Abstract
Background:
In the recent past, algorithms and recommendations to standardize the morphological, immunohistochemical and molecular classification of lung cancers on cytology specimens have been proposed, and several organizations have recommended cell blocks (CBs) as the preferred modality for molecular testing. Based on the literature, there are several different techniques available for CB preparation-suggesting that there is no standard. The aim of this study was to conduct a survey of CB preparation techniques utilized in various practice settings and analyze current issues, if any.
Materials and Methods:
A single E-mail with a link to an electronic survey was distributed to members of the American Society of Cytopathology and other pathologists. Questions pertaining to the participants’ practice setting and CBs-volume, method, quality and satisfaction-were included.
Results:
Of 95 respondents, 90/95 (94%) completed the survey and comprise the study group. Most participants practice in a community hospital/private practice (44%) or academic center (41%). On average, 14 CBs (range 0-50; median 10) are prepared by a laboratory daily. Over 10 methods are utilized: Plasma thrombin (33%), HistoGel (27%), Cellient automated cell block system (8%) and others (31%) respectively. Forty of 90 (44%) respondents are either unsatisfied or sometimes satisfied with their CB quality, with low-cellular yield being the leading cause of dissatisfaction. There was no statistical significance between the three most common CB preparation methods and satisfaction with quality.
Discussion:
Many are dissatisfied with their current method of CB preparation, and there is no consistent method to prepare CBs. In today's era of personalized medicine with an increasing array of molecular tests being applied to cytological specimens, there is a need for a standardized protocol for CB optimization to enhance cellularity.
Keywords: Cell block, cytopathology, fine-needle aspiration, lung cancer, molecular testing, personalized medicine
INTRODUCTION
Classically, cytomorphologic diagnoses have been rendered on Diff-Quik and Papanicolaou-stained smears. Other techniques have been introduced to this traditional method, including cytospins following specimen concentration, thin layer preparations with selective cellular enhancement and cell blocks (CBs) from sample consolidation. Each modality and stain offers its advantages.
Though other preparations may impart greater cytomorphological detail, CBs are recognized for their semblance to histology, including potential to identify architectural features similar to those observed in histological sections,[1,2] especially in the presence of tissue fragments. Furthermore, CBs have the capacity to yield multiple tissue sections for ancillary tests, including special stains, immunohistochemical (IHC) stains with co-ordination of immunoreactivity pattern and molecular diagnostics. Recent expert consensus opinion on molecular guidelines for selection of lung cancer patients for epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) tyrosine kinase inhibitors issued by the College of American Pathologists, International Association for the Study of Lung Cancer and Association for Molecular Pathology states that CBs are preferred over smear preparations for polymerase chain reaction-based EGFR testing and fluorescence in situ hybridization assay for ALK.[3] For these reasons, CBs, which have served as adjuncts to the conventional approaches for diagnosis, are now considered an integral component of the repertoire of cytology preparations, particularly for lung cancer specimens.[3,4]
Several advances in other aspects of medicine have heightened clinical awareness and utility of CBs.[5] There is a strong trend toward the clinical use of minimally-invasive procedures. Not only are they less invasive than surgical alternatives, but they can be performed on an outpatient basis with fewer resources. For patients with (suspected) lung cancer in particular, endobronchial ultrasound-guided fine-needle aspirations (FNAs) have the capacity to provide diagnostic and staging information at the same time.[6] With the capabilities of minimally-invasive procedures and a concurrent emphasis on personalized medicine and molecular diagnostics, the provided specimen size has decreased while the information required from the sample has increased. Because greater numbers of ancillary tests are routinely requested and CBs, comprised of fewer cells than their histological resection counterparts, may represent the only diagnostic tissue ever attained from a patient, a greater burden has been placed on pathologists.[7] Fortunately, progress in molecular techniques enables the use of less tissue, including cytological samples.[8] In fact, ancillary tests results paralleling those reported on histological resections have been reported on CBs; the outcomes are not uniform across studies, however. Though there are several possible contributing factors to the variations, the aim of the current study was to focus on CB-related issues.
MATERIALS AND METHODS
Institutional Review Board approval was not obtained for this research, since it did not require review of any patient information. A link to an electronic survey was distributed through electronic mail (e-mail) to members of the American Society of Cytopathology and other pathologists. Only a single e-mail and no repeat reminder E-mails or incentives, were sent. Questions included in the survey are listed in Figure 1. All questions, except one, were in a multiple-choice format, with 2-8 responses for each. Respondents were permitted to choose only one answer for some and >1 for the remaining. Furthermore, provided was an “Other” option with free text capability for some questions. The one question without multiple choices had a slider with a value scale ranging from 0 to 50. All responses were tabulated and replies to the “Other” options were recorded.
