Abstract
The purpose of this study was to determine the mechanism by which 1α, 25-dihydroxyvitamin D (1,25(OH)2D) alters hypoxia inducible factor-1α (HIF-1α) protein in untransformed and Harvey-ras (H-ras) oncogene transfected MCF10A breast epithelial cells. Treatment with1,25(OH)2D (10 nM) increased both mRNA (2.55±0.6 fold vs. vehicle, p=0.03) and protein levels (2.37±0.3 fold vs. vehicle, p<0.0001) of HIF-1α in MCF10A cells in 12 hours, which remained elevated at 24 hours. However, in H-ras transfected MCF10A cells, 1,25(OH)2D treatment increased HIF-1α protein level (2.08±0.38 fold vs. vehicle, p= 0.05) at 12 hrs, with no change in mRNA level and HIF-1α protein level returned to baseline after 24 hours. A transcription inhibitor prevented the 1,25(OH)2D induction of HIF-1α protein and mRNA levels in MCF10A cells, but failed to alter the induction of HIF-1α protein level in H-ras transfected MCF10A cells. On the other hand, inhibition of proteasomal degradation prevented the 1,25(OH)2D-induced HIF-1α protein level in H-ras transfected MCF10A but not in MCF10A cells. These results support that 1,25(OH)2D regulates HIF-1α protein level via transcriptional regulation in MCF10A cells in contrast to through proteosomal degradation with the presence of H-ras oncogene in MCF10A cells.
Keywords: 1,25-Dihydroxyvitamin D; 25-hydroxyvitamin D; Hypoxia-inducible factor; ras; Vitamin D Receptor
Introduction
Breast cancer is the second leading number of diagnosed cancers among U.S. women [1]. According to the American Cancer Society, 192,370 new cases of invasive breast cancer were estimated to occur among women in the US and 40,610 patients would die of breast cancer during 2009. Epidemiological studies have linked vitamin D to reduced risk for breast cancer [2; 3]. Higher incidence or mortality rates for breast cancer occur in those areas with higher latitude and lower sunlight, which is associated with a low vitamin D synthesis in the skin upon UV exposure [4]. Further, in a review by Garland and colleagues of studies from 1966–2006, a higher level of serum 25 hydroxyvitamin D was associated with lower risk of breast cancer [5].
Vitamin D, obtained from sun exposure, food, and supplements, is metabolized in multiple tissues. Its circulating form, 25 hydroxyvitamin D (25(OH)D), is generated in the liver by the enzyme 25-hydroxylase. The 25(OH)D metabolite is further hydroxylated by the 1α-hydroxylase enzyme to the active form of vitamin D, 1α, 25-dihydroxyvitamin D (1,25(OH)2D), which acts as a ligand to the vitamin D receptor (VDR) to control serum calcium homeostasis. Research supports that vitamin D also has anti-neoplastic effects in colon, prostate, ovarian and breast cancer [6]. One mechanism by which vitamin D may play a role in breast cancer prevention is through regulation of angiogenesis. Angiogenesis is a normal physiologic process in embryo development and wound healing [7] and hypoxia is a key signal for the induction of angiogenesis. However, hypoxia leading to an angiogenic response occurs in different types of solid tumors, including breast cancer [8]. The cellular response to hypoxia is mediated through, at least in part, the family of transcriptional factors hypoxia inducible factor (HIFs). In human breast tumors, the expression of hypoxia inducible factor 1 (HIF-1) is correlated with tumor grade and vascularization [9]. HIF-1 has been shown to induce the gene expression of vascular endothelial growth factor (VEGF) [10], glycolytic enzymes [11], inducible nitric oxide synthase (NOS) [12] and erythropoietin [13], as well as a variety of other proteins involved in physiological processes including energy metabolism. In mammary tissue or cells, the target genes of HIF-1 are involved in several crucial biological processes such as tumor cell survive and proliferation, migration and metastasis, including regulation of angiogenic factors [14].
HIF-1 is composed of a heterodimer of α and β subunits [15]. HIF-1β is constitutively expressed in all cell types. The biological activity of HIF is primarily determined by the abundance of HIF-1α protein, which is altered by oncogenes such as ErbB2 (human epidermal growth factor receptor-2) [16]. In breast cancer, HIF-1α over-expression is seen at a high frequency especially in hereditary breast carcinogenesis [17]. Under normoxic conditions, oxygen-dependent prolyl hydroxylases (PHD) modify HIF-1α to promote recognition by the von Hippel-Lindau tumor suppressor (VHL) to form an E3 ubiquitin ligase complex that mediates the HIF-1α proteasome dependent degradation [18]. Under hypoxic conditions, prolyl hydroxylation is inhibited, leading to stabilization of the HIF-1α protein. Further, factors such as TNFα can increase HIF-1α protein expression through enhancing the induction of mRNA expression of HIF-1α [19]. Due to the multiple roles that HIF-1 plays in breast cancer, understanding the regulation of HIF-1α will provide information that may guide the development of targeted strategies for breast cancer prevention. 1,25(OH)2D has been shown to inhibit angiogenesis in breast tumors in vivo [20] and decrease HIF-1α protein levels in several breast cancer cells [7]. In order to establish strategies for cancer prevention that do not lead to adverse effects, it is critical to identify the mechanisms of vitamin D action that allow normal growth and development as well as to determine if it inhibits production of angiogenic factors in cells containing specific oncogenes such as the activated ras gene.
