M. L. (2000) Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and Tris (HotSHOT). BioTechniques 29, 52–54 [DOI] [PubMed] [Google Scholar]
- 47. Hibi T., Hattori M. (2009) The N-terminal fragment of Reelin is generated after endocytosis and released through the pathway regulated by Rab11. FEBS Lett. 583, 1299–1303 [DOI] [PubMed] [Google Scholar]
- 48. Chameau P., Inta D., Vitalis T., Monyer H., Wadman W. J., van Hooft J. A. (2009) The N-terminal region of Reelin regulates postnatal dendritic maturation of cortical pyramidal neurons. Proc. Natl. Acad. Sci. U.S.A. 106, 7227–7232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Absil P., Pinxten R., Balthazart J., Eens M. (2003) Effects of testosterone on Reelin expression in the brain of male European starlings. Cell Tissue Res. 312, 81–93 [DOI] [PubMed] [Google Scholar]
- 50. Tortorella M. D., Liu R. Q., Burn T., Newton R. C., Arner E. (2002) Characterization of human aggrecanase 2 (ADAM-TS5): substrate specificity studies and comparison with aggrecanase 1 (ADAM-TS4). Matrix Biol. 21, 499–511 [DOI] [PubMed] [Google Scholar]
- 51. Viapiano M. S., Hockfield S., Matthews R. T. (2008) BEHAB/brevican requires ADAMTS-mediated proteolytic cleavage to promote glioma invasion. J. Neurooncol. 88, 261–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Durigova M., Soucy P., Fushimi K., Nagase H., Mort J. S., Roughley P. J. (2008) Characterization of an ADAMTS-5-mediated cleavage site in aggrecan in OSM-stimulated bovine cartilage. Osteoarthritis Cartilage 16, 1245–1252 [DOI] [PubMed] [Google Scholar]
- 53. Pujadas L., Gruart A., Bosch C., Delgado L., Teixeira C. M., Rossi D., de Lecea L., Martínez A., Delgado-García J. M., Soriano E. (2010) Reelin regulates postnatal neurogenesis and enhances spine hypertrophy and long-term potentiation. J. Neurosci. 30, 4636–4649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rogers J. T., Zhao L., Trotter J. H., Rusiana I., Peters M. M., Li Q., Donaldson E., Banko J. L., Keenoy K. E., Rebeck G. W., Hoe H. S., D'Arcangelo G., Weeber E. J. (2013) Reelin supplementation recovers sensorimotor gating, synaptic plasticity and associative learning deficits in the heterozygous reeler mouse. J. Psychopharmacol. 27, 386–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yap C. C., Winckler B. (2012) Harnessing the power of the endosome to regulate neural development. Neuron 74, 440–451 [DOI] [PMC free article] [PubMed] [Google Scholar]