Figure 1.
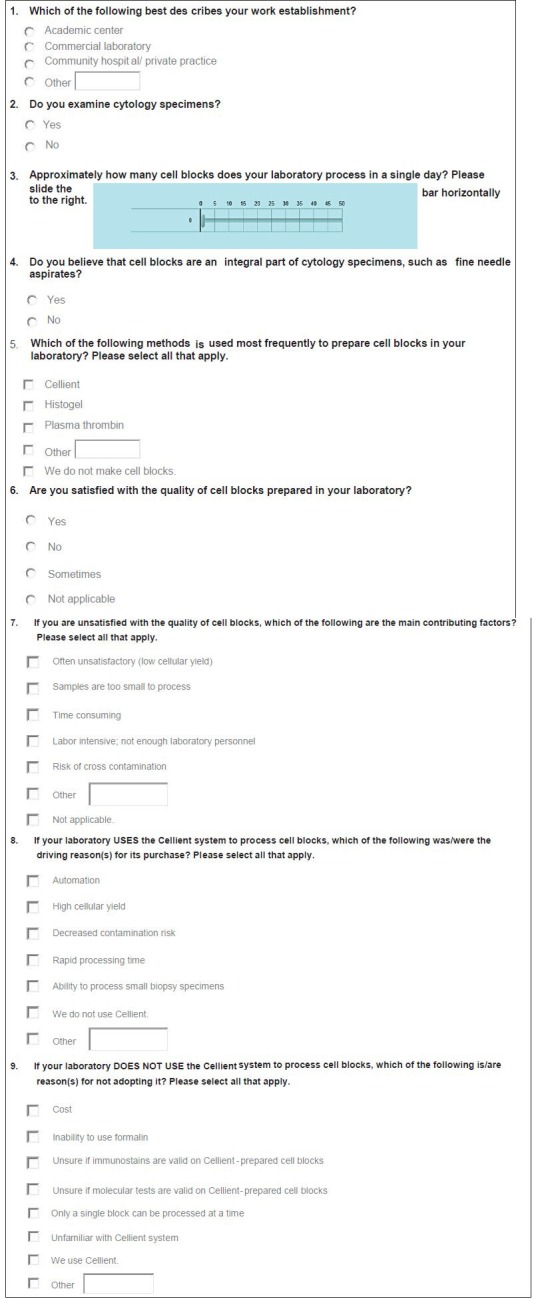
Survey questions
Statistical analysis was calculated on Excel using t-statistic two-tailed test and P values were calculated using 0.05 significance.
RESULTS
Of 95 respondents, 90 (95%) completed the survey and comprise the basis of this study. Most participants work in a community hospital/private practice (44%) or an academic center (41%); 7% work at a commercial laboratory and the remaining at an independent lab, military facility, or VA Medical Center.
Eighty-nine (99%) of respondents examine cytology specimens and consider CBs to be an integral part of cytological specimens. On average, 14 CBs (range 0-50; median 10) are prepared by a laboratory daily. Many participants (n = 40; 44%) are either unsatisfied or sometimes satisfied with the quality of CBs prepared in their laboratories, with low-cellular yield being the leading cause of dissatisfaction (33%) [Figure 2].
Figure 2.
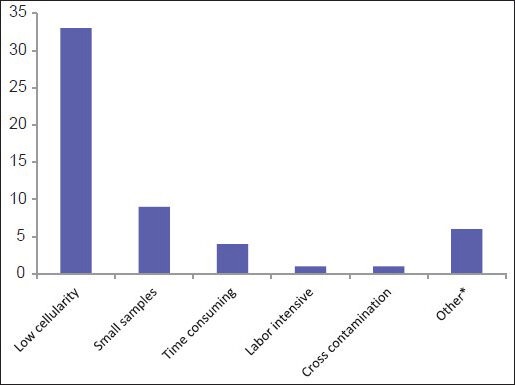
Main contributing factors for being unsatisfied with the quality of cell blocks (*Other: Variable cellularity/variable technicians, inter-operator variability, issues with clotting/low cellularity, poor morphology/antigenicity loss)
Over 10 different methods are utilized to make CBs and include plasma thrombin (33%), HistoGel (27%) (Thermo Scientific Richard-Allan Scientific; Kalamazoo, MI, USA), the Cellient automated cell block system (CACBS) (8%) (Hologic) and other methods (31%); only one (1%) respondent did not make CBs and some used >1 method [Table 1]. HistoGel and plasma thrombin were used almost equivalently at academic centers and in community hospitals/private practices [Figure 3].