The effects of 1,25(OH)2D on HIF-1α have not been studied in models of carcinogenesis including untransformed breast epithelial cells and cells of the same parentage that contain the H-ras oncogene. In the current study, a breast epithelial cell line with or without the H-ras oncogene was employed as a model for carcinogenesis to examine the function of 1,25(OH)2D in regulation of HIF-1α expression. The hypothesis of the study is that 1,25(OH)2D up-regulates HIF-1α protein level via transcriptional regulation in untransformed breast epithelial cells, but does not alter HIF-1α protein expression in cells transfected with the H-ras oncogene. These results will help to understand how 1,25(OH)2D acts on the breast tissue during cancer development.
Materials and Methods
Chemicals and Reagents
1,25(OH)2D and 25(OH) D were purchased from Biomol (Plymouth Meeting, PA). Dulbecco’s modified Eagle medium (DMEM/F-12), horse serum, trypsin and penicillin/streptomycin were from Life Technologies, Gibco-BRL (Rockville, MD). Cholera toxin was from Calbiochem (Darmstadt, Germany). Protein assay reagents were obtained from Pierce (Rockford, IL). Protease inhibitor cocktail, transcription inhibitor, actinomycin D, proteasome inhibitor, MG132, trypan blue, insulin, epidermal growth factor, and hydrocortisone were from Sigma (St. Lois, MO). Tris-HCl Bio-Rad Ready Gels were purchased from Bio-Rad Laboratories (Hercules, CA, USA).
Cell culture
MCF10A human epithelial breast cells and MCF10A cells stably transfected with the Harvey ras oncogene (MCF10A-ras cells) were a gift from Dr. Michael Kinch, Purdue University [21]. MCF10A and MCF10A-ras were cultured in DMEM/F12 (1:1, v:v) containing 5% house serum and supplemented with 10 mg/L insulin, 20 μg/L epidermal growth factor, 50 μg/L cholera toxin, 50 mg/L hydrocortisone, 100 units/ml penicillin, and 0.1 mg/mL streptomycin in a humidified environment at 37°C with 5% CO2. Cells were maintained in linear growth. 1,25(OH)2D was delivered to cells in 100% ethanol at a final ethanol concentration < 1%.
RNA isolation and analysis
MCF10A or MCF10A-ras cells were treated with either vehicle or 10 nM 1,25(OH)2D for time indicated prior to harvest and RNA was isolated using TriReagent (Molecular Research Center, Inc., Cincinnati, OH) following the manufacturer’s instructions. Total RNA (1 μg) was reverse transcribed using MMLV reverse transcriptase (Promega Corp., Madison, WI). Realtime PCR was performed using the Brilliant SYBR Green QPCR Master Mix (Stratagene, La Jolla, CA). HIF-1α mRNA abundance was determined from the threshold cycle (Ct) value. HIF-1α mRNA expression was normalized to 18S mRNA expression and results were expressed as arbitrary units. Primers used for HIF-1α are: HIF-1α forward, 5′-TGCTGAAGACACAGAAGCAAA-3′ and reverse, 5′-AAAGCGCAAGTCCTCAAAGC-3′; 18S forward, 5′-TTAGAGTGTTCAAAGCAG GCCCGA-3′ and reverse, 5′-TCTTGGCAA ATGCTTTCGCTCTGG-3′.
Western blot analysis
Cells were washed with calcium/magnesium free phosphate buffered saline (CMF-PBS, pH=7.4) and harvested on ice into a buffer containing 25 mmol/L HEPES, 150 mmol/L NaCl, 5 mmol/L EDTA, 0.1% Triton, and 1% protease inhibitor cocktail and phosphatase inhibitor cocktail (Sigma, St. Lois, MO). Cells were briefly sonicated and cell debris was removed by centrifugation at 12,000 RPM for 15 min at 4°C. Protein concentration was determined using the bicinchoninic acid protein assay (Pierce, Rockford, IL, USA). Proteins (20–30 μg) were resolved by SDS-PAGE on 10% Tris-HCl gels (Bio-Rad Laboratories, Hercules, CA), transferred onto nitrocellulose membrane (Bio-Rad Laboratories, Hercules, CA) and probed with specific antibody against HIF-1α (Abcam Inc., Cambridge, MA), VDR (Affinity Bioreagent, Neshanic Station, NJ), 1α-hydroxylase and actin antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA). Antigen-antibody complexes were detected using the Lumiglo Reagent (Cell Signaling Technology, Inc., Danvers, MA). Density of immunoreactive bands was assessed by Scion Image (Frederick, MD). Densities of the bands were in the linear range of delectability. Results are expressed as fold change of HIF-1α/actin compared to vehicle control.