Table 1.
Methods most commonly used to prepare cell blocks
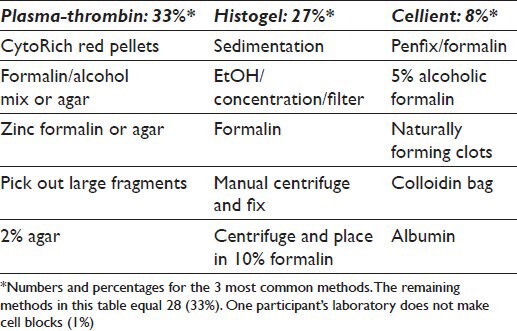
Figure 3.
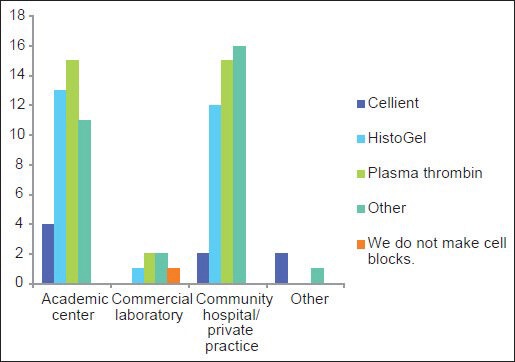
Most frequently used methods to prepare cell blocks in different types of work establishments
Among the three most common methodologies (plasma thrombin, HistoGel and CACBS) analysis of satisfaction of CB quality (differences in the proportion of “Yes” [satisfied] and “No/Sometimes” [unsatisfied/sometimes satisfied] responses) demonstrates that HistoGel scores the lowest, but the results are not statistically significant (P = 0.09) [Figure 4]. The main driving factors for utilization of CACBS by the eight participants include enhanced cellular yield, rapid processing time and ability to process small biopsies [Figure 5]. High cost is the main reason for not employing CACBS [Figure 6].
Figure 4.
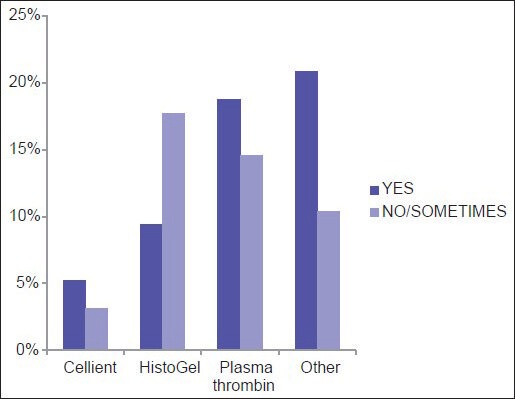
Satisfaction with the quality of cell blocks prepared via different methods
Figure 5.
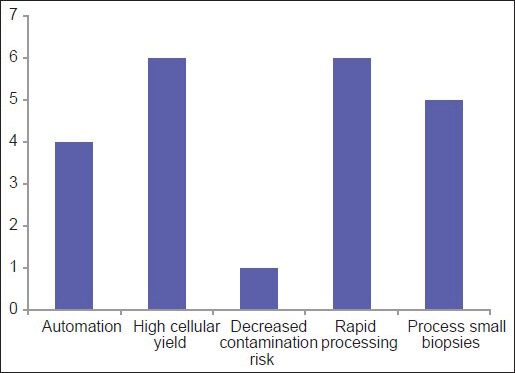
For laboratories using the Cellient system, this graph shows the driving reasons for purchasing the system to prepare cell blocks
Figure 6.
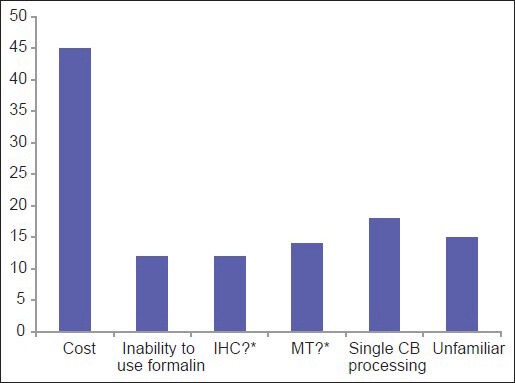
For laboratories not using the Cellient system, this graph shows the driving reasons for not adopting the system to prepare cell blocks (*IHC = Immunohistochemical stains; MT = Molecular testing. Question marks = Unsure if immunohistochemical stains and molecular tests are valid on Cellient-prepared cell blocks)
DISCUSSION
Morphological diagnosis is no longer sufficient.[2,4] Now, more than ever, with the identification of novel predictive and prognostic markers, management is dictated by a combination of morphological and ancillary test results,[2] especially for patients with lung carcinomas. These are not just used to make therapeutic choices, but also represent the current standards of care.[1,3,9] These changes have coincided with the performance of increasing numbers of minimally-invasive procedures, such as ultrasound-guided, computed tomographic-guided and navigational bronchoscopic-guided FNAs[4] for sampling lung, pancreas, other gastrointestinal lesions and/or lymph nodes. For patients with locally advanced and/or metastatic cancer,[2] cytology specimens, such as pleural fluids, peritoneal fluids or FNAs,[3] may provide the only source of diagnostic tissue upon which therapeutic decisions are rendered[1] and selection for clinical trials is determined.[2] This has culminated in greater expectations from pathologists, particularly those practicing cytopathology, to perform an increasing array of ancillary tests-IHC and molecular tests-on smaller cytology specimens and provide satisfactory results to guide therapeutic decisions.[4,10,11]
In the past, there have been no standardized guidelines dictating the type of cytology specimen that should be utilized for such ancillary testing. Moreover, there are studies describing EGFR testing on various cytological preparations. This provides a range of options but also limits standardization.[12] Current expert consensus opinion,[3,11,13] including by the International Association for the Study of Lung Cancer, American Thoracic Society, European Respiratory Society and College of American Pathologists,[3,14] however, advocates CBs over direct smears to carry out these analyses.[3,14]
Usually >1 different preparations, including smears, cytospins, liquid-based preparations and/or CBs are routinely used for cytomorphological diagnosis. CBs, first introduced in the late 1960s and early 1970s,[15,16] offer several advantages, including architectural organization correlating to histology and recognition of intercellular bridges of squamous cell carcinomas not readily seen on other cytological preparations.[1] Also, by being able to view the morphology, the cells of interest can be correlated from level to level and the tumor content of the specimen can be assessed, specifically the tumor volume and percentage,[3,4] in order to determine if the sample is adequate for molecular testing (MT). Residual material from the ThinPrep vial offers an alternative, but determining the ratio of tumor to total cellularity may be difficult and has potential to lead to interpretive errors when the ratio is low. In addition, CBs serve as a source of additional material,[3] so that diagnostic preparations (e.g., smears) do not have to be compromised for ancillary testing, which can have possible downstream medicolegal implications.
Like formalin-fixed histology paraffin blocks, CBs allow for long-term specimen preservation and supply archival deoxyribonucleic acid (DNA) for future diagnostic studies[3,9] and research. CB sections furthermore provide the opportunity to have material that is fixed in 10% neutral-buffered formalin, on which a majority of molecular assays have been optimized and validated on surgical pathology specimens.[3] Finally, because they can be processed like paraffin-embedded sections, CBs easily integrate into existing methods for histochemical, IHC and MT[3,4] and the results are similar to those obtained on paraffin-embedded tissue sections.[17] Despite the numerous advantages of CBs, the cellular yield of CBs is inconsistent and varies widely.[18,19,20]
Given the recent emphasis, the aim of the current study was to focus on the current status of CBs. The survey was completed by 90 pathologists and cytotechnologists from various work settings, ranging from academic centers and commercial laboratories to private practices and thus provides a representative cross-section. Based on the survey, 95% (90/95) of respondents practice cytology and consider CBs to be an integral part of cytological specimens; one respondent who works in a commercial laboratory did not process CBs. The different CB preparations are used comparably in academic centers, community hospitals and private practices [Figure 3]. On average, 14 CBs (range 0-50; median 10) are prepared by a laboratory daily. Currently laboratories use a variety (>10) of methods to prepare CBs [Table 1]. These techniques, each with its unique traits, protocol and advantages, have been described.[2,18,21,22,23,24]
Many survey participants (n = 40; 44%) are either unsatisfied or sometimes satisfied with the quality of CBs prepared in their laboratories, with low-cellular yield being a leading cause of dissatisfaction (33%) [Figure 2]. Review of the literature shows similar variability in the results of adequacy of CBs,[2] including for histologic typing and ancillary tests.[7,9,25,26]
Comparison of CB techniques
Comparison of the three most common techniques (plasma thrombin, HistoGel and CACBS) shows that HistoGel has the lowest rate of satisfaction, but the results are not statistically significant (P = 0.09). Similar results have been cited by others.[27,28,29] Benkovich et al. in their study have reported that plasma thrombin CBs were more cellular and had better cellularity, morphology, cell distribution and pellet size relative to those prepared with HistoGel.[27,28] A second study also demonstrated that cellularity for plasma thrombin CBs was greater than for HistoGel CBs, but the latter had better cellular preservation and architecture.[28] The plasma thrombin technique also yielded better cellularity, cell distribution and background quality relative to other methods, including inverted filter sedimentation,[30] albumin method and simple sedimentation.