Inhibitors
To assess the impact of inhibition of transcription, MCF10A or MCF10A-ras cells were pretreated with the transcriptional inhibitor actinomycin D (2 μg/mL) or vehicle (0.1% ethanol) for 2 h, follow by treatment with either vehicle (0.08% ethanol) or 1,25(OH)2D (10 nM) for 12 h before harvesting. To determine the effect of proteasome inhibition, MCF10A or MCF10A-ras cells were treated with vehicle (0.09% ethanol) or 1,25(OH)2D (10 nM) alone or in combination with proteasome inhibitor MG132 (1 μM) for 12 h prior to harvest.
Statistical Analysis
Values presented are means±SEM. Results were compared with student T Tests (LSD) or by analysis of variance (ANOVA) with P<0.05 considered statistically significant.
Results
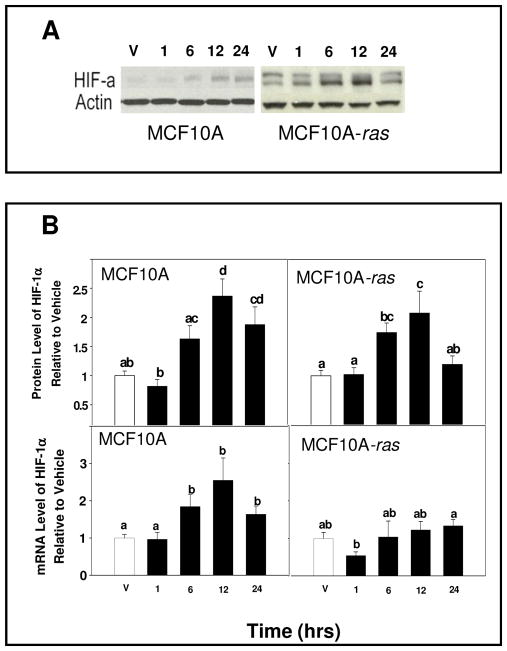
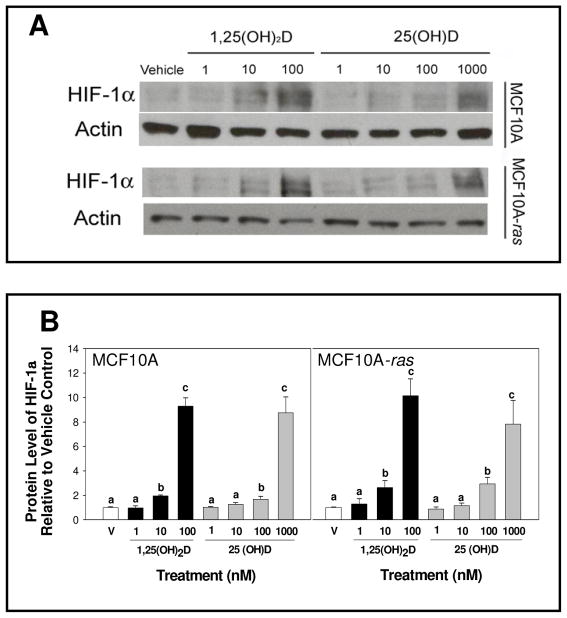
To determine if 1,25(OH)2D alters HIF-1α expression in MCF10A and MCF10A-ras cells, cells were treated with 1,25(OH)2D (10 nM) and mRNA abundance and relative protein level were determined. Following 1,25(OH)2D treatment of MCF10A and MCF10A-ras cells, the protein level of HIF-1α was increased over vehicle within 12 h (Figure 1A and B). In MCF10A cells, the HIF-1α protein level remained elevated while in MCF10A-ras cells, the HIF-1α protein level returned to baseline level after 24 h (Figure 1A and B). In addition, 1,25(OH)2D induction of HIF-1α protein after 12 h in both cells lines was observed in MCF10A and MCF10A-ras cells under hypoxic condition (data not shown). HIF-1α mRNA abundance was increased in MCF10A cells compared to vehicle within 12 h (Figure 1B). In contrast, HIF-1α mRNA was not changed in MCF10A-ras cells at 12 h (Figure 1B). Both 1,25(OH)2D and 25(OH) D treatment increased HIF-1α protein dose-dependently in MCF10A and MCF10A-ras cells (Figure 2). In both cell lines, the concentration at which 25(OH) D induced HIF-1α protein expression is only 10 fold higher than the level of 1,25(OH)2D that induced HIF-1α protein expression, suggesting that the 1α-hydroxylase to convert 25(OH) D to 1,25(OH)2D is active in the cells. These results indicate that 1,25(OH)2D stimulated both gene and protein levels of HIF-1α in MCF10A cells. In contrast, 1,25(OH)2D treatment induced a transient increase in HIF-1α protein level without a concurrent increase in mRNA abundance in MCF10A-ras cells.
Figure 1. 1,25(OH)2D regulation of HIF-1α protein and mRNA levels.
MCF10A and MCF10A-ras cells were treated with either vehicle (open bars) for 12hrs or with 1,25(OH)2D (10 nM) (black bars) for indicated times. Following harvest, lysates were used for either Western blot analysis or mRNA isolation as described. A representative immunoblot of HIF-1α and actin protein level is shown in panel A. The quantification of the immunoblots is shown in panel B (top left) for MCF10A cells and MCF10A-ras cells (panel B, top right). Values are expressed as HIF-1α protein/actin protein for each sample relative to vehicle. The mRNA abundance of HIF-1α, determined by RT-PCR is shown in panel B (bottom right) for MCF10A cells and MCF10A-ras cells (panel B, bottom left). Values are expressed as HIF-1α mRNA/18S mRNA. Results are expressed as mean ± SEM. (n ≥ 5). Bars with common letter superscripts are not significantly different (p< 0.05). Data are representative of three independent experiments.