“Other” techniques may potentially provide greater satisfaction. An alternative solution that outperformed plasma thrombin and HistoGel is the collodion bag technique which has been suggested as a high cellular yield method.[28,31,32,33] The so-called tissue coagulum clot method has been shown to significantly increase the efficiency and cellular yield when compared with conventional saline rinse.[2]
Only a minority of respondents utilized CACBS in our survey; 5 were satisfied and 3 were not entirely satisfied. Though the sample size is small, there is no statistical significance (P = 0.11) between CACBS and the other non-automated “traditional” methods. A similar observation was reported, when comparing CBs prepared with CACBS and “traditional” (non-automated) methods.[33]
Interestingly, the data show that 17% respondents are unfamiliar with CACBS [Figure 6]. Those who are familiar with CACBS use it for both cytology and small biopsy specimens. For CBs, high cellular yield and decreased risk of contamination were the two most cited reasons for using CACBS; high cost was the leading reason for not using it. Other reasons for not using CACBS included an inability to use formalin and uncertainty about results of immunohistochemistry and MT. In a study comparing traditional CBs to CACBS, the majority of IHC stains showed identical results with the two methods, although there were a limited number of overall cases (17 patients; 56 IHC stains).[33]
Specimen fixation
Though IHC stains and MT may be performed on samples fixed in alcohol, most pathology laboratories have standardized immunohistochemistry protocols for formalin-fixed material.[33] When MT[3] or immunohistochemistry is performed on alcohol-fixed specimens, the laboratory needs to conduct appropriate validation studies so as to avoid false negative or false positive results. In addition, proper duration of fixation is required to obtain reliable IHC stain results. For example, at least 6-8 hours of formalin-fixation time for breast biopsies is required to obtain reliable estrogen receptor determination by immunohistochemistry;[34] inadequate fixation may lead to false negative results.[34]
Increasing cellular yield of CBs
While one or more factors may lead to suboptimal results,[6] including operators’ skills,[6] inadequate lesional tissue acquisition, nature and location of the lesion,[2] inappropriate triage of the sample and lack of technical expertise in processing small specimens, the current study demonstrates that variability in CB processing techniques is likely a contributing factor. For instance, five different methods to process CBs using HistoGel were outlined by one group.[27] The literature also highlights variations in fixation,[21,26] concentration and congealing techniques.[13] Several modifications have been described. For instance, concentration of diagnostic cellular material along the cutting surface, with provision to control the depth of cutting by the histotechnologist, has been reported to yield results in 133 out of 134 liquid based cytology specimens of cervical cytology. Similar results are expected with this method using any specimen with low cellularity with loosely cohesive or singly scattered cells such as serous effusions and FNA needle rinses in saline or Roswell Park Memorial Institute (RPMI) culture medium (fresh unfixed cells) or needle rinses directly in 10% formalin.[31,32] Also, a focus on the specimen collection may play a role. Several CB preparation methods, including adding thrombin, plasma, agar gel and use of commercial assay kits, are designed primarily to concentrate cellular material after it is collected from aspiration needles. There has been interest, as with the tissue coagulum method,[2] to protect such material from loss before the preparative procedure.[2,23,35,36]
Though CBs, including FNA-derived CBs, have proven to be valid,[4] low cellular yield leads to suboptimal efficiency of CBs,[2] especially for MT. Such testing requires high tumor content (10-100 ng of DNA without necrosis) and percentage of tumor cells (>40% tumor cells).[4] Even though these can be enhanced by microdissection and the thresholds of minimum tumor content may decrease as the field of MT evolves, with scant cellularity, CBs are still likely to have low yield.
Dedicated current procedural terminology (CPT) code for processing CBs
CB preparation requires significantly greater efforts and more resources with special laboratory setup than simple grossing steps involved for routine biopsy specimens. Nevertheless, the current technical component of the CPT code for CBs is 88305, which is the same as for routine biopsies. Investment in generation of high quality and cellular CBs with incentive to use the best resources would be facilitated by introducing a special CPT code with higher remuneration for the investment. Increasing demand for better quality CBs demands expedited efforts to introduce a dedicated CPT code for its processing.
CONCLUSIONS
Accurate histological classification and MT on small samples are becoming the norm. The clinical demand for ancillary tests to guide targeted therapies is likely to grow, which means that the numbers of tests requested on cytology specimens is likely to increase also.[4] CBs provide an important medium to conduct these tests, but their cellular yield needs to be improved. Though the survey has a limited number of respondents, it demonstrates that even within a small sample, there is no consistent method to prepare CBs, and many pathologists and cytotechnologists are dissatisfied with their current method (s) of CB preparation.
The results stress the need for a better methodical approach for optimization of CBs to enhance cellularity in today's era of personalized medicine.[2,32,37] This study serves as a baseline to launch further investigation of the pros and cons of different CB preparation techniques, as comparative literature in this topic is limited. Additional studies with an in depth analysis to determine the appropriate method(s) is necessary.
Determining and standardizing the most effective technique may alleviate the variability and provide consistency. As it adapted to the introduction of immunohistochemistry and flow cytometry, cytology has to align itself with the multitude of molecular diagnostic tests.[4]
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The author (s) declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
Each author has participated sufficiently in the work and takes public responsibility for appropriate portions of the content of this article. JPC analyzed the data and drafted the study. JJH interpreted the data and revised it for intellectual content. SM and AN contributed to the design of the study and revised it for intellectual content. AS conceived the study, interpreted the data and drafted the content. All authors read and approved the final manuscript. Each author acknowledges that this final version was read and approved.
ETHICS STATEMENT BY ALL AUTHORS
The authors followed the ethics statement requirement(s).
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double blind model (authors are blinded for reviewers and vice versa) through automatic online system.
ACKNOWLEDGMENT
The authors are grateful to Professor David Juran for his assistance with statistical analysis.
Contributor Information
John P. Crapanzano, Email: jpc2141@cumc.columbia.edu.
Jonas J. Heymann, Email: jjh2110@columbia.edu.
Sara Monaco, Email: monacose@upmc.edu.
Aziza Nassar, Email: nassar.aziza@mayo.edu.
Anjali Saqi, Email: aas177@cumc.columbia.edu.
REFERENCES
- 1.Loukeris K, Vazquez MF, Sica G, Wagner P, Yankelevitz DF, Henschke CI, et al. Cytological cell blocks: Predictors of squamous cell carcinoma and adenocarcinoma subtypes. Diagn Cytopathol. 2012;40:380–7. doi: 10.1002/dc.21519. [DOI] [PubMed] [Google Scholar]
- 2.Yung RC, Otell S, Illei P, Clark DP, Feller-Kopman D, Yarmus L, et al. Improvement of cellularity on cell block preparations using the so-called tissue coagulum clot method during endobronchial ultrasound-guided transbronchial fine-needle aspiration. Cancer Cytopathol. 2012;120:185–95. doi: 10.1002/cncy.20199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindeman NI, Cagle PT, Beasley MB, Chitale DA, Dacic S, Giaccone G, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: Guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol. 2013;8:823–59. doi: 10.1097/JTO.0b013e318290868f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dumur CI, Idowu MO, Powers CN. Targeting tyrosine kinases in cancer: The converging roles of cytopathology and molecular pathology in the era of genomic medicine. Cancer Cytopathol. 2013;121:61–71. doi: 10.1002/cncy.21225. [DOI] [PubMed] [Google Scholar]