Figure 2. Does dependent induction of HIF-1α protein by 1,25(OH)2D and 25(OH) D.
MCF10A and MCF10A-ras cells were treated with either vehicle (open bars) or different doses of 1,25(OH)2D (black bars) or 25(OH) D (gray bars) for 12 h. Following harvest, lysates were used for Western blot analysis. A representative immunoblot of HIF-1α and actin protein level is shown in panel A. The quantification of the immunoblots is shown in panel B for MCF10A cells (left) and MCF10A-ras cells (right). Values are expressed as HIF-1α protein/actin protein for each sample relative to vehicle. Bars with common letter superscripts are not significantly different (p< 0.05). Data are representative of three independent experiments.
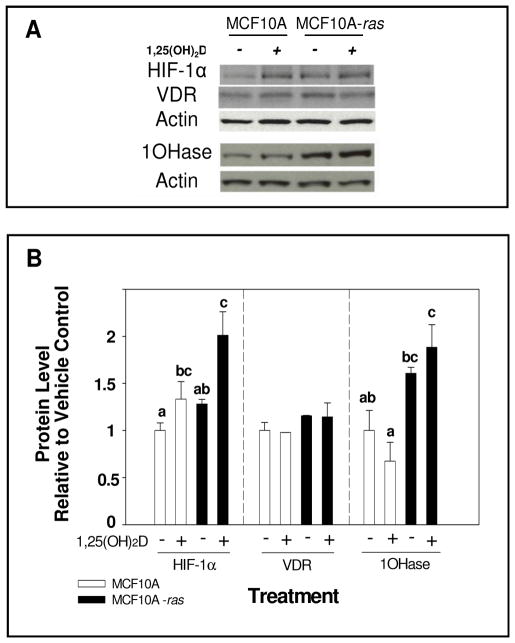
In order to determine if the level of VDR protein is a mechanism underlying the differential responses of HIF-1α mRNA abundance to 1,25(OH)2D treatment between the two cell types, the level of VDR protein was assessed. Although 1,25(OH)2D treatment induced HIF-1α protein accumulation in both cell types, the basal HIF-1α protein levels were similar between the two cell types (Figure 3A and B). Further, neither the basal nor 1,25(OH)2D stimulated VDR protein level were different in the MCF10A or MCF10A-ras cells (Figure 3A and B). In addition, the protein levels of 1α-hydroxylase were investigated in both cells. MCF10A-ras cells expressed significantly higher level of 1α-hydroxylase compared to MCF10A cells. However, 1,25(OH)2D did not significantly induce 1α-hydroxylase protein compared to vehicle in either cell line (Figure 3A and B).
Figure 3. HIF-1α, VDR and 1α-hydroxylase protein level in MCF10A and MCF10A-ras cells.
MCF10A and MCF10A-ras cells were treated for 12 h with vehicle or 1,25(OH)2D (10 nM). A representative immunoblot of HIF-1α, VDR, 1α-hydroxylase (1OHase) and actin protein levels are shown in panel A. The quantification of the immunoblots is shown in panel B. Open bars represent MCF10A cells and black bars represent MCF10A-ras cells. Values are expressed as protein/actin protein for each sample relative to vehicle. Results are expressed as mean ± SEM. (n ≥ 5). Bars with common letter superscripts are not significantly different (p< 0.05). Data are representative of three independent experiments.
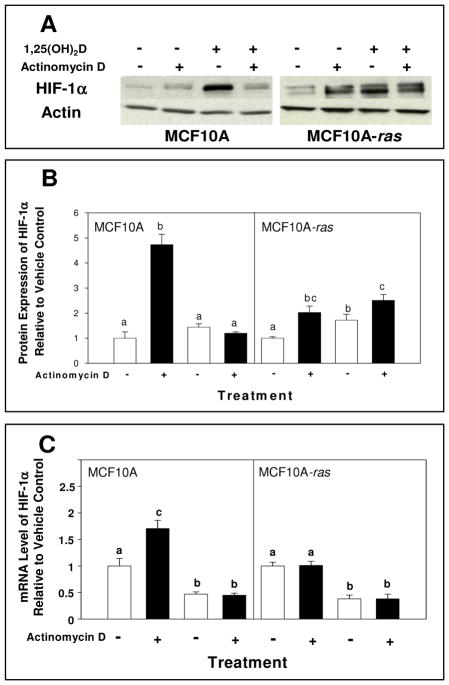
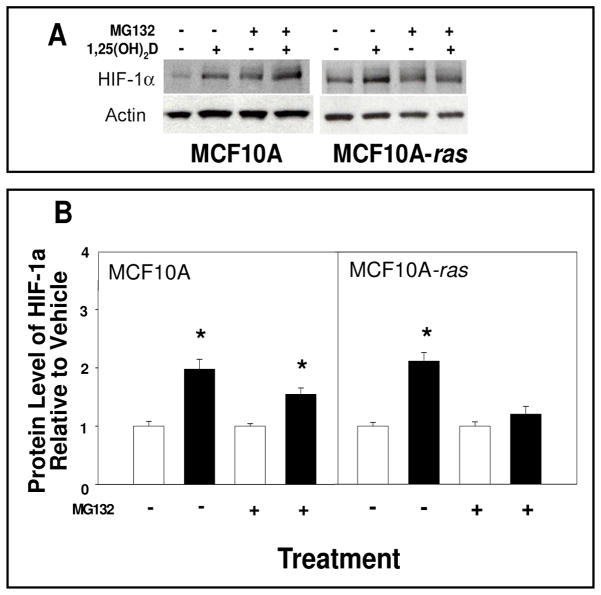
The potential mechanisms through which 1,25(OH)2D induces HIF-1α in MCF10A cells and MCF10A-ras cells were explored. In MCF10A cells, the 1,25(OH)2D induction of HIF-1α protein level was suppressed in the presence of a transcriptional inhibitor, with no effect in the MCF10A-ras cells (Figure 4A and 4B). The 1,25(OH)2D induced mRNA level of HIF-1α was also prevented by transcriptional inhibitor in MCF10A cells (Figure 4C). Furthermore, a proteasomal inhibitor prevented the 1,25(OH)2D induction of HIF-1α protein level in MCF10A-ras cells, but not in untransformed MCF10A cells (Figure 5).
Figure 4. Effects of inhibition of transcription on 1,25(OH)2D-induced HIF-1α protein and mRNA levels in MCF10A and MCF10A-ras cells.
MCF10A and MCF10A-ras cells were pre-treated for 2 h with actinomycin D (2 mg/mL) followed by a 12 h treatment with either vehicle or 1,25(OH)2D (10 nM). Following harvest, lysates were used for Western blot analysis or mRNA isolation. A representative immunoblot of HIF-1α and actin protein level is shown in panel A. The quantification of the immunoblots is shown in panel B and mRNA level of HIF-1α is shown in panel C, indicated with vehicle (open bars) and 1,25(OH)2D treatment (black bars). Values are expressed as HIF-1α protein/actin protein or HIF-1α mRNA relative to 18S mRNA for each sample relative to vehicle control. Results are expressed as mean ± SEM. (n ≥ 5). Bars with common letter superscripts are not significantly different (p< 0.05). Data are representative of three independent experiments.
Figure 5. Effects of inhibition of protein degradation on 1,25(OH)2D-induced HIF-1α protein level in MCF10A and MCF10A-ras cells.
MCF10A and MCF10A-ras cells were treated with either vehicle or 1,25(OH)2D (10 nM) with or without MG132 (1 mM) for 12 h. A representative immunoblot of HIF-1α and actin protein level is shown in panel A. The quantification of the immunoblots is shown in panel B indicated with vehicle (open bars) and 1,25(OH)2D treatment (black bars). Values are expressed as HIF-1α protein/actin protein for each sample relative to vehicle control. Results are expressed as mean ± SEM. (n ≥ 5). Asterisk indicates significant differences between vehicle and 1,25(OH)2D treatments. (p< 0.05). Data are representative of three independent experiments.
Discussion
In the current study, the effect of 1,25(OH)2D treatment on HIF-1α protein level was explored in untransformed and H-ras oncogene-transfected MCF10A cells, a human breast epithelial cell line and a model for breast cancer progression. The results show that 1,25(OH)2D increased HIF-1α protein level in untransformed MCF10A cells dose dependently within 12 hours, which was maintained at 24 hours, through regulation of gene transcription. On the other hand, results suggest that 1,25(OH)2D induced HIF-1α protein level at 12 h in a dose dependent manner, through regulation of protein degradation and independent of regulation of gene transcription, with subsequent down regulation at 24 h in H-ras transfected MCF10A cells. These results suggest that 1,25(OH)2D regulates the protein expression of HIF-1α by different mechanisms in untransformed and H-ras transfected MCF10A cells.
The activity of HIF-1 is primarily regulated by changes in the HIF-1α isoform protein expression. HIF-1α protein expression is regulated by multiple mechanisms including gene transcription, mRNA stability and protein stability level [12]. In addition, Ben-Shoshan and colleagues found that 1,25(OH)2D reduced the levels of HIF-1α protein in a dose-dependent manner in prostate cancer cell lines and colon cancer cells [7]. In the current studies, 1,25(OH)2D increased HIF-1α mRNA and protein expression compared to vehicle control at 12 h in MCF10A cells. Further, the transcriptional inhibitor eliminated 1,25(OH)2D-induced HIF-1α protein and inhibition of protein degradation did not prevent 1,25(OH)2D-induced HIF-1α protein and mRNA. These results indicate that transcriptional regulation of HIF-1α protein by 1,25(OH)2D in MCF10A cells is likely mediated through the VDR, potentially via vitamin D response elements (VDRE). Our results are consistent with the results of Wang et al., who identified a putative VDRE in the HIF-1α promoter (−2910) and demonstrated VDR association with this site using chromatin immunoprecipitation, although the functionality of the site has not been confirmed [22].