- 5.Kalhor N, Wistuba II. Perfecting the fine-needle aspirate cell block. Cancer Cytopathol. 2013;121:109–10. doi: 10.1002/cncy.21284. [DOI] [PubMed] [Google Scholar]
- 6.Feller-Kopman D, Yung RC, Burroughs F, Li QK. Cytology of endobronchial ultrasound-guided transbronchial needle aspiration: A retrospective study with histology correlation. Cancer. 2009;117:482–90. doi: 10.1002/cncy.20049. [DOI] [PubMed] [Google Scholar]
- 7.Esterbrook G, Anathhanam S, Plant PK. Adequacy of endobronchial ultrasound transbronchial needle aspiration samples in the subtyping of non-small cell lung cancer. Lung Cancer. 2013;80:30–4. doi: 10.1016/j.lungcan.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 8.Saieg MA, Geddie WR, Boerner SL, Bailey D, Crump M, da Cunha Santos G. EZH2 and CD79B mutational status over time in B-cell non-Hodgkin lymphomas detected by high-throughput sequencing using minimal samples. Cancer Cytopathol. 2013;121:377–86. doi: 10.1002/cncy.21262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bulman W, Saqi A, Powell CA. Acquisition and processing of endobronchial ultrasound-guided transbronchial needle aspiration specimens in the era of targeted lung cancer chemotherapy. Am J Respir Crit Care Med. 2012;185:606–11. doi: 10.1164/rccm.201107-1199CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirsch FR, Wynes MW, Gandara DR, Bunn PA., Jr The tissue is the issue: Personalized medicine for non-small cell lung cancer. Clin Cancer Res. 2010;16:4909–11. doi: 10.1158/1078-0432.CCR-10-2005. [DOI] [PubMed] [Google Scholar]
- 11.Cagle PT, Allen TC, Dacic S, Beasley MB, Borczuk AC, Chirieac LR, et al. Revolution in lung cancer: New challenges for the surgical pathologist. Arch Pathol Lab Med. 2011;135:110–6. doi: 10.5858/2010-0567-RA.1. [DOI] [PubMed] [Google Scholar]
- 12.da Cunha Santos G. Standardizing preanalytical variables for molecular cytopathology. Cancer Cytopathol. 2013;121:341–3. doi: 10.1002/cncy.21290. [DOI] [PubMed] [Google Scholar]
- 13.Khan S, Omar T, Michelow P. Effectiveness of the cell block technique in diagnostic cytopathology. J Cytol. 2012;29:177–82. doi: 10.4103/0970-9371.101167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–85. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Constantian HM, De Girolami E. Urothelial tumors detected by cytology: New cell block technique. J Urol. 1973;109:304–7. doi: 10.1016/s0022-5347(17)60412-9. [DOI] [PubMed] [Google Scholar]
- 16.De Girolami E. Applications of plasma-thrombin cell block in diagnostic cytology, Part II: II: Digestive and Respiratory tracts, breast and effusions. Pathol Annu. 1977;12(Pt 2):91–110. [PubMed] [Google Scholar]
- 17.Shidham VB, Lindholm PF, Kajdacsy-Balla A, Chang CC, Komorowski R. Methods of cytologic smear preparation and fixation. Effect on the immunoreactivity of commonly used anticytokeratin antibody AE1/AE3. Acta Cytol. 2000;44:1015–22. doi: 10.1159/000328590. [DOI] [PubMed] [Google Scholar]
- 18.Burt AD, Smillie D, Cowan MD, Adams FG. Fine needle aspiration cytology: Experience with a cell block technique. J Clin Pathol. 1986;39:114–5. doi: 10.1136/jcp.39.1.114-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olson NJ, Gogel HK, Williams WL, Mettler FA., Jr Processing of aspiration cytology samples. An alternative method. Acta Cytol. 1986;30:409–12. [PubMed] [Google Scholar]
- 20.Rosell A, Monsó E, Lores L, Vilà X, Llatjós M, Ruiz J, et al. Cytology of bronchial biopsy rinse fluid to improve the diagnostic yield for lung cancer. Eur Respir J. 1998;12:1415–8. doi: 10.1183/09031936.98.12061415. [DOI] [PubMed] [Google Scholar]