On the other hand, previous results suggest that the H-ras oncogene reduces 1,25(OH)2D-induced transactivation of the VDR in keratinocytes and fibroblast cells [23; 24]. Results from our laboratory show that the oncogenic Ras protein interferes with 1,25(OH)2D mediated VDR transcriptional regulation through the PI3K signaling pathway to reduce DNA association of VDR/RXR complex [25]. Other evidence supports that phosphorylation of the RXR by the constitutively activated ERK1/2 pathway [26]. Consistent with this, in the current study, 1,25(OH)2D induced HIF-1α protein level within 12 h without a concurrent increase in mRNA levels and the increase was not altered by inhibition of transcription in the H-ras oncogene transfected MCF10A cells in contrast to the cells without the H-ras oncogene. Our results support an alternative mechanism of a transient 1,25(OH)2D induction of HIF-1α protein through protein degradation in H-ras containing cells. These results suggest the mechanism of 1,25(OH)2D regulation of HIF-1α accumulation in H-ras transfected MCF10A cells is likely through regulation of HIF-1α protein stability, which is similar to the effects of growth factors on activation of HIF-1α at a posttransciptional level in cancer cells [27; 28].
The signaling pathways that oncogenic ras activates seem to be cell type dependent, including phosphatidylinositol 3-kinase/Akt pathway and the Raf/MEK/ERK pathways [29; 30]. Activation of ras/ERK signaling has been shown to increase HIF-1α transactivation in cancer cells without altering mRNA level of HIF-1α [31]. In the current study, there was no significant difference between HIF-1α protein levels in MCF10A cells compared to MCF10A-ras cells. However, the transcriptional inhibitor alone increased HIF-1α protein in MCF10A-ras cells while it did not alter HIF-1α protein in MCF10A cells, which indicates that activated ras protein may potentially reduce the cellular level of HIF-1α gene transcription in MCF10A cells. To our knowledge, this is the first evidence that supports that oncogenic Ras protein may down-regulate the gene transcription of HIF-1α during cancer progression. Further research is necessary to explore this observation.
The enzyme, 1α-hydroxylase, is expressed in other breast cells [32], however, to our knowledge, our results are the first to demonstrate the expression and potential activity of the 1α-hydroxylase in MCF10A cells. Our data supports not only 1α-hydroxylase is present in breast epithelial cells, but it may be functional in converting 25(OH) D to 1,25(OH)2D in breast tissue. In addition, the 1α-hydroxylase protein level was higher in MCF10A-ras cells, suggesting that with the presence of H-ras oncogene, 25(OH)D may be effectively activated.
The VDR protein expression varies during cancer progression. McCarthy et al. demonstrated that breast tumors from humans have higher VDR expression [33]. Studies with the VDR knockout mouse model show deletion of VDR gene alters the balance between proliferation and apoptosis in the mammary gland, which ultimately enhances its susceptibility to carcinogenesis [34; 35]. In the current study, there was no difference in VDR protein expression between MCF10A cells and H-ras transfected MCF10A cells. This discrepancy with the results from other cell lines or animal models may be due to the stage of cancer progression, or cell type specificity. Our results support that 1,25(OH)2D up-regulates HIF-1α through VDR transcriptional regulation in untransformed mammary epithelial cells but 1,25(OH)2D only induces HIF-1α protein transiently, independent of transcription in premalignant cells, although VDR protein levels are similar between the two cells types. These results suggest that during cancer progression, alternative mechanisms may occur to regulate proteins. Further, the 1,25(OH)2D induction of HIF-1α is only transient in the ras-transfected cells, supporting that an improved vitamin D status would not induce HIF-1α expression during cancer progression.
Conclusions
Overall, the results of the current study support that 1,25(OH)2D upregulated the HIF-1α protein level in untransformed cells through transcriptional regulation. In contrast, the results suggest that 1,25(OH)2D only transiently induced HIF-1α protein expression in H-ras-transfected cells through increased protein stability. These results indicate that increasing local 1,25(OH)2D with higher vitamin D status, and thus higher serum 25(OH) D level, may enhance HIF-1α expression, which would aid in normal development. In addition, the results show that 1,25(OH)2D induces a transient increase in HIF-1α protein level through an alternative pathway involving protein stability in the ras-transfected cells compared to untransformed cells. These results also support that better vitamin D status may enhance HIF-1α protein level in untransformed tissue, but not in cells during cancer progression.
Acknowledgments
The authors thank Dr. Michael S. Kinch (Department of Basic Medical Sciences, Purdue University, West Lafayette, IN 47905, USA.) for providing MCF10-ras cell line as a gift. The work was supported by grant NIH RCA128770A from the National Institute of Health.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Yan Jiang, Email: Jiang19@purdue.edu.
Wei Zheng, Email: Zheng138@purdue.edu.
References
- 1.National Cancer Institute. Study Shows link Between Antibiotic Use and Increased Risk of Breast Cancer. U.S. National Institutes of Health; Dec 29, 2004. < http://www.cancer.gov/cancertopics/factsheet/breast-antibotic-link-qa>. [Google Scholar]
- 2.Hiatt RA, Krieger N, Lobaugh B, Drezner MK, Vogelman JH, Orentreich N. Prediagnostic serum vitamin D and breast cancer. J Natl Cancer Inst. 1998;90:461–3. doi: 10.1093/jnci/90.6.461. [DOI] [PubMed] [Google Scholar]
- 3.Janowsky EC, Lester GE, Weinberg CR, Millikan RC, Schildkraut JM, Garrett PA, Hulka BS. Association between low levels of 1,25-dihydroxyvitamin D and breast cancer risk. Public Health Nutr. 1999;2:283–91. doi: 10.1017/s1368980099000385. [DOI] [PubMed] [Google Scholar]
- 4.Garland CF, Garland FC, Gorham ED, Lipkin M, Newmark H, Mohr SB, Holick MF. The role of vitamin D in cancer prevention. Am J Public Health. 2006;96:252–61. doi: 10.2105/AJPH.2004.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garland CF, Gorham ED, Mohr SB, Grant WB, Giovannucci EL, Lipkin M, Newmark H, Holick MF, Garland FC. Vitamin D and prevention of breast cancer: pooled analysis. J Steroid Biochem Mol Biol. 2007;103:708–11. doi: 10.1016/j.jsbmb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Garland CF, Gorham ED, Mohr SB, Garland FC. Vitamin D for cancer prevention: global perspective. Ann Epidemiol. 2009;19:468–83. doi: 10.1016/j.annepidem.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Ben-Shoshan M, Amir S, Dang DT, Dang LH, Weisman Y, Mabjeesh NJ. 1alpha,25-dihydroxyvitamin D3 (Calcitriol) inhibits hypoxia-inducible factor-1/vascular endothelial growth factor pathway in human cancer cells. Mol Cancer Ther. 2007;6:1433–9. doi: 10.1158/1535-7163.MCT-06-0677. [DOI] [PubMed] [Google Scholar]
- 8.Vleugel MM, Greijer AE, Shvarts A, van der Groep P, van Berkel M, Aarbodem Y, van Tinteren H, Harris AL, van Diest PJ, van der Wall E. Differential prognostic impact of hypoxia induced and diffuse HIF-1alpha expression in invasive breast cancer. J Clin Pathol. 2005;58:172–7. doi: 10.1136/jcp.2004.019885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Semenza GL. HIF-1: using two hands to flip the angiogenic switch. Cancer Metastasis Rev. 2000;19:59–65. doi: 10.1023/a:1026544214667. [DOI] [PubMed] [Google Scholar]
- 10.Blancher C, Moore JW, Talks KL, Houlbrook S, Harris AL. Relationship of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha expression to vascular endothelial growth factor induction and hypoxia survival in human breast cancer cell lines. Cancer Res. 2000;60:7106–13. [PubMed] [Google Scholar]
- 11.Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994;269:23757–63. [PubMed] [Google Scholar]
- 12.Lee JW, Bae SH, Jeong JW, Kim SH, Kim KW. Hypoxia-inducible factor (HIF-1)alpha: its protein stability and biological functions. Exp Mol Med. 2004;36:1–12. doi: 10.1038/emm.2004.1. [DOI] [PubMed] [Google Scholar]
- 13.Pugh CW, Gleadle J, Maxwell PH. Hypoxia and oxidative stress in breast cancer. Hypoxia signalling pathways. Breast Cancer Res. 2001;3:313–7. doi: 10.1186/bcr313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan DA, Giaccia AJ. Hypoxia, gene expression, and metastasis. Cancer Metastasis Rev. 2007;26:333–9. doi: 10.1007/s10555-007-9063-1. [DOI] [PubMed] [Google Scholar]
- 15.Semenza GL, Agani F, Booth G, Forsythe J, Iyer N, Jiang BH, Leung S, Roe R, Wiener C, Yu A. Structural and functional analysis of hypoxia-inducible factor 1. Kidney Int. 1997;51:553–5. doi: 10.1038/ki.1997.77. [DOI] [PubMed] [Google Scholar]
- 16.Giatromanolaki A, Koukourakis MI, Simopoulos C, Polychronidis A, Gatter KC, Harris AL, Sivridis E. c-erbB-2 related aggressiveness in breast cancer is hypoxia inducible factor-1alpha dependent. Clin Cancer Res. 2004;10:7972–7. doi: 10.1158/1078-0432.CCR-04-1068. [DOI] [PubMed] [Google Scholar]
- 17.van der Groep P, Bouter A, Menko FH, van der Wall E, van Diest PJ. High frequency of HIF-1alpha overexpression in BRCA1 related breast cancer. Breast Cancer Res Treat. 2008;111:475–80. doi: 10.1007/s10549-007-9817-z. [DOI] [PubMed] [Google Scholar]