- 21.Shidham VB, Atkinson BF. Collection and processing of effusion fluids for cytopathologic evaluation. In: Schmitt WR, editor. Cytopathologic Diagnosis of Serous Fluids. 1st ed. Philadelphia: Saunders Elsevier; 2007. pp. 213–33. [Google Scholar]
- 22.Karnauchow PN, Bonin RE. “Cell-block” technique for fine needle aspiration biopsy. J Clin Pathol. 1982;35:688. doi: 10.1136/jcp.35.6.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kulkarni MB, Desai SB, Ajit D, Chinoy RF. Utility of the thromboplastin-plasma cell-block technique for fine-needle aspiration and serous effusions. Diagn Cytopathol. 2009;37:86–90. doi: 10.1002/dc.20963. [DOI] [PubMed] [Google Scholar]
- 24.Musso C, Silva-Santos MC, Pereira FE. Cotton block method: One-step method of cell block preparation after fine needle aspiration. Acta Cytol. 2005;49:22–6. doi: 10.1159/000326090. [DOI] [PubMed] [Google Scholar]
- 25.Sanz-Santos J, Serra P, Andreo F, Llatjós M, Castellà E, Monsó E. Contribution of cell blocks obtained through endobronchial ultrasound-guided transbronchial needle aspiration to the diagnosis of lung cancer. BMC Cancer. 2012;12:34. doi: 10.1186/1471-2407-12-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nathan NA, Narayan E, Smith MM, Horn MJ. Cell block cytology. Improved preparation and its efficacy in diagnostic cytology. Am J Clin Pathol. 2000;114:599–606. doi: 10.1309/G035-P2MM-D1TM-T5QE. [DOI] [PubMed] [Google Scholar]
- 27.Benkovich V, Cuda J, Khalbuss W, Pantanowitz L, Palekar A, Monaco S. Comparison of cell block prepartion using histogel and plasma thrombin techniques. J Am Soc Cytopathol. 2012;1:S114–5. [Google Scholar]
- 28.Balassanian R, Ono JC, Wool GD, Olejnik-Nave J, Mah MM, Sweeney BJ, et al. A superior technique for cell block preparation for fine needle aspiration. Mod Pathol. 2013;26:83A. doi: 10.1002/cncy.21722. [DOI] [PubMed] [Google Scholar]
- 29.Nigro K, Tynski Z, Wasman J, Abdul-Karim F, Wang N. Comparison of cell block preparation methods for nongynecologic ThinPrep specimens. Diagn Cytopathol. 2007;35:640–3. doi: 10.1002/dc.20713. [DOI] [PubMed] [Google Scholar]
- 30.Diaz-Rosario LA, Kabawat SE. Cell block preparation by inverted filter sedimentation is useful in the differential diagnosis of atypical glandular cells of undetermined significance in ThinPrep specimens. Cancer. 2000;90:265–72. [PubMed] [Google Scholar]
- 31.Shidham VB, Mehrotra R, Varsegi G, D’Amore KL, Hunt B, Narayan R. p16 immunocytochemistry on cell blocks as an adjunct to cervical cytology: Potential reflex testing on specially prepared cell blocks from residual liquid-based cytology specimens. Cytojournal. 2011;8:1. doi: 10.4103/1742-6413.76379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varsegi GM, Shidham V. Cell block preparation from cytology specimen with predominance of individually scattered cells. J Vis Exp. 2009:29. doi: 10.3791/1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wagner DG, Russell DK, Benson JM, Schneider AE, Hoda RS, Bonfiglio TA. Cellient™ automated cell block versus traditional cell block preparation: A comparison of morphologic features and immunohistochemical staining. Diagn Cytopathol. 2011;39:730–6. doi: 10.1002/dc.21457. [DOI] [PubMed] [Google Scholar]
- 34.Gown AM. Current issues in ER and HER2 testing by IHC in breast cancer. Mod Pathol. 2008;21(Suppl 2):S8–15. doi: 10.1038/modpathol.2008.34. [DOI] [PubMed] [Google Scholar]
- 35.Kaneko C, Kobayashi TK, Hasegawa K, Udagawa Y, Iwai M. A cell-block preparation using glucomannan extracted from Amorphophallus konjac. Diagn Cytopathol. 2010;38:652–6. doi: 10.1002/dc.21280. [DOI] [PubMed] [Google Scholar]
- 36.Zito FA, Gadaleta CD, Salvatore C, Filotico R, Labriola A, Marzullo A, et al. A modified cell block technique for fine needle aspiration cytology. Acta Cytol. 1995;39:93–9. [PubMed] [Google Scholar]
- 37.Nakajima T, Yasufuku K. How I do it-Optimal methodology for multidirectional analysis of endobronchial ultrasound-guided transbronchial needle aspiration samples. J Thorac Oncol. 2011;6:203–6. doi: 10.1097/JTO.0b013e318200f496. [DOI] [PubMed] [Google Scholar]