- 18.Chun YS, Kim MS, Park JW. Oxygen-dependent and -independent regulation of HIF-1alpha. J Korean Med Sci. 2002;17:581–8. doi: 10.3346/jkms.2002.17.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Uden P, Kenneth NS, Rocha S. Regulation of hypoxia-inducible factor-1alpha by NF-kappaB. Biochem J. 2008;412:477–84. doi: 10.1042/BJ20080476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mantell DJ, Owens PE, Bundred NJ, Mawer EB, Canfield AE. 1 alpha,25-dihydroxyvitamin D(3) inhibits angiogenesis in vitro and in vivo. Circ Res. 2000;87:214–20. doi: 10.1161/01.res.87.3.214. [DOI] [PubMed] [Google Scholar]
- 21.Zantek ND, Walker-Daniels J, Stewart J, Hansen RK, Robinson D, Miao H, Wang B, Kung HJ, Bissell MJ, Kinch MS. MCF-10A-NeoST: a new cell system for studying cell-ECM and cell-cell interactions in breast cancer. Clin Cancer Res. 2001;7:3640–8. [PubMed] [Google Scholar]
- 22.Wang TT, Tavera-Mendoza LE, Laperriere D, Libby E, MacLeod NB, Nagai Y, Bourdeau V, Konstorum A, Lallemant B, Zhang R, Mader S, White JH. Large-scale in silico and microarray-based identification of direct 1,25-dihydroxyvitamin D3 target genes. Mol Endocrinol. 2005;19:2685–95. doi: 10.1210/me.2005-0106. [DOI] [PubMed] [Google Scholar]
- 23.Solomon C, Sebag M, White JH, Rhim J, Kremer R. Disruption of vitamin D receptor-retinoid X receptor heterodimer formation following ras transformation of human keratinocytes. J Biol Chem. 1998;273:17573–8. doi: 10.1074/jbc.273.28.17573. [DOI] [PubMed] [Google Scholar]
- 24.Stedman L, Nickel KP, Castillo SS, Andrade J, Burgess JR, Teegarden D. 1,25-dihydroxyvitamin D inhibits vitamin E succinate-induced apoptosis in C3H10T1/2 cells but not Harvey ras-transfected cells. Nutr Cancer. 2003;45:93–100. doi: 10.1207/S15327914NC4501_11. [DOI] [PubMed] [Google Scholar]
- 25.Taber LM, Adams LS, Teegarden D. Mechanisms of nuclear vitamin D receptor resistance in Harvey-ras-transfected cells. J Nutr Biochem. 2009;20:629–37. doi: 10.1016/j.jnutbio.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solomon C, White JH, Kremer R. Mitogen-activated protein kinase inhibits 1,25-dihydroxyvitamin D3-dependent signal transduction by phosphorylating human retinoid X receptor alpha. J Clin Invest. 1999;103:1729–35. doi: 10.1172/JCI6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang BH, Jiang G, Zheng JZ, Lu Z, Hunter T, Vogt PK. Phosphatidylinositol 3-kinase signaling controls levels of hypoxia-inducible factor 1. Cell Growth Differ. 2001;12:363–9. [PubMed] [Google Scholar]
- 28.Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu MM, Simons JW, Semenza GL. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60:1541–5. [PubMed] [Google Scholar]
- 29.Rak J, Mitsuhashi Y, Sheehan C, Tamir A, Viloria-Petit A, Filmus J, Mansour SJ, Ahn NG, Kerbel RS. Oncogenes and tumor angiogenesis: differential modes of vascular endothelial growth factor up-regulation in ras-transformed epithelial cells and fibroblasts. Cancer Res. 2000;60:490–8. [PubMed] [Google Scholar]
- 30.Sodhi A, Montaner S, Miyazaki H, Gutkind JS. MAPK and Akt act cooperatively but independently on hypoxia inducible factor-1alpha in rasV12 upregulation of VEGF. Biochem Biophys Res Commun. 2001;287:292–300. doi: 10.1006/bbrc.2001.5532. [DOI] [PubMed] [Google Scholar]
- 31.Blancher C, Moore JW, Robertson N, Harris AL. Effects of ras and von Hippel-Lindau (VHL) gene mutations on hypoxia-inducible factor (HIF)-1alpha, HIF-2alpha, and vascular endothelial growth factor expression and their regulation by the phosphatidylinositol 3′-kinase/Akt signaling pathway. Cancer Res. 2001;61:7349–55. [PubMed] [Google Scholar]
- 32.Fischer D, Becker S, Cordes T, Bucker B, Diedrich K, Friedrich M, Salehin D, Thill M. Vitamin D-24-hydroxylase in benign and malignant breast tissue and cell lines. Anticancer Res. 2009;29:3641–5. [PubMed] [Google Scholar]
- 33.McCarthy K, Laban C, Bustin SA, Ogunkolade W, Khalaf S, Carpenter R, Jenkins PJ. Expression of 25-hydroxyvitamin D-1-alpha-hydroxylase, and vitamin D receptor mRNA in normal and malignant breast tissue. Anticancer Res. 2009;29:155–7. [PubMed] [Google Scholar]
- 34.Zinser GM, McEleney K, Welsh J. Characterization of mammary tumor cell lines from wild type and vitamin D3 receptor knockout mice. Mol Cell Endocrinol. 2003;200:67–80. doi: 10.1016/s0303-7207(02)00416-1. [DOI] [PubMed] [Google Scholar]
- 35.Zinser GM, Welsh J. Effect of Vitamin D3 receptor ablation on murine mammary gland development and tumorigenesis. J Steroid Biochem Mol Biol. 2004;89–90:433–6. doi: 10.1016/j.jsbmb.2004.03.012. [DOI] [PubMed] [Google Scholar]